Nanomaterials for CO2 Capture from Gas Streams
Abstract
:1. Introduction
2. Nanomaterials and CO2 Absorption
3. Nanomaterials and CO2 Adsorption
4. CO2 Capture by Membrane Technologies
5. Conclusions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Yuan, Z.; Tang, J.; Chen, D.; Li, Y.; Hong, Z.; He, X. Membranes for hydrogen rainbow toward industrial decarbonization: Status, challenges and perspectives from materials to processes. Chem. Eng. J. 2023, 470, 144328. [Google Scholar] [CrossRef]
- Ahmad, I.; Alayande, A.B.; Jee, H.; Wang, Z.; Park, Y.-J.; Im, K.S.; Nam, S.Y. Recent progress of MXene-based membranes for high-performance and efficient gas separation. Diam. Relat. Mater. 2023, 135, 109883. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, Z.; Luo, W.; Wang, Y.; Mu, P.; Li, J. A review on the recent advances in composite membranes for CO2 capture processes. Sep. Purif. Technol. 2023, 307, 122752. [Google Scholar] [CrossRef]
- Luo, W.; Li, F.; Li, H.; Zhang, Z.; Zhang, X.; Liang, Y.; Huang, G. From 0D to 3D nanomaterial-based composite membranes for CO2 capture: Recent advances and perspectives. J. Environ. Chem. Eng. 2023, 11, 110657. [Google Scholar] [CrossRef]
- Dai, Y.; Niu, Z.; Wang, Y.; Zhong, S.; Mu, P.; Li, J. Recent advances and prospect of emerging microporous membranes for high-performance CO2 capture. Sep. Purif. Technol. 2023, 318, 123992. [Google Scholar] [CrossRef]
- Alli, Y.A.; Oladoye, P.O.; Ejeromedoghene, O.; Bankole, O.M.; Alimi, O.A.; Omotola, E.O.; Olanrewaju, C.A.; Philippot, K.; Adeleye, A.S.; Ogunlaja, A.S. Nanomaterials as catalysts for CO2 transformation into value-added products: A review. Sci. Total Environ. 2023, 868, 161547. [Google Scholar] [CrossRef]
- Baena-Moreno, F.M.; Leventaki, E.; Riddell, A.; Wojtasz-Mucha, J.; Bernin, D. Effluents and residues from industrial sites for carbon dioxide capture: A review. Environ. Chem. Lett. 2023, 21, 319–337. [Google Scholar] [CrossRef]
- Hanifa, M.; Agarwal, R.; Sharma, U.; Thapliyal, P.C.; Singh, L.P. A review on CO2 capture and sequestration in the construction industry: Emerging approaches and commercialised technologies. J. CO2 Util. 2023, 67, 102292. [Google Scholar] [CrossRef]
- Li, H. CO2 capture by various nanoparticles: Recent development and prospective. J. Clean. Prod. 2023, 414, 137679. [Google Scholar] [CrossRef]
- Segneri, V.; Trinca, A.; Libardi, N.; Colelli, L.; Micciancio, M.; Vilardi, G. Nanoparticles used for CO2 capture by adsorption: A review. Chem. Eng. Trans. 2023, 101, 133–138. [Google Scholar] [CrossRef]
- Youns, Y.T.; Manshad, A.K.; Ali, J.A. Sustainable aspects behind the application of nanotechnology in CO2 sequestration. Fuel 2023, 349, 128680. [Google Scholar] [CrossRef]
- Azni Farhana Mazri, N.; Arifutzzaman, A.; Kheireddine Aroua, M.; Ekhlasur Rahman, M.; Ali Mazari, S. Graphene and its tailoring as emerging 2D nanomaterials in efficient CO2 absorption: A state-of-the-art interpretative review. Alex. Eng. J. 2023, 77, 479–502. [Google Scholar] [CrossRef]
- Chen, H.; Zheng, Y.; Li, J.; Li, L.; Wang, X. AI for nanomaterials development in clean energy and carbon capture, utilization and storage (CCUS). ACS Nano 2023, 17, 9763–9792. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.; Yang, T.; Han, J.; Zhang, Y.; Zhao, L.; Zhao, J.; Li, R.; Huang, Y.; Gu, Z.; Wu, J. The application of mineral kaolinite for environment decontamination: A review. Catalysts 2023, 13, 123. [Google Scholar] [CrossRef]
- Cui, Y.; Zhu, J.; Tong, H.; Zou, R. Advanced perspectives on MXene composite nanomaterials: Types synthetic methods, thermal energy utilization and 3D-printed techniques. iScience 2023, 26, 105824. [Google Scholar] [CrossRef] [PubMed]
- Younis, M.; Ahmad, S.; Atiq, A.; Farooq, M.A.; Huang, M.; Abbas, M. Recent progress in azobenzene-based supramolecular materials and applications. Chem. Rec. 2023, 23, e202300126. [Google Scholar] [CrossRef] [PubMed]
- Zhu, W.; Yue, Y.; Wang, H.; Zhang, B.; Hou, R.; Xiao, J.; Huang, X.; Ishag, A.; Sun, Y. Recent advances on energy and environmental application of graphitic carbón nitride (g-C3N4)-based photocatalysts: A review. J. Environ. Chem. Eng. 2023, 12, 110164. [Google Scholar] [CrossRef]
