Optimization of Gallic Acid-Rich Extract from Mango (Mangifera indica) Seed Kernels through Ultrasound-Assisted Extraction
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Sample Material
2.3. Sample Preparation via UAE
2.4. Experimental Design
2.4.1. Single-Factor Experiments
2.4.2. Multiple-Factor Experiment
2.5. HPLC Analysis
2.6. Characterization of Optimized MSK Extract in Comparison with Conventional Extraction Method
2.6.1. Determination of TPC
2.6.2. Determination of TFC
2.6.3. Determination of Radical Scavenging Activity
2.6.4. Determination of Antimicrobial Activity
2.7. Statistical Analysis
3. Results and Discussion
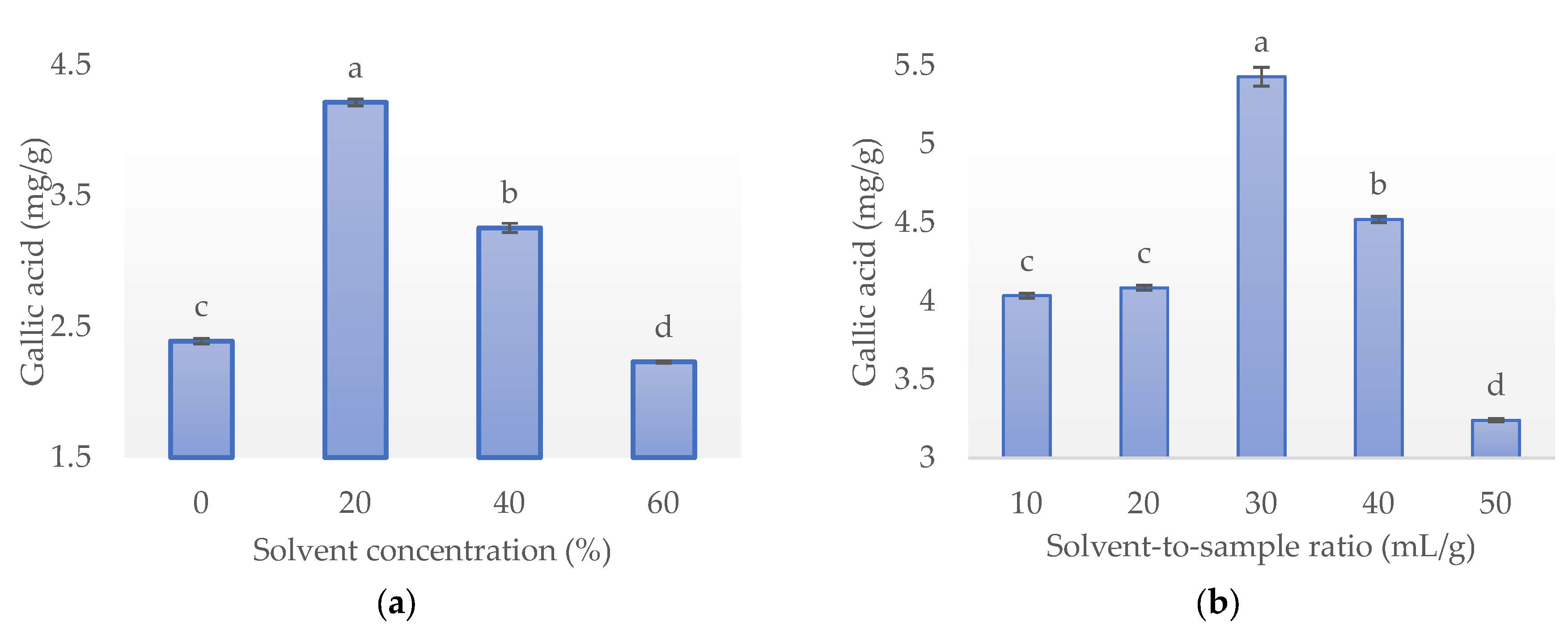
3.1. Single-Factor Experiments
3.1.1. Effect of Solvent Concentration
3.1.2. Effect of Solvent-to-Sample Ratio
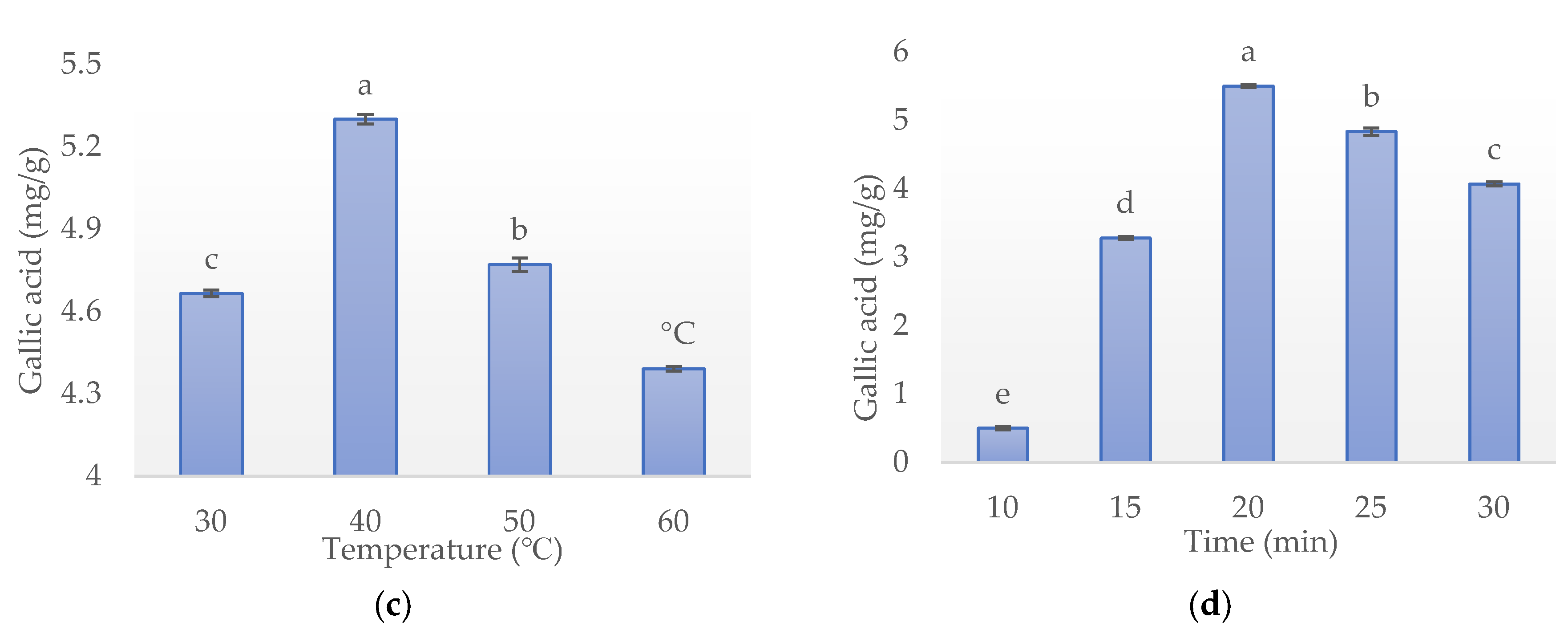
3.1.3. Effect of Temperature
3.1.4. Effect of Time
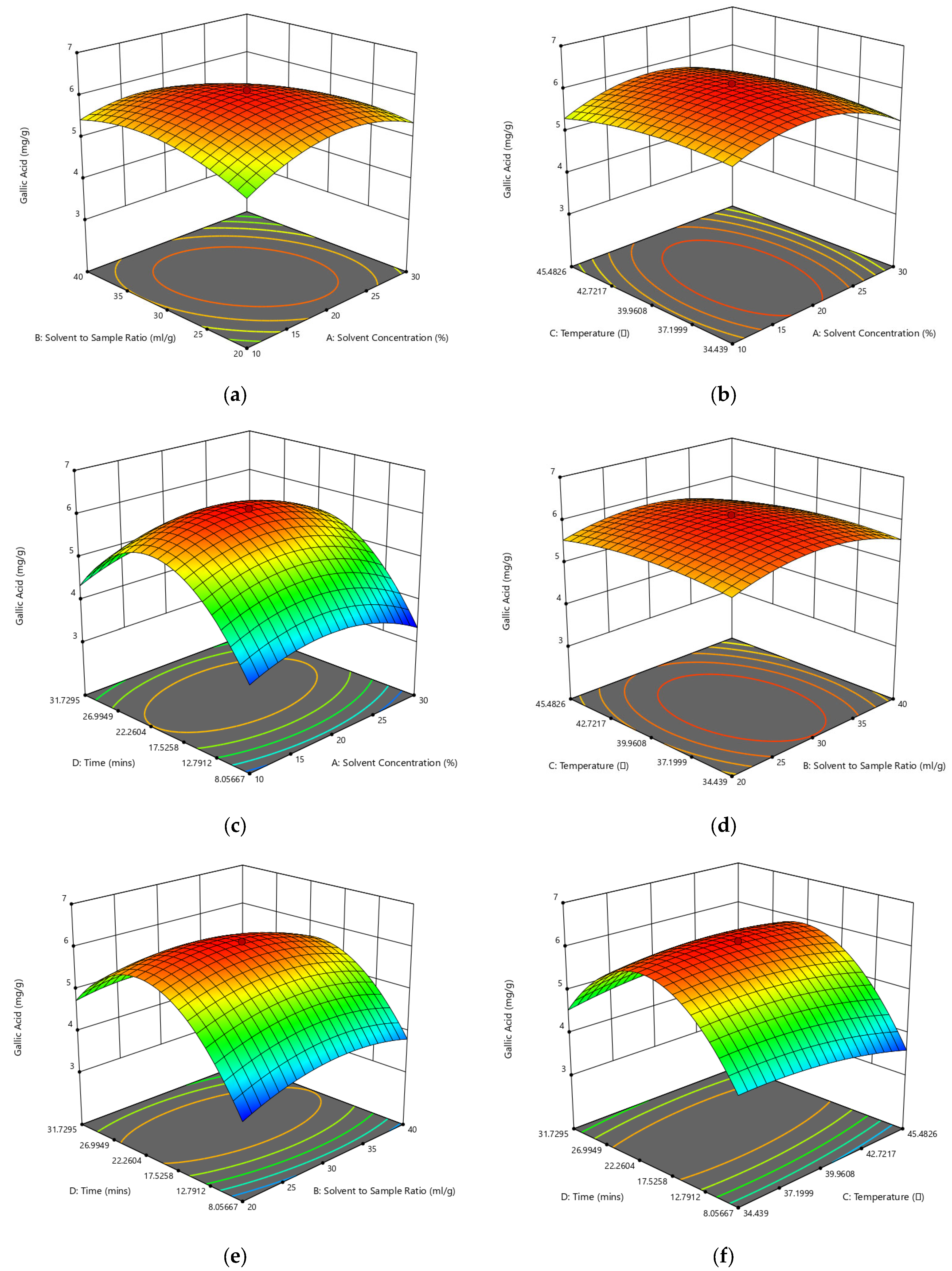
3.2. Optimization via Response Surface Methodology (RSM)
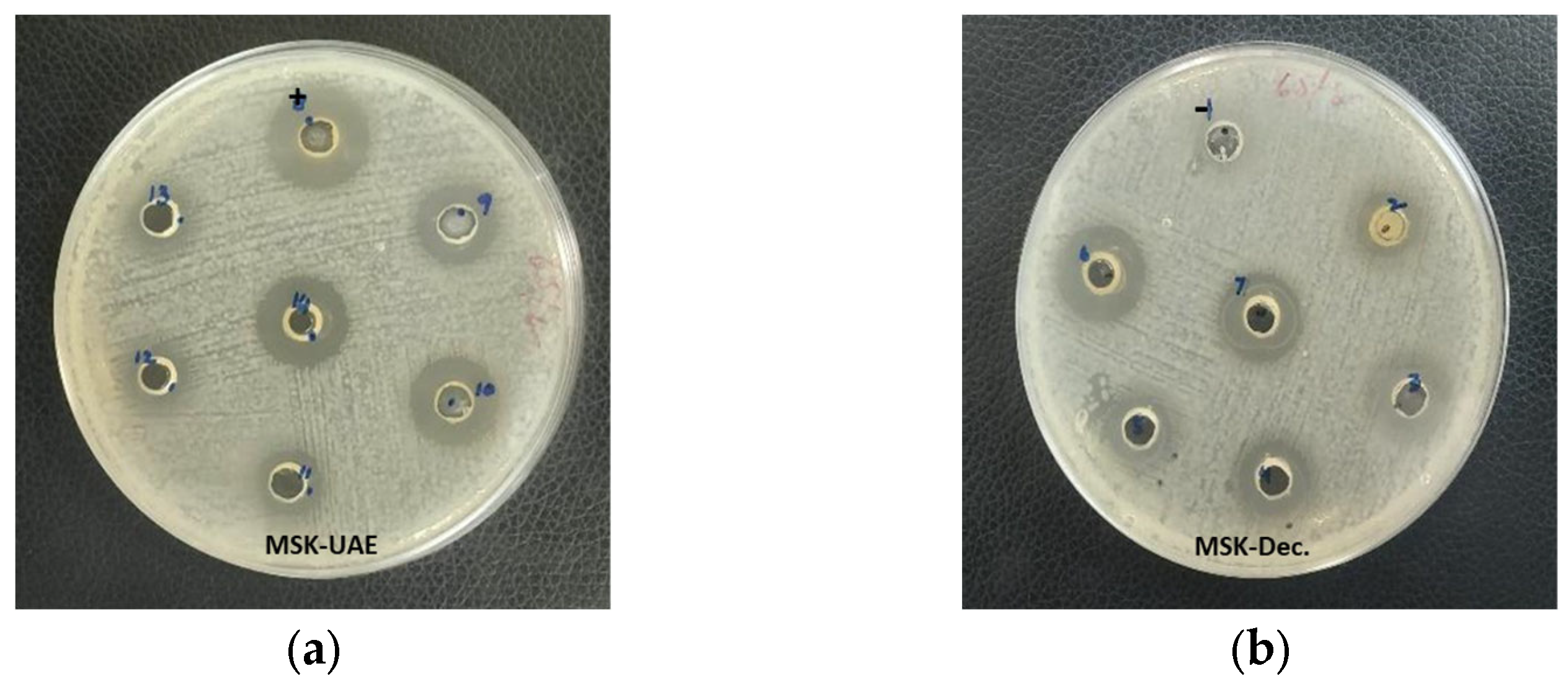
3.3. Characterization of Optimized MSK Extract in Comparison with Conventional Extraction Method
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Kobayashi, M.; Matsui-Yuasa, I.; Fukuda-Shimizu, M.; Mandai, Y.; Tabuchi, M.; Munakata, H.; Kojima-Yuasa, A. Effect of mango seed kernel extract on the adipogenesis in 3T3-L1 adipocytes and in rats fed a high fat diet. Health 2013, 5, 9–15. [Google Scholar] [CrossRef]
- Diarra, S.S. Potential of mango (Mangifera indica L.) seed kernel as a feed ingredient for poultry: A review. World’s Poult. Sci. J. 2014, 70, 279–288. [Google Scholar] [CrossRef]
- Masibo, M.; He, Q. Major mango polyphenols and their potential significance to human health. Compr. Rev. Food Sci. Food Saf. 2008, 7, 309–319. [Google Scholar] [CrossRef] [PubMed]
- Castro-Vargas, H.I.; Ballesteros Vivas, D.; Ortega Barbosa, J.; Morantes Medina, S.J.; Aristizabal Gutiérrez, F.; Parada-Alfonso, F. Bioactive phenolic compounds from the agroindustrial waste of Colombian mango cultivars ‘Sugar Mango’and ‘Tommy Atkins’—An alternative for their use and valorization. Antioxidants 2019, 8, 41. [Google Scholar] [CrossRef] [PubMed]
