Abstract
A comparative study of a Schiff base reaction involving benzaldehyde and n-butylamine was carried out to improve the yield of the resulting imine. This reaction was carried out at different temperatures without and with the elimination of the water produced during the process by the pervaporation (PV) technique using a typical cylindrical cell. To reach this goal, different dense membranes made of crosslinked poly(vinyl alcohol) with different oxalic acid (crosslinker) contents were prepared by the solvent casting method. Different parameters influencing the performance of the membrane in the separation process including swellability, diffusivity, crosslinking density, and thermal properties were investigated. The total and partial cumulative transmembranar fluxes as well as the separation factor were studied and the separation process was monitored by HPLC analysis. The n-butyl-1-phenylmethanimine produced was characterized by FTIR and 1HNMR analyses. The results obtained were a clear improvement in the yield of the reaction. For example, the yield obtained from the Schiff base reaction occurring without assistance by PV varied from 58 to 84 wt% when the temperature changed from 5 to 45 °C. On the other hand, when the PV process was used to eliminate water from this reaction mixture, the yield went from 90.4 to 98.6% by weight in this same temperature order. The cumulative total and partial fluxes significantly decreased with time. On the other hand, the separation factor reached a maximum at about one hour at 5, 15, and 45 °C. At 25 °C, the maximum total flux was reached at about 2 h of the PV process. The best selectivity of the PVA-0.5 membrane with regard to water was obtained at 15 °C. It was also revealed from the results obtained that the cumulative total and partial flux decreased rapidly with time and the separation factor reached a maximum at one hour into the PV process, in which 1.51 × 104 was reached at 15 °C, 6.25 × 103 and 3.50 × 103 at one hour of the separation process, and 10.23 × 103 at 25 °C at 2 h of the water removal by PV.
1. Introduction
Researchers are currently facing new challenges in reducing chemical waste, reaction times, economically efficient chemicals, and harmful chemicals in organic transformations. Green chemistry plays the most important role in synthetic chemistry, maximizing the yield of reaction products while limiting side products by using less hazardous chemicals [1].
As we know, Schiff base is a chemical compound formed when primary amines condense in equilibrium with aldehydes or ketones. It is distinguished from other chemical compounds by an imine (>C=N-) or azomethine (-HC=N-) group. Hugo Schiff was the first scientist to discover this reaction in 1864, and this is how it received its name [2].
The amazing biological activity of Schiff’s base has attracted much attention from researchers for its use as an antifungal and antibacterial agent [3], and properties such as being anti-inflammatory [4], anti-tubercular [5], analgesic [6], anti-cancer [7], anti-helminthic, and anti-oxidant [8]. On the other side, in the industrial domain, Schiff bases are used as corrosion inhibitors [9], catalysts [10], fluorescence properties [11], pigments, dyes [12], polymer stabilizers, and intermediates in the synthesis of organic components [13,14].
Since water is a byproduct of the reversible reaction that creates Schiff bases, a dehydrating agent such as molecular sieve, trimethyl orthoformate, tetramethyl orthosilicate, or distillation using the Dean and Stark apparatus can be used to remove water to increase the efficiency of reactions producing Schiff’s base [15,16].
Pervaporation (PV) is a technology that belongs to membrane techniques and is used to selectively separate volatile organic or hydro-organic solutes from their mixtures. This clean, energy-saving technique that is easy to integrate into production lines is constantly evolving in the petrochemical, pharmaceutical, and agri-food industries [17,18,19].
Widely used processes involving PV, which uses dense (non-porous), asymmetric membranes, are mainly used for the dehydration of organic solvents (bio-ethanol) and the shift of reaction equilibria by the removal of water as a by-product (e.g., the production of pure methyl tertbutyl ether (MTBE)) [20].
In the context of breaking the equilibrium of reactions to force them to selectively produce ester, Khdsange and Wasewar [21] used the PV method to selectively extract water from the esterification reaction involving butyric acid and n-propanol and obtained propyl butyrate, and the results obtained were satisfactory. This example is taken by these researchers as a model reaction for the study and to test the performance of a pervaporation reactor using, as a separation membrane, a poly(vinyl alcohol)/polyethersulfone blend. The results obtained revealed that the best performance in terms of yield (96.41%) was obtained when the reaction temperature was 80 °C, the molar ratio was 2, and the catalyst content was 2.5% in weight for 7 h of reaction.
This membrane played an essential role in improving conversion by selective water extraction. These authors highlighted that the esterification reaction coupled with pervaporation is considered a better choice compared to conventional methods of producing esters from a carboxylic acid and an alcohol.
Polyvinyl alcohol (PVA), which is synthesized by the dehydration of poly(vinyl acetate), is a water-soluble, semi-crystalline synthetic polymer [22]. This polymer is widely used in the industrial field due to its multiple characteristics, including its non-toxicity, biocompatibility, and biodegradability properties, as well as its high barrier properties against oxygen and aromas [23,24,25,26].
Indeed, PVA presents excellent hydrophilicity, biocompatibility, and physicochemical stability [27]. This material, which contains many hydroxyl groups, is easy to crosslink into an insoluble network structure. This polymer was industrialized as a pervaporation membrane for the first time by GFT in 1982. According to GFT [28], PVA membranes are capable of producing a total flux of 50 to 1000 g·m−2·h−1 with a separation factor between 50 and 250 when the concentration of the water in the feed varied between 10 and 20 wt%. PV as a green method has also been used by different researchers to remove water as a byproduct of the esterification reaction [29,30,31,32].
The development of processes based on the integration of new technologies to assist chemical reactions attracts a large number of researchers in the field of process engineering. Recently, considerable efforts have been focused on the design of reactors equipped with membrane systems used to improve the yields of equilibrated reactions.
For example, Chandane et al. [29] investigated the esterification reaction assisted by pervaporation involving propanoic acid and isobutyl alcohol to improve the yield of the isobutyl propionate obtained. A hydrophilic polymer membrane poly(vinyl alcohol)-poly(ether sulfone) (PVA-PES) was used in this study to shift to the right the equilibrium of the esterification reaction, eliminating the water produced during the reaction. The results obtained revealed that the pervaporation-assisted esterification process gave more conversion than the batch esterification process. The membrane showed high selectivity to the removal of water from the reaction mixture. The pervaporation-assisted esterification of oleic acid by n-butanol in the presence of Lipozyme(R) was also investigated by Kwon et al. [30]. It was found, from the results obtained, that the initial reaction rate as well as the equilibrium conversion decreased when the initial water activity increased. These authors also reported that the elimination of the water produced during the reaction was essential to obtain a significant yield. The membrane used in this work was prepared from a dense cellulose acetate and the results obtained in terms of yield and selectivity were very encouraging. Recently, Ameri et al. [32], to improve the conversion of esters produced by the reaction between propionic acid and isopropanol, used Amberlyst 15 (Rohm and Haas) as a catalyst and PERVAP®2201 (Sulzer) as a hydrophilic membrane to eliminate the steam of water from the reaction mixture. From the results obtained, it was revealed that 12 wt% of catalyst added to the reaction mixture increased the acid conversion by 39 wt% without the removal of water and to about 90 wt% with the removal of water by PV during 3 h of the separation process.
The simplest aldehyde is benzaldehyde, with the chemical formula C6H5CHO. This molecule and its derivatives are used in cosmetic formulas as a flavoring, denaturing, and perfuming agent. Its maximum recorded use concentration is 0.5% in perfumes. All organs are exposed to benzaldehyde through respiration and also through skin absorption, and it does not accumulate in any particular type of tissue because this type of molecule is metabolized into benzoic acid, which is ultimately eliminated by urine after forming conjugates with glycine or glucuronic acid.
The dermal irritation and the allergy data that are currently available indicate that benzoic acid has no harmful effects, which is seen as evidence that benzaldehyde is safe. So, in the United States, benzaldehyde is generally recognized as a safe (GRAS) food additive, and in the European Union, it is recognized as a flavoring agent [33].
Benzaldehyde or its derivatives react with a primary amine to form a Schiff base. These substances are utilized for developing drugs that treat a variety of illnesses due to their DNA interaction, anticancer, and antibacterial properties [34,35].
The aliphatic organic amine molecule, n-butylamine, with the chemical formula CH3(CH2)3NH2, reacts with benzaldehyde to produce the Schiff base n-butyl-1-phenylmethanimine. As long as this type of reaction is balanced, the yield of imine obtained remains incomplete. In this situation, it is necessary to remove water as a byproduct to force the reaction to produce the imine only. To eliminate water from the reaction mixture during the reaction, some authors [36] use the distillation method, and this is not without ambiguity when the water formed presents an azeotrope with one of the components of the mixture. The Dean–Stark apparatus is also used to remove water from the reaction mixture. In addition, the reaction has been carried out successfully by using dehydration agents such as sodium sulfate and a molecular sieve [37]. Moreover, methods using solvents, such as tetramethyl orthosilicate or trimethyl orthoformate, which remove water in the reaction medium, have also been separately reported by Love et al. [16] and Look et al. [15]. n-Butylamine is used in many procedures both in industry and in medicine [38,39].
In this present work, the yield of n-butyl-1-phenylmethanimine produced through a Schiff base reaction involving benzaldehyde and n-butylamine was enhanced by removing the water produced during this reaction by the pervaporation method using, for the first time, a cylindrical cell. The advantages of using such a cell are to have a thinner membrane than those used by the classic method using flat cells and also to avoid its breakage due to its mechanical fragility once placed and attached to the porous support.
In this process, the equilibrium should be broken and the reaction forced to produce only n-butyl-1-phenylmethanimine. To reach this goal, a series of membranes based on poly(vinyl alcohol) crosslinked by different oxalic acid amounts were prepared by a solvent casting method and characterized by differential scanning calorimetry (DSC). It is well known from the literature [40,41] that a membrane made of poly(vinyl alcohol) (PVA) is very selective for extracting water from alcohols or other organic products. As long as we are dealing with a reaction mixture that contains n-butylamine, benzaldehyde, water, n-butyl-1-phenylmethanimine, and methanol as solvent, all these components except water are not appreciated by the membrane. This is the reason why the PVA was chosen as the membrane in this technique. The oxalic acid was added to the manufacture of the membrane as a crosslinker, which links the chains together through catalytic esterification reactions in the presence of HCl.
The diffusivity of the pure reactants and products through these membranes was studied through the variation in the swelling degree versus time. For comparison, the condensation reaction of n-butylamine on benzaldehyde was carried out with and without removing water by pervaporation. The production of n-butyl-1-phenylmethanimine was confirmed by Fourier transform infrared (FTIR) and proton nuclear magnetic resonance (1HNMR).
2. Materials and Methods
2.1. Chemicals
Benzaldehyde (purity, 99%), n-butylamine (purity, 98%), chloroform (purity, 99.5%), and poly(vinyl alcohol) (PVA) ( = 20,000 g∙mol−1) and methanol (purity, 98%) were supplied by Sigma Aldrich (Darmstadt, Germany). All chemicals were used as received.
2.2. Schiff Base Reaction
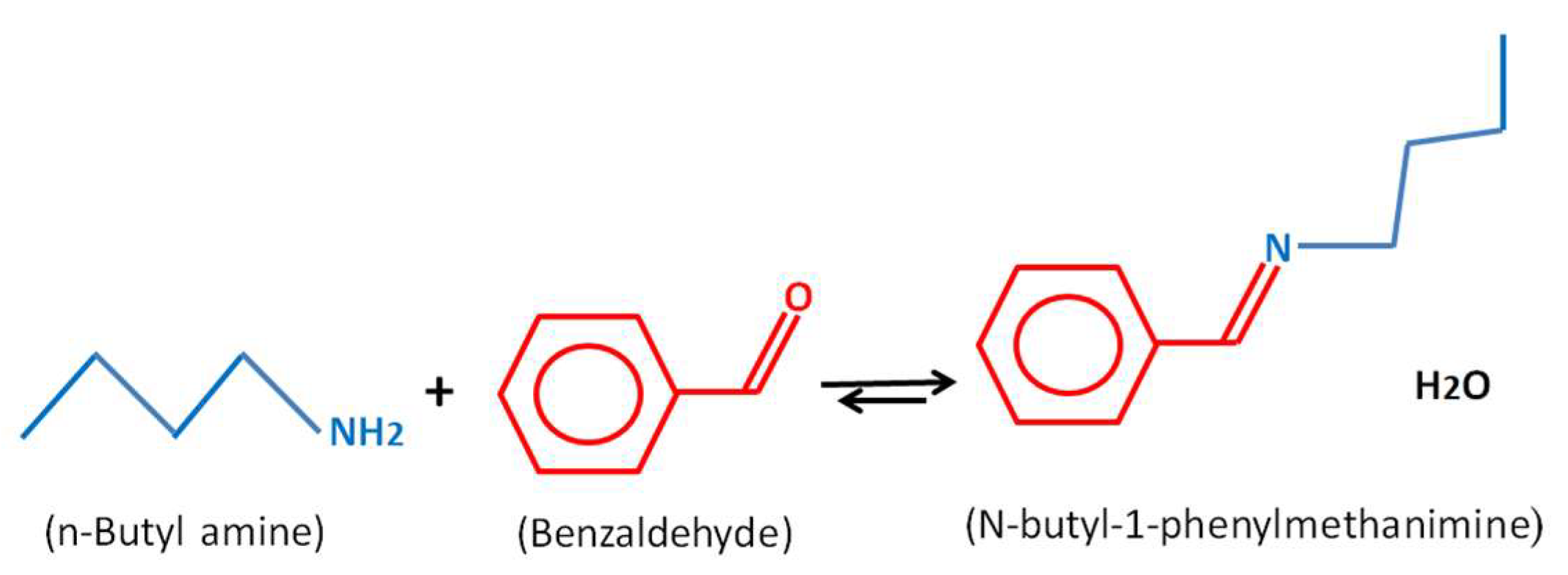
An amount of 0.05 moles (5.35 g) of benzaldehyde and an equivalent mole number of n-butylamine (3.66 g) was placed in a 150 mL three-neck flask containing 20 mL of methanol. The solution was placed into a thermostatically controlled bath at a known temperature. The reaction (Scheme 1) was exothermic and took place with stirring for 1.30 h. Aliquots of 0.5 mL of the reaction mixture were taken at intervals and analyzed by high-performance liquid chromatography (HPLC). This same procedure was applied in the pervaporation cell using a condensation reactor.
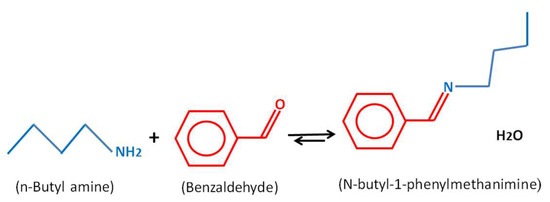
Scheme 1.
Schiff base reaction between n-butylamine and benzaldehyde.
2.3. Membrane Preparation
In a 150 mL beaker, a known amount of PVA was dissolved in a minimum of bi-distilled water with stirring at 90 °C to form a very viscous solution. The resulting solution was then allowed to cool to approximately 35 °C, and then a known quantity of oxalic acid (crosslinker) containing 3 to 5 drops of HCl (conc) was added to this polymeric solution. The inter-chain esterification reaction involving the hydroxyl groups of PVA and the two carboxylic groups of these di-acids was triggered at this time. A series of five PVA films containing different contents of oxalic acid were prepared by the same method and the preparation conditions are presented in Table 1.

Table 1.
Preparation conditions of the PVA-x membrane.
For the swelling experiments, the crosslinked PVA film obtained after total evaporation of the solvent was cut into small, square-shaped pieces of known dimensions.
