Enhancement of Photocatalytic Rhodamine B Degradation over Magnesium–Manganese Baring Extracted Iron Oxalate from Converter Slag
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Iron Oxalate from Converter Slag (FeOX-Slag) and Iron Oxalate from Iron(III) Oxide (FeOX-Fe2O3)
2.3. Characterization
2.4. Photocatalytic Rhodamine B (RhB) Removal
3. Results and Discussion
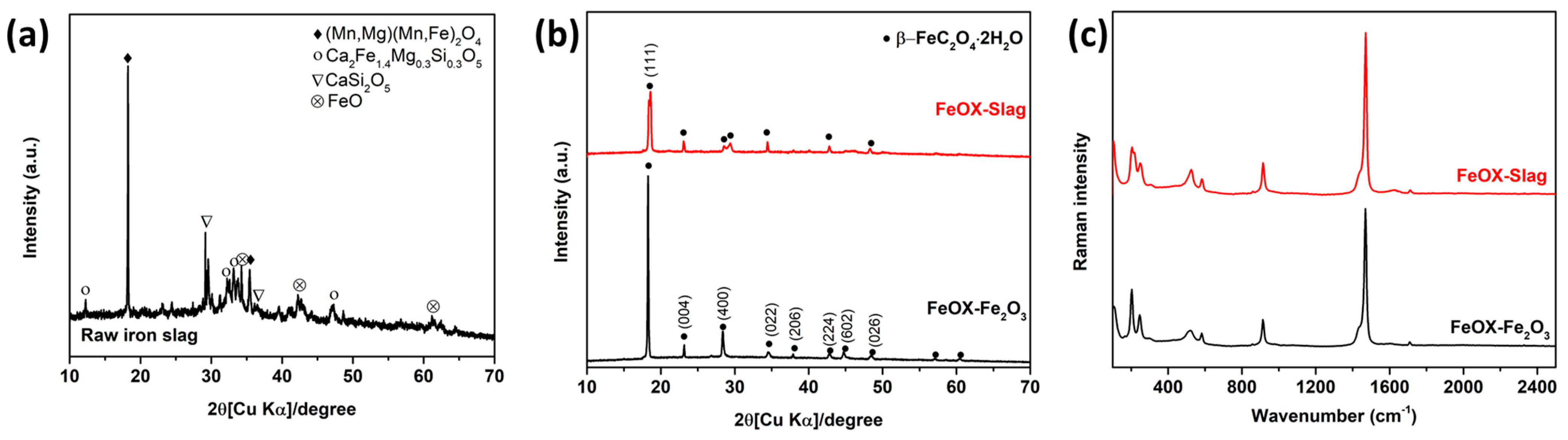
3.1. Exploration of the Phase Composition and Chemical Characteristics
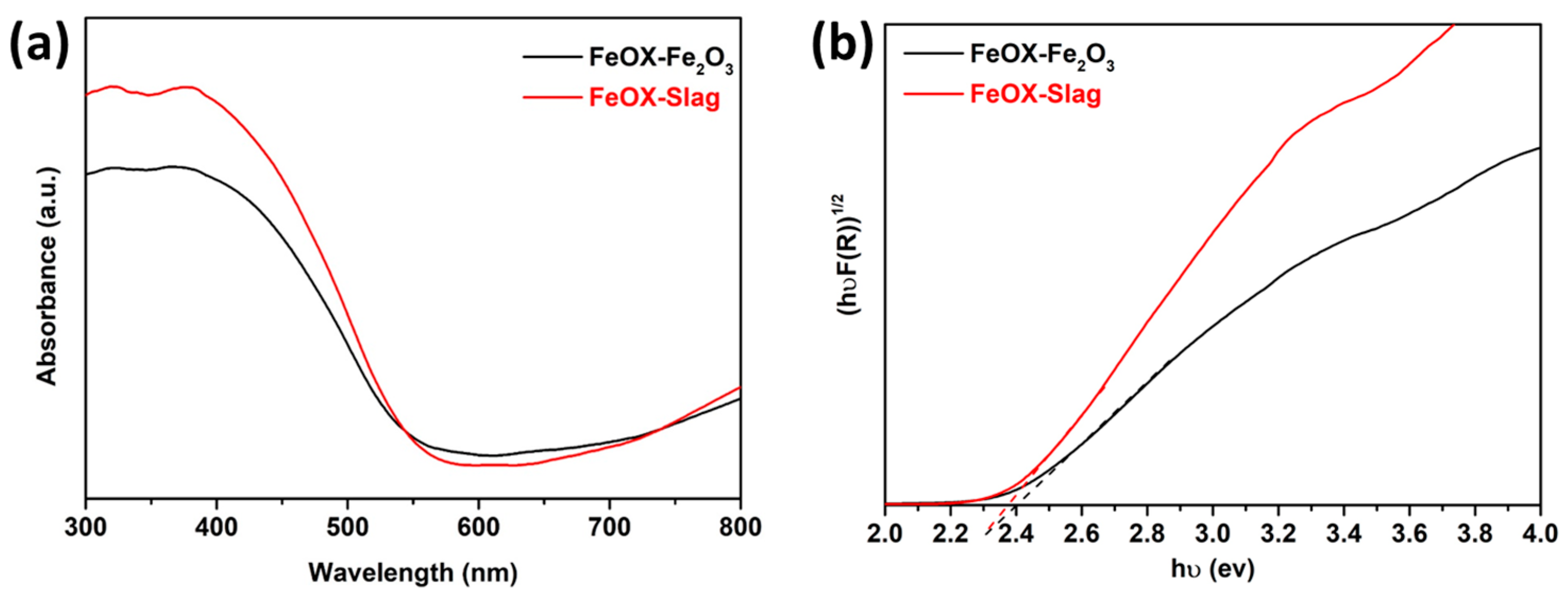
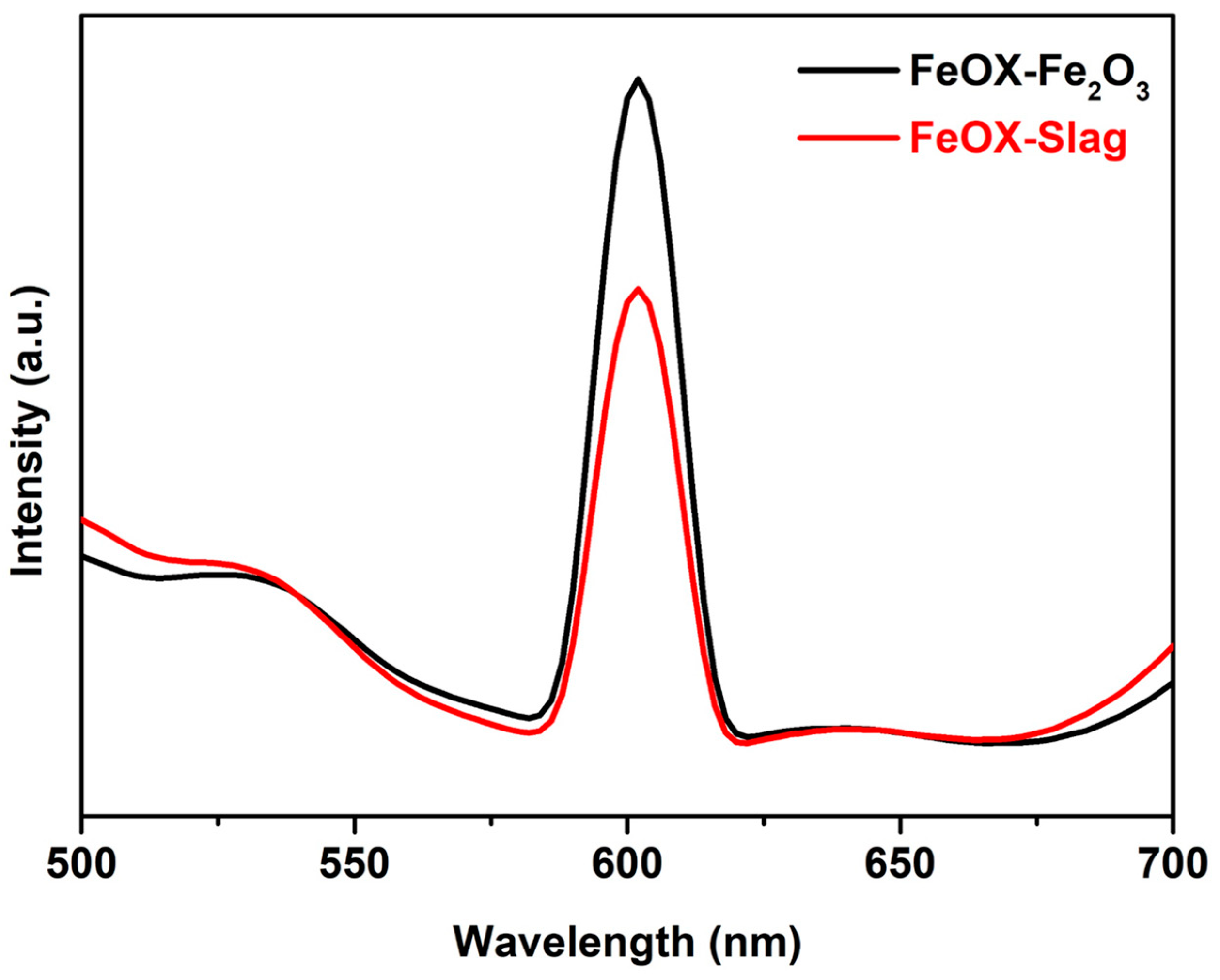
3.2. Optical Characteristics
3.3. Morphology Investigation
3.4. XPS Results
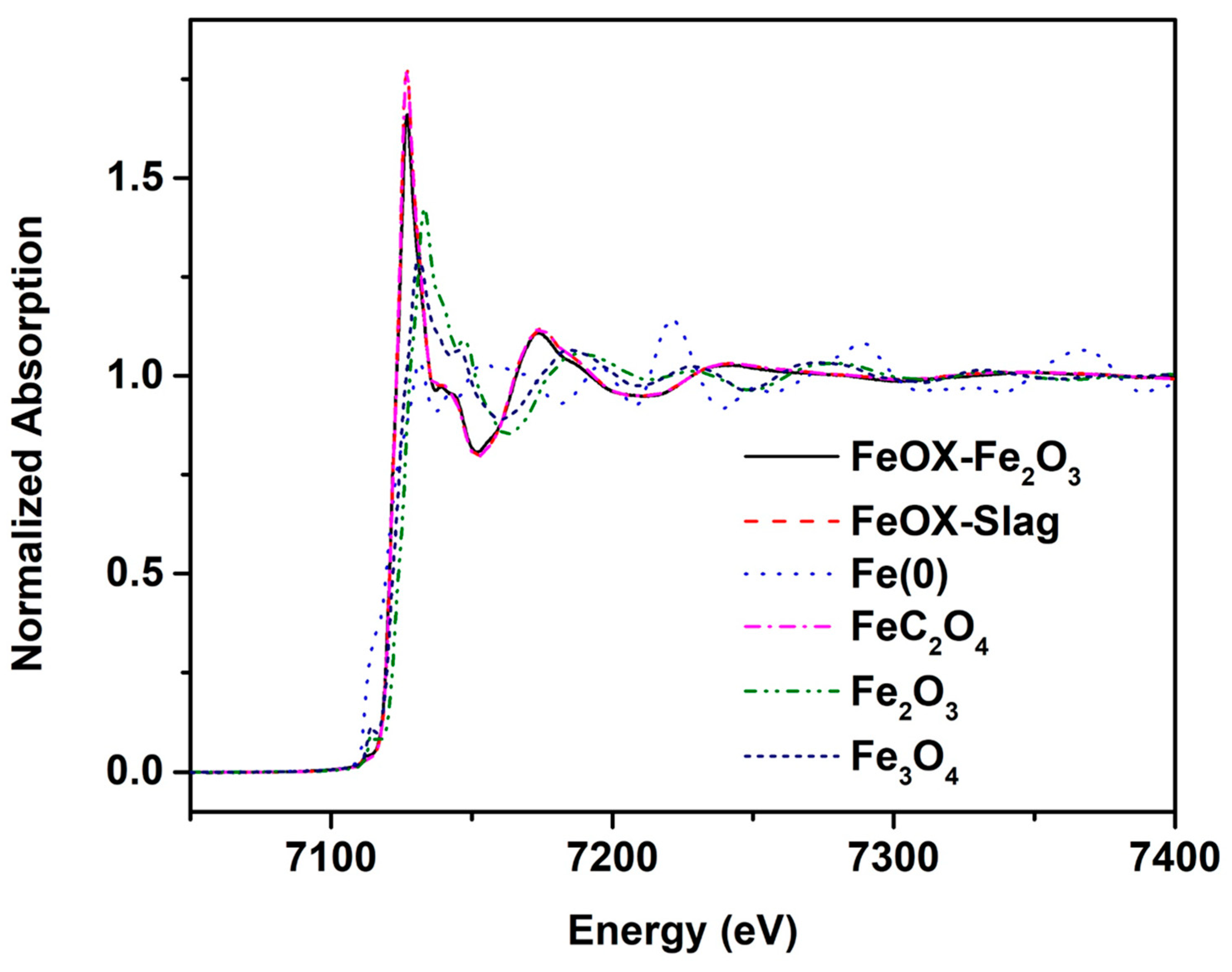
3.5. X-ray Absorption near Edge Structure (XANES) Result
3.6. Charge Separation Properties
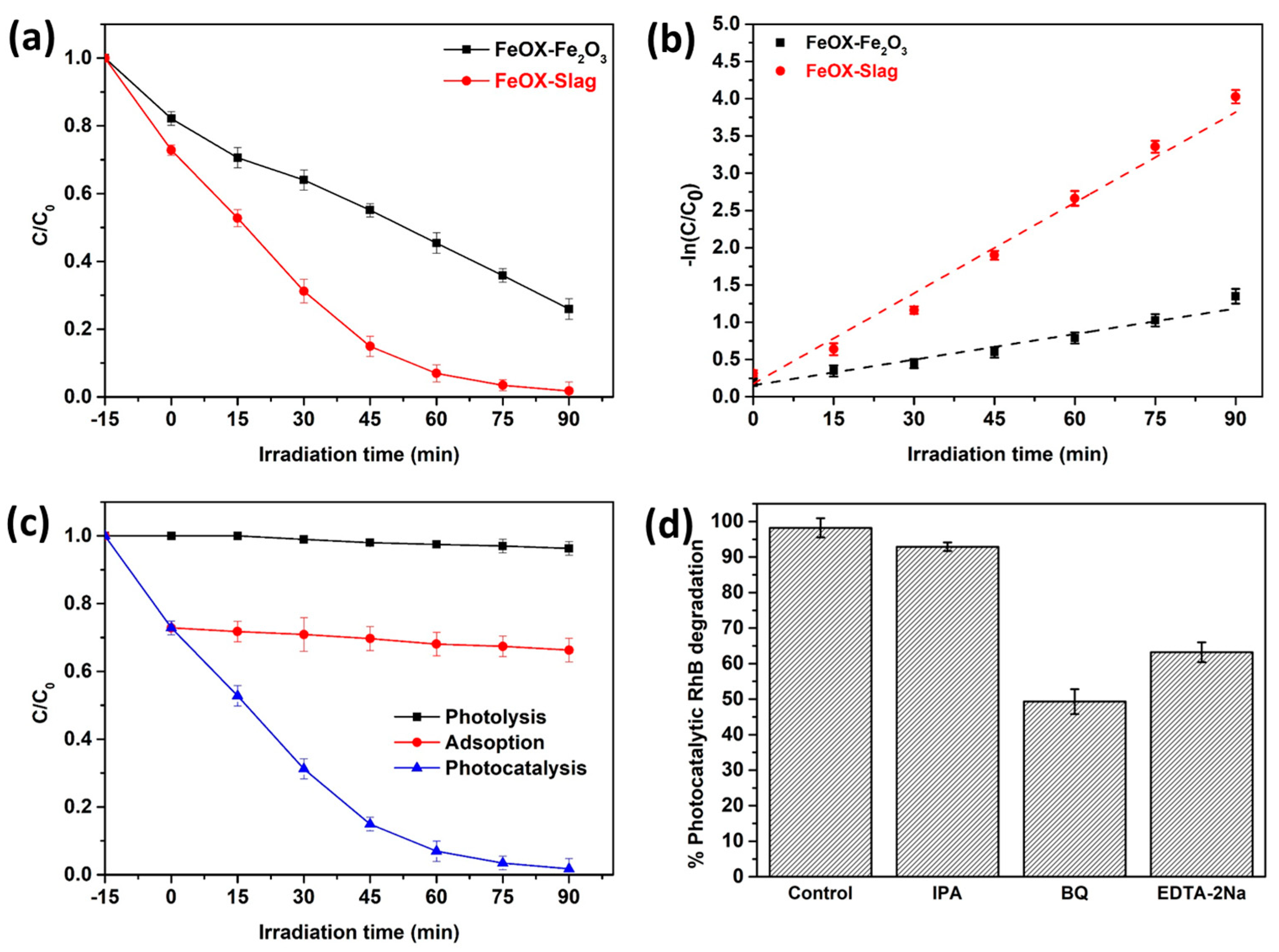
3.7. Photocatalytic RhB Degradation
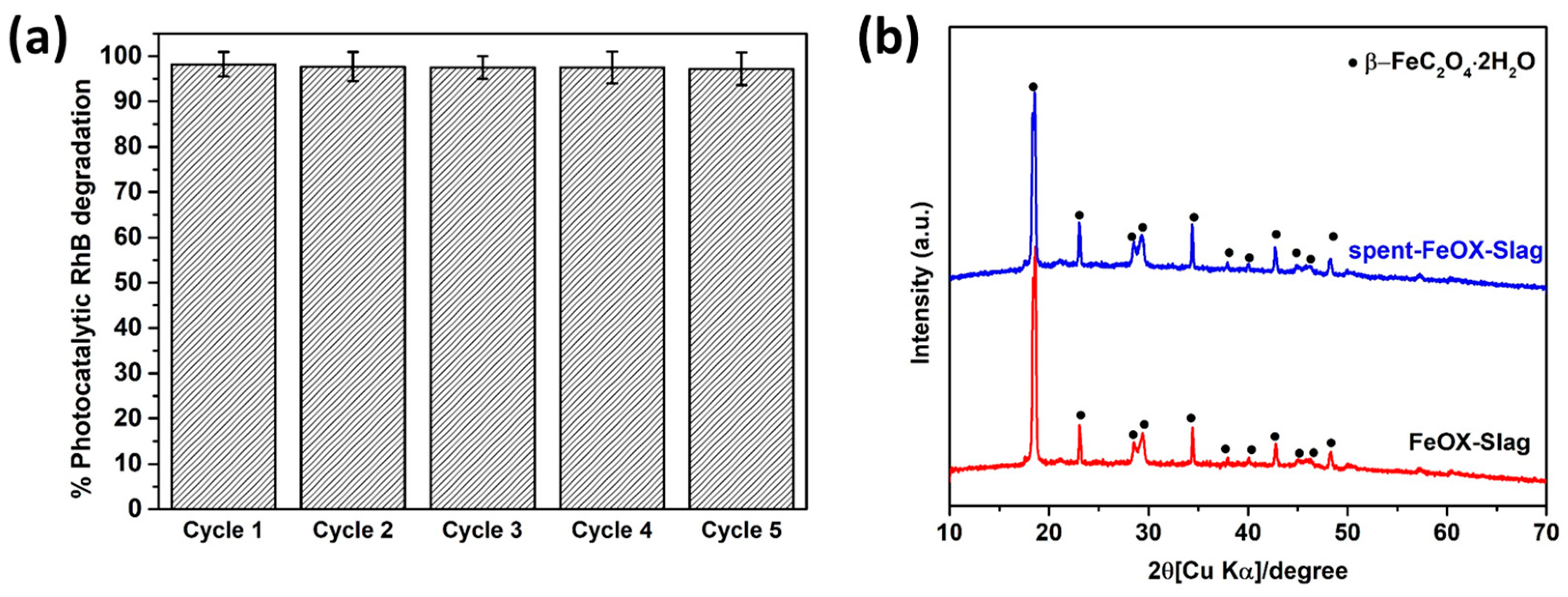
3.8. Stability
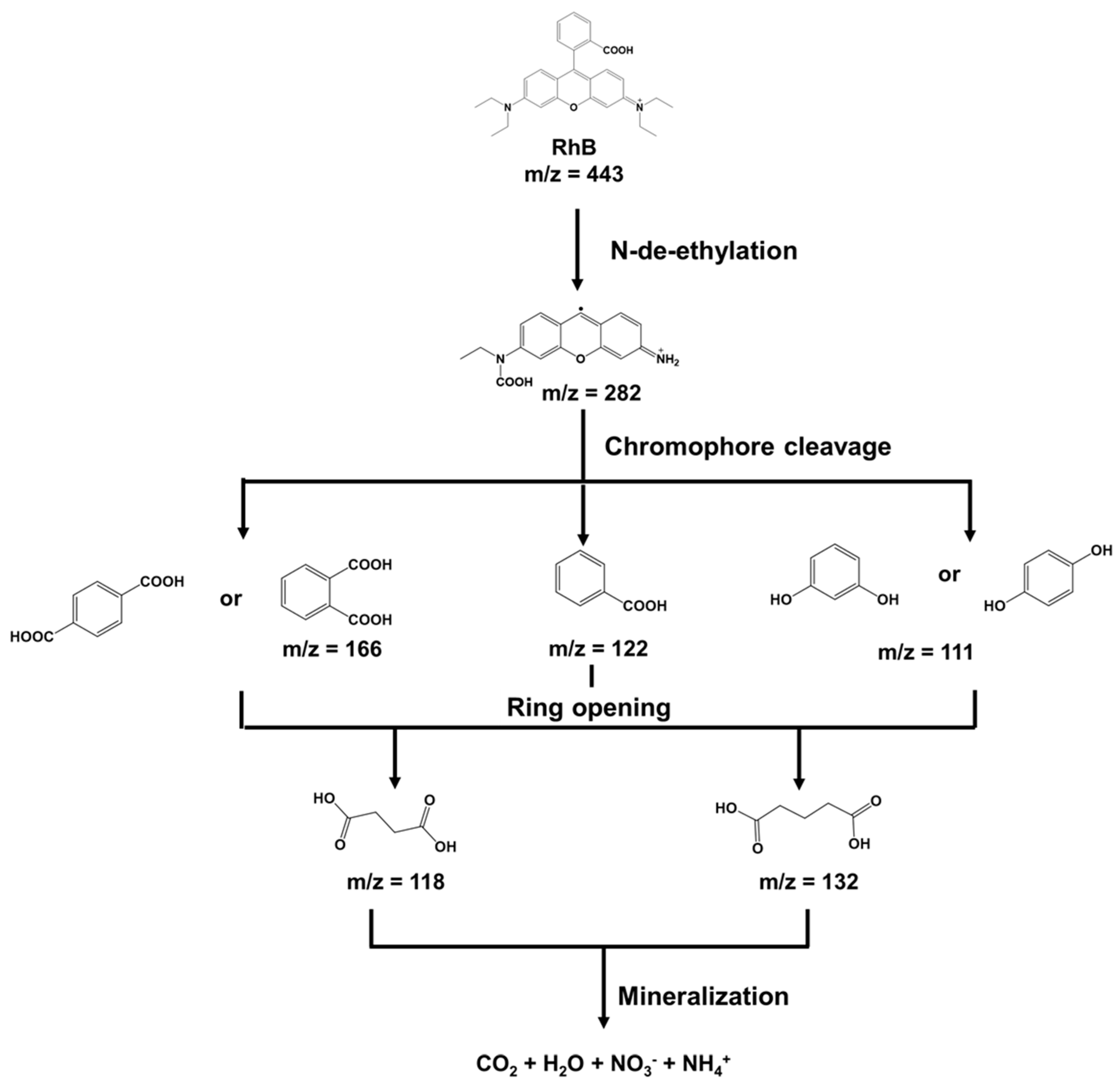
3.9. Possible RhB Removal Process
3.10. Photocatalytic Mechanism
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Liu, T.; Wang, Y.; Li, J.; Yu, Q.; Wang, X.; Gao, D.; Wang, F.; Cai, S.; Zeng, Y. Effects from Converter Slag and Electric Arc Furnace Slag on Chlorophyll a Accumulation of Nannochloropsis sp. Appl. Sci. 2021, 11, 9127. [Google Scholar] [CrossRef]
