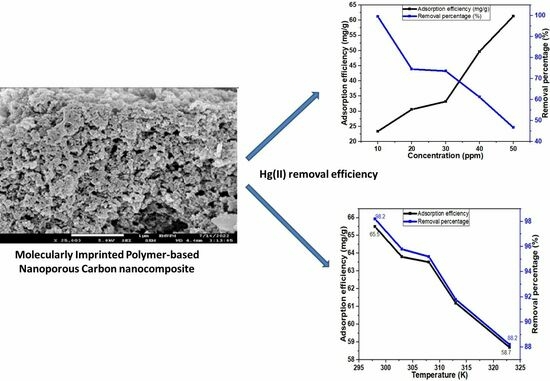
Molecularly Imprinted Polymer-Based Nanoporous Carbon Nanocomposite for Effective Adsorption of Hg(II) Ions from Aqueous Suspensions
Abstract
:1. Introduction
2. Experimental
2.1. Materials
2.2. Preparation of MIP/NC Nanocomposite
2.3. Sample Composite Characterization
2.4. Batch Adsorption Set-Up
2.5. pH Optimization
2.6. Optimization of Adsorbent Dosage
2.7. Adsorption Isotherms
2.8. Adsorption Kinetics
2.9. Adsorption Thermodynamics
3. Results and Discussion
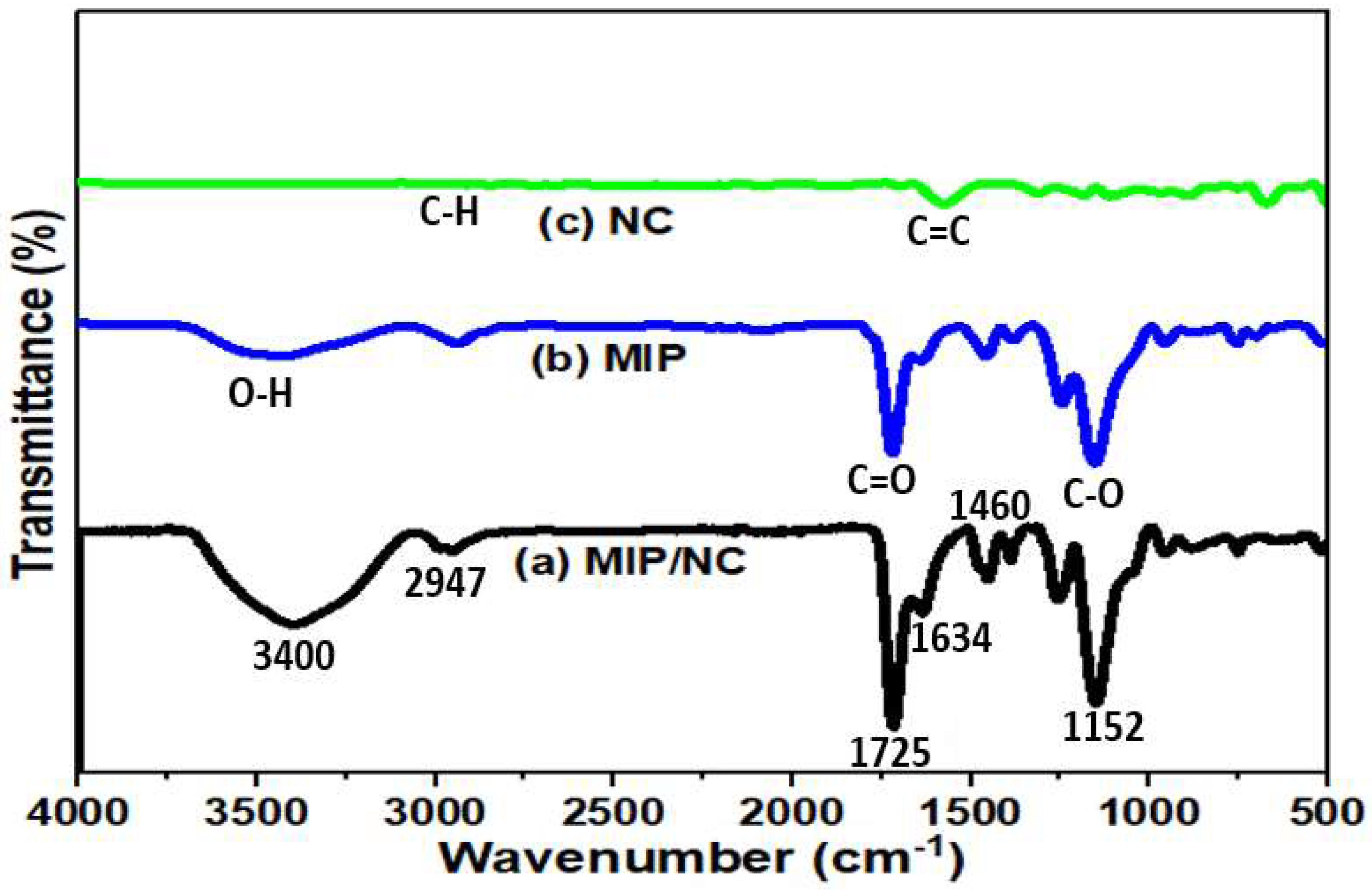
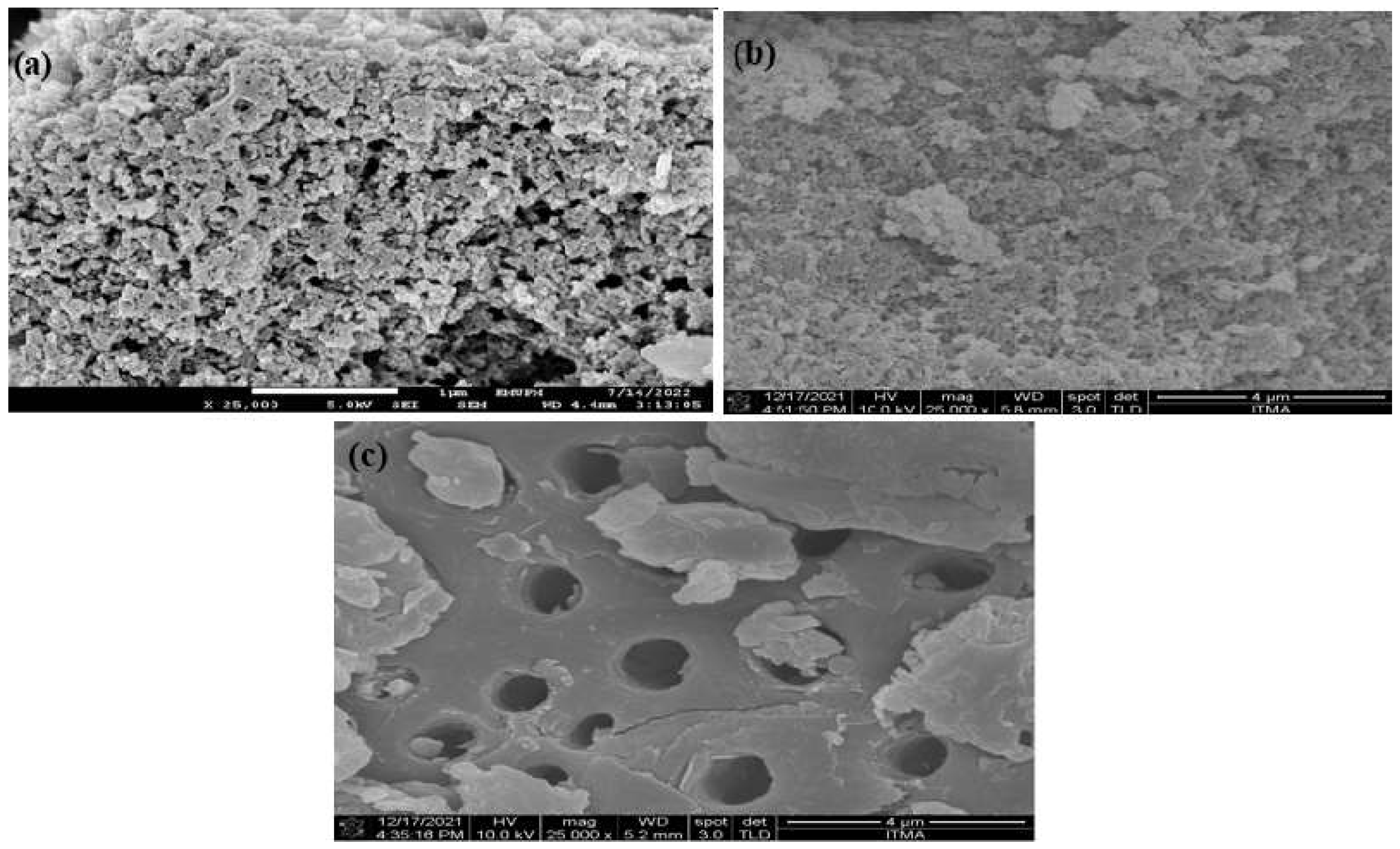
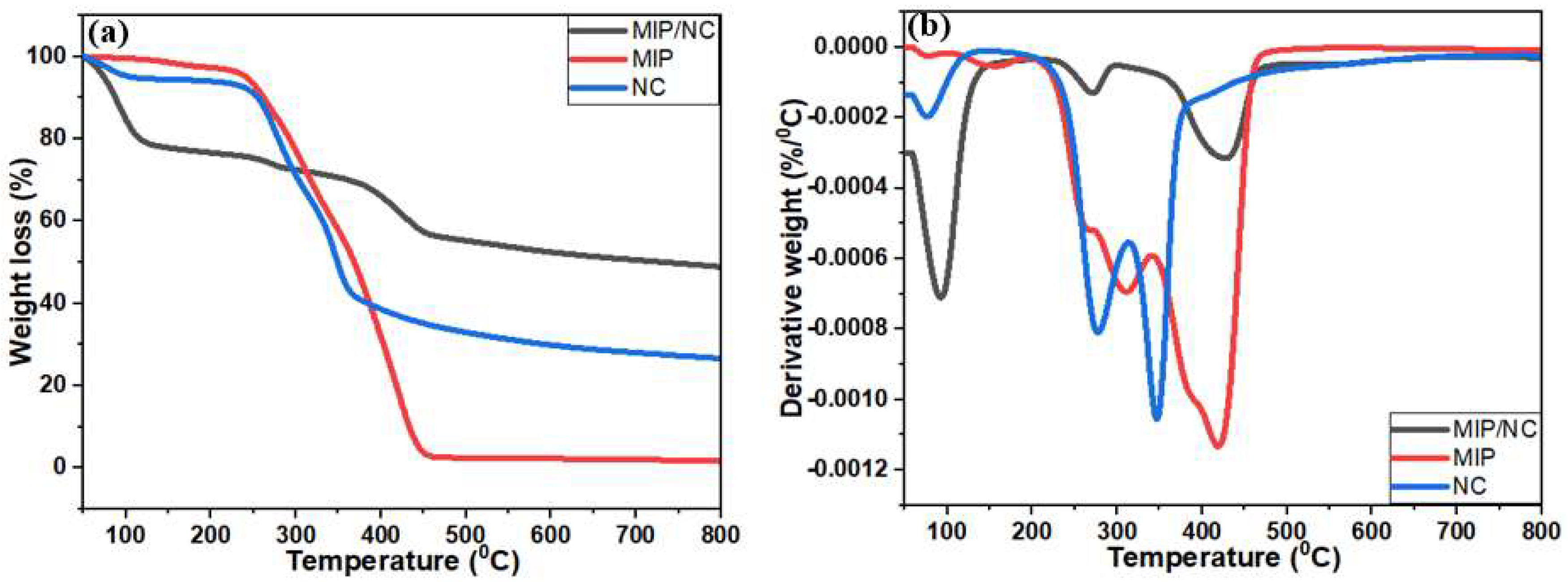
3.1. Physicochemical Characterization of MIP/NC Nanocomposite
3.2. Batch Studies
