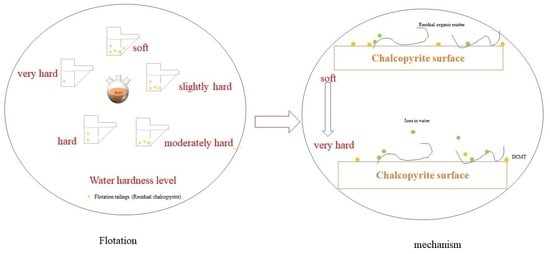
Effect of the Water Hardness Level on Chalcopyrite Flotation Inhibition by the Disodium Carboxymethyl Trithiocarbonate
Abstract
1. Introduction
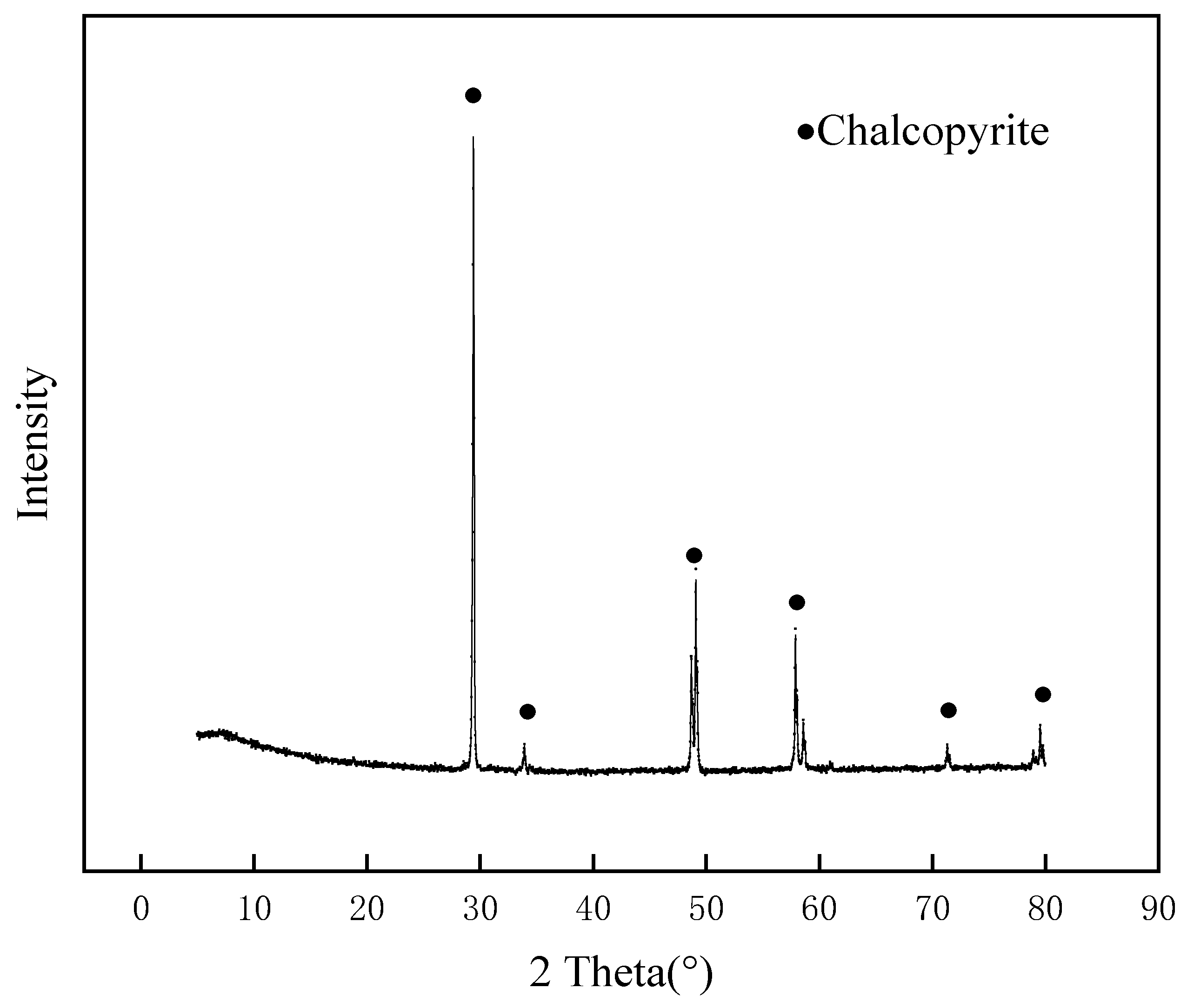
2. Materials and Methods
2.1. Materials and Reagents
2.2. Methods
2.2.1. Grinding and Flotation
2.2.2. Contact Angle
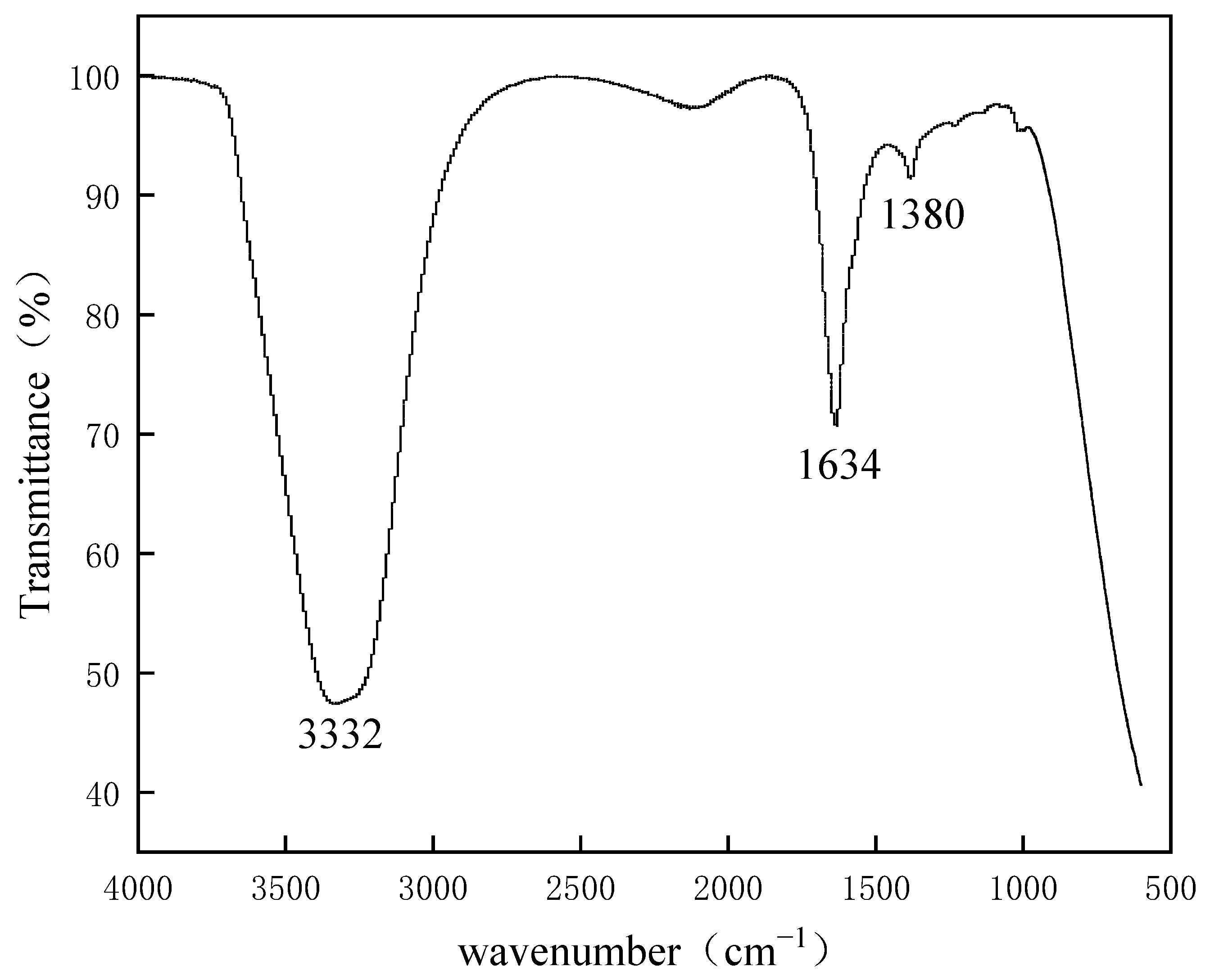
2.2.3. FT-IR Spectroscopy
2.2.4. Raman Spectroscopy
3. Results and Discussion
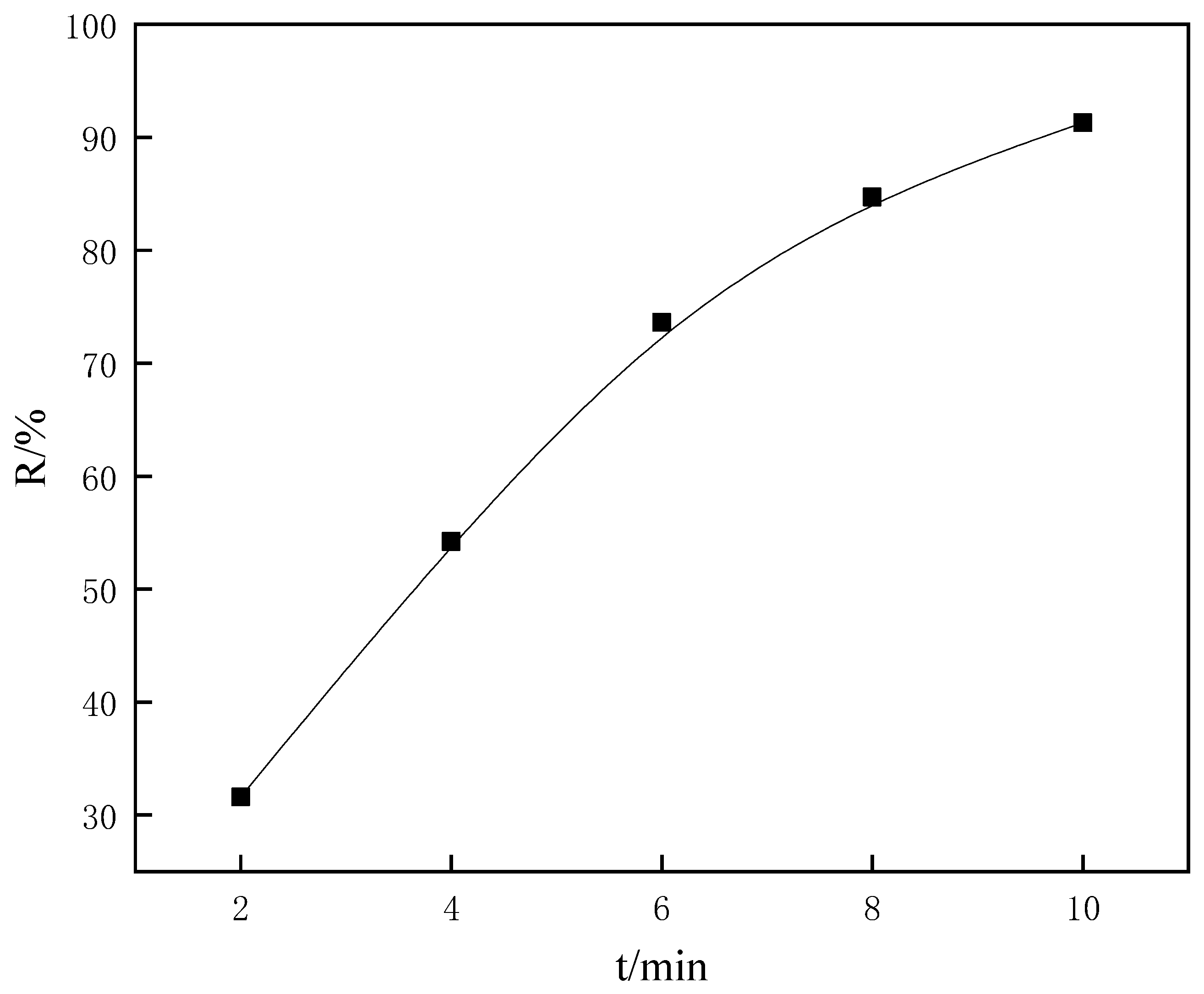
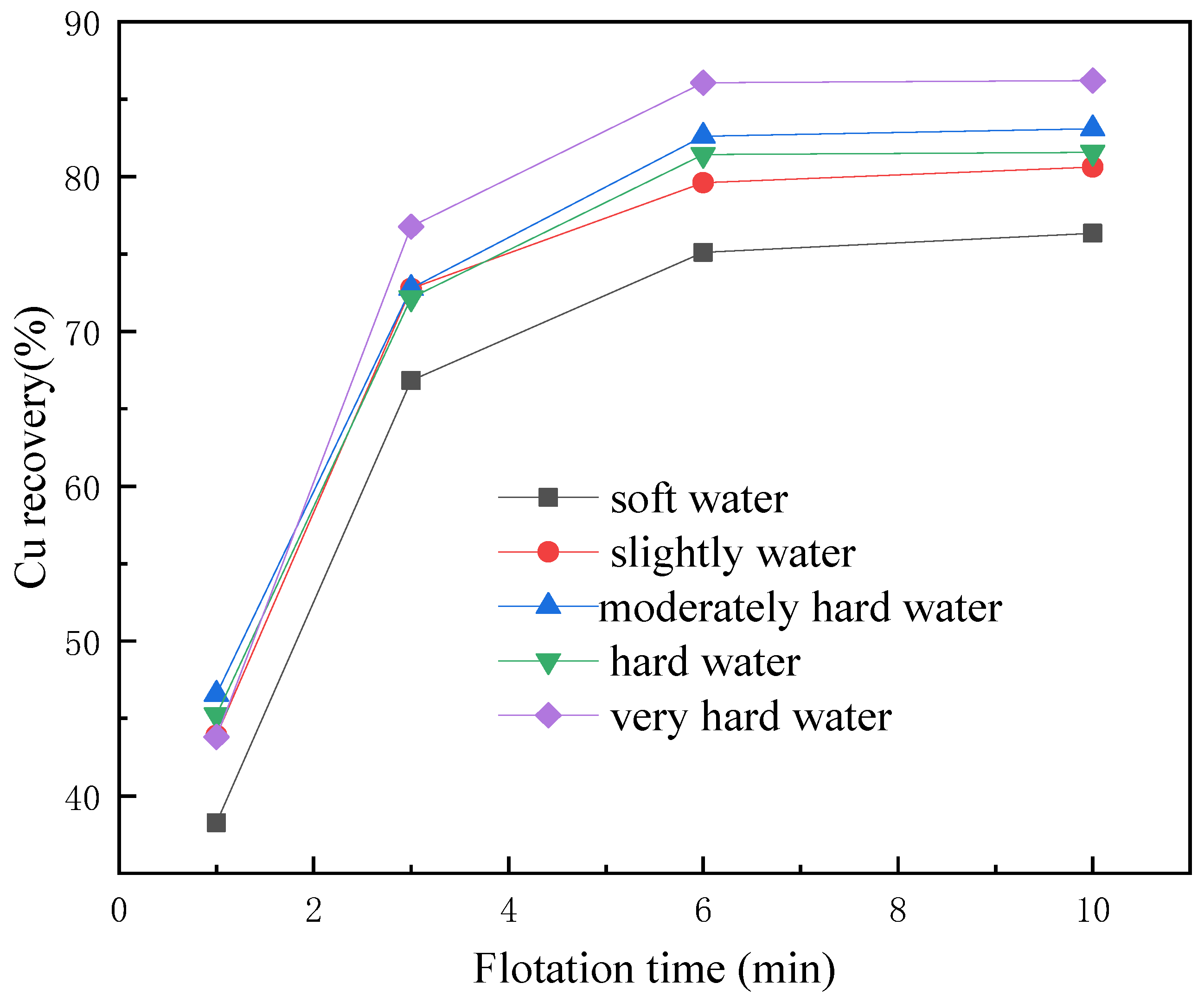
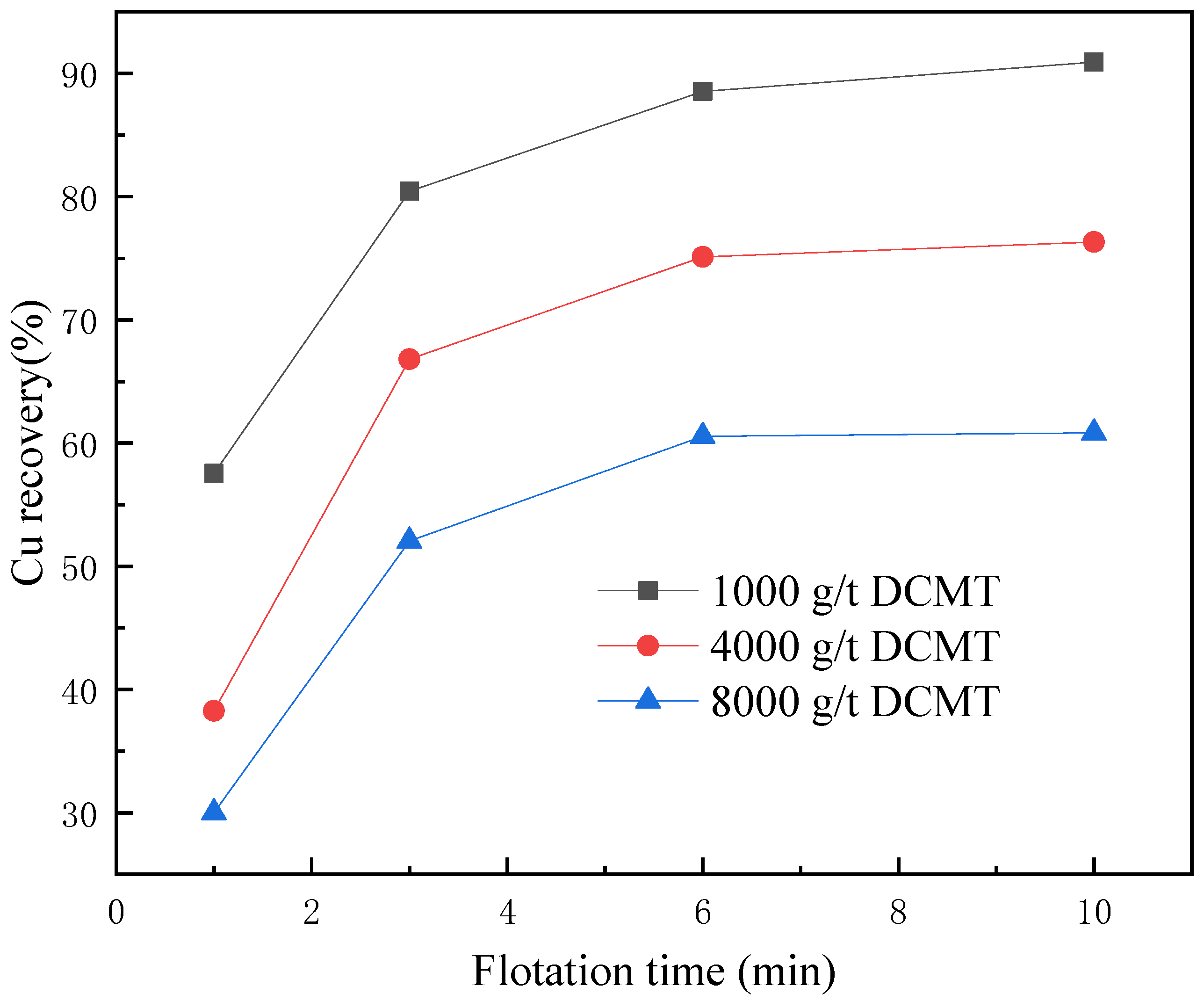
3.1. Flotation Tests
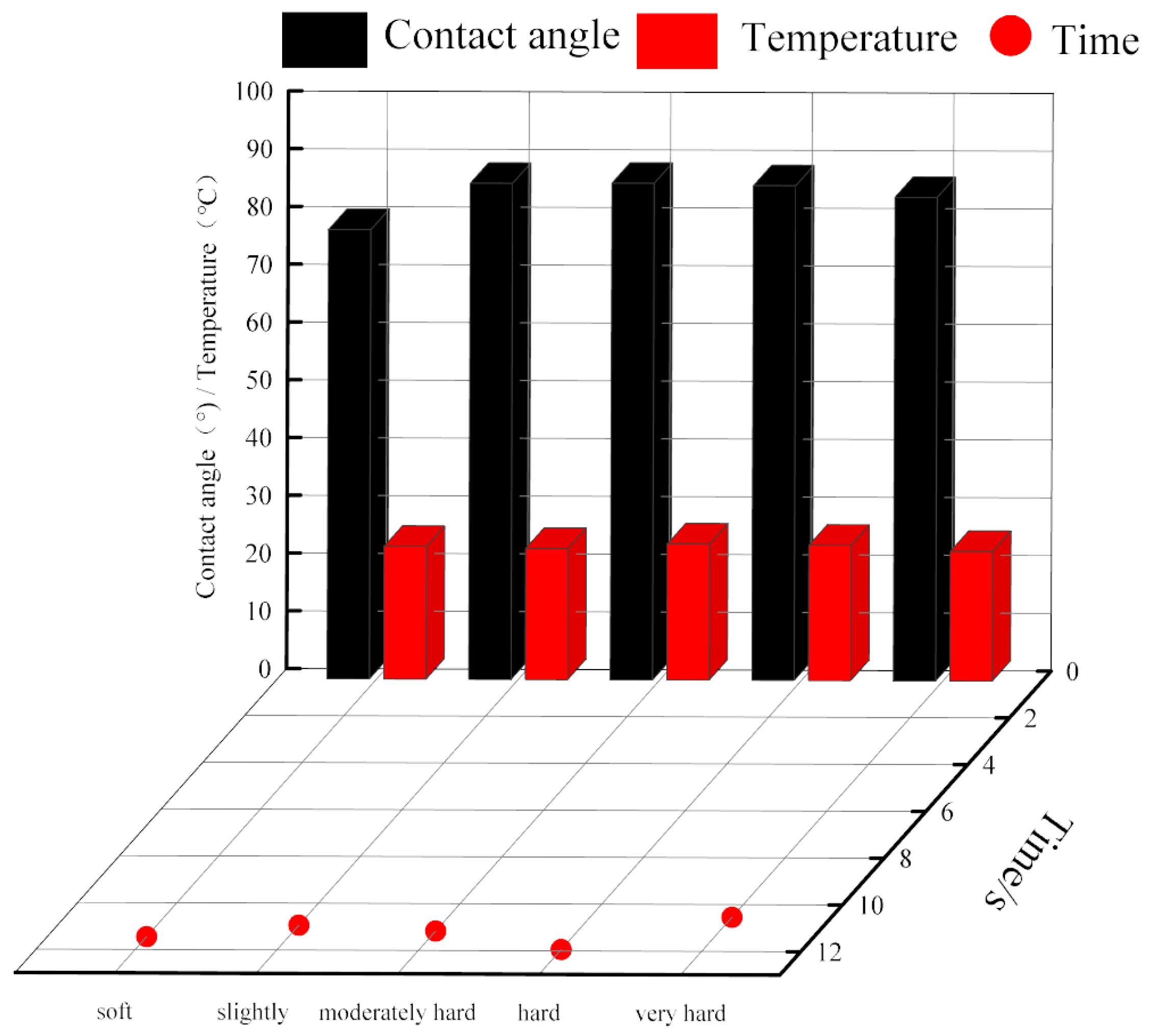
3.2. Powder Contact Angle
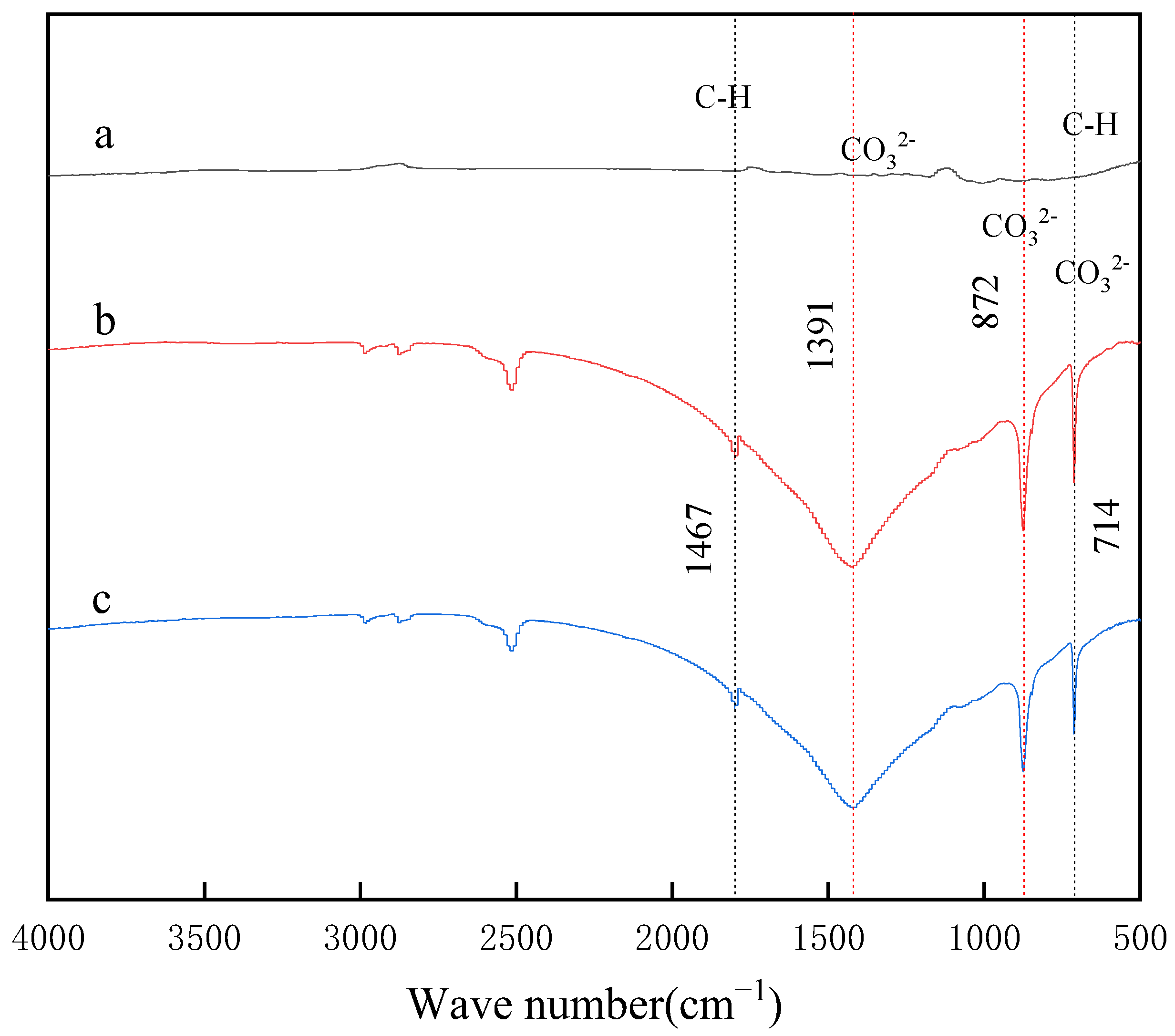
3.3. FT-IR and Raman Spectra
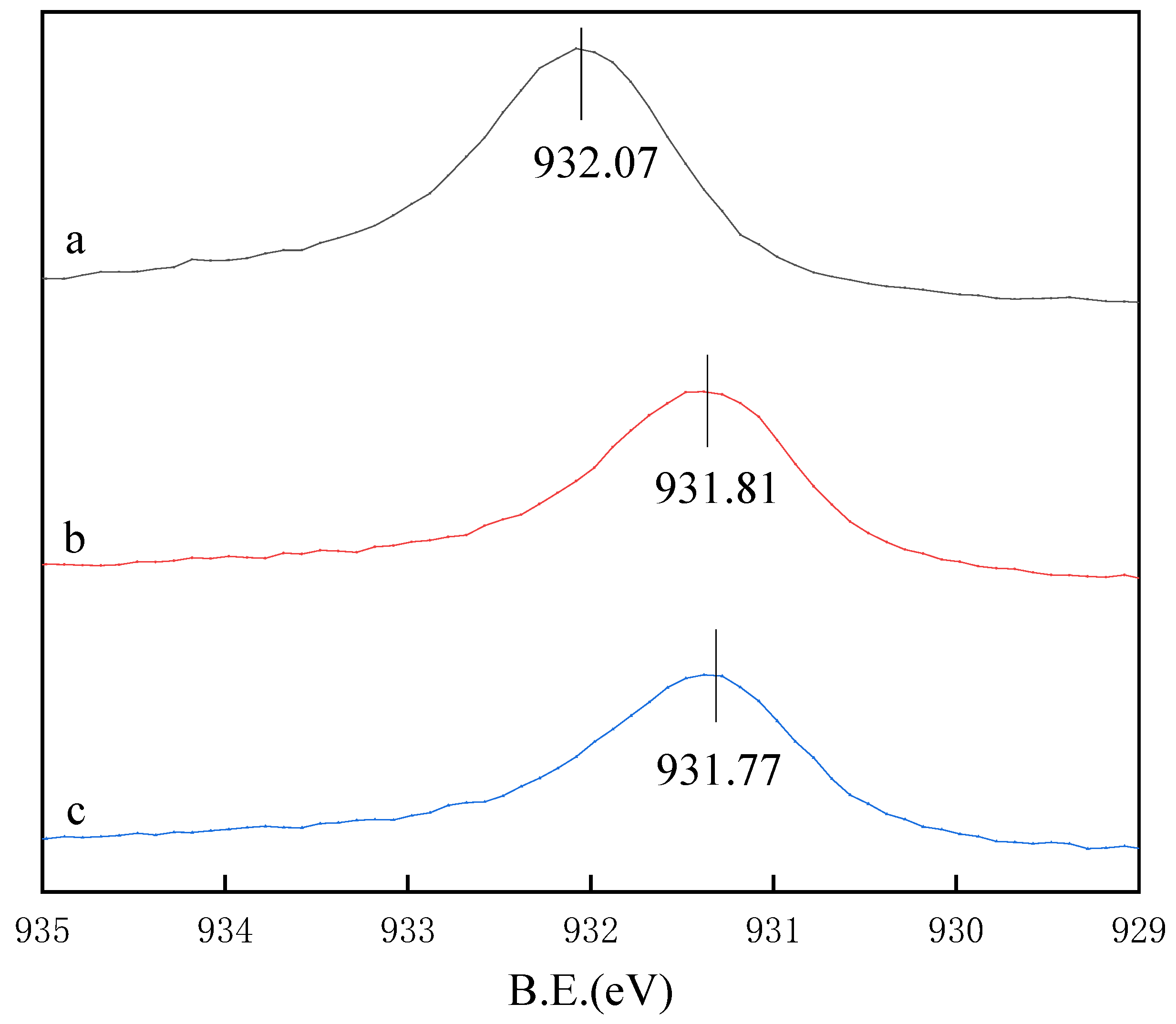
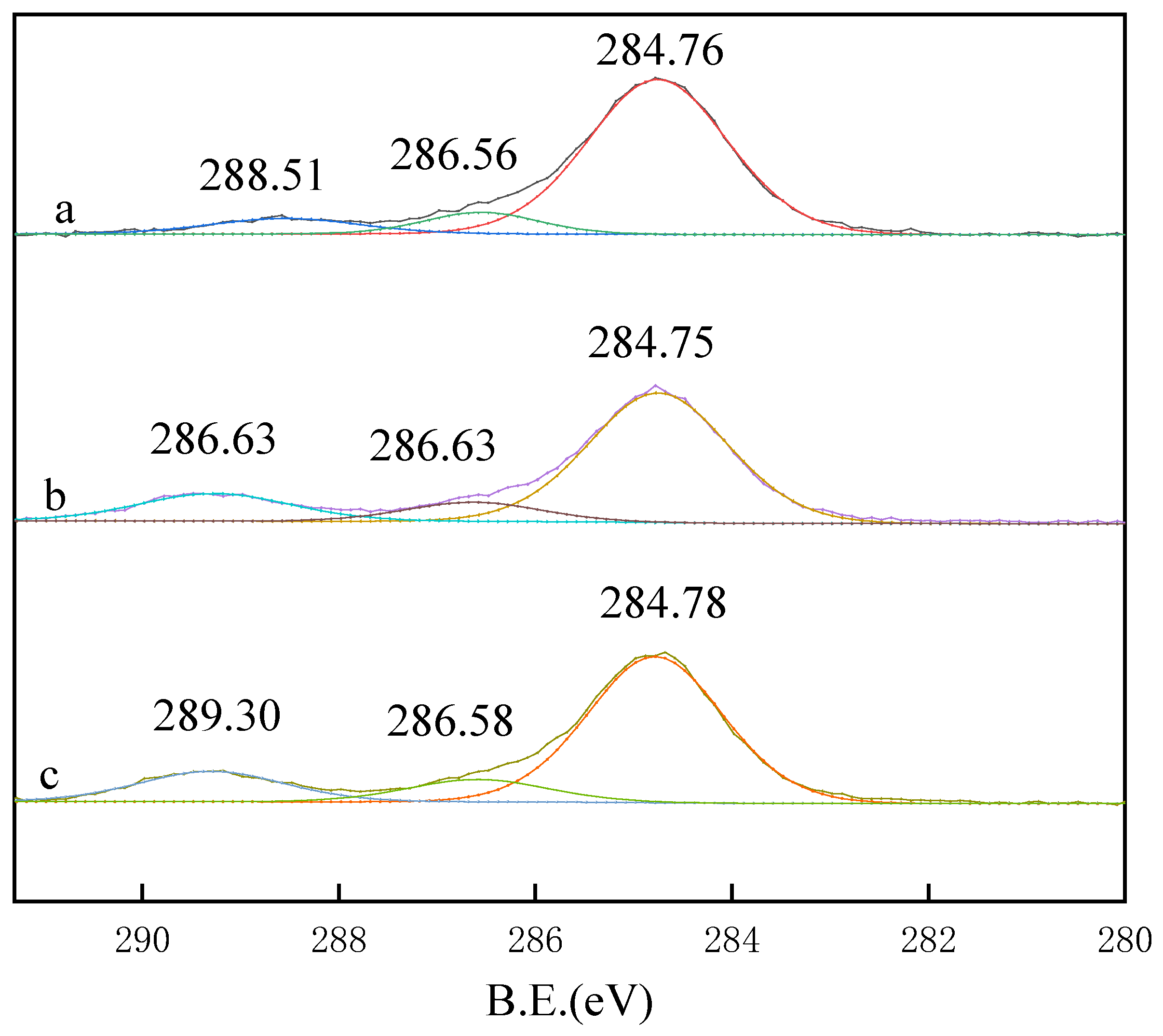
3.4. XPS
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Ozkan, S.G.; Acar, A. Investigation of impact of water type on borate ore flotation. Water Res. 2004, 38, 1773–1778. [Google Scholar] [CrossRef]
- Aliaskari, M.; Schaefer, A.I. Nitrate, arsenic and fluoride removal by electrodialysis from brackish groundwater. Water Res. 2022, 190, 116683. [Google Scholar] [CrossRef]
- Zhiyao, Z.; Yuqin, Z.; Xiaoyi, W.; Zhaoyang, W.; Yuting, B. Water quality evolution mechanism modeling and health risk assessment based on stochastic hybrid dynamic systems. Expert Syst. Appl. 2022, 193, 116406. [Google Scholar] [CrossRef]
