Abstract
Constructed wetlands (CWs) are a kind of green environmental protection technology, which are widely used in sewage treatment. Traditional CWs are faced with the problem of a low treatment effect of high-concentration sewage. In recent years, biochar, as a new type of adsorption material, has been used in CWs because of its advantages of large specific surface area, strong adsorption capacity, and wide material sources. This paper systematically summarized the characteristics of biochar and the preparation of biochar by studying the changes in microorganisms added to CWs and compared the effects of different treatment methods coupled with biochar on the treatment performance of CWs. The effects of biochar coupled with CWs on enzyme activity, functional genes, metabolites, and microbial communities were investigated. This review summarizes how different preparation methods affect the properties of biochar and how these biochar properties cause changes in the microorganisms added to CWs. It provides a new theoretical basis for the treatment of pollutants in CWs.
1. Introduction
Most developing countries face serious water pollution problems, especially in underdeveloped rural areas [1]. A large amount of nitrogen and phosphorus sewage is put into natural water bodies, which has a serious impact on people’s lives. Constructed wetlands (CWs), as a low-cost ecosystem, have the advantages of small area, simple operation, green and easy maintenance, and are widely used in sewage treatment [2]. CWs have a high removal rate of chemical oxygen demand (COD), biochemical oxygen demand (BOD), ammonia nitrogen (NH4+-N), nitrate nitrogen (NO3−-N), total nitrogen (TN), and total phosphorous (TP) [3]. However, CWs often face the problems of insufficient carbon sources, insufficient dissolved oxygen, and low treatment effects at low temperatures [1]. Therefore, some improvements are often carried out in CWs at present, such as the addition of carbon sources, electrochemical system coupling, intermittent aeration, and biochar enhancement.
Biochar has been widely used in pollutant treatment in CWs due to its large specific surface area, high porosity, and strong adsorption performance, and its porous property has a high ability to intercept ammonia nitrogen [4]. It also has a good removal effect on heavy metal ions (such as Cd2+, Cr2+, and Pb2+) [5]. Due to its high specific surface area and high porous structure, granular biochar has been found to have a high adsorption capacity for micropollutants compared with gravel and other substrates [6]. Traditional CW nitrogen removal is generally through nitrification and denitrification. Denitrification generally faces the problem of insufficient carbon sources. Biochar can provide a carbon source to promote its reaction, and biochar has a strong adsorption capacity to absorb NH4+-N [7]. Biological nitrification and denitrification are the main ways of nitrogen removal and are largely dependent on environmental factors. Nitrification is the conversion of NH4+-N into NO3−-N, which is strictly dependent on the dissolved oxygen (DO) concentration. Denitrification is a heterotrophic process in which microorganisms convert NO3−-N into nitrogen or nitrous oxide, which is considered to be an important process for nitrogen removal in CWs and is dependent on carbon sources [8]. Biochar can also increase the denitrification capacity of microorganisms by providing electrons and facilitating electron transfer [9]. The presence of biochar in a CW-coupled electrochemical system can reduce the consumption of external organic compounds and enhance the denitrification process. In addition, biochar can be modified by Fe3+ produced by an anode [10]. Biochar can also promote the growth of plants in CWs [11]. For example, biochar provides a large area for microbes to attach and form biofilms, which enhances the removal of biological contaminants. Biochar can absorb pollutants (nitrogen and phosphorus) and then slowly release them and other chemicals for plant growth [12]. Figure 1 shows the trend in the number of papers published on constructed wetlands in the last ten years. Biochar can also sorb non-biodegradable and toxic organic contaminants present in wastewater and contribute to an improved treatment efficiency and lower toxicity of the wastewater matrix to the microbial community.
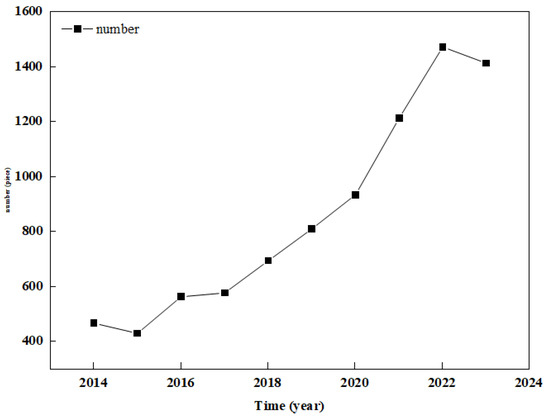
Figure 1.
The trend in the number of papers published on constructed wetlands in the last ten years.
This paper first reviews the coupling of biochar and four ways to enhance the removal of nitrogen and heavy metals. While biochar enhances denitrification, it produces some greenhouse gases and some functional microorganisms, such as Bacillus and Lactococcus. The mechanism underlying microbial community change is still unclear and needs further study, which can then provide a scientific basis to improve performance.
2. Improving the Performance of Constructed Wetlands Using Biochar
Biochar is a carbon-rich material. The raw materials of biochar production are waste biological materials, compost, manure, and sludge. The raw material for biochar can also come from plants grown in CWs. These waste and reusable raw materials are of great significance for the mitigation of environmental pollution [13]. Biochar is a kind of solid material rich in carbon obtained with the pyrolysis of biomass under the conditions of high temperature and oxygen deficiency [14].
The adsorption capacity of biochar made of different materials is also different [15]. The hydraulic retention time affects the adsorption capacity of biochar [16]. Under different hydraulic conditions, coir shell and shell with the highest biochar content in the matrix also have better removal effects on pollutants and stronger wastewater purification capacity [17]. Biochar produced with different methods also has different abilities to fix metals [18]. The physical and chemical properties of biochar are mainly determined by the temperature of biomass materials and pyrolysis. Temperature determines the aromatization of biochar, the number of surface functional groups, and the size of the surface area [19]. With an increase in temperature, the number of surface functional groups of biochar decreases. The surface oxygen-containing functional groups include a hydroxyl group, a carboxyl group, and a phenol group [14].
Biochar in CWs has different removal processes for different pollutants. The adsorption of heavy metal ions by biochar is a physical process, and the adsorption capacity depends on the pore structure and surface chemical properties of biochar. It includes specific surface area, pore size distribution, and the types and quantities of surface functional groups. The highly developed pore structure enhances the adsorption capacity by increasing the reaction contact area, and the functional groups absorb metal ions through chemical interactions [20]. The adsorption of ammonium ions by biochar is mainly controlled by cation exchange, surface complexation with oxygen-containing functional groups, and the formation of ammonium magnesium phosphate compounds [21]. Under the conditions of intermolecular hydrogen bonds and direct electrostatic attraction, COD in CWs added with biochar has a good removal effect [22]. At the same time, due to the action of π-π, both greenhouse gases and total nitrogen have a good removal effect [23]. Strong intermolecular π bonds, hydrogen bonds, and electrostatic attraction have been proven to be the main reasons for the high adsorption of pollutants by biochar [12]. Some studies have also found that the higher the concentration of biochar, the better the absorption effect of NH4+-N [24]. CWs produce greenhouse gases during operation [25]. The addition of biochar can reduce greenhouse gas emissions due to its adsorption [23]. It also has a good removal effect on phosphorus [26]. Dissolved organic matter (DOM) will be produced in the process of biochar preparation at high temperatures, and the ability of biochar to release DOM is closely related to the efficiency of nitrogen removal [27].
2.1. Performance Enhancement with Biochar and Immobilized Microorganisms
Microbial immobilization is a process that restricts the movement of microbial cells within a certain range, and the enzyme activity and stability of microorganisms can be improved with immobilization [28].