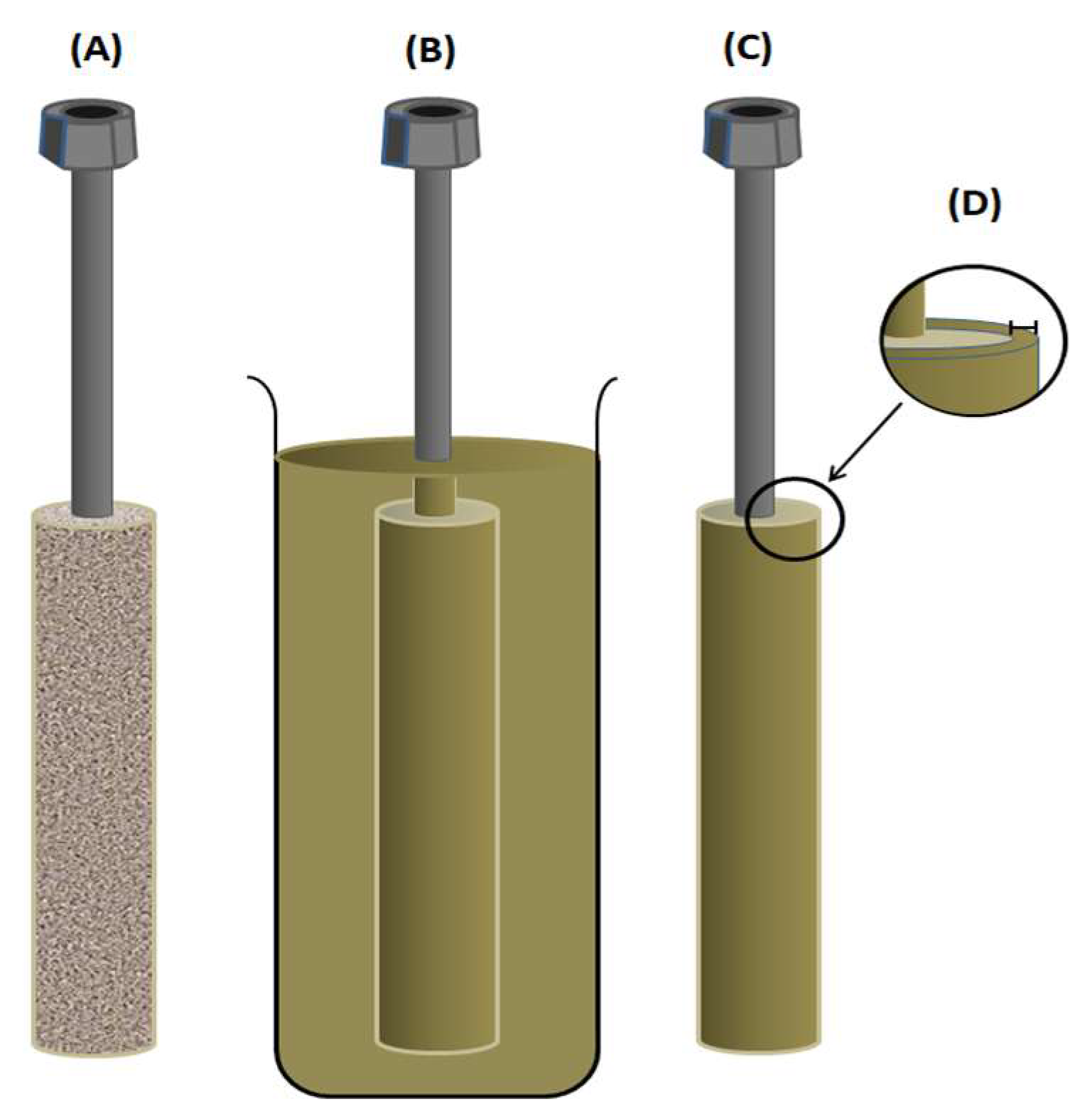
For the pervaporation process, the cell used in this work consists of a solid, porous brass cylinder with a pore diameter varying between 10 and 20 µm covered with a thin film of PVA constituting the membrane (Scheme 2A). The thin film was obtained by immersing this cylinder in the PVA solution prepared in the previous step, as shown in Scheme 2B, then removed and allowed to air dry for 12 h (Scheme 2C). A thin PVA crosslinked film covering the pervaporation cell was obtained after the total evaporation of the solvent and its heating in an oven maintained at 40 °C for 2 h.
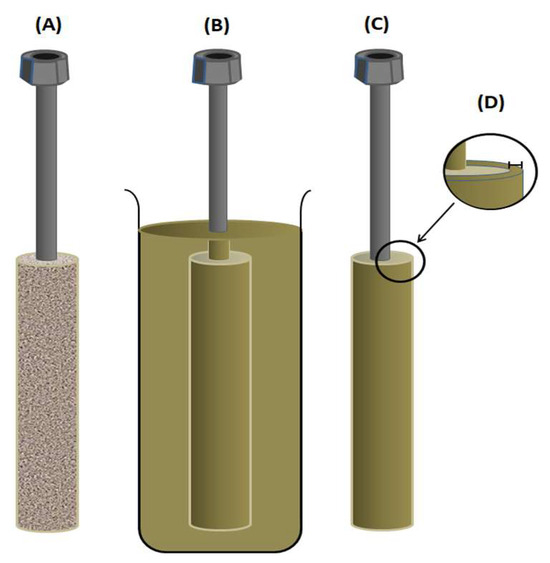
Scheme 2.
(A) Pervaporation cell; (B) cell immersed in the silicone solution; (C) cell coated; and (D) membrane thickness.
The assembly (cylinder covered with PVA) was then placed in a vacuum oven maintained at 90 °C in order to finish the crosslinking reaction and to eliminate all traces of residual reagents and solvent until reaching a constant weight. The thickness of the membrane (Scheme 2D) was taken from the arithmetic average of 5 measurements taken at different locations of the film obtained after having been easily detached from the pervaporation cell (cylinder) by dipping it again in water at 60 °C for 30 min. Five PVA-x membranes with different crosslinking densities (1.89 × 10−2, 3.23 × 10−2, 3.81 × 10−2, 4.03 × 10−2, and 4.50 × 10−2), with a thickness of 35 ± 5 μm measured using a Mitutoyo 293-340-30 digital micrometer, were prepared using the same method by varying the percentage of crosslinking agent in the PVA membrane. Among the advantages of this assembly is obtaining very low thicknesses of a few micrometers, which are not technically easy to obtain when using conventional flat cells due to the excessive fragility and elasticity of the polymer, plus the effect of the electrostatic forces exerted on the two surfaces of the film, which do not facilitate the detachment of the membrane from its mold.
2.4. Pervaporation Setup
The pervaporation system used in this investigation is shown in Scheme 3. The pervaporation cell has a capacity of 120 mL. In static mode, the temperature of the load is kept constant during the process thanks to the circulation of a thermostatic fluid (water) passing between the walls of the cell containing the load (1). The pervaporation process operates in static mode and the temperature of the feed in the tank (2) is kept constant using a thermostatic coil immersed in the mixture. In all cases, the homogenization of the food was ensured by stirring using a magnetic stirrer.
2.5. Characterization
The structure of the synthesized N-butyl-1-phenylmethanimine was highlighted by Fourier transform infrared (FTIR) analysis and by proton nuclear magnetic resonance (1H NMR). The purity of this compound was characterized by differential scanning calorimetry analysis (DSC). The evolution of the reaction over time is followed by high-performance liquid chromatography (HPLC). The thermal properties of PVA and PVA-x films were also analyzed by DSC analysis.
2.5.1. FTIR
The FTIR spectra of N-butyl-1-phenylmethanimine and its reactants were performed on a Perkin Elmer Spectrum GX FTIR spectrometer (Shelton, CT, USA) operating in attenuated total reflection mode in a wave number ranging between 4000 and 650 cm−1, 32 cycles of scanning, and a resolution of 2 cm−1. The samples were analyzed as a thin liquid film placed between two transparent dehydrated KBR pellets.
2.5.2. NMR
The 1H NMR spectra of the N-butyl-1-phenylmethanimine Schiff base and its reactants benzaldehyde and butylamine in CDCl3 were recorded on a JEOL FX 90 Q NMR spectrometer (Tokyo, Japan) at 500 MHz at room temperature (~25 °C).
2.5.3. DSC
The DSC thermograms of polymers, reactants, and products were plotted on a DSC device (Shimadsu DSC 60, Tokyo, Japan) previously calibrated with indium. An amount of 10 mg of samples were placed in aluminum DSC capsules before being placed in the DSC cell. Samples were scanned by heating from −25 to 250 °C with a heating rate of 10 °C∙min−1. The glass transition temperatures, Tgs, of polymers were taken from the inflection point of the thermal curves, which characterized the sliding of about 50% of the polymer chains. The melting temperature, Tm, of PVA-x was determined from the endothermic peak corresponding to its enthalpy of fusion of pure reactants, and products were taken from the summit of the endothermic peak corresponding to their enthalpies of fusion.
2.5.4. HPLC
The qualitative and quantitative analysis of the retentate and the permeate was carried out on an Agilent 1100 Series high-performance liquid chromatograph (HPLC) (San Diego, CA, USA) equipped with a binary pump, degasser, autosampler, UV-diode array detector (UV-DAD), Macheray–Nagel column nucleodur C18 gravity 100 × 3.0 mm, particle size 3.0 mm, mobile phase: methanol/water 90:10, flow rate 0.3 mL·min−1, column temperature 40 °C. The UV detection was measured at 210 and 254 nm and the sample volume injected was 1.0 μL.
2.5.5. The Crosslinking Density
The crosslinking density of the prepared PVA network was estimated from the average molecular weight between two adjacent crosslinks, Mc, using the Flory–Rehner equation [42,43]. This theory states that Mc values increased with the swelling ratio of the hydrogel. According to this approach, the molecular weight between adjacent crosslinks is calculated using Equation (1) [44]
where χ is the PVA–solvent interaction parameter (=0.49) [45]. and are, respectively, the molar volume of water and the volume fraction of polymer in the swollen mass, which is calculated from Equation (2). symbolizes the density of the PVA (1.2 g·cm−3) [45].
where Qm is the mass swollen ratio. The crosslinked density, q, used to characterize the polymer membrane, is given by Equation (3) [46].
where is the molar mass of the monomeric unit of the polymer, which is obtained by the following Equation (4):
where mp and mK-L are the masses of the polymer and the crosslinker, respectively. and are the molar masses of the polymer and crosslinker, respectively.
2.5.6. Mass Transfer
The swelling dynamics of the PVA-x membranes in the pure components of the reaction mixture were carried out at 30 °C. Five dried PVA-x films of known masses measuring 4.5 cm × 4.5 cm with a thickness of 35 ± 5 μm were measured and then immersed separately in each pure component and allowed to swell with continuous stirring until saturation (equilibrium). The film samples were removed at regular intervals, wiped gently on both sides with tissue paper, and then weighed on a US Solid 200 × 0.0001 g Analytical Balance—Density and Dynamic Weighing, 0.1 Digital Laboratory Balance mg.
This experimentation was duplicated and the results obtained were taken from the arithmetic average, and the degree of swelling (DS) of each film sample at a certain time t of the swelling process was calculated using Equation (5) [47,48]:
where and are the weights of the swollen film at time t and the initial weight of the dry film sample.
According to the literature [49], the diffusion of small molecules through a polymer material for a short period of diffusion and when the wt/ is less than 0.5 is expressed by the following relationship:
where and are the weight of the sorbed molecules by the film sample during a time t and the total mass absorbed, respectively. l and D are the polymer film thickness and the diffusion coefficient of the molecules sorbed, respectively. The curve profile obtained from the change in the wt/ versus the square of time describes a straight line, and the slope of the linear portion obtained gives the D value of the absorbate through the PVA-x membrane. The basic equation that allows us to obtain the mass of molecules absorbed by a polymer material is given by the following equation [50].
where n and k are the type of the diffusion mechanism and a constant relating the swelling rate, which depends on the film thickness and the diffusion coefficient, respectively. By analogy with expression (7), the k value is obtained from the following relationship.
2.6. Reaction Preparation
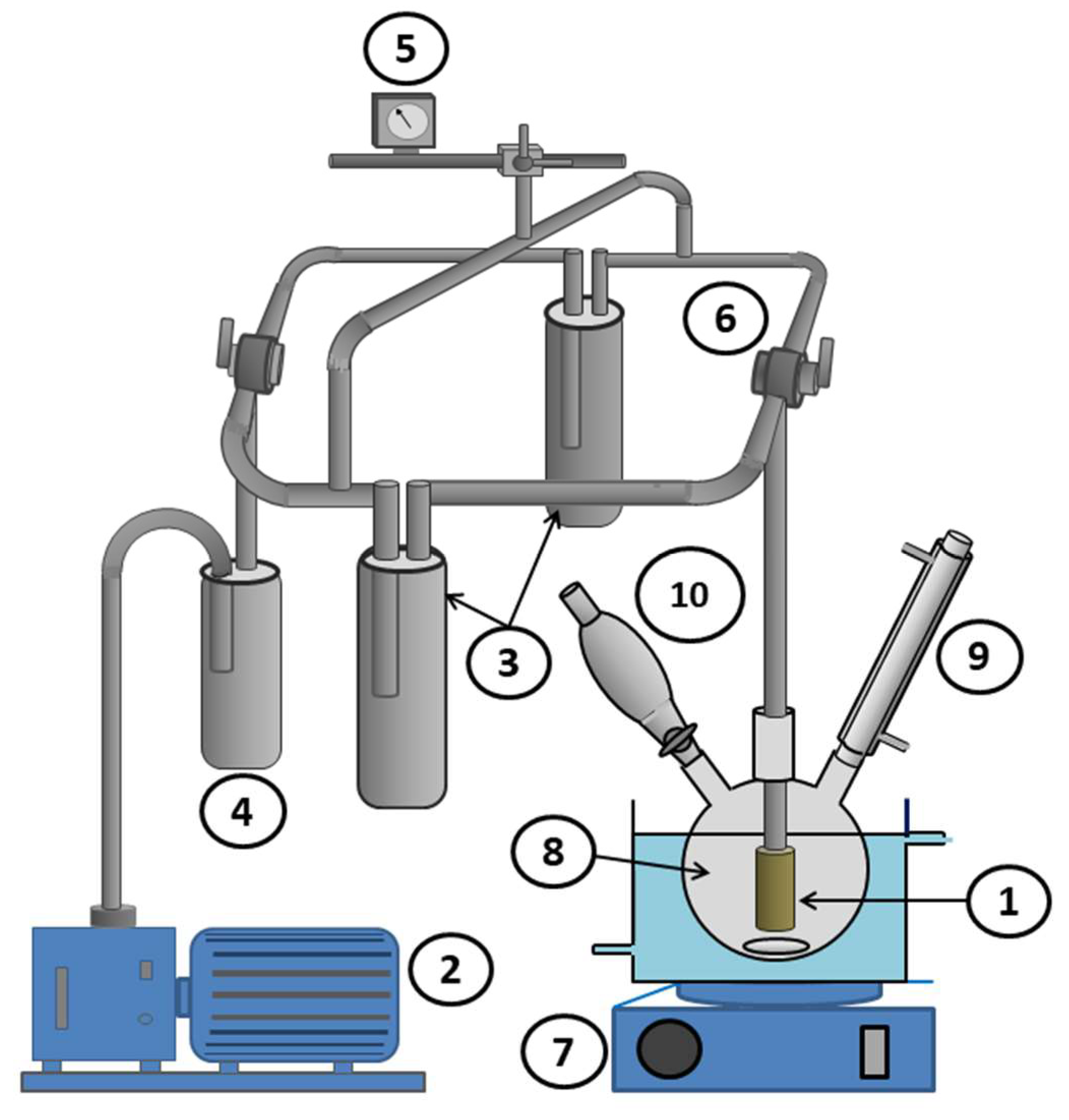
In a two-necked flask connected to a condenser and maintained at a temperature of 5 °C using a thermostat, 10 mL of methanol (solvent) and 0.10 moles (10.612 g) of benzaldehyde were introduced under moderate stirring until thermal equilibrium, which was reached after about 3 min. Given the exothermic nature of Schiff base reactions, an equivalent amount in moles (7.314 g) of n-butylamine was added dropwise to the previous solution while maintaining the reaction temperature constant at the desired value. The pervaporation-assisted Schiff base reaction was carried out in the same device used previously by immersing the cell (Scheme 2C) in the reaction mixture through the other opening of the flask, as indicated in Scheme 3.
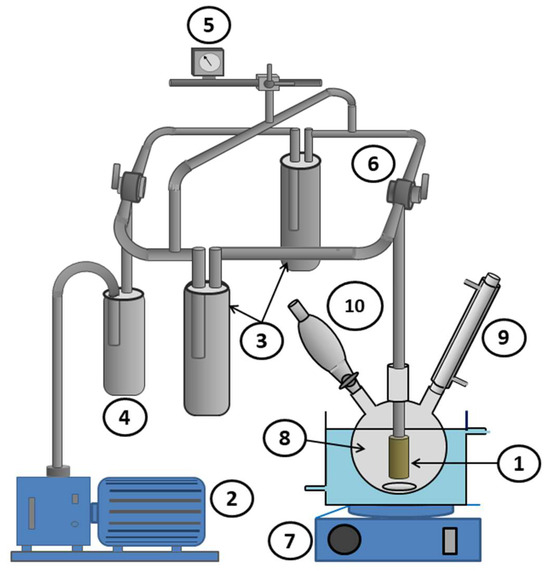
Scheme 3.
