Heavy Metal Removal from Water Using Graphene Oxide in Magnetic-Assisted Adsorption Systems: Characterization, Adsorption Properties, and Modelling
Abstract
1. Introduction
2. Materials and Methods
2.1. Synthesis of Graphene Oxide
2.2. Characterization of GO Samples
2.3. Heavy Metal Adsorption Studies with and without the Presence of a Magnetic Field
3. Results and Discussion
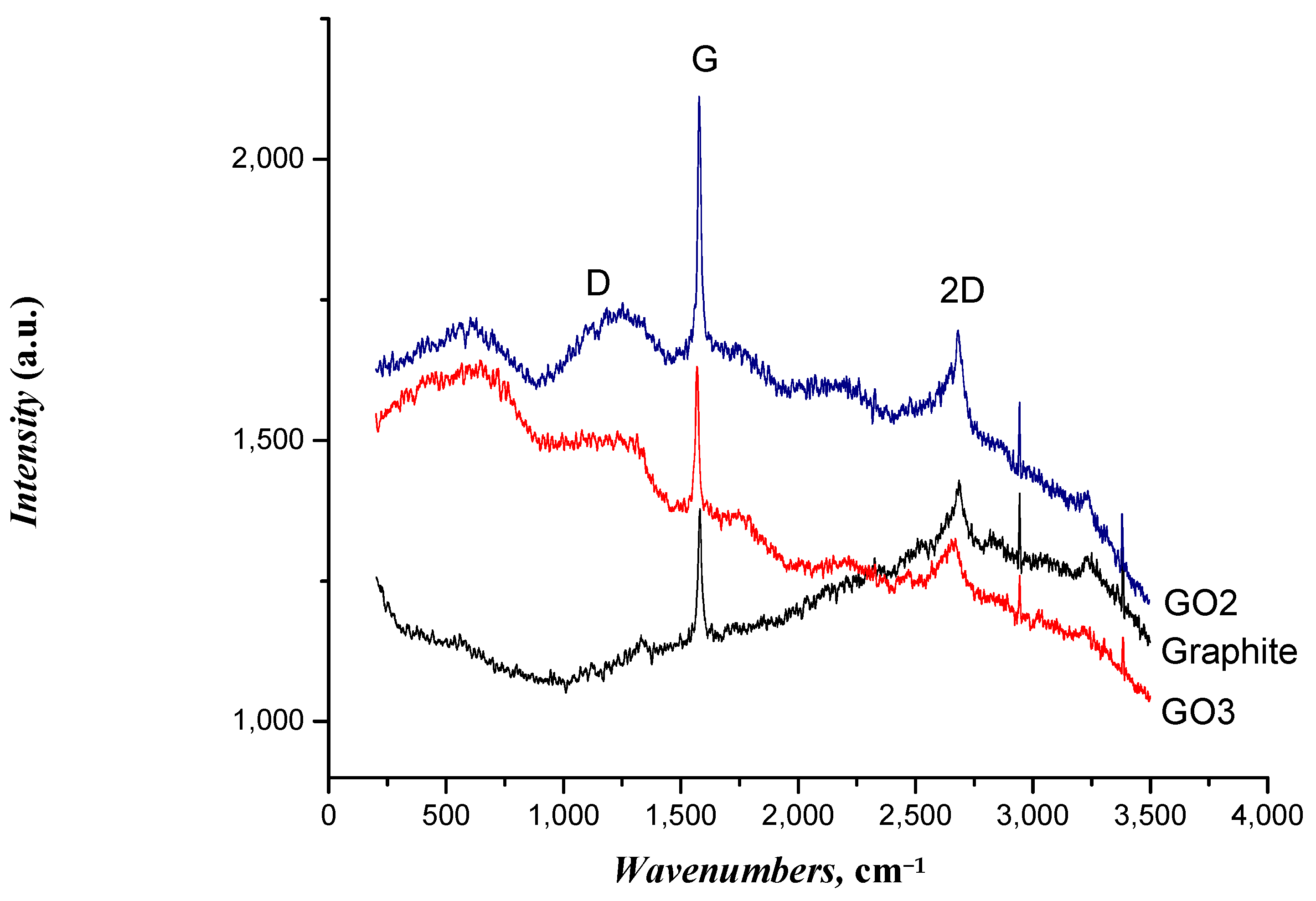
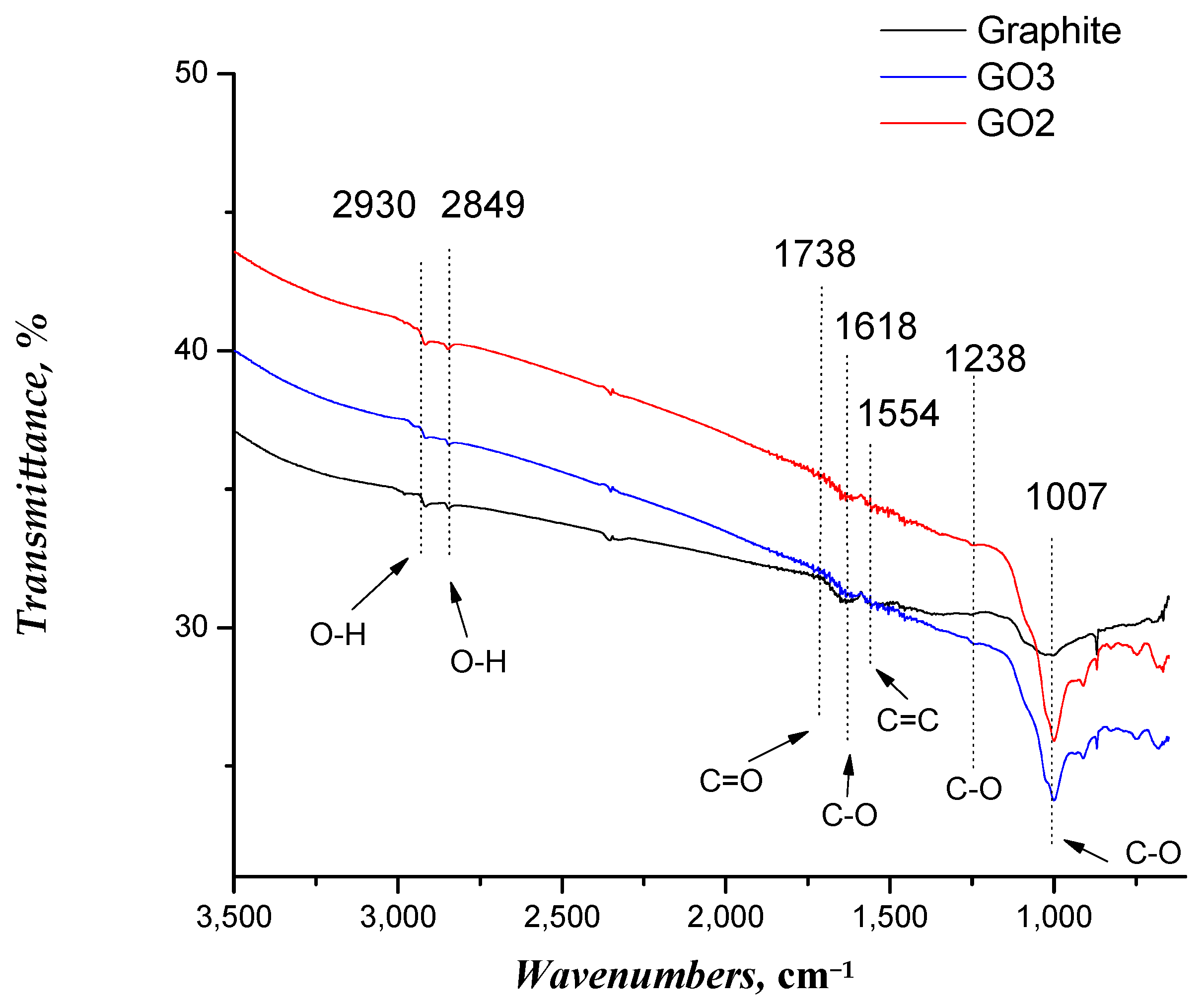
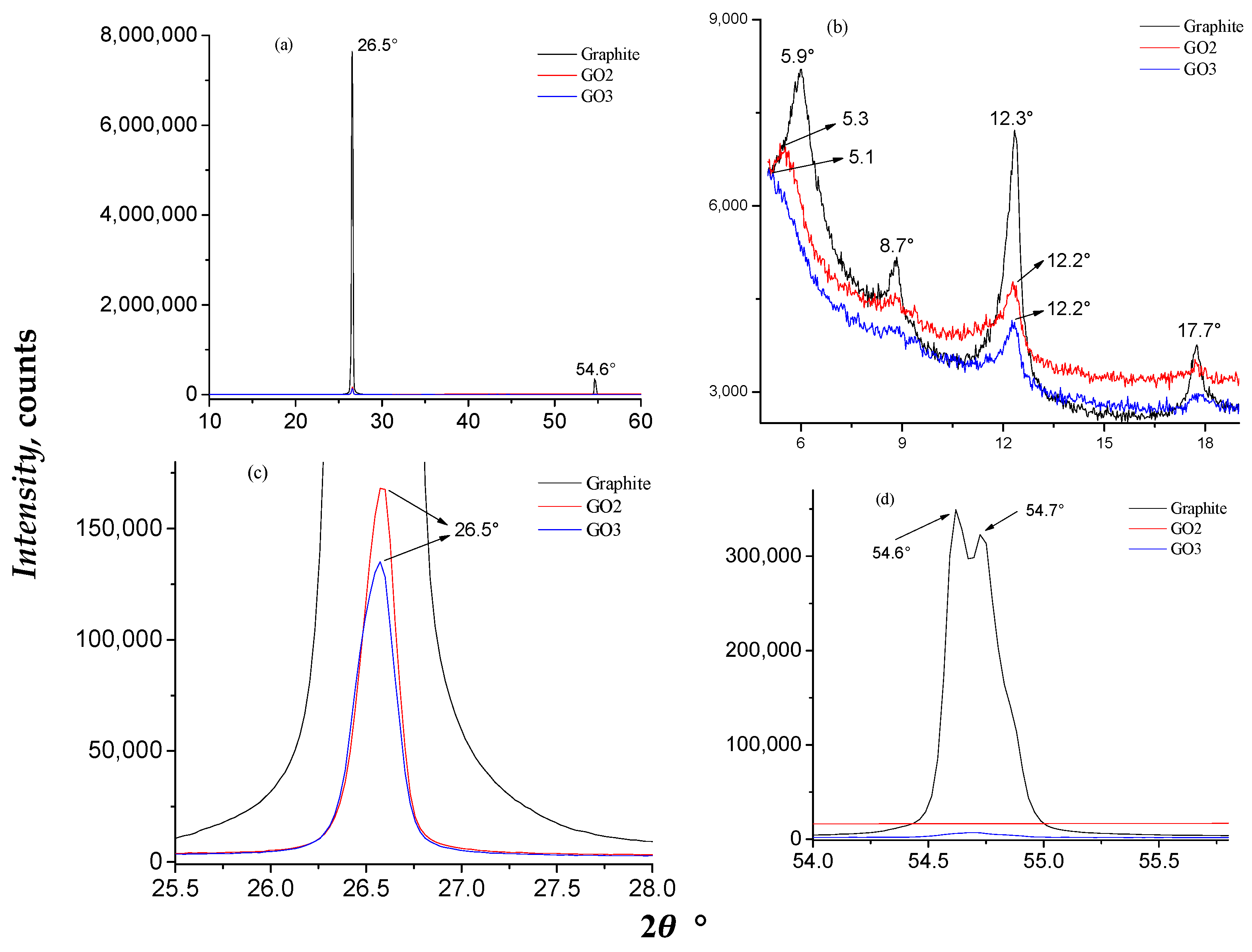
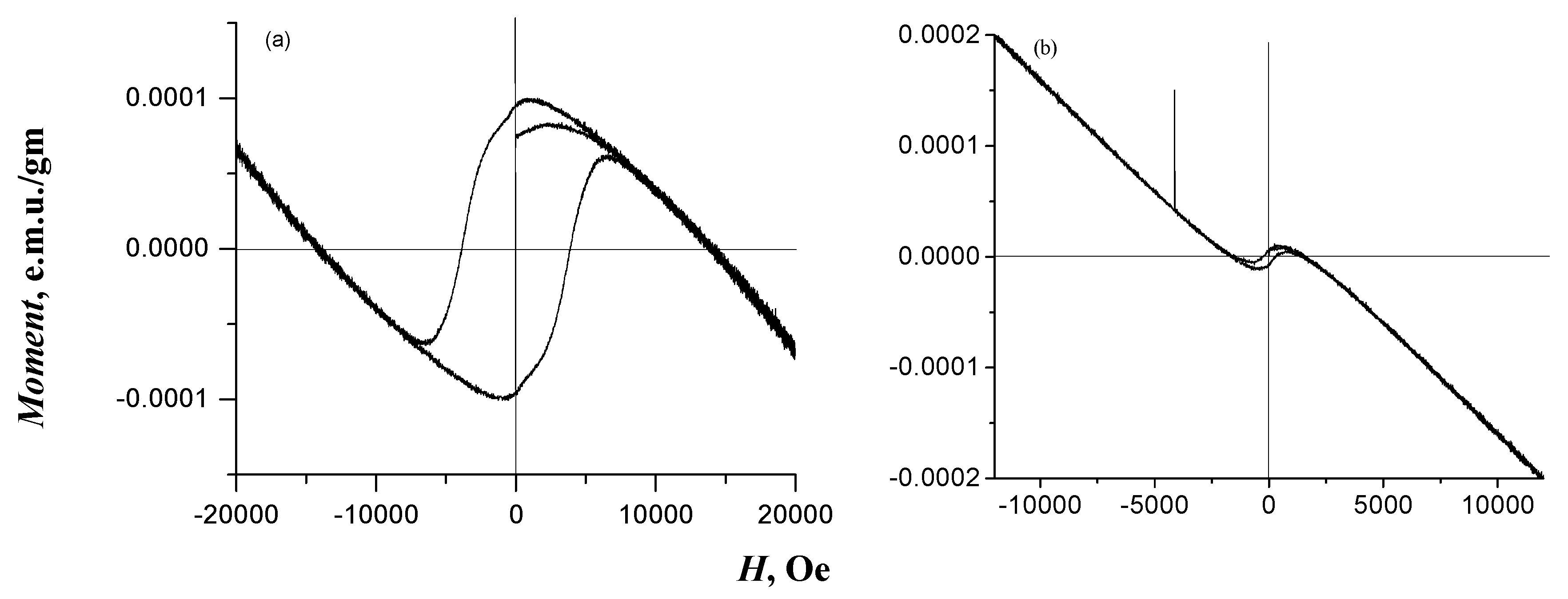
3.1. Characterization of GO Samples
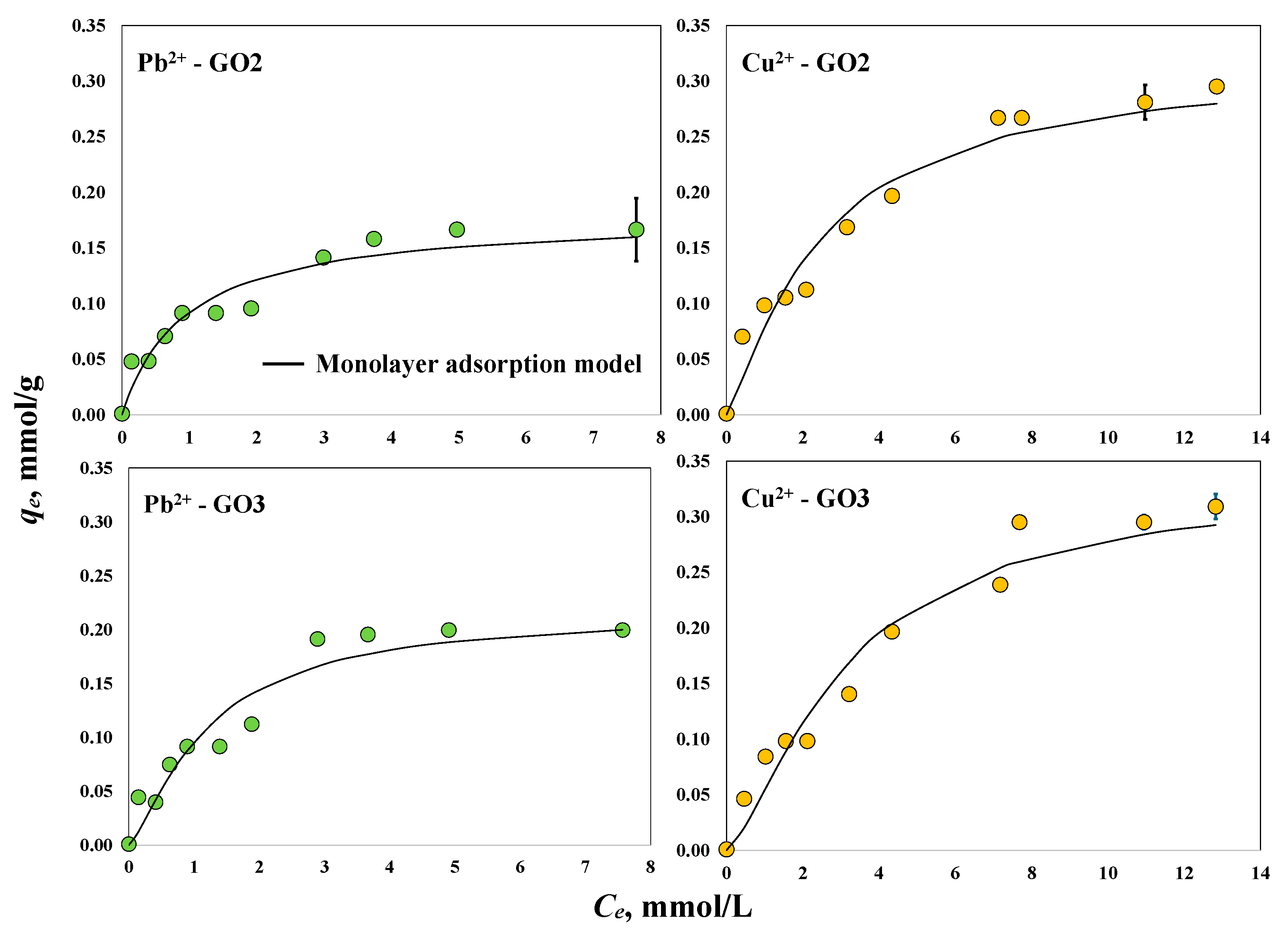
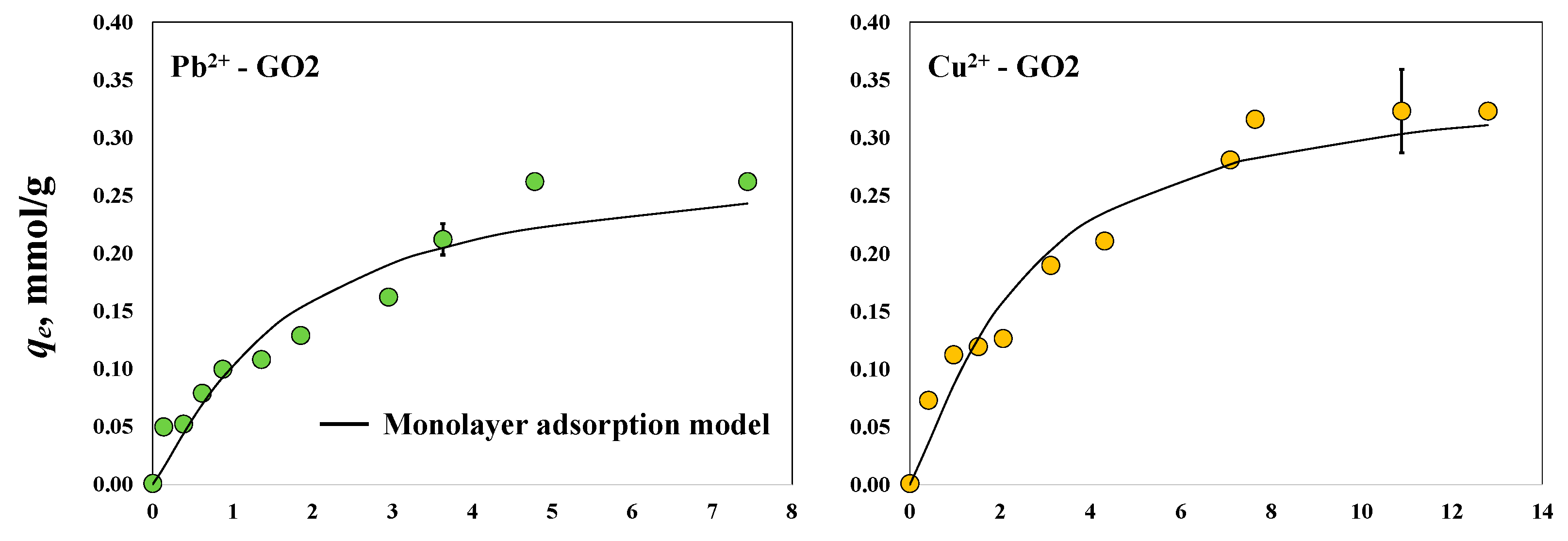
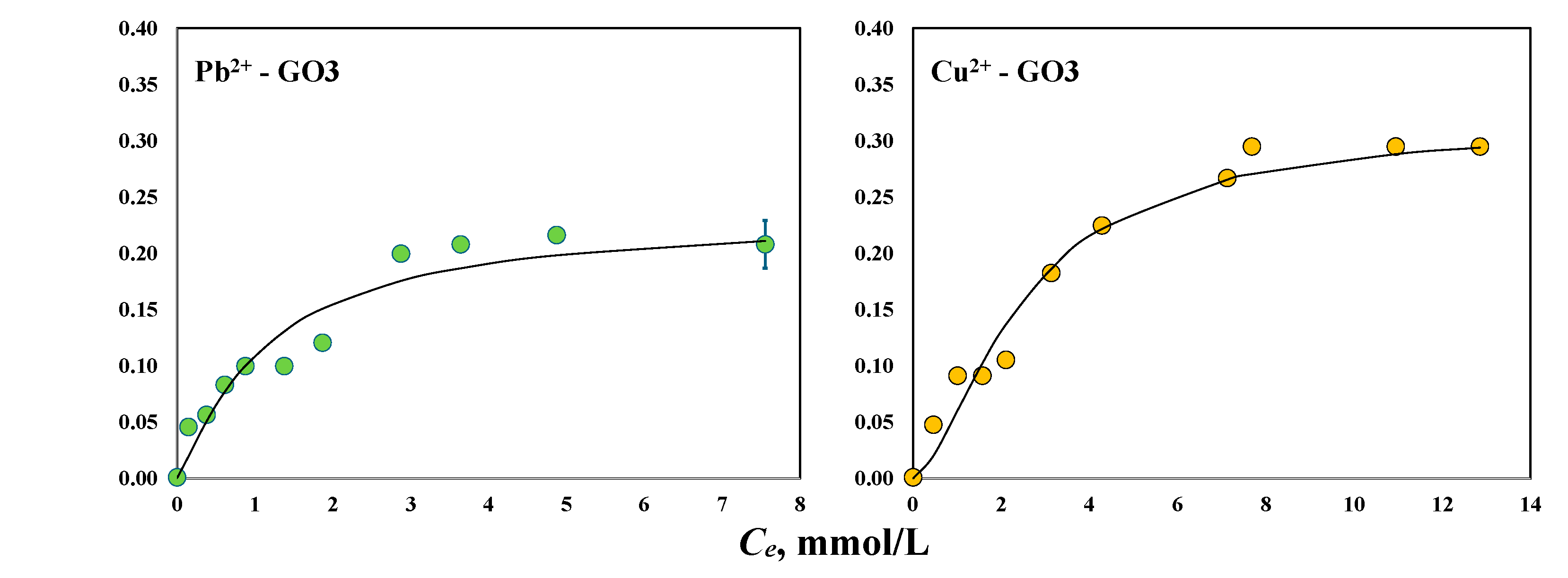
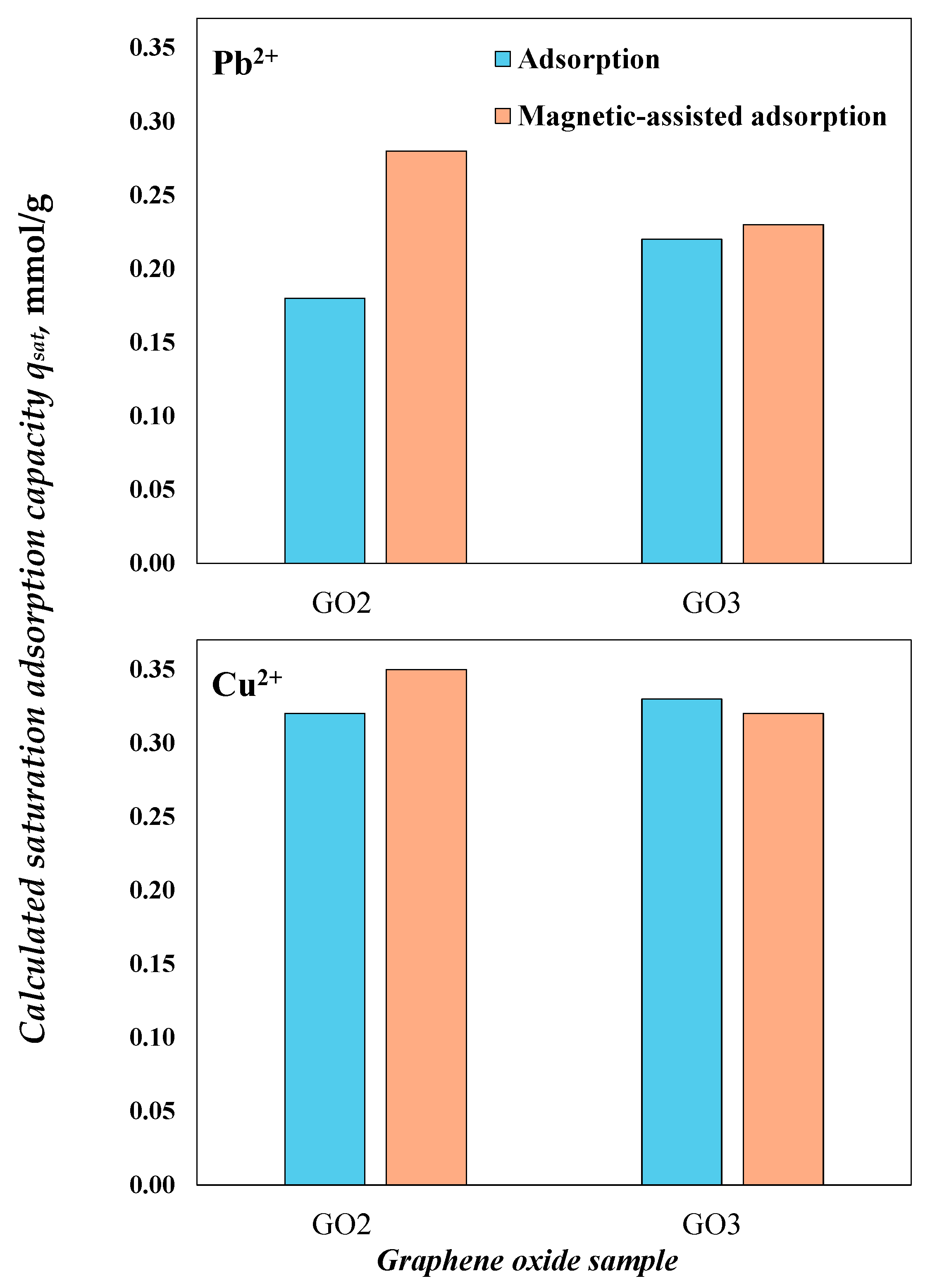
3.2. Adsorption Properties of GO Samples to Remove Pb2+and Cu2+ with and without Magnetic Field
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Ain, Q.U.; Farooq, M.U.; Jalees, M.I. Application of Magnetic Graphene Oxide for Water Purification: Heavy Metals Removal and Disinfection. J. Water Process Eng. 2020, 33, 101044. [Google Scholar] [CrossRef]
- Jin, J.; Sun, J.; Lv, K.; Huang, X.; Wang, J.; Liu, J.; Bai, Y.; Guo, X.; Zhao, J.; Liu, J.; et al. Magnetic-responsive CNT/chitosan composite as stabilizer and adsorbent for organic contaminants and heavy metal removal. J. Mol. Liq. 2021, 334, 116087. [Google Scholar] [CrossRef]
- Majdoub, M.; Amedlous, A.; Anfar, Z.; Jada, A.; El Alem, N. Engineering of amine-based binding chemistry on functionalized graphene oxide/alginate hybrids for simultaneous and efficient removal of trace heavy metals: Towards drinking water. J. Colloid Interface Sci. 2021, 589, 511–524. [Google Scholar] [CrossRef] [PubMed]
- Priya, V.N.; Rajkumar, M.; Mobika, J.; Sibi, S.P.L. Adsorption of As (V) ions from aqueous solution by carboxymethyl cellulose incorporated layered double hydroxide/reduced graphene oxide nanocomposites: Isotherm and kinetic studies. Environ. Technol. Innov. 2022, 26, 102268. [Google Scholar] [CrossRef]
- Abbasi, M.; Safari, E.; Baghdadi, M.; Janmohammadi, M. Enhanced adsorption of heavy metals in groundwater using sand columns enriched with graphene oxide: Lab-scale experiments and process modeling. J. Water Process Eng. 2021, 40, 101961. [Google Scholar] [CrossRef]
- Yao, M.; Wang, Z.; Liu, Y.; Yang, G.; Chen, J. Preparation of dialdehyde cellulose graftead graphene oxide composite and its adsorption behavior for heavy metals from aqueous solution. Carbohydr. Polym. 2019, 212, 345–351. [Google Scholar] [CrossRef]
- Xiao, X.; Yu, Y.; Sun, Y.; Zheng, X.; Chen, A. Heavy metal removal from aqueous solutions by chitosan-based magnetic composite flocculants. J. Environ. Sci. 2021, 108, 22–32. [Google Scholar] [CrossRef]
