Breaking the Equilibrium and Improving the Yield of Schiff Base Reactions by Pervaporation: Application to a Reaction Involving n-butylamine and Benzaldehyde
Abstract
:1. Introduction
2. Materials and Methods
2.1. Chemicals
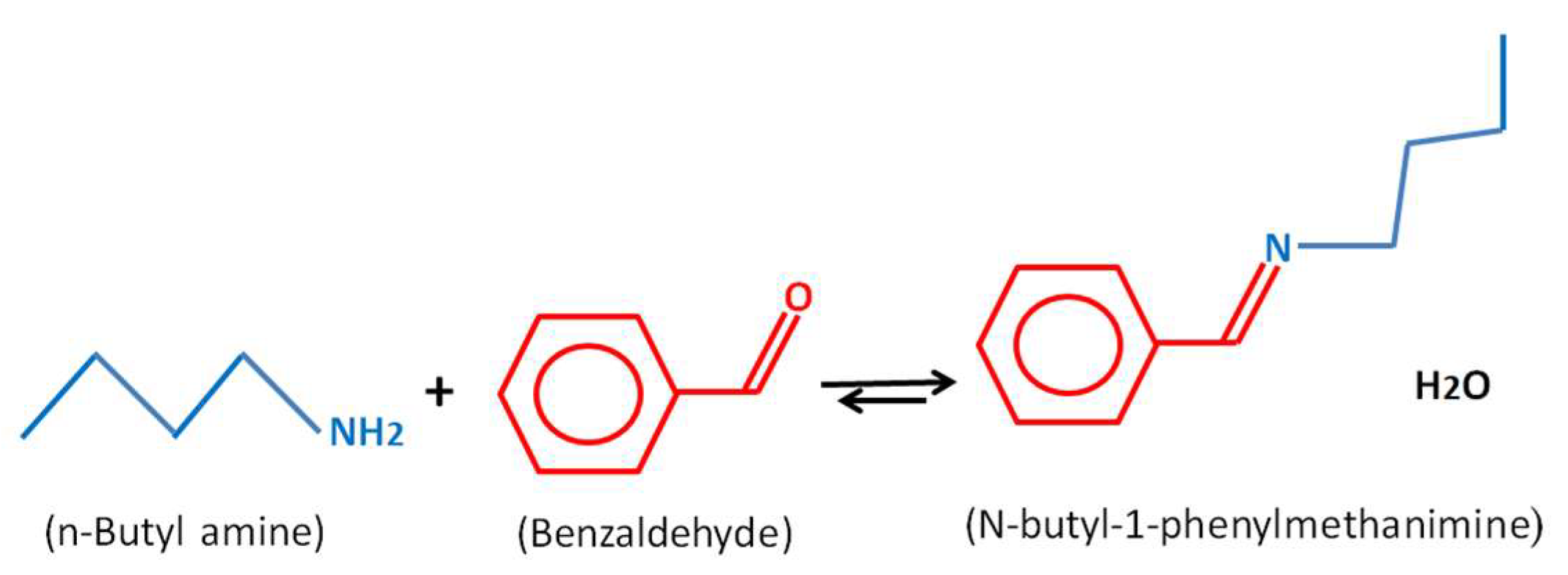
2.2. Schiff Base Reaction
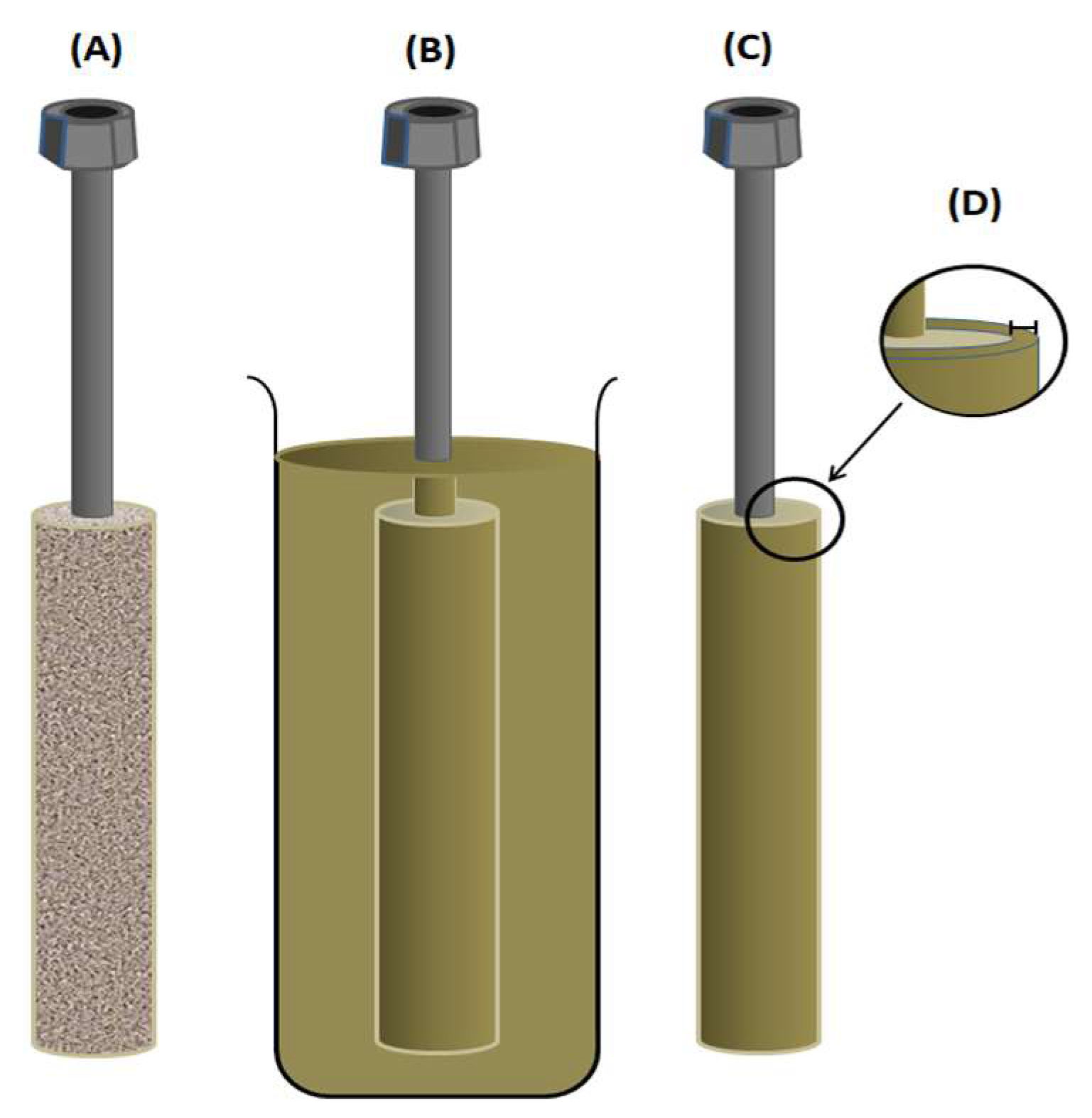
2.3. Membrane Preparation
2.4. Pervaporation Setup
2.5. Characterization
2.5.1. FTIR
2.5.2. NMR
2.5.3. DSC
2.5.4. HPLC
2.5.5. The Crosslinking Density
2.5.6. Mass Transfer
2.6. Reaction Preparation
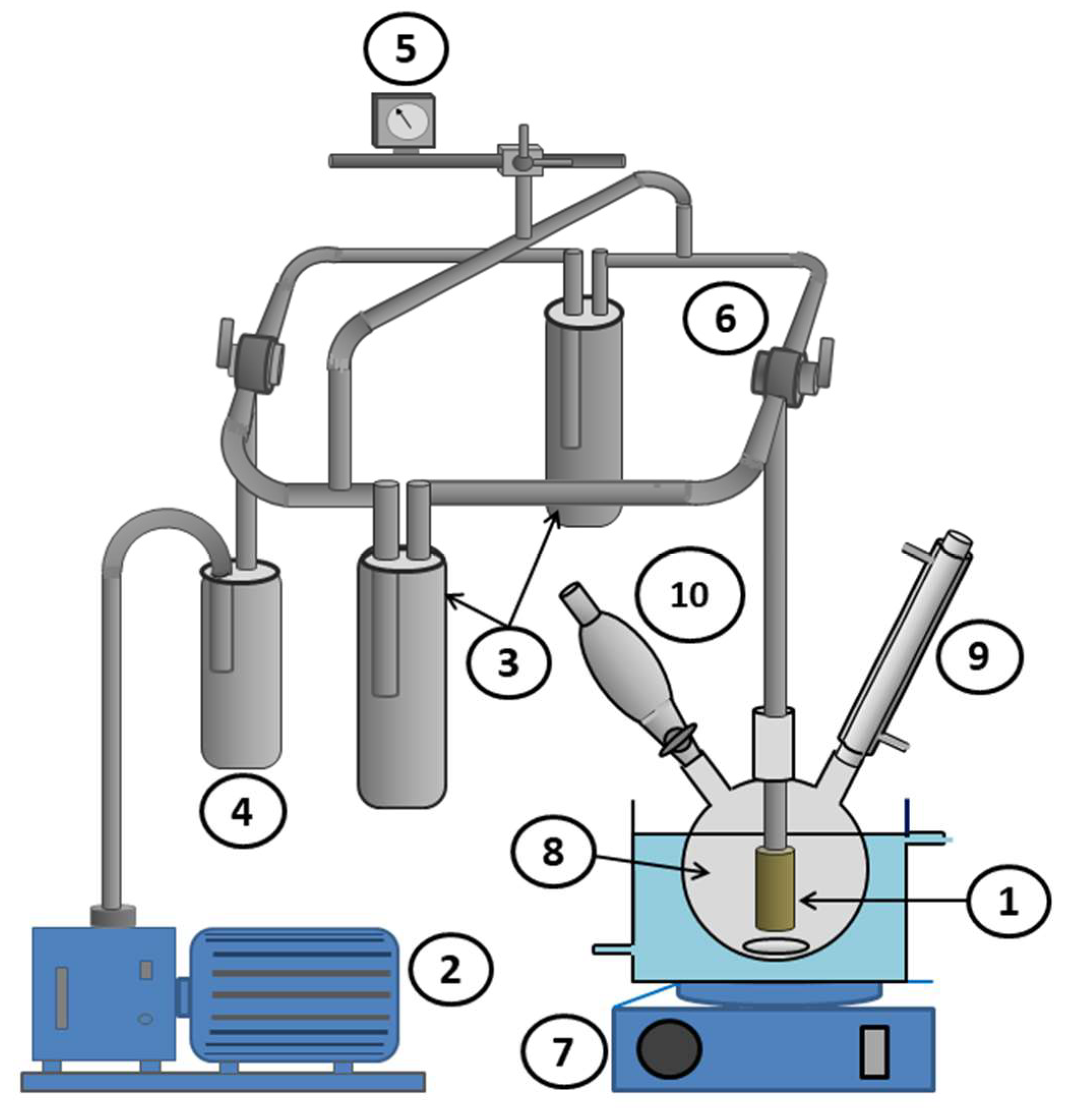
2.7. Pervaporative Parameters
3. Results and Discussion
3.1. Characterization
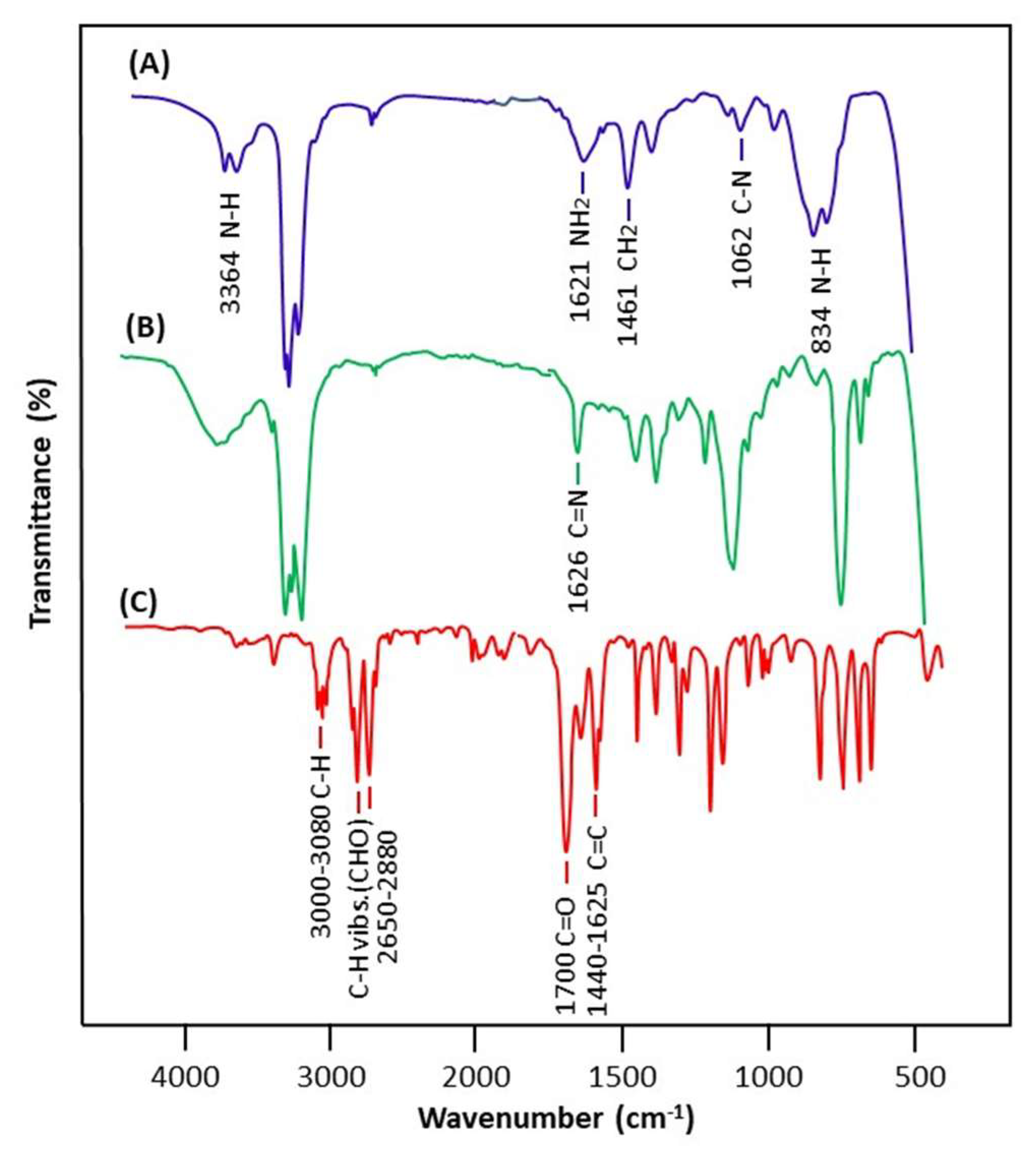
3.1.1. FTIR
3.1.2. 1H NMR
3.1.3. DSC
3.1.4. The Crosslinking Density of the PVA-x Networks
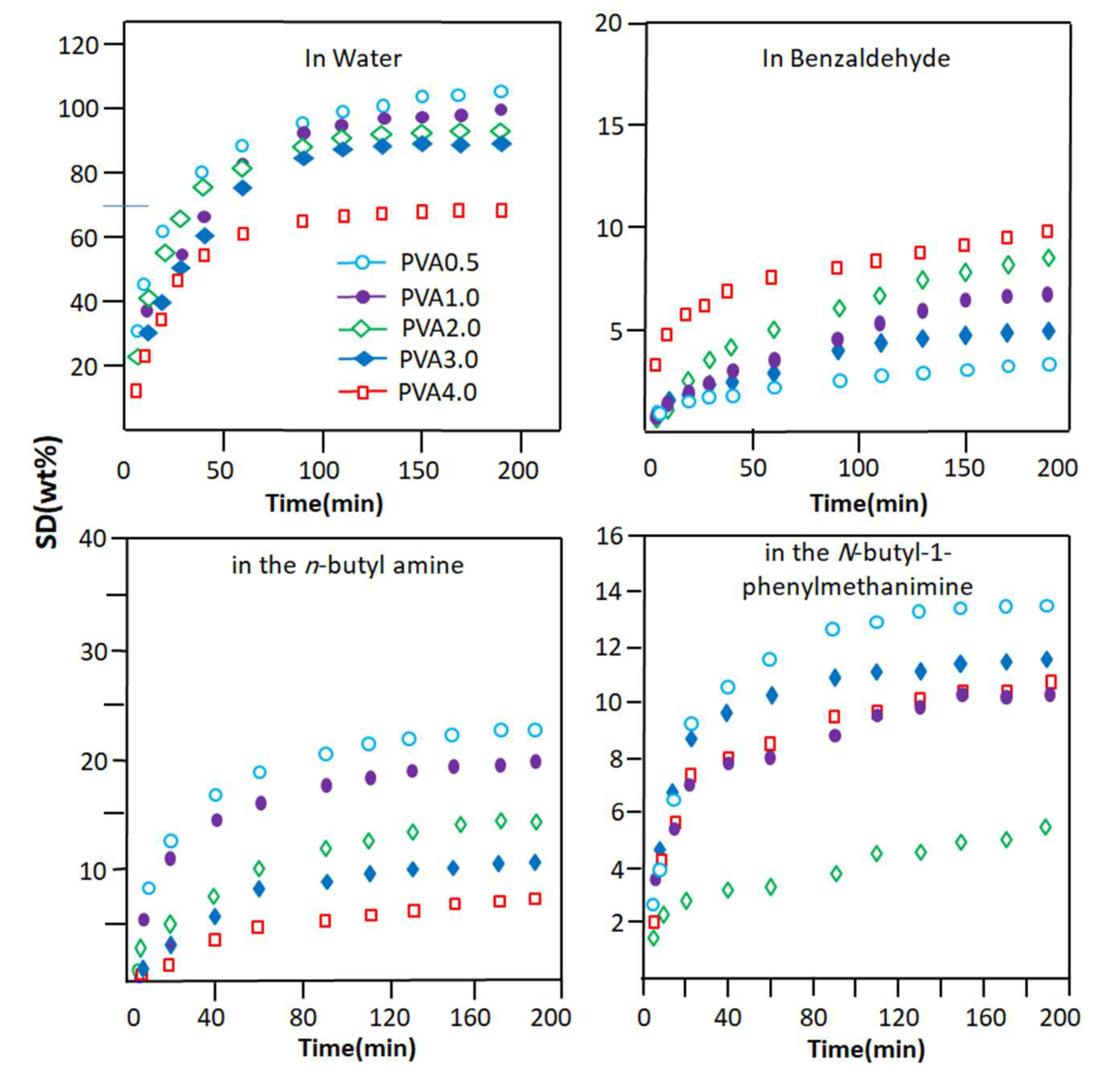
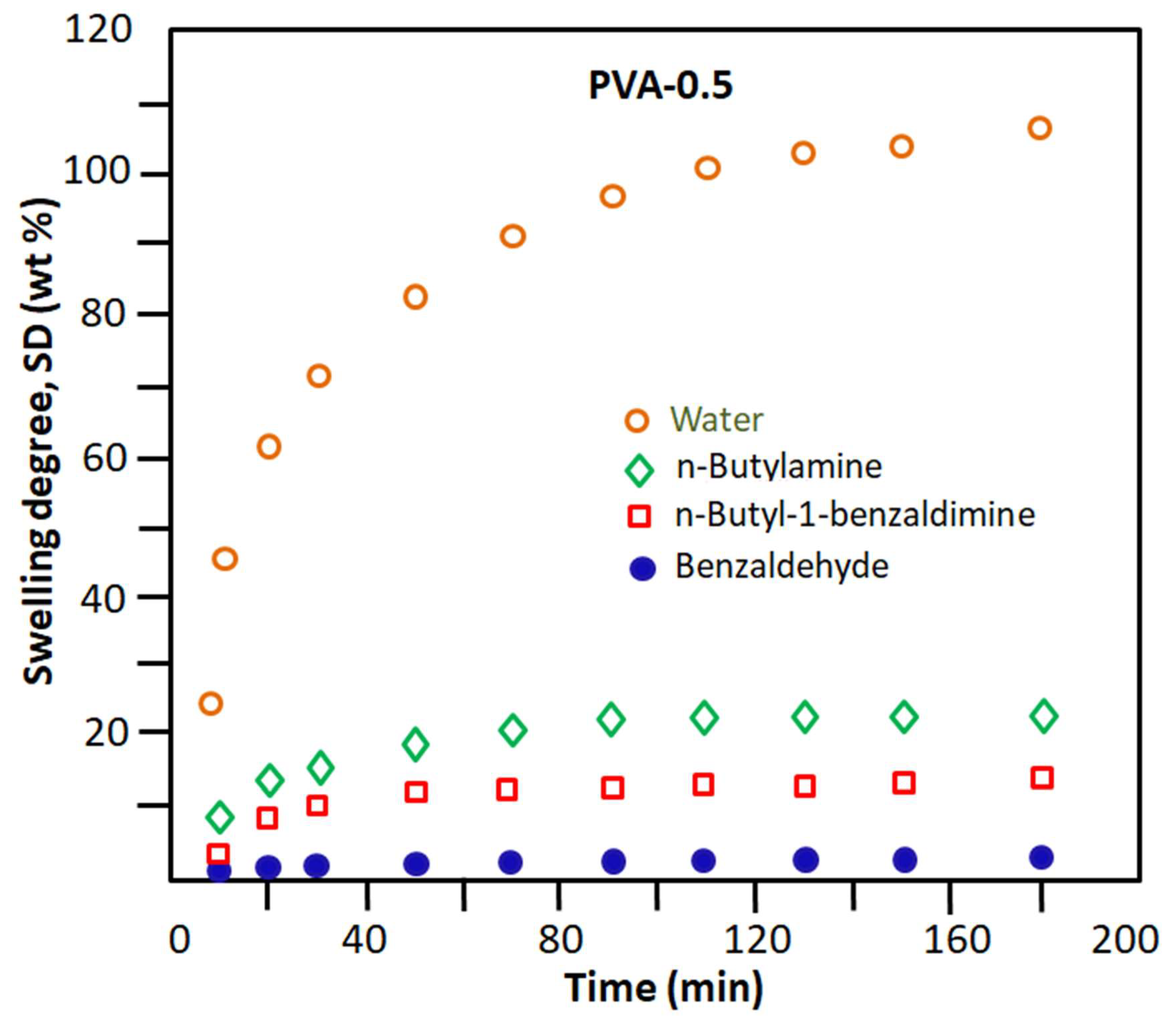
3.1.5. Swellability of PVA-x Membrane
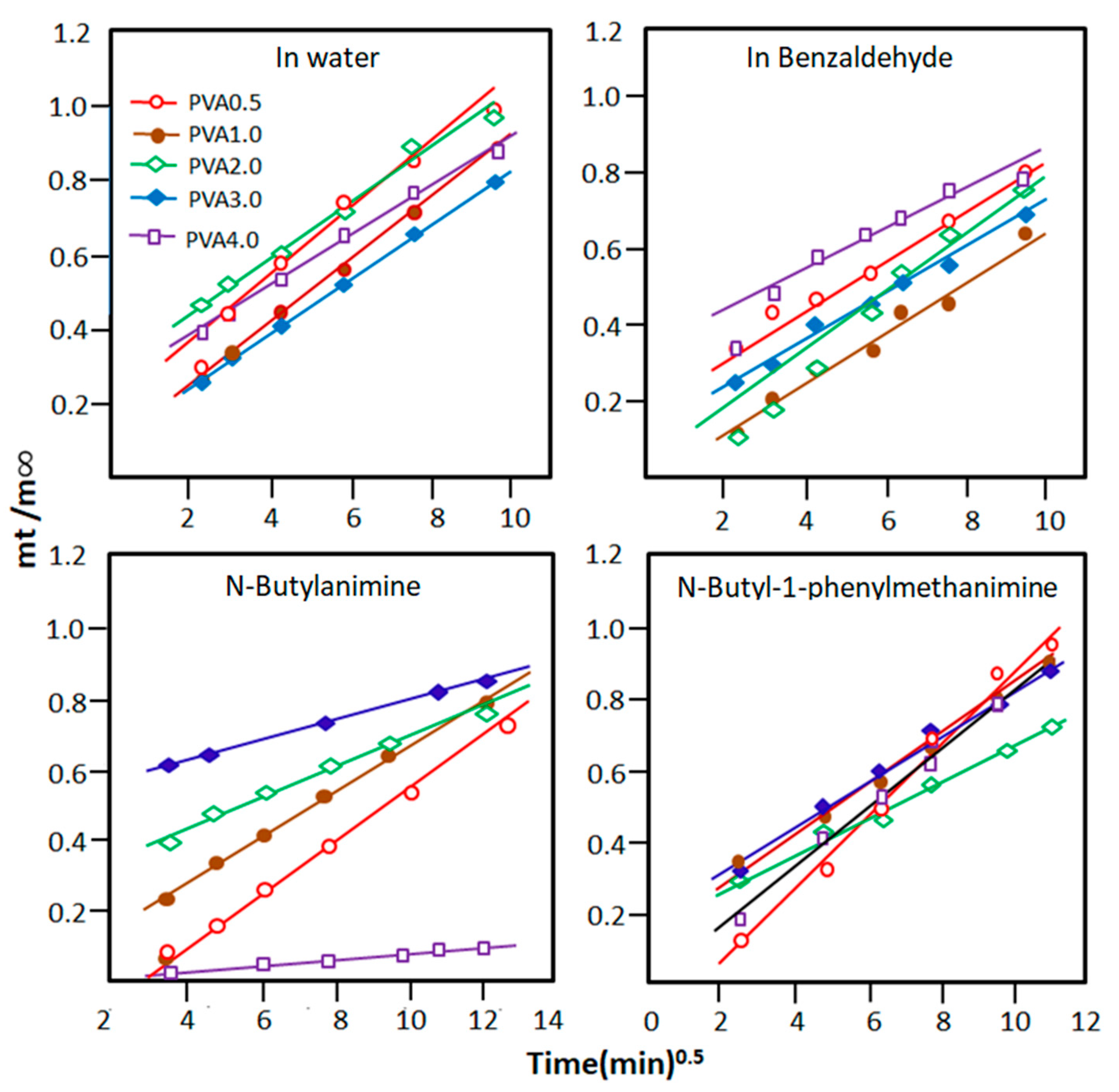
3.1.6. The Diffusion Properties of PVA-x Membrane
3.2. Schiff Base Reaction
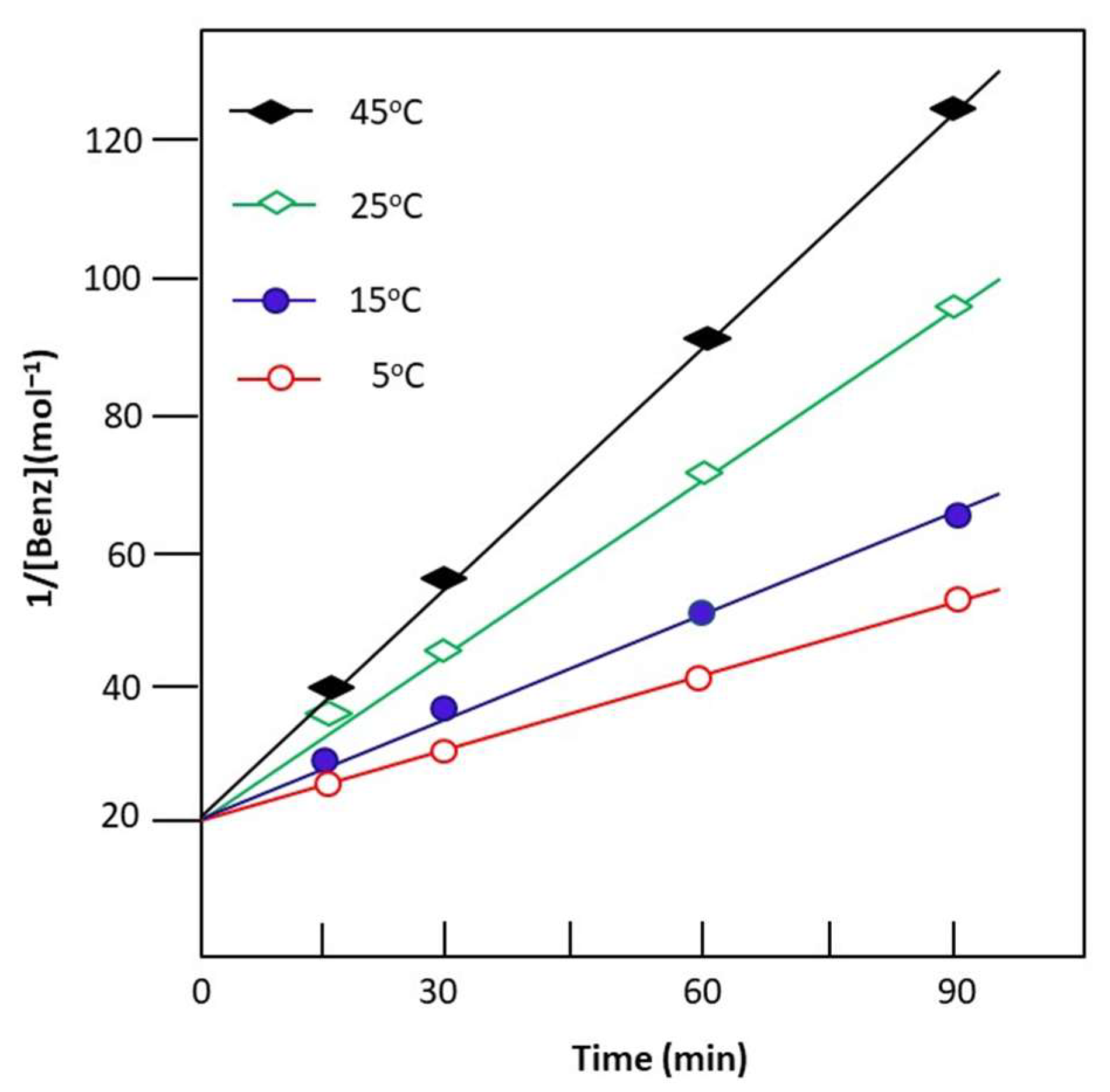
3.2.1. Reaction Non-Assisted by Pervaporation
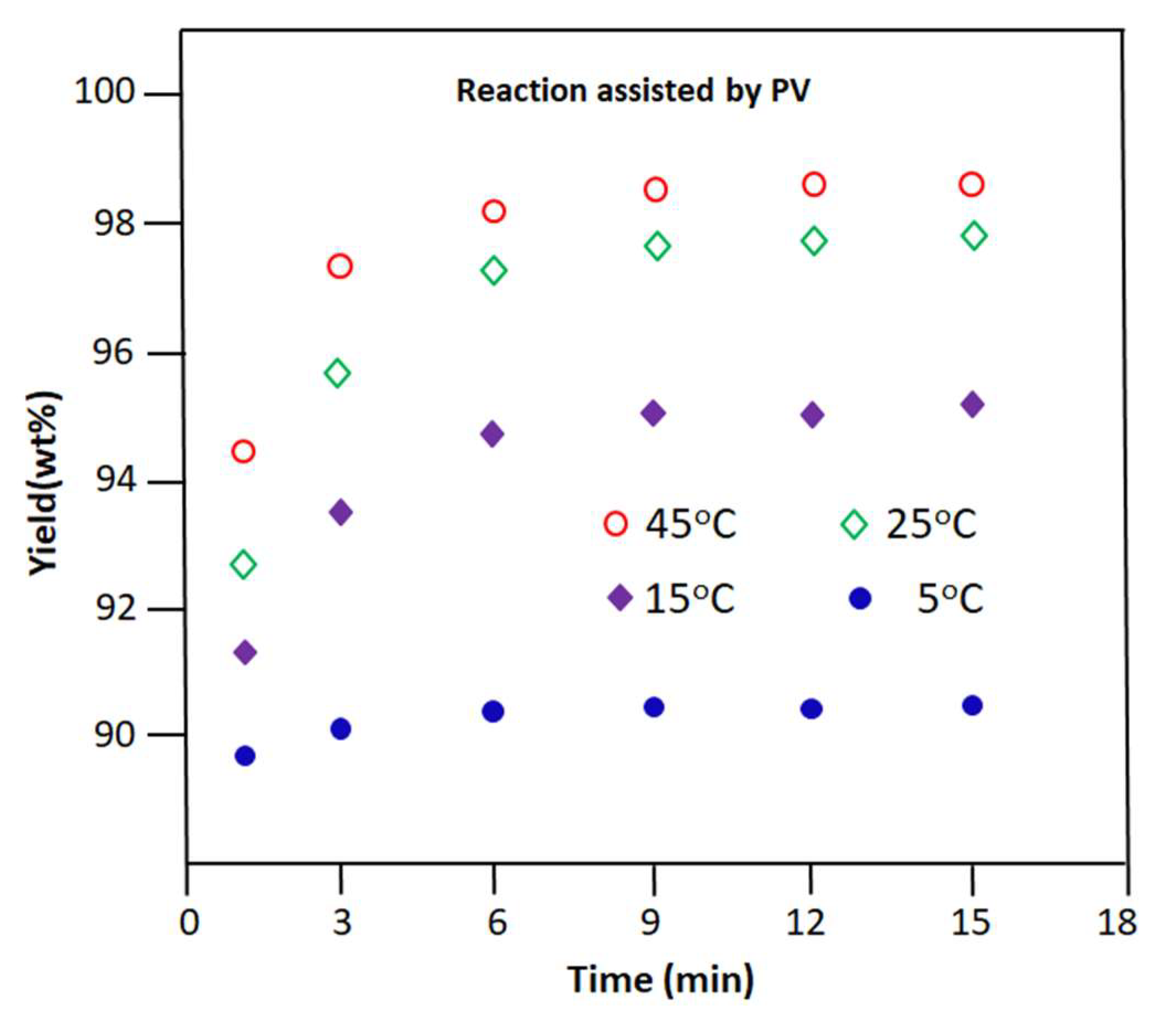
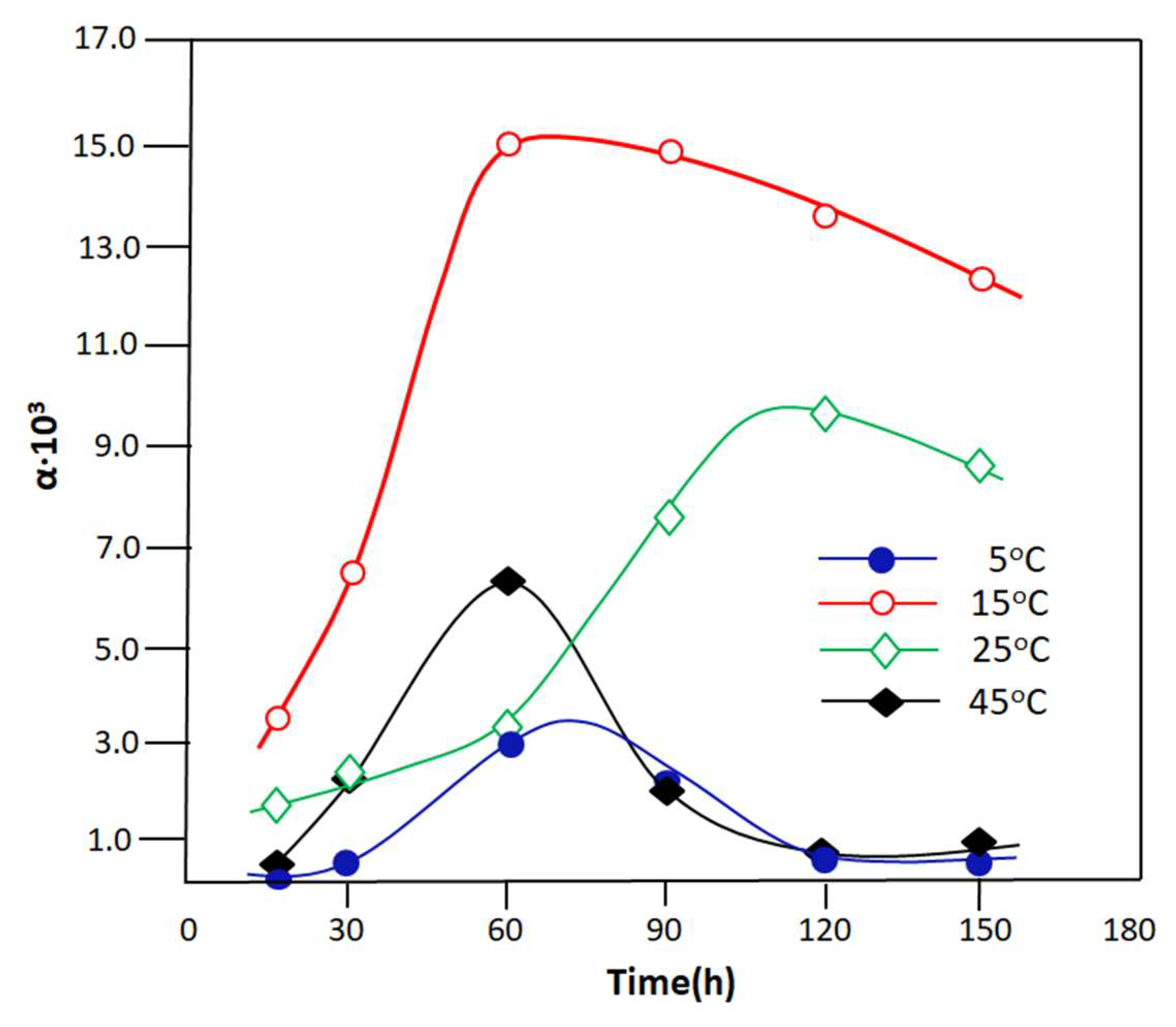
3.2.2. Reaction Assisted by PV
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Verma, R.; Lamba, N.P.; Dandia, A.; Srivastava, A.; Modi, K.; Chauhan, M.S.; Prasad, J. Synthesis of N-Benzylideneaniline by Schiff base reaction using Kinnow peel powder as Green catalyst and comparative study of derivatives through ANOVA techniques. Sci. Rep. 2022, 12, 9636. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.; Manzur, C.; Novoa, N.; Celedón, S.; Carrillo, D.; Hamon, J.-R. Multidentate unsymmetrically-substituted Schiff bases and their metal complexes: Synthesis, functional materials properties, and applications to catalysis. Coord. Chem. Rev. 2018, 357, 144–172. [Google Scholar] [CrossRef]
- Da Silva, C.M.; da Silva, D.L.; Modolo, L.V.; Alves, R.B.; de Resende, M.A.; Martins, C.V.; de Fátima, Â. Schiff bases: A short review of their antimicrobial activities. J. Adv. Res. 2011, 2, 1–8. [Google Scholar] [CrossRef]
- Shawky, A.M.; Abourehab, M.A.; Abdalla, A.N.; Gouda, A.M. Optimization of pyrrolizine-based Schiff bases with 4-thiazolidinone motif: Design, synthesis and investigation of cytotoxicity and anti-inflammatory potency. Eur. J. Med. Chem. 2020, 185, 111780. [Google Scholar] [CrossRef] [PubMed]