- Nong, X.; Shao, D.; Zhong, H.; Liang, J. Evaluation of water quality in the South-to-North Water Diversion Project of China using the water quality index (WQI) method. Water Res. 2020, 178, 115781. [Google Scholar] [CrossRef]
- Wang, J.; Liao, R.; Tao, L.; Zhao, H.; Zhai, R.; Qin, W.; Qiu, G. A comprehensive utilization of silver-bearing solid wastes in chalcopyrite bioleaching. Hydrometallurgy 2017, 169, 152–157. [Google Scholar] [CrossRef]
- Ukubuiwe, A.C.; Olayemi, I.K.; Arimoro, F.O.; Omalu IC, J.; Ukubuiwe, C.C.; Baba, B.M.; Sule, B.U. Effects of water hardness level on metabolic reserves of post-embryonic life stages of Culex quinquefasciatus Say 1826 (Diptera: Culicidae). Int. J. Trop. Insect Sci. 2021, 42, 1297–1306. [Google Scholar] [CrossRef]
- Wonder, C.; Yongjun, P. Selective inhibition of kaolinite entrainment during chalcopyrite flotation in saline water. Miner. Eng. 2022, 184, 107637. [Google Scholar]
- Chen, Y.; Chen, X.; Peng, Y. The effect of sodium hydrosulfide on molybdenite flotation in seawater and diluted seawater. Miner. Eng. 2020, 158, 106589. [Google Scholar] [CrossRef]
- Hamilton, T.; Huai, Y.; Peng, Y. Lead adsorption on copper sulphides and the relevance to its contamination in copper concentrates. Miner. Eng. 2020, 154, 106381. [Google Scholar] [CrossRef]
- Weiming, W.; Tao, C.; Yanhai, S.; Guohua, Y.; Xiong, T. The flotation separation of molybdenite from talc using zinc sulfate in sodium silicate system and related mechanism. Colloids Surf. A Physicochem. Eng. Asp. 2022, 641, 128451. [Google Scholar]