3.2.1. Effect of pH
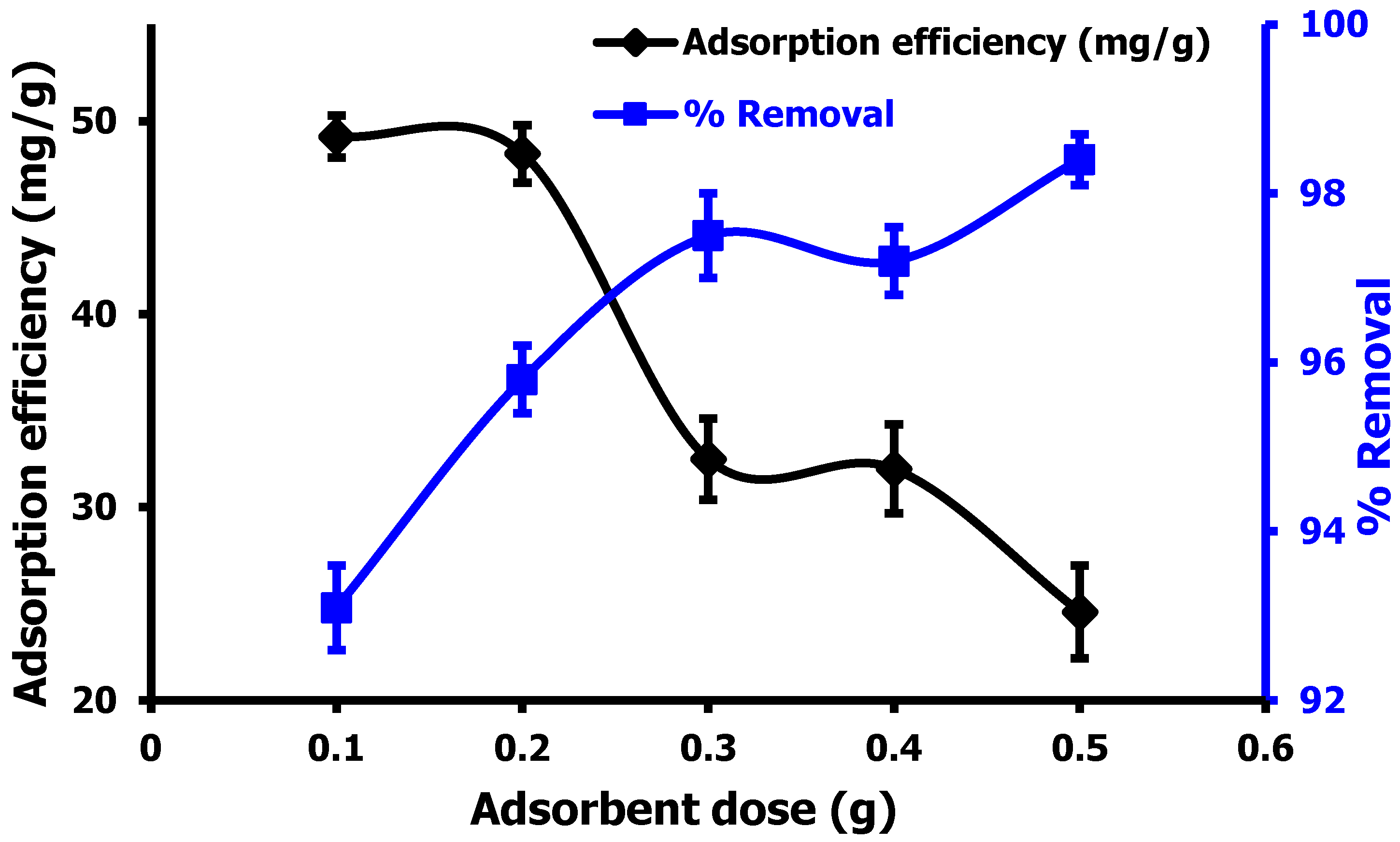
3.2.2. Effect of Absorbent Dosage
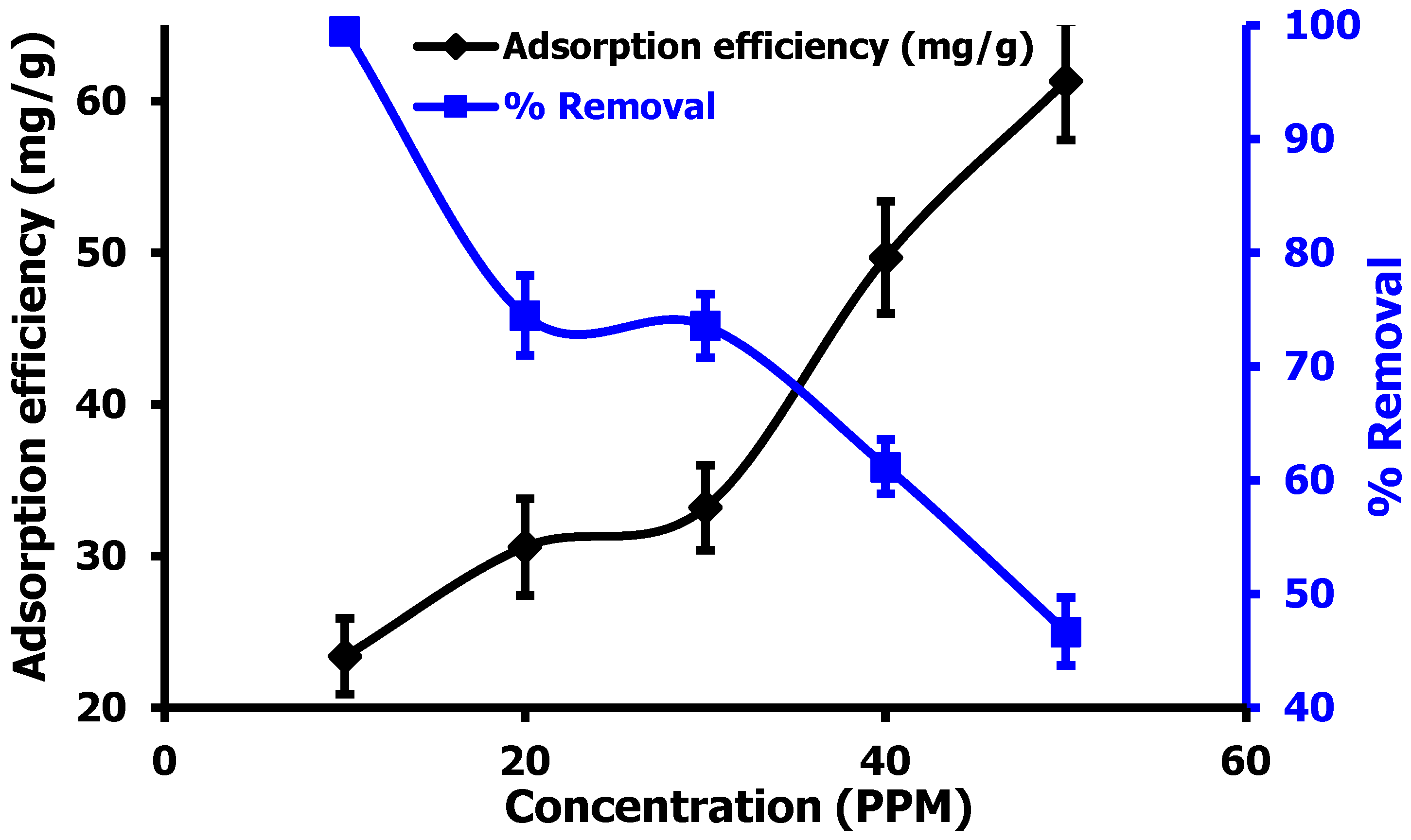
3.2.3. Effect of Hg(II) Ion Concentration
3.2.4. Effect of Contact Time
3.3. Studies of Hg(II) Adsorption Isotherm
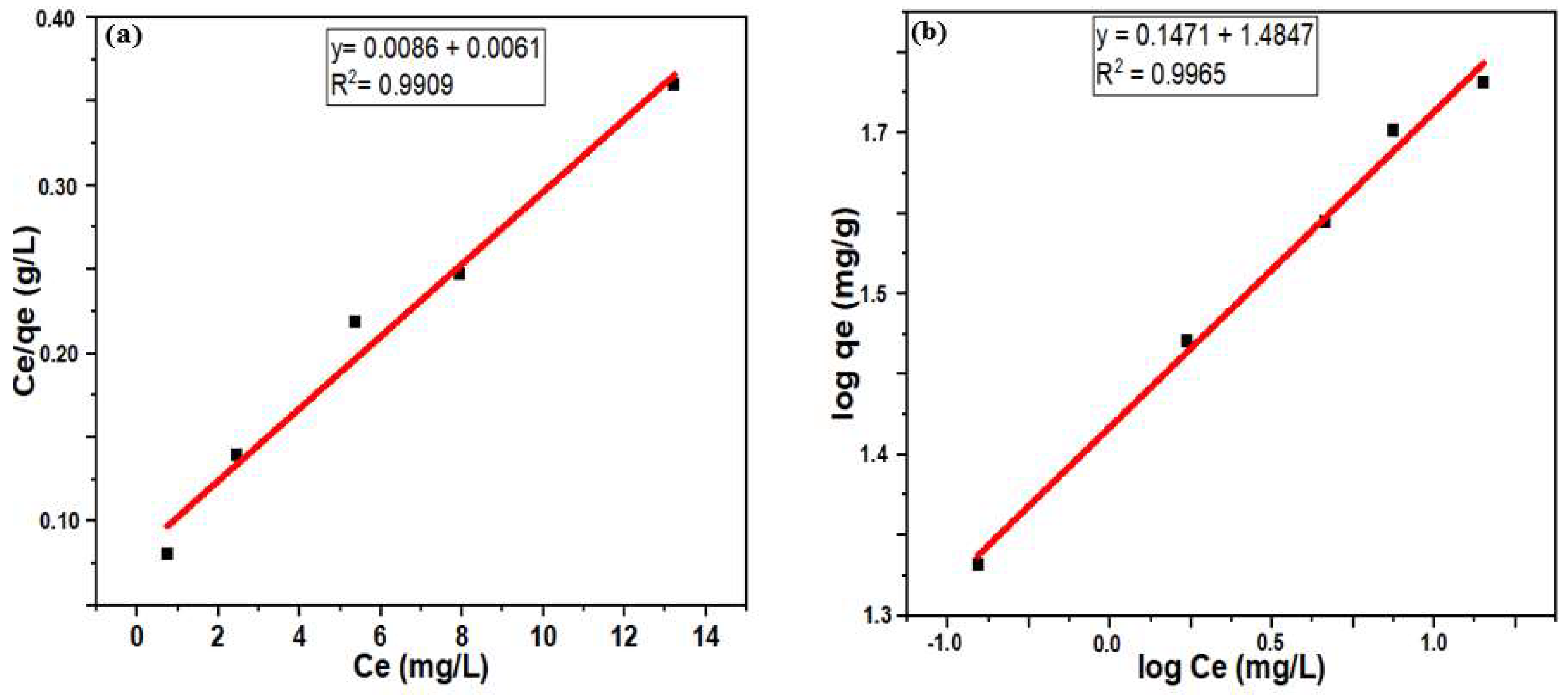
3.4. Kinetics of Hg(II) Adsorption
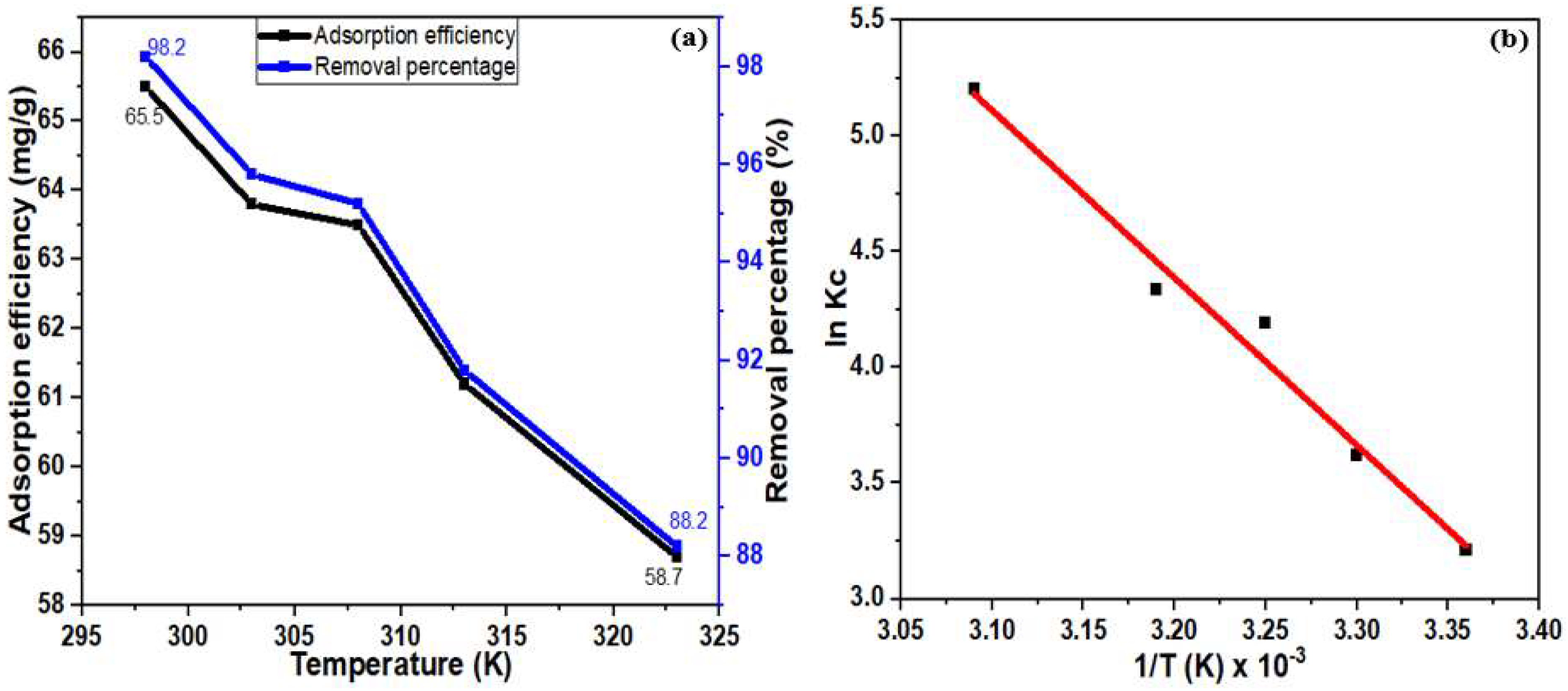
3.5. Thermodynamics of Hg(II) Adsorption
3.6. Application of MIP/NC on Condensate Real Sample
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Awang, N.A.; Wan Salleh, W.N.; Aziz, F.; Yusof, N.; Ismail, A.F. A review on preparation, surface enhancement and adsorption mechanism of biochar-supported nano zero-valent iron adsorbent for hazardous heavy metals. J. Chem. Technol. Biotechnol. 