Studies have shown that the combination of immobilized enzymes and biochar can greatly improve the efficiency of micropollutant removal in CWs [29]. The porous structure of biochar can provide carriers for these immobilized enzymes, and the immobilized enzymes combined with biochar can greatly improve the removal efficiency of micropollutants [30]. For example, the fixation of arbuscular mycorrhizal fungi with biochar promoted the removal of ibuprofen and diclofenac in CWs [31].
Combining immobilized denitrifying bacteria with a carbon source while maintaining the activity of the immobilized denitrifying bacteria can enhance the denitrification process [32]. The use of external carbon sources alone or immobilization of denitrifying bacteria can improve the removal of organic matter and nutrients in a CW, but there are few studies on the combination of carbon sources and denitrifying fixation. Yu’s study showed that denitrifying bacteria can maintain their biological activity and stability when the particles made from the joint immobilization of rice husk and Pseudomonas fluorescence are put into a horizontal subsurface flow constructed wetland (HSFCW) [33]. Stable proliferation improves the ratio of carbon to nitrogen in CW wastewater and alleviates the problem of insufficient carbon sources in CWs. Meanwhile, COD, NH4+-N, and TN all have high removal rates [34].
When biochar is combined with bacteria, biochar can enhance the microbial interpretation of pollutants, and fixing denitrifying bacteria on biochar can significantly improve the removal efficiency of nitrate, showing a synergistic effect in the binding process. However, the properties of biochar, such as porosity, surface properties, and microbial stability, all have certain effects on the binding of biochar and bacteria [35]. The release of nutrients, organic carbon, and organic nitrogen compounds from biochar can also accelerate the growth of bacteria on biochar [9]. The adsorption and immobilization effects of sludge biochar on low-temperature mixed bacteria were studied. It was found that the addition of sludge biochar increased the specific surface area of immobilized particles and protected microorganisms from the interference of hydraulic erosion [14]. The combination of biochar and microbial compound agents has a better treatment effect on heavy metals such as arsenic in CW [24]. The combination of anaerobic ammox bacteria and biochar also has a good treatment effect on landfill leachate [36].
2.2. Combined Enhancement with Biochar and the Oxygen Supply
Nitrification and denitrification are important methods for nitrogen removal in CWs. In order to better remove TN, alternate aerobic and anoxic environments need to be provided for denitrifying bacteria. A low concentration of DO will inhibit the conversion of ammonia nitrogen by nitrifying bacteria into nitric nitrogen [4]. Traditional CW nitrogen removal generally removes nitrogen in water with nitrification and denitrification under the action of nitrifying bacteria and denitrifying bacteria. Nitrifying bacteria are aerobic bacteria, and denitrifying bacteria are anaerobic bacteria. Nitrification is essential for the removal of TN, and efficient nitrification usually requires sufficient dissolved oxygen [37]. Therefore, controlling the oxygen concentration is a crucial factor for efficient nitrogen removal in CWs. Two common ways to control the oxygen concentration are intermittent aeration and tidal flow. By combining biochar with intermittent aeration and tidal flow phase, it is found that both methods can promote the removal of pollutants. But the latter is more practical and effective and can reduce greenhouse gas emissions [1].
Intermittent aeration can provide sufficient DO to promote nitrification and denitrification, and it can also provide an anaerobic environment in the interval of aeration, with lower energy consumption and cost. Biochar can provide a carbon source for the reaction while also reducing greenhouse gas production [38]. Biochar combined with intermittent aeration made of different materials also has different efficiency against pollutants. Biochar made from cow dung with taro as the matrix has a high treatment effect on COD, NO3−-N, NH4+-N, SO42−, and PO43− when it was put into a CW with intermittent aeration. In particular, the average removal rate of total coliform reached 97% [39]. Aeration can significantly reduce DOM (humus, protein, tryptophan) in CWs [40]. High DO concentrations promote the growth of heterotrophic bacteria and increase the thickness of the substrate surface biofilm, thereby increasing the risk of substrate clogging [41].
Different C/N ratios also have different impacts on nitrogen removal in CWs [42]. A higher C/N ratio will have a negative impact on NH4+-N removal in CWs, and a higher C/N ratio will produce higher oxygen consumption, inhibit the growth of autotrophic bacteria, and lead to a decrease in nitrification efficiency [43]. Intermittent aeration technology can better provide an alternating aerobic environment and anaerobic environment for nitrification and denitrification reactions, and biochar combined intermittent aeration has a good effect on the treatment of wastewater with a low carbon–nitrogen ratio. The combined operation of biochar and intermittent aeration was applied to subsurface flow-constructed wetlands (SFCWs) with different nitrogen ratios. It was found that when C/N was less than 7, there was a significant difference in the TN removal effect between adding biochar and not adding biochar because porous biochar could provide an anaerobic environment, which was conducive to the biofilm formation of denitrifying bacteria. As the C/N ratio increased from 10, there was no significant difference in the removal of TN with or without biochar because the carbon source was sufficient. In these CWs with biochar, organic matter is rapidly reduced by hydrogen bonding, hydrophobic attraction, and electrostatic attraction [7]. A CW with biochar combined intermittent aeration under low influent intensity has no significant improvement in the COD removal rate compared with a CW with simple intermittent aeration. Under the condition of sufficient oxygen, although biochar has adsorbability, the adsorption rate of biochar for COD is much lower than the degradation rate of aerobic microorganisms [38]. Table 1 lists the effects of different biochar combined intermittent aeration on COD and nitrogen. However, at high influent intensity, the removal efficiency of the former is more obvious, possibly because the existence of the π-π skeleton on the surface of biochar leads to a high COD removal rate and because organic molecules can be easily adsorbed by electrostatic attraction and intermolecular hydrogen bonds [34]. At the same time, the nitrogen removal efficiency of both intermittent aeration and tidal flow was found to decrease with an increase in influent intensity, indicating that at low influent intensity, weak denitrification would limit nitrogen removal, while high influent intensity would lead to low nitrification [44].

Table 1.
Effect of different biochar combined intermittent aerations on COD and nitrogen.
2.3. Performance Enhancement with Biochar Electrochemical Coupling
Electrochemical-coupled biochar CW is a feasible and ecologically sustainable technology for treating the tail water of wastewater treatment plants with a low carbon-to-nitrogen ratio. The electrochemical and biochar coupling system has a significant nitrogen removal effect, mainly because, in the autotrophic denitrification process, H2 and Fe2+ provided by the cathode and anode are used as electron donors, and the addition of biochar as substrate can improve the activity, diversity, and abundance of microorganisms. The electrochemical system can reduce the redox potential and DO in a CW, and the ferrous ions produced can promote the circulation of iron and nitrogen. At the same time, the iron ions in the system can also combine with biochar.
The CW microbial electrochemical system includes a CW microbial fuel cell and a CW microbial electrolytic battery. The CW and microbial electrolytic cells mainly rely on applied voltage to promote the transfer and utilization of electrons to achieve an efficient redox process [46]. The application of an electrochemical system in a CW can reduce the oxidation–reduction potential (ORP) value and the DO value, provide a reduction environment, and improve nitrogen removal efficiency [47].
Microbial electrochemical technology in wastewater treatment requires a large number of conductive materials to promote extracellular electron transfer and biodegradation. The biochar surface has a large number of electroactive surface oxygen-containing functional groups, which can reversibly exchange electrons [48]. As a conductive substrate, biochar can provide enough specific surface area for the growth of electroactive microorganisms and promote electron transfer. The efficiency of denitrification can be improved by promoting the activity of denitrification enzyme [10].