- Fusco, C.; Casiello, M.; Pisani, P.; Monopoli, A.; Fanelli, F.; Oberhauser, W.; Attrotto, R.; Nacci, A.; D’Accolti, L. Steel slag as low-cost catalyst for artificial photosynthesis to convert CO2 and water into hydrogen and methanol. Sci. Rep. 2022, 12, 11378. [Google Scholar] [CrossRef]
- Huo, B.; Li, B.; Huang, S.; Chen, C.; Zhang, Y.; Banthia, N. Hydration and soundness properties of phosphoric acid modified steel slag powder. Constr. Build. Mater. 2020, 254, 119319. [Google Scholar] [CrossRef]
- Shu, K.; Sasaki, K. Occurrence of steel converter slag and its high value-added conversion for environmental restoration in China: A review. J. Clean. Prod. 2022, 373, 133876. [Google Scholar] [CrossRef]
- Inoue, T.; Chuaicham, C.; Saito, N.; Ohtani, B.; Sasaki, K. Z-scheme heterojunction of graphitic carbon nitride and calcium ferrite in converter slag for the photocatalytic imidacloprid degradation and hydrogen evolution. J. Photochem. Photobiol. A Chem. 2023, 440, 114644. [Google Scholar] [CrossRef]
- Zhang, Z.; Lü, H.; Li, X.; Li, X.; Ran, S.; Chen, Z.; Yang, Y.; Wu, X.; Li, L. Conversion of CaTi1–xMnxO3–δ-Based Photocatalyst for Photocatalytic Reduction of NO via Structure-Reforming of Ti-Bearing Blast Furnace Slag. ACS Sustain. Chem. Eng. 2019, 7, 10299–10309. [Google Scholar] [CrossRef]
- Liu, G.; Schollbach, K.; van der Laan, S.; Tang, P.; Florea, M.V.A.; Brouwers, H.J.H. Recycling and utilization of high volume converter steel slag into CO2 activated mortars—The role of slag particle size. Resour. Conserv. Recycl. 2020, 160, 104883. [Google Scholar] [CrossRef]
