On the Specific Diclofenac–Iron Cation Interaction for Selective Diclofenac Removal from a Water Solution
Abstract
1. Introduction
2. Materials and Methods
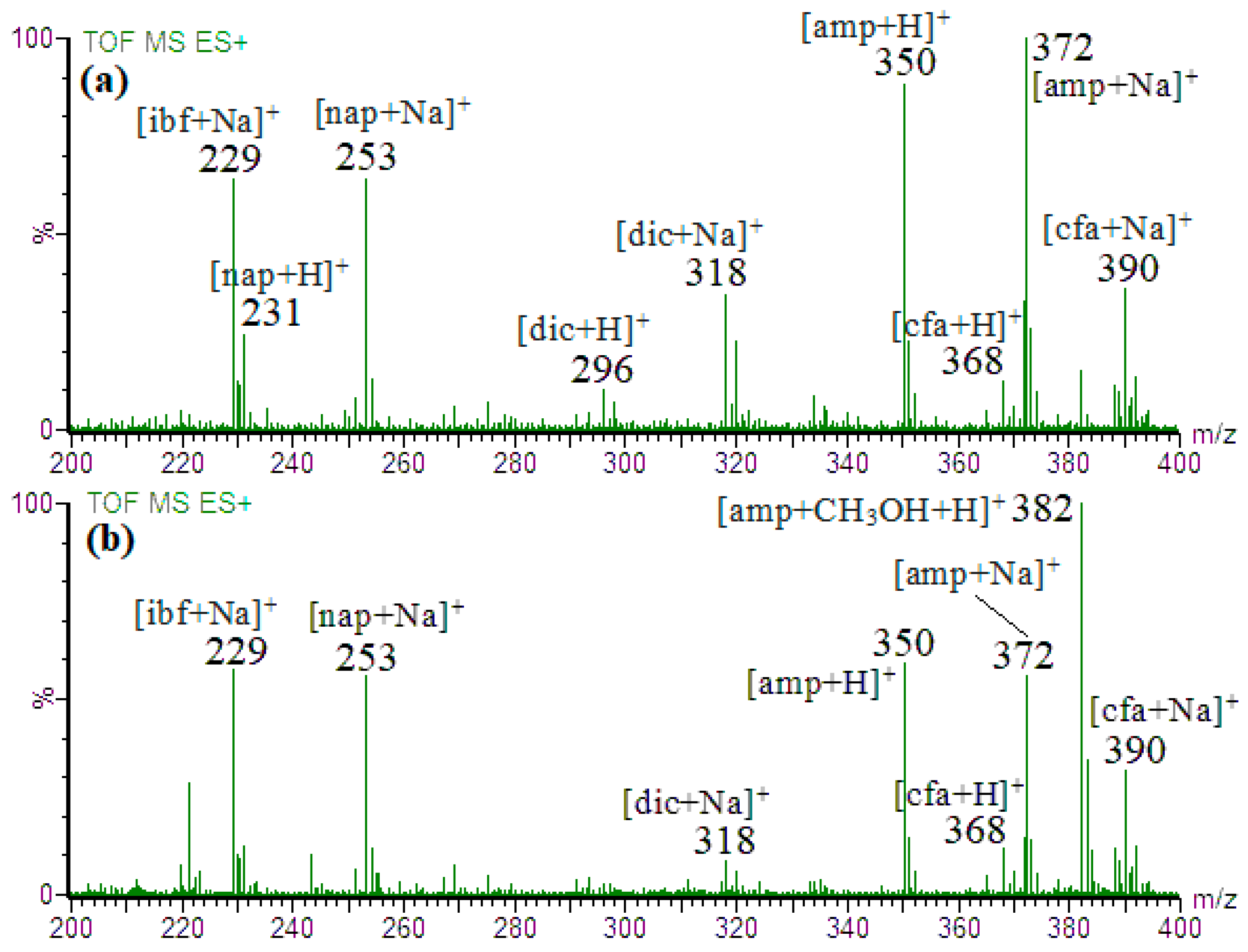
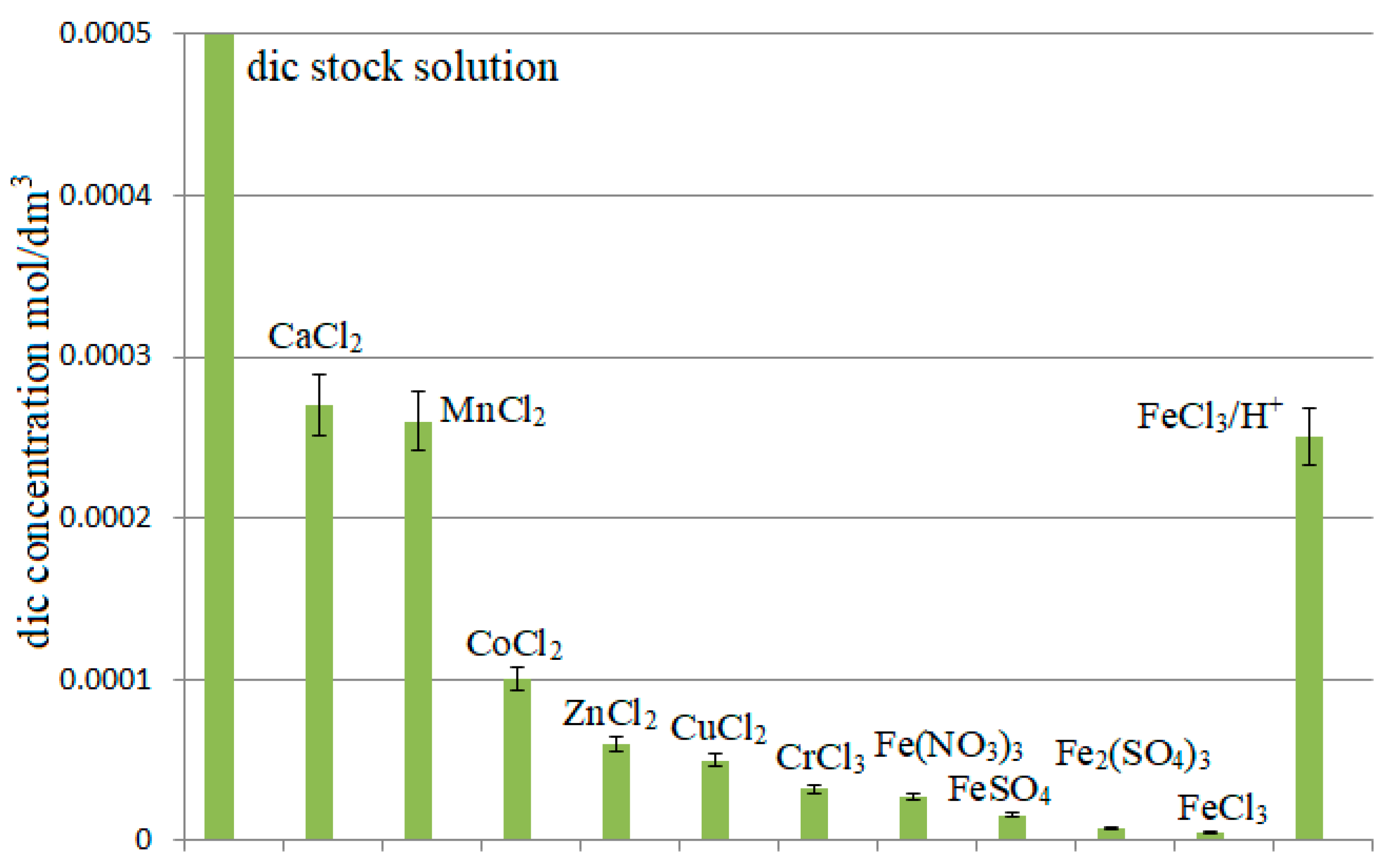
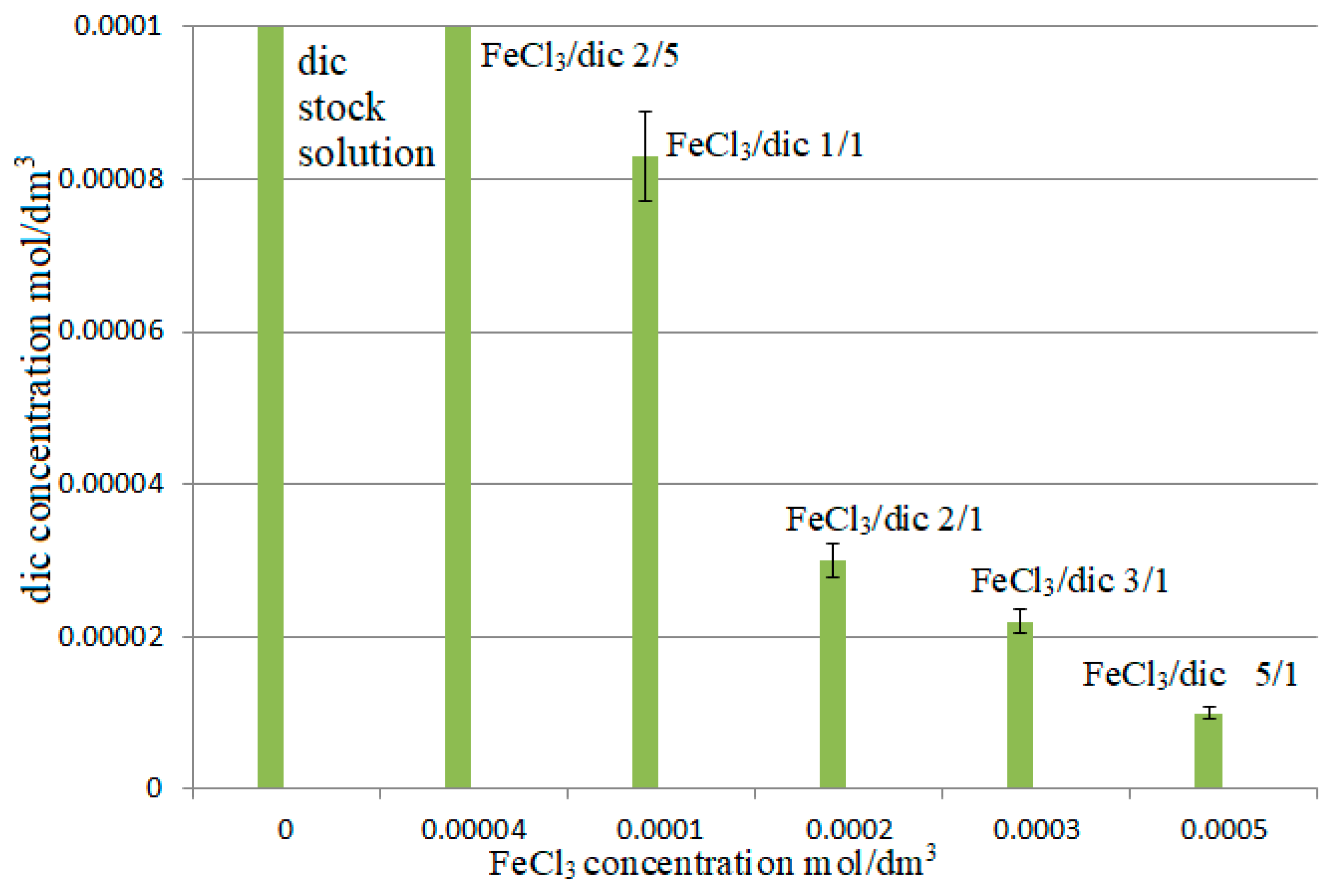
3. Results and Discussion
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Batucan, N.S.P.; Tremblay, L.A.; Northcott, G.L.; Matthaei, C.D. Medicating the environment? A critical review on the risks of carbamazepine, diclofenac and ibuprofen to aquatic organisms. Environ. Adv. 2021, 7, 100164. [Google Scholar] [CrossRef]
- Bonnefille, B.; Gomez, E.; Courant, F.; Escande, A.; Fenet, H. Diclofenac in the marine environment: A review of its occurrence and effects. Mar. Pollut. Bull. 2018, 131, 496–506. [Google Scholar] [CrossRef] [PubMed]
- Alessandretti, I.; Rigueto, C.V.T.; Nazari, M.T.; Rosseto, M.; Dettmer, A. Removal of diclofenac from wastewater: A comprehensive review of detection, characteristics and tertiary treatment techniques. J. Environ. Chem. Eng. 2021, 9, 106743. [Google Scholar] [CrossRef]
- Zhang, Y.; Geißen, S.U.; Gal, C. Carbamazepine and diclofenac: Removal in wastewater treatment plants and occurrence in water bodies. Chemosphere 2008, 73, 1151–1161. [Google Scholar] [CrossRef] [PubMed]
- de Carvalho Filho, J.A.A.; da Cruz, H.M.; Fernandes, B.S.; Motteran, F.; de Paiva, A.L.R.; Cabral, J.J.D.S.P. Efficiency of the bank filtration technique for diclofenac removal: A review. Environ. Pollut. 2022, 300, 118916. [Google Scholar] [CrossRef]
- Wang, Z.; Fu, Y.; Peng, Y.; Wang, S.; Liu, Y. HCO3−/CO32− enhanced degradation of diclofenac by Cu(II)-activated peracetic acid: Efficiency and mechanism. Sep. Purif. Technol. 2021, 277, 119434. [Google Scholar] [CrossRef]