Pervaporation apparatus used in this work: (1) pervaporation cylindrical cell (surface area 117.75 cm2); (2) vacuum pump; (3) condenser (trap); (4) guard trap; (5) Pirani gauge; (6) two-way Rotaflow valve; (7) magnetic stirrer; (8) flask; (9) refrigerant; and (10) addition funnel.
Scheme 3.
Pervaporation apparatus used in this work: (1) pervaporation cylindrical cell (surface area 117.75 cm2); (2) vacuum pump; (3) condenser (trap); (4) guard trap; (5) Pirani gauge; (6) two-way Rotaflow valve; (7) magnetic stirrer; (8) flask; (9) refrigerant; and (10) addition funnel.
2.7. Pervaporative Parameters
The performance of a pervaporation membrane for a given feed composition and temperature is most often reported in terms of the cumulative total flux, J (Kg∙m2∙h−1) (Equation (9)), and the separation factor of the pervaporation membrane, component (i), βi (Equation (10)). This parameter is also defined as the purity of the permeate in terms of the weight fraction of component i.
where m stands for the total weight of the permeate through the effective surface area A at time t of the separation process. X and Y are the mass fractions of species I and j in permeate and feed, respectively.
3. Results and Discussion
3.1. Characterization
The structure of n-butyl-1-phenylmethanimine was characterized by FTIR, 1HNMR, and DSC analyses. The crosslinking of PVA was demonstrated by a water solubility test (θ—solvent) at 90 °C (the temperature at which the corresponding non-crosslinked PVA dissolves, called the θ temperature). The crosslinking of PVA was also confirmed by its glass transition and melting temperatures using the DSC method.
The absorption capacity of the prepared membranes in each pure component of the Schiff base reaction mixture was evaluated by measuring the degree of swelling at equilibrium (saturation). This parameter made it possible to determine the crosslinking density of the PVA-x network using the Flory–Rehner equation and the diffusion coefficient for each component in this material. The detailed results obtained are presented in the following sections.
3.1.1. FTIR
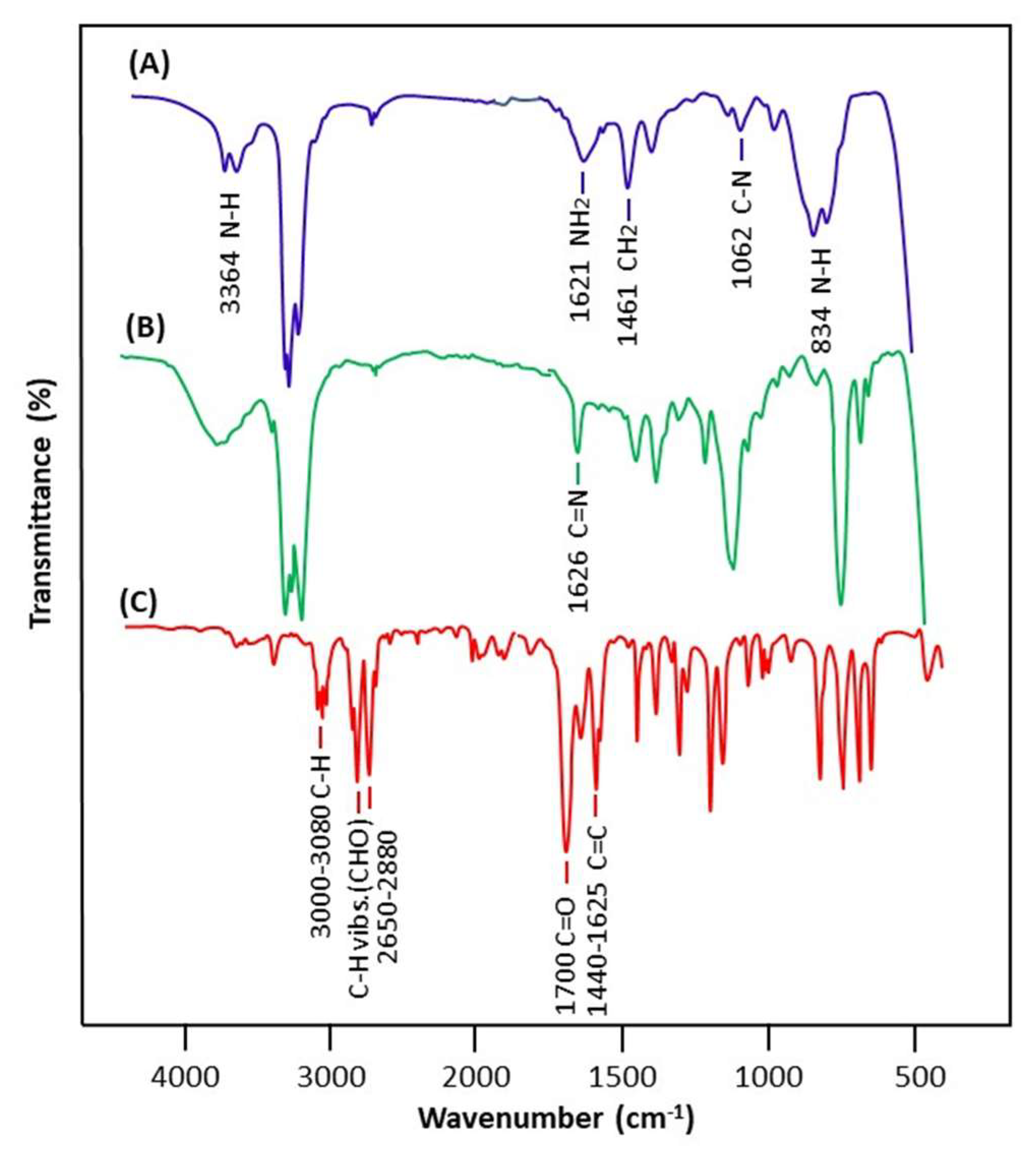
The FTIR spectra of imine as well as those of benzaldehyde and n-butylamine reactants are shown in Figure 1. The comparison of the n-butyl-1-phenylmethanimine spectrum with those of the two components confirms the structure of this compound as the product of the reaction by the appearance on its spectrum of all the signals attributed to both the reagents, such as those of n-butylamine at 3364, 1062, and 1461 cm−1 attributed to the N-H, C-N-, and –CH2- groups, and those of benzaldehyde at 1440–1625, and 1750 cm−1 assigned to –C=C- and their overtones, except that of the carbonyl at 1735 cm−1 and that of the amine group at 1621 cm−1, respectively, which are replaced by a new weak signal at 1626 cm−1 assigned to the C=N stretch of n-butyl-1-phenylmethanimine [51].
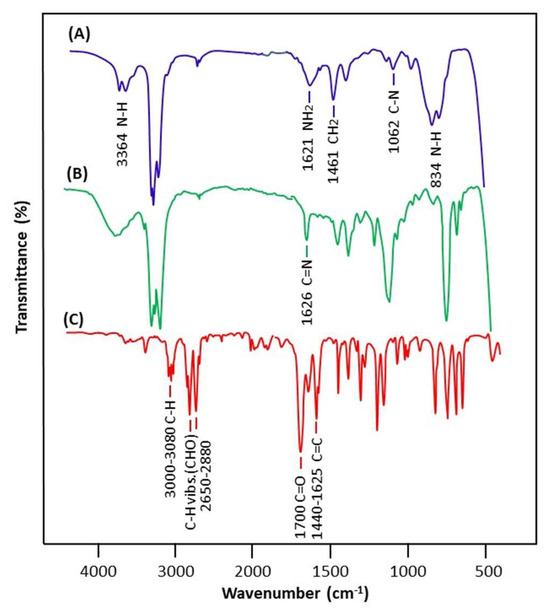
Figure 1.
Comparative FTIR spectra of n-butylamine (A), n-butyl-1-phenylmethanimine (B), and benzaldehyde (C).
3.1.2. 1H NMR
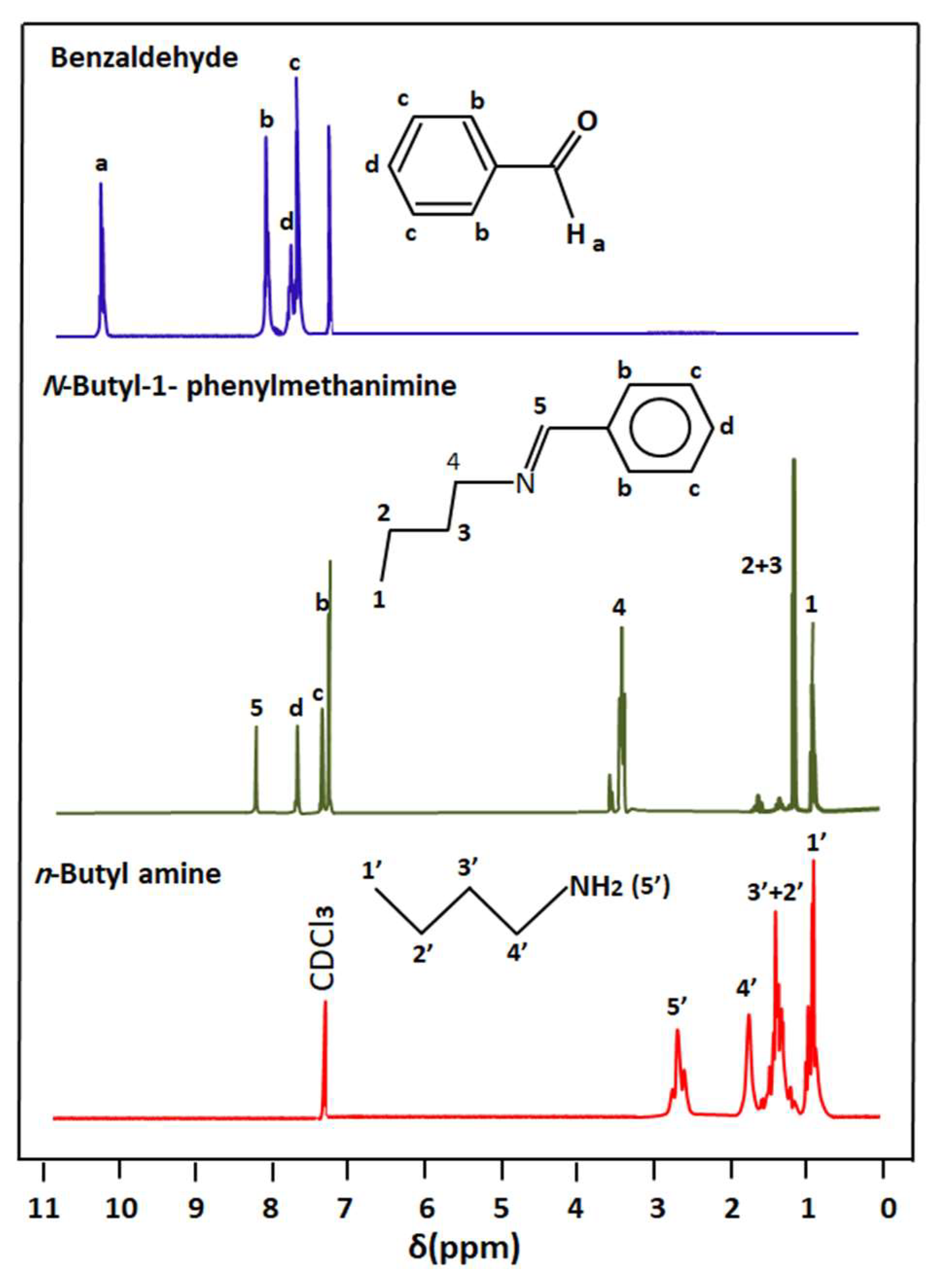
The comparison of the 1HNMR spectrum of n-butyl-1-phenylmethanimine with those of the reagents is presented in Figure 2. As can be observed from these signals, the structure of this product is evidenced by the appearance of the proton (5) at 8.25 ppm belonging to the carbon adjacent to the nitrogen of the imine function (HC=N-) and simultaneously the disappearance of signals (a) and (5′) at 10.25 and 2, 72 ppm, attributed to the protons of the starting aldehyde and amine, respectively. The displacement of the signals belonging to the protons (b) and (2′, 3′, and 4′) attributed, respectively, to the structures of benzaldehyde and n-butylamine is also proof of obtaining n-butyl-1-phenylmethanimine.
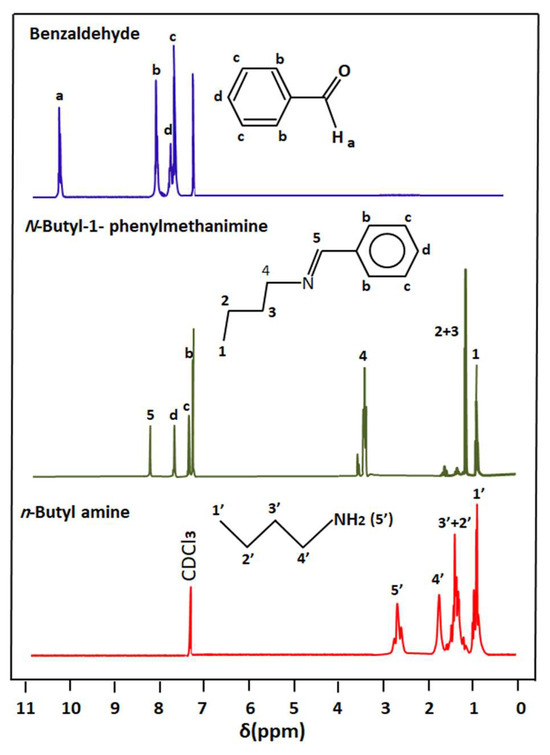
Figure 2.
Comparison of the 1H NMR spectra of n-butyl-1-phenylmethanimine with its reactants benzaldehyde and n-butylamine.
3.1.3. DSC
The melting temperature of n-butyl-1-phenylmethanimine was determined by the DSC analysis, which we do not present in this work, from the top of the endothermic peak, indicating the enthalpy of fusion of this compound and the result obtained after the first and the second run, indicating only one Tm at 141 °C. It is also revealed that no benzaldehyde and n-butylamine residual reactants are present with the synthesized imine.