- Changtao, W.; Runqing, L.; Meirong, W.; Zhijie, X.; Mengjie, T.; Zhigang, Y.; Wei, S.; Chenyang, Z. Flotation separation of molybdenite from chalcopyrite using rhodanine-3-acetic acid as a novel and effective depressant. Miner. Eng. 2021, 162, 106747. [Google Scholar]
- Ikumapayi, F.; Rao, K.H. Recycling Process Water in Complex Sulfide Ore Flotation: Effect of Calcium and Sulfate on Sulfide Minerals Recovery. Miner. Process. Extr. Metall. Rev. 2015, 36, 45–64. [Google Scholar] [CrossRef]
- Chen, J.-H.; Lan, L.-H.; Liao, X.-J. Depression effect of pseudo glycolythiourea acid in flotation separation of copper–molybdenum. Trans. Nonferrous Met. Soc. China 2013, 23, 824–831. [Google Scholar] [CrossRef]
- Zhigang, Y.; Yuehua, H.; Wei, S.; Chenyang, Z.; Jianyong, H.; Zhijie, X.; Jingxiang, Z.; Changping, G.; Chenhu, Z.; Qingjun, G.; et al. Adsorption Mechanism of 4-Amino-5-mercapto-1,2,4-triazole as Flotation Reagent on Chalcopyrite. Langmuir ACS J. Surf. Colloids 2018, 34, 4071–4083. [Google Scholar]
- Yin, Z.-G.; Sun, W.; Hu, Y.-H.; Guan, Q.-J.; Zhang, C.-H.; Gao, Y.-S.; Zhai, J.-H. Depressing behaviors and mechanism of disodium bis (carboxymethyl) trithiocarbonate on separation of chalcopyrite and molybdenite. Trans. Nonferrous Met. Soc. China 2017, 27, 883–890. [Google Scholar] [CrossRef]
- Roman, O.; Lesyk, R. Synthesis and anticancer activity in vitro of some 2-thioxo-4-thiazolidone derivatives. Farmacia 2007, 55, 640–648. [Google Scholar]
- Bresson, C.R.; Parlman, R.M.; Kimble, J.B. Trithiocarbonate Flotation Reagents. U.S. Patent No 4,561,984, 31 December 1985. [Google Scholar]