- Cordeiro, R.; Kachroo, M. Synthesis and biological evaluation of anti-tubercular activity of Schiff bases of 2-Amino thiazoles. Bioorganic Med. Chem. Lett. 2020, 30, 127655. [Google Scholar] [CrossRef] [PubMed]
- Afridi, H.H.; Shoaib, M.; Al-Joufi, F.A.; Shah, S.W.A.; Hussain, H.; Ullah, A.; Zahoor, M.; Mughal, E.U. Synthesis and Investigation of the Analgesic Potential of Enantiomerically Pure Schiff Bases: A Mechanistic Approach. Molecules 2022, 27, 5206. [Google Scholar] [CrossRef]
- Matela, G. Schiff bases and complexes: A review on anti-cancer activity. Anti-Cancer Agents Med. Chem. (Former. Curr. Med. Chem. -Anti-Cancer Agents) 2020, 20, 1908–1917. [Google Scholar] [CrossRef]
- Yaqoob, M.; Jamil, W.; Taha, M.; Solangi, S. Synthesis, Characterization, Anti-Glycation, and Anti-Oxidant Activities of Sulfanilamide Schiff Base Metal Chelates. Acta Chim. Slov. 2022, 69, 772–778. [Google Scholar] [CrossRef]
- Verma, C.; Quraishi, M. Recent progresses in Schiff bases as aqueous phase corrosion inhibitors: Design and applications. Coord. Chem. Rev. 2021, 446, 214105. [Google Scholar] [CrossRef]
- Ambroziak, K.; Pelech, R.; Milchert, E.; Dziembowska, T.; Rozwadowski, Z. New dioxomolybdenum (VI) complexes of tetradentate Schiff base as catalysts for epoxidation of olefins. J. Mol. Catal. A Chem. 2004, 211, 9–16. [Google Scholar] [CrossRef]
- Li, D.; Liang, X.; Zhang, F.; Li, J.; Zhang, Z.; Wang, S.; Li, Z.; Xing, Y.; Guo, K. Imine bond orientation manipulates AIEgen derived Schiff base isomers through the intramolecular hydrogen bond effect for different fluorescence properties and applications. J. Mater. Chem. C 2022, 10, 11016–11026. [Google Scholar] [CrossRef]
- Abuamer, K.M.; Maihub, A.A.; El-Ajaily, M.M.; Etorki, A.M.; Abou-Krisha, M.M.; Almagani, M.A. The role of aromatic Schiff bases in the dyes techniques. Int. J. Org. Chem. 2014, 4, 43362. [Google Scholar] [CrossRef]
- Al-Tikrity, E.T.; Yaseen, A.A.; Yousif, E.; Ahmed, D.S.; Al-Mashhadani, M.H. Impact on Poly (Vinyl chloride) of trimethoprim schiff bases as stabilizers. Polym. Polym. Compos. 2022, 30, 09673911221094020. [Google Scholar] [CrossRef]
- Kajal, A.; Bala, S.; Kamboj, S.; Sharma, N.; Saini, V. Schiff bases: A versatile pharmacophore. J. Catal. 2013, 2013, 893512. [Google Scholar] [CrossRef]
- Look, G.C.; Murphy, M.M.; Campbell, D.A.; Gallop, M.A. Trimethylorthoformate: A mild and effective dehydrating reagent for solution and solid phase imine formation. Tetrahedron Lett. 1995, 36, 2937–2940. [Google Scholar] [CrossRef]
