Effects of Catha edulis (Khat) on the Pharmacokinetics of Metformin in Diabetic Rats Using UPLC/MS/MS Analysis and Its Impact on Hepatic CYP450 Enzymes
Abstract
1. Introduction
2. Materials and Methods
2.1. Plant Material
2.2. Drugs, Chemicals, Solvents, and Reagents
2.3. Apparatuses
- For drying the fresh aerial part of the plant: Labconco Freeze Dryer (Prospect Avenue, NY, USA).
- For powdering the dried plant: Huge Grinder Machine (Sujiayuan, Zhejiang, China).
- For extract evaporation: A rotary evaporator (BUCHI Labortechnik AG, Flawil, Switzerland).
- For taking different volumes from the separated plasma samples: Transferpette variable-volume micropipettes.
- For measurement of blood glucose levels: OneTouch glucometer (New Brunswick, NJ, USA).
- For mixing the collected plasma samples: SYBRON Thermolyne vortex mixer (Dubuque, IA, USA).
- For centrifugation and removal of the precipitated plasma protein: High-speed Eppendorf centrifuge (Freshwater Blvd, Enfield, CT, USA).
- For sample weighting: Mettler digital balance (Greifensee, Zurich, Switzerland).
- For florescence measurements: BioTek Microplate plate reader (Winooski, VT, USA).
- For the UPLC-MS/MS method: UPLC-MS/MS (Waters Acquity, Milford, CT, USA).
2.4. Plant Extraction
2.5. Animals
2.6. Interaction of KT with MT in Diabetic Rats
2.6.1. Induction of Diabetes
2.6.2. Animal Experiments and Drug Administration
2.6.3. Preparation of the Collected Plasma Samples and Pharmacokinetic Study
2.6.4. Estimation of Rat Blood Glucose Levels
2.6.5. Chromatographic and Mass Spectrometric Conditions
2.6.6. Preparation of Standard Stocks, Calibrators, and Spiking Solutions
2.6.7. Pretreatment of the Collected Plasma Samples
2.7. In Vitro Study of KT’s Effects on CYP450 Enzyme
2.7.1. Assessment of the Cytotoxicity of KT on HepG2 Liver Cancer Cells by MTT Assay
2.7.2. CYP3A4 Inhibitor Screening
2.7.3. Reagents Preparation
2.7.4. KT and CYP3A4 Enzyme Preparation
2.7.5. Reaction and Measurements
3. Results
3.1. Interactions of KT with MT in Diabetic Rats
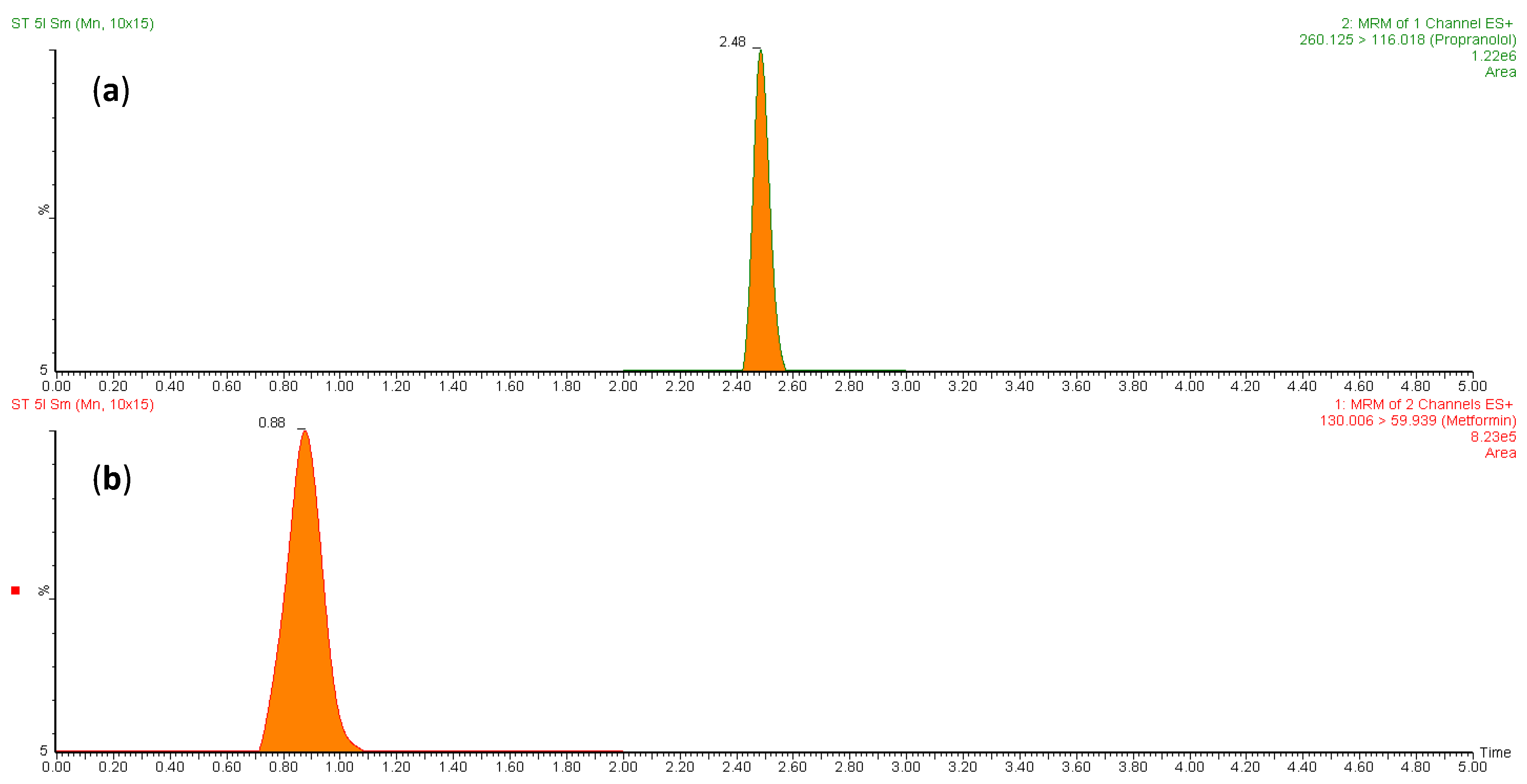
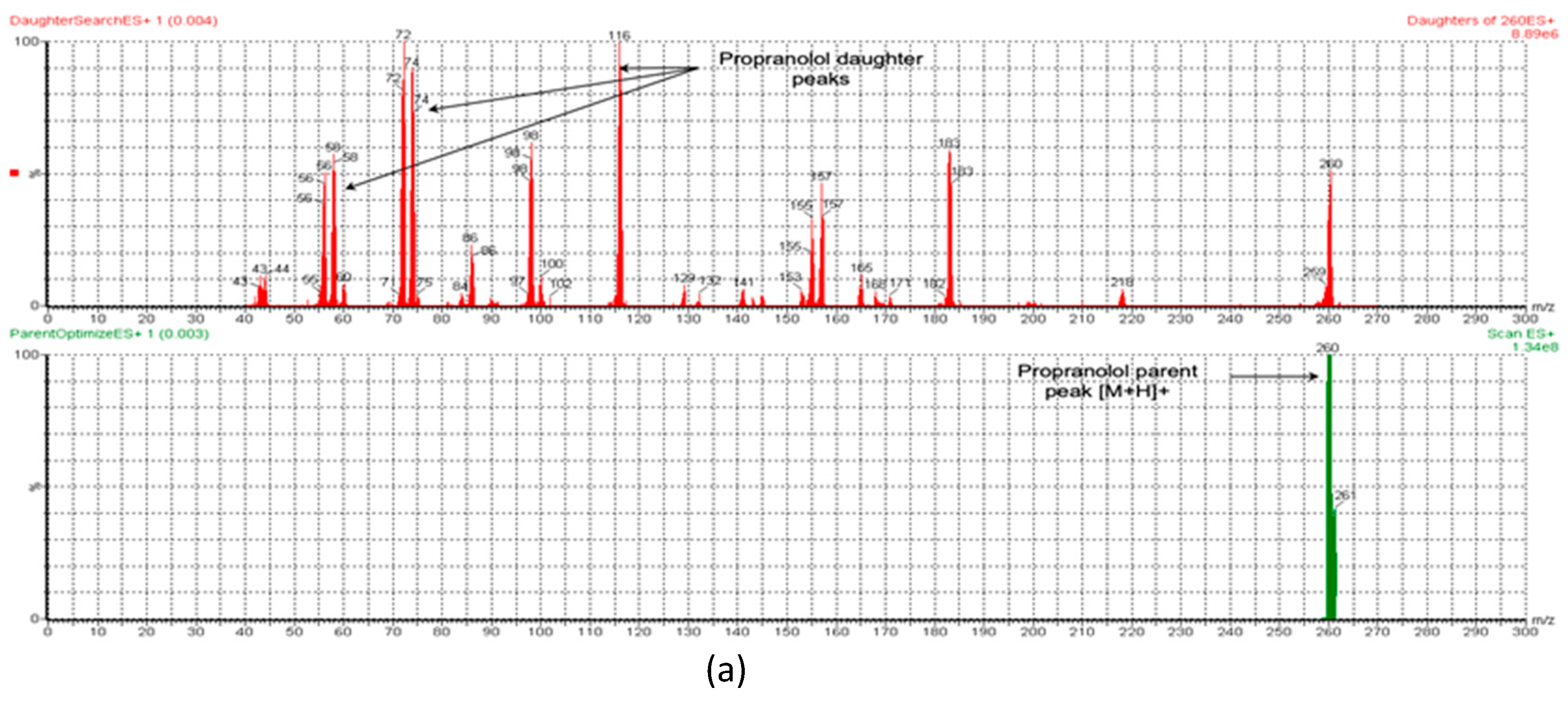
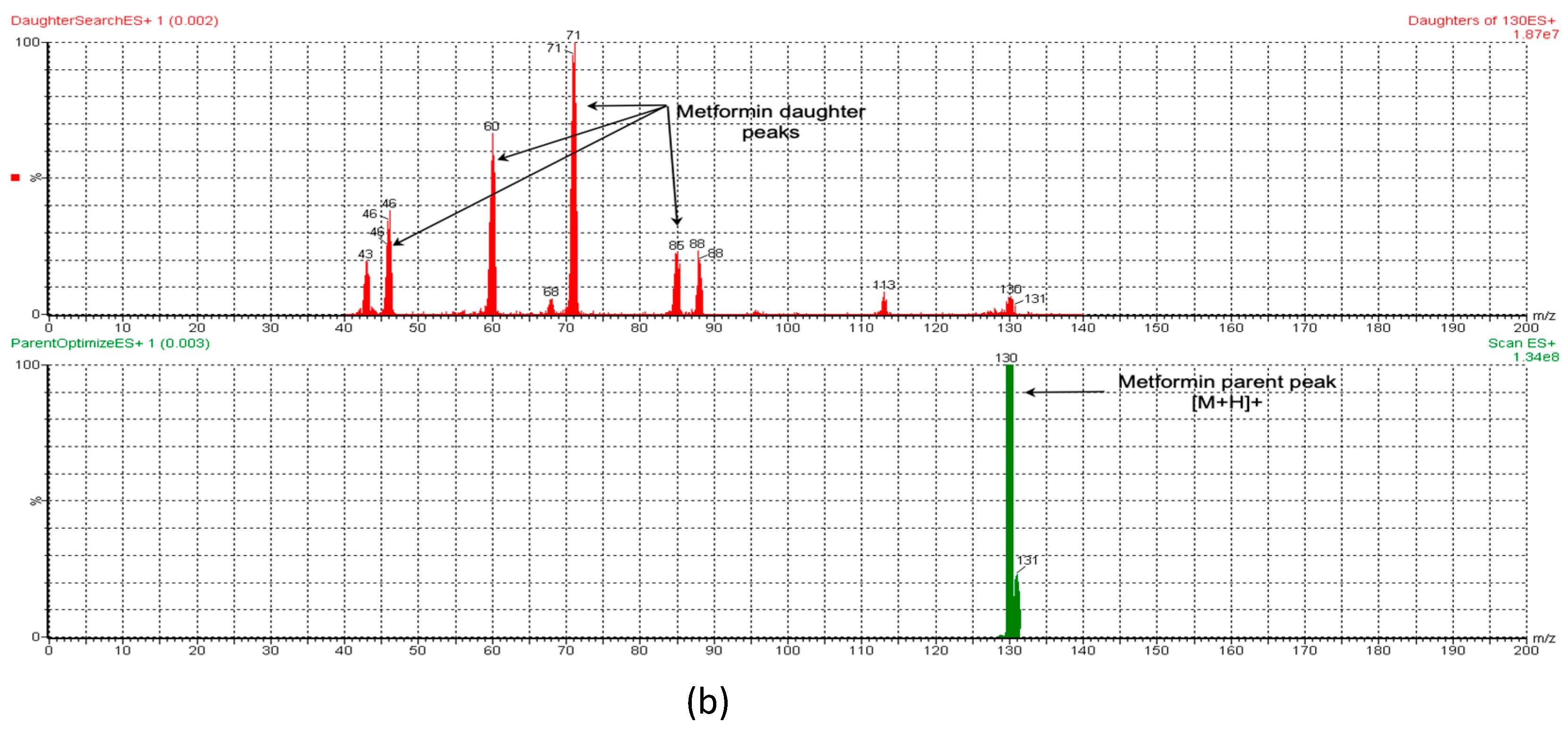
3.1.1. UPLC-MS/MS Method for MT
3.1.2. Calibration Curve and Method Validation
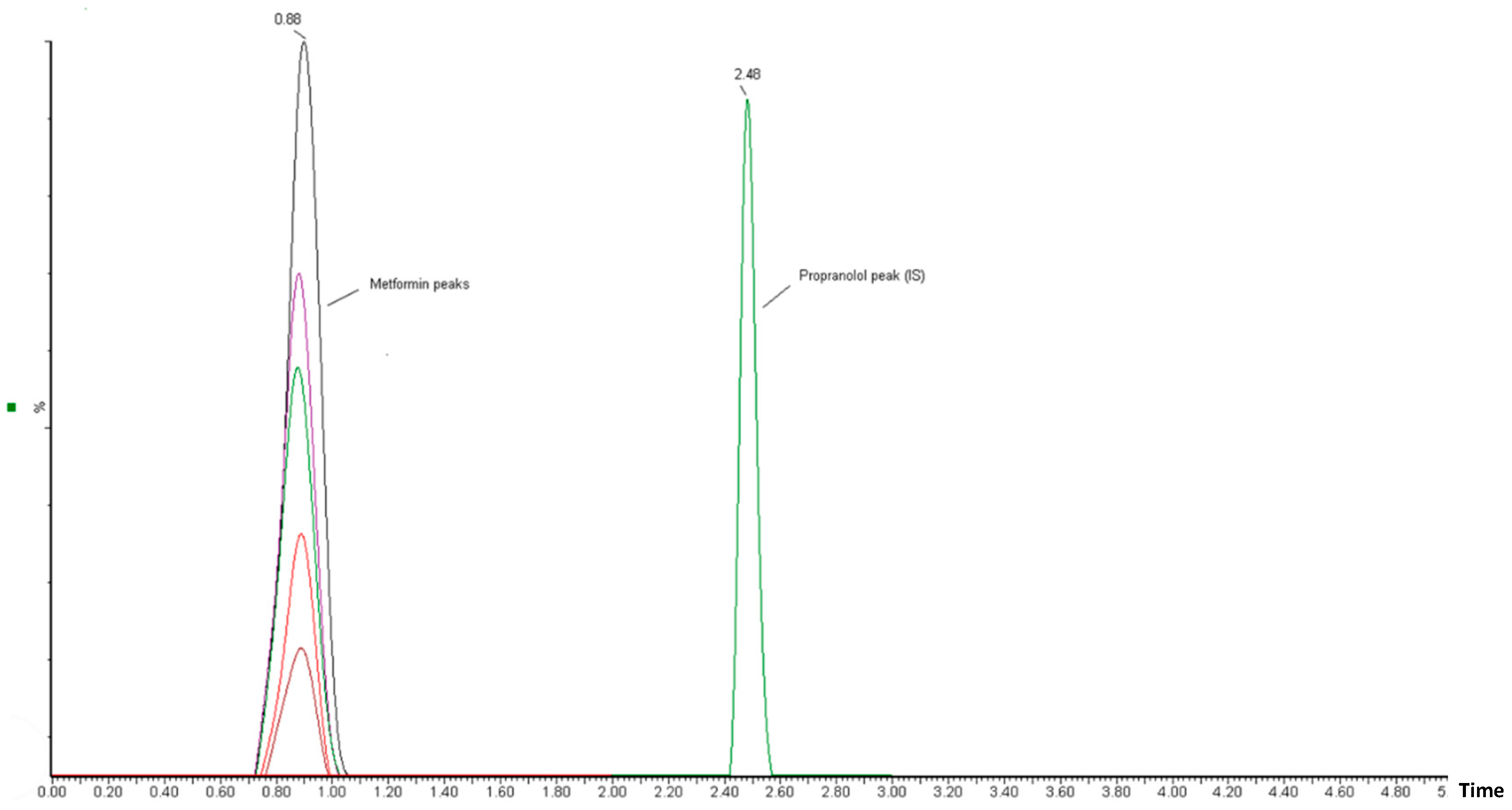
3.1.3. Effect of KT Extract on MT Pharmacokinetics
3.1.4. Effect of MT Alone and in Combination with KT Extract on Fasting Blood Glucose Levels
3.2. In Vitro Study of KT’s Impact on CYP450 Enzyme
3.2.1. Effect of KT on HepG2 Cells
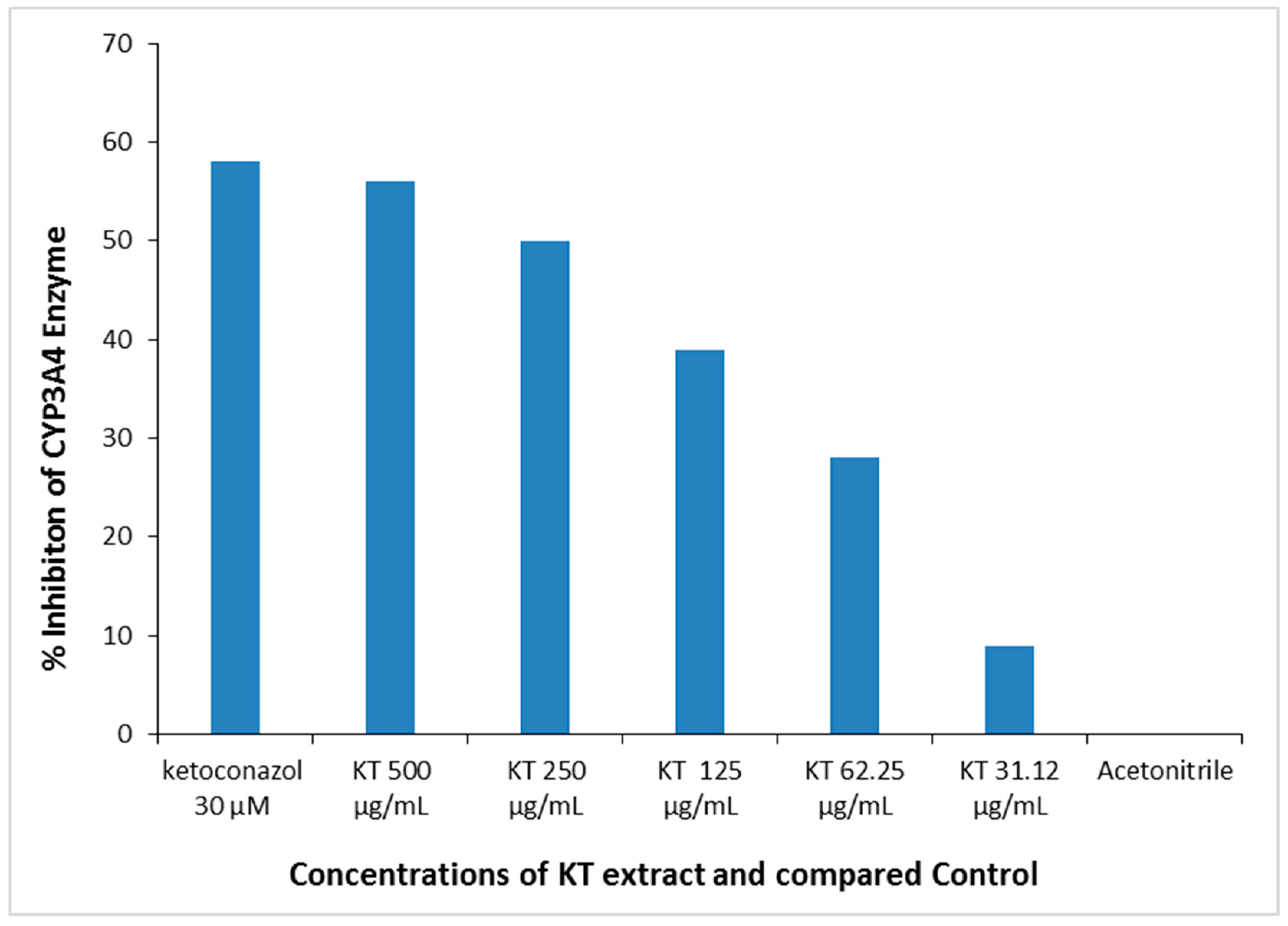
3.2.2. Effect of KT on the CYP3A4 Enzyme