- Timbillah, S. Mechanism for Disodium Carboxymethyl Trithiocarbonate (Orfom® D8) Depression in Chalcopyrite-Molybdenite Flotation Systems. Ph.D. Thesis, Montana Technological University, Butte, MT, USA, 2019. [Google Scholar]
- Timbillah, S.; Fosu, B.; Lan, P.L.; Sosa-Blanco, C.; Hill, L. Towards an Understanding of the Use of Disodium Carboxymethyl Trithiocarbonate (DCMT) as an Alternative Depressant in Cu/Mo Sulfide Flotation. Min. Metall. Explor. 2021, 38, 1–14. [Google Scholar] [CrossRef]
- Yin, Z.; Sun, W.; Hu, Y.; Zhai, J.; Qingjun, G. Evaluation of the replacement of NaCN with depressant mixtures in the separation of copper–molybdenum sulphide ore by flotation. Sep. Purif. Technol. 2017, 173, 9–16. [Google Scholar] [CrossRef]
- Timbillah, S.; Young, C.; Das, A. Understanding the Electrochemical Behavior of Di-Sodium Carboxymethyl Trithiocarbonate (Orfom® D8) Depressant on Copper Metal and Chalcopyrite Surfaces. Meet. Abstr. 2018, MA2018–01, 2153. [Google Scholar] [CrossRef]
- Christian, U.A.; Kayode, O.I.; James, O.I.C.; Ofurum, A.F.; Catherine, U.C. Morphometric diagnosis of the effects of water hardness on development of immature life stages and adult vectorial fitness of Culex quinquefasciatus (Diptera: Culicidae) mosquito. Zoomorphology 2018, 137, 511–518. [Google Scholar]
- Zhang, X.; Lu, L.; Cao, Y.; Yang, J.; Che, W.; Jiongtian, L. The flotation separation of molybdenite from chalcopyrite using a polymer depressant and insights to its adsorption mechanism. Chem. Eng. J. 2020, 395, 125137. [Google Scholar] [CrossRef]
