Abstract
The gas–liquid multiphase process plays a crucial role in the chemical industry, and the utilization of packed beds enhances separation efficiency by increasing the contact area and promoting effective gas–liquid interaction during the separation process. This paper primarily reviews the progress from fundamental research to practical application of gas–liquid multiphase processes in packed bed reactors, focusing on advancements in fluid mechanics (flow patterns, liquid holdup, and pressure drop) and the mechanisms governing gas–liquid interactions within these reactors. Firstly, we present an overview of recent developments in understanding gas–liquid flow patterns; subsequently we summarize liquid holdup and pressure drop characteristics within packed beds. Furthermore, we analyze the underlying mechanisms involved in bubble breakup and coalescence phenomena occurring during continuous flow of gas–liquid dispersions, providing insights for reactor design and operation strategies. Finally, we summarize applications of packed bed reactors in carbon dioxide absorption, chemical reactions, and wastewater treatment while offering future perspectives. These findings serve as valuable references for optimizing gas–liquid separation processes.
1. Introduction
Gas–liquid systems are widely used in various applications, such as chemical reactions [1,2,3], extraction [4,5], gas separation [6,7], and material synthesis [8,9]. The packed bed reactor has the advantages of flexibility, simple operation, and large processing capacity, and has been extensively utilized in the chemical industry, including the petroleum refining [10,11,12,13,14], petrochemical [15,16], biochemical [17,18], environmental [19,20], electrochemical [21], fine chemical, and pharmaceutical industries [22,23]. The packed bed improves separation efficiency in the gas–liquid separation process by increasing the contact area and promoting effective gas–liquid interaction. The design and arrangement of fillers can enhance the interaction between gas and liquid and improve the separation efficiency. They help to improve the uniformity of gas–liquid distribution and reduce pressure drop while increasing processing capacity and reducing energy consumption. However, due to the uneven distribution of liquid and incomplete wetting of fillers, the packed bed reactor also suffers from some problems, such as an uncontrollable reaction process, low production efficiency, and unavailable by-products, which limit its further application in the chemical industry. Therefore, enhancing reaction performance within packed bed reactors is essential for efficient application to meet green chemistry requirements. Furthermore, even minor improvements can lead to significant cost savings along with economic benefits, making it crucial to design packed bed reactors while considering the hydrodynamic aspects of gas–liquid dispersion systems.
The flow through the packing exhibits complex two-phase behavior, resulting in various forms of two-phase flow. including bubbly flow, trickling flow, pulsing flow, and spray flow. Generally, the characteristics of the flow are determined by the specific two-phase flow pattern [24,25]. Each state of the flow possesses distinct features in relation to the pressure drop, gas fraction, mass transfer, momentum transfer, and energy exchange between phases [26,27]. The transition from one flow state to another is highly dependent on the gas–liquid interactions. Therefore, to better design and understand the two-phase flow through the packing, it is essential to study the interaction mechanism of bubbles in the packing. In the continuous flow of gas–liquid dispersion, the key bubble interaction is bubble fragmentation and coalescence. For gas–liquid turbulence in pipelines, the mechanism of bubble fragmentation and coalescence can be ascribed to bubble–bubble collisions and bubble–turbulent eddy current collisions [28,29,30,31]. However, in cases involving two-phase flows through packed beds, collision between bubbles and solid particles must also be taken into consideration when studying bubble rupture and coalescence processes [31]. Due to the presence of fillers in the flow channels, the mechanisms of bubble rupture and coalescence found in the packing bed may be quite different from those found in turbulent dispersed flows.
Liquid holdup is a significant parameter in packed bed reactors with gas–liquid two-phase flow [32,33], as it affects flow distribution, pressure drop, and separation efficiency in packed towers [34]. However, due to the heterogeneous bed structure, liquid holdup varies locally. Local information about liquid holdup is crucial for comprehensive analysis of fluid dynamics and mass transfer efficiency. In general, liquid holdups in industrial-scale drop-bed reactors are in the range of 10–20%. The liquid phase generally forms a thin film around catalyst particles, which often hinders mass transfer between the gas phase and the outer surface of the catalyst.
Accurate estimation of pressure drop is also important in many industrial applications. Precise assessment of pressure drop plays a pivotal role in the design and optimization of pumping power, as well as the size and cost considerations for any pumping system [35,36]. Furthermore, pressure drop affects material transfer efficiency; a high-pressure drop can lead to uneven fluid distribution, which affects the reaction performance. Optimizing the pressure drop can improve the overall operating efficiency and economy of the packed bed reactor.
In this paper, the hydrodynamics of gas and liquid in a packed bed reactor were reviewed to provide a reference for intensifying the gas–liquid separation process in a packed bed reactor. Figure 1 shows an overview of this review. First, advancements in gas–liquid flow patterns are discussed, followed by a summary of liquid holdup and pressure drop in a packed bed. In order to optimize the design and operation of the packed bed reactor, the basic mechanism of bubble breaking and coalescence in the continuous flow of gas–liquid dispersion in the packed bed is analyzed. The applications of a packed bed reactor in carbon dioxide absorption, chemical reaction, and wastewater treatment are summarized. Finally, a summary and future prospects are provided.
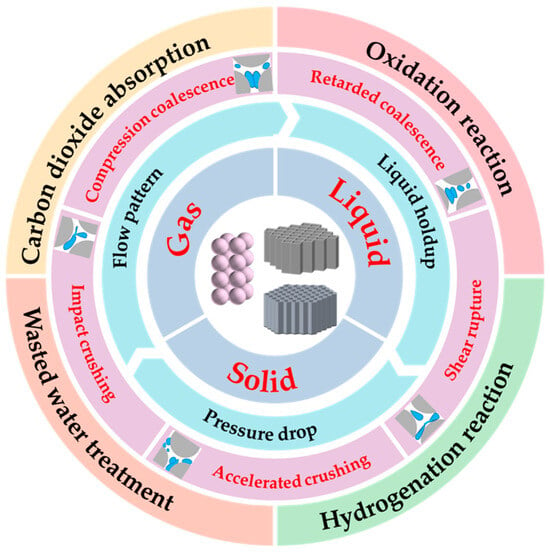
Figure 1.
Overview covered in this review. From inside to outside: (1) schematic diagram of several fillers; (2) the interaction of gas, liquid, and solid; (3) the dynamics within the packed bed; (4) the fundamental mechanism of continuous gas–liquid dispersion flow in the packing; (5) the application of gas–liquid systems in the packed bed.
2. Gas–Liquid Flow Dynamics
2.1. Gas–Liquid Flow Pattern
The flow patterns in reactors vary depending on the flow rates, the characteristics of the packed bed, and the physical properties of the fluids involved. Indeed, flow pattern diagrams illustrating the boundaries and operational ranges of various flow patterns have been documented in the literature [37,38]. In a packed bed reactor, the flow patterns can be classified as bubbly flow (low UG, high UL), trickling flow (low to moderate UG and UL), pulsing flow (moderate UG, high UL), and spray flow (high UG, low UL). Each flow pattern exhibits distinctive hydrodynamic characteristics, including the pressure drop, liquid holdup, mass transfer, and heat transfer rates [24,25,32,39].
Charpentier and Favier [40] proposed a flowchart based on Beck coordinates, which has been widely used in conventional packed bed reactors. Figure 2a describes the flow patterns and the transition from one structure to the other in foaming and non-foaming systems. The lines separating flow patterns in these graphs are actually transitional flow patterns, not abrupt points from one flow pattern to another. In both figures, the lines separating the trickle from the other flow patterns are the same. According to Charpentier and Favier [40], it is the transition line between the small gas–liquid interaction states (trickling flow) and the large gas–liquid interaction states (bubbly flow, pulsing flow, and spray flow) defined by Satterfield (1975) [41] and Charpentier et al. (1971) [42]. The transition line was previously obtained with an air–water system for 3 mm packing in a 10 cm column by Charpentier et al. (1971) [42]. Tomoya Inoue et al. (2015) [38] designed and fabricated a parallel packed bed microreactor appropriate for the direct synthesis of hydrogen peroxide from hydrogen and oxygen. The reactor uses a microfluidic design to uniformly distribute the reaction fluid to each catalyst packed bed, and the design is verified by reactor performance and gas–liquid flow visualization. Figure 2b shows the flow visualization schemes of the 1 ch (top) and 8 ch (bottom) reactors, respectively, and Figure 2c,d show the flow visualization results of the 1 ch and 8 ch reactors on the Charpentier–Favier coordinate diagram, as well as the time slice images of pulse flow and trickle flow, respectively. Typically, it is difficult to visualize this two-phase flow behavior in a normal packed bed, even if the test part is made of a transparent material, due to light reflection/refraction and/or bubble overlap. To avoid bubble overlap and clearly identify flow patterns, Yasugi et al. (2021) [43] fabricated a test cross section simulating a two-dimensional porous structure network. Using high-speed imaging techniques on two-dimensional network models, the two-phase flow pattern in the porous structure was determined. The two-phase flow characteristics of the quasi-two-dimensional test section were observed using a high-speed camera. In order to observe the bubbles more clearly, the two-phase flow image was normalized in the liquid phase image and the obtained typical images of bubbles, slug flows, and ring flows.
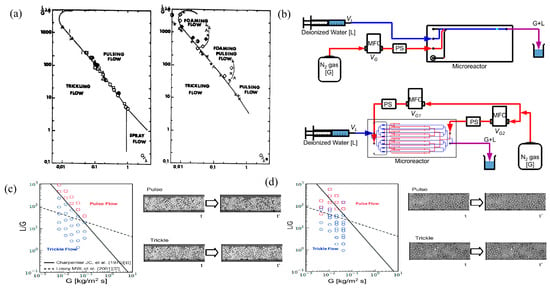
Figure 2.
(a) Flow pattern of the macroscopic packed bed reactor flow. Reprinted with permission from Ref. [40]. 1975 American Institute of Chemical Engineers. (b) Flow visualization schemes for the 1 ch reactor and the 8 ch reactor. Reprinted with permission from Ref. [38]. 2014 Elsevier B.V. MFC: mass flow controller; PS: pressure sensor. (c) From the flow visualization study of the 1 ch reactor mapped onto the Charpentier–Favier coordinate system, including time-sliced images of both pulse and trickle flow. Reprinted with permission from Ref. [38]. 2014 Elsevier B.V. (d) From the flow visualization study of the 8 ch reactor mapped onto the Charpentier–Favier coordinate system, including time-sliced images of both pulse and trickle flow. Reprinted with permission from Ref. [38]. 2014 Elsevier B.V.