- Masibo, M.; He, Q. Mango bioactive compounds and related nutraceutical properties—A review. Food Rev. Int. 2009, 25, 346–370. [Google Scholar] [CrossRef]
- Abdalla, A.E.; Darwish, S.M.; Ayad, E.H.; El-Hamahmy, R.M. Egyptian mango by-product 1. Compositional quality of mango seed kernel. Food Chem. 2007, 103, 1134–1140. [Google Scholar] [CrossRef]
- Ruales, J.; Baenas, N.; Moreno, D.A.; Stinco, C.M.; Meléndez-Martínez, A.J.; García-Ruiz, A. Biological active ecuadorian mango ‘tommy atkins’ ingredients—An opportunity to reduce agrowaste. Nutrients 2018, 10, 1138. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aty, A.M.; Salama, W.H.; Hamed, M.B.; Fahmy, A.S.; Mohamed, S.A. Phenolic-antioxidant capacity of mango seed kernels: Therapeutic effect against viper venoms. Rev. Bras. Farmacogn. 2018, 28, 594–601. [Google Scholar] [CrossRef]
- Jung, S.; Choe, J.H.; Kim, B.; Yun, H.; Kruk, Z.A.; Jo, C. Effect of dietary mixture of gallic acid and linoleic acid on antioxidative potential and quality of breast meat from broilers. Meat Sci. 2010, 86, 520–526. [Google Scholar] [CrossRef]
- Lee, K.H.; Jung, S.; Kim, H.J.; Kim, I.S.; Lee, J.H.; Jo, C. Effect of dietary supplementation of the combination of gallic and linoleic acid in thigh meat of broilers. Asian-Australas. J. Anim. Sci. 2012, 25, 1641. [Google Scholar] [CrossRef]
- Kim, D.-O.; Lee, K.W.; Lee, H.J.; Lee, C.Y. Vitamin C equivalent antioxidant capacity (VCEAC) of phenolic phytochemicals. J. Agric. Food Chem. 2002, 50, 3713–3717. [Google Scholar] [CrossRef]
- Özçelik, B.; Kartal, M.; Orhan, I. Cytotoxicity, antiviral and antimicrobial activities of alkaloids, flavonoids, and phenolic acids. Pharm. Biol. 2011, 49, 396–402. [Google Scholar] [CrossRef]
- Rawdkuen, S.; Sai-Ut, S.; Benjakul, S. Optimizing the tyrosinase inhibitory and antioxidant activity of mango seed kernels with a response surface methodology. Food Anal. Methods 2016, 9, 3032–3043. [Google Scholar] [CrossRef]
- Dorta, E.; Lobo, M.G.; González, M. Optimization of factors affecting extraction of antioxidants from mango seed. Food Bioprocess Technol. 2013, 6, 1067–1081. [Google Scholar] [CrossRef]
- Tiwari, B.K. Ultrasound: A clean, green extraction technology. TrAC Trends Anal. Chem. 2015, 71, 100–109. [Google Scholar] [CrossRef]