- Liao, R.; Feng, Q.; Wen, S.; Liu, J. Flotation separation of molybdenite from chalcopyrite using ferrate(VI) as selective depressant in the absence of a collector. Miner. Eng. 2020, 152, 106369. [Google Scholar] [CrossRef]
- Guangsheng, Z.; Leming, O.; Wencai, Z.; Yuteng, Z. Effects of Sodium Alginate on the Flotation Separation of Molybdenite From Chalcopyrite Using Kerosene as Collector. Front. Chem. 2020, 8, 242. [Google Scholar]
- Fu, Y.; Zhu, Z.; Yao, J.; Han, H.; Yin, W.; Yang, B. Improved depression of talc in chalcopyrite flotation using a novel depressant combination of calcium ions and sodium lignosulfonate. Colloids Surf. A Physicochem. Eng. Asp. 2018, 558, 88–94. [Google Scholar] [CrossRef]
- Binbin, L.; Dezhi, L.; Saizhen, J.; Qingyun, L.; Zejun, W. The effect of nascent calcium carbonate inhibiting the flotation behavior of calcite. Miner. Eng. 2022, 180, 107478. [Google Scholar]
- Changbin, L.; Qing, S.; Guofan, Z.; Hongjiang, L.; Haigang, F. Effect of aluminum ions on the adsorption of carboxymethyl cellulose onto talc. Miner. Eng. 2021, 170, 107018. [Google Scholar]
- Yann, F.; Lev, F.; Inna, F.; Michael, B. The Challenge of Tungsten Skarn Processing by Froth Flotation: A Review. Front. Chem. 2020, 8, 230. [Google Scholar]
- Chimonyo, W.; Fletcher, B.; Peng, Y. The differential depression of an oxidized starch on the flotation of chalcopyrite and graphite. Miner. Eng. 2020, 146, 106114. [Google Scholar] [CrossRef]