In addition to being an electron donor, iron can also maintain reducing hypoxia conditions for denitrification, and dissolved iron ions can promote bacterial growth and precipitation of phosphate groups. Based on redox theory, negative electrode materials or microbial fuel cells are introduced into the CW system to improve the electron transfer efficiency [41].
Biochar and electrochemical coupling, while supplementing organic matter as an external carbon source, can comprehensively improve nitrogen removal efficiency with autotrophic and heterogeneous nitrogen removal. Functional groups as electron donors provide organic carbon sources for anaerobic denitrification. Fe2+ and H2 produced by the anode and cathode can be used as electron donors to enhance the removal of nitrite. At the same time, iron can be combined with biochar to improve the adsorption capacity of biochar. Therefore, the absorption capacity of NO3−-N is improved [10]. A microbial fuel cell consists of an anode, a cathode, and an external resistor through which electrons are transferred to the anode. The anodes usually undergo oxidation by microorganisms, converting organic pollutants into free electrons, protons, and carbon dioxide [49]. Modification of the anode surface can enhance the enrichment of electroactivated bacteria. Zeng Li et al. pointed out that biosynthesized FeS/BC hybrid particles can enhance the activity of electroactivated bacteria [50]. The removal effect of ammonia nitrogen was better when adding biochar to CWs and microbial batteries at low temperatures. The study showed that electricity itself contributes to the oxidation of NH4+, and NH4+ can be removed directly and indirectly with non-biological electrochemical oxidation. Using the fuel cell, supplementary electrons can be generated in degradation kinetics, and the study found that the combination of tidal flow and a microbial fuel cell has a significantly better removal effect on ammonia nitrogen than a single tidal flow constructed wetland [51]. Figure 2 shows the mechanism of electrochemically coupled biochar.
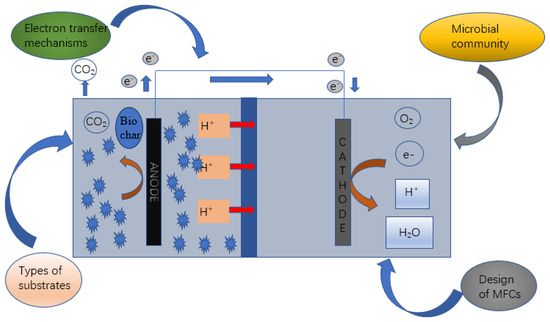
Figure 2.
Mechanism diagram of electrochemically coupled biochar for wastewater treatment in CWs.
2.4. Performance Enhancement with Biochar Modification
The sorption ability of biochar could be further raised using diverse modification methods. Among them, the microbial N removal process (nitrification, denitrification, and anammox) could be facilitated with Fe in different chemical forms and valences. Fe-supported biochar could effectively promote microbial denitrification in various systems to improve NO3−-N removal [52]. It was demonstrated that Fe-modified biochar could enhance microbial quantity and activity and then influence the N removal process. Thus, biochar modified with Fe was considered to be the most promising amendment in CWs to enhance N removal. Under low-temperature conditions, the performance of CWs to treat pollutants will be greatly reduced, and the addition of biochar alone cannot achieve a good effect. A large number of studies have found that modified biochar has a good effect on the treatment of pollutants in CWs at low temperatures [53]. The adsorption capacity of biochar for nitrate is limited, and modified methods are usually used to improve the adsorption capacity of biochar in CWs [54]. Biochar can be physically, chemically, or biologically modified to functionalize or activate it, mainly in terms of surface area, hydrophobicity, pore volume, pore number, and the number of functional groups [13]. Modified biochar can increase the adsorption capacity of nitrogen, phosphorus, and other micro-pollutants in CWs [28]. The commonly used modification methods are metal modification, hydrochloric acid modification, and so on. Metal modification methods generally include magnesium biochar adsorption of phosphate with functional group action and complexation, and biochar can also be modified with Fe2+, Fe3+, Fe2O3, zero-valent iron, etc. Iron-modified biochar can increase the positive charge on its surface and reduce the negative charge [55]. The surface of biochar modified with iron contains a large number of active functional groups of iron oxide, which enhances the adsorption capacity of pollutants [56].
The adsorption capacity of biochar was greatly improved with the heat activation of biochar with concentrated hydrochloric acid. There were hydroxyl, phenolic hydroxyl, and other oxygen-containing groups in the modified biochar. The existence of these groups led to different surface hydrophilicity and surface acidity of the biochar, resulting in different surface charges [34]. The surface negative charge of biochar modified with concentrated hydrochloric acid is greatly reduced, and the positive charge is increased, which enhances the adsorption of negatively charged nitrate ions [42]. At the same time, in the process of heat activation, a large amount of cellulose decomposes, the surface sediment is reduced, and micro-pores are formed on the surface of biochar [38]. In the process of high-temperature activation, the surface area of biochar is increased, the surface adsorption point of biochar is increased, and the adsorption of nitrate by biochar is increased [57]. Substrate modification and bacterial modification were applied to a vertical flow constructed wetland (VFCW) to treat NH4+-N, NO3−-N, TN, TP, and COD. At the same time, the adsorption capacity of the matrix was greatly improved after the improved VFCW [58]. Humic acid (HA) doped into activated carbon of biomass waste and modified biochar with phosphoric acid activation also had a good removal effect on heavy metals in water [59]. The surface function and adsorption capacity of activated carbon can be improved using phosphoric acid to activate biochar and in situ modification [20]. The coupled CW with chemical reduction and denitrification of biochar and iron microorganisms has a good effect on nitrate removal. When biochar was added to a CW, the quantity and activity of microorganisms were further increased [22]. During the modification process, iron will react with the surface of biochar to form a large number of particles, resulting in a rough surface on the biochar and improved exchange capacity. At the same time, iron may block the pore structure of biochar and affect the adhesion of microorganisms, but large particles will form a thin layer of iron oxide on the surface of biochar, strengthening the adsorption and fixation of anions [60]. Hydrochloric acid can reduce the negative charge on the surface of biochar, increase the positive charge, and improve the adsorption of anionic nitrate by enhancing the electrostatic attraction [55]. Iron-modified biochar also has a strong capacity for the nitrification of NH4+-N and microorganisms. At the same time, due to its physical and chemical properties, Fe-B can trap nitrite nitrogen well and improve its processing capacity [22]. However, the iron ions produced by the modified biochar prepared with the pyrolysis of ferric chloride will affect the water environment, and the excessive iron content will lead to the proliferation of iron bacteria, resulting in pipeline blockage [57]. Modified biochar can also improve the quality of soil in CWs, promote the absorption of pollutants by CW plants, and promote the growth of wetland organisms [61]. The DOM released from porous biochar provides a good living environment and carbon source for microorganisms [62]. Substrate adsorption and microbial degradation are the main mechanisms for pollutant removal in CWs, and the removal effect of TN in CWs is better with matrix modification using biochar in the aerobic zone and microbial modification using denitrifying bacteria in the saturated zone [63]. The addition of amorphous Fe(OH)3 to a biochar-modified system can provide a stronger performance than the wood chip system, reduce SO42− production, and increase the removal rate of nitrate nitrogen [64]. Figure 3 shows the correlation mechanism of iron-modified biochar on CWs.