2023, 98, 22–44. [Google Scholar] [CrossRef]
- Schlögl, S.; Diendorfer, P.; Baldermann, A.; Vollprecht, D. Use of industrial residues for heavy metals immobilization in contaminated site remediation: A brief review. Int. J. Environ. Sci. Technol. 2023, 20, 2313–2326. [Google Scholar] [CrossRef]
- Demarco, C.F.; Quadro, M.S.; Selau Carlos, F.; Pieniz, S.; Morselli, L.B.G.A.; Andreazza, R. Bioremediation of Aquatic Environments Contaminated with Heavy Metals: A Review of Mechanisms, Solutions and Perspectives. Sustainability 2023, 15, 1411. [Google Scholar] [CrossRef]
- Chakraborty, R.; Asthana, A.; Singh, A.K.; Jain, B.; Susan, A.B.H. Adsorption of heavy metal ions by various low-cost adsorbents: A review. Int. J. Environ. Anal. Chem. 2022, 102, 342–379. [Google Scholar] [CrossRef]
- Arora, R. Adsorption of heavy metals—A review. Mater. Today Proc. 2019, 18 Pt 7, 4745–4750. [Google Scholar] [CrossRef]
- Da’na, E. Adsorption of heavy metals on functionalized-mesoporous silica: A review. Microporous Mesoporous Mater. 2017, 247, 145–157. [Google Scholar] [CrossRef]
- Economic and Social Commission for Asia and Pacific (ESCAP). SDG 6: Clean Water and Sanitation: Ensure Availability and Sustainable Management of Water and Sanitation for All. 2018. Available online: https://hdl.handle.net/20.500.12870/874 (accessed on 24 May 2023).