Recently, there has been a considerable increase in interest in the field of packed bed compact reactors with internal diameters in the millimeter or micron ranges. The study of flow patterns in a micro-packed bed reactor (μPBR) presents a number of significant challenges, largely due to microscale limitations and the inherent complexity of capillary force effects. The presence of small interstitial channels between small particles and numerous particles gives rise to a multitude of complex internal states, which ultimately affect the ability to recognize and accurately assess gas–liquid two-phase flow behavior in a μPBR.
Krishnamurthy et al. (2006) [44] conducted an experimental study on the adiabatic nitrogen water two-phase flow through a group of staggered circular microcolumns with a diameter of 100 μm and a pitch to diameter ratio of 1.5 within the Reynolds number range of 5 to 50, and discussed the flow mode. The results were compared with those obtained from large-scale and micro channels. Based on flow visualization, four types of two-phase flow modes were observed in the μPBR: bubble slug flow (Figure 3(a1)), gas slug flow, bridge flow (Figure 3(a2)), and annular flow (Figure 3(a3)). Wada et al. (2006) [45] performed flow visualization using oxygen and ethyl acetate to investigate the multiphase flow patterns under reaction conditions (Figure 3b). At low liquid–gas flow rates under all gas flow rates, an “annular flow (Figure 3(b3))” pattern was observed where the liquid preferentially distributed along the wall while the gas passed through the center of the channel. At high liquid–gas ratios, there was a “slug flow (Figure 3(b1))” pattern with a channel across the gas plug passing through a continuous liquid phase. At high gas–liquid flow rates, rapid fluctuations in shape at their interface were exhibited, which are represented here as “agitated flow (Figure 3(b2)).” Faridkhou and Larach (2012) [46] observed the wall fluid dynamics of gas–liquid co-flow in a micro-packed bed by using inverted microscope technology, and identified two main flow patterns: a low interaction flow pattern with small movement at the gas–liquid boundary and highly interacting flow patterns, characterized by rapid displacement of the gas–liquid phase, causing fluctuations in the defined feature lengths. Al-Rifai et al. (2016) [47] investigated the gas–liquid hydrodynamics of benzyl alcohol oxidation in a packed bed microreactor using Au-Pd /TiO2 as the catalyst. As the gas flow rate increased, the fluid mechanics transitioned from a liquid-dominated slug flow to a separate and continuous gas flow. Yang et al. (2015) [48] visualized liquid–liquid and gas–liquid flows across a broad range of operating conditions, using a microreactor with a column internal field as an example, and classified the flow modes into four types: annulus, slug/annulus, slug, and stirred. In 2019, Márquez et al. (2019) [49] designed microcolumns of different sizes and arrangement types in a microcolumn packed bed to obtain more chaotic systems and simulate a real μPBR. The results showed that the fluid surface velocity and reactor size had significant effects on the flow state.
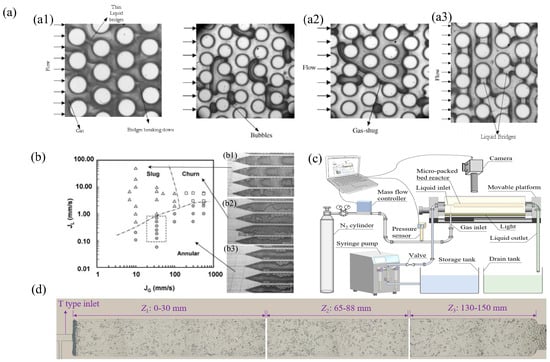
Figure 3.
(a) (a1) Images of bubbly gas slug flow. Reprinted with permission from Ref. [45]. 2007 AIP Publishing. (a2) Image of gas slug flow with elongated gas slugs. Reprinted with permission from Ref. [45]. 2007 AIP Publishing. (a3) Image of liquid bridge flow. Reprinted with permission from Ref. [45]. 2007 AIP Publishing. (b) Gas–liquid flow state observed in multichannel microreactors by Wada et al. (2006) [44]. This work is openly licensed via CC BY 4.0. (b1) Slug flow (gas is the dark region); (b2) agitated flow (interface fluctuation is rapid, liquid periodically throughout the channel); (b3) annular flow (gas flow in the center of the reactor). (c) Annular flow showing the thinning of liquid bridges and their breakage. Reprinted with permission from Ref. [50]. 2022 American Institute of Chemical Engineers. (d) μPBR filled with tiny glass bead packing used in this work. Reprinted with permission from Ref. [50]. 2022 American Institute of Chemical Engineers.
In the above studies, there were obvious differences in the internal structure of the μPBR, leading to differences in the description and definition of the convective type. A simplified μPBR based on the microcolumn structure [44,45,48,49] yields many results, and the study method is almost all in the near-wall region. There are few cases where multiple methods are used to make a comprehensive judgment at the same time. The flow pattern in real μPBR systems is not yet fully understood, hindering the comprehension of gas–liquid flow behavior and gas–solid reaction performance.
In 2022, Liu et al. (2022) [50] designed and fabricated a two-dimensional μPBR filled with glass microbeads (Figure 3d), using high-performance cameras and microscopic lenses to systematically capture gas–liquid flow behaviors with sufficient spatial and temporal resolution in the μPBR under different operating conditions (Figure 3c). They studied the gas–liquid two-phase flow behavior in a two-dimensional packed bed filled with microbeads, identified four typical flow patterns and characteristics (channel flow, stirred flow, quasi-static flow, and parallel flow), and revealed the flow pattern transformation in the μPBR based on visual studies and pressure drop fluctuations. On the basis of the previous research results, the relation of mixing flow to quasi-static flow in μPBR is proposed for the first time. When the air surface velocity is low, the pressure drop is large, which makes it easy to induce channel flow. As the gas surface velocity increases, the gas phase gradually changes from channel flow to stirred flow or quasi-static flow and gradually from the disperse phase to the continuous phase.
2.2. Liquid Holdup
Liquid holdup represents a significant hydrodynamic parameter in the context of reactor design, scale-up, and operation. The term “liquid holdup” is defined as the volume fraction of the bed that the liquid occupies under specific operating conditions. The total liquid holdup encompasses both static liquid holdup and operating liquid holdup [34,51,52]. Shulman et al. (1955) [51] were the first researchers to differentiate these two concepts. Static holdup is defined as the quantity of liquid that is maintained at the contact points between the fillers and on the surface of the fillers when the liquid supply to the tower is halted. Conversely, the operating holdup represents the liquid that will be drained from the filler. Currently, the available methodologies for measuring holdup encompass the gravity method for total holdup estimation [53] and the chromatography method for cross-sectional holdup assessment [54,55].
Yin et al. (2002) [56] utilized non-invasive gamma ray tomography to determine the distribution of liquid (water) holdup in a large packed column with a diameter of 0.6 m, which was filled with 25.4 mm metallic pall rings. Two vertical locations 400 mm apart were selected for horizontal scans at both liquid flow rates. Three different liquid distributor designs were employed to investigate the influence of inlet liquid distribution on the content distribution of the tower. The spatial variation of liquid holdup on the column section was calculated using the chromatographic reconstruction algorithm. The results indicated that the liquid holdup distribution was uneven and that the design of the liquid distributor significantly influences this distribution.
Olujic and Behrens (2006) [57] conducted a comparative experimental study to ascertain the hydraulic properties of a novel modular catalytic filler (Sulzer’s Katapak®-SP 12) and a mixed filler bed (a catalytic filler segment between two sections consisting of a high-capacity structured filler (MellapakPlus 752.Y)). Air–water experiments were conducted under ambient conditions using a plexiglass column with an inner diameter of 0.45 m. The liquid holdup and pressure drop in the mixed filler bed fell between those of pure catalytic packing and structural packing. However, the mixed filler bed had a significant upper limit of gas load.
Zakeri et al. (2012) [58] used air/water and an air/water/sugar solution in a 0.5 m I.D. absorption column with a packing height of 5 m. The liquid holdups in three distinct structural fillers (Mellapak 2X from Sulzer, Koch-Glitsch Flexipac 2Y HC, and Montz-Pak B1-250M) were investigated. As anticipated, the liquid holdup exhibited minimal variation with respect to gas flow, remaining nearly constant for a given liquid load, and demonstrating an increase at high gas velocities. In general, the Sulzer filler had a higher liquid content than the other two fillers.
Mohammed et al. (2013) [59] studied the hydrodynamic characteristics of foam fillers in gas–liquid two-phase co-flow conditions. They developed a model to serve as a predictive tool for calculating liquid holdup in tubular reactors containing solid foam fillers.
Sang et al. (2020) [60] conducted a study on the liquid holdup of micro-packed bed reactors (μPBRs) with metal foam packings, utilizing an automated experimental platform. The researchers parameterized the gas–liquid surface velocity, pore size, and physical properties of the foam filler. They then established empirical relationships for the liquid content in the foam-filler μPBR. The predicted values were found to closely match the experimental values. Table 1 summarizes the empirical relationships of liquid holdup.

Table 1.
Summary of the empirical relationships of liquid holdup.
2.3. Pressure Drop
Another crucial parameter in the design of gas–liquid packed beds is the pressure drop. Hutter et al. (2011) [64] examined the axial dispersion characteristics of metal foam and laser sintering piles. They compared commercial metal foams with pore densities of 20 and 30 ppi to specifically designed downflow cycle structures. Their findings revealed that the relationship between pressure drop and interstitial velocity aligns with the Forchheimer equation (Forchheimer et al. (1901) [65]), which effectively describes the behavior of all studied porous media according to the conventional Ergun model.
Mohammed et al. (2013) [59] studied the hydrodynamic properties of foam fillers under gas–liquid two-phase co-flow conditions. They developed a model to provide a simple predictive model to calculate the pressure drop in tubular reactors with solid foam fillers. They created a straightforward predictive model to calculate the pressure drop in tubular reactors containing solid foam fillers. Accordingly, the correlations are proposed as Equation (1).
where dp/dz is pressure drop, kPa; ρL is liquid density, kg/m3; g is acceleration of gravity, m/s2; ReL is liquid Reynolds number; ReG is gas Reynolds number; as is specific surface area, m2/m3; dw is window (pore) diameter, m; and ε is porosity.
Sang et al. (2019) [60] used an automated experimental platform to study the pressure drop of a metal foam-packed μPBR. The experimental results demonstrated that the μPBRs with foam packing exhibited a superior mass transfer rate and a pressure drop that was 10 times lower than that of microparticles. They established the empirical relationships of pressure drop in the foam-packed μPBR (Equations (2) and (3)), and the predicted values were in good agreement with the experimental values.
where ΔP/L is unit pressure drop, kPa/m; f is friction factor; ρL is liquid density, kg/m3; uL is liquid velocity, m/s; dp is pore diameter of packing, m; ReG is gas Reynolds number; ReL is liquid Reynolds number; WeL is liquid Weber number; and dc is inner diameter of micro-packed bed reactor, m.