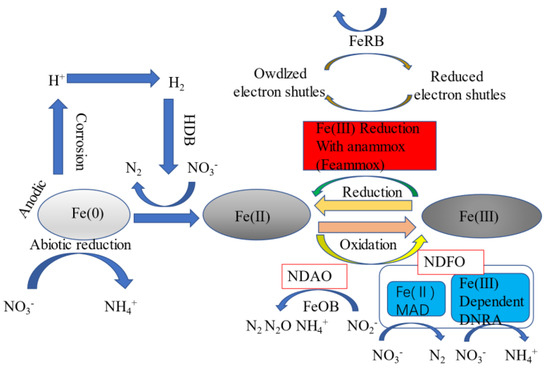
Figure 3.
Mechanism diagram of nitrogen-containing wastewater treatment using iron-modified biochar in CWs.
3. Effects of Biochar on Microbial Communities in Constructed Wetlands
3.1. Effects of Biochar on the Composition and Structure of EPSs
Extracellular polymers (EPSs) are macromolecular polymers secreted or released by microbial cells (bacteria, archaea, and micro-eukaryotes), and the secretion of EPS is considered to be a basic microbial adaptation [8]. The substances released by biochar are mainly tryptophan-like substances, and the DOM released by biochar immobilizes heavy metals in water [55].
The composition and physicochemical properties of extracellular polymers contribute to the maintenance of biological structure and the treatment of wastewater [42]. The high adsorbability of EPSs can increase the removal rate of ammonia nitrogen, nitrite, and nitrate [56]. The addition of biochar significantly reduced the total content of EPSs, resulting in a change in EPS composition [57]. At the same time, the smaller the matrix particle size, the more EPS production because the smaller the matrix particle size, the larger the specific surface area and the more adsorption sites provided [42].
Biochar addition can significantly change the composition, functional groups, and molecular weight distribution of EPSs, which may indirectly affect the treatment effect of CWs [8]. Intermittent aeration results in EPS reduction [58].
3.2. Effect of Biochar on Enzyme Activity
Substrate enzymes in CWs are catalysts with biological functions, which play an important role in the conversion of nitrogen and organic matter in wastewater [59]. Common substrate enzymes include ammonia nitrogen oxygenase (AMO), nitrate reductase (NAR), dehydrogenase (DHA), and phosphatase (PST), among which nitrate reductase, a key enzyme in the first step of denitrification, shows different activities in different artificial CWs. With an increase in biochar content, the activity of nitrate reductase continues to increase. But when the biochar content is too high, it drops slightly [2].
Ferric is a necessary factor in the synthesis of dehydrogenated coenzyme, nitrite reductase, and nitrate reductase. Fe-modified biochar is beneficial for the enrichment of reducing agents and the enhancement of N-cycle enzyme activity, thus improving the removal of nitrate nitrogen in CWs. In the process of bacterial binding with biochar, the electrons generated by metabolic processes are directly transferred to nitric reductase through biochar [9]. The use of modified biochar to treat domestic sewage with different carbon-to-nitrogen ratios can reduce N2, mainly because modified biochar can promote the nitrification and denitrification processes, support more denitrifying bacteria containing nosZ, and promote the activity of N2O reductase in denitrification [13]. Denitrifying bacteria containing the nosZ enzyme will increase after the addition of biochar in CWs, and N2O production will decrease [54].
3.3. Effects of Biochar on Functional Genes
Denitrifying bacteria are a large group, and nitrite reductase genes (nirS and nirk) and nitrous oxide reductase genes (norZ) are commonly used as functional genes to study the denitrification process [60]. Figure 4 is a genetic map of nitrogen removal. The combination of biochar and bacteria showed that the expression of the narG gene was significantly increased. Biochar is an adsorbent of nitrate, and its high porosity and large specific surface area provide conditions for the formation of denitrifying bacteria microbial biofilms, which are mainly reflected in an increase in the nirS gene and the nirK gene abundance. During the operation of CWs, the greenhouse gas CH4 is produced, and the production of CH4 is usually related to the abundance of mcrA genes. The abundance of nirK, nirS, and nosZ genes in modified biochar was found to be significantly higher than that in unmodified biochar. At the same time, the low emission of N2O also confirmed that biochar can induce norZ gene transcription [61]. At a high C/N ratio, the abundance of denitrification functional genes increases significantly [62]. The acidic gene group content of biochar modified with humic acid is higher than that of modified biochar because the acidic group binds to the carbon surface and improves its tensile strength in the π-π interaction during the preparation of active biochar [63].
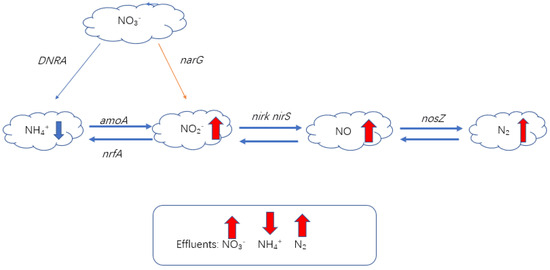
Figure 4.
Gene map of nitrogen removal from nitrogen-containing wastewater treated with CWs coupled with biochar.
The denitrification process is carried out mainly by heterotrophic denitrification bacteria (HDB) and autotrophic denitrification bacteria (ADB), by which nitrite or nitrate could be transformed into nitrogen gases ultimately. The abundance of relevant functional genes can be determined with the quantitative polymerase chain reaction. Nitrite reductase genes (nirS and nirK) and nitrous oxide reductase genes (norZ) are used to study denitrification processes [42], and the czcA gene is associated with cadmium removal. Zhang et al. found that the combined use of zeolite and iron carbon-based had a high removal rate of Cd, and the czcA gene was significantly increased after the addition of zeolite and iron carbon-based in CWs [41]. Bimetallic Fe-Cu/polyvinylpyrrolidone-modified biochar can increase the abundance of denitrification functional genes in wetlands so as to improve the denitrification capacity of wetlands and convert more nitrate into N2 [52]. The addition of FeO can reduce NO3−-N by providing electrons. An increase in the denitrification function genes narG, nirK, and nirS confirmed that the addition of iron and copper can increase the electrons for denitrification and the removal rate of nitrate nitrogen [64].
3.4. Effects of Biochar on Microbial Alpha Diversity
When biochar was added, the diversity and abundance of microbial communities decreased, but functional bacteria associated with nitrogen and phosphorus removal increased [63]. In CWs, the dominant bacteria are divided into three phyla, namely, Proteobacteria, Saccharomycetes, and Gonorrhoea. Proteobacteria are the dominant bacteria species in all CW systems. The abundant Geobacter genus on biochar in Proteobacteria can degrade aromatics released by biochar and reduce the production of CH4 [1]. The enrichment degree of functional bacteria will be improved after the addition of composite biochar made from sewage sludge, food waste, and straw, such as Bacillus and Lactococcus genera [64]. The phyla related to nitrogen removal changed significantly after the addition of biochar, such as Proteobacteria, Actinobacteria, and Chloroplecta [65].
After the addition of biochar, the abundance of denitrifying bacteria increased significantly. Microbial diversity and abundance in CWs with biochar added have no effect on various C/N ratios [22]. In different CWs, the evolution of microbial communities showed that Actinomycetes, Proteobacteria, and Bacteroidetes played an important role in the elimination of antibiotic resistance [40]. The addition of biochar usually can increase the abundance of functional microorganisms related to organic matter degradation and nitrogen removal in the treatment of CWs, such as Dow, Dechloromonas, Thiobacillus, Hydrophilia, Pseudomonas, Nitrospira, and Nitrosomonas, etc., and enhance the organic matter removal and nitrogen removal capacity of CWs by increasing the abundance of these bacteria [13]. After biochar was added to SFCWs, the microbial community showed significant changes, and the relative abundance of Dow, Candida Albicans, Dechloromonas, Bacillus Desulphuricum, Chlorococcus, and Thiobacillus all increased [8].