- Zhou, W.; Liu, X.; Lyu, X.; Gao, W.; Su, H.; Li, C. Extraction and separation of copper and iron from copper smelting slag: A review. J. Clean. Prod. 2022, 368, 133095. [Google Scholar] [CrossRef]
- Liu, F.; Peng, C.; Wilson, B.P.; Lundström, M. Oxalic Acid Recovery from High Iron Oxalate Waste Solution by a Combination of Ultrasound-Assisted Conversion and Cooling Crystallization. ACS Sustain. Chem. Eng. 2019, 7, 17372–17378. [Google Scholar] [CrossRef]
- Santawaja, P.; Kudo, S.; Mori, A.; Tahara, A.; Asano, S.; Hayashi, J.-I. Sustainable Iron-Making Using Oxalic Acid: The Concept, A Brief Review of Key Reactions, and An Experimental Demonstration of the Iron-Making Process. ACS Sustain. Chem. Eng. 2020, 8, 13292–13301. [Google Scholar] [CrossRef]
- Kim, E.J.; Baek, K. Selective recovery of ferrous oxalate and removal of arsenic and other metals from soil-washing wastewater using a reduction reaction. J. Clean. Prod. 2019, 221, 635–643. [Google Scholar] [CrossRef]
- Fu, L.; Huang, Z.; Zhou, X.; Deng, L.; Liao, M.; Yang, S.; Chen, S.; Wang, H.; Wang, L. Ferrous-Oxalate-Modified Aramid Nanofibers Heterogeneous Fenton Catalyst for Methylene Blue Degradation. Polymers 2022, 14, 3491. [Google Scholar] [CrossRef]