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Galicia-Garcia, U.; Benito-Vicente, A.; Jebari, S.; Larrea-Sebal, A.; Siddiqi, H.; Uribe, K.B.; Ostolaza, H.; Martín, C. Pathophysiology of type 2 diabetes mellitus. Int. J. Mol. Sci. 2020, 21, 6275. [Google Scholar] [CrossRef] [PubMed]
- Gupta, R.; Chang, D.; Nammi, S.; Bensoussan, A.; Bilinski, K.; Roufogalis, B. Interactions between antidiabetic drugs and herbs: An overview of mechanisms of action and clinical implications. Diabetol. Metab. Syndr. 2017, 9, 59. [Google Scholar] [CrossRef] [PubMed]
- Baker, C.; Retzik-Stahr, C.; Singh, V.; Plomondon, R.; Anderson, V.; Rasouli, N. Should metformin remain the first-line therapy for treatment of type 2 diabetes? Ther. Adv. Endocrinol. Metab. 2021, 12, 2042018820980225. [Google Scholar] [CrossRef]
- Ye, S.; Hu, J.; Liu, Z.; Liang, M. Progress and research trends on Catha edulis (Vahl) Endl.(Catha edulis): A review and bibliometric analysis. Front. Pharmacol. 2021, 12, 705376. [Google Scholar] [CrossRef] [PubMed]
- Getasetegn, M. Chemical composition of Catha edulis (khat): A review. Phytochem. Rev. 2016, 15, 907–920. [Google Scholar] [CrossRef]
- Gholami, M.; Esmaeilzadeh Bahabadi, S. Kaurene as the major constituent of the essential oils of the narcotic plant, Khat (Catha edulis Forsk). Nat. Prod. Res. 2019, 33, 126–129. [Google Scholar] [CrossRef]
- Ageely, H.M. Health and socio-economic hazards associated with khat consumption. J. Fam. Community Med. 2008, 15, 3. [Google Scholar]
- Thomas, S.; Williams, T. Khat (Catha edulis): A systematic review of evidence and literature pertaining to its harms to UK users and society. Drug Sci. Policy Law 2013, 1, 2050324513498332. [Google Scholar] [CrossRef]
- Chang, H.Y.; Wallis, M.; Tiralongo, E. Use of complementary and alternative medicine among people living with diabetes: Literature review. J. Adv. Nurs. 2007, 58, 307–319. [Google Scholar] [CrossRef]
- Chang, H.-Y.A.; Wallis, M.; Tiralongo, E. Use of complementary and alternative medicine among people with type 2 diabetes in Taiwan: A cross-sectional survey. Evid.-Based Complement. Altern. Med. 2010, 2011, 983792. [Google Scholar] [CrossRef]
- Mahfouz, M.S.; Rahim, B.-E.E.; Solan, Y.M.; Makeen, A.M.; Alsanosy, R.M. Khat chewing habits in the population of the Jazan region, Saudi Arabia: Prevalence and associated factors. PloS ONE 2015, 10, e0134545. [Google Scholar] [CrossRef] [PubMed]
- Alsalahi, A.; Chik, Z.; Mohamed, Z.; Giribabu, N.; Alshawsh, M.A. Cathinone: An alkaloid of Catha edulis (Khat) exacerbated hyperglycemia in diabetes-induced rats. Saudi J. Biol. Sci. 2021, 28, 4633–4643. [Google Scholar] [CrossRef] [PubMed]