- Love, B.E.; Ren, J. Synthesis of sterically hindered imines. J. Org. Chem. 1993, 58, 5556–5557. [Google Scholar] [CrossRef]
- La Rocca, T.; Carretier, E.; Dhaler, D.; Louradour, E.; Truong, T.; Moulin, P. Purification of pharmaceutical solvents by pervaporation through hybrid silica membranes. Membranes 2019, 9, 76. [Google Scholar] [CrossRef]
- Karlsson, H.O.; Tragardh, G. Applications of pervaporation in food processing. Trends Food Sci. Technol. 1996, 7, 78–83. [Google Scholar] [CrossRef]
- Drioli, E.; Giorno, L. Encyclopedia of Membranes; Springer: Berlin/Heidelberg, Germany, 2016. [Google Scholar]
- Zhou, K.; Zhang, Q.G.; Han, G.L.; Zhu, A.M.; Liu, Q.L. Pervaporation of water–ethanol and methanol–MTBE mixtures using poly (vinyl alcohol)/cellulose acetate blended membranes. J. Membr. Sci. 2013, 448, 93–101. [Google Scholar] [CrossRef]
- Khudsange, C.R.; Wasewar, K.L. Process intensification of esterification reaction for the production of propyl butyrate by pervaporation. Resour.-Effic. Technol. 2017, 3, 88–93. [Google Scholar] [CrossRef]
- Lyoo, W.; Lee, H. Synthesis of high-molecular-weight poly (vinyl alcohol) with high yield by novel one-batch suspension polymerization of vinyl acetate and saponification. Colloid. Polym. Sci. 2002, 280, 835–840. [Google Scholar]
- Martínez-Felipe, A.; Moliner-Estopiñán, C.; Imrie, C.T.; Ribes-Greus, A. Characterization of crosslinked poly (vinyl alcohol)-based membranes with different hydrolysis degrees for their use as electrolytes in direct methanol fuel cells. J. Appl. Polym. Sci. 2012, 124, 1000–1011. [Google Scholar] [CrossRef]
- Rhim, J.-W.; Park, H.B.; Lee, C.-S.; Jun, J.-H.; Kim, D.S.; Lee, Y.M. Crosslinked poly (vinyl alcohol) membranes containing sulfonic acid group: Proton and methanol transport through membranes. J. Membr. Sci. 2004, 238, 143–151. [Google Scholar] [CrossRef]
- Chan, L.W.; Hao, J.S.; Heng, P.W.S. Evaluation of permeability and mechanical properties of composite polyvinyl alcohol films. Chem. Pharm. Bull. 1999, 47, 1412–1416. [Google Scholar] [CrossRef]
- Mohammad Mahdi Dadfar, S.; Kavoosi, G.; Mohammad Ali Dadfar, S. Investigation of mechanical properties, antibacterial features, and water vapor permeability of polyvinyl alcohol thin films reinforced by glutaraldehyde and multiwalled carbon nanotube. Polym. Compos. 2014, 35, 1736–1743. [Google Scholar] [CrossRef]
