Study on Treatment Performance of Desulfurization Wastewater by Zero-Valent Iron Fenton-like Process
Abstract
1. Introduction
2. Materials and Methods
2.1. Experimental Equipment and Apparatus
2.2. Water Quality Conditions and Testing Indicators
2.3. Experimental Materials and Reagents
2.4. ZVI Fenton-like Method for Desulfurization Wastewater
2.5. Experimental Design
3. Results and Discussion
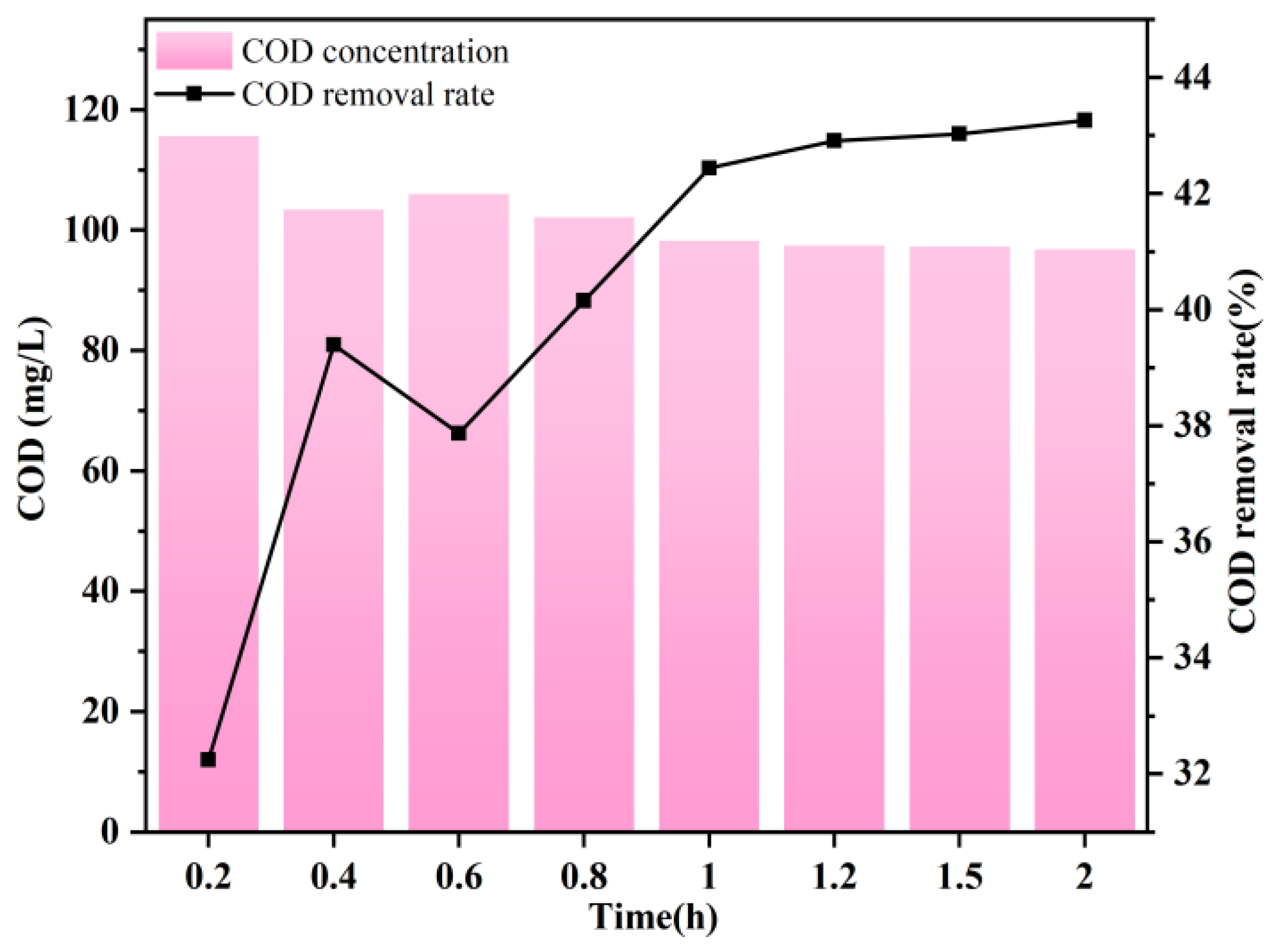
3.1. Impact of Reaction Time on COD Removal
3.2. Impact of H2O2 Dosage on COD Removal
3.3. Impact of Fe Powder Dosage on COD Removal
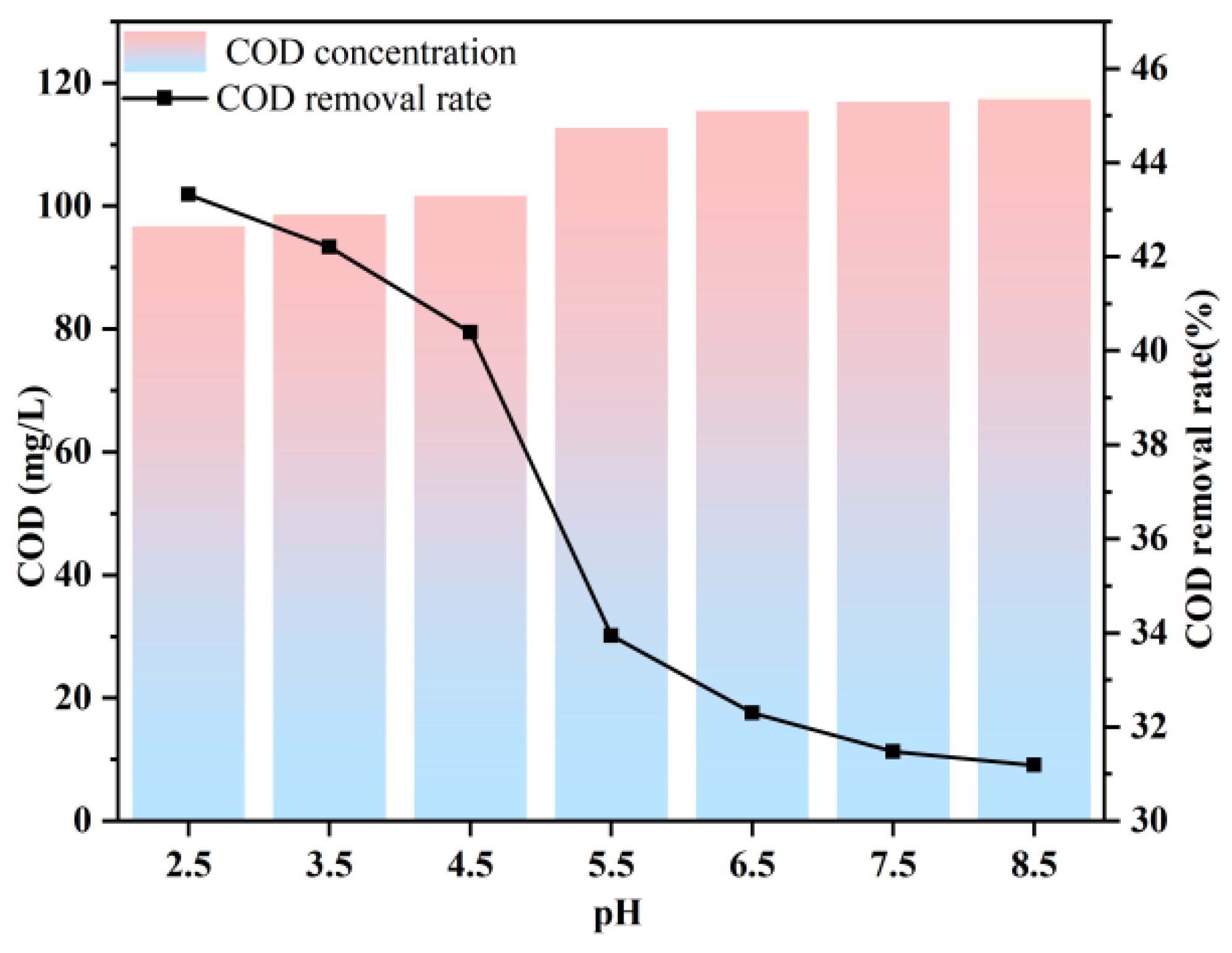
3.4. Impact of pH on COD Removal
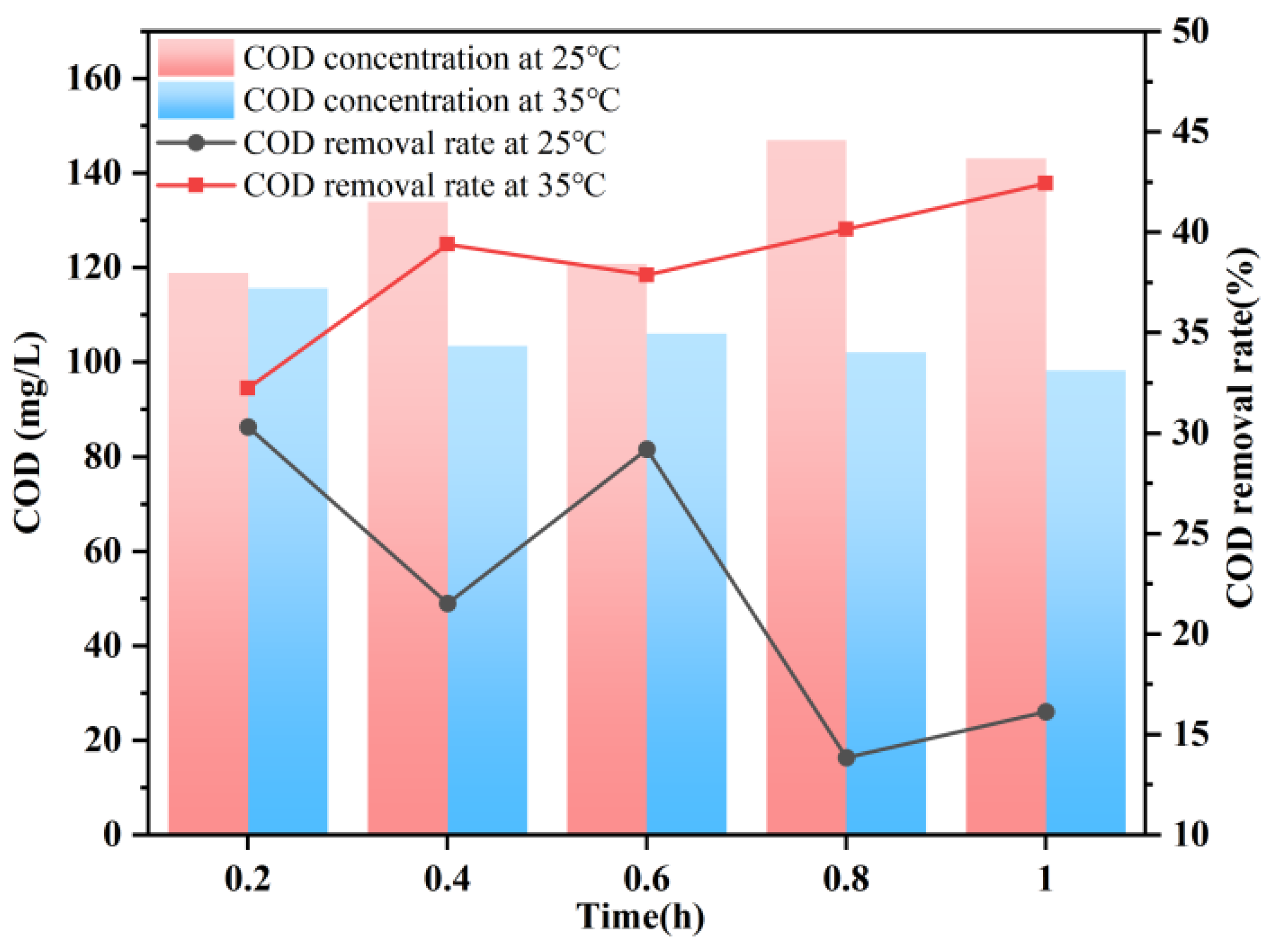
3.5. Impact of Reaction Temperature on COD Removal
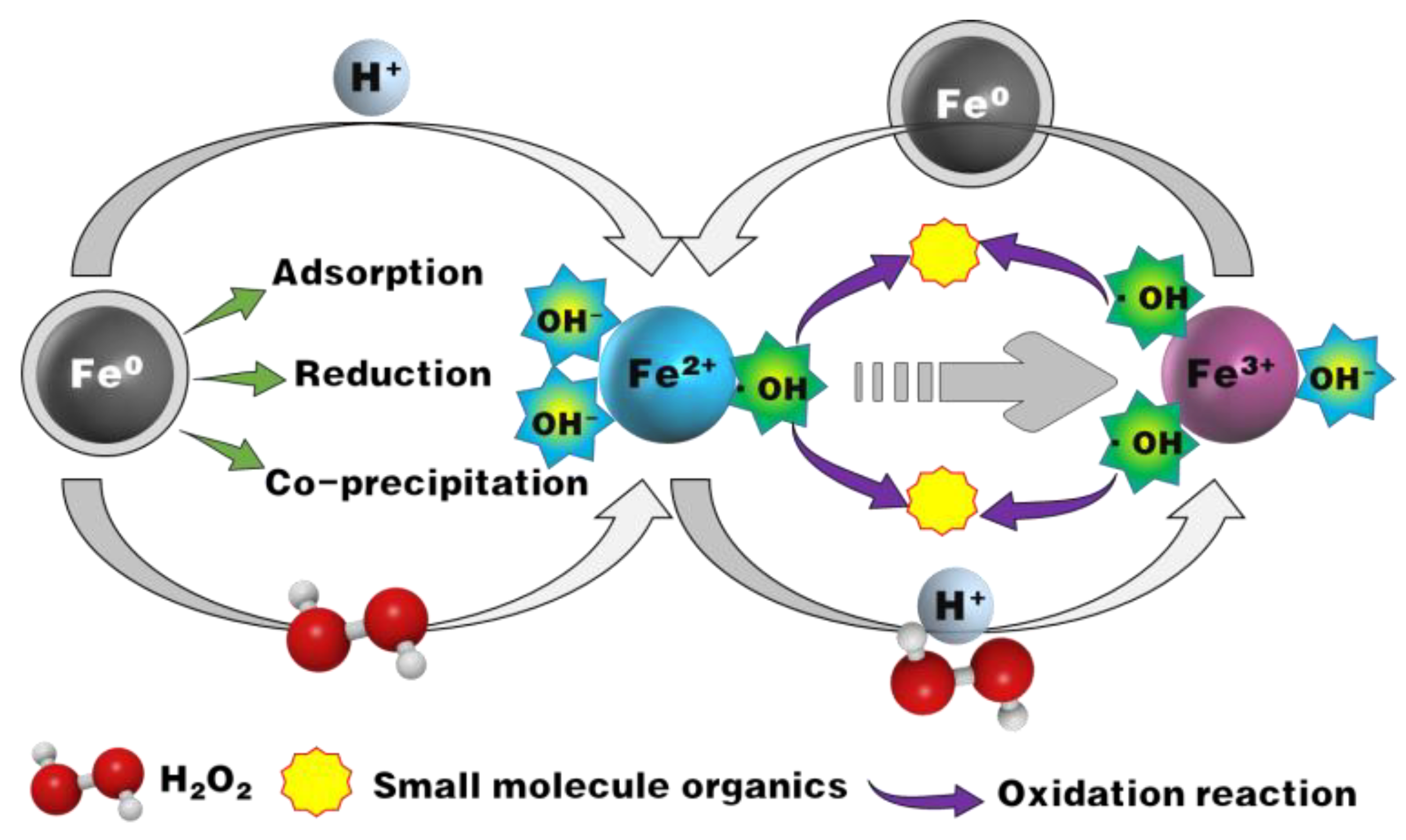
3.6. Mechnism of COD Removal by ZVI Fenton-like Process
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- de Gisi, S.; Molino, A.; Notarnicola, M. Enhancing the recovery of gypsum in limestone-based wet flue gas desulfurization with high energy ball milling process: A feasibility study. Process Saf. Environ. Prot. 2017, 109, 117–129. [Google Scholar] [CrossRef]
- Ma, S.; Chai, J.; Chen, G.; Yu, W.; Zhu, S. Research on desulfurization wastewater evaporation: Present and future perspectives. Renew. Sustain. Energy Rev. 2016, 58, 1143–1151. [Google Scholar] [CrossRef]
- Dong, Y.; Li, Y.; Zhang, L.; Cui, L.; Zhang, B.; Dong, Y. Novel Method of Ultralow SO2 Emission for CFB Boilers: Combination of Limestone Injection and Activated Carbon Adsorption. Energy Fuels 2017, 31, 11481–11488. [Google Scholar] [CrossRef]