- Abdelwahab, N.S.; Morsi, A.; Ahmed, Y.M.; Hassan, H.M.; AboulMagd, A.M. Ecological HPLC method for analyzing an antidiabetic drug in real rat plasma samples and studying the effects of concurrently administered fenugreek extract on its pharmacokinetics. RSC Adv. 2021, 11, 4740–4750. [Google Scholar] [CrossRef] [PubMed]
- Lynch, T.; Neff, A.P. The effect of cytochrome P450 metabolism on drug response, interactions, and adverse effects. Am. Fam. Physician 2007, 76, 391–396. [Google Scholar]
- Lim, S.Y.M.; Binti Azidin, A.R.; Ung, Y.T.; Al-Shagga, M.; Alshawsh, M.A.; Mohamed, Z.; Ong, C.E.; Pan, Y. Effect of 95% ethanol khat extract and cathinone on in vitro human recombinant cytochrome P450 (CYP) 2C9, CYP2D6, and CYP3A4 activity. Eur. J. Drug Metab. Pharmacokinet. 2019, 44, 423–431. [Google Scholar] [CrossRef]
- Ketema, T.; Yohannes, M.; Alemayehu, E.; Ambelu, A. Evaluation of immunomodulatory activities of methanolic extract of khat (Catha edulis, Forsk) and cathinone in Swiss albino mice. BMC Immunol. 2015, 16, 9. [Google Scholar] [CrossRef]
- Barik, R.; Jain, S.; Qwatra, D.; Joshi, A.; Tripathi, G.S.; Goyal, R. Antidiabetic activity of aqueous root extract of Ichnocarpus frutescens in streptozotocin-nicotinamide induced type-II diabetes in rats. Indian J. Pharm. 2008, 40, 19–22. [Google Scholar] [CrossRef]
- Dallak, M.A.; Bin-Jaliah, I.; Al-Khateeb, M.A.; Nwoye, L.O.; Shatoor, A.S.; Soliman, H.S.; Al-Hashem, F.H. In vivo acute effects of orally administered hydro-ethanol extract of Catha edulis on blood glucose levels in normal, glucose-fed hyperglycemic, and alloxan-induced diabetic rats. Saudi Med. J. 2010, 31, 627–633. [Google Scholar]
- Kelly, J.P. Cathinone derivatives: A review of their chemistry, pharmacology and toxicology. Drug Test. Anal. 2011, 3, 439–453. [Google Scholar] [CrossRef]
- Nasr, F.A.; Noman, O.M.; Alqahtani, A.S.; Qamar, W.; Ahamad, S.R.; Al-Mishari, A.A.; Alyhya, N.; Farooq, M. Phytochemical constituents and anticancer activities of Tarchonanthus camphoratus essential oils grown in Saudi Arabia. Saudi Pharm. J. 2020, 28, 1474–1480. [Google Scholar] [CrossRef]
- Mohamed, D.; Elshahed, M.S.; Nasr, T.; Aboutaleb, N.; Zakaria, O. Novel LC–MS/MS method for analysis of metformin and canagliflozin in human plasma: Application to a pharmacokinetic study. BMC Chem. 2019, 13, 82. [Google Scholar] [CrossRef]
- Patel, O.; Muller, C.J.F.; Joubert, E.; Rosenkranz, B.; Taylor, M.J.C.; Louw, J.; Awortwe, C. Pharmacokinetic Interaction of Green Rooibos Extract With Atorvastatin and Metformin in Rats. Front. Pharm. 2019, 10, 1243. [Google Scholar] [CrossRef] [PubMed]