- Nasalapure, A.V.; Chalannavar, R.K.; Kasai, D.R.; Reddy, K.R.; Raghu, A.V. Novel polymeric hydrogel composites: Synthesis, physicochemical, mechanical and biocompatible properties. Nano Express 2021, 2, 030003. [Google Scholar] [CrossRef]
- Wang, M.; Cheng, X.; Jiang, G.; Xie, J.; Cai, W.; Li, J.; Wang, Y. Preparation and pervaporation performance of PVA membrane with biomimetic modified silica nanoparticles as coating. J. Membr. Sci. 2022, 653, 120535. [Google Scholar] [CrossRef]
- Chandane, V.S.; Rathod, A.P.; Wasewar, K.L. Enhancement of esterification conversion using pervaporation membrane reactor. Resour. -Effic. Technol. 2016, 2, S47–S52. [Google Scholar] [CrossRef]
- Kwon, S.J.; Song, K.M.; Hong, W.H.; Rhee, J.S. Removal of water produced from lipase-catalyzed esterification in organic solvent by pervaporation. Biotechnol. Bioeng. 1995, 46, 393–395. [Google Scholar] [CrossRef]
- Cannilla, C.; Bonura, G.; Frusteri, F. Potential of pervaporation and vapor separation with water selective membranes for an optimized production of biofuels—A review. Catalysts 2017, 7, 187. [Google Scholar] [CrossRef]
- Ameri, E.; Moheb, A. Improvement of Isopropyl Propionate Esterification Reaction using a Vapor Permeation Membrane Reactor. Iran. J. Catal. 2022, 12, 399–405. [Google Scholar]
- Andersen, A. Final report on the safety assessment of benzaldehyde. Int. J. Toxicol. 2006, 25, 11–27. [Google Scholar] [PubMed]
- Mohapatra, R.K.; Das, P.K.; Pradhan, M.K.; Maihub, A.A.; El-ajaily, M.M. Biological aspects of Schiff base–metal complexes derived from benzaldehydes: An overview. J. Iran. Chem. Soc. 2018, 15, 2193–2227. [Google Scholar] [CrossRef]
- Matar, S.A.; Talib, W.H.; Mustafa, M.S.; Mubarak, M.S.; AlDamen, M.A. Synthesis, characterization, and antimicrobial activity of Schiff bases derived from benzaldehydes and 3, 3′-diaminodipropylamine. Arab. J. Chem. 2015, 8, 850–857. [Google Scholar] [CrossRef]
- Taylor, C.M.; Kilah, N.L. Synthesis of [2 + 2] Schiff base macrocycles by a solvent templating strategy and halogen bonding directed assembly. J. Incl. Phenom. Macrocycl. Chem. 2022, 102, 543–555. [Google Scholar] [CrossRef]
- Westheimer, F.; Taguchi, K. Catalysis by molecular sieves in the preparation of ketimines and enamines. J. Org. Chem. 1971, 36, 1570–1572. [Google Scholar] [CrossRef]