- Huang, Y.; Lin, J.; Zou, J.; Xu, J.; Wang, M.; Cai, H.; Yuan, B. ABTS as an electron shuttle to accelerate the degradation of diclofenac with horseradish peroxidase-catalyzed hydrogen peroxide oxidation. Sci. Total Environ. 2021, 798, 149276. [Google Scholar] [CrossRef]
- Huang, Y.; Zou, J.; Lin, J.; Yang, H.; Wang, M.; Li, J.; Cao, W.; Yuan, B.; Ma, J. ABTS as Both Activator and Electron Shuttle to Activate Persulfate for Diclofenac Degradation: Formation and Contributions of ABTS●+, SO●−, and ●OH. Environ. Sci. Technol. 2023, 57, 18420–18432. [Google Scholar] [CrossRef]
- Poorsharbaf Ghavi, F.; Raouf, F.; Dadvand Koohi, A. A review on diclofenac removal from aqueous solution, emphasizing on adsorption method. Iran J. Chem. Chem. Eng. 2020, 39, 141–154. [Google Scholar]
- Mestre, A.S.; Carvalho, A.P. Photocatalytic degradation of pharmaceuticals carbamazepine, diclofenac, and sulfamethoxazole by semiconductor and carbon materials: A review. Molecules 2019, 24, 3702. [Google Scholar] [CrossRef]
- Oliveira, K.M.; Honorato, J.; Goncalves, G.R.; Cominetti, M.R.; Batista, A.A.; Correa, R.S. Ru(II)/diclofenac-based complexes: DNA, BSA interaction and their anticancer evaluation against lung and breast tumor cells. Dalton Trans. 2020, 49, 12643–12652. [Google Scholar] [CrossRef] [PubMed]
- García-García, A.; Mendez-Arriaga, J.M.; Martín-Escolano, R.; Cepeda, J.; Gómez-Ruiz, S.; Salinas-Castillo, A.; Seco, J.M.; Sánchez-Moreno, M.; Choquesillo-Lazarte, D.; Ruiz-Muelle, A.B.; et al. In vitro evaluation of leishmanicidal properties of a new family of monodimensional coordination polymers based on diclofenac ligand. Polyhedron 2020, 184, 114570. [Google Scholar] [CrossRef]