- Anuma, S.; Mishra, P.; Bhat, B.R. Polypyrrole functionalized Cobalt oxide Graphene (COPYGO) nanocomposite for the efficient removal of dyes and heavy metal pollutants from aqueous effluents. J. Hazard. Mater. 2021, 416, 125929. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, Y.G.; Mu, X.; Wu, D.B.; Ye, W.M. Graphene oxide-modified organic Gaomiaozi bentonite for Yb(III) adsorption from aqueous solutions. Mater. Chem. Phys. 2021, 274, 125176. [Google Scholar] [CrossRef]
- Chaabane, L.; Beyou, E.; El Ghali, A.; Baouab, M.H.V. Comparative studies on the adsorption of metal ions from aqueous solutions using various functionalized graphene oxide sheets as supported adsorbents. J. Hazard. Mater. 2020, 389, 121839. [Google Scholar] [CrossRef]
- Weng, X.; Wu, J.; Ma, L.; Owens, G.; Chen, Z. Impact of synthesis conditions on Pb(II) removal efficiency from aqueous solution by green tea extract reduced graphene oxide. Chem. Eng. J. 2019, 359, 976–981. [Google Scholar] [CrossRef]
- Bilal, M.; Ihsanullah, I.; Younas, M.; Shah, M.U.H. Recent advances in applications of low-cost adsorbents for the removal of heavy metals from water: A critical review. Sep. Purif. Technol. 2021, 278, 119510. [Google Scholar] [CrossRef]
- Ibebunjo, K.; El Ouardi, Y.; Bediako, J.K.; Iurchenkova, A.; Repo, E. Functionalization of recycled polymer and 3D printing into porous structures for selective recovery of copper from copper tailings. Chem. Eng. Sci. 2024, 286, 119664. [Google Scholar] [CrossRef]
- Wu, H.Y.; Wu, W.K.; Chen, B.; Kong, Q.P.; Lian, J.J. Co-adsorption of tetracycline and copper by the thiourea-modified porous sodium alginate microspheres. Desalination Water Treat. 2024, 317, 100096. [Google Scholar] [CrossRef]
- Abidli, A.; Huang, Y.; Rejeb, Z.B.; Zaoui, A.; Park, C.B. Sustainable and efficient technologies for removal and recovery of toxic and valuable metals from wastewater: Recent progress, challenges, and future perspectives. Chemosphere 2022, 292, 133102. [Google Scholar] [CrossRef]
- Duyen, L.T.; Bac, B.H. Adsorption–desorption behavior of halloysite clay for Cu2+ ions and recovery of copper by electrodeposition method. Desalination Water Treat. 2024, 317, 100207. [Google Scholar] [CrossRef]
- Kumaran, S.; Vetrivelan, V.; Muthu, S.; Al-Saadi, A.A. Computational analysis of anti-cancer drug hydroxyurea adsorption on nanocages of gold, silver and copper: SERS and DFT assessment. Heliyon 2024, 10, e24475. [Google Scholar] [CrossRef]
- Liu, C.; Yan, X.; Zhang, H.-X.; Yang, J.; Yoon, K.-B. Silicone-modified black peanut shell (BPS) biochar adsorbents: Preparation and their adsorptions for copper(II) from water. Heliyon 2024, 10, e35169. [Google Scholar] [CrossRef] [PubMed]