The diversity index of the microbial community was also affected by different biochar supplemental levels. The relative abundance of nitrosomonas and nitrospirochetes increased after the addition of biochar in a CW, and the diversity index of the microbial community increased from 7.54 to 7.81 when the biochar supplemental level was 20.0% [12]. At the same time, the Shannon index and Simpson index of CWs with added biochar are higher than those without added biochar, and the chao and Ace indexes are almost higher than those without added biochar [43].
When coupled electrochemically to biochar, biochar can increase microbial diversity, while cellulose has the opposite effect. In an electrochemically coupled biochar CW, the number of Proteobacteria and Firmicutes will increase, and the abundance of Spirillum nitrate will also increase [10]. In a CW and microbial electrolytic cell, electricity enhanced the denitrification of the cathode, and the abundance of Bacillus, Methylperoxide, and Aeromonas Hydrophila was higher, while the abundance of Thiobacillus and Pseudomonas was also higher [66]. The number of Proteobacteria and Firmicutes was increased and the number of bacteroidetes and acidobacteria was decreased. Meanwhile, the abundance of Spirillum nitrate was also increased after the addition of biochar. Denitrifying bacteria are the main heterogeneous denitrifying bacteria in the CW system [10].
DO is a key determinant of microbial community composition, as it can influence the relative abundance of aerobic and anaerobic groups as well as the overall diversity of microorganisms [66]. In a biochar CW with aeration, the proportion of nitrifying bacteria and denitrifying bacteria increased significantly, tidal flow promoted the growth of bacteria, and intermittent aeration reduced the number of bacteria [1]. The combined use of intermittent aeration and biochar increased the microbial abundance of CWs but also decreased the microbial diversity. If the number of ammonia-oxidizing bacteria and nitrite-oxidizing bacteria increased significantly under intermittent aeration, the abundance of actinomyces in CWs would increase [42]. The relative abundance of CD-tolerant bacteria (Bacteroidetes) and denitrifying bacteria (Proteobacteria and Bacteroidetes) increased significantly after the addition of iron carbon-based groups to CWs based on zeolite [51]. Zhang et al. explored the effects of the amount of biochar added on the treatment performance, enzyme activity, and microbial community of domestic sewage with a low carbon-to-nitrogen ratio treated with aerated CWs and found that in their system, the phylum-level dominant microorganisms were Proteobacteria, Discobacteria, and Phytoplankton [2]. The pathway of CH4 production was changed by increasing the abundance of geobacterium in CWs [43].
The addition of bimetallic-modified biochar also increased the abundance of proteus associated with nitrogen removal. The abundance of iron-oxidizing bacteria and iron-reducing bacteria was increased when modified biochar was added to a CW [67]. After iron modification, the abundance of Proteobacteria and Methanobacteria was increased under alternating aerobic and anoxic conditions [56].
3.5. Effects of Biochar on Microbial Community Structure
The type of substrate in CWs can affect the establishment and growth of the microbial community [31]. The mutual transformation of microorganisms in CWs is crucial for the operation of CWs and the removal of pollutants because microbial community composition is closely related to wastewater treatment performance, functional stability, and greenhouse gas emissions. Therefore, the analysis of microbial community structure is conducive to improving the design and operation of CWs [1]. Biochar made of different materials has different effects on the changes in microbial communities [68]. The coupling between nitrogen-converting microbes usually results in higher energy and material conversion efficiency [47].
When biochar was added to a CW, different bacterial community structures were generated in phyla, classes, families, and genera [42]. The addition of biochar will change the microbial community structure of CWs, and the dissolved organic matter released by biochar can enrich denitrifying bacteria [42]. Researchers explored the microbial communities of CWs by studying phyla, classes, and genera and found that the phyla were mainly composed of Proteobacteria, Firmicutes, and Bacteroides. Proteobacteria is an electrochemically active phylum in CWs, which plays a certain role in the removal of nitrogen, phosphorus, and organic matter in CWs. At the group level, the dominant groups included α-Proteobacteria, γ-Proteobacteria, Clostridium, and Bacteroides, among which bacteroides were more abundant in iron-rich regions. At the same time, biochar, as a conductive material, can enhance the electron transfer between methanogens and geobacteriaceae and promote the production of CH4 [43]. Complex biochar will also affect the CW community. Studies have found that functional bacteria such as Bacillus and Lactococcus will be enriched when biochar made from sewage sludge, food waste, and straw co-fermentation products is added to CWs [54]. The metabolic functions of wetland substrate bacteria mainly focus on chemical heterotrophic degradation, nitrate reduction, nitrite reduction, and aromatic hydrocarbon degradation [49]. The surface charge of biochar can promote the binding of microbial cells and wastewater compounds and promote the growth of microorganisms in the voids [50].
Limited oxygen in CWs can inhibit the activity of heterotrophic microbial communities, which are essential for COD removal in CWs [69]. Therefore, the concentration of DO is one of the key factors affecting microbial community structure, and the combination of the two oxygen supply modes and biochar can affect microbial community structure and enhance important microbial metabolism. Tidal flow creates alternating wet and dry conditions during operation and has a greater impact on microbial communities than intermittent aeration [1]. The combined use of intermittent aeration and microorganisms can affect the community structure of microorganisms [48].
The voids on the surface of biochar can provide a suitable environment for the propagation of microorganisms, increase the removal rate of ammonia nitrogen, and increase the redox potential to enhance nitrification [42]. The mutual transformation of microorganisms plays an important role in the removal of pollutants in CWs, and the composition of the microbial community plays an important role in the wastewater treatment performance and functional stability of CWs. The porous structure of biochar promotes the growth of plants and the attachment and reproduction of microorganisms in wetlands by fixing nutrients and trace elements [1]. Denitrification intensity is closely related to microbial activity. At the same time, when modified biochar is added to a CW, the microbial community will change, and iron-related bacteria will replace denitrifying bacteria [70].
4. Conclusions
This paper reviews the research progress on microbial changes in CWs after biochar addition. The source and preparation method of biochar will affect the treatment performance of biochar. When biochar is combined with different treatment technologies, the removal efficiency of ammonia nitrogen, total nitrogen, and COD will increase, the production of greenhouse gases in CWs will decrease, and the change in microbial community structure will also have a significant impact. The combined use of biochar and intermittent aeration has the highest pollutant removal efficiency, and the control of oxygen concentration is the key factor in achieving efficient nitrogen removal in CWs. The combination of biochar and these treatment techniques increases the diversity of nitrogen-removing microorganisms in the microbial community.
The combined use of each treatment technology and biochar has different effects on plant growth in CWs, and further studies on the removal of pollutants in CWs and the changes in the number of community microorganisms are needed. At the same time, different treatment technologies combined with biochar will also affect the growth of CW plants. Therefore, the next step will be to explore and analyze the leaf growth, plant height, and fresh weight of wetland plants in biochar CWs. The path of removing heavy metals and toxic pollutants from water with modified biochar has not been fully studied, which is also a research area that needs to be improved in the future. At the same time, CWs face underground treatment effects in the winter, which is easy to block. How to ensure a high treatment effect at low temperatures in future research is also a problem worth discussing.