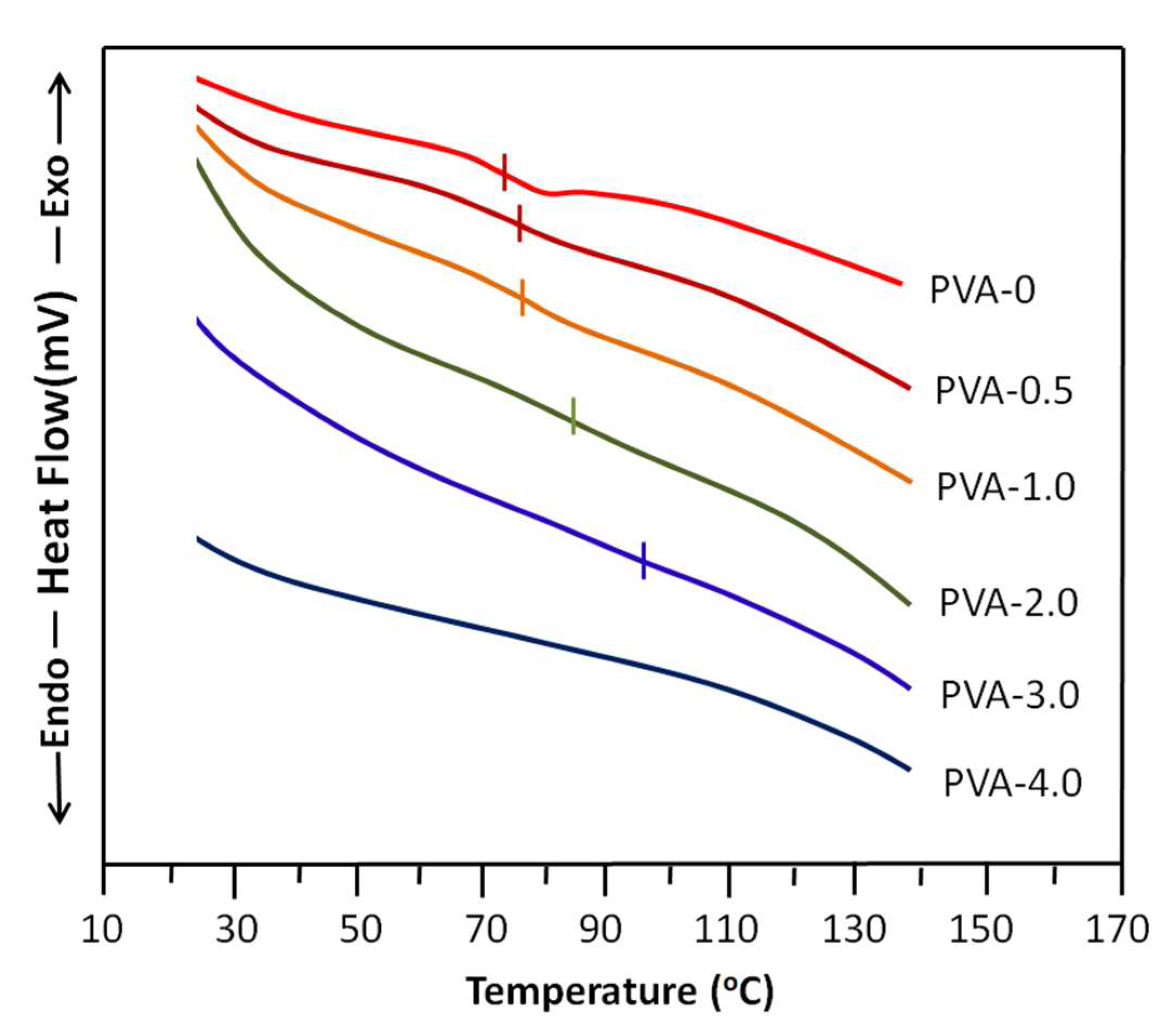
The DSC thermal curves of PVA-x before and after the crosslinking process are shown in Figure 3. The thermogram of non-crosslinked PVA (PVA-0) shows a glass transition temperature, Tg, of 73 ± 3 °C and a melting temperature of 212 ± 3 °C, which agree with the literature [52]. Adding to that, the Tg of PVA, according to the literature, is contradictory due to several factors that directly or indirectly influence the thermal properties of this material, such as the degree of acetylation or the degree of hydroxylation, the molecular weight [53], and the moisture degree, in which the water molecules incrusted in the PVA matrix play a plasticizer role, leading to a decrease in the Tg value [54] of this polymer. On the other hand, as shown by the thermograms of this same figure, the Tg of this polymer tends to disappear with the addition of the crosslinker until complete erasure when the oxalic acid in the membrane is 4 wt% (PVA-4.0). This confirms the crosslinking of PVA. The shift of the Tg to the right observed on the shape of these thermal curves, which increases with the crosslinker content, also supports this conclusion. Because the knots connecting the chains resulting from the crosslinking reaction increasingly reduce the sliding of the chains between them, consequently, with the increase in the degree of crosslinking in the polymer, this leads more and more to the reduction in the movement of the PVA chains and therefore shifts the Tg toward high temperatures.
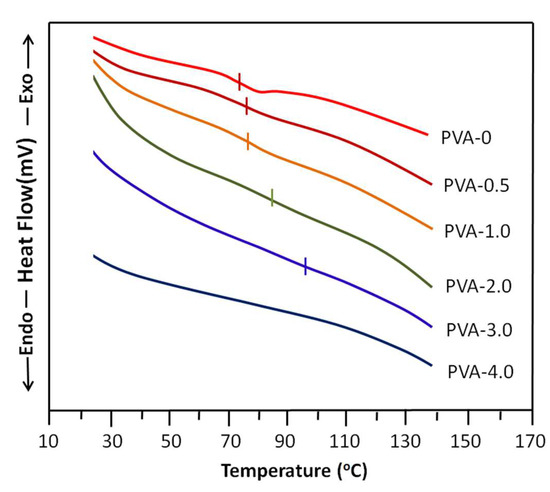
Figure 3.
DSC thermograms of non-crosslinked PVA and crosslinked PVA-x with different oxalic acid contents.
3.1.4. The Crosslinking Density of the PVA-x Networks
The values of the degree of crosslinking of the PVA-x network are estimated from the average molecular weight between two adjacent crosslinks, Mc, and those of the crosslinking density, q, which characterize the hydrogel membranes calculated from Equations (3) and (4), respectively, and the results obtained are presented in Table 2 for comparison with the values of , χ, and .

Table 2.
The values of Mc, Mr, and q of PVA-x calculated using Equation (4).
As these data show, the Mc and q values are in good agreement with the composition of the crosslinking agent in the initial oxalic acid/PVA ratio, which increases as this ratio increases.
3.1.5. Swellability of PVA-x Membrane
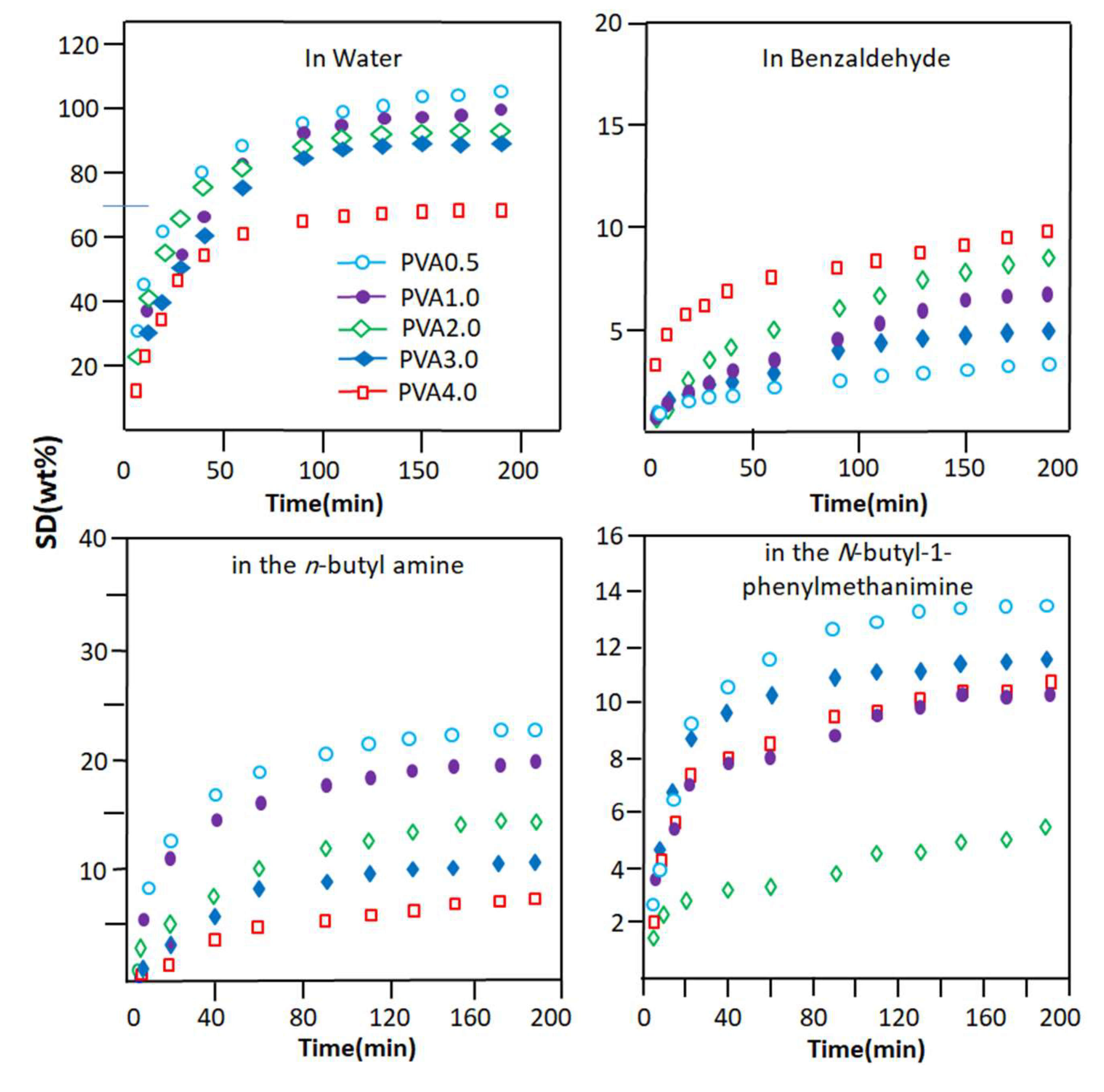
The evolution of the degree of swelling of the PVA-x membranes in each pure component of the reaction mixture as a function of time is plotted in Figure 4, and the swelling capacities of these materials deducted from the maximum swelling of the PVA membrane are shown in Table 3. These results are usual logarithmic curves obtained regardless of the degree of crosslinking of the PVA and the component absorbed. These data reveal that the efficient swelling of PVA-x in water is obtained with the PVA-0.5 membrane, which contains the minimum ratio of oxalic acid (0.5% by weight). This has also been observed by different researchers using other crosslinking agents [55,56]. Indeed, this material is capable of absorbing 107.0 ± 2.3% by weight of water at equilibrium within 3 h of the swelling process. Meanwhile, when this same membrane is immersed in pure n-butyl-1-phenylmethanimine as the main product resulting from the Schiff base reaction, its swelling capacity is far behind, with an absorption capacity that does not exceed 13.4 ± 2.3% by weight. This same material, when immersed in pure n-butylamine (polar molecule) and benzaldehyde (poorly polar molecule), absorbs a maximum of 22.8 ± 2.5 and 3.1 ± 1.1 wt% of these compounds, respectively. However, the swelling capacity of this same membrane, when immersed in pure N-butyl-1-phenylmethanimine, is far behind that in water with an absorption capacity that does not exceed 13.4 wt%. This same material, when immersed in pure n-butylamine (polar molecule) and benzaldehyde (low polar molecule), showed swelling capacities of 22.8 ± 2.5 and 3.1 ± 1.1 wt%, respectively.
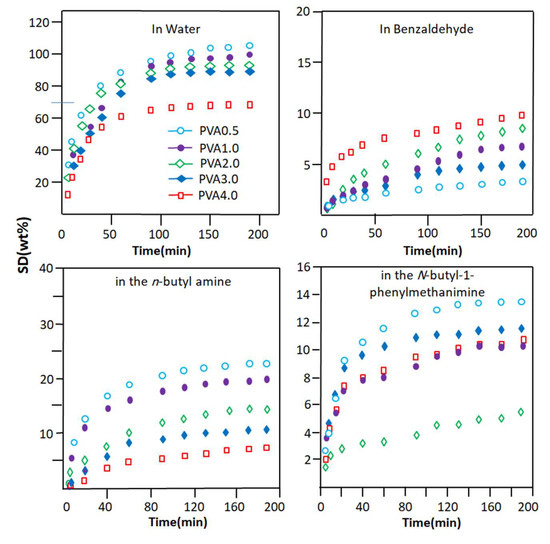
Figure 4.
Change in the swelling degree of PVA-x membranes with different crosslinker contents in the pure components of the Schiff base reaction versus time.

Table 3.
Swelling capacity of PVA-x in the pure components of the reaction mixture taken at 25 °C.
To conclude this part of this study, it was revealed from these data that the swelling capacity of the PVA-0.5 membrane in the pure components of the reaction mixture decreased in the following order:
Water >> n-butylamine > n-butyl-1-phenylmethanimine > benzaldehyde
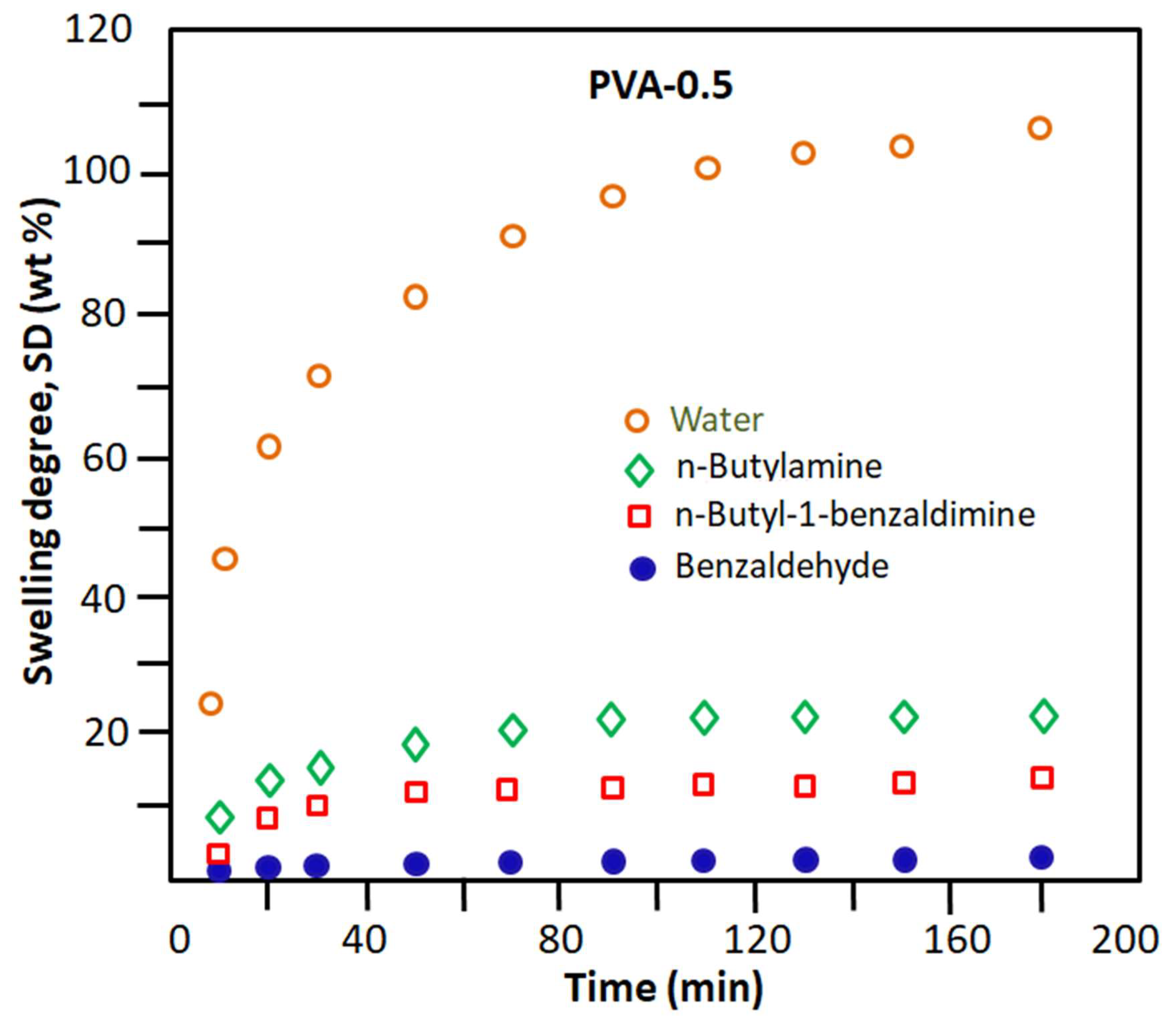
These results seem to be obvious because PVA has a very polar character, regardless of its crosslinking rate. This is due to the nature of the hydroxyl substituents of the corresponding monomeric unit. This goes in the direction of promoting the development of hydrogen bonds with water molecules (very polar) and to a lesser extent the hydrophilic/hydrophobic balance, which goes in the direction of increasing the hydrophobic character of this n-butylamine. This is due to the highly hydrophobic nature of the n-butyl radical. For the rest of the components, the hydrophilicity of these molecules decreases in the direction indicated above. The reduction in the degree of swelling of the PVA-x membrane in these pure organic components as the percentage of crosslinker content in the PVA increases is due to the reduction in the free volume existing between the polymer chains. This allows the passage of a reduced number of penetrating molecules depending on their affinity, size, and nature. The comparison between the results obtained in this part of this study, as shown in Figure 5, places the PVA-0.5 membrane as the most efficient candidate for the elimination of water from the other components of the Shiff base reaction mixture. To be convinced of this, it is enough to take the value of the degree of swelling of PVA-0.5 at one hour of the process, which indicates 87% by weight for water and values less than 21% by weight for the organic components. Indeed, these results predict that, when this membrane is used in the extraction of water produced during the reaction between benzaldehyde and n-butylamine, the result obtained will be very selective for water.