3. Basic Gas–Liquid Dispersion Mechanism in Continuous flow Packed Beds
In order to optimize the design and operation of the packed bed reactor, it is essential to gain a comprehensive understanding of the gas–liquid interaction. To elucidate the flow behavior of the packed bed reactor, it is imperative to meticulously examine the local flow mechanism within the pores through the utilization of local measurement techniques. Given that the pore diameter is in the order of millimeters and the residence time of the bubble within the pore is in the order of milliseconds, it is inherently challenging to conduct studies within the pore.
Melli et al. (1990) [66] investigated the relationship between flow patterns at the macro scale or bed scale and flow mechanisms in the “void space” or pores where the “particle scale” or “micro scale” flow patterns are situated. This marks the inaugural observation of the flow state at the pore level of the fill layer in minute detail. The experiments were conducted in a transparent, two-dimensional network (Figure 3c) that was designed to simulate the void space that fills the bed. However, it should be noted that the research was conducted in a highly idealized experimental environment. In lieu of particles, the researchers fixed O-rings between parallel transparent walls to “mimic the void space that fills the bed,” thereby facilitating the use of high-speed cameras. Notwithstanding the geometric oversimplification, the qualitatively obtained flow patterns were reported to be similar to those observed in a fine-flow bed, most notably the absence of particle-to-particle contact points. Tsochatzidis and Karabelas (1994) [67] employed a uniform glass sphere with a diameter of 6 mm to investigate the fundamental elements of flow behavior at the microscale and to relate these findings to patterns observed at the macroscale. Their research confirmed that the dominant flow pattern identified by Melli et al. (1990) [66] in a two-dimensional bed closely resembles that found in a typical cylindrical packed bed filled with spheroids of the same diameter. In parallel, Benkrid et al. (2003) [68] and Rode et al. (2003) [69] conducted statistical analyses of bubble number density and the mean bubble size in a two-dimensional packed bed, revealing that the average bubble size is influenced by the hydraulic diameter. Additionally, Bordas et al. (2006) [70] performed microscale experiments using exponential matching techniques, demonstrating that the bubble number density in a typical bed with packing sizes ranging from 2 to 6 mm is dependent solely on the pore size. Their studies, along with those of Benkrid et al. (2003) [69] and Bordas et al. (2006) [70], indicated that the bubble number density distribution exhibits a single peak. Notably, the characteristic length of a two-dimensional bed is defined by the hydraulic diameter, while that of a three-dimensional bed is determined by the pore size. Consequently, the flow mechanisms in two-dimensional beds can be effectively compared to those found in standard three-dimensional cylindrical packed beds.
Bubble breaking and coalescence are fundamental mechanisms in the continuous flow of gas–liquid dispersions. In the context of gas–liquid turbulence within tubes, these mechanisms primarily arise from bubble–bubble collisions and bubble–eddy interactions. In the packed bed two-phase flow, the dynamics undergo a shift; collisions between bubbles and solid particles significantly influence bubble fragmentation and coalescence. The presence of packing alters the coalescence and rupture mechanisms compared to those observed in the turbulent dispersion. First, the flow path is constrained by the packing material, resulting in wall shear forces acting on the bubbles as they navigate through the pores. Second, local flow velocities vary in narrow channels and pores, causing bubbles to accelerate, decelerate, or even come to a complete stop at certain positions. Third, bubbles do not achieve a stable final velocity, which limits the potential for bubble entrainment in the wake region. Lastly, interactions with solid particles lead to collisions that can cause bubbles to burst or merge. These factors collectively highlight the complexity of bubble dynamics in packed bed reactors.
3.1. Coalescence Mechanisms
The first coalescence mechanism, known as compression coalescence, occurs when two bubbles come into contact while passing through a narrow channel. In this process, the liquid film between the bubbles undergoes a substantial thinning. Figure 4(a1) illustrates a typical example of compression merging, where two bubbles merge into one within a vertically narrow channel. This mechanism relies heavily on the contact time between the bubbles. Specifically, if the contact time is insufficient to reduce the liquid film to a critical thickness, coalescence will not take place. Research on bubble columns and two-phase flows provides critical film thickness and contact time data. For instance, Kim and Lee (1987) [71] estimate that the critical film thickness for coalescence in air–water systems is approximately 1 × 10−8 m. Additionally, Kirkpatrick and Lockett (1974) [72] report that the contact time required for bubbles with a diameter of 5 mm to coalesce is less than 0.016 s. In the scenario depicted in Figure 4(a1), the contact time between bubbles in the pore channel is about 0.008 s, which is roughly half the contact time needed for bubbles in the pipe flow. The narrow channel compresses the bubbles, thereby accelerating the thinning of the liquid film. This rapid thinning leads to film rupture and subsequent bubble merger.
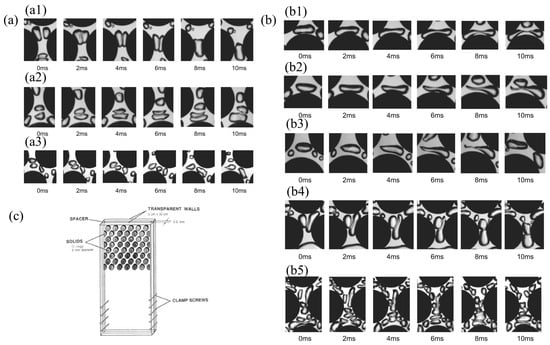
Figure 4.
(a) Coalescence mechanisms. Reprinted with permission from Ref. [31]. 2009 Elsevier. (a1) Compression coalescence mechanism at a vertical channel, (a2) decelerating coalescence mechanism at the outlet of a vertical channel, and (a3) decelerating coalescence mechanism at the outlet of a horizontal channel. (b) Coalescence mechanisms. Reprinted with permission from Ref. [31]. 2009 Elsevier. (b1) Shear breakup mechanism by equal shear forces, (b2) shear breakup mechanism by unequal shear forces, (b3) acceleration breakup mechanism, (b4) impact breakup mechanism at lower superficial velocities, and (b5) impact breakup mechanism at higher superficial velocities. (c) “Two-dimensional” packed bed from Melli et al. (1990) [66]. This work is openly licensed via CC BY 4.0.
The second coalescence mechanism, known as retarded coalescence, occurs when the leading bubble experiences a sudden decrease in speed. A typical example is illustrated in Figure 4(a2), where the front and rear bubbles coalesce. As the front bubble approaches its stagnation point, it slows down, while the rear bubble continues to move relatively quickly. This results in the rear bubble colliding with the front bubble, causing the liquid film between them to thin and ultimately merge. Retarded coalescence can take place at two distinct locations: (1) at the top of the filler, where a stagnation point is present (Figure 4(a2)); and (2) at the exit of a narrow channel, where the flow decelerates (Figure 4(a3)). If the bubbles do not remain in contact long enough for the liquid film to thin to a critical thickness, coalescence will not occur. In piping flow systems, bubble–bubble coalescence mechanisms necessitate longer contact times and higher relative velocities between bubbles (Kirkpatrick and Lockett, 1974) [72]. From Figure 4(a2), it is evident that the contact time for bubbles coalescing at the stagnation point is less than 10 ms. In contrast, for bubbles coalescing in narrow channels, the contact time is under 5 ms, as shown in Figure 4(a3). Notably, the relative velocity between the two bubbles in Figure 4(a3) is lower than that in Figure 4(a2). These findings align with the observations made by Kirkpatrick and Lockett (1974) [72] in pipeline flow. Consequently, the local bubble relative velocity emerges as a critical parameter influencing decelerated coalescence.
3.2. Breakup Mechanisms
Three primary fracture mechanisms have been identified in two-dimensional packed beds. The first mechanism is shear rupture, which arises from shear forces exerted on both sides of the bubble. Bubbles enter through a vertically narrow channel and move toward the filler, where they encounter a stagnation point. As a bubble approaches this point, it deforms and stretches in both directions. There are two stagnation points: one at the bottom of the bubble and the other at the top of the filler. The presence of these two stagnation points causes bubbles to avoid direct collisions with the packing center. When a bubble is pulled from either side of the stagnation point, it stretches through the filler. If the bubble is stretched evenly from both sides of the centerline, it is more likely to break symmetrically, resulting in two bubbles of similar size (Figure 4(b1)). In this scenario, the centerline of the bubble is positioned near the stagnation point, where the shear forces acting on either side are balanced. Conversely, if the centerline is not near the stagnation point, the bubble will produce one large daughter bubble and one small daughter bubble (Figure 4(b2)). This asymmetry arises because unequal shear forces act on either side of the bubble. When the centerline is far from the stagnation point, the shear force on one side becomes excessive, causing the bubble to move toward the side with the greater force. Consequently, the speed at which the bubble approaches the packing is a critical parameter influencing the occurrence of this rupture mechanism.
The second crushing mechanism is accelerated crushing, which is induced by bubble acceleration, as illustrated in Figure 4(b3). In this process, when a bubble enters the convergence region, its head accelerates first, followed by the tail. This differential acceleration causes the tail to separate from the main body of the bubble. Although this rupture mechanism is relatively infrequent, it is significant because it generates tiny bubbles. The intensity of the bubble’s acceleration directly influences the production of these small sub-bubbles; stronger acceleration facilitates their formation. Notably, the average size of the daughter bubble produced through this mechanism is approximately 10 times smaller than that of the larger daughter bubble. Consequently, local flow acceleration emerges as the most critical parameter in this mechanism.
The third crushing mechanism is known as impact crushing, which occurs when bubbles collide directly within a narrow channel. This mechanism can take place in both vertical and horizontal channels. As illustrated in Figure 4(b4,b5), two distinct impact crushing conditions arise at varying gas–liquid surface velocities. In impact crushing, one bubble behaves like a solid particle, and its collision with another bubble can cause the latter to split into two smaller bubbles. Generally, smaller bubbles exhibit a stronger surface structure due to increased surface tension. In turbulent dispersion, bubbles bounce off one another when their relative velocity exceeds a critical threshold. Kirkpatrick and Lockett (1974) [72] identified this critical relative velocity as 12 cm/s for bubble–bubble merger. However, in a packed bed scenario, a bubble with a robust surface structure that collides with another bubble near the packed wall will produce a sub-bubble if its relative velocity surpasses the critical velocity. The presence of solid fillers prevents the bubbles from separating, leading to smaller bubbles breaking through rather than rebounding. In this context, the inhibiting force associated with the bubbles is supplanted by the impact strength. Consequently, as shown in Figure 4(b4,b5), impact breakage occurs more rapidly at high surface velocities than at low surface velocities, as greater relative bubble velocities result in increased impact forces.