- Gude, V.G. Energy and water autarky of wastewater treatment and power generation systems. Renew. Sustain. Energy Rev. 2015, 45, 52–68. [Google Scholar] [CrossRef]
- Han, X.; Yuan, T.; Zhang, D.; Dai, Y.; Liu, J.; Yan, J. Waste heat utilization from boiler exhaust gases for zero liquid discharge of desulphurization wastewater in coal-fired power plants: Thermodynamic and economic analysis. J. Clean. Prod. 2021, 308, 127328. [Google Scholar] [CrossRef]
- Sun, Z.; Chen, H.; Zhao, N.; Feng, Y.; Liu, F.; Cai, C.; Che, G.; Yang, L. Experimental research and engineering application on the treatment of desulfurization wastewater from coal-fired power plants by spray evaporation. J. Water Process Eng. 2021, 40, 101960. [Google Scholar] [CrossRef]
- Ma, S.; Chai, J.; Chen, G.; Wu, K.; Xiang, Y.; Wan, Z.; Zhang, J.; Zhu, H. Partitioning characteristic of chlorine ion in gas and solid phases in process of desulfurization wastewater evaporation: Model development and calculation. Environ. Sci. Pollut. Res. 2019, 26, 8257–8265. [Google Scholar] [CrossRef]
- Azam, K.; Raza, R.; Shezad, N.; Shabir, M.; Yang, W.; Ahmad, N.; Shafiq, I.; Akhter, P.; Razzaq, A.; Hussain, M. Development of recoverable magnetic mesoporous carbon adsorbent for removal of methyl blue and methyl orange from wastewater. J. Environ. Chem. Eng. 2020, 8, 104220. [Google Scholar] [CrossRef]
- Yan, J.; Yuan, W.; Liu, J.; Ye, W.; Lin, J.; Xie, J.; Huang, X.; Gao, S.; Xie, J.; Liu, S.; et al. An integrated process of chemical precipitation and sulfate reduction for treatment of flue gas desulphurization wastewater from coal-fired power plant. J. Clean. Prod. 2019, 228, 63–72. [Google Scholar] [CrossRef]