- Rath, R.K.; Subramanian, S.; Pradeep, T. Surface Chemical Studies on Pyrite in the Presence of Polysaccharide-Based Flotation Depressants. J. Colloid Interface Sci. 2000, 229, 82–91. [Google Scholar] [CrossRef]
- Feng, B.; Luo, X.-P. The solution chemistry of carbonate and implications for pyrite flotation. Miner. Eng. 2013, 53, 181–183. [Google Scholar] [CrossRef]
- Wang, X.; Zhang, Q. Role of surface roughness in the wettability, surface energy and flotation kinetics of calcite. Powder Technol. 2020, 371, 55–63. [Google Scholar] [CrossRef]
- Taylor, E.A.; Mileti, C.J.; Ganesan, S.; Kim, J.H.; Donnelly, E. Measures of Bone Mineral Carbonate Content and Mineral Maturity/Crystallinity for FT-IR and Raman Spectroscopic Imaging Differentially Relate to Physical–Chemical Properties of Carbonate-Substituted Hydroxyapatite. Calcif. Tissue Int. 2021, 109, 77–91. [Google Scholar] [CrossRef]
- Serna, C.J.; White, J.L.; Hem, S.L. Structural Survey of Carbonate-Containing Antacids. J. Pharm. Sci. 1978, 67, 324–327. [Google Scholar] [CrossRef] [PubMed]
- Young, R.D.; Hill, A.F.; Hillier, W.; Ball, G.E. Transition metal-alkane θ-complexes with oxygen donor Co-ligands. J. Am. Chem. Soc. 2011, 133, 13806–13809. [Google Scholar] [CrossRef]
- Williams, S.D.; Johnson, T.J.; Sharpe, S.W.; Oates, R.P.; Brauer, C.S. Quantitative vapor-phase IR intensities and DFT computations to predict absolute IR spectra based on molecular structure: I. Alkanes. J. Quant. Spectrosc. Radiat. Transf. 2013, 129, 298–307. [Google Scholar] [CrossRef]