4. Applications of Gas–Liquid Separation
4.1. CO2 Absorption
With the rapid development of industrialization, the extensive use of fossil fuels has led to a significant increase in CO2 emissions, which has a serious impact on the ecological environment and people’s lives. Therefore, the topic of CO2 capture, utilization, and storage technology has become a hot topic for academic research and industrial application, particularly in the context of carbon neutrality [73]. CO2 absorption is a typical gas–liquid process, and efficient gas–liquid contact equipment is crucial for its effective implementation. The packing inside the packed bed can greatly increase the contact area between the fluids, and the contact area of gas–liquid is where reactions or mass transfer occur. Therefore, packed beds are effective gas–liquid contact mass transfer devices and have been widely used in CO2 absorption processes [74]. In terms of absorbents, organic amine solutions represented by MEA have a high CO2 absorption rate, strong selectivity, and easy regeneration, which are widely used in CO2 absorption process. However, a high degradation rate, high energy consumption of solvent regeneration, and corrosiveness are the main problems of MEA as absorbents in industrial applications. In order to solve these problems, researchers have been constantly improving and developing new absorbents. Khan et al. [75] used monoethanolamine (MEA) and a steric amine 2-amino-2-methyl-1-propanol (AMP) to conduct experiments on packed columns under different process conditions to investigate the CO2 absorption characteristics of the two amines in flue gas. The solvent regeneration was studied in the temperature range of 368–382 K. From the overall experimental study, it can be seen that although the absorption rate of MEA was higher, AMP had a higher absorption capacity and a higher regeneration efficiency. Consequently, compared with MEA, AMP is a more favorable solvent and can be widely used as a CO2 absorber. Gao et al. [76] conducted tests on a new solution of 30 wt% MEA-methanol combined with a 30 wt% MEA aqueous solution in a pilot plant that encompassed the complete absorption and desorption processes. The experiment maintained a constant CO2 removal rate of 90% by adjusting the regenerative energy in the desorber, allowing for systematic variations in the liquid/gas ratio, flue gas flow rate, and absorber height. The results indicate that the MEA-methanol solution exhibited a faster CO2 absorption rate and lower regeneration energy consumption compared to the MEA aqueous solution. The heat load of regeneration of the MEA-methanol solvent was found to be significantly lower than that of the aqueous solution (3.22 MJ/kg CO2 and 4.04 MJ/kg CO2, respectively). This indicates that the MEA-methanol solvent has the potential to replace the MEA aqueous solution in an industrial CO2 pilot plant. The experimental setup is shown in Figure 5a.
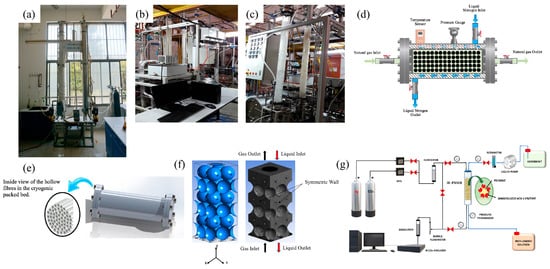
Figure 5.
(a) Photo of CO2 capture equipment in the lab of East China University of Science and Technology. Reprinted with permission from Ref. [76]. 2016 Elsevier. (b,c) Pilot-scale CO2 absorption/desorption unit in the University of Malaya. Reprinted with permission from Ref. [77]. 2020 American Chemical Society. (d,e) Cryogenic packed bed used in this research work. Reprinted with permission from Ref. [78]. 2019 Elsevier. (f) 3D geometry and meshed model [79]. 2018 this work is openly licensed via CC BY 4.0. (g) IE-PBMR experimental setup. Reprinted with permission from Ref. [80]. 2022 Elsevier.
Mirzaei et al. [77] employed monoethanolamine (MEA) (10 wt%), glycerol (10 wt%) aqueous solution, and a combination of monoethanolamine (10 wt%) and glycerol (10 wt%) to examine the removal of COCO2 from a COCO2-NCO2 mixture at a pilot-scale packed bed level. They utilized the ASPEN PLUS simulator to simulate the absorption process, with the simulation results subsequently validated against experimental data obtained from a pilot column. The experimental results showed that the CO2 absorption performance of MEA-glycerol mixture is better than that of MEA aqueous solution. Therefore, using MEA solvent for post-combustion CO2 capture can use glycerol as a promoter to improve CO2 absorption capacity. The experimental setup is shown in Figure 5b,c. Jaberi et al. [81] produced a novel adsorbent CC-hydrochar/ZIF-8-MOF for CO2 capture, which was prepared using (cigarette carbonite) CC-hydrochar as a carrier for ZIF-8-MOF growth. Nanofluids were prepared by dispersion in 30 wt% MEA solution. The CO2 capture performance of the prepared nanofluids was evaluated on a packed bed column. The results showed that the CO2 capture efficiency of CC-hydrochar/ZIF-8-MOF-based fluids could be significantly increased to more than 32.36% under optimal conditions, while the capture efficiency of pure MEA solution was 30%. Therefore, adding a small amount of CC-hydrochar/ZIF-8-MOF to the base solution can greatly improve CO2 capture efficiency, and the capture efficiency can reach 99.15%.
In addition, some researchers have developed new fillers from the aspect of equipment, aiming to obtain better CO2 absorption performance. Babar et al. [78] improved the performance of low-temperature packed beds by using new filler materials, such as cellulose acetate and CA/NH2-MIL-101(Al). Their study investigated CO2 capture efficiency, axial temperature distribution during cooling, and CO2 recovery steps for three different types of hollow fibers: spherical glass beads, CA hollow fibers, and CA/NH2-MIL-101(Al) composite hollow fibers. The results demonstrated that the CO2 capture efficiency of composite hollow fibers is 141.9% higher than that of spherical glass beads and 9.5% higher than that of pure CA hollow fibers. Additionally, temperature profile analysis revealed that pure CA and composite CA/NH2-MIL-101(Al) hollow fibers required less energy for cooling and achieved longer bed saturation times compared to glass beads. The NH2-MIL-101(Al) hollow fibers also helped reduce pressure drop, lower capital costs, and enhance CO2 capture efficiency. The experimental setup is illustrated in Figure 5d,e.
The fluid dynamics within the countercurrent packed bed are essential to gain an understanding of the design and operational parameters that may affect reactor and reaction efficiency during post-combustion CO2 capture. Li et al. [79] developed computational fluid dynamics (CFD) models to precisely examine the intricate details of flow within the packed bed. The simulation results clearly illustrated the evolution of the liquid distribution, wetting area, and film thickness at different gas–liquid flow rates. The influence of the number of liquid inlets on the flow behavior was also determined. By comparing the pressure drop measured by an experiment with the pressure drop determined by a simulation, it was found that there was a close correspondence and similar trend between the experimental and simulation data. The results show that the simulation model is effective and can reasonably predict the flow dynamics in countercurrent packed beds, which is shown in Figure 5f. Eduardo et al. [82] developed a 3D-printed structured packed bed device to enhance mass and heat transfer in a multiphase flow system, specifically investigating its impact on the CO2 absorption process using aqueous amines. The design, fabrication, and functional characteristics of the device are comprehensively reported. Hydrodynamic performance measurements were conducted, along with discussions on the heat transfer characteristics of this process-enhancing apparatus. Furthermore, temperature distribution within the system was thoroughly examined. A comprehensive heat transfer model was developed utilizing MFIX, a computational fluid dynamics software for multiphase systems; comparison between modeling results and experimental data validated the effectiveness of the MFIX model in predicting thermal behavior within an irrigating absorbent column setup. Future studies may explore the potential of this model to forecast thermal behavior in MEA-CO2 absorption systems.
The substantial dispersive particle size restricts the gas–liquid mass transfer rate of conventional absorption apparatus to a considerable extent. Micro-reaction technology, which facilitates gas–liquid processes at the microscale, addresses the limitations of traditional gas–liquid equipment, resulting in a significant enhancement in mass transfer performance by several orders of magnitude [83]. The mass transfer properties of different chemical devices are shown in Figure 6. Iliuta et al. [80] proposed a method to immobilize carbonic anhydrase II (hCAII) to enhance CO2 capture in a packed bed microreactor (IE-PBMR), as shown in Figure 5g. The hCAII enzyme was covalently immobilized on the surface of the filler (polyethylene solid particles) by glutaraldehyde (prior amine functionalization by co-deposition of polydopamine and polyethylenimine). Their study on enzyme-mediated CO2 capture revealed that an IE-PBMR, which can be incorporated into modular units, is a promising reactor device for CO2 removal. It holds considerable potential for environmentally friendly CO2 capture, especially in mitigating emissions from the residential, commercial, agricultural, and transportation sectors.

Figure 6.
Mass transfer properties of different chemical devices (where kLa is the mass transfer coefficient). Reprinted with permission from Ref. [74]. 2023 Elsevier.
4.2. Oxidation/Hydrogenation Reactions
The μPBR is representative of the multiphase packed bed due to its simple structure, low running cost, high heat and mass transfer efficiency, and favorable safety characteristics. It has been successfully applied to oxidation, hydrogenation, Fischer–Tropsch synthesis and other reactions [50].
Zhang et al. [84] developed a continuous μPBR platform (Figure 7a) to investigate the kinetics of the aerobic oxidation of ethylbenzene using the organic catalyst NHPI. The microreactor system improved gas–liquid mass transfer performance, resulting in a space–time yield of ethylbenzene oxidation that was enhanced by two orders of magnitude compared to the traditional batch reactor. In addition, the organic catalyst NHPI was covalently attached to commercial activated carbon through a simulated reaction. The synthesized heterogeneous catalyst exhibited remarkable efficacy, with a product yield of 87.4% in the continuous aerobic oxidation of ethylbenzene. Moreover, a continuous μPBR reactor platform was also developed to determine the kinetic parameters of the aerobic oxidation of ethyl lactate catalyzed by Cu/keto-ABNO [85]. The different operating parameters in the catalytic system were investigated, and their effects on the oxidation performance were quantified. Compared with the batch reactor, the micro-packed bed reactor enhanced the gas–liquid mass transfer, shortened the reaction time to 1/15, and increased the space–time yield by six times. Ultimately, the kinetic parameters of the reaction were established, and the applicability and accuracy of the kinetic model were validated through experimental tests. Cao et al. [86] used a continuous ozonation system (Figure 7b) based on a μPBR to improve the dissolution rate of ozone and facilitate the effective degradation of refractory organic pollutants. Yang et al. [87] used a μPBR reactor to achieve an efficient and ultrafast continuous-flow synthesis of FDCA. Using Au/CeO2 as the catalyst and O2 as the green oxidant, 100% HMF conversion and 90% FDCA selectivity were achieved within 41 s, which is 1–2 orders of magnitude higher than the space–time yield of conventional reactors. Their study not only provides an effective strategy for the synthesis of FDCA from HMF, but also opens up a new way to enhance the heterogeneous reaction under limited mass transfer conditions.