- de Morais, M.G.; Vargas, B.P.; da Silva Vaz, B.; Cardias, B.B.; Costa, J.A.V. Advances in the synthesis and applications of nanomaterials to increase CO2 biofixation in microalgal cultivation. Clean Technol. Environ. Policy 2023, 25, 617–632. [Google Scholar] [CrossRef]
- Waseem, M.; Al-Marzouqi, M.; Ghasem, N. A review of catalytically enhanced CO2-rich amine solutions regeneration. J. Environ. Chem. Eng. 2023, 11, 110188. [Google Scholar] [CrossRef]
- Chowdhury, S.; Kumar, Y.; Shrivastava, S.; Patel, S.K.; Sangwai, J.S. A review on the recent scientific and commercial progress on the direct air capture technology to manage atmospheric CO2 concentrations and future erspectives. Energy Fuels 2023, 37, 10733–10757. [Google Scholar] [CrossRef]
- Jaiswar, G.; Dabas, N.; Chaudhary, S.; Jain, V.P. Progress in absorption of environmental carbon dioxide using nanoparticles and membrane technology. Int. J. Environ. Sci. Technol. 2023, 20, 10385–10404. [Google Scholar] [CrossRef]
- Peu, S.D.; Das, A.; Hossain, M.S.; Akanda, M.A.; Akanda, M.M.; Rahman, M.; Miah, M.N.; Das, B.K.; Islam, A.R.; Salah, M.M. A comprehensive review on recent advancements in absorption-based post combustion carbon capture technologies to obtain a sustainable energy sector with clean environment. Sustainability 2023, 15, 5827. [Google Scholar] [CrossRef]
- Zhang, C.; Zhang, X.; Su, T.; Zhang, Y.; Wang, L.; Zhu, X. Modification schemes of efficient sorbents for trace CO2 capture. Renew. Sustain. Energy Rev. 2023, 18, 113473. [Google Scholar] [CrossRef]
- Boldoo, T.; Ham, J.; Cho, H. Evaluation of CO2 absorption characteristics of low cost Al2O3/MeOH nanoabsorbent using porous nickel foam for high efficiency CO2 absorption system. J. Clean. Prod. 2023, 384, 135624. [Google Scholar] [CrossRef]
- Ge, B.-B.; Yan, J.; Zhong, D.-L.; Lu, Y.-Y.; Li, X.-Y. CO2 capture enhancement by forming tetra-n-butyl ammonium bromide semiclathrate in graphite nanofluids. Can. J. Chem. Eng. 2023, 101, 4128–4137. [Google Scholar] [CrossRef]
- Geng, Z.; Yang, Y.; Wang, Y.; Zhu, T.; Xu, W. Catalytic regeneration of amine-based absorbents for CO2 capture: The effect of acidic sites and accessibility. Sep. Purif. Technol. 2023, 327, 124889. [Google Scholar] [CrossRef]
- Itas, Y.S.; Razali, R.; Tata, S.; Kolo, M.; Lawal, A.; Alrub, S.A.; El Ghoul, J.; Khandaker, M.U. DFT studies on the effects of C vacancy on the CO2 capture mechanism of silicon carbide anotubes photocatalyst (Si12C12-X; X = 1; 2). Silicon 2023, 1–11. [Google Scholar] [CrossRef]
- Jin, G.; Wang, H.; Zhang, K.; Zhang, H.; Fan, J.; Wang, J.; Guo, D.; Wang, Z. ZIF-8 based porous liquids with high hydrothermal stability for carbon capture. Mater. Today Commun. 2023, 36, 106820. [Google Scholar] [CrossRef]
- Li, Y.; Lu, H.; Liu, Y.; Wu, K.; Zhu, Y.; Liang, B. CO2 absorption and desorption enhancement by nano-SiO2 in DBU-glycerol solution with high viscosity. Sep. Purif. Technol. 2023, 309, 122983. [Google Scholar] [CrossRef]
- Mahdavi, H.; Sadiq, M.M.; Smith, S.J.D.; Mulet, X.; Hill, M.R. Underlying potential evaluation of the real-process applications of magnetic porous liquids. J. Mater. Chem. A 2023, 11, 16846–16853. [Google Scholar] [CrossRef]
- Tengku Hassan, T.N.A.; Mohd Shariff, A.; Abd Aziz, N.F.; Mustafa, N.F.A.; Tan, L.S.; Abdul Halim, H.N.; Mohamed, M.; Hermansyah, H. Aqueous potassium salt of L-cysteine as potential CO2 removal solvent: An investigation on physicochemical properties and CO2 loading capacity. Sustainability 2023, 15, 11558. [Google Scholar] [CrossRef]
- Wang, S.-L.; Xiao, Y.-Y.; Zhou, S.-D.; Jiang, K.; Yu, Y.-S.; Rao, Y.-C. Synergistic effect of water-soluble hydroxylated multi-wall carbon nanotubes and graphene nanoribbons coupled with tetra butyl ammonium bromide on kinetics of carbon dioxide hydrate formation. Energies 2023, 16, 5831. [Google Scholar] [CrossRef]
- Wang, X.; Bao, Z.; Akhmedov, N.G.; Hopkinson, D.; Hoffman, J.; Duan, Y.; Egbebi, A.; Resnik, K.; Li, B. Unique biological amino acids turn CO2 emission into novel nanomaterials with three switchable product pathways. Environ. Technol. Innov. 2023, 32, 103279. [Google Scholar] [CrossRef]