- Koralegedara, N.H.; Pinto, P.X.; Dionysiou, D.D.; Al-Abed, S.R. Recent advances in flue gas desulfurization gypsum processes and applications—A review. J. Environ. Manag. 2019, 251, 109572. [Google Scholar] [CrossRef]
- Han, X.; Zhang, D.; Yan, J.; Zhao, S.; Liu, J. Process development of flue gas desulphurization wastewater treatment in coal-fired power plants towards zero liquid discharge: Energetic, economic and environmental analyses. J. Clean. Prod. 2020, 261, 121144. [Google Scholar] [CrossRef]
- An, W.; Zhao, J.; Lu, J.; Han, Y.; Li, D. Zero-liquid discharge technologies for desulfurization wastewater: A review. J. Environ. Manag. 2022, 321, 115953. [Google Scholar] [CrossRef]
- Zhu, Y.; Fan, W.; Feng, W.; Wang, Y.; Liu, S.; Dong, Z.; Li, X. A critical review on metal complexes removal from water using methods based on Fenton-like reactions: Analysis and comparison of methods and mechanisms. J. Hazard. Mater. 2021, 414, 125517. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Li, J.; Huang, T.; Guan, X. The influences of iron characteristics, operating conditions and solution chemistry on contaminants removal by zero-valent iron: A review. Water Res. 2016, 100, 277–295. [Google Scholar] [CrossRef] [PubMed]
- Fu, F.; Xie, L.; Tang, B.; Wang, Q.; Jiang, S. Application of a novel strategy-Advanced Fenton-chemical precipitation to the treatment of strong stability chelated heavy metal containing wastewater. Chem. Eng. J. 2012, 189, 283–287. [Google Scholar] [CrossRef]
- Pliego, G.; Zazo, J.A.; Garcia-Munoz, P.; Munoz, M.; Casas, J.A.; Rodriguez, J.J. Trends in the Intensification of the Fenton Process for Wastewater Treatment: An Overview. Crit. Rev. Environ. Sci. Technol. 2015, 45, 2611–2692. [Google Scholar] [CrossRef]