- Lee, J.; Kim, J.; Choi, W. Oxidation of aquatic pollutants by ferrous-oxalate complexes under dark aerobic conditions. J. Hazard. Mater. 2014, 274, 79–86. [Google Scholar] [CrossRef]
- Wang, G.; Zhou, A.; Xu, Q. α-Ferrous oxalate with different micro scale: Synthesis and catalytic degradation effect to rhodamine B. Solid State Sci. 2019, 91, 54–60. [Google Scholar] [CrossRef]
- Sarmah, K.; Pratihar, S. Synthesis, Characterization, and Photocatalytic Application of Iron Oxalate Capped Fe, Fe–Cu, Fe–Co, and Fe–Mn Oxide Nanomaterial. ACS Sustain. Chem. Eng. 2016, 5, 310–324. [Google Scholar] [CrossRef]
- Liu, Z.-J.; Liu, W.; Wang, Y.; Guo, M.-L. Preparation of β-ferrous oxalate dihydrate layered nanosheets by mechanochemical method and its visible-light-driven photocatalytic performance. Mater. Lett. 2016, 178, 83–86. [Google Scholar] [CrossRef]
- Conde-Morales, I.I.; Hinojosa-Reyes, L.; Guzmán-Mar, J.L.; Hernández-Ramírez, A.; Sáenz-Tavera, I.d.C.; Villanueva-Rodríguez, M. Different Iron Oxalate Sources as Catalysts on Pyrazinamide Degradation by the Photo-Fenton Process at Different pH Values. Water Air Soil Pollut. 2020, 231, 425. [Google Scholar] [CrossRef]
- Luo, Z.; Min, Y.; Qu, L.; Song, Y.; Hong, Y. Remediation of phenanthrene contaminated soil by ferrous oxalate and its phytotoxicity evaluation. Chemosphere 2021, 265, 129070. [Google Scholar] [CrossRef]