Author Contributions
J.H., writing—original draft preparation; H.C. and J.C.; resources; G.Y.; supervision; S.G.; validation; J.L. and D.Z.; formal analysis. All authors have read and agreed to the published version of the manuscript.
Funding
This research was funded by the Hunan Provincial Natural Science Foundation of China (No. 2021JJ30728), the Scientific Research Projects of Ecology and Environment Department of Hunan (No. HBKT-2021012), the Water Conservancy Science and Technology Project of Hunan Province (No. XSKJ2022068-03), and the Scientific Research Fund of Hunan Provincial Education Department (Project Contract No.: 22A0206).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare that they have no known competing financial interest or personal relationships that could have appeared to influence the work reported in this paper.
References
- Ji, B.; Chen, J.; Mei, J.; Chang, J.; Li, X.; Jia, W.; Qu, Y. Roles of biochar media and oxygen supply strategies in treatment performance, greenhouse gas emissions, and bacterial community features of subsurface-flow constructed wetlands. Bioresour. Technol. 2020, 302, 122890. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, M.; Dong, L.; Han, C.; Li, M.; Wu, H. Effects of biochar dosage on treatment performance, enzyme activity and microbial community in aerated constructed wetlands for treating low C/N domestic sewage. Environ. Technol. Innov. 2021, 24, 101919. [Google Scholar] [CrossRef]
- Parde, D.; Patwa, A.; Shukla, A.; Vijay, R.; Killedar, D.J.; Kumar, R. A review of constructed wetland on type, treatment and technology of wastewater. Environ. Technol. Innov. 2021, 21, 101261. [Google Scholar] [CrossRef]
- Feng, L.K.; Wang, R.G.; Jia, L.X.; Wu, H.M. Can biochar application improve nitrogen removal in constructed wetlands for treating anaerobically-digested swine wastewater? Chem. Eng. J. 2020, 379, 122273. [Google Scholar] [CrossRef]
- Zhang, J.; Cheng, S.; He, F.; Liang, W.; Wu, Z. Effects of Cd2+ and Pb2+ on the substrate bioflms in the integrated vertical-flow constructed wetland. J. Environ. Sci. 2008, 20, 900–906. [Google Scholar] [CrossRef] [PubMed]
- Lei, Y.; Langenhoff, A.; Bruning, H.; Rijnaarts, H. Sorption of micropollutants on selected constructed wetland support matrices. Chemosphere 2021, 275, 130050. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, R.; Liu, H.; Wu, S.; Wu, H. Nitrogen removal responses to biochar addition in intermittent-aerated subsurface flow constructed wetland microcosms: Enhancing role and mechanism. Ecol. Eng. 2019, 128, 57–65. [Google Scholar] [CrossRef]
- Deng, C.; Huang, L.; Liang, Y.; Xiang, H.; Jiang, J.; Wang, Q.; Hou, J.; Chen, Y. Response of microbes to biochar strengthen nitrogen removal in subsurface flow constructed wetlands: Microbial community structure and metabolite characteristics. Sci. Total Environ. 2019, 694, 133687. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, S.; Yang, Z.; Xiao, L. Synergetic effects of biochars and denitrifier on nitrate removal. Bioresour. Technol. 2021, 335, 125245. [Google Scholar] [CrossRef]
- Zhong, L.; Yang, S.-S.; Ding, J.; Wang, G.-Y.; Chen, C.-X.; Xie, G.-J.; Xu, W.; Yuan, F.; Ren, N.-Q. Enhanced nitrogen removal in an electrochemically coupled biochar-amended constructed wetland microcosms: The interactive effects of biochar and electrochemistry. Sci. Total Environ. 2021, 789, 147761. [Google Scholar] [CrossRef]
- Li, J.; Fan, J.; Zhang, J.; Hu, Z.; Liang, S. Preparation and evaluation of wetland plant-based biochar for nitrogen removal enhancement in surface flow constructed wetlands. Environ. Sci. Pollut. Res. 2018, 25, 13929–13937. [Google Scholar] [CrossRef] [PubMed]
- Zhuang, L.-L.; Li, M.; Li, Y.; Zhang, L.; Xu, X.; Wu, H.; Liang, S.; Su, C.; Zhang, J. The performance and mechanism of biochar-enhanced constructed wetland for wastewater treatment. J. Water Process Eng. 2022, 45, 102522. [Google Scholar] [CrossRef]
- Deng, S.; Chen, J.; Chang, J. Application of biochar as an innovative substrate in constructed wetlands/biofilters for wastewater treatment: Performance and ecological benefits. J. Clean. Prod. 2021, 293, 126156. [Google Scholar] [CrossRef]
- Xing, C.; Xu, X.; Xu, Z.; Wang, R.; Xu, L. Study on the Decontamination Effect of Biochar-Constructed Wetland under Different Hydraulic Conditions. Water 2021, 13, 893. [Google Scholar] [CrossRef]
- Feng, L.; Liu, Y.; Zhang, J.; Li, C.; Wu, H. Dynamic variation in nitrogen removal of constructed wetlands modified by biochar for treating secondary livestock effluent under varying oxygen supplying conditions. J. Environ. Manag. 2020, 260, 110152. [Google Scholar] [CrossRef]
- Hamada, M.S.; Ibaid, Z.Z.; Shatat, M. Performance of citrus charcoal and olivepomace charcoal as natural substrates in the treatment of municipal wastewater by vertical flow subsurface constructed wetlands. Bioresour. Technol. Rep. 2021, 15, 100801. [Google Scholar] [CrossRef]
- Zhang, Z.; Solaiman, Z.M.; Meney, K.; Murphy, D.V.; Rengel, Z. Biochars immobilize soil cadmium, but do not improve growth of emergent wetland species Juncus subsecundus in cadmium-contaminated soil. J. Soils Sediments 2013, 13, 140–151. [Google Scholar] [CrossRef]
- Feng, L.; Gao, Z.; Hu, T.; He, S.; Liu, Y.; Jiang, J.; Zhao, Q.; Wei, L. Performance and mechanisms of biochar-based materials additive in constructed wetlands for enhancing wastewater treatment efficiency: A review. Chem. Eng. J. 2023, 471, 144772. [Google Scholar] [CrossRef]
- Liu, H.; Cheng, C.; Wu, H. Sustainable utilization of wetland biomass for activated carbon production: A review on recent advances in modification and activation methods. Sci. Total Environ. 2021, 790, 148214. [Google Scholar] [CrossRef]
- Cui, X.; Hao, H.; Zhang, C.; He, Z.; Yang, X. Capacity and mechanisms of ammonium and cadmium sorption on different wetland-plant derived biochars. Sci. Total Environ. 2016, 539, 566–575. [Google Scholar] [CrossRef]
- Hou, W.; Wang, S.; Li, Y.; Hao, Z.; Zhang, Y.; Kong, F. Influence of modified biochar supported Fe-Cu/polyvinylpyrrolidone on nitrate removal and high selectivity towards nitrogen in constructed wetlands. Environ. Pollut. 2021, 289, 117812. [Google Scholar] [CrossRef]