- Yi, Q.; Zhao, C.; Lv, C.; Wan, G.; Meng, M.; Sun, L. CO2 absorption enhancement in low transition temperature mixtures-based nanofluids: Experiments and modeling. Sep. Purif. Technol. 2023, 325, 124584. [Google Scholar] [CrossRef]
- Zabelina, A.; Dedek, J.; Guselnikova, O.; Zabelin, D.; Trelin, A.; Miliutina, E.; Kolska, Z.; Siegel, J.; Svorcik, V.; Vana, J.; et al. Photoinduced CO2 conversion under Arctic conditions─the high potential of plasmon chemistry under low temperature. ACS Catal. 2023, 13, 3830–3840. [Google Scholar] [CrossRef]
- Zare, A.; Darvishi, P.; Lashanizadegan, A.; Zerafat, M. Theoretical and experimental investigation of CO2 solubility in nanofluids containing NaP zeolite nanocrystals and [C12mim][Cl] ionic liquid. Can. J. Chem. Eng. 2023, 101, 3925–3936. [Google Scholar] [CrossRef]
- Zarei, F.; Keshavarz, P. Intensification of CO2 absorption and desorption by metal/non-metal oxide nanoparticles in bubble columns. Environ. Sci. Pollut. Res. 2023, 30, 19278–19291. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Ding, Y.; Ma, L.; Zhu, X.; Wang, H.; Cheng, M.; Liao, Q. An amine-functionalized strategy to enhance the CO2 absorption of type III porous liquids. Energy 2023, 279, 127975. [Google Scholar] [CrossRef]
- Khanmohammadian, E.; Mohammadi, M.; Hashemi, R.; Eslami, S.; Reza Ehsani, M. Improvement of gas hydrate-based CO2 capture from CH4/CO2 mixture using silica and modified silica nanoparticles in the presence of potassium hydroxide. Fuel 2023, 334, 126458. [Google Scholar] [CrossRef]
- Lu, T.; Li, Z.; Du, L. Enhanced CO2 geological sequestration using silica aerogel nanofluid: Experimental and molecular dynamics insights. Chem. J. 2023, 474, 145566. [Google Scholar] [CrossRef]
- Ali, N.; Babar, A.A.; Wang, X.; Yu, J.; Ding, B. Hollow, porous, and flexible Co3O4-doped carbon nanofibers for efficient CO2 capture. Adv. Eng. Mater. 2023, 25, 2201335. [Google Scholar] [CrossRef]
- Anagnostopoulou, M.; Zindrou, A.; Cottineau, T.; Kafizas, A.; Marchal, C.; Deligiannakis, Y.; Keller, V.; Christoforidis, K.C. MOF-derived defective Co3O4 nanosheets in carbon nitride nanocomposites for CO2 photoreduction and H2 production. ACS Appl. Mater. Interfaces 2023, 15, 6817–6830. [Google Scholar] [CrossRef] [PubMed]
- Arifutzzaman, A.; Musa, I.N.; Aroua, M.K.; Saidur, R. MXene based activated carbon novel nano-sandwich for efficient CO2 adsorption in fixed-bed column. J. CO2 Util. 2023, 68, 102353. [Google Scholar] [CrossRef]
- Butt, F.S.; Lewis, A.; Rea, R.; Mazlan, N.A.; Chen, T.; Radacsi, N.; Mangano, E.; Fan, X.; Yang, Y.; Yang, S.; et al. Highly-controlled soft-templating synthesis of hollow ZIF-8 nanospheres for selective CO2 separation and storage. ACS Appl. Mater. Interfaces 2023, 15, 31740–317545. [Google Scholar] [CrossRef] [PubMed]
- Chen, P.-B.; Yang, J.-W.; Rao, Z.-X.; Wang, Q.; Tang, H.-T.; Pan, Y.-M.; Liang, Y. Efficient in-situ conversion of low-concentration carbon dioxide in exhaust gas using silver nanoparticles in N-heterocyclic carbene polymer. J. Colloid Interface Sci. 2023, 652, 866–877. [Google Scholar] [CrossRef] [PubMed]
- Chi, S.; Ye, Y.; Zhao, X.; Liu, J.; Jin, J.; Du, L.; Mi, J. Porous molecular sieve polymer composite with high CO2 adsorption efficiency and hydrophobicity. Sep. Purif. Technol. 2023, 307, 122738. [Google Scholar] [CrossRef]
- Giraldo, L.J.; Medina, O.E.; Ortiz-Perez, V.; Franco, C.A.; Cortes, F.B. Enhanced carbon storage process from flue gas streams using rice husk silica nanoparticles: An approach in shallow coal bed methane reservoirs. Energy Fuels 2023, 37, 2945–2959. [Google Scholar] [CrossRef]
- Gu, Y.-M.; Wang, Y.-H.; Zhao, S.-S.; Fan, H.-J.; Liu, X.-W.; Lai, Z.; Wang, S.-D. N-donating and water-resistant Zn-carboxylate frameworks for humid carbon dioxide capture from flue gas. Fuel 2023, 336, 126793. [Google Scholar] [CrossRef]
- He, Y.; Wang, Z.; Cao, A.; Xu, X.; Li, J.; Zhang, B.; Kang, L. Construction of graphene oxide-coated zinc tetraphenyporphyrin nanostructures for photocatalytic CO2 reduction to highly selective CH4 product. J. Colloid Interface Sci. 2023, 638, 123–134. [Google Scholar] [CrossRef]