- Peng, Y.; Xu, J.; Liu, T.; Mao, Y.-G. Controlled synthesis of one-dimensional BiOBr with exposed (110) facets and enhanced photocatalytic activity. CrystEngComm 2017, 19, 6473–6480. [Google Scholar] [CrossRef]
- Zhang, N.; Zhang, G.; Huang, T.; Chong, S.; Liu, Y. Fe3O4 and Fe3O4/Fe2+/Fe-0 catalyzed Fenton-like process for advanced treatment of pharmaceutical wastewater. Desalination Water Treat. 2017, 93, 100–108. [Google Scholar] [CrossRef]
- Quintana, G.O.; Fagnani, E.; Candello, F.P.; Guimaraes, J.R. The Dichromate Method versus the Photoelectrochemical Method: The Synergistic Influence of Turbidity and Chlorides on Chemical Oxygen Demand Analysis. J. Braz. Chem. Soc. 2018, 29, 490–498. [Google Scholar] [CrossRef]
- Guo, M.; Weng, X.; Wang, T.; Chen, Z. Biosynthesized iron-based nanoparticles used as a heterogeneous catalyst for the removal of 2,4-dichlorophenol. Sep. Purif. Technol. 2017, 175, 222–228. [Google Scholar] [CrossRef]
- Rezaei, F.; Vione, D. Effect of pH on Zero Valent Iron Performance in Heterogeneous Fenton and Fenton-like Processes: A Review. Molecules 2018, 23, 3127. [Google Scholar] [CrossRef]
- Ramos, P.B.; Vitale, P.; Barreto, G.P.; Aparicio, F.; Dublan, M.Á.; Eylera, G.N. Treatment of real non-biodegradable wastewater: Feasibility analysis of a zero-valent iron/H2O2 process. J. Environ. Chem. Eng. 2020, 8, 103954. [Google Scholar] [CrossRef]
- Galdames, A.; Ruiz-Rubio, L.; Orueta, M.; Sanchez-Arzalluz, M.; Luis Vilas-Vilela, J. Zero-Valent Iron Nanoparticles for Soil and Groundwater Remediation. Int. J. Environ. Res. Public Health 2020, 17, 5817. [Google Scholar] [CrossRef]