For the hydrogenation reaction, Duan et al. [88] established a continuous-flow selective hydrogenation system based on a μPBR reactor, using 5-nitroisoquinoline and 5-amino-1,2,3,4-tetrahydroisoquinoline as model reactions. Under optimal reaction conditions, maximum yields of 99.9% for 5-aminoisoquinoline and 99.3% for 5-amino-1,2,3,4-tetrahydroisoquinoline were achieved. The system has excellent performance in selective hydrogenation of related heterocyclic nitroaromatic hydrocarbons, and the yield exceeds 97.5%. Fan et al. [89] reported a continuous hydrogenation process of N-ethyl carbazole in a μPBR reactor (Figure 7c). Under the mild reaction condition of 80 °C and 4 Mpa, 1 wt% Ru/Al2O3 showed the best catalytic performance, achieving a N-ethyl carbazole conversion of 100% and a selectivity of dodecahydro-N-ethylcarbazole of 99.41% with hydrogen storage of 5.79 wt%. The hydrogenation kinetic model of N-ethyl carbazole was established, showing a good fit for each product. Wang et al. [90] developed a continuous-flow system based on a μPBR for hydrogenation of nitrile to primary amines and selected benzonitrile hydrogenation as the model reaction. Under optimal reaction conditions, benzonitrile showed the highest conversion (100%) and selectivity (99.1%) for benzylamine. The reaction kinetics were also assessed after addressing both internal and external diffusion limitations. The continuous-flow system utilizing the μPBR has shown good substrate applicability in other nitrile hydrogenation systems. In addition, researchers have also established kinetic models for partial hydrogenation. Duan et al. [91] developed a continuous-flow system based on a μPBR reactor (Figure 7d) and successfully established a kinetic model for the hydrogenation of m-dinitrobenzene. The activation energy and pre-exponential factor were determined using the kinetic network, and the kinetic model effectively fit the transformation of each substance. The kinetic model of hydrogenation of halogenated nitrobenzene was established by Duan et al. [92]. The accuracy of the kinetic model was validated through comparison with literature values, and the established model offers valuable insights for optimizing the hydrogenation process of halogenated nitroaromatic hydrocarbons in continuous-flow systems.
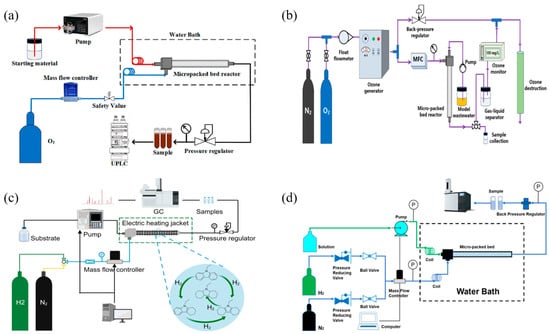
Figure 7.
(a) Schematic diagram of the continuous flow experimental apparatus. Reprinted with permission from Ref. [84]. 2023 Elsevier. (b) Schematic diagram of the continuous ozonation experimental apparatus. Reprinted with permission from Ref. [86]. 2020 Elsevier. (c) Schematic diagram of the N-ethylcarbazole continuous-flow hydrogenation system. Reprinted with permission from Ref. [89]. 2024 Elsevier. (d) Schematic diagram of the m-dinitrobenzene continuous-flow hydrogenation system. Reprinted with permission from Ref. [91]. 2021 Elsevier.
4.3. Wastewater Treatment
As conventional treatment in municipal wastewater treatment plants (WWTPs) has difficulty in removing many emerging contaminants (CECs), alternative treatment methods, including adsorption, membrane separation, photocatalysis [93], and ozonation, are currently under investigation. Of the oxidation processes used in this study, ozonation has proven to be a highly effective treatment method because ozone reacts directly with some organic matter and ozone decomposes (mainly at alkaline pH) to hydroxyl radical (HO·), which is a strongly non-selective oxidizing radical (ROS). The packed bed can make the gas–liquid contact more sufficient, greatly increasing the reaction efficiency [94].
Thorat et al. [95] proposed a method for separation and treatment of crude oil, desalination wastewater, acidic wastewater, alkaline wastewater, and oily wastewater in refineries. Combined systems such as ozonation + moving bed biofilm reactor (MBR) and photocatalysis + packed bed biofilm reactor (PBR) were discussed for the treatment of low biochemical index wastewaters. The packed bed ozonation process used in tertiary wastewater treatment has the advantages of a very short reaction time (usually a few minutes), high efficiency, and pollutant mineralization and has been widely used in wastewater treatment. Tabar et al. [96] treated switchgrass with ozone, which proved that ozone could selectively oxidize lignin with a good performance. It showed that a packed bed combined with ozone oxidation technology has a good effect on the treatment of paper mill effluent. Cao et al.’s [86] study based on a micro-packed bed reactor’s continuous ozonation system improved the dissolution rate of ozone and realized the rapid and efficient degradation of refractory organic pollutants. Chen et al. [97] synthesized easily separated carbon-silicon film (Mn-CSM) with excellent catalytic performance for refractory organic compounds by electrospinning. A Mn-CSM packed bed reactor was established for catalytic ozonation.
Pollution from tannery effluent is very common, producing about 30–40 m3 of effluent per ton of raw material processed, and represents one of the challenging issues confronted by numerous countries [98]. Vedaraman et al. [99] used a packed bed reactor to ozonize the tanning dye Acid Black 52 and studied the effects of the operating variables pH, dye concentration, and exposure time on the oxidation effect. Experiments were conducted in a PBR with a diameter of 5.5 cm and a height of 110 cm, featuring a working volume of 0.5 L of synthetic dye solution. The entire experimental setup utilized Teflon tubing. The process description of the experimental setup is given in Figure 8a.
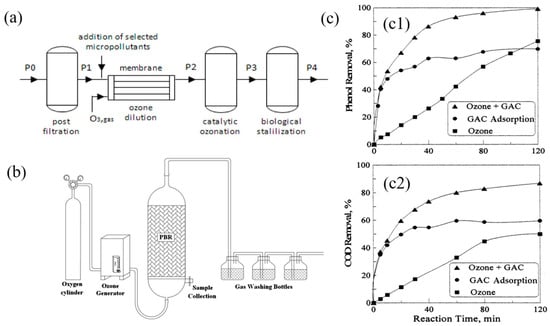
Figure 8.
(a) Schematic flowchart of the pre-industrial-scale unit from Vedaraman et al. [99]. 2024. This work is openly licensed via CC BY 4.0. (b) Schematic of experimental setup. Ozonation process in PBR for removal of AB 52 dye. Reprinted with permission from Ref. [99]. 2012 Springer-Verlag Berlin Heidelberg. (c) Phenol (c1) and COD (c2) removal for ozonation, GAC adsorption, and combined GAC adsorption and ozonation from Lin et al. [100]. 2003. This work is openly licensed via CC BY 4.0.
The vast majority of wastewater is toxic and cannot be discharged into the environment without tertiary treatment. Figure 8b is a pre-industrial-scale device; this unit comprises four distinct operational phases: post-filtration, ozone dilution, catalytic ozonation, and biological stabilization. To improve the biodegradability of poorly biodegradable industrial effluents and to break down refractory or toxic organic matter, ozone oxidation appears to be a promising advanced oxidation process.
In a packed bed of granular activated carbon (GAC), the combination of GAC adsorption, catalytic action, and ozonation demonstrated synergistic effects in enhancing phenol decomposition and reducing chemical oxygen demand (COD). This resulted from the facilitated adsorption and potential catalytic reactions by GAC. Ozonation not only improved phenol and COD removal but also regenerated GAC in situ, making it advantageous for industrial adsorption processes. Figure 8c shows that phenol removal increased steadily with ozonation alone, while rapid initial removal followed by a plateau was observed with GAC adsorption alone after 40 min. When GAC adsorption and ozonation were combined, the initial phenol removal matched that of GAC alone, indicating the dominant role played by GAC at this stage with minimal contribution from ozonation. However, as the process continued, both liquid- and solid-phase phenol oxidation were significantly impacted by ozonation alongside heterogeneous oxidation facilitated by GAC adsorption, accelerating phenol removal.
5. Development Prospects
Process intensification is widely recognized as a promising development path for the chemical engineering industry and a significant area of research. One of the key pieces of equipment for achieving process intensification is the packed bed, which is extensively used in chemical engineering, environmental engineering, and the process industry. The selection and design of filler materials are crucial for the performance of packed beds. Inappropriate fillers can lead to inefficient reactions or even complete loss of responsiveness.
The porosity, specific surface area, and chemical properties of the filler significantly influence fluid flow and reaction conduct. Poorly designed packed beds may cause uneven fluid distribution, increased local flow resistance, and reduced overall reaction rates. Advances in materials science have led to the development and application of new filler materials, such as functional nanomaterials, composites, and high-performance plastics. These materials offer enhanced corrosion resistance, high-temperature resistance, and improved chemical stability, thereby boosting the overall performance of packed beds. In bioreactors and wastewater treatment, the use of biological fillers has gained increasing attention. These fillers facilitate the adhesion and growth of microorganisms, enhancing biodegradation efficiency. The design of more complex porous fillers can increase surface area, optimize gas–liquid or liquid–solid contact, and improve reaction rates and separation efficiency. For instance, fillers with high specific surface area and high porosity are employed to enhance hydrodynamic characteristics and mass transfer performance.
Micro-packed beds have many advantages over macroscopic devices, namely, higher surface volume ratios leading to higher heat and mass transfer coefficients. This allows the reaction to be carried out under more aggressive conditions at a higher yield, which is not possible in large reactors. In addition, the rapid screening of many catalysts and the safe synthesis of dangerous substances can be considered as other advantages that prompt more research into microreactors. Micro-packed beds are a promising tool that have the potential to push industry portfolios in new application directions [101]. Today, many industry-renowned processes, such as hydrogenation [102], hydrodesulfurization [103], and Fischer–Tropsch synthesis [104,105,106], are carried out in multiphase micro-packed beds.