- Hosseini, S.R.; Omidkhah, M.; Mehri Lihgyan, Z.; Norouzbahari, S.; Ghadimi, A. Synthesis, characterization, and gas adsorption performance of an efficient hierarchical ZIF-11@ZIF-8 core–shell metal–organic framework (MOF). Sep. Purif. Technol. 2023, 307, 122679. [Google Scholar] [CrossRef]
- Iranvandi, M.; Tahmasebpoor, M.; Azimi, B.; Heidari, M.; Pevida, C. The novel SiO2-decorated highly robust waste-derived activated carbon with homogeneous fluidity for the CO2 capture process. Sep. Purif. Technol. 2023, 306, 122625. [Google Scholar] [CrossRef]
- Issa, G.; Kormunda, M.; Tumurbaatar, O.; Szegedi, A.; Kovacheva, D.; Karashanova, D.; Popova, M. Impact of Ce/Zr ratio in the nanostructured ceria and zirconia composites on the selective CO2 adsorption. Nanomaterials 2023, 13, 2428. [Google Scholar] [CrossRef] [PubMed]
- Jin, Z.; Zhang, J.; Qiu, J.; Hu, Y.; Di, T.; Wang, T. Nitrogen vacancy-induced spin polarization of ultrathin zinc porphyrin nanosheets for efficient photocatalytic CO2 reduction. J. Colloid Interface Sci. 2023, 652, 122–131. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Lu, Y.; Wang, C.; Zhang, Y.; Jin, X.; Wu, J.; Wang, Y.; Zeng, J.; Yan, Z.; Sun, H.; et al. MOF-derived nano CaO for highly efficient CO2 fast adsorption. Fuel 2023, 340, 127476. [Google Scholar] [CrossRef]
- Liu, M.; Ma, C.; Cheng, X.; Gao, K.; Zhang, G.; Wang, D.; Liu, F. New insight into multiple hydrogen-bond networks of functional organosilicas system for collaborative transformation of CO2 under mild conditions. Sep. Purif. Technol. 2023, 317, 123937. [Google Scholar] [CrossRef]
- Lopez-Arias, M.; Moro, C.; Francioso, V.; Elgaali, H.H.; Velay-Lizancos, M. Effect of nanomodification of cement pastes on the CO2 uptake rate. Constr. Build. Mater. 2023, 404, 133165. [Google Scholar] [CrossRef]
- Mahdipoor, H.R.; Ebrahimi, R.; Ganji Babakhani, E.; Halladj, R.; Safari, N.; Ganji, H. Investigating the selective adsorption of CO2 by MIL-101(Cr)-NH2 and modeling the equilibrium data using a new three-parameter isotherm. Colloids Surf. A Physicochem. Eng. Asp. 2023, 675, 131971. [Google Scholar] [CrossRef]
- Medina, O.E.; Galeano-Caro, D.; Brattekås, B.; Pérez-Cadenas, A.; Carrasco-Marín, F.; Cortés, F.B.; Franco, C.A. Simultaneous CO2 adsorption and conversion over Ni-Pd supported CeO2 nanoparticles during catalytic n-C7 asphaltene gasification. Fuel 2023, 342, 127733. [Google Scholar] [CrossRef]
- Nabipour, H.; Wang, X.; Song, L.; Hu, Y. Synthesis of a bio-based and intrinsically anti-flammable epoxy thermoset and the application of its carbonized foam as an efficient CO2 capture adsorbent. Mater. Today Sustain. 2023, 21, 100265. [Google Scholar] [CrossRef]
- Noorani, N.; Moghaddasfar, A.; Mehrdad, A.; Darbandi, M. Improved the CO2 adsorption performance in cobalt oxide nanoparticles in the presence of DES. New J. Chem. 2023, 47, 16748–16755. [Google Scholar] [CrossRef]
- Norouzbahari, S.; Mehri Lighyan, Z.; Ghadimi, A.; Sadatnia, B. ZIF-8@Zn-MOF-74 core–shell metal–organic framework (MOF) with open metal sites: Synthesis, characterization, and gas adsorption performance. Fuel 2023, 339, 127463. [Google Scholar] [CrossRef]
- Panayotov, D.; Zdravkova, V.; Lagunov, O.; Andonova, S.; Spassova, I.; Nihtianova, D.; Atanasova, G.; Drenchev, N.; Ivanova, E.; Mihaylov, M.; et al. Capturing CO2 by ceria and ceria-zirconia nanomaterials of different origin. Phys. Chem. Chem. Phys. 2023, 25, 17154–17175. [Google Scholar] [CrossRef] [PubMed]
- Ping, R.; Ma, C.; Shen, Z.; Zhang, G.; Wang, D.; Liu, F.; Liu, M. Metalloporphyrin and triazine integrated nitrogen-rich frameworks as high-performance platform for CO2 adsorption and conversion under ambient pressure. Sep. Purif. Technol. 2023, 310, 123151. [Google Scholar] [CrossRef]
- Qiu, L.-Q.; Li, H.-R.; He, L.-N. Incorporating catalytic units into nanomaterials: Rational design of multipurpose catalysts for CO2 valorization. Acc. Chem. Res. 2023, 56, 2225–2240. [Google Scholar] [CrossRef]