- Maslov, D.L.; Trifonova, O.P.; Balashova, E.E.; Lokhov, P.G. N-butylamine for improving the efficiency of untargeted mass spectrometry analysis of plasma metabolite composition. Int. J. Mol. Sci. 2019, 20, 5957. [Google Scholar] [CrossRef]
- Wang, C.; Ma, B.; Zhang, L. Experimental study on CO2 capture by using n-butylamine to plug the gas channeling to enhanced oil recovery. J. Pet. Explor. Prod. Technol. 2022, 12, 2523–2531. [Google Scholar] [CrossRef]
- Kanse, N.G.; Dawande, S. Separation of ethanol/water (azeotropic mixture) by pervaporation using PVA membrane. Mater. Today Proc. 2017, 4, 10520–10523. [Google Scholar] [CrossRef]
- Sapalidis, A.A. Porous Polyvinyl alcohol membranes: Preparation methods and applications. Symmetry 2020, 12, 960. [Google Scholar] [CrossRef]
- Erdödi, G.; Iván, B. Novel amphiphilic conetworks composed of telechelic poly (ethylene oxide) and three-arm star polyisobutylene. Chem. Mater. 2004, 16, 959–962. [Google Scholar] [CrossRef]
- Haraszti, M.; Tóth, E.; Iván, B. Poly (methacrylic acid)-l-polyisobutylene: A novel polyelectrolyte amphiphilic conetwork. Chem. Mater. 2006, 18, 4952–4958. [Google Scholar] [CrossRef]
- Gupta, S.; Webster, T.J.; Sinha, A. Evolution of PVA gels prepared without crosslinking agents as a cell adhesive surface. J. Mater. Sci. Mater. Med. 2011, 22, 1763–1772. [Google Scholar] [CrossRef] [PubMed]
- Yang, M.-H.; Chu, T.-J. The determination of interaction parameter χ1 for polyvinyl alcohol and water from the diffusion data. Polym. Test. 1993, 12, 57–64. [Google Scholar] [CrossRef]
- Peppas, N.A.; Bures, P.; Leobandung, W.; Ichikawa, H. Hydrogels in pharmaceutical formulations. Eur. J. Pharm. Biopharm. 2000, 50, 27–46. [Google Scholar] [CrossRef] [PubMed]
- Ranjha, N.M.; Ayub, G.; Naseem, S.; Ansari, M.T. Preparation and characterization of hybrid pH-sensitive hydrogels of chitosan-co-acrylic acid for controlled release of verapamil. J. Mater. Sci. Mater. Med. 2010, 21, 2805–2816. [Google Scholar] [CrossRef] [PubMed]
- Denkbaş, E.B.; Kilicay, E.; Birlikseven, C.; Öztürk, E. Magnetic chitosan microspheres: Preparation and characterization. React. Funct. Polym. 2002, 50, 225–232. [Google Scholar] [CrossRef]
- Comyn, J. Introduction to polymer permeability and the mathematics of diffusion. In Polymer Permeability; Springer: Cham, Switzerland, 1985; pp. 1–10. [Google Scholar]
- Masaro, L.; Zhu, X. Physical models of diffusion for polymer solutions, gels and solids. Progress. Polym. Sci. 1999, 24, 731–775. [Google Scholar] [CrossRef]