- Chen, X.; Zhu, H.; Bañuelos, G.; Shutes, B.; Yan, B.; Cheng, R. Biochar reduces nitrous oxide but increases methane emissions in batch wetland mesocosms. Chem. Eng. J. 2020, 392, 124842. [Google Scholar] [CrossRef]
- Guo, X.F.; Cui, X.Y.; Li, H.S. Effects of fillers combined with biosorbents on nutrient and heavy metal removal from biogas slurry in constructed wetlands. Sci. Total Environ. 2020, 703, 134788. [Google Scholar] [CrossRef]
- Bonetti, G.; Trevathan-Tackett, S.M.; Hebert, N.; Carnell, P.E.; Macreadie, P.I. Microbial community dynamics behind major release of methane in constructed wetlands. Appl. Soil Ecol. 2021, 167, 104163. [Google Scholar] [CrossRef]
- Bolton, L.; Joseph, S.; Greenway, M.; Donne, S.; Munroe, P.; Marjo, C.E. Phosphorus adsorption onto an enriched biochar substrate in constructed wetlands treating wastewater. Ecol. Eng. 2019, 142, 100005. [Google Scholar] [CrossRef]
- Kizito, S.; Wu, S.; Kirui, W.K.; Lei, M.; Lu, Q.; Bah, H.; Dong, R. Evaluation of slow pyrolyzed wood and rice husks biochar for adsorption of ammonium nitrogen from piggery manure anaerobic digestate slurry. Sci. Total Environ. 2015, 505, 102–112. [Google Scholar] [CrossRef] [PubMed]
- Ajibade, F.O.; Yin, W.-X.; Guadie, A.; Ajibade, T.F.; Liu, Y.; Kumwimba, M.N.; Liu, W.-Z.; Han, J.-L.; Wang, H.-C.; Wang, A.-J. Impact of biochar amendment on antibiotic removal and ARGs accumulation in constructed wetlands for low C/N wastewater treatment. Chem. Eng. J. 2023, 459, 141541. [Google Scholar] [CrossRef]
- Madadi, R.; Bester, K. Fungi and biochar applications in bioremediation of organic micropollutants from aquatic media. Mar. Pollut. Bull. 2021, 166, 112247. [Google Scholar] [CrossRef] [PubMed]
- Hu, B.; Hu, S.S.; Vymazal, J.; Chen, Z.B. Arbuscular mycorrhizal symbiosis in constructed wetlands with different substrates: Effects on the phytoremediation of ibuprofen and diclofenac. J. Environ. Manag. 2021, 296, 113217. [Google Scholar] [CrossRef] [PubMed]
- El Barkaoui, S.; Mandi, L.; Aziz, F.; Del Bubba, M.; Ouazzani, N. A critical review on using biochar as constructed wetland substrate: Characteristics, feedstock, design and pollutants removal mechanisms. Ecol. Eng. 2023, 190, 106927. [Google Scholar] [CrossRef]
- Lei, Y.; Wagner, T.; Rijnaarts, H.; de Wilde, V.; Langenhoff, A. The removal of micropollutants from treated effluent by batch-operated pilot-scale constructed wetlands. Water Res. 2023, 230, 119494. [Google Scholar] [CrossRef] [PubMed]
- Yu, G.; Peng, H.; Fu, Y.; Yan, X.; Du, C.; Chen, H. Enhanced nitrogen removal of low C/N wastewater in constructed wetlands with co-immobilizing solid carbon source and denitrifying bacteria. Bioresour. Technol. 2019, 280, 337–344. [Google Scholar] [CrossRef] [PubMed]
- Yu, D.; Niu, J.; Zhong, L.; Chen, K.; Wang, G.; Yan, M.; Li, D.; Yao, Z. Biochar raw material selection and application in the food chain: A review. Sci. Total Environ. 2022, 836, 155571. [Google Scholar] [CrossRef] [PubMed]
- Jia, W.; Yang, Y.C.; Yang, L.Y.; Gao, Y. High-efficient nitrogen removal and its microbiological mechanism of a novel carbon self-sufficient constructed wetland. Sci. Total Environ. 2021, 775, 145901. [Google Scholar] [CrossRef]
- Jia, L.; Wang, R.; Feng, L.; Zhou, X.; Lv, J.; Wu, H. Intensified nitrogen removal in intermittently-aerated vertical flow constructed wetlands with agricultural biomass: Effect of influent C/N ratios. Chem. Eng. J. 2018, 345, 22–30. [Google Scholar] [CrossRef]
- Zhou, X.; Wang, X.; Zhang, H.; Wu, H. Enhanced nitrogen removal of low C/N domestic wastewater using a biochar-amended aerated vertical flow constructed wetland. Bioresour. Technol. 2017, 241, 269–275. [Google Scholar] [CrossRef]
- Chand, N.; Suthar, S.; Kumar, K.; Tyagi, V.K. Enhanced removal of nutrients and coliforms from domestic wastewater in cattle dung biochar-packed Colocasia esculenta-based vertical subsurface flow constructed wetland. J. Water Process Eng. 2021, 41, 101994. [Google Scholar] [CrossRef]
- Feng, L.K.; Wu, H.M.; Zhang, J.; Brix, H. Simultaneous elimination of antibiotics resistance genes and dissolved organic matter in treatment wetlands: Characteristics and associated relationship. Chem. Eng. J. 2021, 415, 128966. [Google Scholar] [CrossRef]
- Zhuang, L.-L.; Yang, T.; Zhang, J.; Li, X. The configuration, purification effect and mechanism of intensified constructed wetland for wastewater treatment from the aspect of nitrogen removal: A review. Bioresour. Technol. 2019, 293, 122086. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Chen, Z.; Li, Z.; Wu, H. Impacts of aeration and biochar addition on extracellular polymeric substances and microbial communities in constructed wetlands for low C/N wastewater treatment: Implications for clogging. Chem. Eng. J. 2020, 396, 125349. [Google Scholar] [CrossRef]
- Guo, Z.Z.; Kang, Y.; Hu, Z.; Liang, S.; Xie, H.J.; Ngo, H.H.; Zhang, J. Removal pathways of benzofluoranthene in a constructed wetland amended with metallic ions embedded carbon. Bioresour. Technol. 2020, 311, 123481. [Google Scholar] [CrossRef] [PubMed]
- Zhou, X.; Jia, L.; Liang, C.; Feng, L.; Wang, R.; Wu, H. Simultaneous enhancement of nitrogen removal and nitrous oxide reduction by a saturated biochar-based intermittent aeration vertical flow constructed wetland: Effects of influent strength. Chem. Eng. J. 2018, 334, 1842–1850. [Google Scholar] [CrossRef]
- Zhou, X.; Gao, L.; Zhang, H.; Wu, H. Determination of the optimal aeration for nitrogen removal in biochar-amended aerated vertical flow constructed wetlands. Bioresour. Technol. 2018, 261, 461–464. [Google Scholar] [CrossRef] [PubMed]
- Yu, B.; Liu, C.; Wang, S.; Wang, W.; Zhao, S.; Zhu, G. Applying constructed wetland-microbial electrochemical system to enhance NH4+ removal at low temperature. Sci. Total Environ. 2020, 724, 138017. [Google Scholar] [CrossRef]
- Prado, A.; Berenguer, R.; Esteve-Núñez, A. Electroactive biochar outperforms highly conductive carbon materials for biodegrading pollutants by enhancing microbial extracellular electron transfer. Carbon 2019, 146, 597–609. [Google Scholar] [CrossRef]