- Rong, N.; Wang, J.; Liu, K.; Han, L.; Mu, Z.; Liao, X.; Meng, W. Enhanced CO2 capture durability and mechanical properties using cellulose-templated CaO-based pellets with steam injection during calcination. Ind. Eng. Chem. Res. 2023, 62, 1533–1541. [Google Scholar] [CrossRef]
- Routier, C.; Vallan, L.; Daguerre, Y.; Juvany, M.; Istif, E.; Mantione, D.; Brochon, C.; Hadziioannou, G.; Strand, Å.; Näsholm, T.; et al. Chitosan-modified polyethyleneimine nanoparticles for enhancing the carboxylation reaction and lants’ CO2 uptake. ACS Nano 2023, 17, 3430–3441. [Google Scholar] [CrossRef] [PubMed]
- Shao, B.; Wang, Z.-Q.; Gong, X.-Q.; Liu, H.; Qian, F.; Hu, P.; Hu, J. Synergistic promotions between CO2 capture and in-situ conversion on Ni-CaO composite catalyst. Nat. Commun. 2023, 14, 996. [Google Scholar] [CrossRef]
- Shi, Y.; Tian, G.; Ni, R.; Zhang, L.; Hu, W.; Zhao, Y. Facile and green lyocell/feather nonwovens with in-situ growth of ZIF-8 as adsorbent for physicochemical CO2 capture. Sep. Purif. Technol. 2023, 322, 124356. [Google Scholar] [CrossRef]
- Tabarkhoon, F.; Abolghasemi, H.; Rashidi, A.; Bazmi, M.; Alivand, M.S.; Tabarkhoon, F.; Farahani, M.V.; Esrafili, M.D. Synthesis of novel and tunable micro-mesoporous carbon nitrides for ultra-high CO2 and H2S capture. Chem. Eng. J. 2023, 456, 140973. [Google Scholar] [CrossRef]
- Tang, X.; Wang, B.; Wang, C.; Chu, S.; Liu, S.; Pei, W.; Li, L.; Wu, J.; Li, W.; Wu, J.; et al. Facile synthesis of Zncluster/NG nanozymes mimicking carbonic anhydrase for CO2 capture. Colloids Surf. A Physicochem. Eng. Asp. 2023, 676, 132201. [Google Scholar] [CrossRef]
- Tian, C.; Liu, X.; Liu, C.; Li, S.; Li, Q.; Sun, N.; Gao, K.; Jiang, Z.; Chang, K.; Xuan, Y. Air to fuel: Direct capture of CO2 from air and in-situ solar-driven conversion into syngas via Nix/NaA nanomaterials. Nano Res. 2023, 16, 10899–10912. [Google Scholar] [CrossRef]
- Wang, H.; Li, J.; Wan, Y.; Nazir, A.; Song, X.; Huo, P.; Wang, H. Synthesis of AgInS2 QDs-MoS2/GO composite with enhanced interfacial charge separation for efficient photocatalytic degradation of tetracycline and CO2 reduction. J. Alloys Compd. 2023, 954, 170159. [Google Scholar] [CrossRef]
- Xie, C.; Xie, Y.; Zhang, C.; Dong, H.; Zhang, L. Explainable machine learning for carbon dioxide adsorption on porous carbon. J. Environ. Chem. Eng. 2023, 11, 109053. [Google Scholar] [CrossRef]
- Yang, Y.; Liu, W.; Wu, B.; Wang, T.; Dong, H.; Fang, M.; Gao, X. Kinetic research on ion exchange regeneration of quaternary ammonium-based CO2 sorbent for direct air capture. Sep. Purif. Technol. 2023, 324, 124504. [Google Scholar] [CrossRef]
- Yin, Q.; Li, X.; Yan, X.; Zhang, X.; Qin, S.; Hao, Y.; Li, N.; Zhu, Z.; Liu, X.; Chang, T. Optimization and kinetics modeling of CO2 fixation into cyclic carbonates using urea-functionalized ionic organic polymers under mild conditions. Mol. Catal. 2023, 550, 113601. [Google Scholar] [CrossRef]
- Zhang, T.; Zhao, X.; Lin, M.; Yang, B.; Yan, J.; Zhuang, Z.; Yu, Y. Surfactant-free synthesis of ordered 1D/2D NiZn-LDH heterostructure through oriented attachment for efficient photocatalytic CO2 reduction with nearly 100% CO selectivity. Sci. China Mater. 2023, 66, 2308–2316. [Google Scholar] [CrossRef]
- Zhang, T.; Zheng, Y.; Zhao, X.; Lin, M.; Yang, B.; Yan, J.; Zhuang, Z.; Yu, Y. Scalable synthesis of holey deficient 2D Co/NiO single-crystal nanomeshes via topological transformation for efficient photocatalytic CO2 reduction. Small 2023, 19, 2206873. [Google Scholar] [CrossRef]
- Zhao, R.; Nie, Y.; Liu, J.; Wang, Y.; Li, N.; Cheng, Q.; Xia, M. New insight into ZnO@ZIFs composite: An efficient photocatalyst with boosted light response ability and stability for CO2 reduction. Environ. Sci. Pollut. Res. 2023, 30, 82672–82685. [Google Scholar] [CrossRef]
- Abdollahi, S.A.; Ranjbar, S.F. Modeling the CO2 separation capability of poly(4-methyl-1-pentane) membrane modified with different nanoparticles by artificial neural networks. Sci. Rep. 2023, 13, 8812. [Google Scholar] [CrossRef]
- Faghih, S.M.; Salimi, M.; Mazaheri, H. Fabrication of Pebax/4A zeolite nanocomposite membrane to enhance CO2 selectivity compared to pure O2, N2, and CH4 gases. Int. J. Eng. Trans. A Basics 2023, 36, 408–419. [Google Scholar] [CrossRef]