- Chuaicham, C.; Trakulmututa, J.; Shu, K.; Shenoy, S.; Srikhaow, A.; Zhang, L.; Mohan, S.; Sekar, K.; Sasaki, K. Recent Clay-Based Photocatalysts for Wastewater Treatment. Separations 2023, 10, 77. [Google Scholar] [CrossRef]
- Trakulmututa, J.; Chuaicham, C.; Shenoy, S.; Srikhaow, A.; Sasaki, K.; Smith, S.M. Effect of transformation temperature toward optical properties of derived CuO/ZnO composite from Cu–Zn hydroxide nitrate for photocatalytic ciprofloxacin degradation. Opt. Mater. 2022, 133, 112941. [Google Scholar] [CrossRef]
- Hao, D.; Huang, Q.; Wei, W.; Bai, X.; Ni, B.-J. A reusable, separation-free and biodegradable calcium alginate/g-C3N4 microsphere for sustainable photocatalytic wastewater treatment. J. Clean. Prod. 2021, 314, 128033. [Google Scholar] [CrossRef]
- Balu, S.; Chuaicham, C.; Balakumar, V.; Rajendran, S.; Sasaki, K.; Sekar, K.; Maruthapillai, A. Recent development on core-shell photo(electro)catalysts for elimination of organic compounds from pharmaceutical wastewater. Chemosphere 2022, 298, 134311. [Google Scholar] [CrossRef]
- Shanmugam, M.; Augustin, A.; Mohan, S.; Honnappa, B.; Chuaicham, C.; Rajendran, S.; Hoang, T.K.A.; Sasaki, K.; Sekar, K. Conducting polymeric nanocomposites: A review in solar fuel applications. Fuel 2022, 325, 124899. [Google Scholar] [CrossRef]
- Edwards, H.G.M.; Russell, N.C. Vibrational spectroscopic study of iron(II) and iron(III) oxalates. J. Mol. Struct. 1998, 443, 223–231. [Google Scholar] [CrossRef]