- Chemat, F.; Vian, M.A.; Cravotto, G. Green extraction of natural products: Concept and principles. Int. J. Mol. Sci. 2012, 13, 8615–8627. [Google Scholar] [CrossRef] [PubMed]
- Gao, Y.; Wang, Z.; Luo, L.; Dai, J.; Li, D. Optimization of ultrasound-assisted aqueous two-phase extraction of polyphenols from mango seed kernel. Trans. Chin. Soc. Agric. Eng. 2012, 28, 255–261. [Google Scholar]
- Ávila, N.S.; Capote, F.P.; De Castro, M.L. Ultrasound-assisted extraction and silylation prior to gas chromatography–mass spectrometry for the characterization of the triterpenic fraction in olive leaves. J. Chromatogr. A 2007, 1165, 158–165. [Google Scholar] [CrossRef] [PubMed]
- Liu, W.; Yu, Y.; Yang, R.; Wan, C.; Xu, B.; Cao, S. Optimization of total flavonoid compound extraction from Gynura medica leaf using response surface methodology and chemical composition analysis. Int. J. Mol. Sci. 2010, 11, 4750–4763. [Google Scholar] [CrossRef] [PubMed]
- Pan, X.; Niu, G.; Liu, H. Microwave-assisted extraction of tea polyphenols and tea caffeine from green tea leaves. Chem. Eng. Process. Process Intensif. 2003, 42, 129–133. [Google Scholar] [CrossRef]
- Aznar-Ramos, M.J.; Razola-Díaz, M.D.C.; Verardo, V.; Gómez-Caravaca, A.M. Comparison between Ultrasonic Bath and Sonotrode Extraction of Phenolic Compounds from Mango Peel By-Products. Horticulturae 2022, 8, 1014. [Google Scholar] [CrossRef]
- Zou, T.-B.; Xia, E.-Q.; He, T.-P.; Huang, M.-Y.; Jia, Q.; Li, H.-W. Ultrasound-assisted extraction of mangiferin from mango (Mangifera indica L.) leaves using response surface methodology. Molecules 2014, 19, 1411–1421. [Google Scholar] [CrossRef]
- Yang, J.; Yan, Z.; Li, L.; Zhang, L.; Zhao, M.; Yi, H.; Wang, Z.; Li, G.; Wang, Z.; Li, M. Green Extraction of Phenolic Compounds from Lotus (Nelumbo nucifera Gaertn) Leaf Using Deep Eutectic Solvents: Process Optimization and Antioxidant Activity. Separations 2023, 10, 272. [Google Scholar] [CrossRef]
- Liyana-Pathirana, C.; Shahidi, F. Optimization of extraction of phenolic compounds from wheat using response surface methodology. Food Chem. 2005, 93, 47–56. [Google Scholar] [CrossRef]