- Silverstein, R.M.; Bassler, G.C. Spectrometric identification of organic compounds. J. Chem. Educ. 1962, 39, 546. [Google Scholar] [CrossRef]
- Freire, T.F.; Quinaz, T.; Fertuzinhos, A.; Quyền, N.T.; de Moura, M.F.; Martins, M.; Zille, A.; Dourado, N. Thermal, mechanical and chemical analysis of poly (vinyl alcohol) multifilament and braided yarns. Polymers 2021, 13, 3644. [Google Scholar] [CrossRef]
- Restrepo, I.; Medina, C.; Meruane, V.; Akbari-Fakhrabadi, A.; Flores, P.; Rodríguez-Llamazares, S. The effect of molecular weight and hydrolysis degree of poly (vinyl alcohol)(PVA) on the thermal and mechanical properties of poly (lactic acid)/PVA blends. Polímeros 2018, 28, 169–177. [Google Scholar] [CrossRef]
- Salazar, J.D.R. Study of Structural, Thermic, µ-Raman and Optic Transformation of PVA/TiO2 Polymeric Membranes. Sci. Et Tech. 2018, 23, 543–552. [Google Scholar]
- Rynkowska, E.; Fatyeyeva, K.; Marais, S.; Kujawa, J.; Kujawski, W. Chemically and thermally crosslinked PVA-based membranes: Effect on swelling and transport behavior. Polymers 2019, 11, 1799. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.-J.; Lee, S.-B.; Han, N.W. Effects of the degree of crosslinking on properties of poly (vinyl alcohol) membranes. Polym. J. 1993, 25, 1295–1302. [Google Scholar] [CrossRef]
- Lima, E.C.; Hosseini-Bandegharaei, A.; Moreno-Piraján, J.C.; Anastopoulos, I. A critical review of the estimation of the thermodynamic parameters on adsorption equilibria. Wrong use of equilibrium constant in the Van’t Hoof equation for calculation of thermodynamic parameters of adsorption. J. Mol. Liq. 2019, 273, 425–434. [Google Scholar] [CrossRef]
- Arrhenius, S. Über die Reaktionsgeschwindigkeit bei der Inversion von Rohrzucker durch Säuren. Z. Für Phys. Chem. 1889, 4, 226–248. [Google Scholar] [CrossRef]
- Crowell, T.I.; Bell, C.E.; O’Brien, D.H. Extrathermodynamic relationships in Schiff base formation. J. Am. Chem. Soc. 1964, 86, 4973–4976. [Google Scholar] [CrossRef]
- Jyoti, G.; Keshav, A.; Anandkumar, J. Review on pervaporation: Theory, membrane performance, and application to intensification of esterification reaction. J. Eng. 2015, 2015, 927068. [Google Scholar] [CrossRef]
- Vane, L.M. Review of pervaporation and vapor permeation process factors affecting the removal of water from industrial solvents. J. Chem. Technol. Biotechnol. 2020, 95, 495–512. [Google Scholar] [CrossRef]
