Supercritical Extraction of Ylang Ylang (Cananga odorata) Essential Oil at the Near-Critical Region
Abstract
1. Introduction
2. Materials and Methods
2.1. Preparation of Raw Material
2.2. Measurement of Moisture Content
2.3. Extraction Unit
2.4. Experimental Program
2.5. Component Analysis
3. Experimental Results
3.1. Moisture Content of Flowers
3.2. Experiments without Co-Solvent
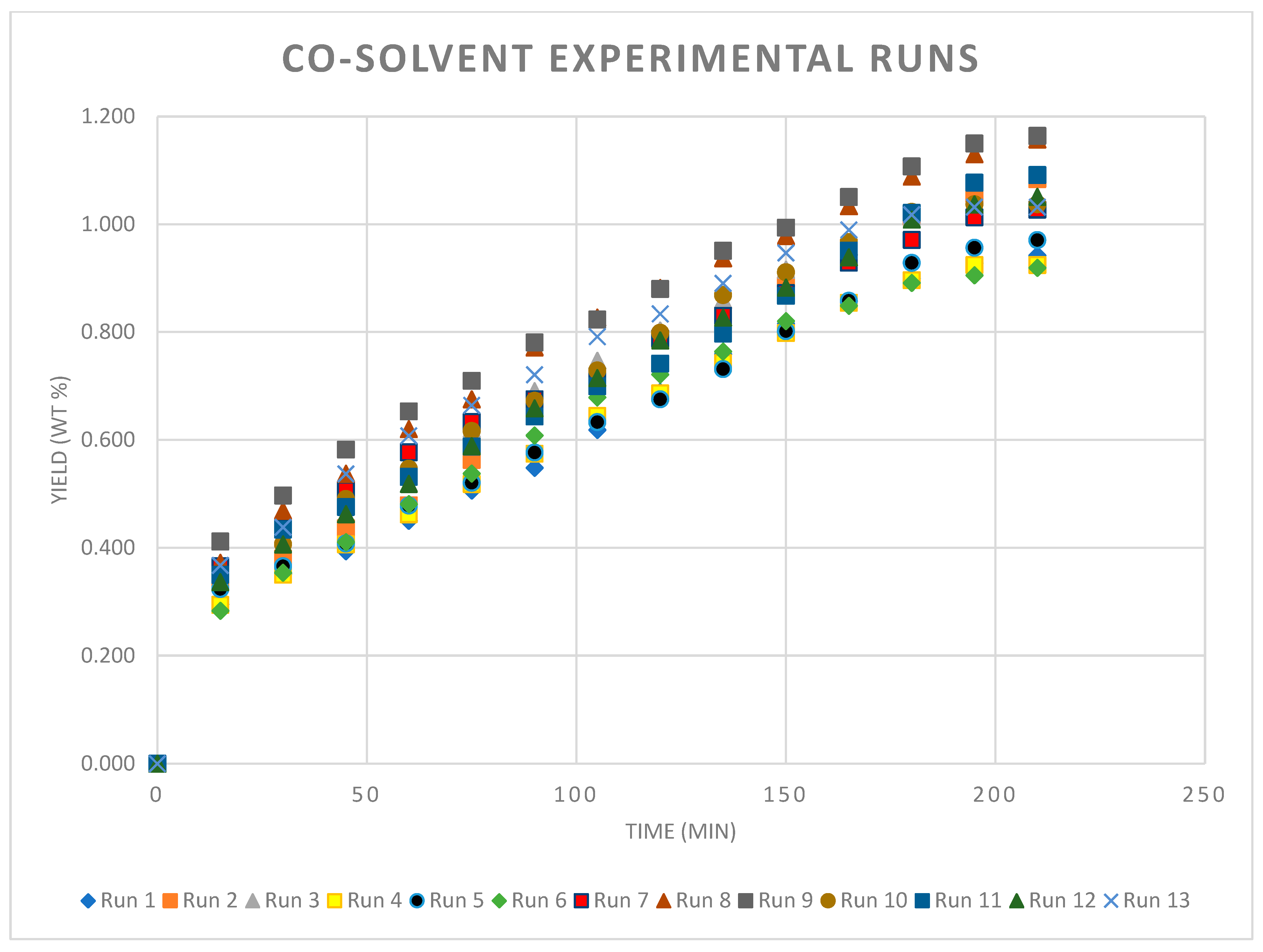
3.3. With Co-Solvent
4. Discussion
4.1. Extraction Curves without Co-Solvent
4.2. Extraction Curves with Co-Solvent
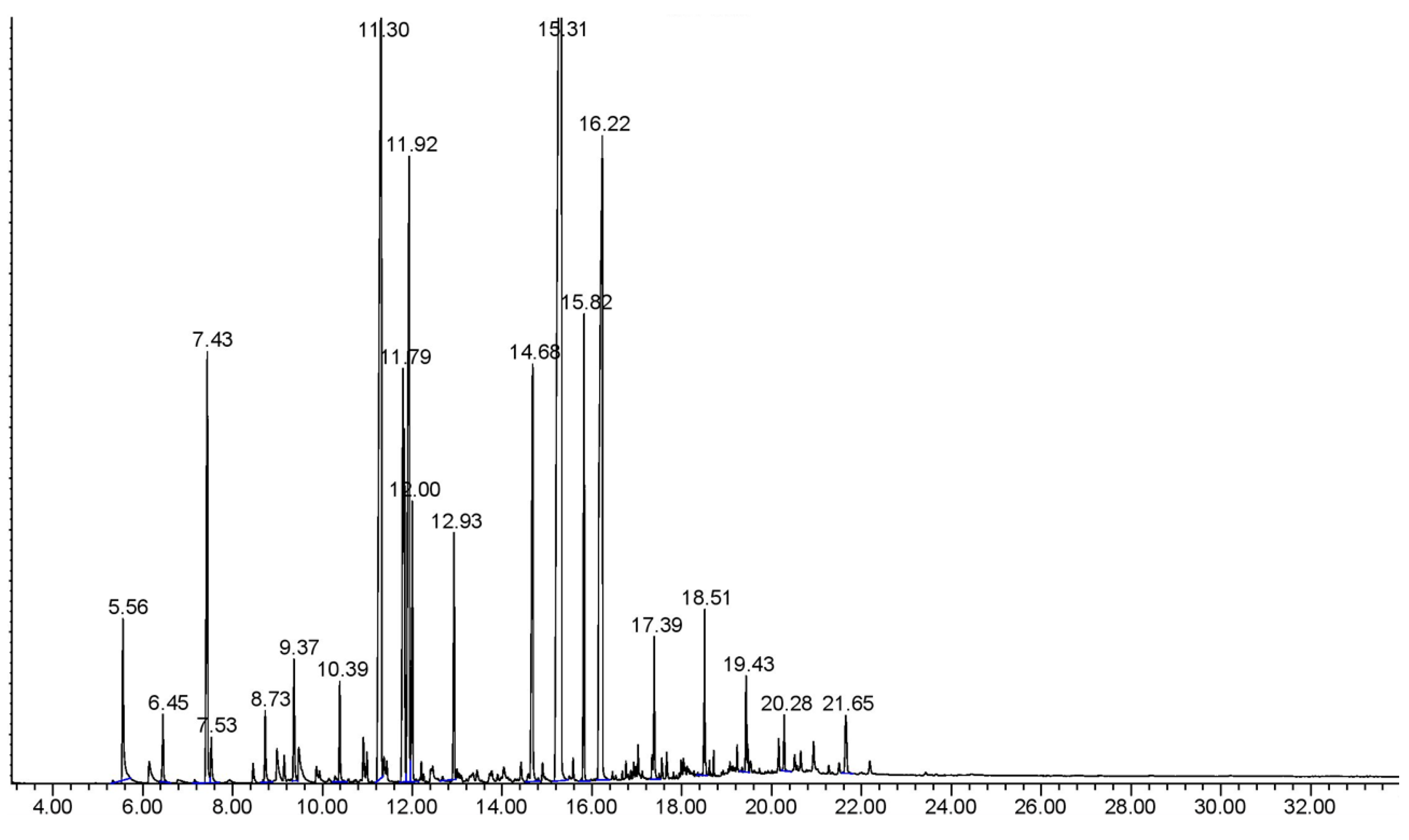
4.3. Comparison of Extract Compositions
4.4. Comparisons with SFE at Higher Pressures
4.5. Potential Commercialization
- (1)
- Larger amounts of extracts be produced for the purpose of making up potentially commercial perfume samples for evaluation and market testing.
- (2)
- An economic feasibility evaluation be carried out to determine the capital and operating costs. This should then be compared to the costs of operating the traditional steam distillation extraction to evaluate if extraction at or around the critical point is commercially viable.