- Bao, S.; Wang, Y.; Yu, Y.; Yang, W.; Sun, Y. Cross-linked sulfydryl-functionalized graphene oxide as ultra-high capacity adsorbent for high selectivity and ppb level removal of mercury from water under wide pH range. Environ. Pollut. 2021, 271, 116378. [Google Scholar] [CrossRef]
- Bao, S.; Wang, Y.; Wei, Z.; Yang, W.; Yu, Y.; Sun, Y. Amino-assisted AHMT anchored on graphene oxide as high performance adsorbent for efficient removal of Cr(VI) and Hg(II) from aqueous solutions under wide pH range. J. Hazard. Mater. 2021, 416, 125825. [Google Scholar] [CrossRef]
- Wong, L.Y.; Lau, S.Y.; Pan, S.; Lam, M.K. 3D graphene-based adsorbents: Synthesis, proportional analysis and potential applications in oil elimination. Chemosphere 2022, 287, 132129. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Fan, X.W.; Li, Y.Z. Efficient removal of a low concentration of Pb(II), Fe(III) and Cu(II) from simulated drinking water by co-immobilization between low-dosages of metal-resistant/adapted fungus Penicillium janthinillum and graphene oxide and activated carbon. Chemosphere 2022, 286, 131591. [Google Scholar] [CrossRef]
- Yadav, S.; Yadav, A.; Bagotia, N.; Sharma, A.K.; Kumar, S. Adsorptive potential of modified plant-based adsorbents for sequestration of dyes and heavy metals from wastewater—A review. J. Water Process Eng. 2021, 42, 102148. [Google Scholar] [CrossRef]
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Fawzy, A.; Alduaij, O.; Al-Bahir, A.; Alshammari, D.A.; Alqarni, N.; Eldesoky, A.; Farag, A.A.; Toghan, A. A comparative study of pyridine and pyrimidine derivatives based formamidine for copper corrosion inhibition in nitric acid: Experimental and computational exploration. Int. J. Electrochem. Sci. 2024, 19, 100403. [Google Scholar] [CrossRef]
- Badran, A.M.; Utra, U.; Yussof, N.S.; Bashir, M.J.K. Advancements in Adsorption Techniques for Sustainable Water Purification: A Focus on Lead Removal. Separations 2023, 10, 565. [Google Scholar] [CrossRef]
- Lai, K.C.; Lee, L.Y.; Hiew, B.Y.Z.; Thangalazhy-Gopakumar, S.; Gan, S. Environmental application of three-dimensional graphene materials as adsorbents for dyes and heavy metals: Review on ice-templating method and adsorption mechanisms. J. Environ. Sci. 2019, 79, 174–199. [Google Scholar] [CrossRef] [PubMed]
- Chaabane, L.; Beyou, E.; Baouab, M.H.V. Preparation of a novel zwitterionic graphene oxide-based adsorbent to remove of heavy metal ions from water: Modeling and comparative studies. Adv. Powder Technol. 2021, 32, 2502–2516. [Google Scholar] [CrossRef]
- Pradhan, S.S.; Konwar, K.; Ghosh, T.N.; Mondal, B.; Sarkar, S.K.; Deb, P. Multifunctional Iron oxide embedded reduced graphene oxide as a versatile adsorbent candidate for effectual arsenic and dye removal. Colloids Interface Sci. Commun. 2020, 39, 100319. [Google Scholar] [CrossRef]