- Perontsis, S.; Dimitriou, A.; Fotiadou, P.; Hatzidimitriou, A.G.; Athanasios, N.; Papadopoulos, A.N.; Psomas, G. Cobalt(II) complexes with the non-steroidal anti-inflammatory drug diclofenac and nitrogen-donor ligands. J. Inorg. Biochem. 2019, 196, 110688. [Google Scholar] [CrossRef]
- Gacki, M.; Kafarska, K.; Wolf, W.M. A supramolecular polymeric chain in the cobalt(II) complex with diclofenac: Synthesis, crystal structure, spectroscopic, thermal and antioxidant activity. J. Coord. Chem. 2019, 72, 3481–3494. [Google Scholar] [CrossRef]
- Dimiza, F.; Perdih, F.; Tangoulis, V.; Turel, I.; Kessissoglou, D.P.; Psomas, G. Interaction of copper(II) with the non-steroidal anti-inflammatory drugs naproxen and diclofenac: Synthesis, structure, DNA-and albumin-binding. J. Inorg. Biochem. 2011, 105, 476–489. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Singh, P. Synthesis, characterization and antiinflammatory effects of Cr(III), Mn(II), Fe(III) and Zn(II) complexes with diclofenac sodium. Indian J. Chem. 2000, 39A, 874–876. [Google Scholar]
- Kovala-Demertzi, D. Transition metal complexes of diclofenac with potentially interesting anti-inflammatory activity. J. Inorg. Biochem. 2000, 79, 153–157. [Google Scholar] [CrossRef]
- Kobelnik, M.; Bernabé, G.A.; Ribeiro, C.A.; Capela, J.M.V.; Fertonani, F.L. Decomposition kinetics of iron (III)-diclofenac compound. J. Therm. Anal. Calorim. 2009, 97, 493–496. [Google Scholar] [CrossRef]
- Kenawi, I.M. Density functional theory assessment of the thermal degradation of diclofenac and its calcium and iron complexes. J. Mol. Struct. 2005, 754, 61–70. [Google Scholar] [CrossRef]