- Hamalova, K.; Neubertova, V.; Vostinakova, M.; Fila, V.; Kolska, Z. Amine-doped PEBA membrane for CO2 capture. Mater. Lett. 2023, 333, 133695. [Google Scholar] [CrossRef]
- Hou, R.; Wang, S.; Wang, L.; Li, C.; Wang, H.; Xu, Y.; Wang, C.; Pan, Y.; Xing, W. Enhanced CO2 separation performance by incorporating KAUST-8 nanosheets into crosslinked poly(ethylene oxide) membrane. Sep. Purif. Technol. 2023, 309, 123057. [Google Scholar] [CrossRef]
- Imad, M.; Castro-Muñoz, R.; Bernauer, M.; Martin, V.; Izak, P.; Fila, V. Zr-based metal-organic framework UiO-66/Ultem® 1000 membranes for effective CO2/H2 separation. Chem. Eng. Technol. 2023, 46, 2046–2053. [Google Scholar] [CrossRef]
- Katare, A.; Mandal, B. Surface engineering of Zr BDC nanoparticles via conjugation with lysine to enhance the CO2/N2 separation performance of chitosan mixed matrix membranes under dry and humid conditions. ACS Appl. Nano Mater. 2023, 6, 4821–4833. [Google Scholar] [CrossRef]
- Widakdo, J.; Kadja, G.T.M.; Anawati, A.; Subrahmanya, T.M.; Austria, H.F.M.; Huang, T.H.; Suharyadi, E.; Hung, W.S. Graphene oxide-melamine nanofilm composite membrane for efficient CO2 gas separation. Sep. Purif. Technol. 2023, 323, 124521. [Google Scholar] [CrossRef]
- Li, S.; Zhang, K.; Liu, C.; Feng, X.; Wang, P.; Wang, S. Nanohybrid Pebax/PEGDA-GPTMS membrane with semi-interpenetrating network structure for enhanced CO2 separations. J. Membr. Sci. 2023, 674, 121516. [Google Scholar] [CrossRef]
- Lin, Z.; Yuan, Z.; Wang, K.; He, X. Synergistic tuning mixed matrix membranes by Ag+-doping in UiO-66-NH2/polymers of intrinsic microporosity for remarkable CO2/N2 separation. J. Membr. Sci. 2023, 681, 121775. [Google Scholar] [CrossRef]
- Maleh, M.S.; Raisi, A. In-situ growth of ZIF-8 nanoparticles in Pebax-2533 for facile preparation of high CO2-selective mixed matrix membranes. Colloids Surf. A Physicochem. Eng. Asp. 2023, 659, 130747. [Google Scholar] [CrossRef]
- Maleh, M.S.; Raisi, A. Experimental and modeling study on interfacial morphology of ZIF-67/Pebax-2533 mixed matrix 99membranes for CO2 separation applications. Surf. Interfaces 2023, 38, 102846. [Google Scholar] [CrossRef]
- Ni, Z.; Cao, Y.; Zhang, X.; Zhang, N.; Xiao, W.; Bao, J.; He, G. Synchronous design of membrane material and process for pre-combustion CO2 capture: A superstructure method integrating membrane type selection. Membranes 2023, 13, 318. [Google Scholar] [CrossRef]
- Nobakht, D.; Abedini, R. A new ternary Pebax®1657/maltitol/ZIF-8 mixed matrix membrane for efficient CO2 separation. Process Saf. Environ. Prot. 2023, 170, 709–719. [Google Scholar] [CrossRef]
- Qi, R.; Li, Z.; Zhang, H.; Fu, H.; Zhang, H.; Gao, D.; Chen, H. CO2 capture performance of ceramic membrane with superhydrophobic modification based on deposited SiO2 particles. Energy 2023, 283, 129202. [Google Scholar] [CrossRef]
- Ren, H.; Ni, J.; Shen, M.; Zhou, D.; Sun, F.; Loke Show, P. Enhanced carbon dioxide fixation of Chlorella vulgaris in micro3algae reactor loaded with nanofiber membrane carried iron oxide nanoparticles. Biores. Technol. 2023, 382, 129176. [Google Scholar] [CrossRef] [PubMed]
- Tabesh, H.; Gholami, M.H.; Marefat, M. The Effect of sweeping media and temperature on aqueous CO2 removal using 4hollow fiber membrane contactor (HFMC): An experimental determination. Int. J. Chem. Eng. 2023, 2023, 3577656. [Google Scholar] [CrossRef]
- Wang, Y.; Niu, Z.; Dai, Y.; Zhong, S.; Li, J. Efficient CO2 separation by ionic liquid nanoconfined in ultra-thin TCOH@Pebax-1657 MMM. Sep. Purif. Technol. 2023, 325, 124667. [Google Scholar] [CrossRef]
- Wang, Y.; Sheng, L.; Zhang, X.; Li, J.; Wang, R. Hybrid carbon molecular sievemembranes having ordered Fe3O4@ZIF-8-derived microporous structure for gas separation. J. Membr. Sci. 2023, 666, 121127. [Google Scholar] [CrossRef]
- Webb, M.T.; Condes, L.C.; Ly, H.G.; Galizia, M.; Razavi, S. Rational design, synthesis, and characterization of facilitated transport membranes exhibiting enhanced permeability, selectivity and stability. J. Membr. Sci. 2023, 685, 121910. [Google Scholar] [CrossRef]