- Kong, Q.; Shi, X.; Ma, W.; Zhang, F.; Yu, T.; Zhao, F.; Zhao, D.; Wei, C. Strategies to improve the adsorption properties of graphene-based adsorbent towards heavy metal ions and their compound pollutants: A review. J. Hazard. Mater. 2021, 415, 125690. [Google Scholar] [CrossRef]
- Ragab, A.H.; Mubarak, M.F.; El-Sabban, H.A.; Kang, J.; El Shahawy, A.; Alshwyeh, H.A.; Hemdan, M. Exploring the sustainable synthesis pathway and comprehensive characterization of magnetic hybrid alumina nanoparticles phase (MHAl-NPsP) as highly efficient adsorbents and selective copper ions removal. Environ. Technol. Innov. 2024, 34, 103628. [Google Scholar] [CrossRef]
- Arshad, F.; Selvaraj, M.; Zain, J.; Banat, F.; Haija, M.A. Polyethylenimine modified graphene oxide hydrogel composite as an efficient adsorbent for heavy metal ions. Sep. Purif. Technol. 2019, 209, 870–880. [Google Scholar] [CrossRef]
- López, S.F.; Virgen, M.M.; Montoya, V.H.; Morán, M.M.; Gómez, R.T.; Vázquez, N.R.; Cruz, M.P.; González, M.E. Effect of an external magnetic field applied in batch adsorption systems: Removal of dyes and heavy metals in binary solutions. J. Mol. Liq. 2018, 269, 450–460. [Google Scholar] [CrossRef]
- Vázquez, O.F.G.; Virgen, M.d.R.M.; Montoya, V.H.; Gómez, R.T.; Flores, J.L.A.; Cruz, M.A.P.; Morán, M.A.M. Adsorption of Heavy Metals in the Presence of a Magnetic Field on Adsorbents with Different Magnetic Properties. Ind. Eng. Chem. Res. 2016, 55, 9323–9331. [Google Scholar] [CrossRef]
- Singh, S.; Anil, A.G.; Khasnabis, S.; Kumar, V.; Nath, B.; Adiga, V.; Naik, T.S.K.; Subramanian, S.; Kumar, V.; Singh, J.; et al. Sustainable removal of Cr(VI) using graphene oxide-zinc oxide nanohybrid: Adsorption kinetics, isotherms and thermodynamics. Environ. Res. 2022, 203, 111891. [Google Scholar] [CrossRef]
- Fu, W.; Huang, Z. Magnetic dithiocarbamate functionalized reduced graphene oxide for the removal of Cu(II), Cd(II), Pb(II), and Hg(II) ions from aqueous solution: Synthesis, adsorption, and regeneration. Chemosphere 2018, 209, 449–456. [Google Scholar] [CrossRef]
- Ren, J.; Zhu, Z.; Qiu, Y.; Yu, F.; Ma, J.; Zhao, J. Magnetic field assisted adsorption of pollutants from an aqueous solution: A review. J. Hazard. Mater. 2021, 408, 124846. [Google Scholar] [CrossRef]
- Tang, T.; Liu, F.; Liu, Y.; Li, X.; Xu, Q.; Feng, Q.; Tang, N.; Du, Y. Identifying the magnetic properties of graphene oxide. Appl. Phys. Lett. 2014, 104, 123104. [Google Scholar] [CrossRef]
- Sarkar, S.K.; Ahmed, M.; Mukadam, M.D.; Yusuf, S.M. Investigation of Graphite Oxide and Reduced Graphene Oxide: Magnetic Properties Revisited. Asian J. Mater. Chem. 2016, 1, 66–74. [Google Scholar] [CrossRef]