- Singh, A.; Thatikonda, T.; Kumar, A.; Wazir, P.; Vijayabhaskar, V.; Nandi, U.; Singh, P.P.; Singh, S.; Gupta, A.P.; Tikoo, M.K. Determination of ZSTK474, a novel Pan PI3K inhibitor in mouse plasma by LC–MS/MS and its application to Pharmacokinetics. J. Pharm. Biomed. Anal. 2018, 149, 387–393. [Google Scholar] [CrossRef]
- Shengule, S.; Kumbhare, K.; Patil, D.; Mishra, S.; Apte, K.; Patwardhan, B. Herb-drug interaction of Nisha Amalaki and Curcuminoids with metformin in normal and diabetic condition: A disease system approach. Biomed. Pharmacother. 2018, 101, 591–598. [Google Scholar] [CrossRef]
- Khare, V.; Singh, A.; Mahajan, G.; Alam, N.; Kour, S.; Gupta, M.; Kumar, A.; Singh, G.; Singh, S.K.; Saxena, A.K. Long-circulatory nanoparticles for gemcitabine delivery: Development and investigation of pharmacokinetics and in-vivo anticancer efficacy. Eur. J. Pharm. Sci. 2016, 92, 183–193. [Google Scholar] [CrossRef]
- Choi, Y.; Lee, U.; Lee, B.; Lee, M. Pharmacokinetic interaction between itraconazole and metformin in rats: Competitive inhibition of metabolism of each drug by each other via hepatic and intestinal CYP3A1/2. Br. J. Pharmacol. 2010, 161, 815–829. [Google Scholar] [CrossRef] [PubMed]
- Hinault, C.; Caroli-Bosc, P.; Bost, F.; Chevalier, N. Critical Overview on Endocrine Disruptors in Diabetes Mellitus. Int. J. Mol. Sci. 2023, 24, 4537. [Google Scholar] [CrossRef] [PubMed]
- Abdulaziz Al Dawish, M.; Alwin Robert, A.; Braham, R.; Abdallah Al Hayek, A.; Al Saeed, A.; Ahmed Ahmed, R.; Sulaiman Al Sabaan, F. Diabetes mellitus in Saudi Arabia: A review of the recent literature. Curr. Diabetes Rev. 2016, 12, 359–368. [Google Scholar] [CrossRef]
- Drzewoski, J.; Hanefeld, M. The current and potential therapeutic use of metformin—The good old drug. Pharmaceuticals 2021, 14, 122. [Google Scholar] [CrossRef]
- Abdelgawad, M.A.; Elmowafy, M.; Musa, A.; Al-Sanea, M.M.; Nayl, A.A.; Ghoneim, M.M.; Ahmed, Y.M.; Hassan, H.M.; AboulMagd, A.M.; Salem, H.F. Development and Greenness Assessment of HPLC Method for Studying the Pharmacokinetics of Co-Administered Metformin and Papaya Extract. Molecules 2022, 27, 375. [Google Scholar] [CrossRef]
- Elango, H.; Ponnusankar, S.; Sundaram, S. Assessment of pharmacodynamic and pharmacokinetic interaction of aqueous extract of Cassia auriculata L. and metformin in rats. Pharmacogn. Mag. 2015, 11, S423. [Google Scholar] [PubMed]
- Jin, S.; Lee, S.; Jeon, J.-H.; Kim, H.; Choi, M.-K.; Song, I.-S. Enhanced intestinal permeability and plasma concentration of metformin in rats by the repeated administration of red ginseng extract. Pharmaceutics 2019, 11, 189. [Google Scholar] [CrossRef]