- Jang, S.; Lee, A.Y.; Lee, A.R.; Choi, G.; Kim, H.K. Optimization of ultrasound-assisted extraction of glycyrrhizic acid from licorice using response surface methodology. Integr. Med. Res. 2017, 6, 388–394. [Google Scholar] [CrossRef]
- Castañeda-Valbuena, D.; Ayora-Talavera, T.; Luján-Hidalgo, C.; Álvarez-Gutiérrez, P.; Martínez-Galero, N.; Meza-Gordillo, R. Ultrasound extraction conditions effect on antioxidant capacity of mango by-product extracts. Food Bioprod. Process. 2021, 127, 212–224. [Google Scholar] [CrossRef]
- Ojeda, G.A.; Sgroppo, S.C.; Sánchez-Moreno, C.; de Ancos, B. Mango ‘criollo’ by-products as a source of polyphenols with antioxidant capacity. Ultrasound assisted extraction evaluated by response surface methodology and HPLC-ESI-QTOF-MS/MS characterization. Food Chem. 2022, 396, 133738. [Google Scholar] [CrossRef] [PubMed]
- Borrás-Enríquez, A.J.; Reyes-Ventura, E.; Villanueva-Rodríguez, S.J.; Moreno-Vilet, L. Effect of ultrasound-assisted extraction parameters on total polyphenols and its antioxidant activity from mango residues (Mangifera indica L. var. Manililla). Separations 2021, 8, 94. [Google Scholar] [CrossRef]
- Soong, Y.-Y.; Barlow, P.J. Quantification of gallic acid and ellagic acid from longan (Dimocarpus longan Lour.) seed and mango (Mangifera indica L.) kernel and their effects on antioxidant activity. Food Chem. 2006, 97, 524–530. [Google Scholar] [CrossRef]
- Ramirez, J.E.; Zambrano, R.; Sepúlveda, B.; Simirgiotis, M.J. Antioxidant properties and hyphenated HPLC-PDA-MS profiling of Chilean Pica mango fruits (Mangifera indica L. Cv. piqueño). Molecules 2014, 19, 438–458. [Google Scholar] [CrossRef]
- Chanda, S.; Amrutiya, N.; Rakholiya, K. Evaluation of antioxidant properties of some Indian vegetable and fruit peels by decoction extraction method. Am. J. Food Technol. 2013, 8, 173–182. [Google Scholar] [CrossRef]
- Hussain, A.I.; Chatha, S.A.; Noor, S.; Khan, Z.A.; Arshad, M.U.; Rathore, H.A.; Sattar, M.Z. Effect of extraction techniques and solvent systems on the extraction of antioxidant components from peanut (Arachis hypogaea L.) hulls. Food Anal. Methods 2012, 5, 890–896. [Google Scholar] [CrossRef]
- Sultana, B.; Anwar, F.; Ashraf, M. Effect of extraction solvent/technique on the antioxidant activity of selected medicinal plant extracts. Molecules 2009, 14, 2167–2180. [Google Scholar] [CrossRef] [PubMed]