- Arrifano, G.D.P.; Augusto-Oliveira, M.; Lopes-Araújo, A.; Santos-Sacramento, L.; Macchi, B.M.; Nascimento, J.L.M.D.; Crespo-Lopez, M.E. Global Human Threat: The Potential Synergism between Mercury Intoxication and COVID-19. Int. J. Environ. Res. Public Health 2023, 20, 4207. [Google Scholar] [CrossRef]
- Saha, S.; Dhara, K.; Chukwuka, A.V.; Pal, P.; Saha, N.C.; Faggio, C. Sub-lethal acute effects of environmental concentrations of inorganic mercury on hematological and biochemical parameters in walking catfish, Clarias batrachus. Comp. Biochem. Physiol. Part C Toxicol. Pharmacol. 2023, 264, 109511. [Google Scholar] [CrossRef]
- Chen, X.; Hossain, M.F.; Duan, C.; Lu, J.; Tsang, Y.F.; Islam, M.S.; Zhou, Y. Isotherm models for adsorption of heavy metals from water-a review. Chemosphere 2022, 307, 135545. [Google Scholar] [CrossRef]
- Fabre, E.; Lopes, C.B.; Vale, C.; Pereira, E.; Silva, C.M. Valuation of banana peels as an effective biosorbent for mercury removal under low environmental concentrations. Sci. Total Environ. 2020, 709, 135883. [Google Scholar] [CrossRef]
- Kurniawan, T.A.; Lo, W.; Liang, X.; Goh, H.H.; Othman, M.H.D.; Chong, K.K.; Chew, K.W. Remediation technologies for contaminated groundwater due to arsenic (As), mercury (Hg), and/or fluoride (F): A critical review and way forward to contribute to carbon neutrality. Sep. Purif. Technol. 2023, 314, 123474. [Google Scholar] [CrossRef]
- Wu, H.; Lin, G.; Liu, C.; Chu, S.; Mo, C.; Liu, X. Progress and challenges in molecularly imprinted polymers for adsorption of heavy metal ions from wastewater. Trends Environ. Anal. Chem. 2022, 36, e00178. [Google Scholar] [CrossRef]
- Metwally, M.G.; Benhawy, A.H.; Khalifa, R.M.; El Nashar, R.M.; Trojanowicz, M. Application of molecularly imprinted polymers in the analysis of waters and wastewaters. Molecules 2021, 26, 6515. [Google Scholar] [CrossRef] [PubMed]
- Diagboya, P.N.; Olu-Owolabi, B.I.; Adebowale, K.O. Synthesis of covalently bonded graphene oxide–iron magnetic nanoparticles and the kinetics of mercury removal. Rsc Adv. 2015, 5, 2536–2542. [Google Scholar] [CrossRef]
- Huang, R.; Ma, X.; Li, X.; Guo, L.; Xie, X.; Zhang, M.; Li, J. A novel ion-imprinted polymer based on graphene oxide-mesoporous silica nanosheet for fast and efficient removal of chromium (VI) from aqueous solution. J. Colloid Interface Sci. 2018, 514, 544–553. [Google Scholar] [CrossRef] [PubMed]
- Lyu, Q.; Liu, Y.; Guan, Y.; Liu, X.; Che, D. DFT study on the mechanisms of mercury removal from natural gas over Se-modified activated carbon. Fuel 2022, 324, 124658. [Google Scholar] [CrossRef]
- Egirani, D.; Latif, M.T.; Wessey, N.; Poyi, N.R.; Shehata, N. Preparation and characterization of powdered and granular activated carbon from Palmae biomass for mercury removal. Appl. Water Sci. 2021, 11, 10. [Google Scholar] [CrossRef]
- Rodriguez, R.; Contrino, D.; Mazyck, D.W. Role of activated carbon precursor in mercury removal. Ind. Eng. Chem. Res. 2020, 59, 17740–17747. [Google Scholar] [CrossRef]
- Gai, K.; Avellan, A.; Hoelen, T.P.; Lopez-Linares, F.; Hatakeyama, E.S.; Lowry, G.V. Impact of mercury speciation on its removal from water by activated carbon and organoclay. Water Res. 2019, 157, 600–609. [Google Scholar] [CrossRef]
- Abdullah, N.S.; Sharifuddin, S.S.; Hussin, M.H. Study on adsorption of mercury from aqueous solution on activated carbons prepared from palm kernel shell. Key Eng. Mater. 2018, 783, 109–114. [Google Scholar] [CrossRef]
- Hadavifar, M.; Bahramifar, N.; Younesi, H.; Li, Q. Adsorption of mercury ions from synthetic and real wastewater aqueous solution by functionalized multi-walled carbon nanotube with both amino and thiolated groups. Chem. Eng. J. 2014, 237, 217–228. [Google Scholar] [CrossRef]
- Jeon, C.; Solis, K.L.; An, H.R.; Hong, Y.; Igalavithana, A.D.; Ok, Y.S. Sustainable removal of Hg (II) by sulfur-modified pine-needle biochar. J. Hazard. Mater. 2020, 388, 122048. [Google Scholar] [CrossRef] [PubMed]
