Effect of Fly Ash on the Mass Transfer Performance of CO2 Removal Using MEA and DEA Solutions in a Packed Tower
Abstract
1. Introduction
2. Experimental Section
2.1. Experimental Setup
2.2. Properties and Principle of Absorbent
2.3. Fly Ash Used in Experiments
2.4. Experimental Methods and Processes
2.5. The Establishment of KGav Mathematical Relationship
3. Result and Discussion
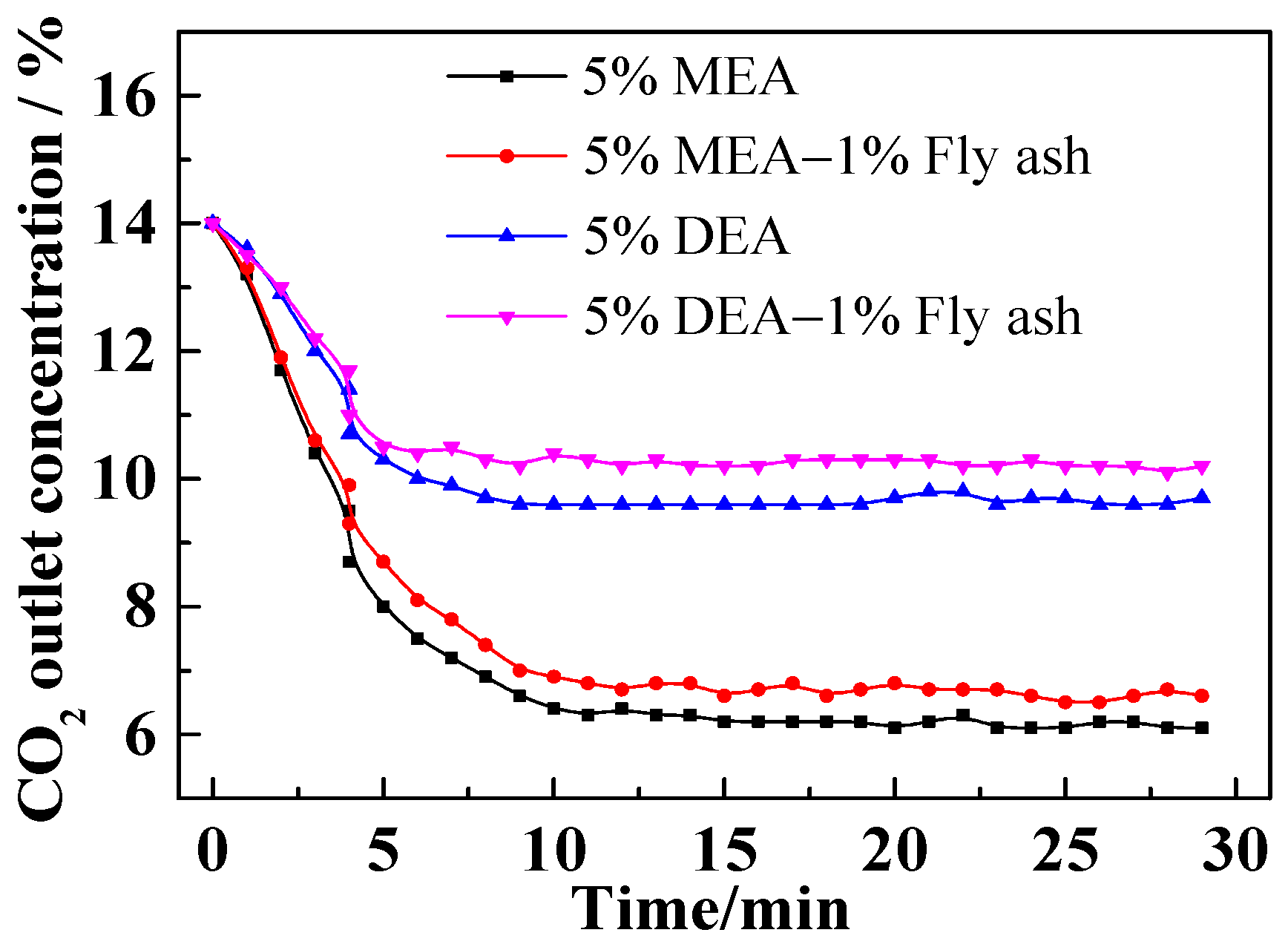
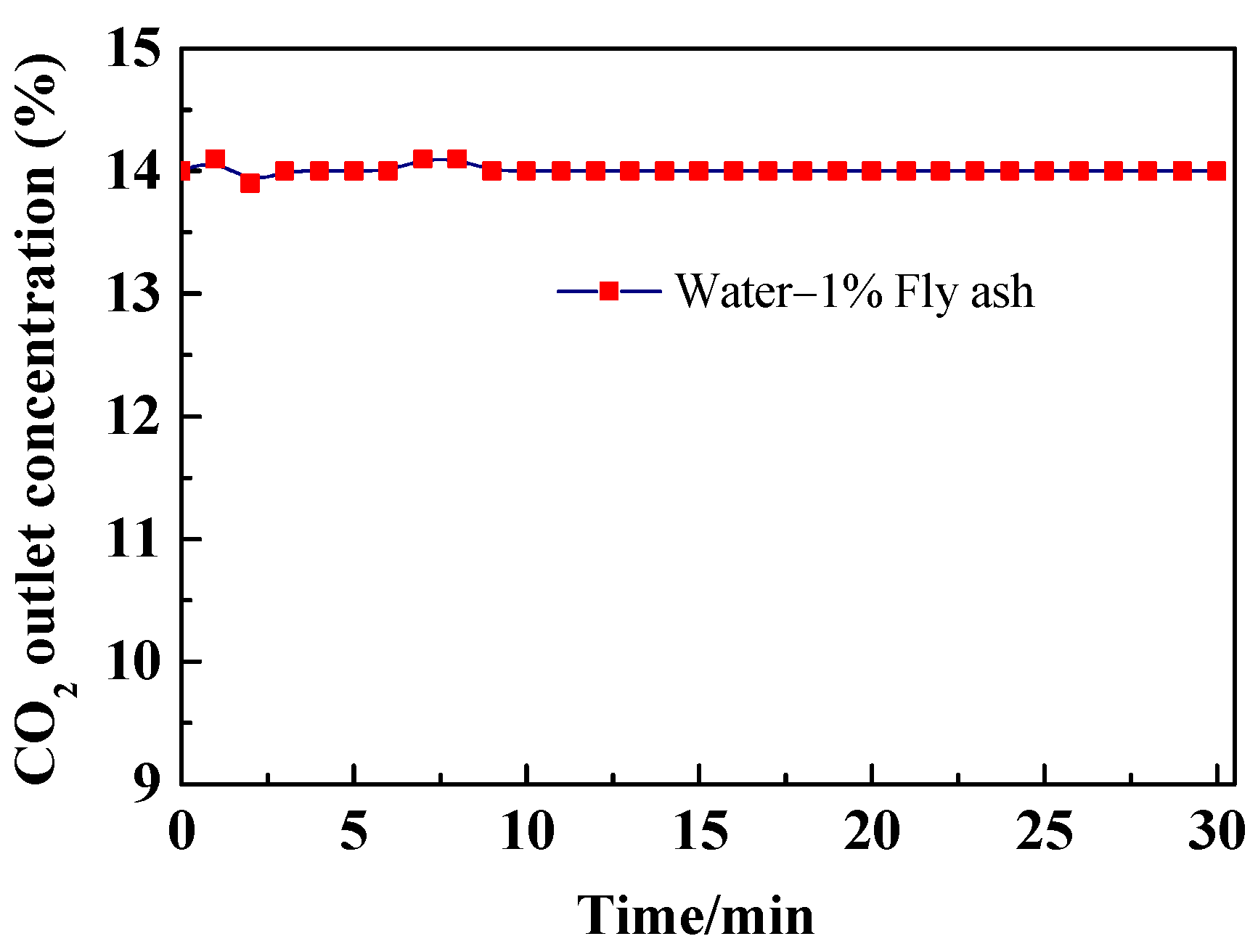
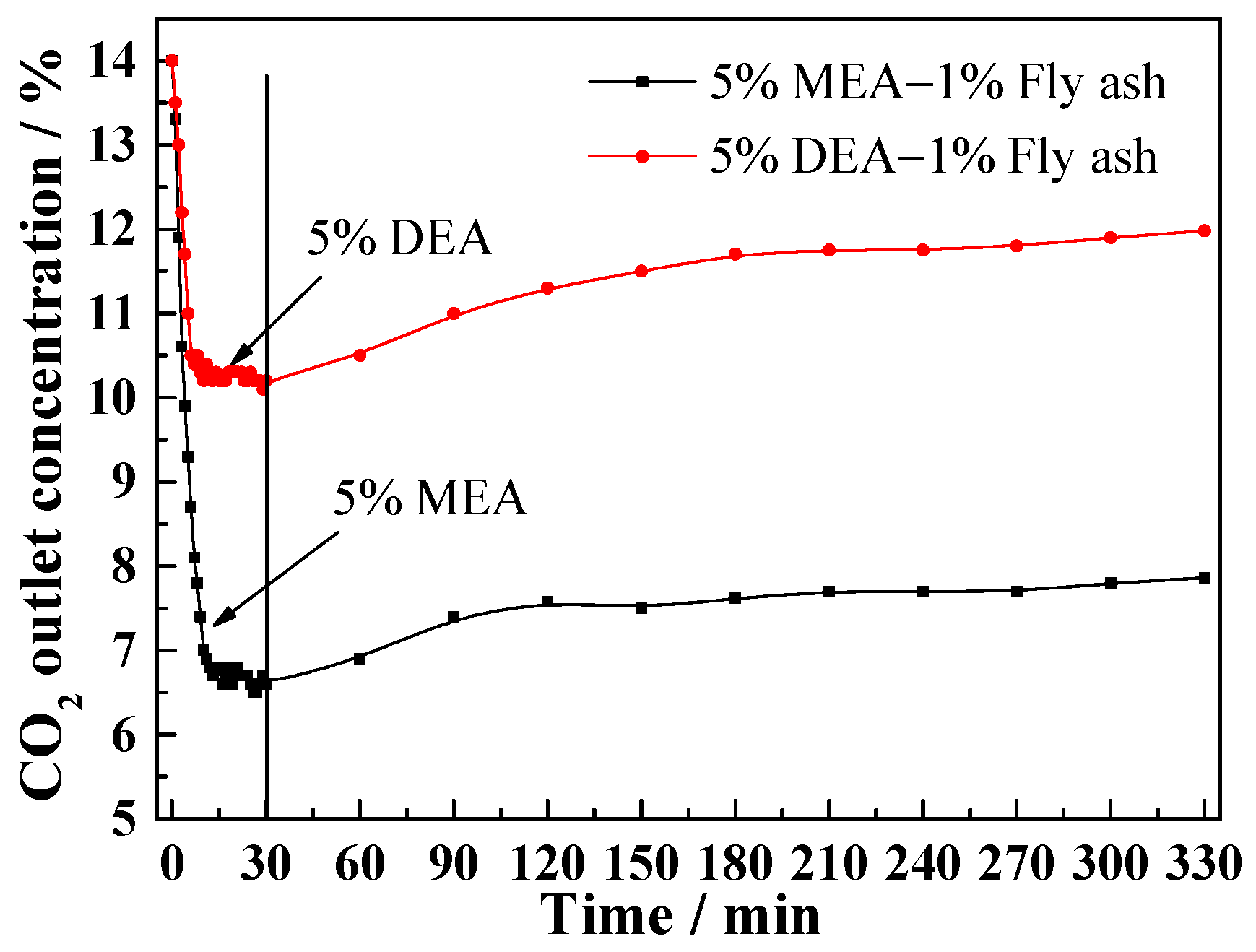