- Bucci, R.; Magrí, A.D.; Magrí, A.L.; Napoli, A. Spectroscopic characteristics and thermal properties of divalent metal complexes of diclofenac. Polyhedron 2000, 19, 2515–2520. [Google Scholar] [CrossRef]
- Agatonović-Kuštrin, S.; Ẑivanović, L.; Zečević, M.; Radulović, D. Spectrophotometric study of diclofenac-Fe(III) complex. J. Pharm. Biomed. Anal. 1997, 16, 147–153. [Google Scholar] [CrossRef] [PubMed]
- Kenawi, I.M.; Barsoum, B.N.; Youssef, M.A. Cetirizine dihydrochloride interaction with some diclofenac complexes. Eur. J. Pharm. Sci. 2005, 26, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Issa, M.M.; Nejem, R.M.; Al-Kholy, M.; El-Abadla, S.N.; Helles, S.R.; Saleh, A.A. An indirect atomic absorption spectrometric determination of ciprofloxacin, amoxycillin and diclofenac sodium in pharmaceutical formulations. J. Serb. Chem. Soc. 2008, 73, 569–576. [Google Scholar] [CrossRef]
- Gao, Y.Q.; Rao, Y.Y.; Ning, H.; Chen, J.-X.; Zeng, Q.; Tian, F.-X.; Gao, N.-Y. Comparative investigation of diclofenac degradation by Fe2+/chlorine and Fe2+/PMS processes. Sep. Pur. Technol. 2022, 297, 121555. [Google Scholar] [CrossRef]
- Zhao, J.; Wang, Q.; Fu, Y.; Peng, B.; Zhou, G. Kinetics and mechanism of diclofenac removal using ferrate(VI): Roles of Fe3+, Fe2+, and Mn2+. Environ. Sci. Pollut. Res. 2018, 25, 22998–23008. [Google Scholar] [CrossRef]
- Lin, J.; Hu, Y.; Xiao, J.; Huang, Y.; Wang, M.; Yang, H.; Zou, J.; Yuan, B.; Ma, J. Enhanced diclofenac elimination in Fe(II)/peracetic acid process by promoting Fe(III)/Fe(II) cycle with ABTS as electron shuttle. Chem. Eng. J. 2021, 420, 129692. [Google Scholar] [CrossRef]