- Hassanzadeh-Taheri, M.; Hassanpour-Fard, M.; Doostabadi, M.; Moodi, H.; Vazifeshenas-Darmiyan, K.; Hosseini, M. Co-administration effects of aqueous extract of turnip leaf and metformin in diabetic rats. J. Tradit. Complement. Med. 2018, 8, 178–183. [Google Scholar] [CrossRef] [PubMed]
- Stage, T.B.; Pedersen, R.S.; Damkier, P.; Christensen, M.M.H.; Feddersen, S.; Larsen, J.T.; Højlund, K.; Brøsen, K. Intake of S t J ohn’s wort improves the glucose tolerance in healthy subjects who ingest metformin compared with metformin alone. Br. J. Clin. Pharmacol. 2015, 79, 298–306. [Google Scholar] [CrossRef] [PubMed]
- Shikha, C.; Tamanna, N.; Soni, L.K. Effect of Allium sativum on the pharmacokinetic of Metformin in rat plasma: A herb-drug interaction study. Der Pharma Chem. 2011, 3, 287–291. [Google Scholar]
- Lim, X.; Chan, J.; Japri, N.; Lee, J.; Tan, T. Carica papaya L. Leaf: A systematic scoping review on biological safety and herb-drug interactions. Evid.-Based Complement. Altern. Med. 2021, 2021, 1–21. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.Y.M.; Loo, J.S.E.; Alshagga, M.; Alshawsh, M.A.; Ong, C.E.; Pan, Y. In vitro and In silico studies of interactions of cathinone with human recombinant cytochrome P450 CYP (1A2), CYP2A6, CYP2B6, CYP2C8, CYP2C19, CYP2E1, CYP2J2, and CYP3A5. Toxicol. Rep. 2022, 9, 759–768. [Google Scholar] [CrossRef]
- Liu, A.; Coleman, S.P. Determination of metformin in human plasma using hydrophilic interaction liquid chromatography–tandem mass spectrometry. J. Chromatogr. B 2009, 877, 3695–3700. [Google Scholar] [CrossRef]
- Ningrum, V.D.A.; Wibowo, A.; Fuaida, I.; Ikawati, Z.; Sadewa, A.H.; Ikhsan, M.R. Validation of an HPLC-UV method for the determination of metformin hydrochloride in spiked-human plasma for the application of therapeutic drug monitoring. Res. J. Pharm. Technol. 2018, 11, 2197–2202. [Google Scholar] [CrossRef]
- Chhetri, H.P.; Thapa, P.; Van Schepdael, A. Simple HPLC-UV method for the quantification of metformin in human plasma with one step protein precipitation. Saudi Pharm. J. 2014, 22, 483–487. [Google Scholar] [CrossRef]
- Rebecca, Y.M.; Sudha, V.; Kumar, A.H. Validated high performance liquid chromatography method for the determination of metformin in human plasma and its application to pharmacokinetic study. Chromatogr. Sep. Technol. J 2019, 2, 119–124. [Google Scholar]
- Koseki, N.; Kawashita, H.; Niina, M.; Nagae, Y.; Masuda, N. Development and validation for high selective quantitative determination of metformin in human plasma by cation exchanging with normal-phase LC/MS/MS. J. Pharm. Biomed. Anal. 2005, 36, 1063–1072. [Google Scholar] [CrossRef] [PubMed]