- Liu, X.; Ma, R.; Wang, X.; Ma, Y.; Yang, Y.; Zhuang, L.; Zhang, S.; Jehan, R.; Chen, J.; Wang, X. Graphene oxide-based materials for efficient removal of heavy metal ions from aqueous solution: A review. Environ. Pollut. 2019, 252, 62–73. [Google Scholar] [CrossRef]
- Sharma, N.; Swami, S.; Shrivastava, V.; Nair, R.; Shrivastava, R. Graphene oxide and functionalized graphene oxide: Robust, 2d material as heterogeneous green catalyst for heterocyclic synthesis. Mater. Today Proc. 2021, 43, 3309–3317. [Google Scholar] [CrossRef]
- Varrla, E.; Paton, K.R.; Backes, C.; Harvey, A.; Smith, R.J.; McCauley, J.; Coleman, J.N. Turbulence-assisted shear exfoliation of graphene using household detergent and a kitchen blender. Nanoscale 2014, 6, 11810–11819. [Google Scholar] [CrossRef] [PubMed]
- Pérez-Álvarez, D.T.; Davies, P.; Stafford, J. Foam flows in turbulent liquid exfoliation of layered materials and implications for graphene production and inline characterisation. Chem. Eng. Res. Des. 2022, 177, 245–254. [Google Scholar] [CrossRef]
- Faria, P.C.C.; Órfão, J.J.M.; Pereira, M.F.R. Adsorption of anionic and cationic dyes on activated carbons with different surface chemistries. Water Res. 2004, 38, 2043–2052. [Google Scholar] [CrossRef]
- Aghamohammadi, H.; Eslami-Farsani, R.; Torabian, M.; Amousa, N. Recent advances in one-pot functionalization of graphene using electrochemical exfoliation of graphite: A review study. Synth. Met. 2020, 269, 116549. [Google Scholar] [CrossRef]
- Nimbalkar, A.S.; Tiwari, S.K.; Ha, S.K.; Hong, C.K. An Efficient Water Saving Step During the Production of Graphene Oxide via Chemical Exfoliation of Graphite. Mater. Today Proc. 2020, 21, 1749–1754. [Google Scholar] [CrossRef]
- Anurag, K.; Kumar, S.R. Synthesis of graphene through electrochemical exfoliation technique in aqueous medium. Mater. Today Proc. 2021, 44, 2695–2699. [Google Scholar] [CrossRef]
- Amrhar, O.; El Gana, L.; Mobarak, M. Calculation of adsorption isotherms by statistical physics models: A review. Environ. Chem. Lett. 2021, 19, 4519–4547. [Google Scholar] [CrossRef]
- George, J.; Upadhyay, S.; Balachandran, M. Exploration of Graphene Layers in Various Carbon Materials by Raman Spectroscopic Techniques. Mapana J. Sci. 2021, 20, 65–81. [Google Scholar] [CrossRef]
- Li, Z.; Cai, W.; Liu, J.; Zhou, M.; Cheng, L.; Yu, H.; Song, L.; Gui, Z.; Hu, Y. One-pot exfoliation and synthesis of hydroxyapatite-functionalized graphene as multifunctional nanomaterials based on electrochemical approach. Compos. Part A Appl. Sci. Manuf. 2021, 149, 106583. [Google Scholar] [CrossRef]
- Rasuli, H.; Rasuli, R.; Alizadeh, M.; BoonTong, G. Microwave-assisted exfoliation and tearing of graphene oxide in the presence of TiO2 nanoparticles. Results Phys. 2020, 18, 103200. [Google Scholar] [CrossRef]