- Hanna, S.; Wojciech, C.; Piotr, T. Starch-metal complexes and metal compounds. J. Sci. Food Agric. 2018, 98, 2845–2856. [Google Scholar]
- Kazuo, N. Infrared and Raman Spectra of Inorganic and Coordination Compounds, 4th ed.; John Wiley & Sons: Hoboken, NJ, USA, 1986. [Google Scholar]
- Mielczarski, J.A.; Cases, J.M.; Alnot, M.; Ehrhardt, J.J. XPS Characterization of Chalcopyrite, Tetrahedrite, and Tennantite Surface Products after Different Conditioning. 1. Aqueous Solution at pH 10. Langmuir 1996, 12, 2519–2530. [Google Scholar] [CrossRef]
- Liu, G.; Qiu, Z.; Wang, J.; Liu, Q.; Xiao, J.; Zeng, H.; Zhong, H.; Xu, Z. Study of N-isopropoxypropyl-N’-ethoxycarbonyl thiourea adsorption on chalcopyrite using in situ SECM, ToF-SIMS and XPS. J. Colloid Interface Sci. 2015, 437, 42–49. [Google Scholar] [CrossRef]
- Zuyuan, T.; Haodong, L.; Qian, W.; Wenqing, Q.; Congren, Y. Effects of redox potential on chalcopyrite leaching: An overview. Miner. Eng. 2021, 172, 107135. [Google Scholar]
- Chastain, J.; King, R.C. (Eds.) Handbook of X-Ray Photoelectron Spectroscopy: A Reference Book of Standard Spectra for Identification and Interpretation of XPS Data; Physical Electronics: Eden Prairie, MN, USA, 1995. [Google Scholar]



| Water Hardness Level | Soft | Slightly Hard | Moderately Hard | Hard | Very Hard |
|---|---|---|---|---|---|
| CaCO3 (mg/L) | <17.0 | 17.0–59 | 60–119 | 120–180 | >180 |
| Element | Cu | Fe | S | Al2O3 | CaO | Bi |
|---|---|---|---|---|---|---|
| Content/% | 34.36 | 30.60 | 35.37 | <0.5 | 0.34 | 0.0069 |