- El-Aneed, A.; Arnason, T.; Michel, D.; Gaunt, M. Development and validation of fast and simple flow injection analysis-tandem mass spectrometry (FIA-MS/MS) for the determination of metformin in dog serum. J. Pharm. Biomed. Anal. 2015, 107, 229–235. [Google Scholar]
- Swales, J.G.; Gallagher, R.; Peter, R.M. Determination of metformin in mouse, rat, dog and human plasma samples by laser diode thermal desorption/atmospheric pressure chemical ionization tandem mass spectrometry. J. Pharm. Biomed. Anal. 2010, 53, 740–744. [Google Scholar] [CrossRef] [PubMed]
- Chaudhari, K.; Wang, J.; Xu, Y.; Winters, A.; Wang, L.; Dong, X.; Cheng, E.Y.; Liu, R.; Yang, S.-H. Determination of metformin bio-distribution by LC-MS/MS in mice treated with a clinically relevant paradigm. PloS ONE 2020, 15, e0234571. [Google Scholar] [CrossRef]
- Uçaktürk, E. The development and validation of a gas chromatography-mass spectrometry method for the determination of metformin in human plasma. Anal. Methods 2013, 5, 4723–4730. [Google Scholar] [CrossRef]
- Zhang, W.; Han, F.; Zhao, H.; Lin, Z.; Huang, Q.; Weng, N. Determination of metformin in rat plasma by HILIC-MS/MS combined with Tecan automation and direct injection. Biomed. Chromatogr. 2012, 26, 1163–1169. [Google Scholar] [CrossRef]
- Balamurugan, K.; Kirtimaya, M.; Suresh, R. Simultaneous estimation of linagliptin and metformin HCl in human plasma by RP-HPLC method. Int. Res. J. Pharm. 2019, 10, 167–170. [Google Scholar]
- Sebaiy, M.M.; El-Adl, S.M.; Baraka, M.M.; Hassan, A.A. Rapid RP-HPLC method for simultaneous estimation of metformin, pioglitazone, and glimepiride in human plasma. Acta Chromatogr. 2020, 32, 16–21. [Google Scholar] [CrossRef]
- Ranetti, M.-C.; Ionescu, M.; Hinescu, L.; Ionica, E.; Anuta, V.; Ranetti, A.E.; Stecoza, C.E.; Mircioiu, C. Validation of a HPLC method for the simultaneous analysis of metformin and gliclazide in human plasma. Farmacia 2009, 57, 728–735. [Google Scholar]
- Shakoor, A.; Ahmed, M.; Ikram, R.; Hussain, S.; Tahir, A.; Jan, B.M.; Adnan, A. Stability-indicating RP-HPLC method for simultaneous determination of metformin hydrochloride and vildagliptin in tablet and biological samples. Acta Chromatogr. 2020, 32, 39–43. [Google Scholar] [CrossRef]
- Mohamed, A.-M.I.; Mohamed, F.A.-F.; Ahmed, S.; Mohamed, Y.A.S. An efficient hydrophilic interaction liquid chromatographic method for the simultaneous determination of metformin and pioglitazone using high-purity silica column. J. Chromatogr. B 2015, 997, 16–22. [Google Scholar] [CrossRef] [PubMed]