- Xue, K.; Zhan, G.; Wu, X.; Zhang, H.; Chen, Z.; Chen, H.; Li, J. Integration of membrane contactors and catalytic solvent regeneration for efficient carbon dioxide capture. J. Membr. Sci. 2023, 684, 121870. [Google Scholar] [CrossRef]
- Yang, H.; Chen, G.; Cheng, L.; Liu, Y.; Cheng, Y.; Yao, H.; Liu, Y.; Liu, G.; Jin, W. Manipulating gas transport channels in graphene oxide membrane with swift heavy ion irradiation. Sep. Purif. Technol. 2023, 320, 124136. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhao, M.; Li, X.; Xin, Q.; Ding, X.; Zhao, L.; Ye, H.; Lin, L.; Li, H.; Zhang, Y. Constructing mixed matrix membranes for CO2 separation based on light lanthanide fluoride nanosheets with mesoporous structure. J. Ind. Eng. Chem. 2023, 125, 200–210. [Google Scholar] [CrossRef]
- Zhao, M.; Guo, J.; Xin, Q.; Zhang, Y.; Li, X.; Ding, X.; Zhang, L.; Zhao, L.; Ye, H.; Li, H.; et al. Novel aminated F-Ce nanosheet mixed matrix membranes with controllable channels for CO2 capture. Sep. Purif. Technol. 2023, 324, 124512. [Google Scholar] [CrossRef]
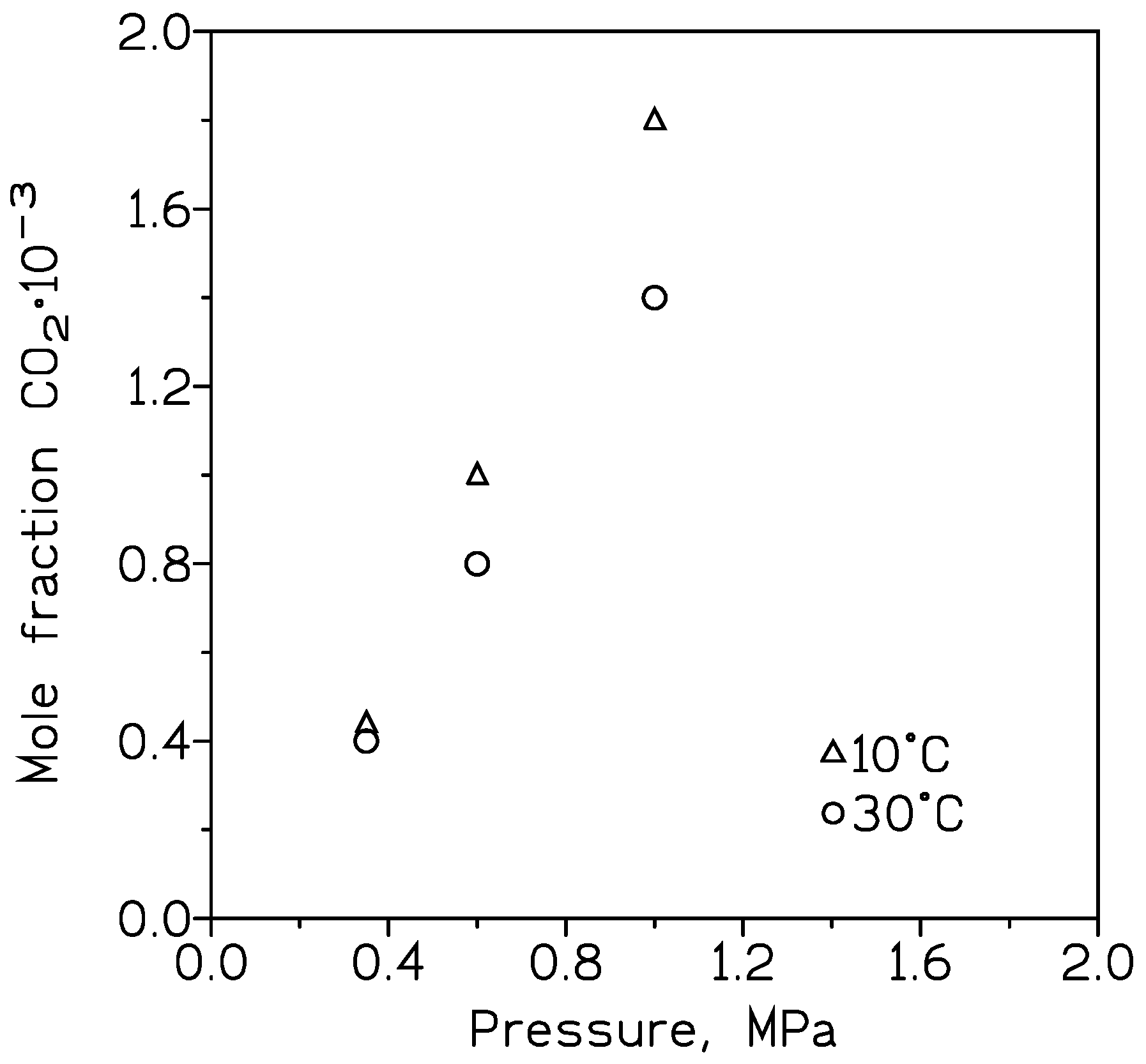
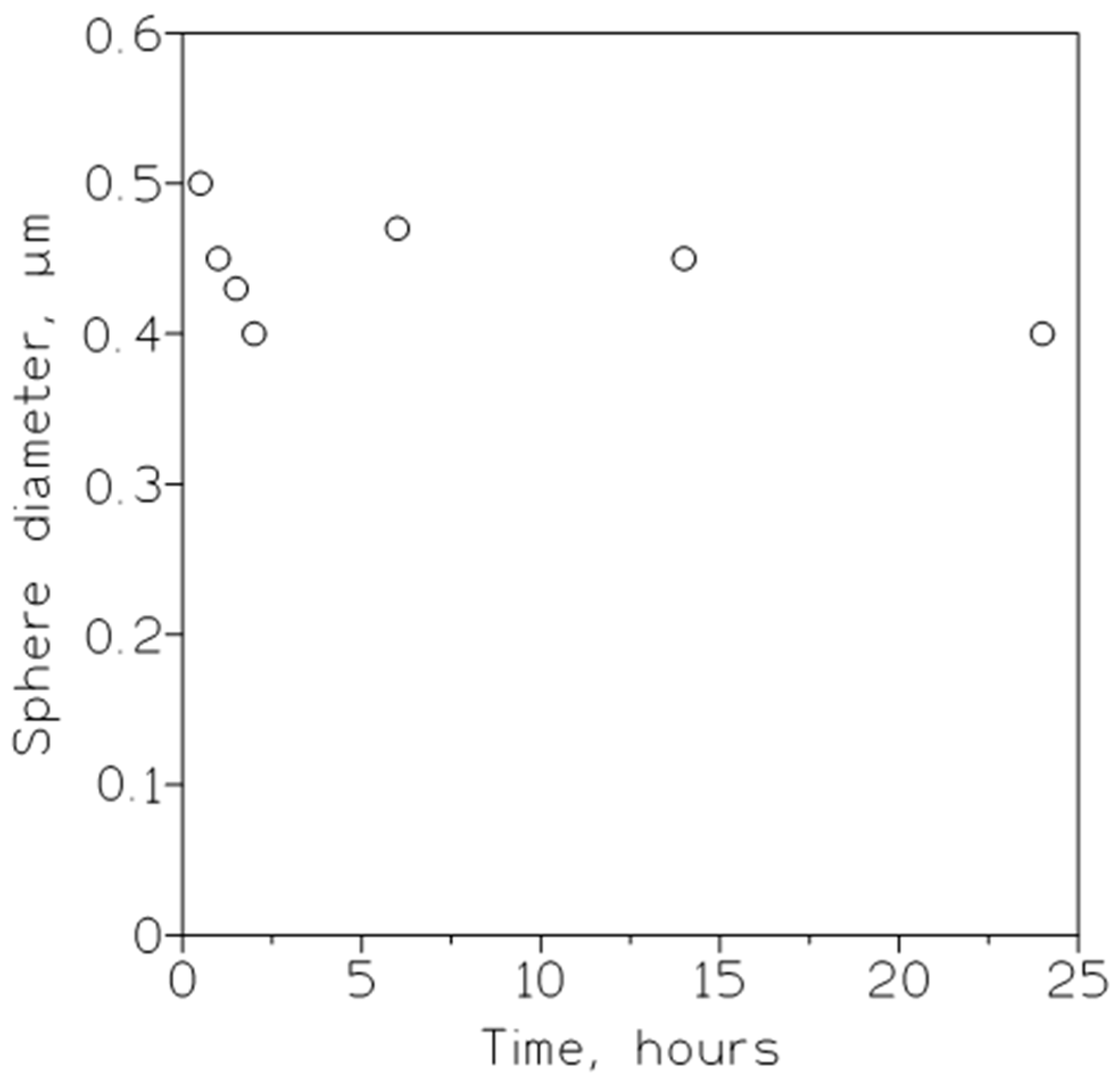
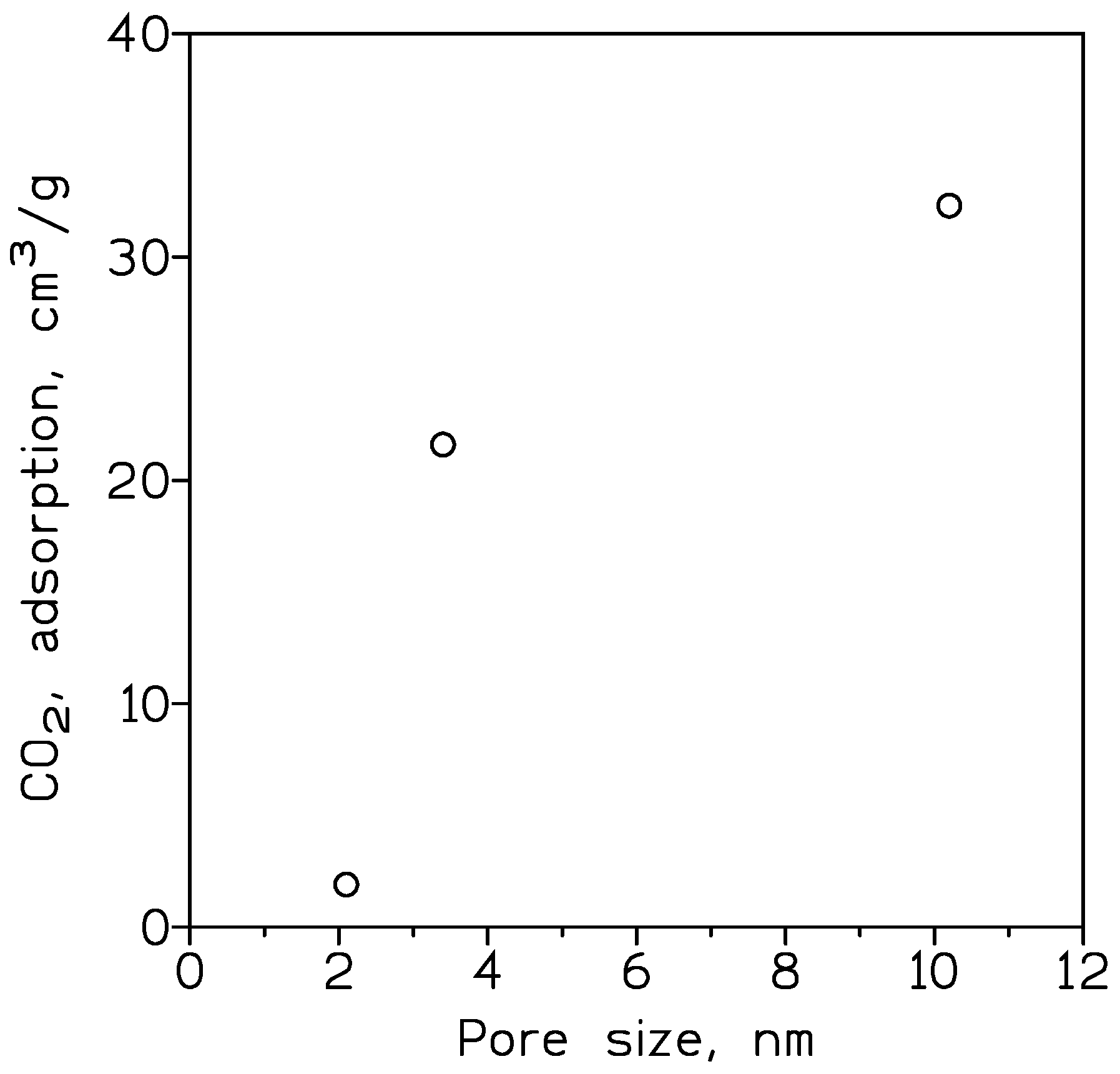

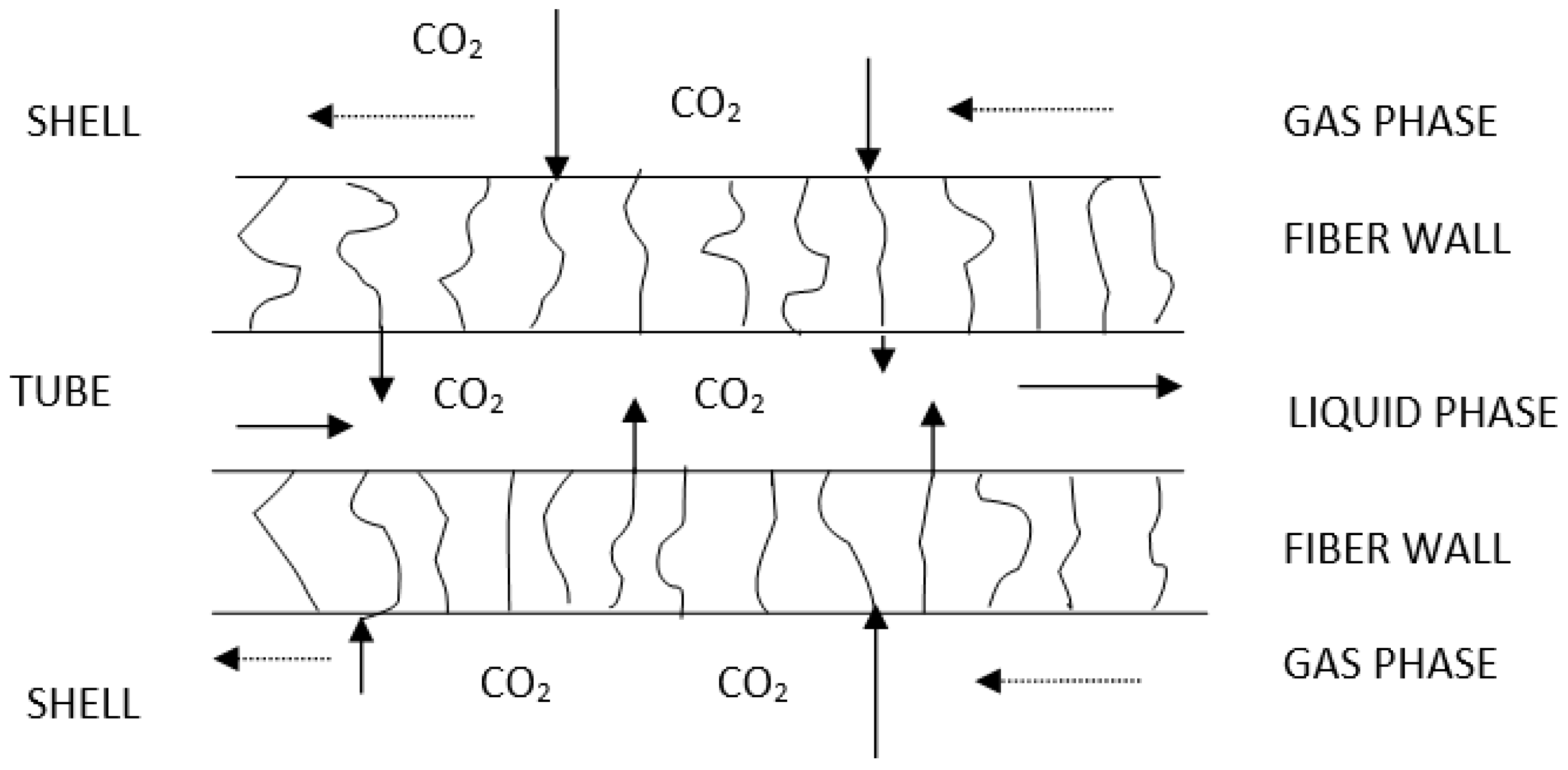
| Cp, kg/m3 | 10 nm | 25 nm | 60 nm |
|---|---|---|---|
| 0 | 1 | 1 | 1 |
| 0.2 | 1.21 | 1.16 | 1.15 |
| 0.4 | 1.23 | 1.22 | 1.21 |
| 0.6 | 1.35 | 1.30 | 1.17 |
| 0.8 | 1.20 | 1.24 | 1.12 |
| 1.0 | 1.15 | 1.10 | 1.10 |
| Ref. | Nanomaterial | Objective |
|---|---|---|
| [24] | Porous Ni foam | CO2 capture |
| [25] | TBAB+graphite nanofluid | CO2 removal |
| [26] | SO42−/ZrTiOx | CO2 desorption rate |
| [27] | Silicon carbide nanotubes | CO2 capture/storage |
| [28] | ZIF-8+DMBI | Improve ZIF-8 thermal stability |
| [29] | Nano-SiO2 and glycerol | CO2 desorption |
| [30] | Nanomagnetic porous liquid | Continuous use up to 3 cycles |
| [31] | K-L-cysteine | Substitution of amines |
| [32] | TBAB+MWCNTs | Formation of CO2 hydrate |
| [33] | Amino-acid-based technology | CO2 conversion to bicarbonate |
| [34] | Nanofluids+TiO2 nanoparticles | CO2 removal |
| [35] | Gold nanoparticles | CO2 capture |
| [36] | Zeolite nanocrystals+ionic liquid | CO2 removal |
| [37] | MDA-Fe3O4 | CO2 removal |
| [38] | Modified ZIF-8 salt | CO2 capture |
| [39] | APTES+silica nanoparticles | CO2/CH4 separation |
| [40] | Silica aerogel nanofluid | CO2 geological sequestration |
| T, °C | C-CeO2 | O-CeO2 | S-CeO2 |
|---|---|---|---|
| 30 | 7 | 6.5 | 5 |
| 50 | 6.8 | 6.2 | 5.8 |
| 100 | 6.5 | 5.6 | 4.6 |
| 200 | 4.4 | 3.8 | 2.7 |
| Gas Mixture | 0.1 Bar | 0.5 Bar | 1 Bar |
|---|---|---|---|
| CO2/N2 50:50 | 32 | 51 | 60 |
| CO2/N2 15:85 | 26 | 40 | 45 |
| CO2/CH4 50:50 | 23 | 32 | 36 |
| CO2/CH4 15:85 | 20 | 25 | 28 |
| Ref. | Nanomaterial | Objective |
|---|---|---|
| [41] | Carbon nanofibers+Co3O4 | CO2 uptake and CO2/N2 separation |
| [42] | Carbon nitride+Co3O4 | CO2 photodegradation |
| [43] | 2DMXenes+activated carbon | CO2 uptake in fixed-bed column |
| [44] | ZIF-8 hollow nanospheres | CO2/N2 separation and CO2 storage |
| [45] | Ag nanoparticles+carbene polymers | CO2 uptake and conversion to CO32− |
| [46] | NaY@polyacrylate matrix | CO2/H2O separation |
| [47] | Doped rice husk silica nanoparticles | CO2 uptake |
| [48] | Zn-N pillar MOFs | CO2 uptake and CO2/N2 separation |
| [49] | GO+Zn/TPP nanocomposite | CO2 capture |
| [50] | ZIF11@ZIF-8 structures | CO2/N2, CO2/CH4 separation |
| [51] | Activated carbon+nanosilica | CO2 removal |
| [52] | Ce-Zr nanocomposites | CO2 loading |
| [53] | Zn/TCPP nanosheets | Intensify photocatalytic CO2 reduction |
| [54] | MOF-derived nano-CaO | CO2 uptake up to 4 cycles |
| [55] | THPMOs | CO2 adsorption at 0 °C |
| [56] | Nano-TiO2+cement pastes | CO2 capture |
| [57] | MIL-101(Cr)-NH2 | CO2/N2, CO2/CH4 separations |
| [58] | Ceria nanoparticles | CO2 uptake |
| [59] | Carbon foams | CO2 capture at 0 °C |
| [60] | Co3O4 nanoparticles | CO2 capture |
| [61] | Metal frameworks | CO2/N2, CO2/CH4 separations |
| [62] | Ceria derivatives | Static and dynamic CO2 capture |
| [63] | Integrated N2-rich frameworks | CO2 uptake and catalytic conversion |