- Bailon, M.X.; Chaudhary, D.K.; Jeon, C.; Ok, Y.S.; Hong, Y. Impact of sulfur-impregnated biochar amendment on microbial communities and mercury methylation in contaminated sediment. J. Hazard. Mater. 2022, 438, 129464. [Google Scholar] [CrossRef]
- Zhang, Y.; Yan, L.; Xu, W.; Guo, X.; Cui, L.; Gao, L.; Du, B. Adsorption of Pb (II) and Hg (II) from aqueous solution using magnetic CoFe2O4-reduced graphene oxide. J. Mol. Liq. 2014, 191, 177–182. [Google Scholar] [CrossRef]
- Du, W.; Yin, L.; Zhuo, Y.; Xu, Q.; Zhang, L.; Chen, C. Catalytic oxidation and adsorption of elemental mercury over CuCl2-impregnated sorbents. Ind. Eng. Chem. Res. 2014, 53, 582–591. [Google Scholar] [CrossRef]
- Rahim, Z.A.; Yusof, N.A.; Ismail, S.; Mohammad, F.; Abdullah, J.; Rahman, N.A.; Abubakar, L.; Soleiman, A.A. Functional nano molecularly imprinted polymer for the detection of Penicillin G in pharmaceutical samples. J. Polym. Res. 2023, 30, 113. [Google Scholar] [CrossRef]
- Bagheri, A.R.; Aramesh, N.; Khan, A.A.; Gul, I.; Ghotekar, S.; Bilal, M. Molecularly imprinted polymers-based adsorption and photocatalytic approaches for mitigation of environmentally-hazardous pollutants—A review. J. Environ. Chem. Eng. 2021, 9, 104879. [Google Scholar] [CrossRef]
- Villarreal-Lucio, D.S.; Vargas-Berrones, K.X.; Díaz de León-Martínez, L.; Flores-Ramíez, R. Molecularly imprinted polymers for environmental adsorption applications. Environ. Sci. Pollut. Res. 2022, 29, 89923–89942. [Google Scholar] [CrossRef]
- Guo, H.; Li, H.; Jing, C.; Wang, X. Soluble polymers with intrinsic porosity for efficient removal of phenolic compounds from water. Microporous Mesoporous Mater. 2021, 319, 111068. [Google Scholar] [CrossRef]
- Wang, X.; Yu, C.; Guo, H.; Cheng, Y.; Li, Y.; Zheng, D.; Feng, S.; Lin, Y. Robust fluorescent detection of iodine vapor by a film sensor based on a polymer of intrinsic microporosity. Chem. Eng. J. 2022, 438, 135641. [Google Scholar] [CrossRef]
- Baby, R.; Hussein, M.Z.; Zainal, Z.; Abdullah, A.H. Preparation of Functionalized Palm Kernel Shell Bio-adsorbent for the treatment of heavy metal-contaminated water. J. Hazard. Mater. Adv. 2023, 10, 100253. [Google Scholar] [CrossRef]
- Uchegbulam, I.; Momoh, E.O.; Agan, S.A. Potentials of Palm Kernel Shell Derivatives: A Critical Review on Waste Recovery for Environmental Sustainability. Clean. Mater. 2022, 6, 100154. [Google Scholar] [CrossRef]
- Singh, G.; Lee, J.M.; Kothandam, G.; Palanisami, T.; Al-Muhtaseb, A.A.H.; Karakoti, A.; Vinu, A. A review on the synthesis and applications of nanoporous carbons for the removal of complex chemical contaminants. Bull. Chem. Soc. Jpn. 2021, 94, 1232–1257. [Google Scholar] [CrossRef]
- Aliyu, M.; Abdullah, A.H.; bin Mohamed Tahir, M.I. Adsorption tetracycline from aqueous solution using a novel polymeric adsorbent derived from the rubber waste. J. Taiwan Inst. Chem. Eng. 2022, 136, 104333. [Google Scholar] [CrossRef]
- Jena, K.K.; Reddy, K.S.K.; Karanikolos, G.N.; Choi, D.S. L-Cysteine and silver nitrate based metal sulfide and Zeolite-Y nano adsorbent for efficient removal of mercury (II) ion from wastewater. Appl. Surf. Sci. 2023, 611, 155777. [Google Scholar] [CrossRef]
- Aliyu, M.; Abdullah, A.H.; bin Mohamed Tahir, M.I. A Potential Approach for Converting Rubber Waste into a Low-Cost Polymeric Adsorbent for Removing Methylene Blue from Aqueous Solutions. Indones. J. Chem. 2022, 22, 653–665. [Google Scholar] [CrossRef]
- Samah, N.A.; Rosli, N.A.M.; Manap, A.H.A.; Aziz, Y.F.A.; Yusoff, M.M. Synthesis & characterization of ion imprinted polymer for arsenic removal from water: A value addition to the groundwater resources. Chem. Eng. J. 2020, 394, 124900. [Google Scholar] [CrossRef]