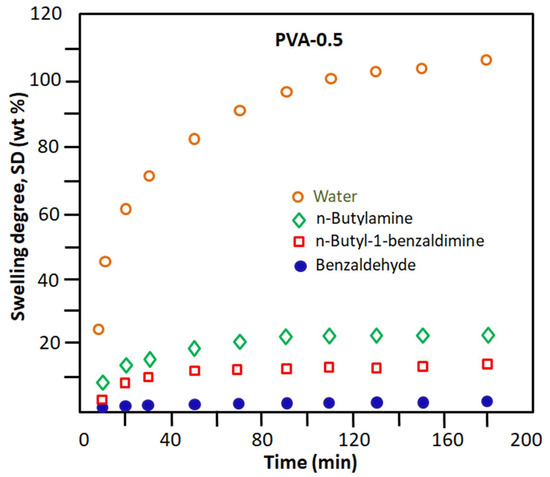
Figure 5.
Change in the swelling degree of the PVA-0.5 membrane in the pure reactants and products involved in the Schiff base reaction.
3.1.6. The Diffusion Properties of PVA-x Membrane
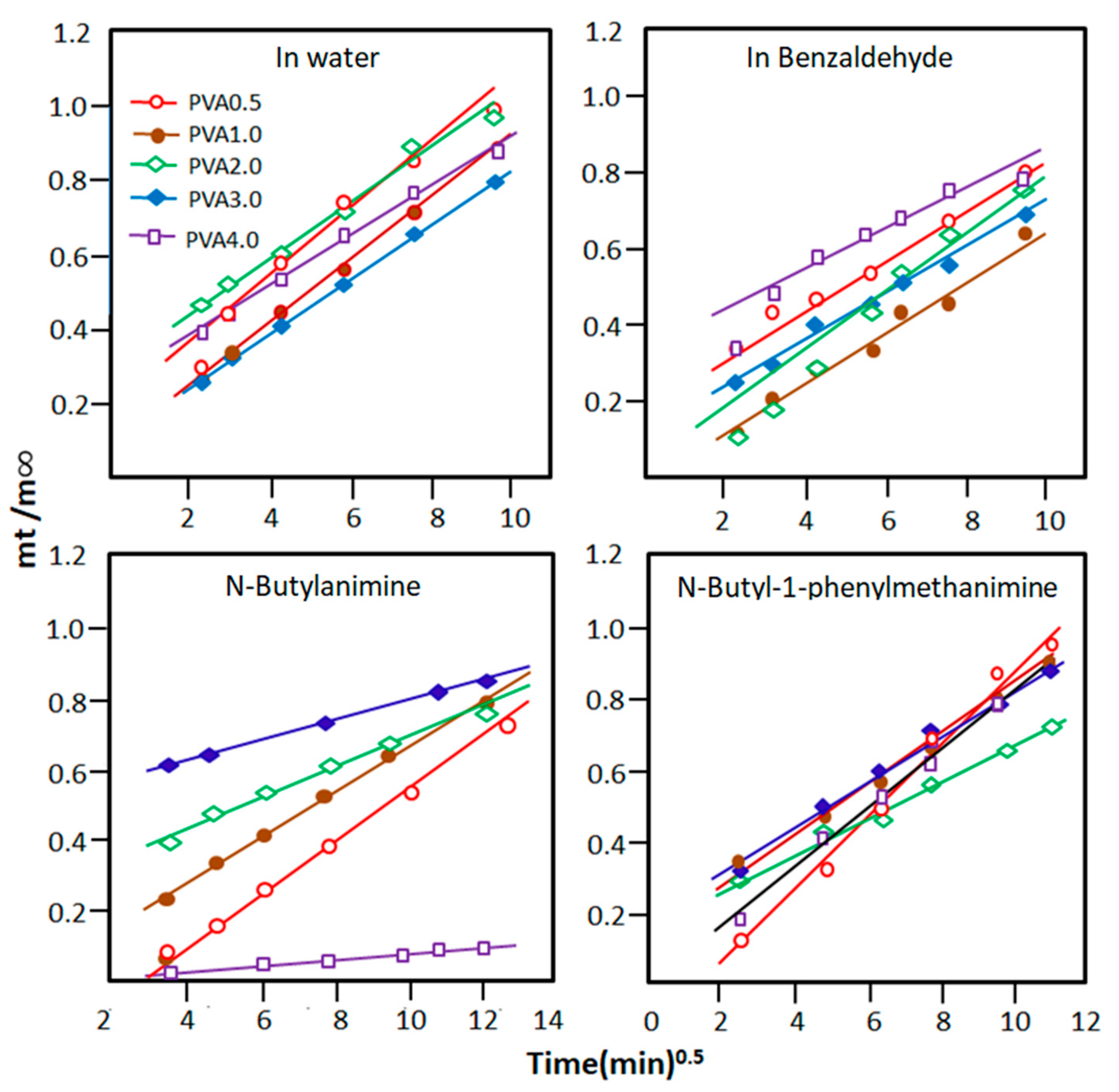
The curves indicating the change in the ratio as a function of the square root of time are plotted for each pure component in Figure 6 and reveal a straight line for all PVA-x/absorbate systems. This indicates that the order of the diffusion process is 0.5 and that the Fick equation fits well with this type of diffusion. The value of the diffusion factor, D, and the proportionality constant, k, for each pure component of the reaction mixture are deduced from the slope and intercept of each curve, respectively, using Equations (3) and (4), and the results obtained are shown in Table 4. As can be read from these data, the diffusion coefficient of water molecules through the PVA-x matrix is much higher than those of n-butyl-1-phenylmethanimine, benzaldehyde, and n-butylamine. These results also reveal that water molecules penetrate the PVA-0.5 membrane more quickly than other materials containing more crosslinks and that water molecules diffuse much more rapidly than other pure components of the mixture. For example, according to the k values, the diffusion rate of water molecules in the PVA-0.5 matrix is 2.69 times higher than that of imine in this same membrane, 1.92 times that of n-butylamine, and 2.37 times that of benzaldehyde.
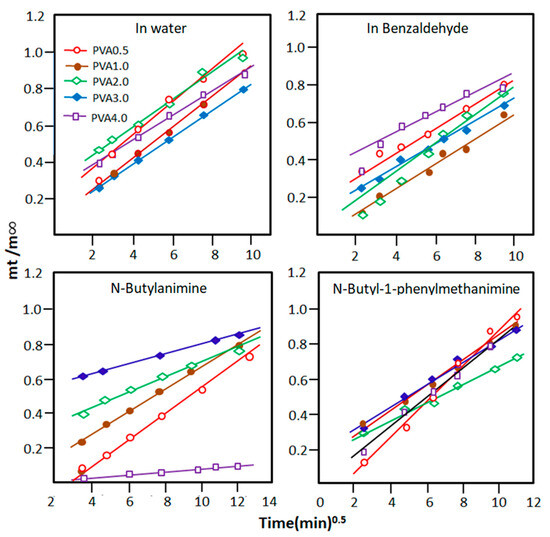
Figure 6.
Variation in the ratio versus the square root of time for PVA-x in the reactants and products involved in the Schiff base reaction.

Table 4.
Diffusion factor, D, and proportional constant, k, of each pure component involved in the reaction mixture.
3.2. Schiff Base Reaction
3.2.1. Reaction Non-Assisted by Pervaporation
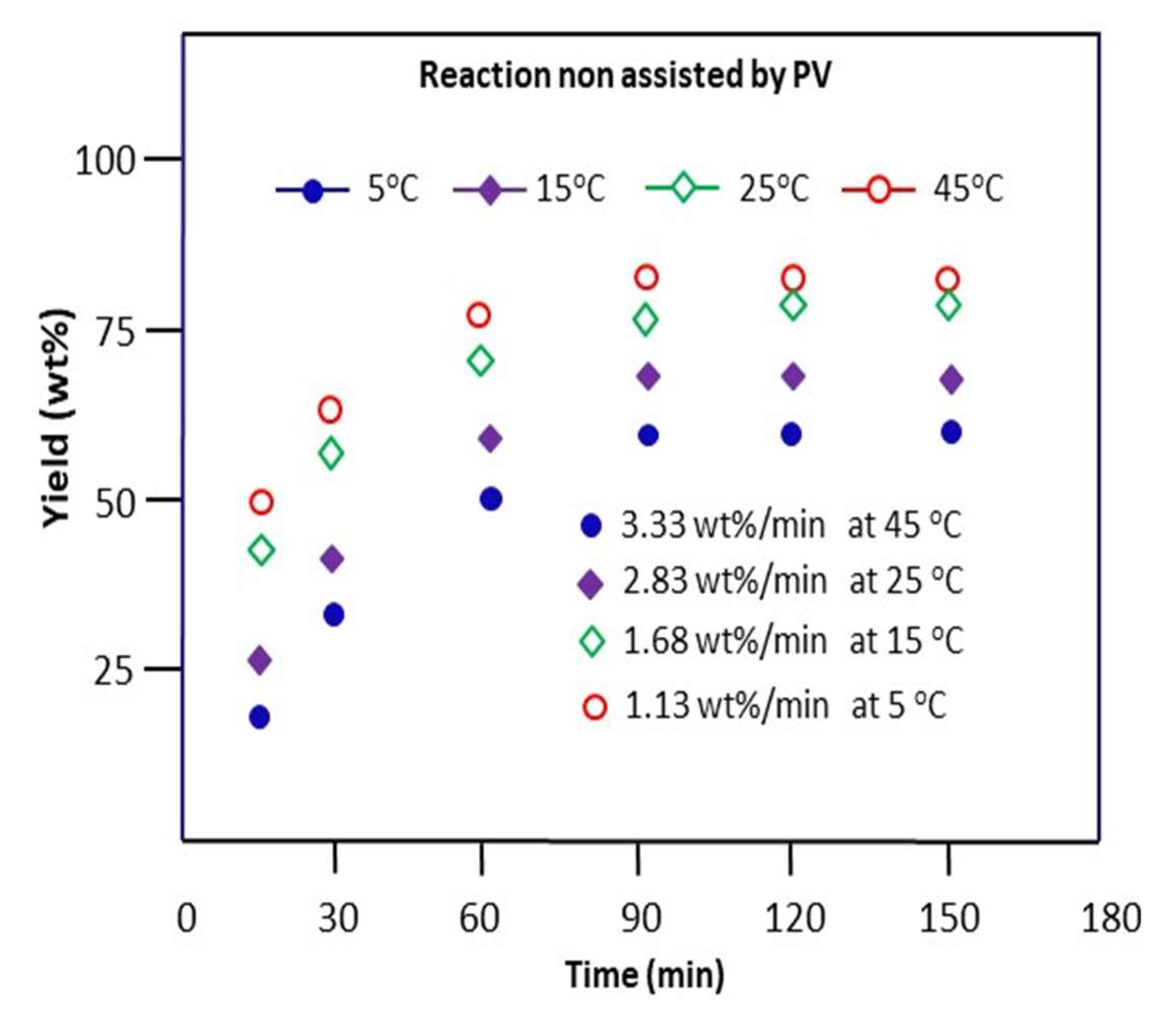
The Schiff base reaction involving benzaldehyde and n-butylamine was carried out in methanol for a time at 5, 15, 25, and 45 °C without the removal of water produced during the reaction process. The variations in the obtained yield of n-butyl-1-phenylmethanimine versus time are plotted and grouped in Figure 7.
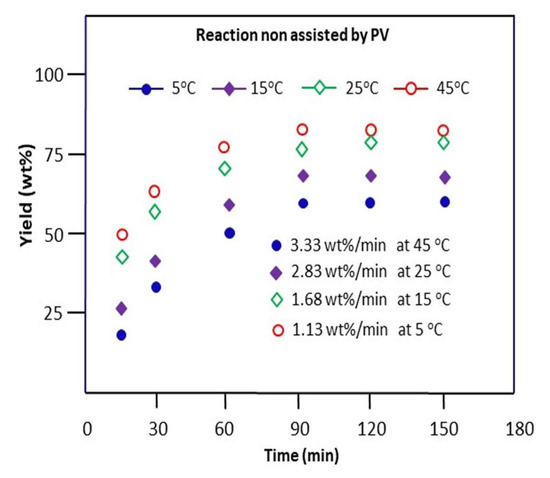
Figure 7.
Variation in the yield of the Schiff base reaction non-assisted by PV between n-butylamine and benzaldehyde at different temperatures.
As these data show, the rate of conversion of benzaldehyde to imine is very rapid during the first 15 min of the reaction, as shown in this figure, and then stabilizes after about 1 h of the process. During this stage, about 80, 65, 53, and 29 wt% of the cumulative total yield are obtained at 5, 15, 25, and 45 °C with a reaction rate of 1.13, 1.68, 2, 83, and 3.33 wt%·min−1, respectively. These results also reveal that the best yield of produced imine is obtained at 45 °C, in which 84.0 wt% of n-butyl-1-phenylmethanimine is obtained at equilibrium (90 min). The kinetic order of the reaction is estimated on the hypothesis of order 2 supported by confirmation according to the following reasoning:
For a second-order reaction:
C6H5COH + C4H9NH2 ⇌ C11H15N + H2O
In this case, the reaction rate is expressed by Equation (11):
where the initial mole numbers of the reactants are equal and the kinetic equation of the reaction is written as follows:
where k is the second-order rate constant. The integration of Equation (12) yields
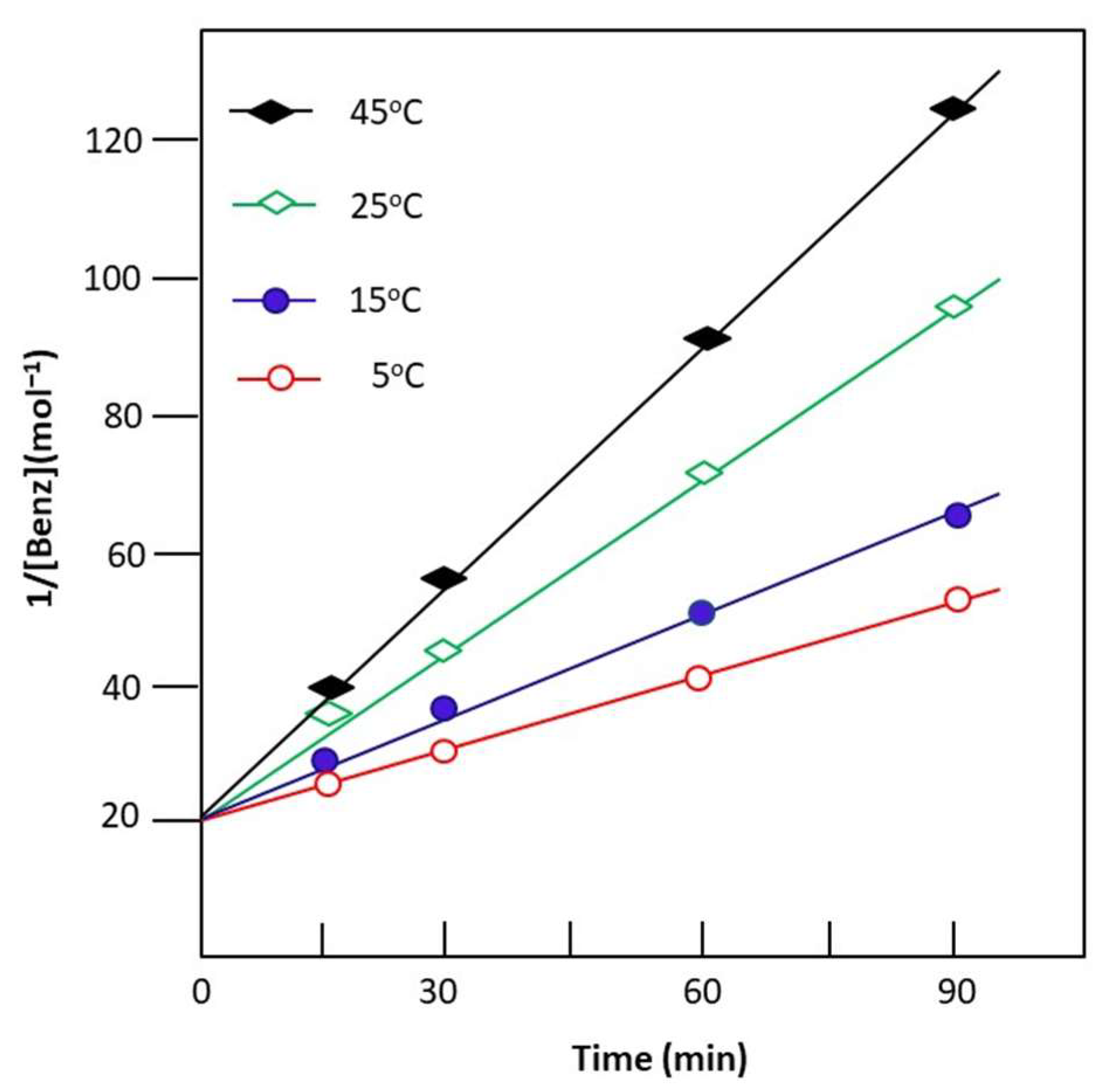
where and are the mole numbers of the benzaldehyde at time zero and t, respectively. The curve indicating the variation in the inverse of the mole number of remaining benzaldehyde or n-butylamine versus time, taken from the data of Figure 8, indicates a straight line for any temperature, and the rate constant k is obtained from the slope of the corresponding curve and Equation (13). The results confirm order 2 of the Schiff base reaction and its intercept gives its rate constant k. Table 5 shows the rate constants of the reaction for different investigated temperatures.