| [64] | Zn, Cu-based MOFs | CO2 adsorption and catalytic conversion |
| [65] | Cellulose-CaO-based pellets | CO2 adsorption after twenty cycles |
| [66] | PEI nanoparticles | CO2 capture |
| [67] | Ni-CaO composite | CO2 capture and catalytic conversion |
| [68] | ZIF-8 derivative | CO2 capture |
| [69] | Dual-pore carbon nitride | CO2/N2 and CO2/CH4 selectivity |
| [70] | Nanozymes | CO2 capture and catalytic conversion |
| [71] | Dual catalyst–adsorbent | CO2 uptake and conversion |
| [72] | Ag-In-Mo composites | Reducing CO and CH4 in CO2 products |
| [73] | Carbon-based material (MLP) | Prediction of CO2 uptake |
| [74] | Various chemicals | Regeneration of CO2 adsorbents |
| [75] | Urea derivatives | Catalytic efficiency in CO2 cycloaddition |
| [76] | Ni-Zn heterostructures | CO2 capture, photocatalytic conversion |
| [77] | 2D metal oxide nanomesh | Photocatalytic CO2–syngas conversion |
| [78] | ZnO@ZIF nanocomposites | CO2 capture and photocatalytic reduction |
| Ref. | Limit | CO2 Uptake, mmol/g |
|---|---|---|
| [69] | Upper Low | 22.9 |
| [50] | 8.2 | |
| [65] | 7.3 | |
| [47] | 0.75 | |
| [71] | 0.38 | |
| [62] | 0.13 |
| Material | Material wt% | CO2 Permeation | N2 Permeation |
|---|---|---|---|
| ZrBOC | 0 | 14 | <0.5 |
| 3 | 25 | <0.5 | |
| 7 | 79 | <0.5 | |
| 10 | 65 | <0.5 | |
| Lys-c-ZrBOC | 0 | 14 | <0.5 |
| 3 | 40 | <0.5 | |
| 7 | 135 | <0.5 | |
| 10 | 132 | <0.5 |
| Membrane | 0.2 | 0.4 | 0.6 | 0.8 | 1.0 |
|---|---|---|---|---|---|
| Original | 62 | 55 | 45 | 35 | 30 |
| Superhydrophobic | 98 | 90 | 75 | 67 | 62 |
| Ref. | Nanomaterial | Objective |
|---|---|---|
| [79] | PMP+metal oxides | Modeling CO2 permeation |
| [80] | Zeolite+Pebax-1657 | CO2/N2, CO2/O2, CO2/CH4 separation |
| [81] | PEBA | CO2 capture |
| [82] | KAUST-8 nanosheets | CO2 uptake, CO2/N2 separation |
| [83] | Nanofillers+PEI | H2/CO2 separation |
| [84] | Zr-MOFs+AA+CS | CO2 permeation, CO2/N2 separation |
| [85] | Graphene oxide+melanine | As above |
| [86] | PEO+SiO2+Pebax1657 | CO2 diffusion, CO2/N2 separation |
| [87] | Silver+UiO66 | CO2/N2 separation |
| [88] | ZIF-8+Pebax-2533 | CO2/N2, CO2/CH4 selectivity |
| [89] | ZIF-67+Pebax-2533 | As above |
| [90] | Superstructure method | Modeling CO2 capture |
| [91] | ZIF-8+Pebax-1657 | CO2/N2, CO2/CH4 separation |
| [92] | Ceramic membranes | CO2 capture |
| [93] | Fe2O3+microalgae | CO2 fixation efficiency |
| [94] | HFM contactor | CO2 exchange in air or water |
| [95] | TCOH+Pebax-1657+IL | CO2/N2, CO2/CH4 separations |
| [96] | Carbon membranes | As above |
| [97] | Silver+Pebax-1657 | CO2 permeation |
| [98] | Membrane contactor | CO2 removal from industrial flue gas |
| [99] | Graphene oxide | CO2 removal |
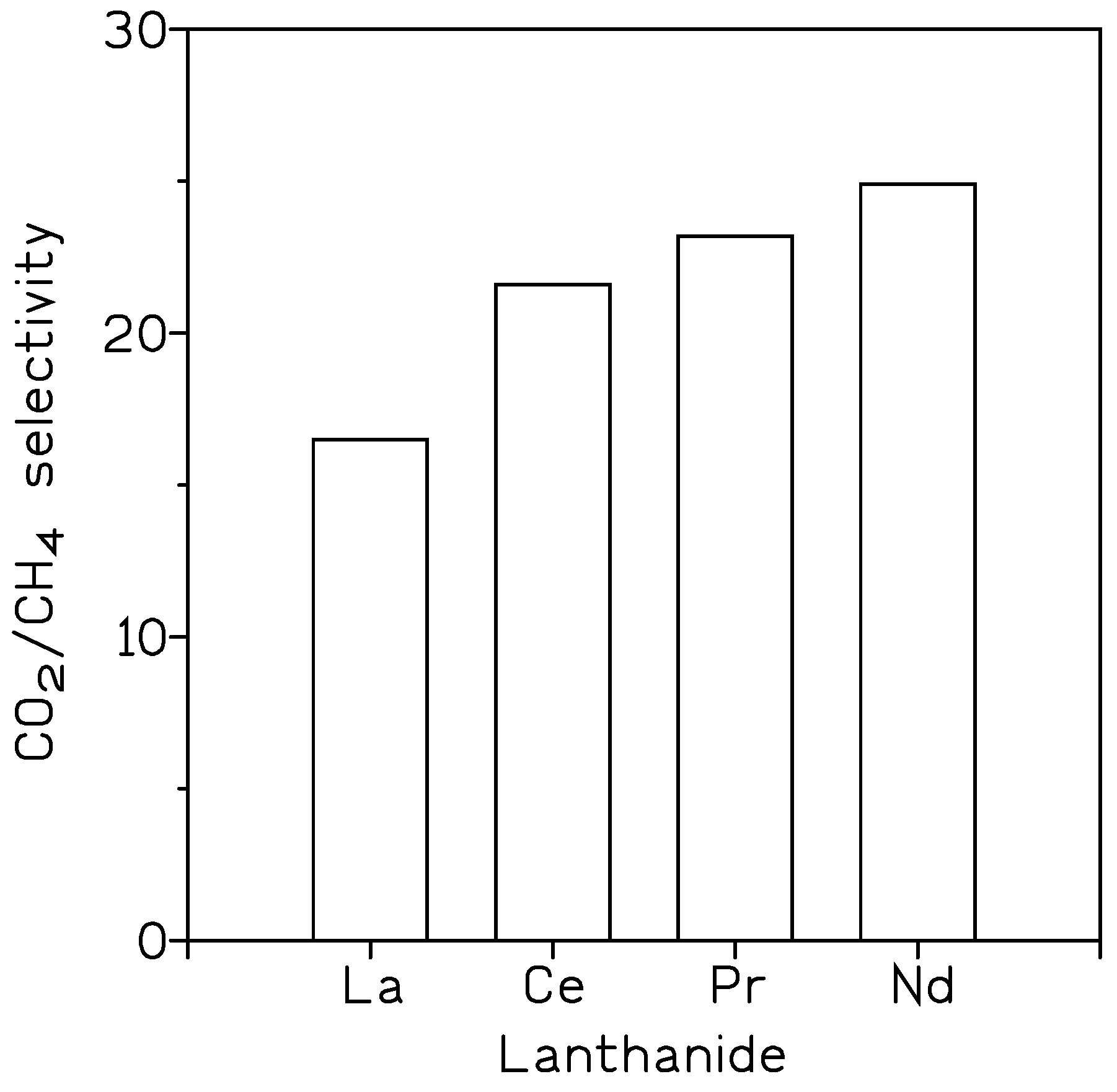
| [100] | Ln+Pebax-1657 | CO2/CH4 selectivity |
| [101] | PEI+cerium | CO2/N2 separation |
| Ref. | CO2/N2 | Ref. | CO2/CH4 |
|---|---|---|---|
| [86] | 79.6 | [92] | 52.9 |
| [84] | 71.3 | [96] | 48.0 |
| [101] | 70.0 | [100] | 36.7 |
| [87] | 30 | [91] | 26.6 |
| [84] | 29.4 | [89] | 22.5 |
| [96] | 29 |
| Technology | Pros | Cons |
|---|---|---|
| Absorption | Established technology | Chemistry of amines, regeneration of solvent, stability of the adsorbent |
| Adsorption | Established technology, elevated gas removal capacity | Possible generation of toxic wastes, stability of the adsorbent |
| Membranes | Modular configuration, adequate surface area per unit volume | Limitations due to gas permeation, resistance due to degradation of membrane |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alguacil, F.J. Nanomaterials for CO2 Capture from Gas Streams. Separations 2024, 11, 1. https://doi.org/10.3390/separations11010001
Alguacil FJ. Nanomaterials for CO2 Capture from Gas Streams. Separations. 2024; 11(1):1. https://doi.org/10.3390/separations11010001
Chicago/Turabian StyleAlguacil, Francisco Jose. 2024. "Nanomaterials for CO2 Capture from Gas Streams" Separations 11, no. 1: 1. https://doi.org/10.3390/separations11010001
APA StyleAlguacil, F. J. (2024). Nanomaterials for CO2 Capture from Gas Streams. Separations, 11(1), 1. https://doi.org/10.3390/separations11010001