- Prathiba, S.; Kumar, P.S.; Vo, D.-V.N. Recent advancements in microbial fuel cells: A review on its electron transfer mechanisms, microbial community, types of substrates and design for bio-electrochemical treatment. Chemosphere 2022, 286, 131856. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Zhang, P.; Qiu, Y.; Zhang, Z.; Wang, X.; Yu, Y.; Feng, Y. Biosynthetic FeS/BC hybrid particles enhanced the electroactive bacteria enrichment in microbial electrochemical systems. Sci. Total Environ. 2021, 762, 143142. [Google Scholar] [CrossRef]
- Saeed, T.; Miah, M.J.; Khan, T. Intensified constructed wetlands for the treatment of municipal wastewater: Experimental investigation and kinetic modelling. Environ. Sci. Pollut. Res. 2021, 28, 30908–30928. [Google Scholar] [CrossRef]
- Jia, W.; Sun, X.; Gao, Y.; Yang, Y.; Yang, L. Fe-modified biochar enhances microbial nitrogen removal capability of constructed wetland. Sci. Total Environ. 2020, 740, 139534. [Google Scholar] [CrossRef]
- Wu, H.; Ma, W.; Kong, Q.; Liu, H. Spatial-temporal dynamics of organics and nitrogen removal in surface flow constructed wetlands for secondary effluent treatment under cold temperature. Chem. Eng. J. 2018, 350, 445–452. [Google Scholar] [CrossRef]
- Wang, B.; Liu, S.-y.; Li, F.-y.; Fan, Z.-p. Removal of nitrate from constructed wetland in winter in high-latitude areas with modified hydrophyte biochars. Korean J. Chem. Eng. 2017, 34, 717–722. [Google Scholar] [CrossRef]
- Wang, H.; Xu, J.; Sheng, L. Preparation of straw biochar and application of constructed wetland in China: A review. J. Clean. Prod. 2020, 273, 123131. [Google Scholar] [CrossRef]
- Sha, N.Q.; Wang, G.H.; Li, Y.H.; Bai, S.Y. Removal of abamectin and conventional pollutants in vertical flow constructed wetlands with Fe-modified biochar. Rsc Adv. 2020, 10, 44171–44182. [Google Scholar] [CrossRef] [PubMed]
- Guo, Z.; Zhang, J.; Kang, Y.; Liu, H. Rapid and efficient removal of Pb(II) from aqueous solutions using biomass-derived activated carbon with humic acid in-situ modification. Ecotoxicol. Environ. Saf. 2017, 145, 442–448. [Google Scholar] [CrossRef] [PubMed]
- Min, L.; Zhongsheng, Z.; Zhe, L.; Haitao, W. Removal of nitrogen and phosphorus pollutants from water by FeCl3-impregnated biochar. Ecol. Eng. 2020, 149, 105792. [Google Scholar] [CrossRef]
- Kasak, K.; Truu, J.; Ostonen, I.; Sarjas, J.; Oopkaup, K.; Paiste, P.; Koiv-Vainik, M.; Mander, U.; Truu, M. Biochar enhances plant growth and nutrient removal in horizontal subsurface flow constructed wetlands. Sci. Total Environ. 2018, 639, 67–74. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Liu, X.; Huang, J.; Huang, M.; Wang, T.; Bao, S.; Tang, W.; Fang, T. The contributions and mechanisms of iron-microbes-biochar in constructed wetlands for nitrate removal from low carbon/nitrogen ratio wastewater. Rsc Adv. 2020, 10, 23212–23220. [Google Scholar] [CrossRef]
- Easton, Z.M.; Rogers, M.; Davis, M.; Wade, J.; Eick, M.; Bock, E. Mitigation of sulfate reduction and nitrous oxide emission in denitrifying environments with amorphous iron oxide and biochar. Ecol. Eng. 2015, 82, 605–613. [Google Scholar] [CrossRef]
- Jia, L.; Wu, W.; Zhang, J.; Wu, H. Insight into heavy metals (Cr and Pb) complexation by dissolved organic matters from biochar: Impact of zero-valent iron. Sci. Total Environ. 2021, 793, 148469. [Google Scholar] [CrossRef]
- Tang, S.; Liao, Y.; Xu, Y.; Dang, Z.; Zhu, X.; Ji, G. Microbial coupling mechanisms of nitrogen removal in constructed wetlands: A review. Bioresour. Technol. 2020, 314, 123759. [Google Scholar] [CrossRef]
- Zhou, Y.; Ji, B.; Jiang, M.; Jin, Y.; Chang, J. Performance and microbial community features of tidal-flow biochar-amended constructed wetlands treating sodium dodecyl sulfate (SDS)-containing greywater. J. Clean. Prod. 2023, 396, 136545. [Google Scholar] [CrossRef]
- Zhang, Y.; Li, Y.; Wang, J.; Wang, X.; Liu, Y.; Wang, S.; Kong, F. Interactions of chlorpyrifos degradation and Cd removal in iron-carbon-based constructed wetlands for treating synthetic farmland wastewater. J. Environ. Manag. 2021, 299, 113559. [Google Scholar] [CrossRef]
- Zheng, C.; Zhang, X.; Gan, L.; He, Z.; Zhu, J.; Zhang, W.; Gao, Y.; Yang, L. Effects of biochar on the growth of Vallisneria natans in surface flow constructed wetland. Environ. Sci. Pollut. Res. 2021, 28, 66158–66170. [Google Scholar] [CrossRef] [PubMed]
- Meng, F.C.; Feng, L.J.; Yin, H.J.; Chen, K.Q.; Hu, G.H.; Yang, G.F.; Zhou, J.H. Assessment of nutrient removal and microbial population dynamics in a non-aerated vertical baffled flow constructed wetland for contaminated water treatment with composite biochar addition. J. Environ. Manag. 2019, 246, 355–361. [Google Scholar] [CrossRef]
- Liang, Y.; Wang, Q.; Huang, L.; Liu, M.; Wang, N.; Chen, Y. Insight into the mechanisms of biochar addition on pollutant removal enhancement and nitrous oxide emission reduction in subsurface flow constructed wetlands: Microbial community structure, functional genes and enzyme activity. Bioresour. Technol. 2020, 307, 123249. [Google Scholar] [CrossRef] [PubMed]
- Gotore, O.; Rameshprabu, R.; Itayama, T. Adsorption performances of corn cob-derived biochar in saturated and semi-saturated vertical-flow constructed wetlands for nutrient removal under erratic oxygen supply. Environ. Chem. Ecotoxicol. 2022, 4, 155–163. [Google Scholar] [CrossRef]
- Peng, Y.; He, S.; Wu, F. Biochemical processes mediated by iron-based materials in water treatement: Enhancing nitrogen and phosphorus removal in low C/N ratio wastewater. Sci. Total Environ. 2021, 775, 145137. [Google Scholar] [CrossRef]
- Jia, L.; Li, C.; Zhang, Y.; Chen, Y.; Li, M.; Wu, S.; Wu, H. Microbial community responses to agricultural biomass addition in aerated constructed wetlands treating low carbon wastewater. J. Environ. Manag. 2020, 270, 110912. [Google Scholar] [CrossRef]
- Shen, X.T.; Zhang, J.; Xie, H.J.; Sun, B.; Liang, S.; Wu, H.M.; Hu, Z.; Ngo, H.H.; Guo, W.S.; Lu, J.X. Electron shuttles enhance phenanthrene removal in constructed wetlands filled with manganese oxides-coated sands. Chem. Eng. J. 2021, 426, 131755. [Google Scholar] [CrossRef]
- Saeed, T.; Haque, I.; Khan, T. Organic matter and nutrients removal in hybrid constructed wetlands: Influence of saturation. Chem. Eng. J. 2019, 371, 154–165. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).