- Dewanto, V.; Wu, X.; Adom, K.K.; Liu, R.H. Thermal processing enhances the nutritional value of tomatoes by increasing total antioxidant activity. J. Agric. Food Chem. 2002, 50, 3010–3014. [Google Scholar] [CrossRef] [PubMed]
- Sogi, D.S.; Siddiq, M.; Greiby, I.; Dolan, K.D. Total phenolics, antioxidant activity, and functional properties of ‘Tommy Atkins’ mango peel and kernel as affected by drying methods. Food Chem. 2013, 141, 2649–2655. [Google Scholar] [CrossRef]
- Bagamboula, C.; Uyttendaele, M.; Debevere, J. Inhibitory effect of thyme and basil essential oils, carvacrol, thymol, estragol, linalool and p-cymene towards Shigella sonnei and S. flexneri. Food Microbiol. 2004, 21, 33–42. [Google Scholar] [CrossRef]
- Du Toit, E.; Rautenbach, M. A sensitive standardised micro-gel well diffusion assay for the determination of antimicrobial activity. J. Microbiol. Methods 2000, 42, 159–165. [Google Scholar] [CrossRef]
- Raju, B.; Ballal, M.; Bairy, I. A novel treatment approach towards emerging multidrug resistant Enteroaggregative Escherichia coli (EAEC) causing acute/persistent diarrhea using medicinal plant extracts. Res. J. Pharm. Biol. Chem. Sci. 2011, 2, 15–23. [Google Scholar]
- Lim, K.J.A.; Cabajar, A.A.; Lobarbio, C.F.Y.; Taboada, E.B.; Lacks, D.J. Extraction of bioactive compounds from mango (Mangifera indica L. var. Carabao) seed kernel with ethanol–water binary solvent systems. J. Food Sci. Technol. 2019, 56, 2536–2544. [Google Scholar] [CrossRef]
- Wayne, P. National committee for clinical laboratory standards. Perform. Stand. Antimicrob. Disc Susceptibility Test. 2002, 12, 1–53. [Google Scholar]
- Li, H.; Chen, B.; Yao, S. Application of ultrasonic technique for extracting chlorogenic acid from Eucommia ulmodies Oliv. (E. ulmodies). Ultrason. Sonochem. 2005, 12, 295–300. [Google Scholar] [CrossRef] [PubMed]
- Zou, T.-B.; Jia, Q.; Li, H.-W.; Wang, C.-X.; Wu, H.-F. Response surface methodology for ultrasound-assisted extraction of astaxanthin from Haematococcus pluvialis. Mar. Drugs 2013, 11, 1644–1655. [Google Scholar] [CrossRef] [PubMed]