5. Conclusions and Recommendations
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Pavela, R.; Maggi, F.; Giordani, C.; Cappellacci, L.; Petrelli, R.; Canale, A. Insecticidal activity of two essential oils used in perfumery (ylang-ylang and frankincense). Nat. Prod. Res. 2021, 35, 4746–4752. [Google Scholar] [CrossRef] [PubMed]
- Tan, L.T.H.; Lee, L.H.; Yin, W.F.; Chan, C.K.; Kadir, H.A.; Chan, K.G.; Goh, B.H. Traditional Uses, Phytochemistry, and Bioactivities of Cananga odorata (Ylang-Ylang). In Evidence-Based Complementary and Alternative Medicine; Hindawi Publishing Corporation: London, UK, 2015; pp. 1–31. [Google Scholar] [CrossRef]
- Parrotta, J.A. Cananga odorata; Wiley-VCH Verlag CmbH & Co.: Weinheim, Germany, 2014; pp. 1–8. [Google Scholar]
- Zhang, Q.W.; Lin, L.G.; Ye, W.C. Techniques for extraction and isolation of natural products: A comprehensive review. Chin. Med. 2018, 13, 20. [Google Scholar] [CrossRef] [PubMed]
- Alexander, P.E.-V.; Galatro, D.; Gonzalez, Y.; Angulo, W.; Álava-Intriago, J.J.; Manzano, P.; Hernández, M.R. Modeling and optimization of the extraction of ylang-ylang essential oils using surrogate models from simulated data, coupled with covariance matrix adaptation evolution strategy. J. Food Eng. 2023, 357, 111637. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavor Materials of Natural Origin; The University of Michigan: Ann Arbor, MI, USA, 1960; p. 665. [Google Scholar]
- Seair Exim Solutions. Ylang Ylang Import Data in USA—Updated Shipment Details Report; Seair Exim Solutions: New Delhi, India, 2022. [Google Scholar]
- Mallavarapu, G.R.; Gurudutt, K.N.; Syamasundar, K.V. Chapter 99—Ylang–Ylang (Cananga odorata) Oils. In Essential Oils in Food Preservation, Flavor and Safety; Preedy, V.R., Ed.; Academic Press: San Diego, CA, USA, 2016; pp. 865–873. [Google Scholar]
- Stashenko, E.E.; Prada, N.Q.; Martínez, J.R. HRGC/FID/NPD and HRGGC/MSD study of Colombian ylang-ylang (Cananga odorata) oils obtained by different extraction techniques. J. High Resolut. Chromatogr. 1996, 19, 353–358. [Google Scholar] [CrossRef]
- Bjorklund, E.; Eskilsson, C.S. Supercritical Fluid Extraction. Encycl. Anal. Sci. 2005, 597–604. [Google Scholar] [CrossRef]
- Sanders, N. Food legislation and the scope for increased use of near-critical fluid extraction operations in the food, flavouring and pharmaceutical industries. In Extraction of Natural Products Using Near-Critical Solvents; King, M.B., Bott, T.R., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 34–49. [Google Scholar] [CrossRef]
- Watson, M.J. Supercritical Fluid Extraction of Ylang Ylang. PhD Thesis, University of the West Indies, Kingston, Jamaica, 2007. [Google Scholar]
- McGaw, D.; Watson, M.; Paltoo, V.; Changyen, I.; Grannum, J. A Comparison of Methods for the Extraction of the Fragrance from Ylang Ylang. In Proceedings of the Conference Paper: 6th International Symposium on Supercritical Fluids, Versailles, France, 28–30 April 2003. [Google Scholar]
- Hawthorne, S.B.; Rickkola, M.-L.; Screnius, K.; Holm, Y.; Hiltunen, R.; Hartonen, K. Comparison of hydrodistillation and supercritical fluid extraction for the determination of essential oils in aromatic plants. J. Chromatogr. A 1993, 634, 297–308. [Google Scholar] [CrossRef]
- Arctander, S. Perfume and Flavour Chemicals; Allured Publishing Corporation: Carol Stream, IL, USA, 1969; Volume 1. [Google Scholar]
- Buccellato, F. Ylang survey, Perfum. Flavor 1982, 7, 9–13. [Google Scholar]