- Pontarolo, R.; Gimenez, A.C.; de Francisco, T.M.G.; Ribeiro, R.P.; Pontes, F.L.D.; Gasparetto, J.C. Simultaneous determination of metformin and vildagliptin in human plasma by a HILIC–MS/MS method. J. Chromatogr. B 2014, 965, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Elgawish, M.S.; Nasser, S.; Salama, I.; Abbas, A.M.; Mostafa, S.M. Liquid chromatography tandem mass spectrometry for the simultaneous determination of metformin and pioglitazone in rat plasma: Application to pharmacokinetic and drug-drug interaction studies. J. Chromatogr. B 2019, 1124, 47–57. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Peng, Y.; Wan, P.; Yin, L.; Wang, G.; Sun, J. Simultaneous determination and pharmacokinetic study of metformin and pioglitazone in dog plasma by LC–MS-MS. J. Chromatogr. Sci. 2014, 52, 52–58. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Jagadeesh, B.; Bharathi, D.V.; Pankaj, C.; Narayana, V.S.; Venkateswarulu, V. Development and validation of highly selective and robust method for simultaneous estimation of pioglitazone, hydroxypioglitazone and metformin in human plasma by LC–MS/MS: Application to a pharmacokinetic study. J. Chromatogr. B 2013, 930, 136–145. [Google Scholar] [CrossRef]
- Polagani, S.R.; Pilli, N.R.; Gajula, R.; Gandu, V. Simultaneous determination of atorvastatin, metformin and glimepiride in human plasma by LC–MS/MS and its application to a human pharmacokinetic study. J. Pharm. Anal. 2013, 3, 9–19. [Google Scholar] [CrossRef]
- Wattamwar, T.; Mungantiwar, A.; Gujar, S.; Pandita, N. Development of LC-MS/MS method for simultaneous determination of Canagliflozin and Metformin in human plasma and its pharmacokinetic application in Indian population under fast and fed conditions. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1154, 122281. [Google Scholar] [CrossRef]
- Hsieh, Y.; Galviz, G.; Hwa, J.J. Ultra-performance hydrophilic interaction LC-MS/MS for the determination of metformin in mouse plasma. Bioanalysis 2009, 1, 1073–1079. [Google Scholar] [CrossRef]
- Abou-Omar, M.N.; Kenawy, M.; Youssef, A.O.; Alharthi, S.; Attia, M.S.; Mohamed, E.H. Validation of a novel UPLC-MS/MS method for estimation of metformin and empagliflozin simultaneously in human plasma using freezing lipid precipitation approach and its application to pharmacokinetic study. J. Pharm. Biomed. Anal. 2021, 200, 114078. [Google Scholar] [CrossRef]
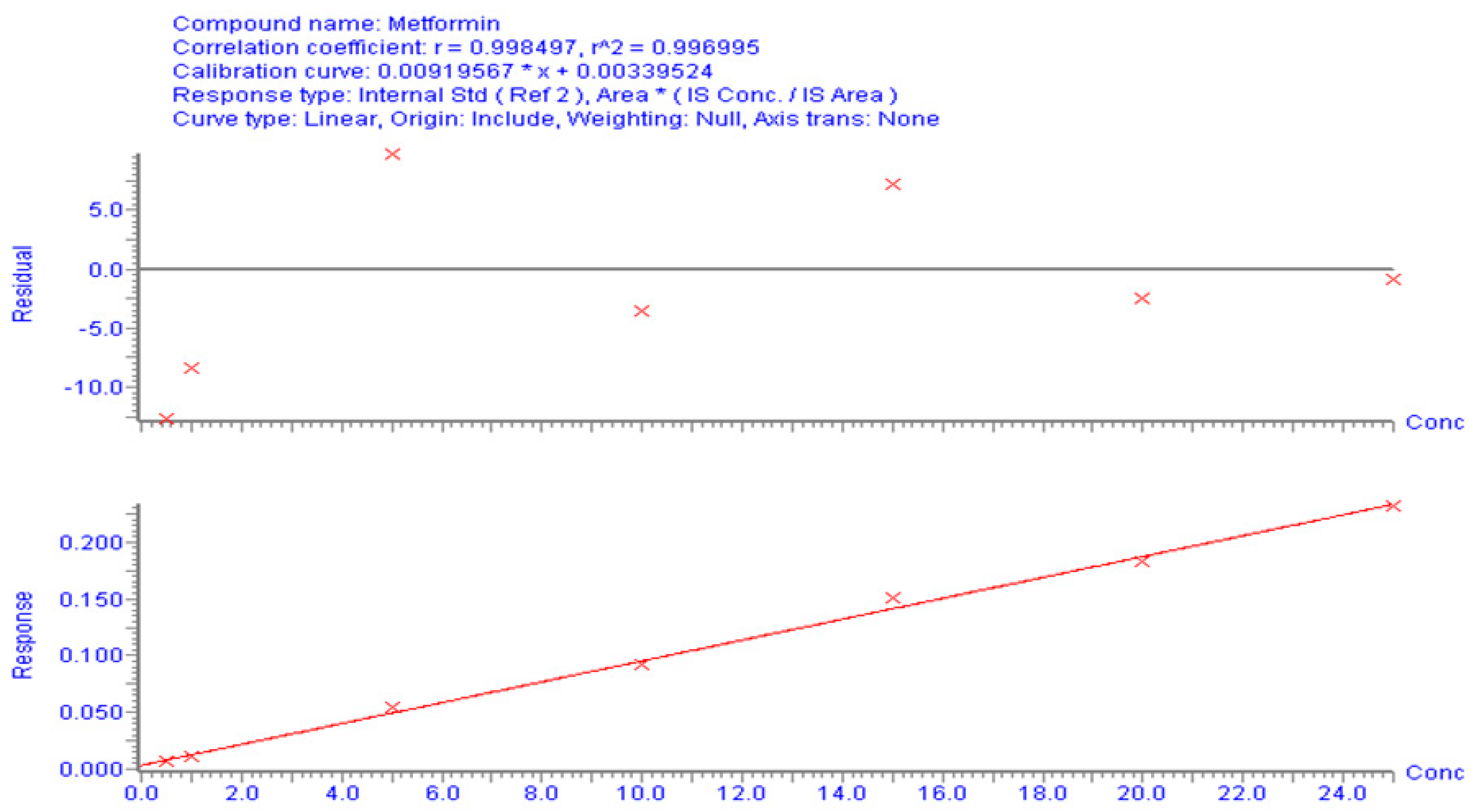
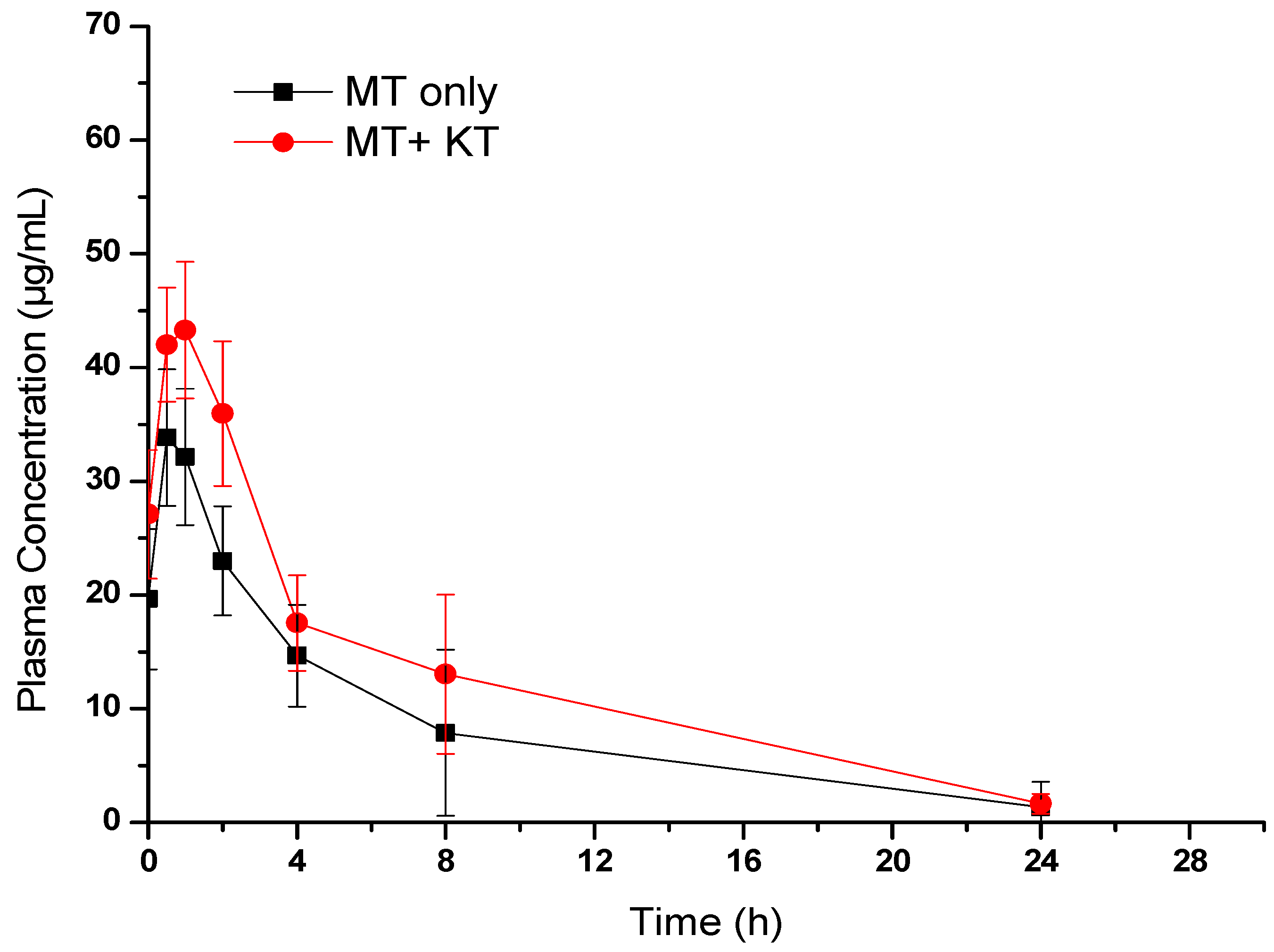
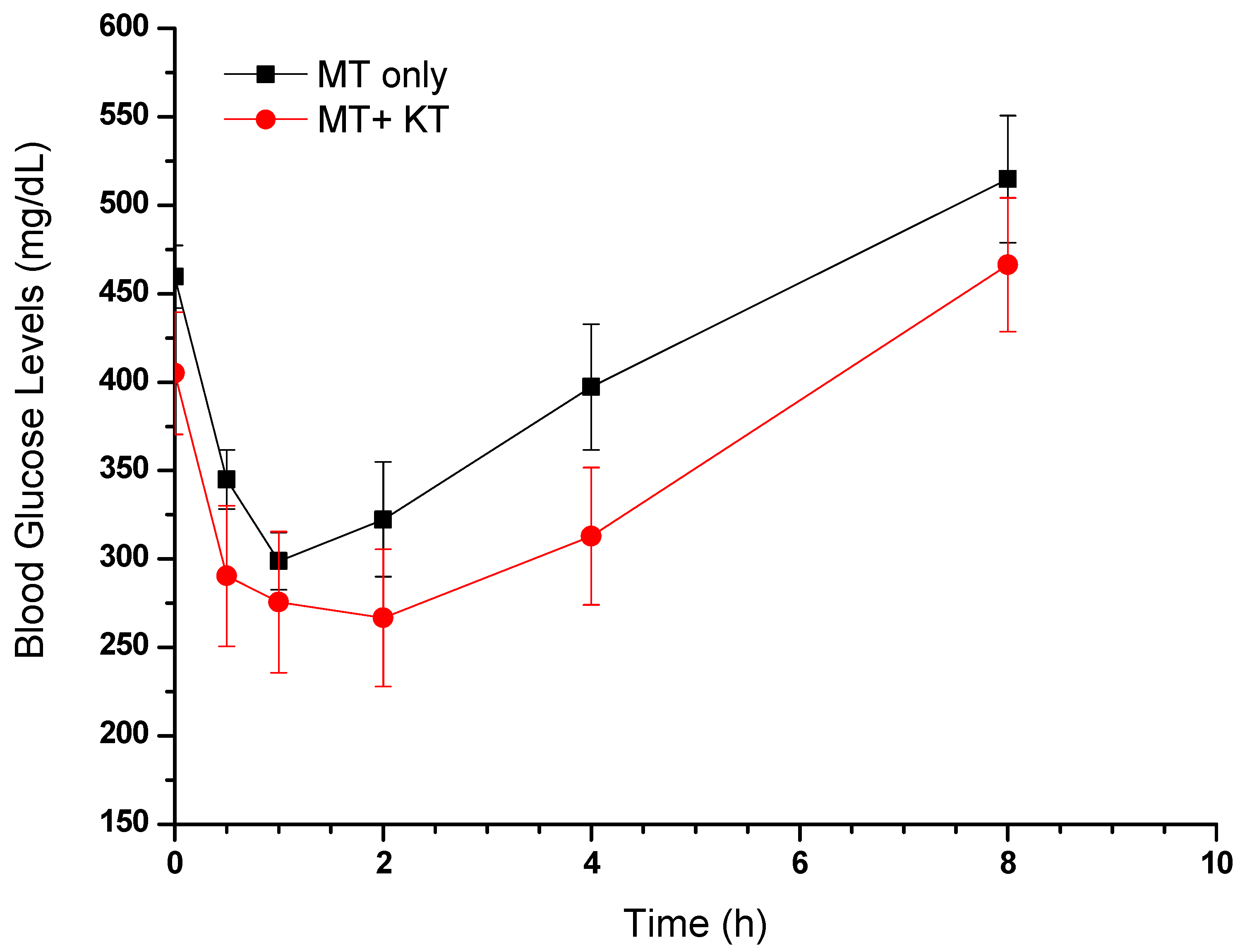
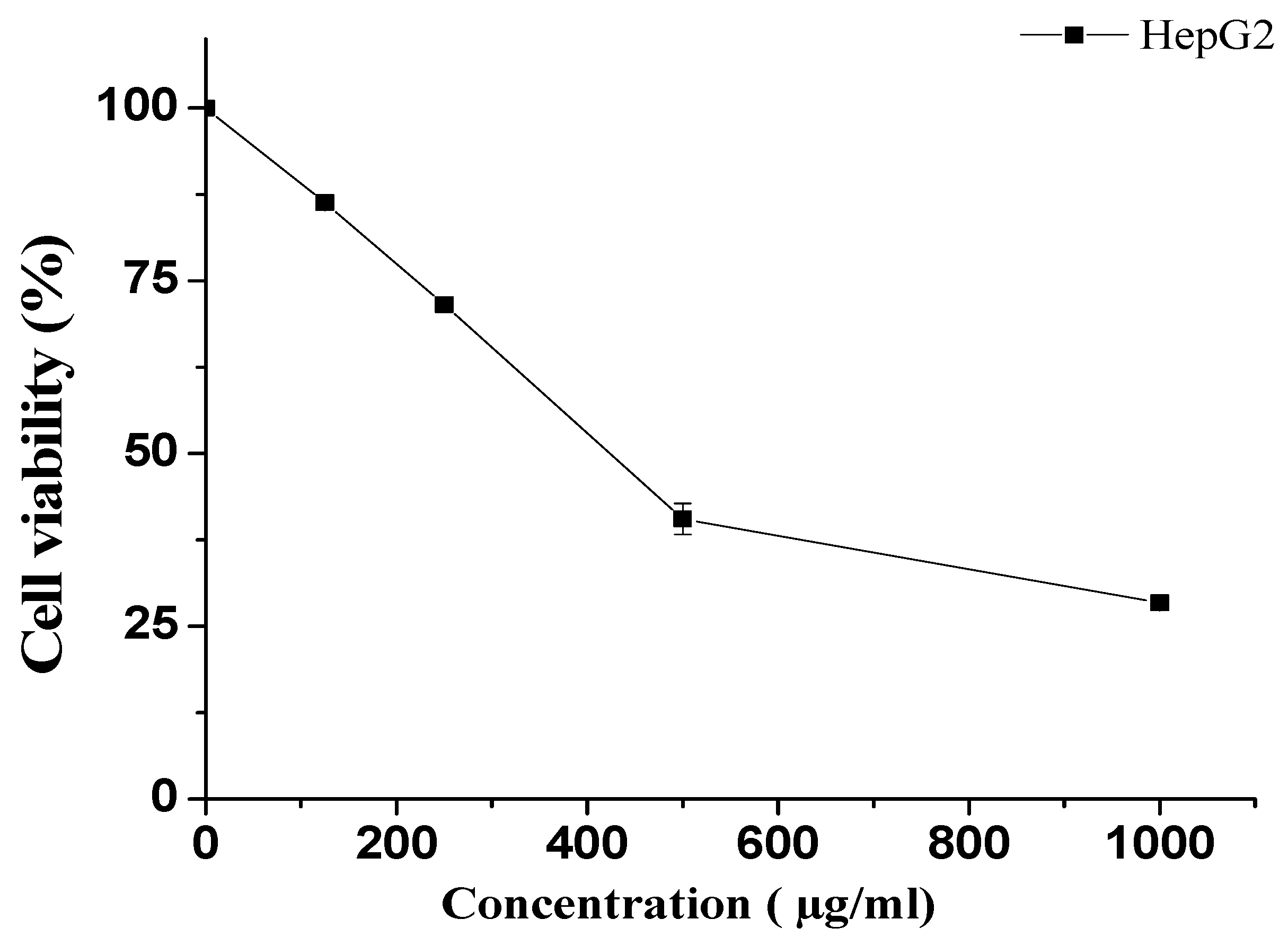
| Pharmacokinetic Parameters | Animals Treated with MT Alone | Animals Treated with MT + KT |