- Setyaningsih, W.; Saputro, I.E.; Palma, M.; Barroso, C.G. Stability of 40 phenolic compounds during ultrasound-assisted extractions (UAE). AIP Conf. Proc. 2016, 1755, 080009. [Google Scholar]
- Tabaraki, R.; Nateghi, A. Optimization of ultrasonic-assisted extraction of natural antioxidants from rice bran using response surface methodology. Ultrason. Sonochem. 2011, 18, 1279–1286. [Google Scholar] [CrossRef] [PubMed]
- Nithitanakool, S.; Pithayanukul, P.; Bavovada, R. Antioxidant and hepatoprotective activities of Thai mango seed kernel extract. Planta Med. 2009, 75, 1118–1123. [Google Scholar] [CrossRef]
- Sultana, B.; Hussain, Z.; Asif, M.; Munir, A. Investigation on the antioxidant activity of leaves, peels, stems bark, and kernel of mango (Mangifera indica L.). J. Food Sci. 2012, 77, C849–C852. [Google Scholar] [CrossRef]
- Dorta, E.; González, M.; Lobo, M.G.; Sánchez-Moreno, C.; de Ancos, B. Screening of phenolic compounds in by-product extracts from mangoes (Mangifera indica L.) by HPLC-ESI-QTOF-MS and multivariate analysis for use as a food ingredient. Food Res. Int. 2014, 57, 51–60. [Google Scholar] [CrossRef]
- Ribeiro, S.M.R.; Schieber, A. Bioactive compounds in mango (Mangifera indica L.). In Bioactive Foods in Promoting Health; Elsevier: London, UK, 2010; pp. 507–523. [Google Scholar]
- Polewski, K.; Kniat, S.; Slawinska, D. Gallic acid, a natural antioxidant, in aqueous and micellar environment: Spectroscopic studies. Curr. Top. Biophys. 2002, 26, 217–227. [Google Scholar]
- Mirghani, M.E.; Yosuf, F.; Kabbashi, N.; Vejayan, J.; Yosuf, Z. Antibacterial activity of mango kernel extracts. J. Appl. Sci. 2009, 9, 3013–3019. [Google Scholar] [CrossRef]
- Alaiya, M.A.; Odeniyi, M.A. Utilisation of Mangifera indica plant extracts and parts in antimicrobial formulations and as a pharmaceutical excipient: A review. Future J. Pharm. Sci. 2023, 9, 29. [Google Scholar] [CrossRef]
| Factors | −2 | −1 | 0 | +1 | +2 |
|---|---|---|---|---|---|
| Solvent concentration (%) | 0 | 10 | 20 | 30 | 40 |
| Solvent-to-sample ratio (mL/g) | 10 | 20 | 30 | 40 | 50 |
| Temperature (°C) | 20 | 30 | 40 | 50 | 60 |
| Time (min) | 10 | 15 | 20 | 25 | 30 |
| Run Order | X1 Solvent Concentration (%) | X2 Solvent-to-Sample Ratio (mL/g) | X3 Temperature (°C) | X4 Time (min) | Y Gallic Acid (mg/g) * |