- Moyler, D.A. Extraction of flavours and fragrances with compressed CO2. In Extraction of Natural Products Using Near-Critical Solvents; King, M.B., Bott, T.R., Eds.; Springer: Dordrecht, The Netherlands, 1993; pp. 140–183. [Google Scholar]


| Non-Co-Solvent Experimental Results Uncoded Variables | ||||
|---|---|---|---|---|
| Runs | Pressure (bar) | Temperature (°C) | Flowrate (L/min) | Yield (wt %) |
| 1 | 80 | 35 | 3 | 0.791 |
| 2 | 120 | 50 | 1.5 | 0.829 |
| 3 | 120 | 35 | 1.5 | 0.826 |
| 4 | 120 | 35 | 3 | 0.965 |
| 5 | 80 | 35 | 1.5 | 0.735 |
| 6 | 120 | 50 | 3 | 0.971 |
| 7 | 80 | 50 | 1.5 | 0.738 |
| 8 | 80 | 50 | 3 | 0.802 |
| Component | Retention Time (min) | Percentage Composition |
|---|---|---|
| Benzyl Alcohol | 5.55 | 0.66–2.90 |
| p-Cresol | 6.12 | 0.65–1.67 |
| Benzyl Acetate | 7.45 | 0.58–14.41 |
| Phenylethyl Acetate | 8.73 | 0.66–1.34 |
| Cinnamyl Acetate | 9.41 | 0.50–13.24 |
| Geranyl Acetate | 10.39 | 0.39–0.95 |
| Beta Caryophyllene | 11.00 | 0.35–1.22 |
| Gamma Cadinene | 11.81 | 6.67–9.82 |
| Isoeugenol | 11.92 | 1.17–5.24 |
| Farnesene | 12.00 | 1.34–4.54 |
| Ocimene | 12.03 | 2.84–7.34 |
| Cubebene | 12.22 | 3.15–9.84 |
| Bourbonene | 12.93 | 0.71–2.07 |
| Farnesol | 14.67 | 2.03–5.40 |
| Benzyl Benzoate | 15.34 | 12.76–24.14 |
| Farnesyl Acetate | 15.84 | 2.97–9.68 |
| Benzyl Salicylate | 16.20 | 12.16–16.15 |
| Co-Solvent Experimental Results Uncoded Variables | ||||
|---|---|---|---|---|
| Runs | Pressure (bar) | Temperature (°C) | Flowrate (L/min) | Yield (wt %) |
| 1 | 80 | 35 | 3 | 0.942 |
| 2 | 120 | 35 | 1.5 | 1.084 |
| 3 | 100 | 42.5 | 2.25 | 1.042 |
| 4 | 80 | 50 | 1.5 | 0.924 |
| 5 | 80 | 50 | 3 | 0.971 |
| 6 | 80 | 35 | 1.5 | 0.919 |
| 7 | 100 | 42.5 | 2.25 | 1.027 |
| 8 | 120 | 35 | 3 | 1.157 |
| 9 | 120 | 50 | 3 | 1.164 |
| 10 | 100 | 42.5 | 2.25 | 1.037 |
| 11 | 120 | 50 | 1.5 | 1.091 |
| 12 | 100 | 42.5 | 2.25 | 1.051 |
| 13 | 100 | 42.5 | 2.25 | 1.032 |
| Component | Retention Time (min) | Percentage Composition |
|---|---|---|
| p-Cresol | 6.12 | 0.13–1.35 |
| Benzyl Alcohol | 5.55 | 0.31–2.58 |
| Benzyl Acetate | 7.45 | 0.55–12.65 |
| Geranyl Acetate | 10.39 | 0.68–1.49 |
| Cinnamyl Acetate | 9.41 | 8.06–15.21 |
| Beta Caryophyllene | 11.00 | 0.30–2.85 |
| Cubebene | 12.22 | 0.70–15.45 |
| Gamma Cadinene | 11.81 | 0.23–22.57 |
| Isoeugenol | 11.92 | 0.47–7.73 |
| Ocimene | 12.03 | 0.25–5.84 |
| Farnesene | 12.00 | 1.75–16.52 |
| Bourbonene | 12.93 | 1.06–2.04 |
| Farnesol | 14.67 | 3.21–5.84 |
| Benzyl Benzoate | 15.34 | 17.43–36.62 |
| Farnesyl Acetate | 15.84 | 1.82–15.13 |
| Benzyl Salicylate | 16.20 | 7.50–18.16 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Mahabir, R.; Maharaj, S.; Watson, M.J.; McGaw, D.R.; Coonai, C. Supercritical Extraction of Ylang Ylang (Cananga odorata) Essential Oil at the Near-Critical Region. Separations 2024, 11, 295. https://doi.org/10.3390/separations11100295
Mahabir R, Maharaj S, Watson MJ, McGaw DR, Coonai C. Supercritical Extraction of Ylang Ylang (Cananga odorata) Essential Oil at the Near-Critical Region. Separations. 2024; 11(10):295. https://doi.org/10.3390/separations11100295
Chicago/Turabian StyleMahabir, Rodney, Sharad Maharaj, Marian J. Watson, David R. McGaw, and Cian Coonai. 2024. "Supercritical Extraction of Ylang Ylang (Cananga odorata) Essential Oil at the Near-Critical Region" Separations 11, no. 10: 295. https://doi.org/10.3390/separations11100295
APA StyleMahabir, R., Maharaj, S., Watson, M. J., McGaw, D. R., & Coonai, C. (2024). Supercritical Extraction of Ylang Ylang (Cananga odorata) Essential Oil at the Near-Critical Region. Separations, 11(10), 295. https://doi.org/10.3390/separations11100295