- Makuła, P.; Pacia, M.; Macyk, W. How To Correctly Determine the Band Gap Energy of Modified Semiconductor Photocatalysts Based on UV–Vis Spectra. J. Phys. Chem. Lett. 2018, 9, 6814–6817. [Google Scholar] [CrossRef]
- Chuaicham, C.; Inoue, T.; Balakumar, V.; Tian, Q.; Ohtani, B.; Sasaki, K. Visible light-driven ZnCr double layer oxide photocatalyst composites with fly ashes for the degradation of ciprofloxacin. J. Environ. Chem. Eng. 2022, 10, 106970. [Google Scholar] [CrossRef]
- Chuaicham, C.; Sekar, K.; Balakumar, V.; Mittraphab, Y.; Shimizu, K.; Ohtani, B.; Sasaki, K. Fabrication of graphitic carbon nitride/ZnTi-mixed metal oxide heterostructure: Robust photocatalytic decomposition of ciprofloxacin. J. Alloys Compd. 2022, 906, 164294. [Google Scholar] [CrossRef]
- Keshavarz, F.; Kadek, M.; Barbiellini, B.; Bansil, A. Anodic Activity of Hydrated and Anhydrous Iron (II) Oxalate in Li-Ion Batteries. Condens. Matter 2022, 7, 8. [Google Scholar]
- Balakumar, V.; Manivannan, R.; Chuaicham, C.; Karthikeyan, S.; Sasaki, K. A simple tactic synthesis of hollow porous graphitic carbon nitride with significantly enhanced photocatalytic performance. Chem. Commun. 2021, 57, 6772–6775. [Google Scholar] [CrossRef]
- Pawar, R.R.; Chuaicham, C.; Sekar, K.; Rajendran, S.; Sasaki, K. Synthesis, characterization, and application of MOF@clay composite as a visible light-driven photocatalyst for Rhodamine B degradation. Chemosphere 2022, 291, 132922. [Google Scholar] [CrossRef]