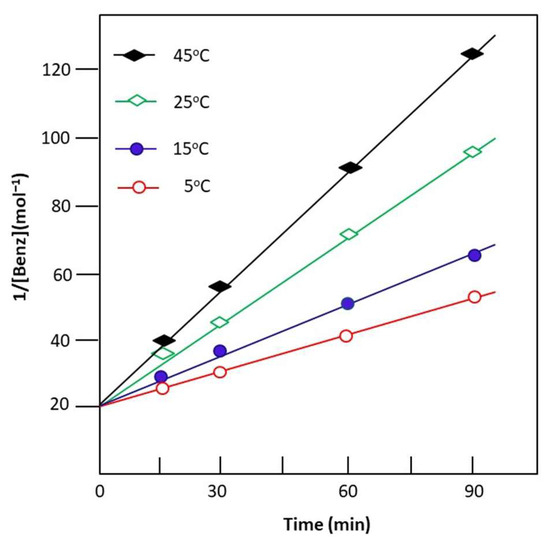
Figure 8.
Change in the inverse of the mole number of unreacted benzaldehyde as a function of the reaction time obtained at different temperatures.

Table 5.
k and keq values of the Schiff base reaction determined at different temperatures.
The equilibrium reaction constant is determined using Equation (14):
where x and are the mole numbers of imine or water obtained at equilibrium and the initial mole number of benzaldehyde or n-butylamine, respectively. The values of calculated at different temperatures from the data of Figure 6 and Equation (14) are gathered for comparison in Table 5.
These results clearly reflect the dependence of the equilibrium constant on temperature, as predicted by the Van-t-Hoff law [57].
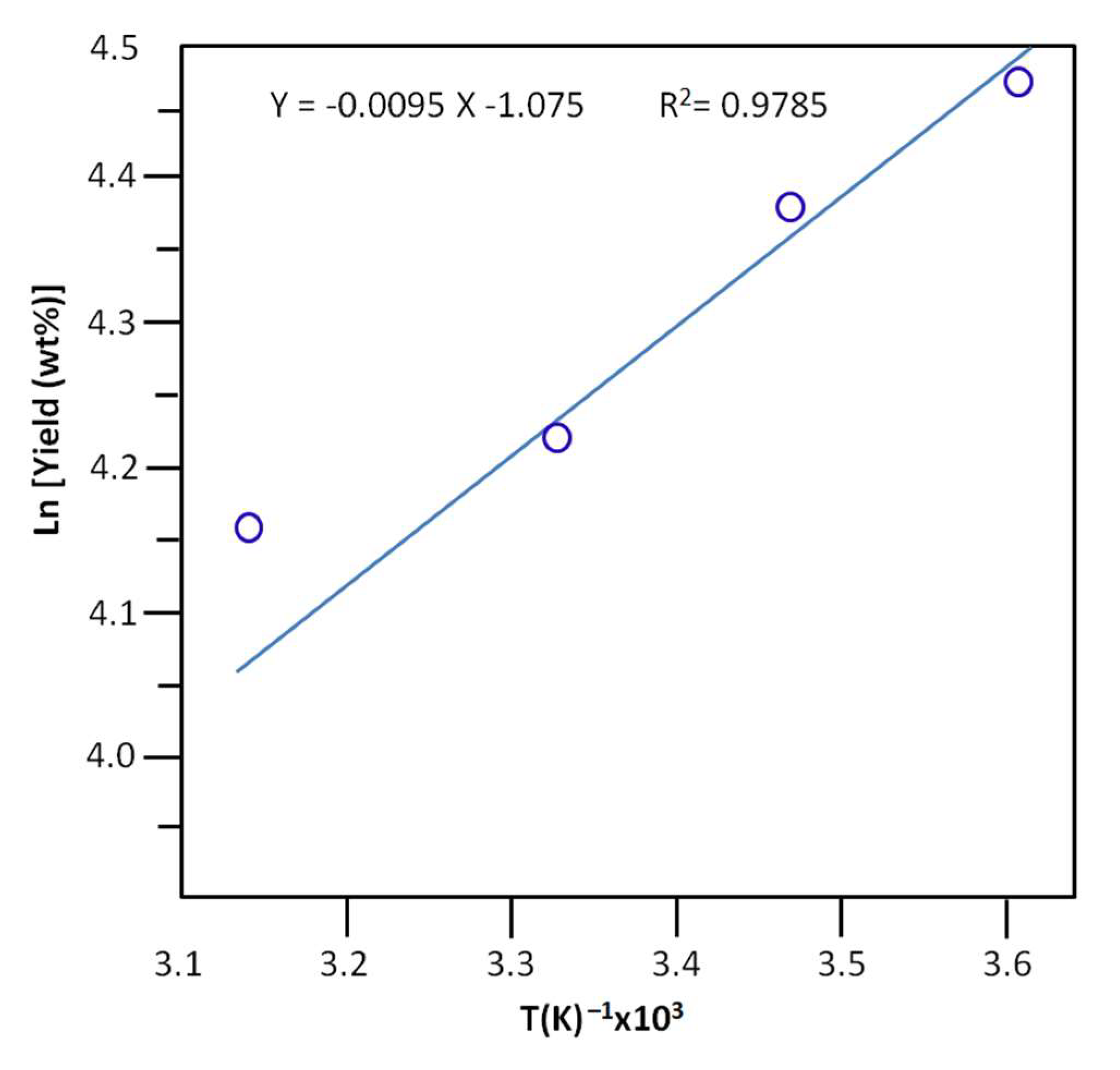
The activation energy, Ea, of the production of n-butyl-1-phenylmethanimine was deduced from the slope of the linear curve indicating the variation in the logarithm of the yield versus the inverse of the temperature and the linearized equation of Arrhenius (15) [58].
where A is the frequency factor expressed in time-1. R(J·K−1·mol−1) and T’(K) are the gas constant and the temperature at which the reaction occurred. As shown in Figure 9, the profile of the curve obtained reveals a straight line, and the value of the activation energy deduced from the average slope of this curve is equal to 7.733 ± 0.067 KJ·mol−1. This value is relatively low compared to that of the literature, which is equal to 7.7 kcal/mol (32.186 kJ·mol−1) [59].
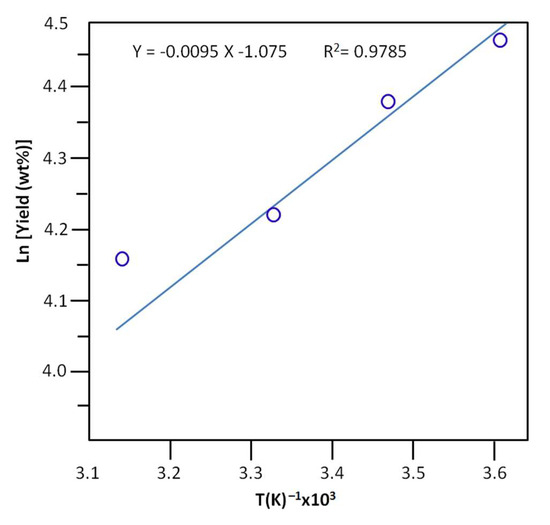
Figure 9.
Arrhenius plots and activation energies for the yield of the Schiff base reaction non‒assisted by PV.
3.2.2. Reaction Assisted by PV
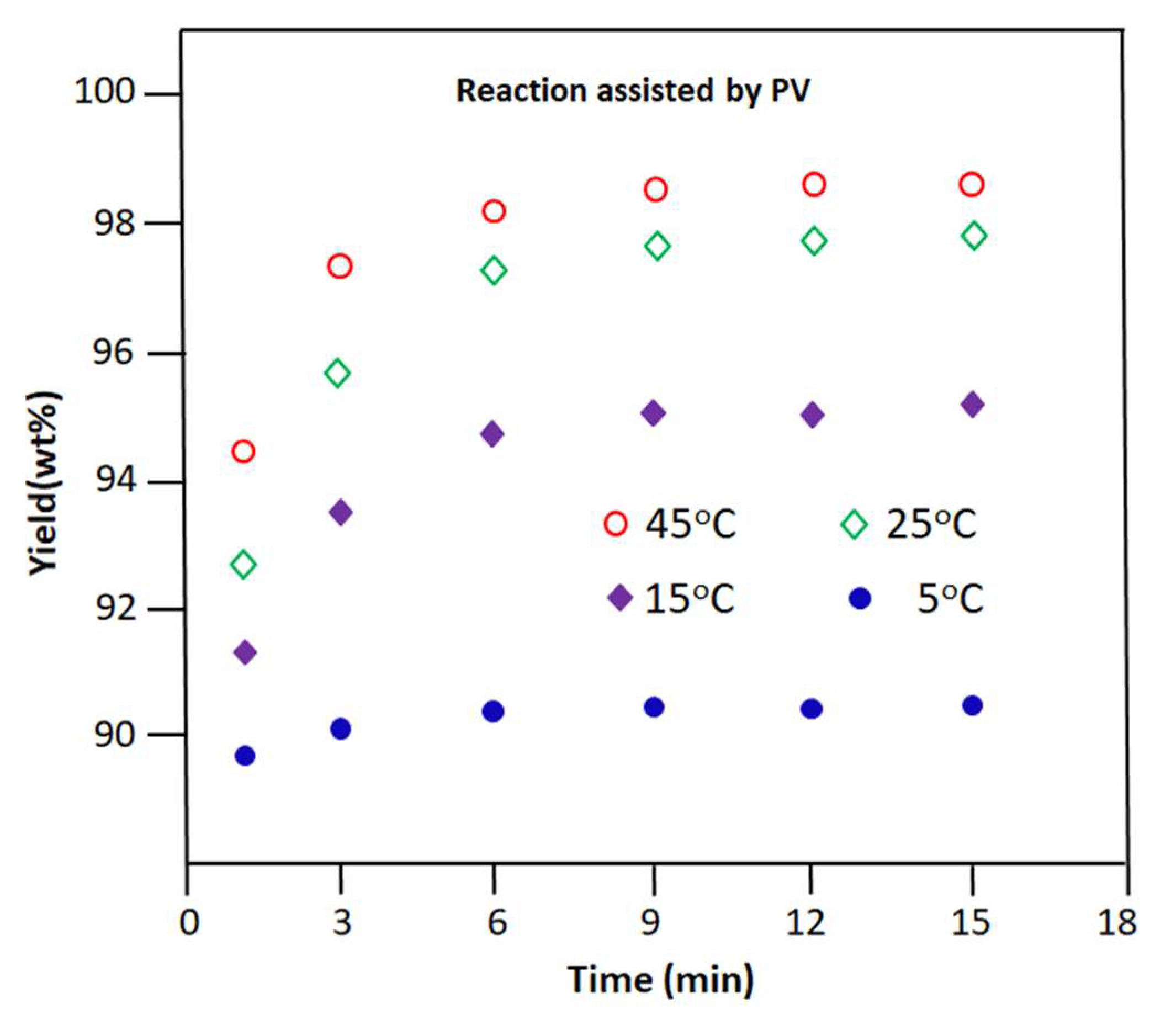
The Schiff base reaction involving benzaldehyde and n-butylamine is carried out under the same conditions as those described in Section 3.2.1 but with the removal of the resulting water as a by-product by PV. Note that PVA-0.5 was selected in the previous part of this study as a more efficient semi-permeable membrane that can meet the requirements to accomplish this task.
The change in the yield of n-butyl-1-phenylmethanimine versus time obtained at different temperatures is plotted in Figure 10. As can be seen from the profile of these curves, as in the case of the reaction carried out without the extraction of water by PV, the production of n-butyl-1-phenylmethanimine is very fast during the first 15 min of the reaction process, in which 99.25, 96.00, 94.74, and 95.84% of the total imine was yielded at 5, 15, 25, and 45 °C, respectively, in the retentate during this period.
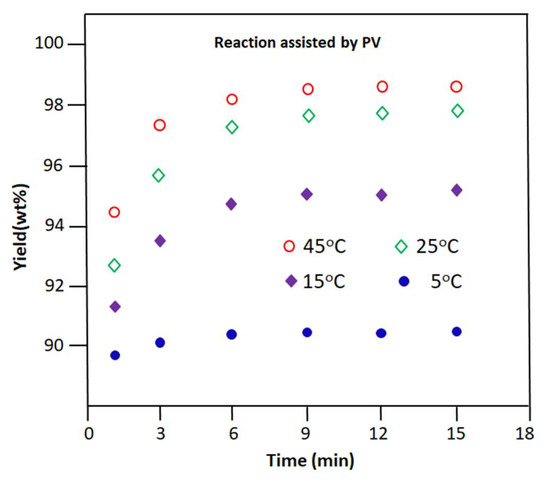
Figure 10.
Variation in the yield of the Schiff base reaction vs. time occurring at different temperatures. Reaction assisted by PV.