- Zhang, W.; Gao, H.; He, J.; Yang, P.; Wang, D.; Ma, T.; Xia, H.; Xu, X. Removal of norfloxacin using coupled synthesized nanoscale zero-valent iron (nZVI) with H2O2 system: Optimization of operating conditions and degradation pathway. Sep. Purif. Technol. 2017, 172, 158–167. [Google Scholar] [CrossRef]
- Chang, M.-C.; Shu, H.-Y.; Yu, H.-H. An integrated technique using zero-valent iron and UV/H2O2 sequential process for complete decolorization and mineralization of C.I. Acid Black 24 wastewater. J. Hazard. Mater. 2006, 138, 574–581. [Google Scholar] [CrossRef] [PubMed]
- Cheng, R.; Cheng, C.; Liu, G.-h.; Zheng, X.; Li, G.; Li, J. Removing pentachlorophenol from water using a nanoscale zero-valent iron/H2O2 system. Chemosphere 2015, 141, 138–143. [Google Scholar] [CrossRef] [PubMed]
- Ali, J.; Shahzad, A.; Wang, J.; Ifthikar, J.; Lei, W.; Aregay, G.G.; Chen, Z.; Chen, Z. Modulating the redox cycles of homogenous Fe-(III)/PMS system through constructing electron rich thiomolybdate centres in confined layered double hydroxides. Chem. Eng. J. 2021, 408, 127242. [Google Scholar] [CrossRef]
- Bae, S.; Hanna, K. Reactivity of Nanoscale Zero-Valent Iron in Unbuffered Systems: Effect of pH and Fe(II) Dissolution. Environ. Sci. Technol. 2015, 49, 10536–10543. [Google Scholar] [CrossRef]
- O’Carroll, D.; Sleep, B.; Krol, M.; Boparai, H.; Kocur, C. Nanoscale zero valent iron and bimetallic particles for contaminated site remediation. Adv. Water Resour. 2013, 51, 104–122. [Google Scholar] [CrossRef]
- Alowitz, M.J.; Scherer, M.M. Kinetics of nitrate, nitrite, and Cr(VI) reduction by iron metal. Environ. Sci. Technol. 2002, 36, 299–306. [Google Scholar] [CrossRef]