- Zhang, N.; Li, J.M.; Liu, G.G.; Chen, X.L.; Jiang, K. Photodegradation of diclofenac in seawater by simulated sunlight irradiation: The comprehensive effect of nitrate, Fe(III) and chloride. Mar. Pollut. Bull. 2017, 117, 386–391. [Google Scholar] [CrossRef]
- Lin, J.; Zou, J.; Cai, H.; Huang, Y.; Li, J.; Xiao, J.; Yuan, B.; Ma, J. Hydroxylamine enhanced Fe (II)-activated peracetic acid process for diclofenac degradation: Efficiency, mechanism and effects of various parameters. Water Res. 2021, 207, 117796. [Google Scholar] [CrossRef]
- Américo-Pinheiro, J.H.P.; Salomão, G.R.; Paschoa, C.V.M.; Cruz, I.A.; Isique, W.D.; Ferreira, L.F.R.; Torres, N.H.; Bilal, M.; Iqbal, H.M.N.; Sillanpää, M.; et al. Effective adsorption of diclofenac and naproxen from water using fixed-bed column loaded with composite of heavy sugarcane ash and polyethylene terephthalate. Environ. Res. 2022, 211, 112971. [Google Scholar] [CrossRef]
- Younes, H.A.; Taha, M.; Mahmoud, R.; Mahmoud, H.M.; Abdelhameed, R.M. High adsorption of sodium diclofenac on post-synthetic modified zirconium-based metal-organic frameworks: Experimental and theoretical studies. J. Colloid. Interface Sci. 2022, 607, 334–346. [Google Scholar] [CrossRef]
- Podniesińska, L.; Frański, R.; Frańska, M. Comparison of the electrospray ionization (ESI) responses of penicillins with ESI responses of their methanolysis products. Eur. J. Mass Spectrom. 2019, 25, 357–361. [Google Scholar] [CrossRef]
- Montoya-Pelaez, P.J.; Brown, R.S. Methanolysis of nitrocefin catalyzed by one and two Zn2+ ions. A simplified model for class B β-lactamases. Inorg. Chem. 2002, 41, 309–316. [Google Scholar] [CrossRef] [PubMed]
- Montoya-Pelaez, P.J.; Gibson, G.T.; Neverov, A.A.; Brown, R.S. La3+-catalyzed methanolysis of N-aryl-β-lactams and nitrocefin. Inorg. Chem. 2003, 42, 8624–8632. [Google Scholar] [CrossRef] [PubMed]