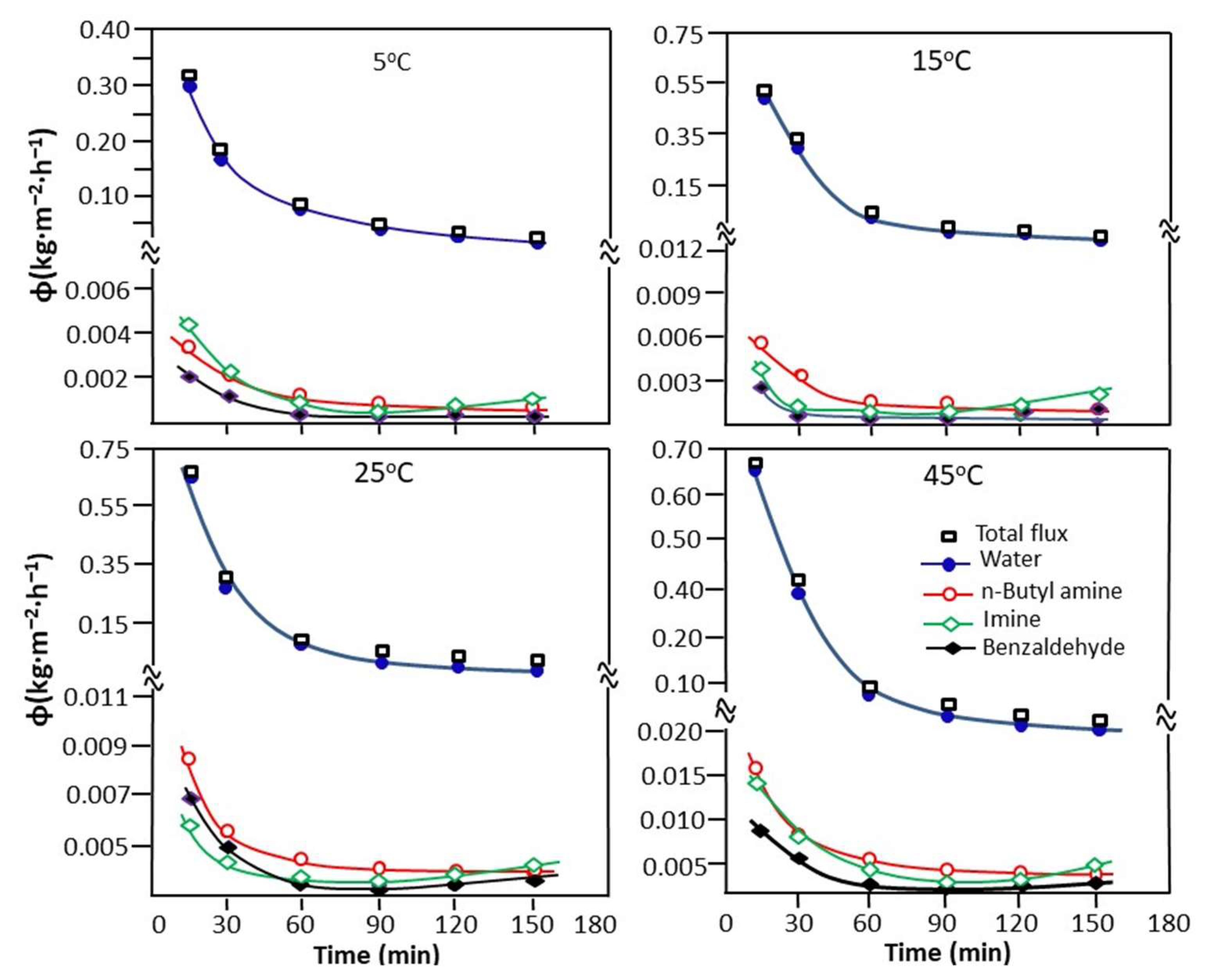
The variation in the cumulative total flux, calculated from Equation (9), and the partial flux of water, n-butylamine, n-butyl-1-phenylmethanimine, and benzaldehyde as a function of time taken at different temperatures are presented in Figure 11. As shown in the profile of the curves obtained, the total cumulative flux decreases rapidly as a function of time, describing an exponential decay curve for any temperature studied.
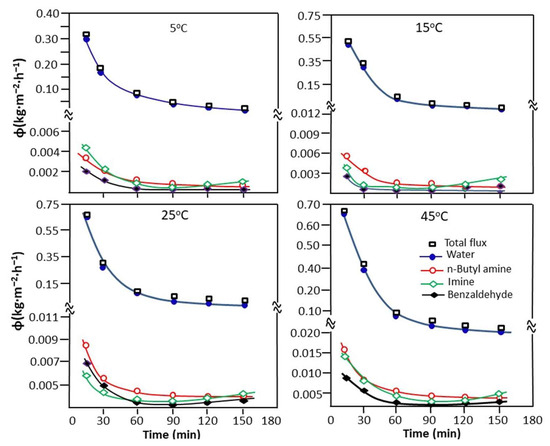
Figure 11.
Change in the cumulative total and partial flux of the reaction mixture taken at different temperatures.
As already noted in the previous experiment (reaction carried out without water extraction), the reaction is faster during the first hour of the reaction. During this period, a large amount of water is released and simultaneously extracted during its production until total exhaustion, characterizing the end of the reaction. According to the results obtained from the variation in the total cumulative flux as a function of temperature presented in Table 6, the best flux (0.421 Kg·m−2.h−1) is obtained at 45 °C. Concerning the partial flux, water occupies the first place and the other components of the reaction mixture are far behind. The classification in descending order of partial flow is as follows:
water >> n-butyl-1-phenylmethanimine > butylamine > benzaldehyde

Table 6.
Cumulative total flux and partial flux of the Schiff base reaction mixture taken at the halfway point of the pervaporation process.
This goes in the same direction as that of the swelling of the PVA-0.5 membrane in these pure compounds (Section 3.2.1), except in the case of the two molecules containing nitrogen, where the order is reversed. This may be due to the interactions between these components when the compounds are together and also the solubility of each component in the other. The slight increase in the flux of n-butyl-1-phenylmethanimine observed at the end of the extraction process is probably due to the increase in the concentration of this product in the feed during the extraction of water, as it is well known in the literature [60,61] that the flux of a component of a mixture increases with its concentration in the feed.
These curves reveal an increase in the yield of n-butyl-1-phenylmethanimine produced when the temperature increases, which is faster when the reaction is accompanied by the elimination of water by pervaporation.
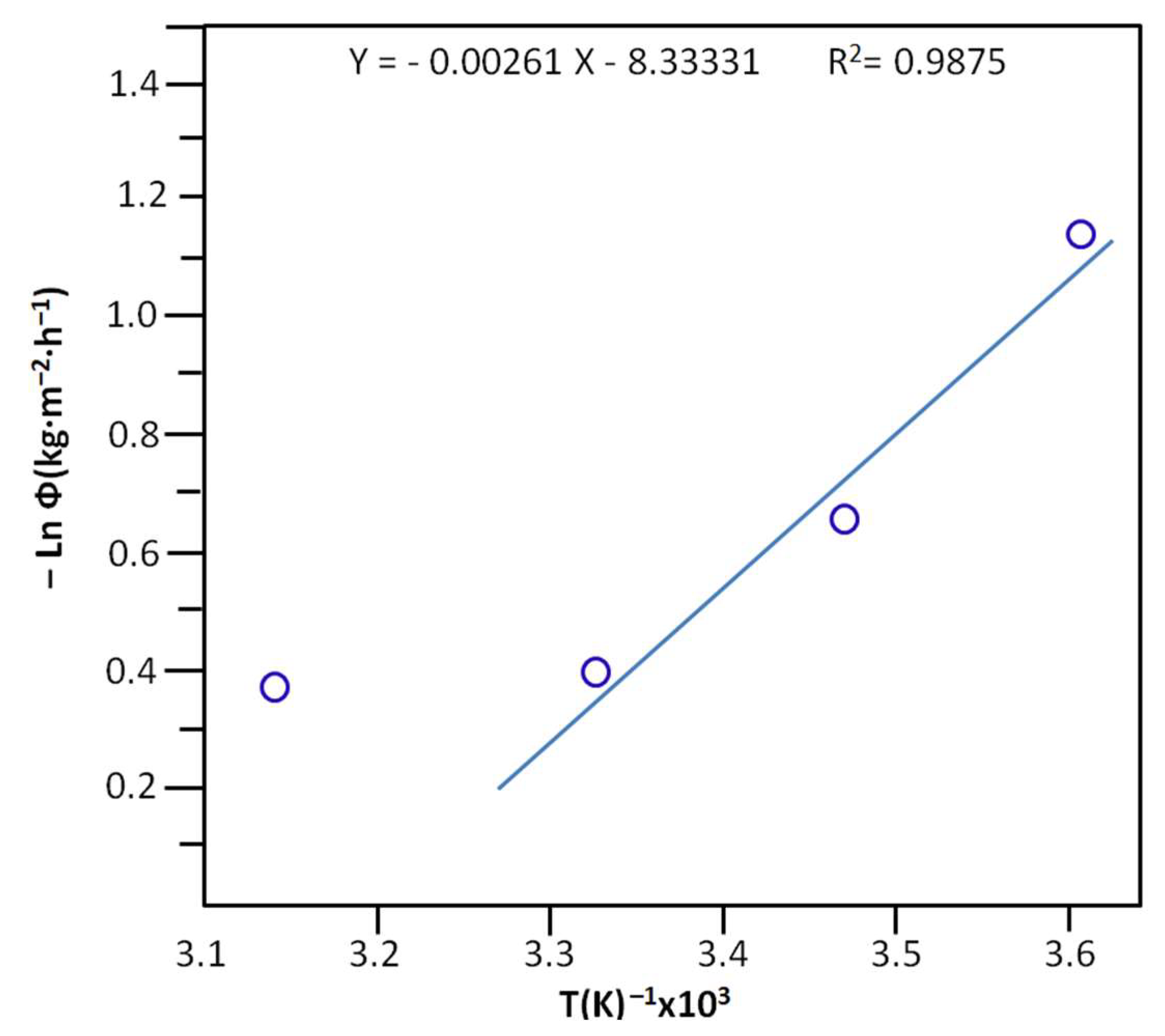
The activation energy, Ea, of the cumulative total flux taken at the halfway point of the extraction of water was deducted from the slope of the linear portion of the curve indicating the logarithm of the maximum cumulative total flux versus the inverse of temperature and Equation (16) [62]:
where A is the frequency factor expressed in time−1. R(J·K−1·mol−1) and T(K) are the gas constant and the temperature at which the separation of water occurs. The results obtained are shown in Figure 12 and reveal an activation energy of 21.62 ± 0.70 KJ·mol−1.
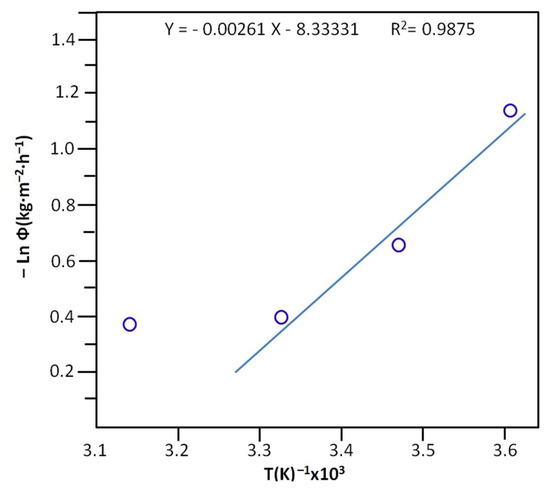
Figure 12.
Arrhenius plots indicating the variation in the Ln (Φ) vs. 1/T, taken at the maximum of the cumulative total flux of the separation process.
Due to the lack of published results in the literature on the subject using the same condensation reaction involving the same or related reagents, the Ea obtained was compared in this work to that obtained by Jiang et al. [63] from an equilibrium esterification reaction involving oleic acid and ethanol assisted by pervaporation to remove the resulting water.
The Ea obtained by these authors was estimated at 15.788 KJ·mol−1, not far from that obtained in this work. This indicates that water extraction by the PV process does not depend much on the nature of the products involved in the reaction nor its kinetics. According to Feng and Huang [64], the activation energy depends on the transmembrane driving force, which is expressed in terms of the partial pressure of the mixture components.
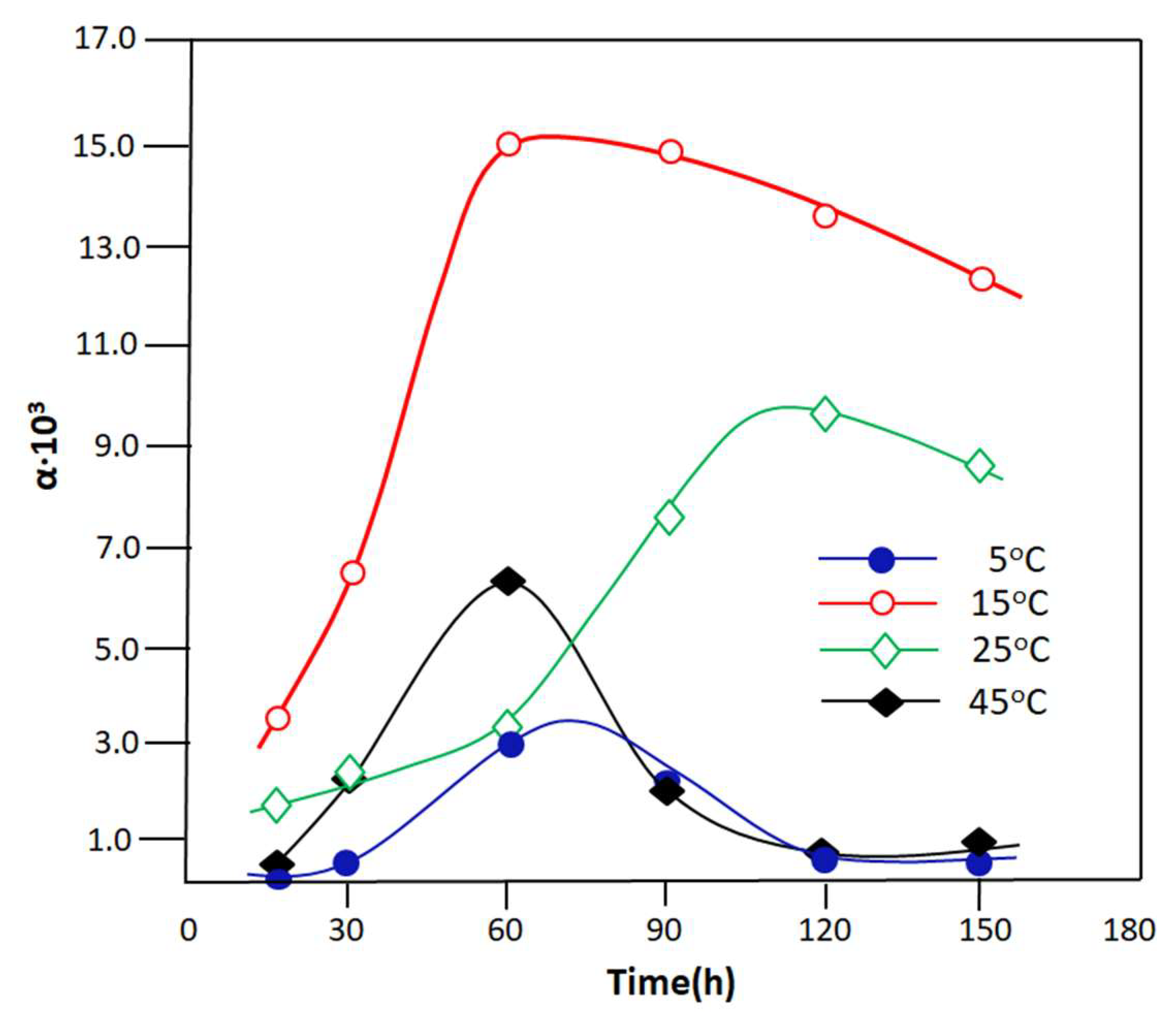
The variation in the separation factor of the PVA-0.5 membrane with respect to water as a function of time studied at different temperatures is shown in Figure 13. The profiles of these curves reveal that the PVA-0.5 membrane is generally very selective for water. In fact, the separation factor of this membrane reached its maximum during one hour of the PV process, during which 1.51 × 104 was reached at 15 °C, 6.25 × 103 and 3.50 × 103, and 10.23 × 103 at 25 °C after 2 h of water extraction. The increase in α at the beginning of the process is probably due to the speed of the reaction, producing a large amount of water, as shown in the kinetic curve of the Schiff base reaction (Figure 7).
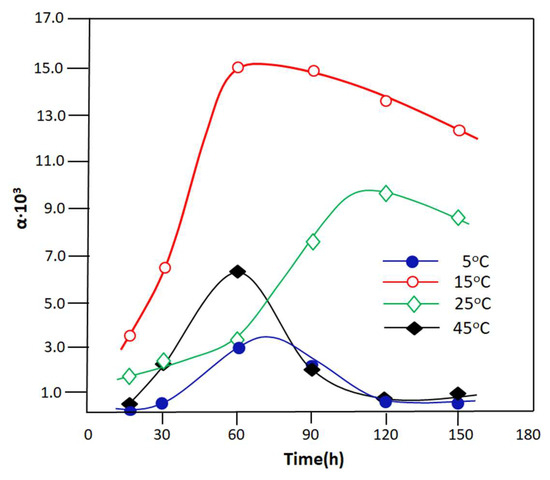
Figure 13.
Variation in separation factor as a function of time taken at different temperatures.
According to the literature [60,61,62], an increase in the concentration of a component in a mixture leads to an increase in its separation factor. As a result, the water concentration in the reactor during this period is high, which results in a larger flux containing mainly water. The decrease in α beyond this period is due simultaneously to the exhaustion of water eliminated from the reaction as a function of time and also to the increase in the amount of the resulting imine. In this case, the concentration of water in the reactor decreases, and this leads to a reduction in the separation factor. The classification in order of selectivity seems difficult in this case because two antagonistic processes are implemented simultaneously, which are, on the one hand, the increase in the yield of the products, which goes in the direction of an increase with temperature, and on the other hand, the selectivity, which goes in the direction of decreasing with the temperature. The best compromise between these two parameters indicates that the separation factor is obtained when the temperature of the reaction–separation process is close to the ambient temperature.