- Guan, X.; Jiang, X.; Qiao, J.; Zhou, G. Decomplexation and subsequent reductive removal of EDTA-chelated Cu-II by zero-valent iron coupled with a weak magnetic field: Performances and mechanisms. J. Hazard. Mater. 2015, 300, 688–694. [Google Scholar] [CrossRef] [PubMed]
- Jiang, X.; Qiao, J.; Lo, I.M.C.; Wang, L.; Guan, X.; Lu, Z.; Zhou, G.; Xu, C. Enhanced paramagnetic Cu2+ ions removal by coupling a weak magnetic field with zero valent iron. J. Hazard. Mater. 2015, 283, 880–887. [Google Scholar] [CrossRef] [PubMed]
- Bae, S.; Lee, W. Influence of Riboflavin on Nanoscale Zero-Valent Iron Reactivity during the Degradation of Carbon Tetrachloride. Environ. Sci. Technol. 2014, 48, 2368–2376. [Google Scholar] [CrossRef]
- Gan, L.; Li, B.; Guo, M.; Weng, X.; Wang, T.; Chen, Z. Mechanism for removing 2,4-dichlorophenol via adsorption and Fenton-like oxidation using iron-based nanoparticles. Chemosphere 2018, 206, 168–174. [Google Scholar] [CrossRef]
- Dulova, N.; Trapido, M.; Dulov, A. Catalytic degradation of picric acid by heterogeneous Fenton-based processes. Environ. Technol. 2011, 32, 439–446. [Google Scholar]
- Bogacki, J.; Marcinowski, P.; Zapalowska, E.; Maksymiec, J.; Naumczyk, J. Cosmetic wastewater treatment by the ZVI/H2O2 process. Environ. Technol. 2017, 38, 2589–2600. [Google Scholar] [CrossRef]
- Guan, X.; Sun, Y.; Qin, H.; Li, J.; Lo, I.M.C.; He, D.; Dong, H. The limitations of applying zero-valent iron technology in contaminants sequestration and the corresponding countermeasures: The development in zero-valent iron technology in the last two decades (1994–2014). Water Res. 2015, 75, 224–248. [Google Scholar] [CrossRef] [PubMed]