|---|---|---|---|---|---|
| 1 | 10 (−1) | 20 (−1) | 30 (−1) | 15 (−1) | 4.33 ± 0.04 |
| 2 | 30 (1) | 40 (1) | 30 (−1) | 25 (1) | 3.85 ± 0.07 |
| 3 | 20 (0) | 30 (0) | 60 (2) | 20 (0) | 3.51 ± 0.10 |
| 4 | 10 (−1) | 40 (1) | 50 (1) | 25 (1) | 4.57 ± 0.04 |
| 5 | 10 (−1) | 20 (−1) | 50 (1) | 25 (1) | 4.45 ± 0.05 |
| 6 | 0 (−2) | 30 (0) | 40 (0) | 20 (0) | 3.86 ± 0.04 |
| 7 | 30 (1) | 40 (1) | 50 (1) | 25 (1) | 4.09 ± 0.10 |
| 8 | 20 (0) | 30 (0) | 40 (0) | 20 (0) | 6.06 ± 0.12 |
| 9 | 20 (0) | 30 (0) | 40 (0) | 20 (0) | 6.13 ± 0.06 |
| 10 | 30 (1) | 20 (−1) | 50 (1) | 25 (1) | 4.90 ± 0.08 |
| 11 | 40 (2) | 30 (0) | 40 (0) | 20 (0) | 3.86 ± 0.07 |
| 12 | 30 (1) | 20 (−1) | 30 (−1) | 15 (−1) | 4.15 ± 0.02 |
| 13 | 30 (1) | 40 (1) | 50 (1) | 15 (−1) | 3.56 ± 0.06 |
| 14 | 10 (−1) | 20 (−1) | 50 (1) | 15 (−1) | 3.61 ± 0.10 |
| 15 | 20 (0) | 30 (0) | 40 (0) | 30 (2) | 5.30 ± 0.36 |
| 16 | 30 (1) | 20 (−1) | 50 (1) | 15 (−1) | 4.24 ± 0.23 |
| 17 | 10 (−1) | 40 (1) | 30 (−1) | 15 (−1) | 5.08 ± 0.08 |
| 18 | 30 (1) | 40 (1) | 30 (−1) | 15 (−1) | 4.00 ± 0.10 |
| 19 | 30 (1) | 20 (−1) | 30 (−1) | 25 (1) | 4.54 ± 0.25 |
| 20 | 10 (−1) | 40 (1) | 50 (1) | 15 (−1) | 4.13 ± 0.31 |
| 21 | 20 (0) | 10 (−2) | 40 (0) | 20 (0) | 4.63 ± 0.23 |
| 22 | 20 (0) | 30 (0) | 20 (−2) | 20 (0) | 4.00 ± 0.05 |
| 23 | 20 (0) | 50 (2) | 40 (0) | 20 (0) | 4.35 ± 0.18 |
| 24 | 10 (−1) | 20 (−1) | 30 (−1) | 25 (1) | 4.36 ± 0.11 |
| 25 | 10 (−1) | 40 (1) | 30 (−1) | 25 (1) | 4.74 ± 0.07 |
| 26 | 20 (0) | 30 (0) | 40 (0) | 20 (0) | 6.07 ± 0.19 |
| 27 | 20 (0) | 30 (0) | 40 (0) | 10 (−2) | 4.51 ± 0.20 |
| Source | Sum of Square | Mean Square | F-Value | p-Value |
|---|---|---|---|---|
| Model | 13.57 | 0.9689 | 72.79 | ˂0.0001 |
| Solvent Concentration | 0.1568 | 0.1568 | 11.78 | 0.0050 |
| Solvent-to-sample ratio | 0.0523 | 0.0523 | 3.93 | 0.0709 |
| Temperature | 0.2563 | 0.2563 | 19.25 | 0.0009 |
| Time | 0.6600 | 0.6600 | 49.58 | <0.0001 |
| Solvent Concentration × Solvent-to-sample ratio | 1.05 | 1.05 | 78.93 | <0.0001 |
| Solvent Concentration × Temperature | 0.2500 | 0.2500 | 18.78 | 0.0010 |
| Solvent Concentration × Time | 0.0132 | 0.0132 | 0.9935 | 0.3386 |
| Solvent-to-sample ratio × Temperature | 0.0812 | 0.0812 | 6.10 | 0.0295 |