3.1. Effect of Fly Ash on CO2 Outlet Concentration
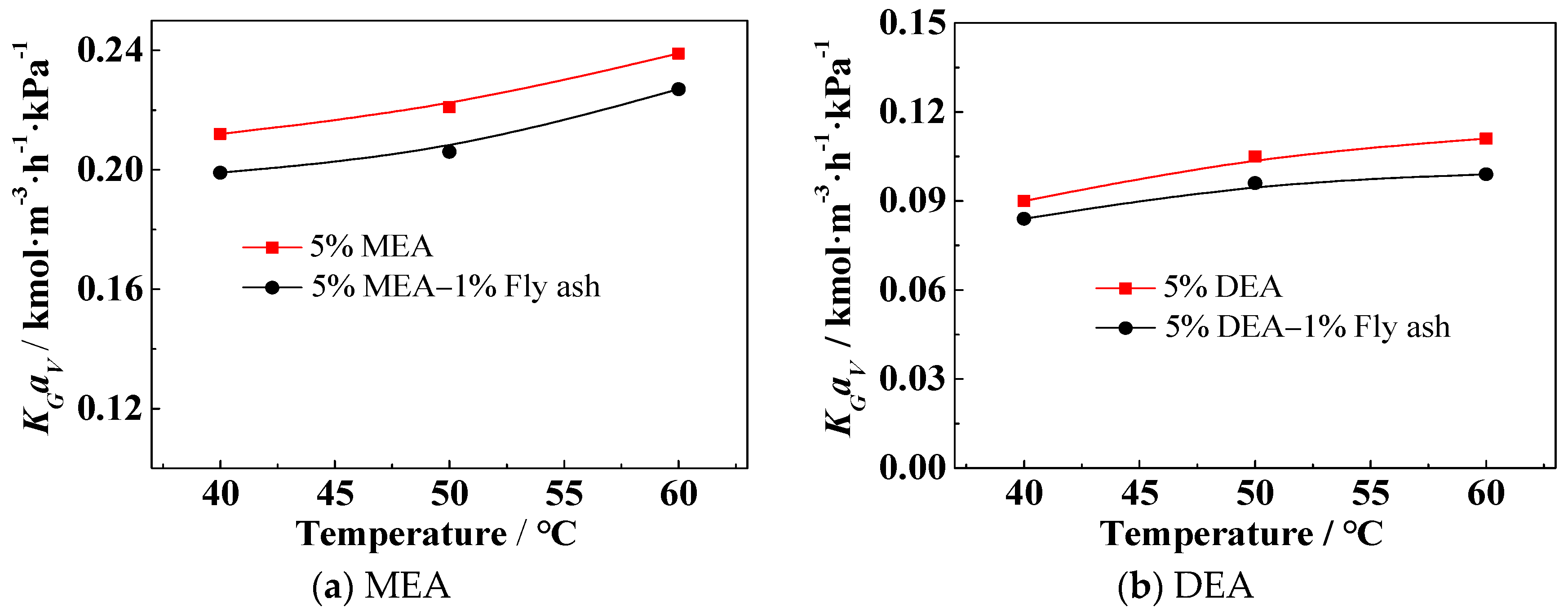
3.2. Effect of the Solution Temperature on KGav
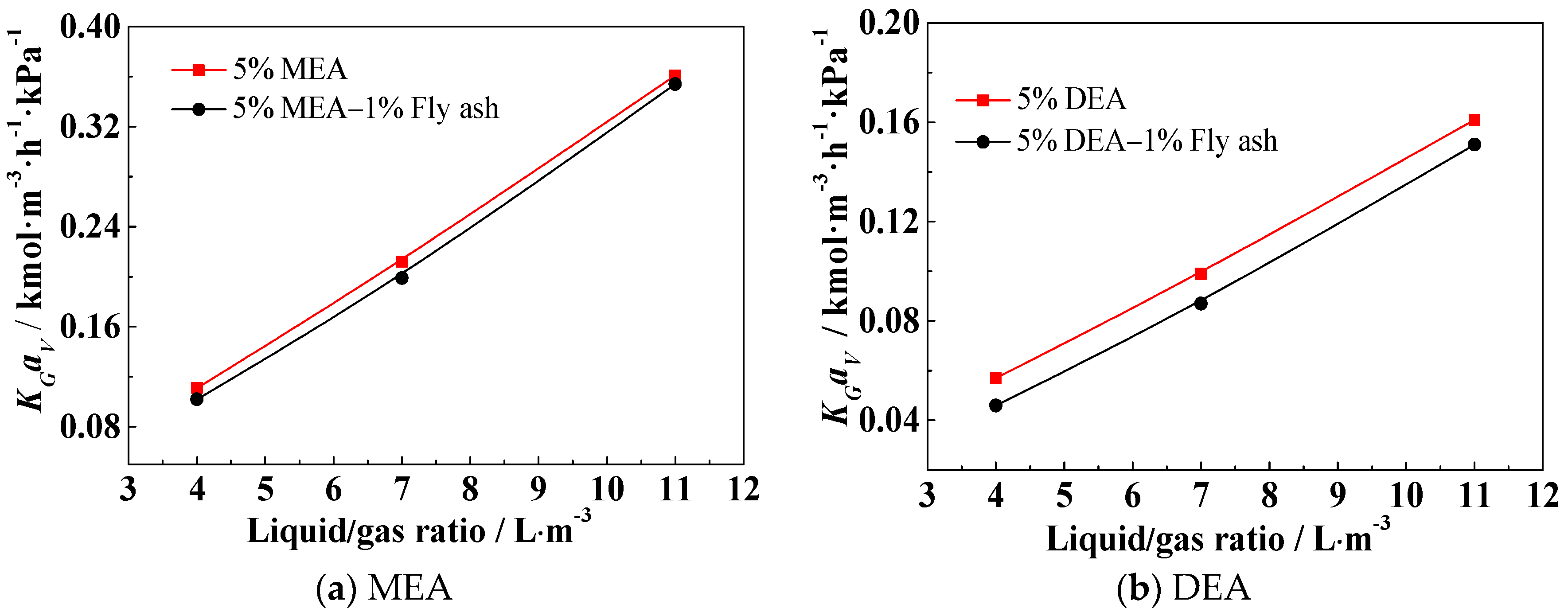
3.3. Effect of the Liquid/Gas Ratio (L/G) on KGav
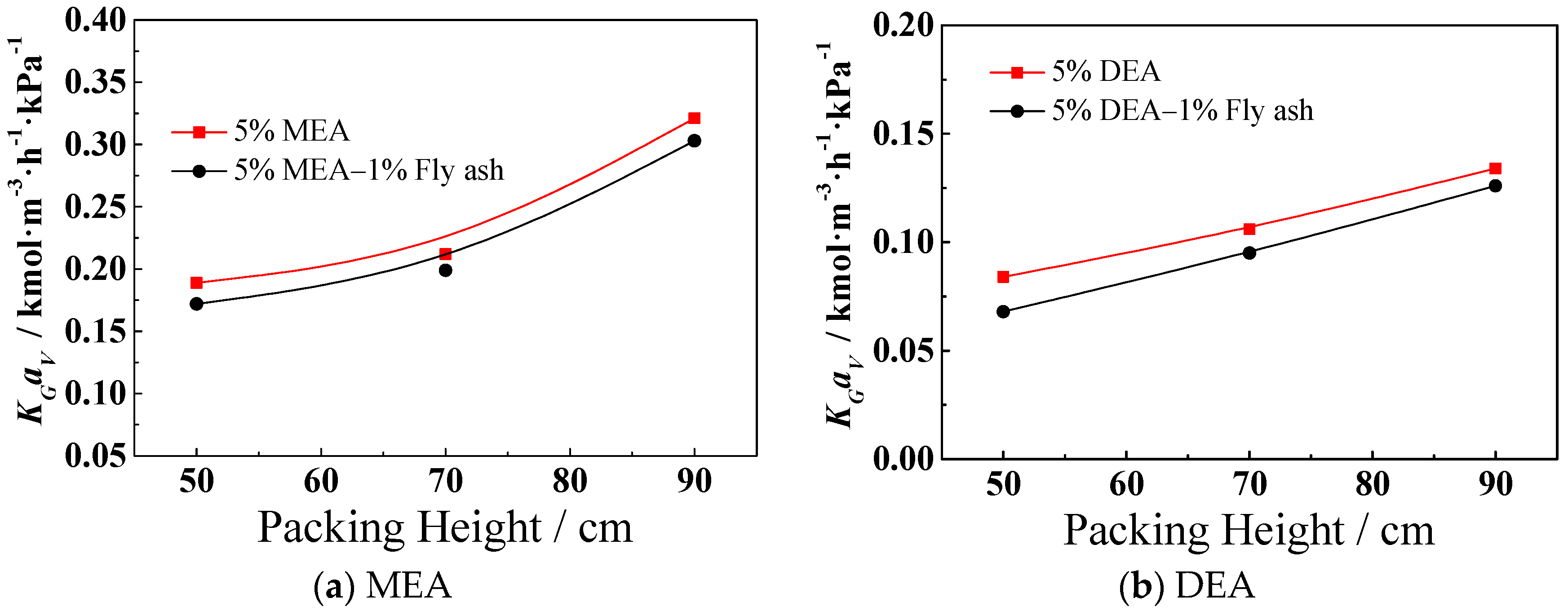