Additionally, the development of intelligent packed bed systems that monitor and automatically adjust operating conditions, such as flow, temperature, and pressure in real time can optimize performance and save energy.
With the continuous advancement of material science, design optimization and technological progress, the application of packed bed in various fields will be more and more extensive. Especially in the fields of environmental protection, energy and emerging technologies, the technological innovation and application expansion of packed beds will bring more opportunities to related industries.
Author Contributions
Conceptualization, C.D. and Z.R.; methodology, C.D.; software, Y.Z., Z.Z. and J.D.; resources, C.D., Y.T. and Z.R.; data curation, Y.Z., Z.Z. and J.D.; writing—original draft preparation, Y.Z., Z.Z. and J.D.; writing—review and editing, Y.Z. and C.D.; visualization, Y.Z. and C.D.; supervision, C.D., Y.T. and Z.R.; funding acquisition, C.D. and Z.R. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the National Natural Science Foundation of China, grant number 22125802, 22478018, 22338001.
Data Availability Statement
No new data were created or analyzed in this study.
Conflicts of Interest
The authors declare no conflicts of interest.
Nomenclature
Symbols: A sectional area of the packed bed, m2; a corrugation inclination angle, rad; ap characteristic geometric specific area, m2/m3; apcc geometric specific area of closed channels, m2/m3; as specific surface area, m2/m3; dc inner diameter of micro-packed bed reactor, m; dp pore diameter of packing, m; dp/dz pressure drop, kPa; dw window (pore) diameter, m; F liquid flow rate, m/s; f friction factor; GaL liquid Galileo number; g acceleration of gravity, m/s2; hL total liquid holdup, m3/m3; kLa mass transfer coefficient; L length, m; ReG gas Reynolds number; ReL liquid Reynolds number; uG gas velocity, m/s; uL liquid velocity, m/s; uLS superficial liquid velocity, m/s; Vb bed volume, m3; Vd liquid distributor volume, m3; Vf droplet volume in free spaces, m3; V1 total liquid volume, m3; VP liquid present in piping, m3; Vs sump volume, m3; WeL liquid Weber number; X open to total flow channel area ratio.
Greek letters: Г fraction of open cross-sectional area; ΔP/L unit pressure drop, kPa/m; ε porosity; μL liquid viscosity, Pa∙m; ρL liquid density, kg/m3.; σL liquid surface tension, N/m; τ liquid residence time in the packed bed determined through the RTD experiments, s; sphericity.
Indices: G gas; L liquid.
References
- Albo, J.; Qadir, M.I.; Samperi, M.; Fernandes, J.A.; de Pedro, I.; Dupont, J. Use of an optofluidic microreactor and Cu nanoparticles synthesized in ionic liquid and embedded in TiO2 for an efficient photoreduction of CO2 to methanol. Chem. Eng. J. 2021, 404, 126643. [Google Scholar] [CrossRef]
- Park, C.P.; Kim, D.-P. Dual-channel microreactor for gas−liquid syntheses. J. Am. Chem. Soc. 2010, 132, 10102–10106. [Google Scholar] [CrossRef] [PubMed]
- Polyzos, A.; O’Brien, M.; Petersen, T.P.; Baxendale, I.R.; Ley, S.V. The continuous-flow synthesis of carboxylic acids using CO2 in a tube-in-tube gas permeable membrane reactor. Angew. Chem.-Int. Ed. 2011, 50, 1190. [Google Scholar] [CrossRef] [PubMed]
- Susanti; Winkelman, J.G.; Schuur, B.; Heeres, H.J.; Yue, J. Lactic acid extraction and mass transfer characteristics in slug flow capillary microreactors. Ind. Eng. Chem. Res. 2016, 55, 4691–4702. [Google Scholar] [CrossRef]
- Wang, K.; Luo, G. Microflow extraction: A review of recent development. Chem. Eng. Sci. 2017, 169, 18–33. [Google Scholar] [CrossRef]
- Nguyen, D.T.; Leho, Y.T.; Esser-Kahn, A.P. A three-dimensional microvascular gas exchange unit for carbon dioxide capture. Lab A Chip 2012, 12, 1246–1250. [Google Scholar] [CrossRef]
- Zhang, Q.; Dong, Z.; Liu, Z.; Chen, G. Effect of ultrasonic waveforms on gas–liquid mass transfer in microreactors. AIChE J. 2022, 68, e17689. [Google Scholar] [CrossRef]
- Han, C.; Hu, Y.; Wang, K.; Luo, G. Preparation and in-situ surface modification of CaCO3 nanoparticles with calcium stearate in a microreaction system. Powder Technol. 2019, 356, 414–422. [Google Scholar] [CrossRef]
- Lu, Y.; Liu, Y.; Zhou, C.; Luo, G. Preparation of Li2CO3 nanoparticles by carbonation reaction using a microfiltration membrane dispersion microreactor. Ind. Eng. Chem. Res. 2014, 53, 11015–11020. [Google Scholar] [CrossRef]
- Yuan, P.; Lei, X.-Q.; Sun, H.-M.; Zhang, H.-W.; Cui, C.-S.; Yue, Y.-Y.; Liu, H.-Y.; Bao, X.-J.; Wang, T. Effects of pore size, mesostructure and aluminum modification on FDU-12 supported NiMo catalysts for hydrodesulfurization. Pet. Sci. 2020, 17, 1737–1751. [Google Scholar] [CrossRef]
- Behnejad, B.; Abdouss, M.; Tavasoli, A. Comparison of performance of Ni–Mo/γ-alumina catalyst in HDS and HDN reactions of main distillate fractions. Pet. Sci. 2019, 16, 645–656. [Google Scholar] [CrossRef]
- Kaiser, M.J. A review of refinery complexity applications. Pet. Sci. 2017, 14, 167–194. [Google Scholar] [CrossRef]
- Ghassabzadeh, H.; Rashidzadeh, M.; Niaei, A. A novel fast evaluation method for mesoporous NiMo/Al2O3 hydrodemetallization (HDM) catalysts: Activity and metal uptake capacity measurements. Mech. Catal. 2020, 130, 381–402. [Google Scholar] [CrossRef]
- Chi, K.; Zhao, Z.; Tian, Z.; Hu, S.; Yan, L.; Li, T.; Wang, B.; Meng, X.; Gao, S.; Tan, M. Hydroisomerization performance of platinum supported on ZSM-22/ZSM-23 intergrowth zeolite catalyst. Pet. Sci. 2013, 10, 242–250. [Google Scholar] [CrossRef]
- Dashliborun, A.M.; Larachi, F.; Hamidipour, M. Cyclic operation strategies in inclined and moving packed beds—Potential marine applications for floating systems. AIChE J. 2016, 62, 4157–4172. [Google Scholar] [CrossRef]
- Gorshkova, E.; Manninen, M.; Alopaeus, V.; Laavi, H.; Koskinen, J.; Simulation, N. Three-phase CFD-model for trickle bed reactors. Int. J. Nonlinear Sci. Numer. Simul. 2012, 13, 397–404. [Google Scholar] [CrossRef]
- Devarapalli, M.; Atiyeh, H.K.; Phillips, J.R.; Lewis, R.S.; Huhnke, R.L. Ethanol production during semi-continuous syngas fermentation in a trickle bed reactor using Clostridium ragsdalei. Bioresour. Technol. 2016, 209, 56–65. [Google Scholar] [CrossRef]
- Karimi, A.; Vahabzadeh, F.; Mohseni, M.; Mehranian, M. Decolorization of Maxilon-Red by Kissiris Immobilized Phanerochaete Chrysosporium in a Trickle-Bed Bioreactor-Involvement of Ligninolytic Enzymes. Iran. J. Chem. Chem. Eng. 2009, 28, 1–13. [Google Scholar]
- Rachbauer, L.; Voitl, G.; Bochmann, G.; Fuchs, W. Biological biogas upgrading capacity of a hydrogenotrophic community in a trickle-bed reactor. Appl. Energy 2016, 180, 483–490. [Google Scholar] [CrossRef]
- Üresin, E.; Saraç, H.İ.; Sarıoğlan, A.; Ay, Ş.; Akgün, F.J.P.S.; Protection, E. An experimental study for H2S and CO2 removal via caustic scrubbing system. Process Saf. Environ. Prot. 2015, 94, 196–202. [Google Scholar] [CrossRef]
- Abdullah, G.H.; Xing, Y. Hydrogen peroxide generation in divided-cell trickle bed electrochemical reactor. Ind. Eng. Chem. Res. 2017, 56, 11058–11064. [Google Scholar] [CrossRef]
- Bonrath, W. New trends in (heterogeneous) catalysis for the fine chemicals industry. Chimia 2014, 68, 485. [Google Scholar] [CrossRef] [PubMed]
- Zhang, T.; Yin, G.; Li, B.; Wang, X.; Jiang, S.; Yuan, Z. Improved process for 2, 3, 5-trimethylhydroquinone manufacture: Highly efficient catalytic hydrogenation of 2, 3, 5-trimethylbenzoquinone. Res. Chem. Intermed. 2015, 41, 663–677. [Google Scholar] [CrossRef]
- Ng, K. A model for flow regime transitions in cocurrent down-flow trickle-bed reactors. AIChE J. 1986, 32, 115–122. [Google Scholar] [CrossRef]
- Revankar, S.T. Pore scale model for flow regime transition in a trickle bed reactor. Chem. Eng. Commun. 2001, 184, 125–138. [Google Scholar] [CrossRef]
- Attou, A.; Ferschneider, G. A two-fluid hydrodynamic model for the transition between trickle and pulse flow in a cocurrent gas–liquid packed-bed reactor. Chem. Eng. Sci. 2000, 55, 491–511. [Google Scholar] [CrossRef]
- Boelhouwer, J.; Piepers, H.; Drinkenburg, A. Liquid-induced pulsing flow in trickle-bed reactors. Chem. Eng. Sci. 2002, 57, 3387–3399. [Google Scholar] [CrossRef]
- Cheaters, A. The Modeling of Coalescence Processes in Fluid-liquid Dispersions. Trans. Inst. Chem. Eng. 1991, 69, 259–270. [Google Scholar]
- Luo, H.; Svendsen, H.F. Theoretical model for drop and bubble breakup in turbulent dispersions. AIChE J. 1996, 42, 1225–1233. [Google Scholar] [CrossRef]