| Chalcopyrite | Atomic Concentration/% | ||||
|---|---|---|---|---|---|
| C1s | O1s | S2p | Cu2p | Ca2p | |
| untreated | 58.52 | 15.69 | 19.46 | 6.34 | - |
| Treated by CaCO3 | 49.54 | 27.20 | 14.00 | 4.26 | 5.00 |
| Treated by CaCO3/DCMT | 51.11 | 25.39 | 12.38 | 3.92 | 5.30 |
| Chalcopyrite | Concentration/% | ||
|---|---|---|---|
| C-C | C-O | C=O | |
| untreated | 81.78 | 8.27 | 9.96 |
| Treated by CaCO3 | 72.64 | 10.41 | 16.95 |
| Treated by CaCO3/DCMT | 72.41 | 11.46 | 16.12 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Wu, W.; Shao, Y.; Qin, W.; Chen, L. Effect of the Water Hardness Level on Chalcopyrite Flotation Inhibition by the Disodium Carboxymethyl Trithiocarbonate. Separations 2023, 10, 375. https://doi.org/10.3390/separations10070375
Wang Y, Wu W, Shao Y, Qin W, Chen L. Effect of the Water Hardness Level on Chalcopyrite Flotation Inhibition by the Disodium Carboxymethyl Trithiocarbonate. Separations. 2023; 10(7):375. https://doi.org/10.3390/separations10070375
Chicago/Turabian StyleWang, Yonghai, Weiming Wu, Yanhai Shao, Wenqing Qin, and Luzheng Chen. 2023. "Effect of the Water Hardness Level on Chalcopyrite Flotation Inhibition by the Disodium Carboxymethyl Trithiocarbonate" Separations 10, no. 7: 375. https://doi.org/10.3390/separations10070375
APA StyleWang, Y., Wu, W., Shao, Y., Qin, W., & Chen, L. (2023). Effect of the Water Hardness Level on Chalcopyrite Flotation Inhibition by the Disodium Carboxymethyl Trithiocarbonate. Separations, 10(7), 375. https://doi.org/10.3390/separations10070375