- Kalahal, P.B.; Sajjan, A.M.; Yunus Khan, T.; Rajhi, A.A.; Achappa, S.; Banapurmath, N.R.; Duhduh, A.A. Novel Polyelectrolyte Complex Membranes Containing Carboxymethyl Cellulose–Gelatin for Pervaporation Dehydration of Azeotropic Bioethanol for Biofuel. Polymers 2022, 14, 5114. [Google Scholar] [CrossRef]
- Jiang, W.; Zhu, J.; Yuan, Z.; Lu, J.; Ding, J. Optimization of the esterification of oleic acid and ethanol in a fixed bed membrane reactor by response surface method. Fuel 2023, 342, 127867. [Google Scholar] [CrossRef]
- Feng, X.; Huang, R.Y. Estimation of activation energy for permeation in pervaporation processes. J. Membr. Sci. 1996, 118, 127–131. [Google Scholar] [CrossRef]




| PVA-x Membrane | PVA (g) | PVA (wt%) | Oxalic Acid (g) | Oxalic Acid (wt%) |
|---|---|---|---|---|
| PVA-0.5 | 2.0 | 99.50 | 0.01 | 0.50 |
| PVA-1.0 | 2.0 | 99.01 | 0.02 | 0.99 |
| PVA-2.0 | 2.0 | 98.04 | 0.04 | 1.96 |
| PVA-3.0 | 2.0 | 97.09 | 0.06 | 2.91 |
| PVA-4.0 | 2.0 | 96.15 | 0.08 | 3.85 |
| PVA-x Membrane | Vp | χ | q | ||
|---|---|---|---|---|---|
| PVA-0.5 | 0.301 | 0.49 | 377.13 | 19,906.77 | 0.0189 |
| PVA-1.0 | 0.342 | 0.49 | 640.56 | 19,806.24 | 0.0323 |
| PVA-2.0 | 0.316 | 0.49 | 748.13 | 19,650.12 | 0.0381 |
| PVA-3.0 | 0.308 | 0.49 | 803.86 | 19,933.77 | 0.0403 |
| PVA-4.0 | 0.303 | 0.49 | 864.83 | 19,234.67 | 0.0450 |
| Membrane | Swelling Capacity (wt%) | |||
|---|---|---|---|---|
| Water | n-Butylamine | Benzaldehyde | n-butyl-1-phenylmethanimine | |
| PVA-0.5 | 107.0 ± 2.3 | 22.8 ± 2.5 | 3.1 ± 1.1 | 13.4 ± 2.3 |
| PVA-1.0 | 100.1 ± 2.0 | 20.3 ± 2.1 | 7.0 ± 1.2 | 10.1 ± 2.0 |
| PVA-2.0 | 93.2 ± 1.3 | 14.9 ± 1.5 | 8.3 ± 1.2 | 3.5 ± 1.7 |
| PVA-3.0 | 90.0 ± 1.8 | 10.5 ± 2.3 | 5.0 ± 1.5 | 11.7 ± 0.8 |
| PVA-4.0 | 69.2 ± 1.3 | 7.5 ± 1.5 | 7.5 ± 1.5 | 10.9 ± 1.5 |
| Sample | Water | Benzaldehyde | n-Butylamine | N-butyl-1-phenylmethanimine | ||||
|---|---|---|---|---|---|---|---|---|
| D (µm2∙min−1) | K (µm∙min−1) | D (µm2∙min−1) | K (µm∙min−1) | D (µm2∙min−1) | K (µm∙min−1) | D (µm2∙min−1) | K (µm∙min−1) | |
| PVA-0.5 | 9.62 ± 0.12 | 10.01 ± 0.13 | 4.06±0.08 | 4.22 ± 0.08 | 5.06 ± 0.05 | 5.26 ± 0.06 | 4.32 ± 0.20 | 4.49 ± 0.21 |
| PVA-1.0 | 7.36 ± 0.07 | 7.65 ± 0.07 | 3.76 ± 0.15 | 3.91 ± 0.15 | 3.76 ± 0.05 | 3.91 ± 0.05 | 3.76 ± 0.05 | 3.90 ± 0.19 |
| PVA-2.0 | 5.41 ± 0.12 | 5.63 ± 0.13 | 3.46 ± 0.22 | 3.60 ± 0.23 | 3.46 ± 0.10 | 3.60 ± 0.10 | 4.32 ± 0.11 | 4.48 ± 0.19 |