- Tyurnina, A.V.; Morton, J.A.; Subroto, T.; Khavari, M.; Maciejewska, B.; Mi, J.; Grobert, N.; Porfyrakis, K.; Tzanakis, I.; Eskin, D.G. Environment friendly dual-frequency ultrasonic exfoliation of few-layer graphene. Carbon 2021, 185, 536–545. [Google Scholar] [CrossRef]
- Yan, Y.; Manickam, S.; Lester, E.; Wu, T.; Pang, C.H. Synthesis of graphene oxide and graphene quantum dots from miscanthus via ultrasound-assisted mechano-chemical cracking method. Ultrason. Sonochem. 2021, 73, 105519. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Perez-Page, M.; Ji, Z.; Zhang, Z.; Guo, Z.; Holmes, S. One step electrochemical exfoliation of natural graphite flakes into graphene oxide for polybenzimidazole composite membranes giving enhanced performance in high temperature fuel cells. J. Power Sources 2021, 491, 229550. [Google Scholar] [CrossRef]
- Raj, C.J.; Manikandan, R.; Thondaiman, P.; Sivakumar, P.; Savariraj, A.D.; Cho, W.-J.; Kim, B.C.; Jung, H. Sonoelectrochemical exfoliation of graphene in various electrolytic environments and their structural and electrochemical properties. Carbon 2021, 184, 266–276. [Google Scholar] [CrossRef]
- Loudiki, A.; Matrouf, M.; Azriouil, M.; Farahi, A.; Lahrich, S.; Bakasse, M.; El Mhammedi, M. Preparation of graphene samples via electrochemical exfoliation of pencil electrode: Physico-electrochemical Characterization. Appl. Surf. Sci. Adv. 2022, 7, 100195. [Google Scholar] [CrossRef]
- Ávila-Crisóstomo, C.E.; Pal, U.; Pérez-Rodríguez, F.; Shelyapina, M.G.; Shmyreva, A.A. Local-field effect on the hybrid ferromagnetic-diamagnetic response of opals with Ni nanoparticles. J. Magn. Magn. Mater. 2020, 514, 167102. [Google Scholar] [CrossRef]
- Zhang, X.; Li, G.; Li, Q.; Shaikh, M.S.; Li, Z. The pure paramagnetism in graphene oxide. Results Phys. 2021, 26, 104407. [Google Scholar] [CrossRef]
- Yang, X.; Wan, Y.; Zheng, Y.; He, F.; Yu, Z.; Huang, J.; Wang, H.; Ok, Y.S.; Jiang, Y.; Gao, B. Surface functional groups of carbon-based adsorbents and their roles in the removal of heavy metals from aqueous solutions: A critical review. Chem. Eng. J. 2019, 366, 608–621. [Google Scholar] [CrossRef]
- Hsu, C.-Y.; Ajaj, Y.; Mahmoud, Z.H.; Ghadir, G.K.; Alani, Z.K.; Hussein, M.M.; Hussein, S.A.; Karim, M.M.; Al-Khalidi, A.; Abbas, J.K.; et al. Adsorption of heavy metal ions use chitosan/graphene nanocomposites: A review study. Results Chem. 2024, 7, 101332. [Google Scholar] [CrossRef]
- Ahmad, I.; Siddiqui, W.A.; Ahmad, T. Synthesis, Characterization of Silica Nanoparticles and Adsorption Removal of Cu2+ Ions in Aqueous Solution. Int. J. Emerg. Technol. Adv. Eng. 2017, 7, 439–445. [Google Scholar]