- Chenakin, S.; Kruse, N. Thermal Decomposition of Nickel Oxalate Dihydrate: A Detailed XPS Insight. J. Phys. Chem. C 2019, 123, 30926–30936. [Google Scholar] [CrossRef]
- Li, Y.; Wu, H.; Wu, Y.; Li, Q. Facile synthesis of mesoporous Co3O4 nanowires for application in supercapacitors. J. Mater. Sci. Mater. Electron. 2017, 28, 16826–16835. [Google Scholar] [CrossRef]
- Li, L.; Ma, P.; Hussain, S.; Jia, L.; Lin, D.; Yin, X.; Lin, Y.; Cheng, Z.; Wang, L. FeS2/carbon hybrids on carbon cloth: A highly efficient and stable counter electrode for dye-sensitized solar cells. Sustain. Energy Fuels 2019, 3, 1749–1756. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, B.; Chuaicham, C.; Sasaki, K. Mechanism analysis of selenium (VI) immobilization using alkaline-earth metal oxides and ferrous salt. Chemosphere 2020, 248, 126123. [Google Scholar] [CrossRef]
- Srikhaow, A.; Smith, S.M.; Uraisin, K.; Suttiponparnit, K.; Kongmark, C.; Chuaicham, C. Catalytic remediation of phenol contaminated wastewater using Cu-Zn hydroxide nitrate. RSC Adv. 2016, 6, 36766–36774. [Google Scholar] [CrossRef]
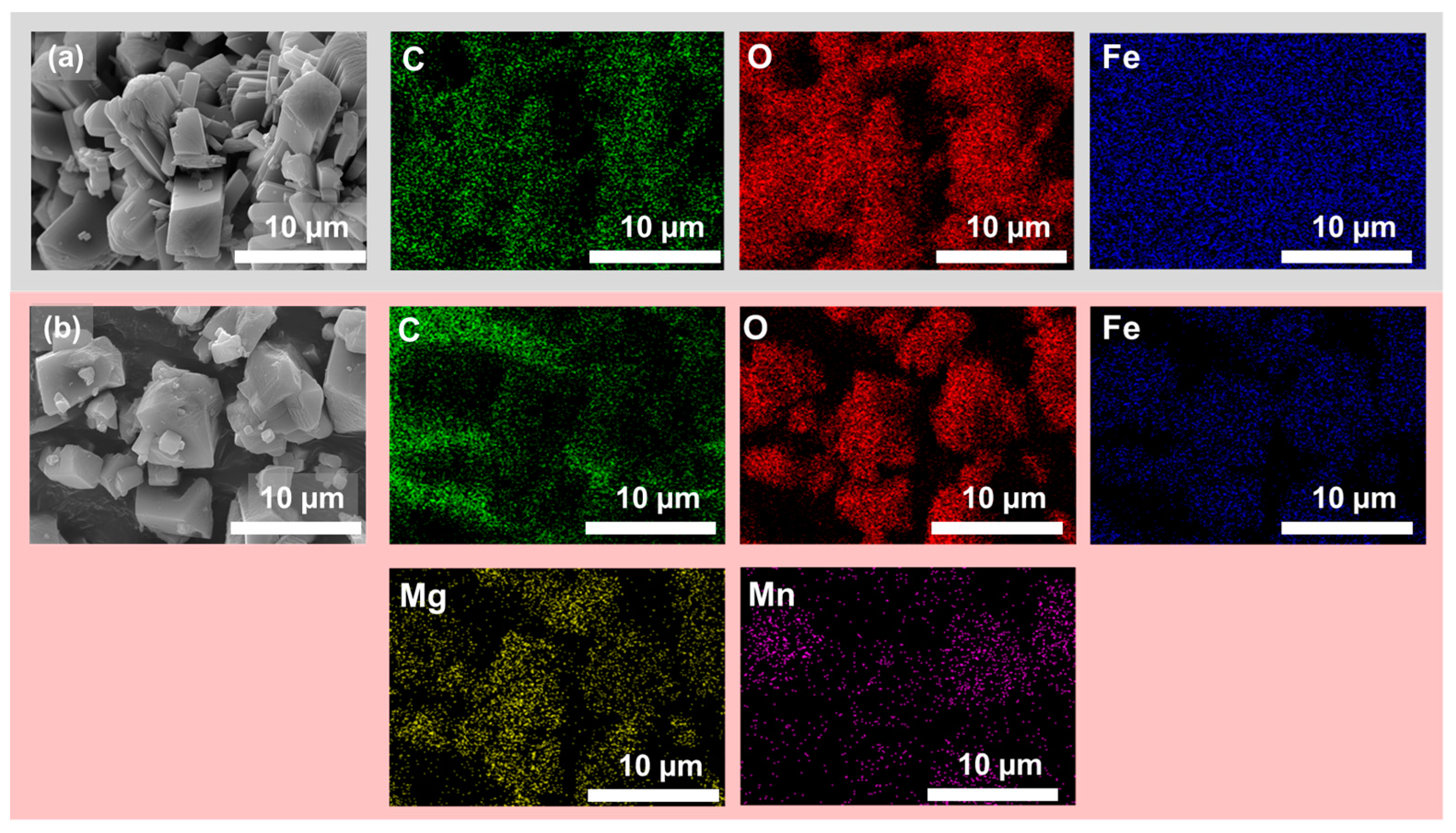


| C | O | Fe | Mg | Mn | |
|---|---|---|---|---|---|
| FeOX-Fe2O3 | 26.4 | 66.3 | 7.3 | n.d. | n.d. |