- Martínez-Bazán, C.; Montanes, J.; Lasheras, J.C. On the breakup of an air bubble injected into a fully developed turbulent flow. Part 1. Breakup frequency. J. Fluid Mech. 1999, 401, 157–182. [Google Scholar] [CrossRef]
- Jo, D.; Revankar, S.T. Bubble mechanisms and characteristics at pore scale in a packed-bed reactor. Chem. Eng. Sci. 2009, 64, 3179–3187. [Google Scholar] [CrossRef]
- Al-Dahhan, M.H.; Larachi, F.; Dudukovic, M.P.; Laurent, A. High-pressure trickle-bed reactors: A review. Ind. Eng. Chem. Res. 1997, 36, 3292–3314. [Google Scholar] [CrossRef]
- Duduković, M.P.; Larachi, F.; Mills, P.L. Multiphase catalytic reactors: A perspective on current knowledge and future trends. Catal. Rev. Sci. Eng. 2002, 44, 123–246. [Google Scholar] [CrossRef]
- Kister, H.Z. Distillation Design; McGraw-Hill: New York, NY, USA, 1992; Volume 1. [Google Scholar]
- Lau, V.; Rezkallah, K. New data on two-phase water-air hydrodynamics in vertical upward and downward tubes. In Proceedings of the annual conference of the Canadian Nuclear Association and 16th annual conference of the Canadian Nuclear Society, Saskatoon, SK, Canada, 28 February–2 March 1995. annual conference; Volume I and II. [Google Scholar]
- Liu, L.; Huang, N.; Liu, D. Divergency and consistency between frictional pressure drops from momentum balance and energy balance for two-phase flow. Chem. Eng. Sci. 2022, 247, 117070. [Google Scholar] [CrossRef]
- Losey, M.W.; Schmidt, M.A.; Jensen, K.F. Microfabricated Multiphase Packed-Bed Reactors: Characterization of Mass Transfer and Reactions. Ind. Eng. Chem. Res. 2001, 40, 2555–2562. [Google Scholar] [CrossRef]
- Inoue, T.; Adachi, J.; Ohtaki, K.; Lu, M.; Murakami, S.; Sun, X.; Wang, D.F. Direct hydrogen peroxide synthesis using glass microfabricated reactor—Paralleled packed bed operation. Chem. Eng. J. 2015, 278, 517–526. [Google Scholar] [CrossRef]
- Jo, D.; Revankar, S.T. Investigation of bubble breakup and coalescence in a packed-bed reactor–Part 1: A comparative study of bubble breakup and coalescence models. Int. J. Multiph. Flow 2011, 37, 995–1002. [Google Scholar] [CrossRef]
- Charpentier, J.C.; Favier, M. Some liquid holdup experimental data in trickle-bed reactors for foaming and nonfoaming hydrocarbons. AIChE J. 1975, 21, 1213–1218. [Google Scholar] [CrossRef]
- Satterfield, C.N. Trickle-bed reactors. AIChE J. 1975, 21, 209–228. [Google Scholar] [CrossRef]
- Charpentier, J.C.; Bakos, M.; Le Goff, P. Hydrodynamics of two-phase concurrent downflow in packed bed reactors: Gas-liquid flow regimes, liquid axial dispersion, and dead zones. In Proceedings of the Second Congress Applied Physical Chemistry, Veszprem, Hungary, 2–5 August 1971; Volume 1. [Google Scholar]
- Yasugi, N.; Fujitsu, A.; Odaira, N.; Ito, D.; Ito, K.; Saito, Y. Characteristics of Two-Phase Flow in Packed Bed Systems. In Proceedings of the International Conference on Nuclear Engineering ICONE, virtual conferenc, 4–6 August 2021; p. V004T014A066. [Google Scholar]
- Krishnamurthy, S.; Peles, Y. Gas-liquid two-phase flow across a bank of micropillars. Phys. Fluids 2007, 19, 043302. [Google Scholar] [CrossRef]
- Wada, Y.; Schmidt, M.A.; Jensen, K.F. Flow distribution and ozonolysis in gas—Liquid multichannel microreactors. Ind. Eng. Chem. Res. 2006, 45, 8036–8042. [Google Scholar] [CrossRef]
- Faridkhou, A.; Larachi, F. Hydrodynamics of Gas–Liquid Cocurrent Flows in Micropacked Beds Wall Visualization Study. Ind. Eng. Chem. Res. 2012, 51, 16495–16504. [Google Scholar] [CrossRef]
- Al-Rifai, N.; Galvanin, F.; Morad, M.; Cao, E.; Cattaneo, S.; Sankar, M.; Dua, V.; Hutchings, G.; Gavriilidis, A. Hydrodynamic effects on three phase micro-packed bed reactor performance–Gold–palladium catalysed benzyl alcohol oxidation. Chem. Eng. Sci. 2016, 149, 129–142. [Google Scholar] [CrossRef]
- Yang, L.; Shi, Y.; Abolhasani, M.; Jensen, K.F. Characterization and modeling of multiphase flow in structured microreactors: A post microreactor case study. Lab Chip 2015, 15, 3232–3241. [Google Scholar] [CrossRef] [PubMed]
- Márquez, N.; Moulijn, J.A.; Makkee, M.; Kreutzer, M.T.; Castaño, P. Tailoring the multiphase flow pattern of gas and liquid through micro-packed bed of pillars. React. Chem. Eng. 2019, 4, 838–851. [Google Scholar] [CrossRef]
- Liu, W.; Xie, B.Q.; Zhang, C.H.; Duan, X.N.; Zhang, J.S. Nature and characteristics of gas-liquid flow regimes in a micro-packed bed reactor. AIChE J. 2023, 69, 13. [Google Scholar] [CrossRef]
- Shulman, H.; Ullrich, C.; Wells, N. Performance of packed columns. I. Total, static, and operating holdups. AIChE J. 1955, 1, 247–253. [Google Scholar] [CrossRef]
- Hoogendoorn, C.; Lips, J. Axial mixing of liquid in gas-liquid flow through packed beds. Can. J. Chem. Eng. 1965, 43, 125–131. [Google Scholar] [CrossRef]
- Nemec, D.; Berčič, G.; Levec, J. Gravimetric method for the determination of liquid holdup in pressurized trickle-bed reactors. Ind. Eng. Chem. Res. 2001, 40, 3418–3422. [Google Scholar] [CrossRef]
- Boyer, C.; Fanget, B. Measurement of liquid flow distribution in trickle bed reactor of large diameter with a new gamma-ray tomographic system. Chem. Eng. Sci. 2002, 57, 1079–1089. [Google Scholar] [CrossRef]
- Chen, J.; Rados, N.; Al-Dahhan, M.H.; Duduković, M.P.; Nguyen, D.; Parimi, K. Particle motion in packed/ebullated beds by CT and CARPT. AIChE J. 2001, 47, 994–1004. [Google Scholar] [CrossRef]
- Yin, F.; Afacan, A.; Nandakumar, K.; Chuang, K.T. Liquid holdup distribution in packed columns: Gamma ray tomography and CFD simulation. Chem. Eng. Process. Process Intensif. 2002, 41, 473–483. [Google Scholar] [CrossRef]
- Olujic, Ž.; Behrens, M. Holdup and pressure drop of packed beds containing a modular catalytic structured packing. Chem. Eng. Technol. 2006, 29, 979–985. [Google Scholar] [CrossRef]
- Zakeri, A.; Einbu, A.; Svendsen, H.F. Experimental investigation of liquid holdup in structured packings. Chem. Eng. Res. Des. 2012, 90, 585–590. [Google Scholar] [CrossRef]
- Mohammed, I.; Bauer, T.; Schubert, M.; Lange, R. Hydrodynamic multiplicity in a tubular reactor with solid foam packings. Chem. Eng. J. 2013, 231, 334–344. [Google Scholar] [CrossRef]
- Sang, L.; Tu, J.; Cheng, H.; Luo, G.; Zhang, J. Hydrodynamics and mass transfer of gas–liquid flow in micropacked bed reactors with metal foam packing. Chem. Eng. J. 2020, 66, e16803. [Google Scholar] [CrossRef]
- Moreira, M.F.; Ferreira, M.C.; Freire, J.T. Total Liquid Saturation in Gas−Liquid Cocurrent Downflow and Upflow through Packed Beds and Analysis of Correlations for Predicting the Total Liquid Saturation. Ind. Eng. Chem. Res. 2004, 43, 1096–1102. [Google Scholar] [CrossRef]
- Kumar, R.K.; Rao, A.; Sankarshana, T.; Khan, A. Liquid holdup in concurrent gas liquid upflow through packed column with random and corrugated structured packing. In Proceedings of the World Congress on Engineering and Computer Science 2012, San Francisco, CA, USA, 23–25 October 2013. [Google Scholar]
- Zhang, J.; Teixeira, A.R.; Kögl, L.T.; Yang, L.; Jensen, K.F. Hydrodynamics of gas–liquid flow in micropacked beds: Pressure drop, liquid holdup, and two-phase model. AIChE J. 2017, 63, 4694–4704. [Google Scholar] [CrossRef]
- Hutter, C.; Zenklusen, A.; Lang, R.; von Rohr, P.R. Axial dispersion in metal foams and streamwise-periodic porous media. Chem. Eng. Sci. 2011, 66, 1132–1141. [Google Scholar] [CrossRef]
- Forchheimer, P. Water movement through ground. Z. Des Vereines Dtsch. Ingenieure 1901, 45, 1736–1741. [Google Scholar]
- Melli, T.R.; De Santos, J.M.; Kolb, W.B.; Scriven, L. Cocurrent downflow in networks of passages. Microscale roots of macroscale flow regimes. Ind.Eng. Chem.Res. 1990, 29, 2367–2379. [Google Scholar] [CrossRef]
- Tsochatzidis, N.A.; Karabelas, A.J. Experiments in trickle beds at the micro-and macroscale. Flow characterization and onset of pulsing. Ind.Eng. Chem.Res. 1994, 33, 1299–1309. [Google Scholar] [CrossRef]
- Benkrid, K.; Rode, S.; Pons, M.N.; Pitiot, P.; Midoux, N. Bubble flow mechanisms in trickle beds—An experimental study using image processing. Chem. Eng. Sci. 2002, 57, 3347–3358. [Google Scholar] [CrossRef]
- Rode, S.; Benkrid, K.; Gillier, T.; Midoux, N. Bubble flow in trickle beds: Investigations using resistive sensors. Chem. Eng. Sci. 2003, 58, 2995–3004. [Google Scholar] [CrossRef]