3.4. Effect of the Packing Height on KGav
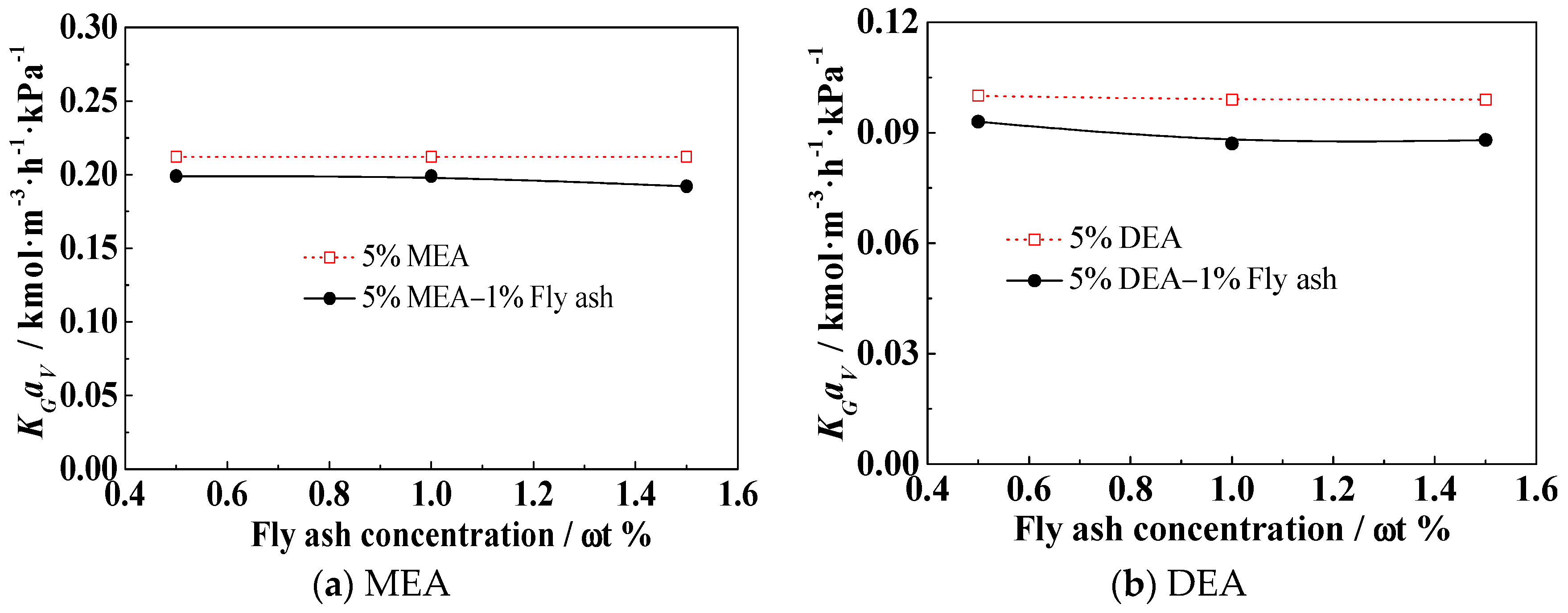
3.5. Effect of the Fly Ash Concentration on KGav
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Heidaryan, E.; Gouran, A.; Nejati, K.; Aghel, B. Enhancement of CO2 capture operation in oscillatory baffled reactor. Sep. Purif. Technol. 2023, 324, 124536. [Google Scholar] [CrossRef]
- IEA CO2 Emissions in 2022. IEA Paris. 2023. Available online: https://www.iea.org/reports/co2-emissions-in-2022 (accessed on 5 July 2023).