|---|---|---|
| Cmax (µg/mL) | 34.91 ± 9.76 | 46.58 ± 10.66 |
| Tmax (h) | 1.0 | 1.0 |
| AUC0–24 (µg·h/mL) | 214.06 ± 172.9 | 310.89 ± 172.9 |
| AUC0–inf (µg·h/mL) | 228.89 ± 171.47 | 324.78 ± 182.88 |
| Kel (h) | 0.15 ± 0.031 | 0.12 ± 0.014 |
| T1/2 (h) | 4.85 ± 1.73 | 5.61 ± 0.66 |
| MRT (h) | 5.50 ± 3.02 | 6.24 ± 1.14 |
| No-Inhibitor | Background Control | Positive Control | KT (500 μg/mL) | KT (250 μg/mL) | KT (125 μg/mL) | KT (62.5 μg/mL) | KT (31.25 μg/mL) | |
|---|---|---|---|---|---|---|---|---|
| CYP3A4 Enzyme Stock | 50 μL | -- | 50 μL | 50 μL | 50 μL | 50 μL | 50 μL | 50 μL |
| KT | -- | -- | -- | 20 μL | 20 μL | 20 μL | 20 μL | 20 μL |
| Ketoconazole 150 μM | -- | -- | 20 μL | -- | -- | -- | -- | -- |
| CYP3A4 Assay Buffer | 20 μL | 70 μL | -- | -- | -- | -- | -- | -- |
| CYP3A4 Substrate/NADP+ (3x) Mixture | 30 μL | 30 μL | 30 μL | 30 μL | 30 μL | 30 μL | 30 μL | 30 μL |
| Total Volume | 100 μL | 100 μL | 100 μL | 100 μL | 100 μl | 100 μL | 100 μL | 100 μL |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alqahtani, A.S.; Parvez, M.K.; Alqahtani, A.M.; Fantoukh, O.I.; Herqash, R.N.; Elzayat, E.M.; Nasr, F.A.; Ezzeldin, E.; Almousallam, M.M.; Raish, M. Effects of Catha edulis (Khat) on the Pharmacokinetics of Metformin in Diabetic Rats Using UPLC/MS/MS Analysis and Its Impact on Hepatic CYP450 Enzymes. Separations 2023, 10, 442. https://doi.org/10.3390/separations10080442
Alqahtani AS, Parvez MK, Alqahtani AM, Fantoukh OI, Herqash RN, Elzayat EM, Nasr FA, Ezzeldin E, Almousallam MM, Raish M. Effects of Catha edulis (Khat) on the Pharmacokinetics of Metformin in Diabetic Rats Using UPLC/MS/MS Analysis and Its Impact on Hepatic CYP450 Enzymes. Separations. 2023; 10(8):442. https://doi.org/10.3390/separations10080442
Chicago/Turabian StyleAlqahtani, Ali S., Mohammad Khalid Parvez, Abdulaziz M. Alqahtani, Omer I. Fantoukh, Rashed N. Herqash, Ehab M. Elzayat, Fahd A. Nasr, Essam Ezzeldin, Mousallam M. Almousallam, and Mohammad Raish. 2023. "Effects of Catha edulis (Khat) on the Pharmacokinetics of Metformin in Diabetic Rats Using UPLC/MS/MS Analysis and Its Impact on Hepatic CYP450 Enzymes" Separations 10, no. 8: 442. https://doi.org/10.3390/separations10080442
APA StyleAlqahtani, A. S., Parvez, M. K., Alqahtani, A. M., Fantoukh, O. I., Herqash, R. N., Elzayat, E. M., Nasr, F. A., Ezzeldin, E., Almousallam, M. M., & Raish, M. (2023). Effects of Catha edulis (Khat) on the Pharmacokinetics of Metformin in Diabetic Rats Using UPLC/MS/MS Analysis and Its Impact on Hepatic CYP450 Enzymes. Separations, 10(8), 442. https://doi.org/10.3390/separations10080442