- Bordas, M.-L.; Cartellier, A.; Séchet, P.; Boyer, C. Bubbly flow through fixed beds: Microscale experiments in the dilute regime and modeling. AIChE J. 2006, 52, 3722–3743. [Google Scholar] [CrossRef]
- KIM, J.W.; LEE, W.K.J. Coalescence behavior of two bubbles in stagnant liquids. J. Chem. Eng. Jpn. 1987, 20, 448–453. [Google Scholar] [CrossRef]
- Kirkpatrick, R.; Lockett, M. The influence of approach velocity on bubble coalescence. Chem. Eng. Sci. 1974, 29, 2363–2373. [Google Scholar] [CrossRef]
- Heldebrant, D.J.; Kothandaraman, J.; Mac Dowell, N.; Brickett, L. Next steps for solvent-based CO2 capture; integration of capture, conversion, and mineralisation. Chem. Sci. 2022, 13, 6445–6456. [Google Scholar] [CrossRef]
- Sheng, L.; Wang, K.; Deng, J.; Chen, G.W.; Luo, G.S. Gas-liquid microdispersion and microflow for carbon dioxide absorption and utilization: A review. Curr. Opin. Chem. Eng. 2023, 40, 100917. [Google Scholar] [CrossRef]
- Khan, A.A.; Halder, G.N.; Saha, A.K. Carbon dioxide capture characteristics from flue gas using aqueous 2-amino-2-methyl-1-propanol (AMP) and monoethanolamine (MEA) solutions in packed bed absorption and regeneration columns. Int. J. Greenh. Gas Control 2015, 32, 15–23. [Google Scholar] [CrossRef]
- Gao, J.; Yin, J.; Zhu, F.F.; Chen, X.; Tong, M.; Kang, W.Z.; Zhou, Y.B.; Lu, J. Integration study of a hybrid solvent MEA-Methanol for post combustion carbon dioxide capture in packed bed absorption and regeneration columns. Sep. Purif. Technol. 2016, 167, 17–23. [Google Scholar] [CrossRef]
- Mirzaei, S.; Shamiri, A.; Aroua, M.K. CO2 Absorption/Desorption in Aqueous Single and Novel Hybrid Solvents of Glycerol and Monoethanolamine in a Pilot-Scale Packed Bed Column. Energy Fuels 2020, 34, 8503–8515. [Google Scholar] [CrossRef]
- Babar, M.; Bustam, M.A.; Ali, A.; Maulud, A.S.; Shafiq, U.; Shariff, A.M.; Man, Z. Eficient CO2 capture using NH2-MIL-101/CA composite cryogenic packed bed column. Cryogenics 2019, 101, 79–88. [Google Scholar] [CrossRef]
- Yang, L.; Liu, F.; Song, Z.C.; Liu, K.L.; Saito, K. 3D Numerical Study of Multiphase Counter-Current Flow within a Packed Bed for Post Combustion Carbon Dioxide Capture. Energies 2018, 11, 1441. [Google Scholar] [CrossRef]
- Iliuta, I.; Rasouli, H.; Iliuta, M.C. Evaluation of intensified CO2 capture in packed-bed microreactors with immobilized carbonic anhydrase by combined theory and experiment. Chem. Eng. J. 2023, 455, 140625. [Google Scholar] [CrossRef]
- Jaberi, H.; Mosleh, S.; Dashtian, K.; Salehi, Z. Fluid based cigarette carbonaceous hydrochar supported ZIF-8 MOF for CO2 capture process: The engineering parameters determination for the packed bed column design. Chem. Eng. Process.-Process Intensif. 2020, 153, 108001. [Google Scholar] [CrossRef]
- Miramontes, E.; Love, L.J.; Lai, C.H.; Sun, X.; Tsouris, C. Additively manufactured packed bed device for process intensification of CO2 absorption and other chemical processes. Chem. Eng. J. 2020, 388, 124092. [Google Scholar] [CrossRef]
- Zhu, K.; Yao, C.Q.; Liu, Y.Y.; Chen, G.W. Using expansion units to improve CO2 absorption for natural gas purification—A study on the hydrodynamics and mass transfer. Chin. J. Chem. Eng. 2021, 29, 35–46. [Google Scholar] [CrossRef]
- Zhang, C.H.; Luo, J.; Xie, B.Q.; Liu, W.; Zhang, J.S. Green and continuous aerobic oxidation of ethylbenzene over homogeneous and heterogeneous NHPI in a micro-packed bed reactor. Chem. Eng. J. 2023, 468, 143674. [Google Scholar] [CrossRef]
- Zhang, C.H.; Luo, J.; Xie, B.Q.; Liu, W.; Zhang, J.S. Continuous Cu/keto-ABNO catalyzed aerobic oxidation of ethyl lactate to ethyl pyruvate in a micro-packed bed reactor: Process optimization and reaction kinetics. Chem. Eng. Sci. 2024, 292, 119985. [Google Scholar] [CrossRef]
- Cao, Q.; Sang, L.; Tu, J.C.; Xiao, Y.S.; Liu, N.; Wu, L.D.; Zhang, J.S. Rapid degradation of refractory organic pollutants by continuous ozonation in a micro-packed bed reactor. Chemosphere 2021, 270, 128621. [Google Scholar] [CrossRef] [PubMed]
- Yang, W.Y.; Tang, X.J.; Li, W.J.; Luo, X.; Zhang, C.Y.; Shen, C. Fast and continuous synthesis of 2,5-furandicarboxylic acid in a micropacked-bed reactor. Chem. Eng. J. 2022, 442, 136110. [Google Scholar] [CrossRef]
- Duan, X.N.; Wang, X.P.; Chen, X.K.; Zhang, J.S. Continuous and Selective Hydrogenation of Heterocyclic Nitroaromatics in a Micropacked Bed Reactor. Org. Process Res. Dev. 2021, 25, 2100–2109. [Google Scholar] [CrossRef]
- Fan, Y.W.; Wang, P.X.; Zhang, J.H.; Huang, M.M.; Liu, W.; Xu, Y.L.; Duan, X.A.; Li, Y.Y.; Zhang, J.S. Continuous hydrogenation of N-ethylcarbazole in a micro-packed bed reactor for hydrogen storage. Chem. Eng. J. 2024, 484, 149404. [Google Scholar] [CrossRef]
- Wang, P.X.; Peng, Z.P.; Wang, X.P.; Lin, Y.; Hong, H.B.; Chen, F.; Chen, X.K.; Zhang, J.S. Continuous hydrogenation of nitriles to primary amines with high selectivity in flow. Chem. Eng. Sci. 2023, 269, 118460. [Google Scholar] [CrossRef]
- Duan, X.N.; Yin, J.B.; Huang, M.M.; Feng, A.X.; Fu, W.S.; Chen, H.X.; Huang, Z.F.; Ding, Y.G.; Zhang, J.S. Hydrogenation kinetics of m-dinitrobenzene in a continuous micro-packed bed reactor. Chem. Eng. Sci. 2022, 248, 117113. [Google Scholar] [CrossRef]
- Duan, X.N.; Yin, J.B.; Huang, M.M.; Wang, P.X.; Zhang, J.S. Hydrogenation kinetics of halogenated nitroaromatics over Pt/C in a continuous Micro-packed bed reactor. Chem. Eng. Sci. 2022, 251, 117483. [Google Scholar] [CrossRef]
- Akach, J.; Kabuba, J.; Ochieng, A.J.I.; Research, E.C. Simulation of the light distribution in a solar photocatalytic bubble column reactor using the Monte Carlo method. Ind. Eng. Chem. Res. 2020, 59, 17708–17719. [Google Scholar] [CrossRef]
- Chavez, A.M.; Ribeiro, A.R.; Moreira, N.F.E.; Silva, A.M.T.; Rey, A.; Alvarez, P.M.; Beltran, F.J. Removal of Organic Micropollutants from a Municipal Wastewater Secondary Effluent by UVA-LED Photocatalytic Ozonation. Catalysts 2019, 9, 472. [Google Scholar] [CrossRef]
- Narayan Thorat, B.; Kumar Sonwani, R. Current technologies and future perspectives for the treatment of complex petroleum refinery wastewater: A review. Bioresour. Technol. 2022, 355, 127263. [Google Scholar] [CrossRef]
- Tabar, I.B. Ozone as Oxidant for Biomass Pretreatment and Nanocellulose Production. Ph.D. Thesis, Purdue University, West Lafayette, IN, USA, 2017. [Google Scholar]
- Chen, S.; Ren, T.; Zhou, Z.; Lu, K.; Huang, X.; Zhang, X. Insights into Mn loaded carbon-silica-membrane based catalytic ozonation process for efficient wastewater treatment: Performance and mechanism. Chem. Eng. J. 2023, 475, 145874. [Google Scholar] [CrossRef]
- Suthanthararajan, R.; Ravindranath, E.; Chits, K.; Umamaheswari, B.; Ramesh, T.; Rajamam, S. Membrane application for recovery and reuse of water from treated tannery wastewater. Desalination 2004, 164, 151–156. [Google Scholar] [CrossRef]
- Vedaraman, N.; Begum, S.S.; Srinivasan, S.V. Response surface methodology for decolourisation of leather dye using ozonation in a packed bed reactor. Clean Technol. Environ. Policy 2013, 15, 607–616. [Google Scholar] [CrossRef]
- Lin, S.H.; Wang, C.H. Ozonation of phenolic wastewater in a gas-induced reactor with a fixed granular activated carbon bed. Ind. Eng. Chem. Res. 2003, 42, 1648–1653. [Google Scholar] [CrossRef]
- Faridkhou, A.; Hamidipour, M.; Larachi, F. Hydrodynamics of gas–liquid micro-fixed beds–measurement approaches and technical challenges. Chem. Eng. J. 2013, 223, 425–435. [Google Scholar] [CrossRef]
- Tadepalli, S.; Qian, D.; Lawal, A. Comparison of performance of microreactor and semi-batch reactor for catalytic hydrogenation of o-nitroanisole. Catal. Today 2007, 125, 64–73. [Google Scholar] [CrossRef]
- Bellos, G.; Papayannakos, N. The use of a three phase microreactor to investigate HDS kinetics. Catal. Today 2003, 79, 349–355. [Google Scholar] [CrossRef]
- Guettel, R.; Turek, T. Assessment of micro-structured fixed-bed reactors for highly exothermic gas-phase reactions. Chem. Eng. Sci. 2010, 65, 1644–1654. [Google Scholar] [CrossRef]
- Knobloch, C.; Güttel, R.; Turek, T. Holdup and pressure drop in micro packed-bed reactors for Fischer-Tropsch synthesis. Chem. Ing. Tech. 2013, 85, 455–460. [Google Scholar] [CrossRef]
- Knochen, J.; Güttel, R.; Knobloch, C.; Turek, T. Fischer–Tropsch synthesis in milli-structured fixed-bed reactors: Experimental study and scale-up considerations. Chem. Eng. Process.-Process Intensif. 2010, 49, 958–964. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).