- Liu, F.; Guan, J.; Qi, Z.; Fang, M.; Shen, Z.; Li, C. Technology route selection for carbon capture utilization and storage in coal fired power plants. J. Huazhong Univ. Sci. Technol. 2022, 50, 1–13. [Google Scholar]
- Yuan, X.; Su, C.W.; Umar, M.; Shao, X.; LobonŢ, O.R. The Race to zero emissions: Can renewable energy be the path to carbon neutrality. Environ. Manag. 2022, 308, 114648. [Google Scholar] [CrossRef]
- Yoshimoto, Y.; Kishimoto, K.; Sen, K.K.; Mochida, T.; Chapman, A. Toward Economically Efficient Carbon Reduction: Contrasting Greening Plastic Supply Chains with Alternative Energy Policy Approaches. Sustainability 2023, 15, 13229. [Google Scholar] [CrossRef]
- British Petroleum. BP Statistical Review of World Energy; British Petroleum: London, UK, 2021. [Google Scholar]
- Liang, Z.; Fu, K.; Idem, R.; Tontiwachwuthikul, P. Review on current advances, future challenges and consideration issues for post-combustion CO2 capture using amine-based absorbents. Chin. J. Chem. Eng. 2016, 24, 278–288. [Google Scholar] [CrossRef]
- Wen, L.; Liu, H.; Rongwong, W.; Liang, Z.; Fu, K.; Idem, R.; Tontiwachwuthikul, P. Comparison of overall gas-phase mass transfer coefficient for CO2 absorption between tertiary amines in a randomly packed column. Chem. Eng. Technol. 2015, 38, 1435–1443. [Google Scholar] [CrossRef]
- Shukla, C.; Mishra, P.; Dash, S.K. A review of process intensified CO2 capture in RPB for sustainability and contribution to industrial net zero. Front. Energy Res. 2023, 11, 1135188. [Google Scholar] [CrossRef]
- Wang, R.; Liu, S.; Li, Q.; Zhang, S.; Wang, L.; An, S. CO2 capture performance and mechanism of blended amine solvents regulated by N-methylcyclohexyamine. Energy 2021, 215, 119209. [Google Scholar] [CrossRef]
- Liu, C.; Zhao, Z.; Shao, L.; Zhu, L.; Xu, F.; Jiang, X.; Zheng, C.; Gao, X. Experimental study and modified modeling on effect of SO2 on CO2 absorption using amine solution. Chem. Eng. J. 2022, 448, 137751. [Google Scholar] [CrossRef]
- Aghel, B.; Sahraie, S.; Heidaryan, E. Carbon dioxide desorption from aqueous solutions of monoethanolamine and diethanolamine in a microchannel reactor. Sep. Purif. Technol. 2020, 237, 116390. [Google Scholar] [CrossRef]
- Aghel, B.; Sahraie, S.; Heidaryan, E. Comparison of aqueous and non-aqueous alkanolamines solutions for carbon dioxide desorption in a microreactor. Energy 2020, 201, 117618. [Google Scholar] [CrossRef]
- Aghel, B.; Maleki, M.; Sahraie, S.; Heidaryan, E. Desorption of carbon dioxide from a mixture of monoethanolamine with alcoholic solvents in a microreactor. Fuel 2021, 306, 121636. [Google Scholar] [CrossRef]
- Islam, M.S.; Yusoff, R.; Ali, B.S.; Islam, M.N.; Chakrabarti, M.H. Degradation studies of amines and alkanolamines during sour gas treatment process. Int. J. Phys. Sci. 2011, 6, 5877–5890. [Google Scholar]
- Lawal, A.O.; Idem, R.O. Effects of operating variables on the product distribution and reaction pathways in the oxidative degradation of CO2-loaded aqueous MEA−MDEA blends during CO2 absorption from flue gas streams. Ind. Eng. Chem. Res. 2005, 44, 986–1003. [Google Scholar] [CrossRef]
- Supap, T.; Idem, R.; Tontiwachwuthikul, P.; Saiwan, C. Kinetics of sulfur dioxide- and oxygen-induced degradation of aqueous monoethanolamine solution during CO2 absorption from power plant flue gas streams. Int. J. Greenh. Gas Control 2009, 3, 133–142. [Google Scholar] [CrossRef]