- Martínez, J.H.; Navarro, P.G.; Garcia, A.A.M.; de las Parras, P.J.M. β-Lactam degradation catalysed by Cd2+ ion in methanol. Int. J. Biol. Macromol. 1999, 25, 337–343. [Google Scholar] [CrossRef] [PubMed]
- Frański, R.; Zalas, M.; Gierczyk, B.; Schroeder, G. Electro-oxidation of diclofenac in methanol as studied by high?performance liquid chromatography/electrospray ionization mass spectrometry. Rapid Commun. Mass Spectrom. 2016, 30, 1662–1666. [Google Scholar] [CrossRef]
- Kosjek, T.; Žigon, D.; Kralj, B.; Heath, E. The use of quadrupole-time-of-flight mass spectrometer for the elucidation of diclofenac biotransformation products in wastewater. J. Chromatogr. A 2008, 1215, 57–63. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Frańska, M.; Grześkowiak, A. On the Specific Diclofenac–Iron Cation Interaction for Selective Diclofenac Removal from a Water Solution. Separations 2024, 11, 285. https://doi.org/10.3390/separations11100285
Frańska M, Grześkowiak A. On the Specific Diclofenac–Iron Cation Interaction for Selective Diclofenac Removal from a Water Solution. Separations. 2024; 11(10):285. https://doi.org/10.3390/separations11100285
Chicago/Turabian StyleFrańska, Magdalena, and Aleksandra Grześkowiak. 2024. "On the Specific Diclofenac–Iron Cation Interaction for Selective Diclofenac Removal from a Water Solution" Separations 11, no. 10: 285. https://doi.org/10.3390/separations11100285
APA StyleFrańska, M., & Grześkowiak, A. (2024). On the Specific Diclofenac–Iron Cation Interaction for Selective Diclofenac Removal from a Water Solution. Separations, 11(10), 285. https://doi.org/10.3390/separations11100285