- Yeboah, M.L. Facile synthesis of micro-mesoporous activated carbon in ambient air via one and two-stage activation of palm kernel shell waste for methylene blue adsorption. Int. J. Environ. Anal. Chem. 2021, 1–19. [Google Scholar] [CrossRef]
- Roushani, M.; Abbasi, S.; Khani, H. Synthesis and application of ion-imprinted polymer nanoparticles for the extraction and preconcentration of mercury in water and food samples employing cold vapor atomic absorption spectrometry. Environ. Monit. Assess. 2015, 173, 266–273. [Google Scholar] [CrossRef]
- Lee, J.; Kim, S.; Shin, H. Hierarchical porous carbon electrodes with sponge-like edge structures for the sensitive electrochemical detection of heavy metals. Sensors 2021, 21, 1346. [Google Scholar] [CrossRef]
- Meléndez-Marmolejo, J.; Díaz de León-Martínez, L.; Galván-Romero, V.; Villarreal-Lucio, S.; Ocampo-Pérez, R.; Medellín-Castillo, N.A.; Flores-Ramírez, R. Design and application of molecularly imprinted polymers for adsorption and environmental assessment of anti-inflammatory drugs in wastewater samples. Environ. Sci. Pollut. Res. 2022, 29, 45885–45902. [Google Scholar] [CrossRef]
- Ensafi, A.A.; Nasr-Esfahani, P.; Rezaei, B. Synthesis of molecularly imprinted polymer on carbon quantum dots as an optical sensor for selective fluorescent determination of promethazine hydrochloride. Sens. Actuators B Chem. 2018, 257, 889–896. [Google Scholar] [CrossRef]
- Elaigwu, S.E.; Greenway, G.M. Characterization of energy-rich hydrochars from microwave-assisted hydrothermal carbonization of coconut shell. Waste Biomass Valorization 2019, 10, 1979–1987. [Google Scholar] [CrossRef]
- Nasir, S.; Hussein, M.Z.; Zainal, Z.; Yusof, N.A.; Mohd Zobir, S.A. Electrochemical energy storage potentials of waste biomass: Oil palm leaf-and palm kernel shell-derived activated carbons. Energies 2018, 11, 3410. [Google Scholar] [CrossRef]
- Li, Y.; Zhang, X.; Yang, R.; Li, G.; Hu, C. The role of H3PO4 in the preparation of activated carbon from NaOH-treated rice husk residue. RSC Adv. 2015, 5, 32626–32636. [Google Scholar] [CrossRef]
- Saleh, T.A.; Sarı, A.; Tuzen, M. Optimization of parameters with experimental design for the adsorption of mercury using polyethylenimine modified-activated carbon. J. Environ. Chem. Eng. 2017, 5, 1079–1088. [Google Scholar] [CrossRef]
- Dima, S.O.; Sarbu, A.; Dobre, T.; Purcar, V.; Nicolae, C.A. Diosgenin selective molecularly imprinted polymers with acrylonitrile-methacrylic acid matrix. Mater. Plast. 2012, 49, 106–113. [Google Scholar] [CrossRef]
- Duan, C.; Ma, T.; Wang, J.; Zhou, Y. Removal of heavy metals from aqueous solution using carbon-based adsorbents: A review. J. Water Process Eng. 2020, 37, 101339. [Google Scholar] [CrossRef]
- Wu, G.; Wang, Z.; Wang, J.; He, C. Hierarchically imprinted organic–inorganic hybrid sorbent for selective separation of mercury ion from aqueous solution. Anal. Chim. Acta 2007, 582, 304–310. [Google Scholar] [CrossRef]
- Martín-Lara, M.A.; Calero, M.; Ronda, A.; Iáñez-Rodríguez, I.; Escudero, C. Adsorptive behavior of an activated carbon for bisphenol A removal in single and binary (bisphenol A—Heavy metal) solutions. Water 2020, 12, 2150. [Google Scholar] [CrossRef]
- Shafqat, S.R.; Bhawani, S.A.; Bakhtiar, S.; Ibrahim, M.N.M. Synthesis of molecularly imprinted polymer for removal of Congo red. BMC Chem. 2020, 14, 27. [Google Scholar] [CrossRef] [PubMed]
- Liu, Z.; Sun, Y.; Xu, X.; Qu, J.; Qu, B. Adsorption of Hg (II) in an aqueous solution by activated carbon prepared from rice husk using KOH activation. ACS Omega 2020, 5, 29231–29242. [Google Scholar] [CrossRef] [PubMed]
- Roy, P.; Dey, U.; Chattoraj, S.; Mukhopadhyay, D.; Mondal, N.K. Modeling of the adsorptive removal of arsenic (III) using plant biomass: A bioremedial approach. Appl. Water Sci. 2017, 7, 1307–1321. [Google Scholar] [CrossRef]