| Solvent-to-sample ratio × Time | 0.1296 | 0.1296 | 9.74 | 0.0089 |
| Temperature × Time | 0.4032 | 0.4032 | 30.29 | 0.0001 |
| Solvent Concentration2 | 6.47 | 6.47 | 486.28 | <0.0001 |
| Solvent-to-sample ratio2 | 3.30 | 3.30 | 247.95 | <0.0001 |
| Temperature2 | 7.10 | 7.10 | 533.73 | <0.0001 |
| Time2 | 1.79 | 1.79 | 134.40 | <0.0001 |
| Lack-of-fit | 0.1569 | 0.0157 | 10.94 | 0.0866 |
| Optimum Conditions | Extraction Yield | ||||
|---|---|---|---|---|---|
| Solvent concentration (%) | Solvent-to-sample ratio (mL/g) | Temperature (°C) | Time (min) | Experimental (mg/g) | Predicted (mg/g) |
| 19.4 | 29.32 | 38.47 | 21.4 | 6.1 ± 0.09 | 6.1 ± 0.11 |
| Extracts | Gallic Acid (mg/g) | TPC as GAE, (mg/g) | TFC as CE, (mg/g) | Radical Scavenging Activity % | Zone of Inhibition (mm) |
|---|---|---|---|---|---|
| MSK-UAE * | 6.11 ± 0.11 a | 64.30 ± 1.48 a | 4.99 ± 0.14 a | 73.50 ± 0.06 a | 18 ± 1 |
| MSK-Dec.** | 2.03 ± 0.01 b | 13.11 ± 0.21 b | 2.97 ± 0.07 b | 53.66 ± 0.14 b | 16 ± 1 |
| p-value | 0.000 | 0.000 | 0.000 | 0.000 | 0.070 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hayat, Z.; Riaz, T.; Saleem, K.; Akram, K.; Ur Rehman, H.; Azam, M. Optimization of Gallic Acid-Rich Extract from Mango (Mangifera indica) Seed Kernels through Ultrasound-Assisted Extraction. Separations 2023, 10, 376. https://doi.org/10.3390/separations10070376
Hayat Z, Riaz T, Saleem K, Akram K, Ur Rehman H, Azam M. Optimization of Gallic Acid-Rich Extract from Mango (Mangifera indica) Seed Kernels through Ultrasound-Assisted Extraction. Separations. 2023; 10(7):376. https://doi.org/10.3390/separations10070376
Chicago/Turabian StyleHayat, Zafar, Tuba Riaz, Kinza Saleem, Kashif Akram, Hafeez Ur Rehman, and Muhammad Azam. 2023. "Optimization of Gallic Acid-Rich Extract from Mango (Mangifera indica) Seed Kernels through Ultrasound-Assisted Extraction" Separations 10, no. 7: 376. https://doi.org/10.3390/separations10070376
APA StyleHayat, Z., Riaz, T., Saleem, K., Akram, K., Ur Rehman, H., & Azam, M. (2023). Optimization of Gallic Acid-Rich Extract from Mango (Mangifera indica) Seed Kernels through Ultrasound-Assisted Extraction. Separations, 10(7), 376. https://doi.org/10.3390/separations10070376