- Yang, J.; Yu, X.; An, L.; Tu, S.T.; Yan, J. CO2 capture with the absorbent of a mixed ionic liquid and amine solution considering the effects of SO2 and O2. Appl. Energy 2017, 194, 9–18. [Google Scholar] [CrossRef]
- Chen, Z.; Yates, D.; Neathery, J.K.; Liu, K. The effect of fly ash on fluid dynamics of CO2 scrubber in coal-fired power plant. Chem. Eng. Res. Des. 2012, 90, 328–335. [Google Scholar] [CrossRef]
- Budzianowski, W.M.; Koziol, A. Stripping of ammonia from aqueous solutions in the presence of carbon dioxide: Effect of negative enhancement of mass transfer. Chem. Eng. Res. Des. 2005, 83, 196–204. [Google Scholar] [CrossRef]
- da Silva, E.F.; Lepaumier, H.; Grimstvedt, A.; Vevelstad, S.J.; Einbu, A.; Vernstad, K.; Svendsen, H.F.; Zahlsen, K. Understanding 2-ethanolamine degradation in postcombustion CO2 capture. Ind. Eng. Chem. Res. 2012, 51, 13329–13338. [Google Scholar] [CrossRef]
- Huang, Q.; Thompson, J.; Bhatnagar, S.; Chandan, P.; Remias, J.E.; Selegue, J.P.; Liu, K. Impact of flue gas contaminants on monoethanolamine thermal degradation. Ind. Eng. Chem. Res. 2013, 53, 553–563. [Google Scholar] [CrossRef]
- Zhang, X.; Zhang, A.; Li, S.; Li, J.; Zhang, Y.; Zhang, X.; He, Y. Analysis and countermeasures on deterioration of amine in CO2 removal system from flue gas. J. Northwest Univ. 2015, 45, 68–72. [Google Scholar]
- Salten, A.H.; Maćkowiak, J.F.; Maćkowiak, J.K.; Kenig, E.Y.A. New hydrodynamic analogy model for the determination of transport phenomena in random packings. Chem. Eng. Sci. 2021, 233, 116246. [Google Scholar] [CrossRef]
- Flagiello, D.; Parisi, A.; Lancia, A.; Di Natale, F. A Review on gas-liquid mass transfer coefficients in packed-bed columns. ChemEngineering 2021, 5, 43. [Google Scholar] [CrossRef]
- Lydonrochelle, M.T. Amine scrubbing for CO2 capture. Science 2009, 325, 1652–1654. [Google Scholar]
- Danckwerts, P.V. The reaction of CO2 with ethanolamines. Chem. Eng. Sci. 1979, 34, 443–446. [Google Scholar] [CrossRef]
- Vaidya, P.D.; Kenig, E.Y. CO2-alkanolamine reaction kinetics: A review of recent studies. Chem. Eng. Technol. 2007, 30, 1467–1474. [Google Scholar] [CrossRef]
- Aroonwilas, A.; Veawab, A.; Tontiwachwuthikul, P. Behavior of the mass-transfer coefficient of structured packings in CO2 absorbers with chemical reactions. Ind. Eng. Chem. Res. 1999, 38, 2044–2050. [Google Scholar] [CrossRef]
- Maneeintr, K.; Idem, R.O.; Tontiwachwuthikul, P.; Wee, A.G. Comparative mass transfer performance studies of CO2 absorption into aqueous solutions of DEAB and MEA. Ind. Eng. Chem. Res. 2010, 49, 2857–2863. [Google Scholar] [CrossRef]
- Treybal, R.E. Mass Transfer Operations; McGraw Hill: Kuala Lumpur, Malaysia, 1980; p. 466. [Google Scholar]
- Liang, Z.H.; Sanpasertparnich, T.; Tontiwachwuthikul, P.P.; Gelowitz, D.; Idem, R. Part 1: Design, modeling and simulation of postcombustion CO2 capture systems using reactive solvents. Carbon Manag. 2011, 2, 265–288. [Google Scholar] [CrossRef]
- Afkhamipour, M.; Mofarahi, M. Review on the mass transfer performance of CO2 absorption by amine-based solvents in low-and high-pressure absorption packed columns. RSC Adv. 2017, 7, 17857–17872. [Google Scholar] [CrossRef]
- Ling, H.; Gao, H.; Liang, Z. Comprehensive solubility of N2O and mass transfer studies on an effective reactive N,N-dimethylethanolamine (DMEA) solvent for post-combustion CO2 capture. Chem. Eng. J. 2019, 355, 369–379. [Google Scholar] [CrossRef]