| PVA-3.0 | 4.71 ± 0.12 | 4.90 ± 0.13 | 2.78 ± 0.24 | 2.89 ± 0.25 | 2.78 ± 0.06 | 2.89 ± 0.06 | 3.46 ± 0.13 | 3.60 ± 0.14 |
| PVA-4.0 | 3.46 ± 0.07 | 3.60 ± 0.07 | 2.40 ± 0.26 | 2.50 ± 0.27 | 2.40 ± 0.05 | 2.50 ± 0.05 | 3.46 ± 0.13 | 3.60 ± 0.14 |
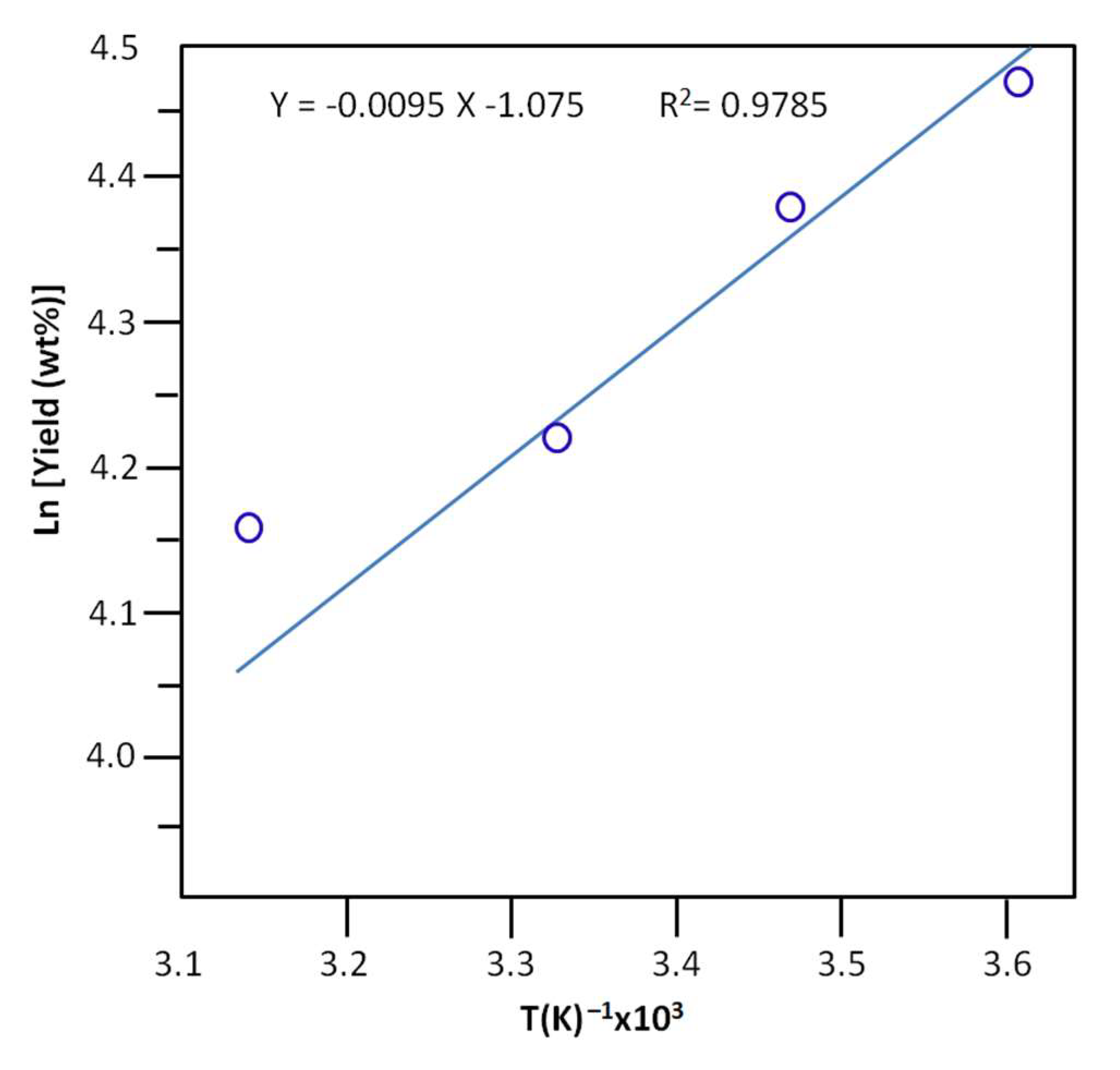
| T (°C) | Order | k (mol.min)−1 | keq |
|---|---|---|---|
| 5 | 2 | 0.339 ± 0.012 | 2.576 ± 0.221 |
| 15 | 2 | 0.508 ± 0.013 | 4.868 ± 0.167 |
| 25 | 2 | 0.800 ± 0.010 | 14.519 ± 1.142 |
| 45 | 2 | 1.102 ± 0.009 | 27.500 ± 1.433 |
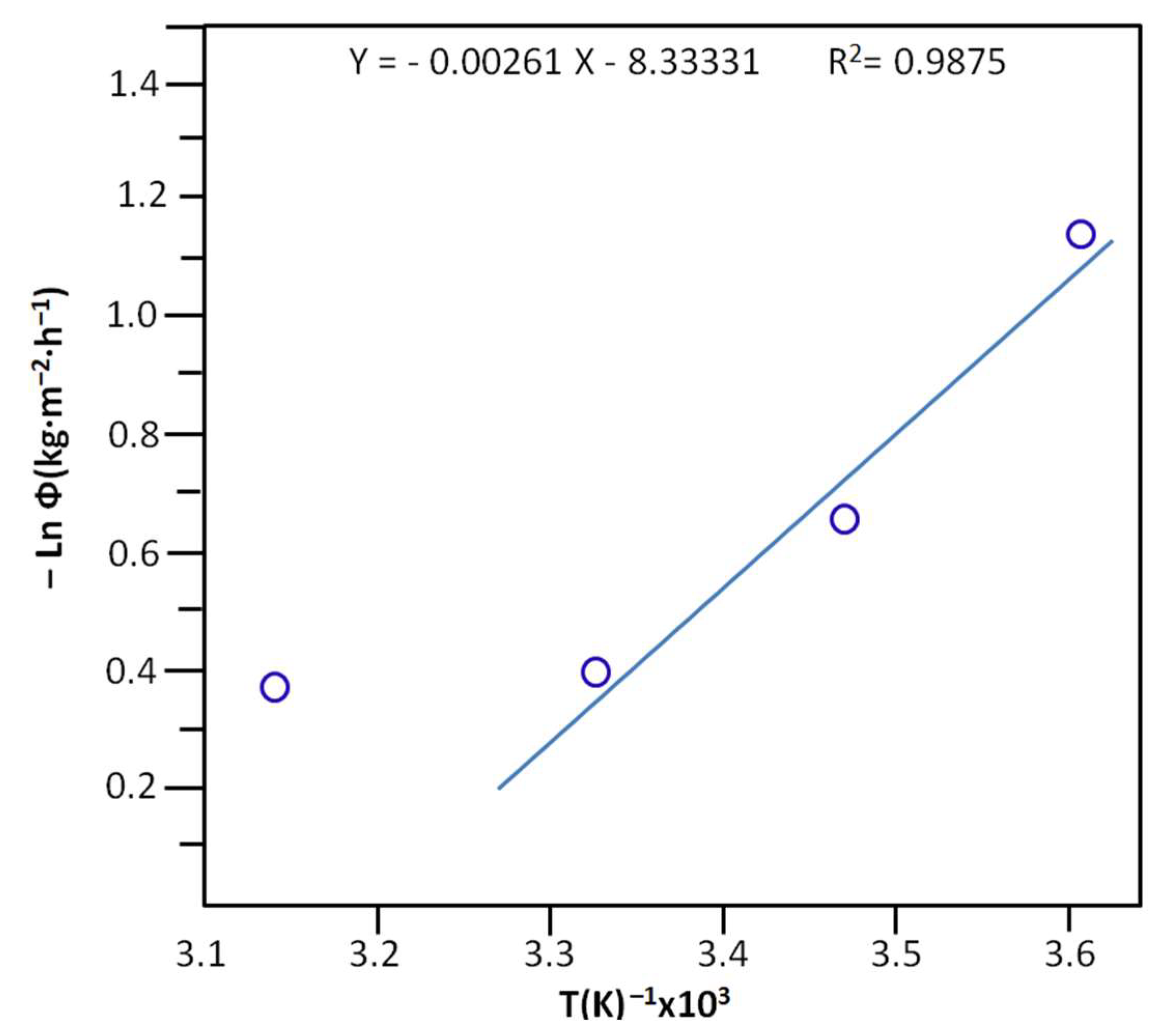
| T (°C) | (Kg·m−2·h−1)103 | (Kg·m−2·h−1)103 | (Kg·m−2·h−1)103 | (Kg·m−2·h−1)103 | (Kg·m−2·h−1)103 |
|---|---|---|---|---|---|
| 5 | 180 ± 11 | 2.0 ± 0.3 | 1.0 ± 0.2 | 2.2± 0.3 | 0.17 ± 0.05 |
| 15 | 348 ± 10 | 5.5 ± 0,3 | 1.0 ± 0.2 | 1.3 ± 0.2 | 0.27 ± 0.07 |
| 25 | 310 ± 12 | 3.2 ± 0.3 | 4.0 ± 0.2 | 4.3 ± 0.2 | 0.27 ± 0.07 |
| 45 | 421 ± 13 | 7.0 ± 0.3 | 5.0 ± 0.3 | 7.0 ± 0.3 | 0.40 ± 0.08 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Al Khulaifi, R.S.; AlShehri, M.M.; Al-Qadsy, I.; Al Jufareen, M.A.; Saeed, W.S.; Badjah-Hadj-Ahmed, A.Y.; Aouak, T. Breaking the Equilibrium and Improving the Yield of Schiff Base Reactions by Pervaporation: Application to a Reaction Involving n-butylamine and Benzaldehyde. Separations 2023, 10, 602. https://doi.org/10.3390/separations10120602
Al Khulaifi RS, AlShehri MM, Al-Qadsy I, Al Jufareen MA, Saeed WS, Badjah-Hadj-Ahmed AY, Aouak T. Breaking the Equilibrium and Improving the Yield of Schiff Base Reactions by Pervaporation: Application to a Reaction Involving n-butylamine and Benzaldehyde. Separations. 2023; 10(12):602. https://doi.org/10.3390/separations10120602
Chicago/Turabian StyleAl Khulaifi, Rana Salem, Mohammed Mousa AlShehri, Inas Al-Qadsy, Mona A. Al Jufareen, Waseem Sharaf Saeed, Ahmed Yacine Badjah-Hadj-Ahmed, and Taieb Aouak. 2023. "Breaking the Equilibrium and Improving the Yield of Schiff Base Reactions by Pervaporation: Application to a Reaction Involving n-butylamine and Benzaldehyde" Separations 10, no. 12: 602. https://doi.org/10.3390/separations10120602
APA StyleAl Khulaifi, R. S., AlShehri, M. M., Al-Qadsy, I., Al Jufareen, M. A., Saeed, W. S., Badjah-Hadj-Ahmed, A. Y., & Aouak, T. (2023). Breaking the Equilibrium and Improving the Yield of Schiff Base Reactions by Pervaporation: Application to a Reaction Involving n-butylamine and Benzaldehyde. Separations, 10(12), 602. https://doi.org/10.3390/separations10120602