- Zhou, T.; Li, Y.; Ji, J.; Wong, F.-S.; Lu, X. Oxidation of 4-chlorophenol in a heterogeneous zero valent iron/H2O2 Fenton-like system: Kinetic, pathway and effect factors. Sep. Purif. Technol. 2008, 62, 551–558. [Google Scholar] [CrossRef]
- Gu, K.; Li, H.; Zhang, J.; Li, J. Performances and Interactions of Contaminants Removal from Water by Sulfidated Zerovalent Iron. Prog. Chem. 2021, 33, 1812–1822. [Google Scholar] [CrossRef]
- Yang, S.; Ren, T.; Zhang, Y.; Zheng, D.; Xin, J. ZVI/Oxidant Systems Applied in Water Environment and Their Electron Transfer Mechanisms. Prog. Chem. 2017, 29, 388–399. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, Z.; Zhou, W.; Liu, X.; Yang, X.; Yang, W.; Zheng, H. Study on Treatment Performance of Desulfurization Wastewater by Zero-Valent Iron Fenton-like Process. Separations 2023, 10, 451. https://doi.org/10.3390/separations10080451
Liu Z, Zhou W, Liu X, Yang X, Yang W, Zheng H. Study on Treatment Performance of Desulfurization Wastewater by Zero-Valent Iron Fenton-like Process. Separations. 2023; 10(8):451. https://doi.org/10.3390/separations10080451
Chicago/Turabian StyleLiu, Ziguo, Wei Zhou, Xianli Liu, Xuefen Yang, Wei Yang, and Han Zheng. 2023. "Study on Treatment Performance of Desulfurization Wastewater by Zero-Valent Iron Fenton-like Process" Separations 10, no. 8: 451. https://doi.org/10.3390/separations10080451
APA StyleLiu, Z., Zhou, W., Liu, X., Yang, X., Yang, W., & Zheng, H. (2023). Study on Treatment Performance of Desulfurization Wastewater by Zero-Valent Iron Fenton-like Process. Separations, 10(8), 451. https://doi.org/10.3390/separations10080451