- Gao, H.; Xu, B.; Han, L.; Luo, X.; Liang, Z. Mass transfer performance and correlations for CO2 absorption into aqueous blended of DEEA/MEA in a random packed column. AIChE J. 2017, 63, 3048–3057. [Google Scholar] [CrossRef]
- Ling, H.; Liu, S.; Wang, T.; Gao, H.; Liang, Z. Characterization and correlations of CO2 absorption performance into aqueous amine blended solution of monoethanolamine (MEA) and N, N-dimethylethanolamine (DMEA) in a packed column. Energy Fuels 2019, 33, 7614–7625. [Google Scholar] [CrossRef]
- Xie, W.; Zhang, J.; Xu, L.; Lv, J.; Zhong, H.; Sheng, C. Influence of particles on mass transfer performance for CO2 absorption using K2CO3 solution in a random θ-ring packed column. Int. J. Greenh. Gas Control 2017, 58, 81–86. [Google Scholar] [CrossRef]
- Zou, L.; Gao, H.; Wu, Z.; Liang, Z. Fast screening of amine/physical solvent systems and mass transfer studies on efficient aqueous hybrid MEA/Sulfolane solution for postcombustion CO2 capture. J. Chem. Technol. Biotechnol. 2020, 95, 649–664. [Google Scholar] [CrossRef]
- Naami, A.; Sema, T.; Edali, M.; Liang, Z.; Idem, R.; Tontiwachwuthikul, P. Analysis and predictive correlation of mass transfer coefficient KGav of blended MDEA-MEA for use in post-combustion CO2 capture. Int. J. Greenh. Gas Control 2013, 19, 3–12. [Google Scholar] [CrossRef]

| Type | Size (mm × mm) | Bulk Density (kg/m3) | Surface Area (m2/m3) | Void Fraction (%) |
|---|---|---|---|---|
| θ-ring | 3 × 3 | 520 | 1800 | 93 |
| Name | Viscosity (298 K)/mPa·s | Absorption Capacity (CO2/amine)/mol·mol−1 | Reaction Constan (298 K)/m3·kmol−1·s−1 |
|---|---|---|---|
| MEA | 18.95 | 0.35 | 5400 |
| DEA | 351.9 | 0.4 | 3000–4000 |
| ω(Na2O) | ω(MgO) | ω(Al2O3) | ω(SiO2) |
| 1.3 | 1.33 | 23.2 | 56.6 |
| ω(CaO) | ω(K2O) | ω(Fe2O3) | ω(others) |
| 6.1 | 2.4 | 7.3 | 1.77 |
| Parameter | Basic Conditions of Experiments | |
|---|---|---|
| Packed tower | Inside diameter (mm) | 35 |
| Height (mm) | 1300 | |
| MEA concentration | 5% (mass fraction) | |
| DEA concentration | 5% (mass fraction) | |
| CO2 initial concentration | 14% (volume fraction) | |
| Concentration of fly ash | 1% | |
| Solution temperature | 40 °C | |
| Packed height | 70 cm | |
| Flue gas flow | 7 L/min | |
| Liquid/gas ratio (L/G) | 7 L/m3 | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xie, W.; Tu, C.; Zhang, J.; Xu, C. Effect of Fly Ash on the Mass Transfer Performance of CO2 Removal Using MEA and DEA Solutions in a Packed Tower. Separations 2024, 11, 20. https://doi.org/10.3390/separations11010020
Xie W, Tu C, Zhang J, Xu C. Effect of Fly Ash on the Mass Transfer Performance of CO2 Removal Using MEA and DEA Solutions in a Packed Tower. Separations. 2024; 11(1):20. https://doi.org/10.3390/separations11010020
Chicago/Turabian StyleXie, Wenxia, Chunmin Tu, Jun Zhang, and Chengwei Xu. 2024. "Effect of Fly Ash on the Mass Transfer Performance of CO2 Removal Using MEA and DEA Solutions in a Packed Tower" Separations 11, no. 1: 20. https://doi.org/10.3390/separations11010020
APA StyleXie, W., Tu, C., Zhang, J., & Xu, C. (2024). Effect of Fly Ash on the Mass Transfer Performance of CO2 Removal Using MEA and DEA Solutions in a Packed Tower. Separations, 11(1), 20. https://doi.org/10.3390/separations11010020