| FeOX-Slag | 35.5 | 56.0 | 6.8 | 1.1 | 0.6 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuaicham, C.; Trakulmututa, J.; Shenoy, S.; Balakumar, V.; Santawaja, P.; Kudo, S.; Sekar, K.; Sasaki, K. Enhancement of Photocatalytic Rhodamine B Degradation over Magnesium–Manganese Baring Extracted Iron Oxalate from Converter Slag. Separations 2023, 10, 440. https://doi.org/10.3390/separations10080440
Chuaicham C, Trakulmututa J, Shenoy S, Balakumar V, Santawaja P, Kudo S, Sekar K, Sasaki K. Enhancement of Photocatalytic Rhodamine B Degradation over Magnesium–Manganese Baring Extracted Iron Oxalate from Converter Slag. Separations. 2023; 10(8):440. https://doi.org/10.3390/separations10080440
Chicago/Turabian StyleChuaicham, Chitiphon, Jirawat Trakulmututa, Sulakshana Shenoy, Vellaichamy Balakumar, Phatchada Santawaja, Shinji Kudo, Karthikeyan Sekar, and Keiko Sasaki. 2023. "Enhancement of Photocatalytic Rhodamine B Degradation over Magnesium–Manganese Baring Extracted Iron Oxalate from Converter Slag" Separations 10, no. 8: 440. https://doi.org/10.3390/separations10080440
APA StyleChuaicham, C., Trakulmututa, J., Shenoy, S., Balakumar, V., Santawaja, P., Kudo, S., Sekar, K., & Sasaki, K. (2023). Enhancement of Photocatalytic Rhodamine B Degradation over Magnesium–Manganese Baring Extracted Iron Oxalate from Converter Slag. Separations, 10(8), 440. https://doi.org/10.3390/separations10080440