- Wang, S.; Xiao, K.; Mo, Y.; Yang, B.; Vincent, T.; Faur, C.; Guibal, E. Selenium(VI) and copper(II) adsorption using polyethyleneimine-based resins: Effect of glutaraldehyde crosslinking and storage condition. J. Hazard. Mater. 2020, 386, 121637. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Liu, X.; Han, H.; Han, R. Adsorption properties of lead and zinc ions onto iminodiacetic acid functionalized loofah from solution. Desalination Water Treat. 2022, 253, 256–269. [Google Scholar] [CrossRef]
- Lima, L.K.S.; Silva, M.G.C.; Vieira, M.G.A. Study of binary and single biosorption by the floating aquatic macrophyte salvinia natans. Braz. J. Chem. Eng. 2016, 33, 649–660. [Google Scholar] [CrossRef]
- Mahdi, Z.; Yu, Q.J.; El Hanandeh, A. Removal of lead(II) from aqueous solution using date seed-derived biochar: Batch and column studies. Appl. Water Sci. 2018, 8, 181. [Google Scholar] [CrossRef]
- Sun, X.; Huang, X.; Liao, X.P.; Shi, B. Adsorptive removal of Cu(II) from aqueous solutions using collagen-tannin resin. J. Hazard. Mater. 2011, 186, 1058–1063. [Google Scholar] [CrossRef] [PubMed]
- Harmita, H.; Karthikeyan, K.G.; Pan, X.J. Copper and cadmium sorption onto kraft and organosolv lignins. Bioresour. Technol. 2009, 100, 6183–6191. [Google Scholar] [CrossRef]
- Xia, L.; Hu, Y.X.; Zhang, B.H. Kinetics and equilibrium adsorption of copper(II) and nickel(II) ions from aqueous solution using sawdust xanthate modified with ethanediamine. Trans. Nonferrous. Met. Soc. China 2014, 24, 868–875. [Google Scholar] [CrossRef]
- Zhang, R.; Zhou, Y.; Gu, X.; Lu, J. Competitive Adsorption of Methylene Blue and Cu2+ onto Citric Acid Modified Pine Sawdust. Clean 2015, 43, 96–103. [Google Scholar] [CrossRef]
- Jiang, S.; Do, H.; Yusuf, A.; Xiao, Z.; Wang, C.; Li, J.; Sun, Y.; Ren, Y.; He, J. Camellia oleifera shell–reduced graphene oxide for adsorption of copper(II). Mater. Chem. Phys. 2024, 314, 128818. [Google Scholar] [CrossRef]
- Zhan, J.; Lei, Z.; Zhang, Y. Non-covalent interactions of graphene surface: Mechanisms and applications. Chem 2022, 8, 947–979. [Google Scholar] [CrossRef]
- Basem, A.; Jasim, D.J.; Majdi, H.S.; Mohammed, R.M.; Ahmed, M.; Al-Rubaye, A.H.; Kianfar, E. Adsorption of heavy metals from wastewater by chitosan: A review. Engineering 2024, 23, 102404. [Google Scholar] [CrossRef]
| Adsorbent | Cation | Removal Conditions | Adsorption Capacity, mmol/g | Reference |
|---|---|---|---|---|
| Polyethyleneimine-glutaraldehyde resin | Pb2+ | 298 K, pH 3 | 0.12 | [62] |
| Iminodiacteic acid-funcionalized loofah | Pb2+ | 303 K, pH 5 | 0.20 | [63] |
| Aquatic macrophyte Salvinia natans | Pb2+ | 303 K, pH 4 | 0.28 | [64] |
| Date seed-derived biochar | Pb2+ | 303 K, pH 6 | 0.36 | [65] |