4. Conclusions
To conclude this work, we can say that the objectives set were successfully achieved. Indeed, the cylindrical pervaporation cell used in this work is well suited to selectively extracting byproducts such as water during balanced reactions involving, for example, benzaldehyde and n-butylamine. This cell is relatively simple to manufacture and practically does not depend on the mechanical properties of the polymer used as a membrane, particularly when it comes to an extra thin film difficult to obtain by conventional techniques. The results obtained from the study of the swelling of PVA-x membranes containing different crosslinking ratios indicate that the diffusion of each pure component of the Schiff base reaction mixture obeys the Fick model. This study also reveals that the swelling of the PVA-0.5 membrane, which contains the lowest rate of crosslinking, swelled significantly more in water than the other pure components. The application of PVA-0.5, chosen as the most effective membrane during the material transfer test, showed very satisfactory results in the extraction of the resulting water during the condensation reaction of the Schiff base. For example, when the PV process is used to remove water from this reaction mixture, the yield of the reaction passed from 84.0 to 98.6 wt% at 45 °C, from 77.0 to 98.6 at 25 °C, from 67 to 95.1, and from 58 to 90.4 at 5 °C. The cumulative total and partial fluxes dramatically decreased with time and the separation factor reached a maximum at about 1 h (at 5, 15, and 45 °C) and at 2 h (at 25 °C). The maximum selectivity of PVA-0.5 with regard to water was obtained at 15 °C.
Author Contributions
Methodology, R.S.A.K., M.M.A., M.A.A.J. and A.Y.B.-H.-A.; Software, R.S.A.K.; Formal analysis, R.S.A.K., M.M.A., Inas Al-Qadsy, M.A.A.J. and A.Y.B.-H.-A.; Investigation, M.M.A.; Resources, W.S.S.; Data curation, R.S.A.K., I.A.-Q., W.S.S. and A.Y.B.-H.-A.; Writing—original draft, I.A.-Q. and T.A.; Writing—review & editing, T.A.; Supervision, T.A.; Project administration, T.A. All authors have read and agreed to the published version of the manuscript.
Funding
The authors extend their appreciation to the Researchers Supporting Project, number (RSPD2023R767), King Saud University, Riyadh, Saudi Arabia.
Data Availability Statement
Data are contained within the article.
Acknowledgments
The authors extend their appreciation to the Researchers Supporting Project, number (RSPD2023R767), King Saud University, Riyadh, Saudi Arabia.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Verma, R.; Lamba, N.P.; Dandia, A.; Srivastava, A.; Modi, K.; Chauhan, M.S.; Prasad, J. Synthesis of N-Benzylideneaniline by Schiff base reaction using Kinnow peel powder as Green catalyst and comparative study of derivatives through ANOVA techniques. Sci. Rep. 2022, 12, 9636. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.-R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Shawky, A.M.; Abourehab, M.A.; Abdalla, A.N.; Gouda, A.M. Optimization of pyrrolizine-based Schiff bases with 4-thiazolidinone motif: Design, synthesis and investigation of cytotoxicity and anti-inflammatory potency. Eur. J. Med. Chem. 2020, 185, 111780. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.; Kachroo, M. Synthesis and biological evaluation of anti-tubercular activity of Schiff bases of 2-Amino thiazoles. Bioorganic Med. Chem. Lett. 2020, 30, 127655. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.H.; Shoaib, M.; Al-Joufi, F.A.; Shah, S.W.A.; Hussain, H.; Ullah, A.; Zahoor, M.; Mughal, E.U. Synthesis and Investigation of the Analgesic Potential of Enantiomerically Pure Schiff Bases: A Mechanistic Approach. Molecules 2022, 27, 5206. [Google Scholar] [CrossRef]
- Matela, G. Schiff bases and complexes: A review on anti-cancer activity. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. -Anti-Cancer Agents) 2020, 20, 1908–1917. [Google Scholar] [CrossRef]
- Yaqoob, M.; Jamil, W.; Taha, M.; Solangi, S. Synthesis, Characterization, Anti-Glycation, and Anti-Oxidant Activities of Sulfanilamide Schiff Base Metal Chelates. Acta Chim. Slov. 2022, 69, 772–778. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M. Recent progresses in Schiff bases as aqueous phase corrosion inhibitors: Design and applications. Coord. Chem. Rev. 2021, 446, 214105. [Google Scholar] [CrossRef]
- Ambroziak, K.; Pelech, R.; Milchert, E.; Dziembowska, T.; Rozwadowski, Z. New dioxomolybdenum (VI) complexes of tetradentate Schiff base as catalysts for epoxidation of olefins. J. Mol. Catal. A Chem. 2004, 211, 9–16. [Google Scholar] [CrossRef]
- Li, D.; Liang, X.; Zhang, F.; Li, J.; Zhang, Z.; Wang, S.; Li, Z.; Xing, Y.; Guo, K. Imine bond orientation manipulates AIEgen derived Schiff base isomers through the intramolecular hydrogen bond effect for different fluorescence properties and applications. J. Mater. Chem. C 2022, 10, 11016–11026. [Google Scholar] [CrossRef]
- Abuamer, K.M.; Maihub, A.A.; El-Ajaily, M.M.; Etorki, A.M.; Abou-Krisha, M.M.; Almagani, M.A. The role of aromatic Schiff bases in the dyes techniques. Int. J. Org. Chem. 2014, 4, 43362. [Google Scholar] [CrossRef]
- Al-Tikrity, E.T.; Yaseen, A.A.; Yousif, E.; Ahmed, D.S.; Al-Mashhadani, M.H. Impact on Poly (Vinyl chloride) of trimethoprim schiff bases as stabilizers. Polym. Polym. Compos. 2022, 30, 09673911221094020. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff bases: A versatile pharmacophore. J. Catal. 2013, 2013, 893512. [Google Scholar] [CrossRef]
- Look, G.C.; Murphy, M.M.; Campbell, D.A.; Gallop, M.A. Trimethylorthoformate: A mild and effective dehydrating reagent for solution and solid phase imine formation. Tetrahedron Lett. 1995, 36, 2937–2940. [Google Scholar] [CrossRef]
- Love, B.E.; Ren, J. Synthesis of sterically hindered imines. J. Org. Chem. 1993, 58, 5556–5557. [Google Scholar] [CrossRef]
- La Rocca, T.; Carretier, E.; Dhaler, D.; Louradour, E.; Truong, T.; Moulin, P. Purification of pharmaceutical solvents by pervaporation through hybrid silica membranes. Membranes 2019, 9, 76. [Google Scholar] [CrossRef]
- Karlsson, H.O.; Tragardh, G. Applications of pervaporation in food processing. Trends Food Sci. Technol. 1996, 7, 78–83. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhou, K.; Zhang, Q.G.; Han, G.L.; Zhu, A.M.; Liu, Q.L. Pervaporation of water–ethanol and methanol–MTBE mixtures using poly (vinyl alcohol)/cellulose acetate blended membranes. J. Membr. Sci. 2013, 448, 93–101. [Google Scholar] [CrossRef]
- Khudsange, C.R.; Wasewar, K.L. Process intensification of esterification reaction for the production of propyl butyrate by pervaporation. Resour.-Effic. Technol. 2017, 3, 88–93. [Google Scholar] [CrossRef]
- Lyoo, W.; Lee, H. Synthesis of high-molecular-weight poly (vinyl alcohol) with high yield by novel one-batch suspension polymerization of vinyl acetate and saponification. Colloid. Polym. Sci. 2002, 280, 835–840. [Google Scholar]
- Martínez-Felipe, A.; Moliner-Estopiñán, C.; Imrie, C.T.; Ribes-Greus, A. Characterization of crosslinked poly (vinyl alcohol)-based membranes with different hydrolysis degrees for their use as electrolytes in direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 124, 1000–1011. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.B.; Lee, C.-S.; Jun, J.-H.; Kim, D.S.; Lee, Y.M. Crosslinked poly (vinyl alcohol) membranes containing sulfonic acid group: Proton and methanol transport through membranes. J. Membr. Sci. 2004, 238, 143–151. [Google Scholar] [CrossRef]
- Chan, L.W.; Hao, J.S.; Heng, P.W.S. Evaluation of permeability and mechanical properties of composite polyvinyl alcohol films. Chem. Pharm. Bull. 1999, 47, 1412–1416. [Google Scholar] [CrossRef]
- Mohammad Mahdi Dadfar, S.; Kavoosi, G.; Mohammad Ali Dadfar, S. Investigation of mechanical properties, antibacterial features, and water vapor permeability of polyvinyl alcohol thin films reinforced by glutaraldehyde and multiwalled carbon nanotube. Polym. Compos. 2014, 35, 1736–1743. [Google Scholar] [CrossRef]
- Nasalapure, A.V.; Chalannavar, R.K.; Kasai, D.R.; Reddy, K.R.; Raghu, A.V. Novel polymeric hydrogel composites: Synthesis, physicochemical, mechanical and biocompatible properties. Nano Express 2021, 2, 030003. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, X.; Jiang, G.; Xie, J.; Cai, W.; Li, J.; Wang, Y. Preparation and pervaporation performance of PVA membrane with biomimetic modified silica nanoparticles as coating. J. Membr. Sci. 2022, 653, 120535. [Google Scholar] [CrossRef]
- Chandane, V.S.; Rathod, A.P.; Wasewar, K.L. Enhancement of esterification conversion using pervaporation membrane reactor. Resour. -Effic. Technol. 2016, 2, S47–S52. [Google Scholar] [CrossRef]
- Kwon, S.J.; Song, K.M.; Hong, W.H.; Rhee, J.S. Removal of water produced from lipase-catalyzed esterification in organic solvent by pervaporation. Biotechnol. Bioeng. 1995, 46, 393–395. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, F. Potential of pervaporation and vapor separation with water selective membranes for an optimized production of biofuels—A review. Catalysts 2017, 7, 187. [Google Scholar] [CrossRef]
- Ameri, E.; Moheb, A. Improvement of Isopropyl Propionate Esterification Reaction using a Vapor Permeation Membrane Reactor. Iran. J. Catal. 2022, 12, 399–405. [Google Scholar]
- Andersen, A. Final report on the safety assessment of benzaldehyde. Int. J. Toxicol. 2006, 25, 11–27. [Google Scholar] [PubMed]
- Mohapatra, R.K.; Das, P.K.; Pradhan, M.K.; Maihub, A.A.; El-ajaily, M.M. Biological aspects of Schiff base–metal complexes derived from benzaldehydes: An overview. J. Iran. Chem. Soc. 2018, 15, 2193–2227. [Google Scholar] [CrossRef]
- Matar, S.A.; Talib, W.H.; Mustafa, M.S.; Mubarak, M.S.; AlDamen, M.A. Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3, 3′-diaminodipropylamine. Arab. J. Chem. 2015, 8, 850–857. [Google Scholar] [CrossRef]
- Taylor, C.M.; Kilah, N.L. Synthesis of [2 + 2] Schiff base macrocycles by a solvent templating strategy and halogen bonding directed assembly. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 543–555. [Google Scholar] [CrossRef]
- Westheimer, F.; Taguchi, K. Catalysis by molecular sieves in the preparation of ketimines and enamines. J. Org. Chem. 1971, 36, 1570–1572. [Google Scholar] [CrossRef]
- Maslov, D.L.; Trifonova, O.P.; Balashova, E.E.; Lokhov, P.G. N-butylamine for improving the efficiency of untargeted mass spectrometry analysis of plasma metabolite composition. Int. J. Mol. Sci. 2019, 20, 5957. [Google Scholar] [CrossRef]
- Wang, C.; Ma, B.; Zhang, L. Experimental study on CO2 capture by using n-butylamine to plug the gas channeling to enhanced oil recovery. J. Pet. Explor. Prod. Technol. 2022, 12, 2523–2531. [Google Scholar] [CrossRef]
- Kanse, N.G.; Dawande, S. Separation of ethanol/water (azeotropic mixture) by pervaporation using PVA membrane. Mater. Today Proc. 2017, 4, 10520–10523. [Google Scholar] [CrossRef]
- Sapalidis, A.A. Porous Polyvinyl alcohol membranes: Preparation methods and applications. Symmetry 2020, 12, 960. [Google Scholar] [CrossRef]
- Erdödi, G.; Iván, B. Novel amphiphilic conetworks composed of telechelic poly (ethylene oxide) and three-arm star polyisobutylene. Chem. Mater. 2004, 16, 959–962. [Google Scholar] [CrossRef]
- Haraszti, M.; Tóth, E.; Iván, B. Poly (methacrylic acid)-l-polyisobutylene: A novel polyelectrolyte amphiphilic conetwork. Chem. Mater. 2006, 18, 4952–4958. [Google Scholar] [CrossRef]
- Gupta, S.; Webster, T.J.; Sinha, A. Evolution of PVA gels prepared without crosslinking agents as a cell adhesive surface. J. Mater. Sci. Mater. Med. 2011, 22, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Chu, T.-J. The determination of interaction parameter χ1 for polyvinyl alcohol and water from the diffusion data. Polym. Test. 1993, 12, 57–64. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, N.M.; Ayub, G.; Naseem, S.; Ansari, M.T. Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J. Mater. Sci. Mater. Med. 2010, 21, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Denkbaş, E.B.; Kilicay, E.; Birlikseven, C.; Öztürk, E. Magnetic chitosan microspheres: Preparation and characterization. React. Funct. Polym. 2002, 50, 225–232. [Google Scholar] [CrossRef]
- Comyn, J. Introduction to polymer permeability and the mathematics of diffusion. In Polymer Permeability; Springer: Cham, Switzerland, 1985; pp. 1–10. [Google Scholar]
- Masaro, L.; Zhu, X. Physical models of diffusion for polymer solutions, gels and solids. Progress. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Freire, T.F.; Quinaz, T.; Fertuzinhos, A.; Quyền, N.T.; de Moura, M.F.; Martins, M.; Zille, A.; Dourado, N. Thermal, mechanical and chemical analysis of poly (vinyl alcohol) multifilament and braided yarns. Polymers 2021, 13, 3644. [Google Scholar] [CrossRef]
- Restrepo, I.; Medina, C.; Meruane, V.; Akbari-Fakhrabadi, A.; Flores, P.; Rodríguez-Llamazares, S. The effect of molecular weight and hydrolysis degree of poly (vinyl alcohol)(PVA) on the thermal and mechanical properties of poly (lactic acid)/PVA blends. Polímeros 2018, 28, 169–177. [Google Scholar] [CrossRef]
- Salazar, J.D.R. Study of Structural, Thermic, µ-Raman and Optic Transformation of PVA/TiO2 Polymeric Membranes. Sci. Et Tech. 2018, 23, 543–552. [Google Scholar]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and thermally crosslinked PVA-based membranes: Effect on swelling and transport behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Lee, S.-B.; Han, N.W. Effects of the degree of crosslinking on properties of poly (vinyl alcohol) membranes. Polym. J. 1993, 25, 1295–1302. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Für Phys. Chem. 1889, 4, 226–248. [Google Scholar] [CrossRef]
- Crowell, T.I.; Bell, C.E.; O’Brien, D.H. Extrathermodynamic relationships in Schiff base formation. J. Am. Chem. Soc. 1964, 86, 4973–4976. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on pervaporation: Theory, membrane performance, and application to intensification of esterification reaction. J. Eng. 2015, 2015, 927068. [Google Scholar] [CrossRef]
- Vane, L.M. Review of pervaporation and vapor permeation process factors affecting the removal of water from industrial solvents. J. Chem. Technol. Biotechnol. 2020, 95, 495–512. [Google Scholar] [CrossRef]
- Kalahal, P.B.; Sajjan, A.M.; Yunus Khan, T.; Rajhi, A.A.; Achappa, S.; Banapurmath, N.R.; Duhduh, A.A. Novel Polyelectrolyte Complex Membranes Containing Carboxymethyl Cellulose–Gelatin for Pervaporation Dehydration of Azeotropic Bioethanol for Biofuel. Polymers 2022, 14, 5114. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, J.; Yuan, Z.; Lu, J.; Ding, J. Optimization of the esterification of oleic acid and ethanol in a fixed bed membrane reactor by response surface method. Fuel 2023, 342, 127867. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y. Estimation of activation energy for permeation in pervaporation processes. J. Membr. Sci. 1996, 118, 127–131. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).