- Gheitasi, F.; Ghammamy, S.; Zendehdel, M.; Semiromi, F.B. Removal of mercury (II) from aqueous solution by powdered activated carbon nanoparticles prepared from beer barley husk modified with Thiol/Fe3O4. J. Mol. Struct. 2022, 1267, 133555. [Google Scholar] [CrossRef]
- Chen, J.; Tan, L.; Cui, Z.; Qu, K.; Wang, J. Graphene oxide molecularly imprinted polymers as novel adsorbents for solid-phase microextraction for selective determination of norfloxacin in the marine environment. Polymers 2022, 14, 1839. [Google Scholar] [CrossRef] [PubMed]
- Bashir, K.; Guo, P.; Chen, G.; Li, Y.; Ge, Y.; Shu, H.; Fu, Q. Synthesis, characterization, and application of griseofulvin surface molecularly imprinted polymers as the selective solid phase extraction sorbent in rat plasma samples. Arab. J. Chem. 2020, 13, 4082–4091. [Google Scholar] [CrossRef]
- Han, X.; Zhang, X.; Zhong, L.; Yu, X.; Zhai, H. Preparation of sulfamethoxazole molecularly imprinted polymers based on magnetic metal–organic frameworks/graphene oxide composites for the selective extraction of sulfonamides in food samples. Microchem. J. 2022, 177, 107259. [Google Scholar] [CrossRef]
- Li, B.; Xiong, H.; Xiao, Y. Progress on synthesis and applications of porous carbon materials. Int. J. Electrochem. Sci 2020, 15, 1363–1377. [Google Scholar] [CrossRef]
- Wieszczycka, K.; Filipowiak, K.; Wojciechowska, I.; Aksamitowski, P. Novel ionic liquid-modified polymers for highly effective adsorption of heavy metals ions. Sep. Purif. Technol. 2020, 236, 116313. [Google Scholar] [CrossRef]
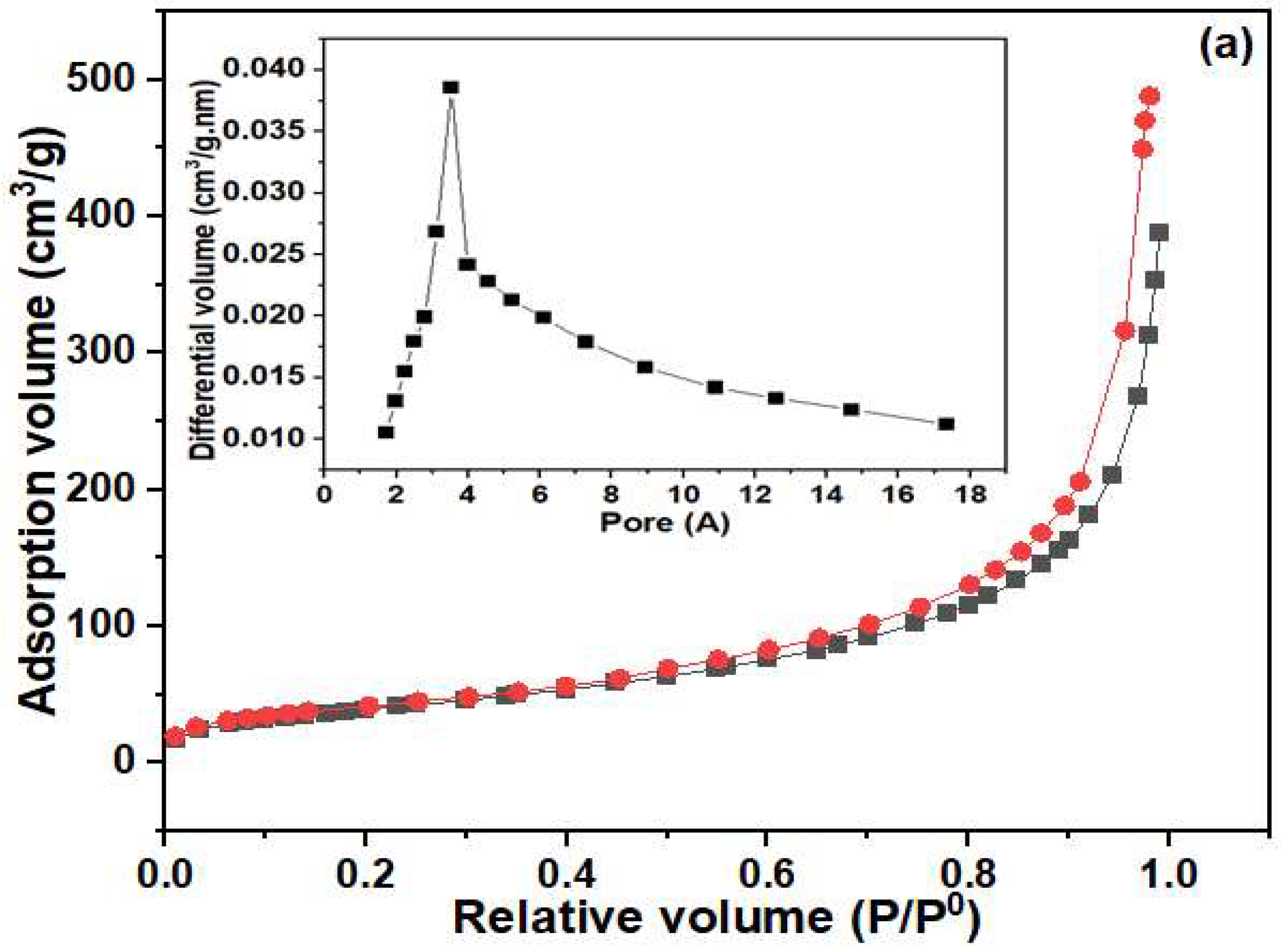
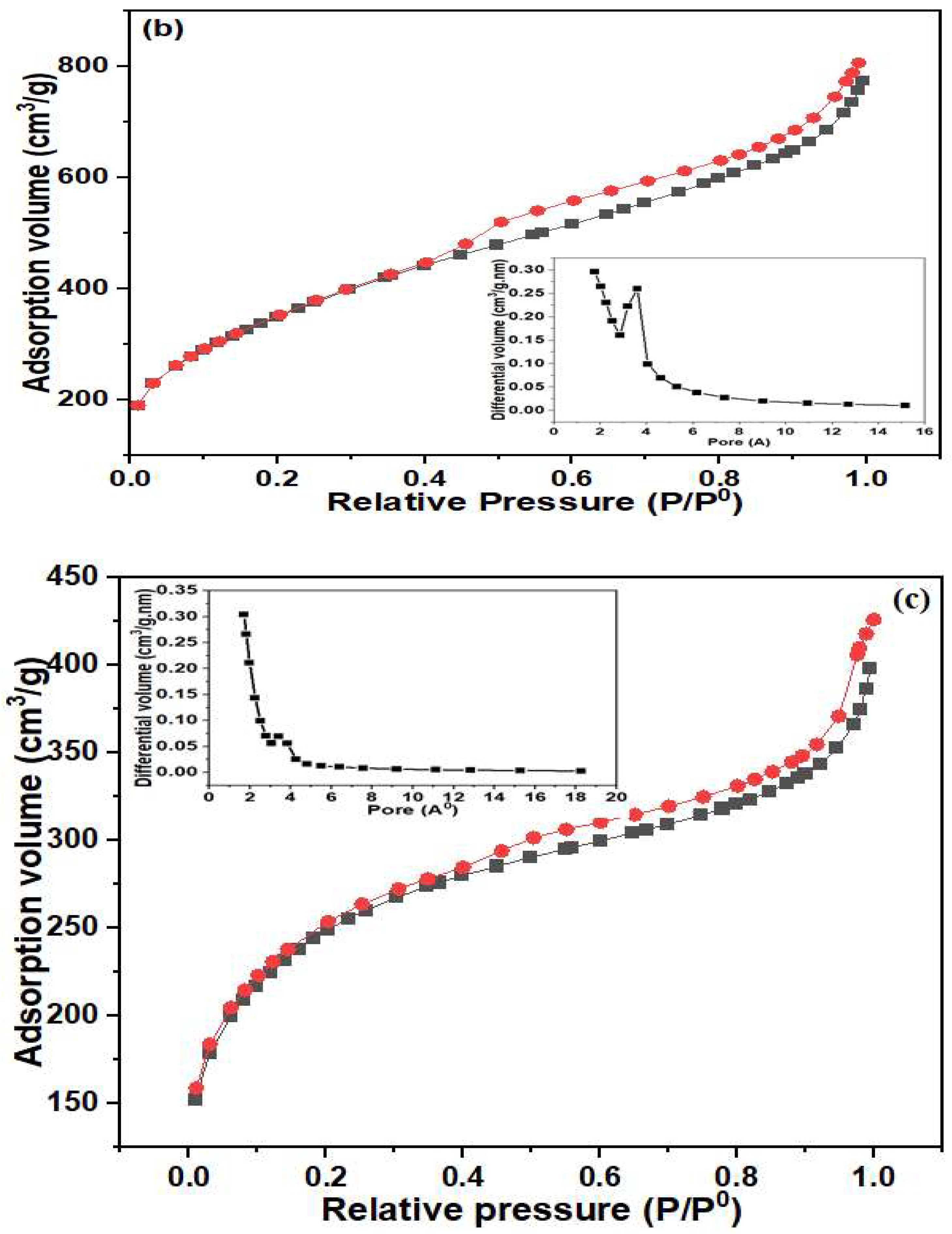



| Sample | BET Surface Area (m2/g) | Average Pore Volume (cm3/g) | Average Pore Size (nm) |
|---|---|---|---|
| NC | 1280 | 0.677 | 2.116 |
| MIP | 146 | 0.163 | 4.468 |
| MIP/NC | 884.9 | 0.389 | 1.760 |
| Langmuir Isotherm | Freundlich Isotherm | ||||
|---|---|---|---|---|---|
| Maximum Adsorption Capacity, (mg/g) | Langmuir Constant, KL (L/mg) | Correlation Coefficient, R2 | Freundlich Constant, KF (mg/g) | Freundlich Constant, n | Correlation Coefficient, R2 |
| 116 | 0.708 | 0.9909 | 4.03 | 6.798 | 0.9965 |
| Kinetic Models | qe Experimental (mg/g) | qe Calculated (mg/g) | Correlation Coefficient R2 |
|---|---|---|---|
| Pseudo-first-order | 61.34 | 0.014 | 0.9620 |
| Pseudo-second-order | 61.34 | 63.60 | 0.9935 |
| Temperature K | ΔS (KJ/mol K) | ΔH (KJ/mol) | ΔG (KJ/mol) |
|---|---|---|---|
| 298 | 34.1 | 10.55 | −7.95 |
| 303 | −9.12 | ||
| 308 | −10.73 | ||
| 313 | −11.28 | ||
| 323 | −13.96 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Abubakar, L.; Yusof, N.A.; Abdullah, A.H.; Mohammad, F.; Wahid, M.H.; Ismail, S.; Abdul Rahim, Z.; Al-Lohedan, H.A.; Soleiman, A.A. Molecularly Imprinted Polymer-Based Nanoporous Carbon Nanocomposite for Effective Adsorption of Hg(II) Ions from Aqueous Suspensions. Separations 2023, 10, 454. https://doi.org/10.3390/separations10080454
Abubakar L, Yusof NA, Abdullah AH, Mohammad F, Wahid MH, Ismail S, Abdul Rahim Z, Al-Lohedan HA, Soleiman AA. Molecularly Imprinted Polymer-Based Nanoporous Carbon Nanocomposite for Effective Adsorption of Hg(II) Ions from Aqueous Suspensions. Separations. 2023; 10(8):454. https://doi.org/10.3390/separations10080454
Chicago/Turabian StyleAbubakar, Lawal, Nor Azah Yusof, Abdul Halim Abdullah, Faruq Mohammad, Mohd Hanif Wahid, Suhainie Ismail, Zulaiha Abdul Rahim, Hamad A. Al-Lohedan, and Ahmed A. Soleiman. 2023. "Molecularly Imprinted Polymer-Based Nanoporous Carbon Nanocomposite for Effective Adsorption of Hg(II) Ions from Aqueous Suspensions" Separations 10, no. 8: 454. https://doi.org/10.3390/separations10080454
APA StyleAbubakar, L., Yusof, N. A., Abdullah, A. H., Mohammad, F., Wahid, M. H., Ismail, S., Abdul Rahim, Z., Al-Lohedan, H. A., & Soleiman, A. A. (2023). Molecularly Imprinted Polymer-Based Nanoporous Carbon Nanocomposite for Effective Adsorption of Hg(II) Ions from Aqueous Suspensions. Separations, 10(8), 454. https://doi.org/10.3390/separations10080454