| Fe3O4-reduced graphene oxide | Pb2+ | 303 K, pH 5 | 0.34 | [30] |
| GO2 without the application of a magnetic field | Pb2+ | 303 K, pH 5 | 0.17 | Present study |
| GO3 without the application of a magnetic field | Pb2+ | 303 K, pH 5 | 0.20 | Present study |
| Collagen–tannin resin | Cu2+ | 303 K, pH 5 | 0.26 | [66] |
| Hancili Green Bentonite | Cu2+ | 298 K, pH 7 | 0.22 | [15] |
| Softwood kraft lignin | Cu2+ | 297 K, pH 5 | 0.11 | [67] |
| Sawdust xanthate modified with ethanediamine | Cu2+ | 298 K, pH 6 | 0.02 | [68] |
| Pine sawdust modified with citric acid | Cu2+ | 298 K, pH 5 | 0.23 | [69] |
| Camellia oleifera shell-reduced graphene oxide | Cu2+ | 298 K, pH 5 | 0.28 | [70] |
| GO2 without the application of a magnetic field | Cu2+ | 303 K, pH 5 | 0.30 | Present study |
| GO3 without the application of a magnetic field | Cu2+ | 303 K, pH 5 | 0.31 | Present study |
| Adsorbent | Cation | nIon | DGO-Ion, mmol/g |
|---|---|---|---|
| GO2 without magnetic field | Pb2+ | 1 | 0.18 |
| Cu2+ | 1.22 | 0.26 | |
| GO3 without magnetic field | Pb2+ | 1.34 | 0.16 |
| Cu2+ | 1.43 | 0.23 | |
| GO2 with magnetic field | Pb2+ | 1.18 | 0.24 |
| Cu2+ | 1.23 | 0.29 | |
| GO3 with magnetic field | Pb2+ | 1.18 | 0.20 |
| Cu2+ | 1.59 | 0.20 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Melchor-Durán, A.P.; Moreno-Virgen, M.R.; Bonilla-Petriciolet, A.; Reynel-Ávila, H.E.; Lucio Ortiz, E.; González-Vázquez, O.F. Heavy Metal Removal from Water Using Graphene Oxide in Magnetic-Assisted Adsorption Systems: Characterization, Adsorption Properties, and Modelling. Separations 2024, 11, 294. https://doi.org/10.3390/separations11100294
Melchor-Durán AP, Moreno-Virgen MR, Bonilla-Petriciolet A, Reynel-Ávila HE, Lucio Ortiz E, González-Vázquez OF. Heavy Metal Removal from Water Using Graphene Oxide in Magnetic-Assisted Adsorption Systems: Characterization, Adsorption Properties, and Modelling. Separations. 2024; 11(10):294. https://doi.org/10.3390/separations11100294
Chicago/Turabian StyleMelchor-Durán, A. P., M. R. Moreno-Virgen, A. Bonilla-Petriciolet, H. E. Reynel-Ávila, E. Lucio Ortiz, and O. F. González-Vázquez. 2024. "Heavy Metal Removal from Water Using Graphene Oxide in Magnetic-Assisted Adsorption Systems: Characterization, Adsorption Properties, and Modelling" Separations 11, no. 10: 294. https://doi.org/10.3390/separations11100294
APA StyleMelchor-Durán, A. P., Moreno-Virgen, M. R., Bonilla-Petriciolet, A., Reynel-Ávila, H. E., Lucio Ortiz, E., & González-Vázquez, O. F. (2024). Heavy Metal Removal from Water Using Graphene Oxide in Magnetic-Assisted Adsorption Systems: Characterization, Adsorption Properties, and Modelling. Separations, 11(10), 294. https://doi.org/10.3390/separations11100294









