Identification and Assessment of Therapeutic Phytoconstituents of Catharanthus roseus through GC-MS Analysis
Abstract
1. Introduction
2. Materials and Methods
2.1. Sample Collection and Identification
2.2. Preparation of Extract from Plant Leaves
2.3. GC-MS Analysis
2.4. Identification of Compounds
2.5. Principle Component Analysis (PCA)
3. Results
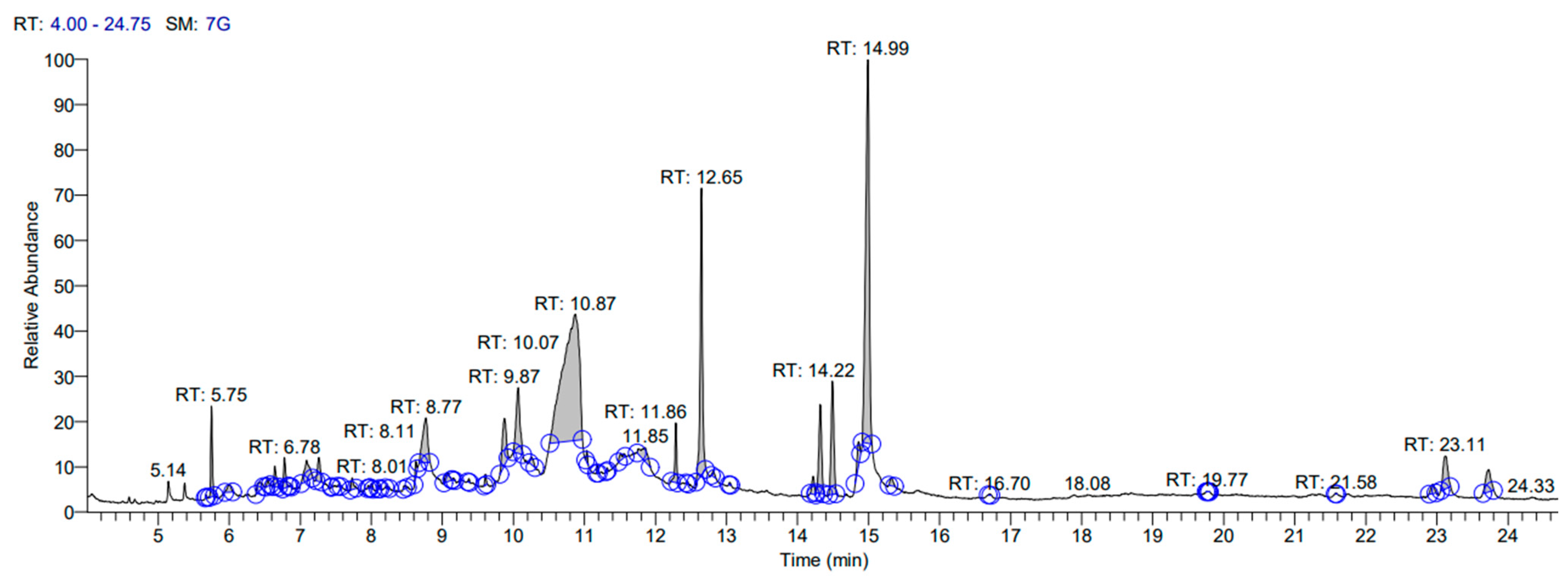
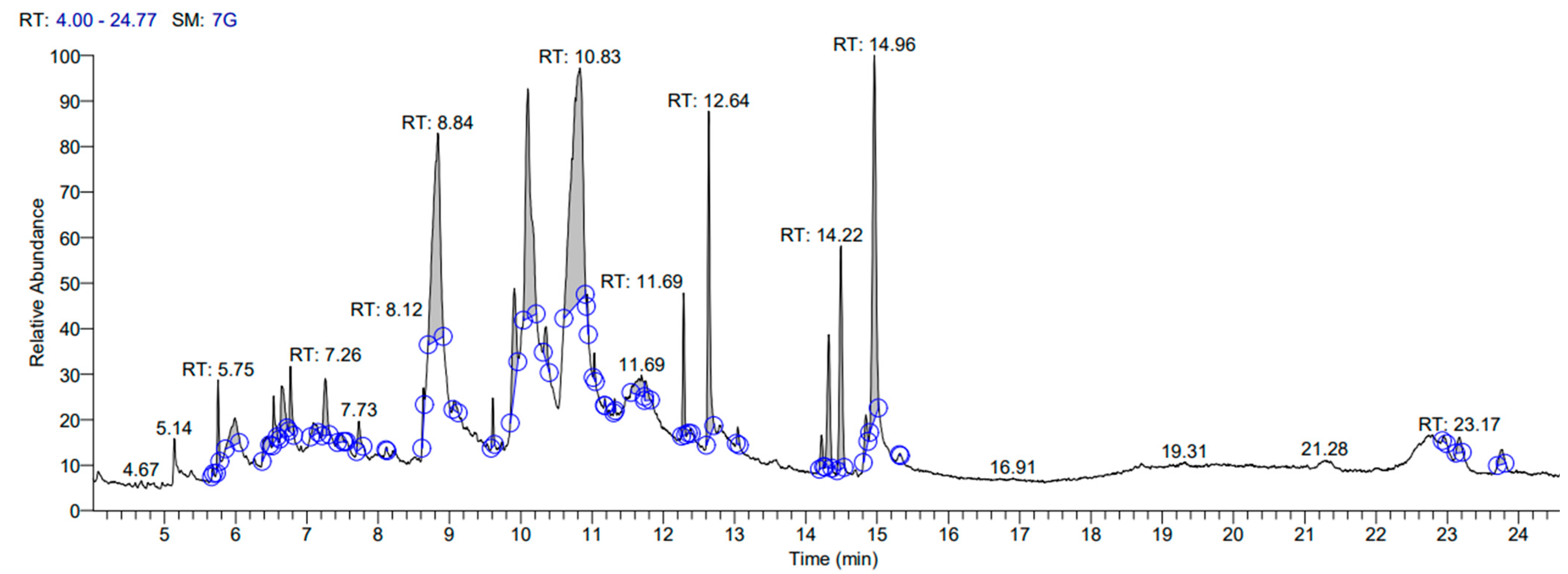
3.1. Gas Chromatography-Mass Spectrophotometry (GC-MS) Analysis
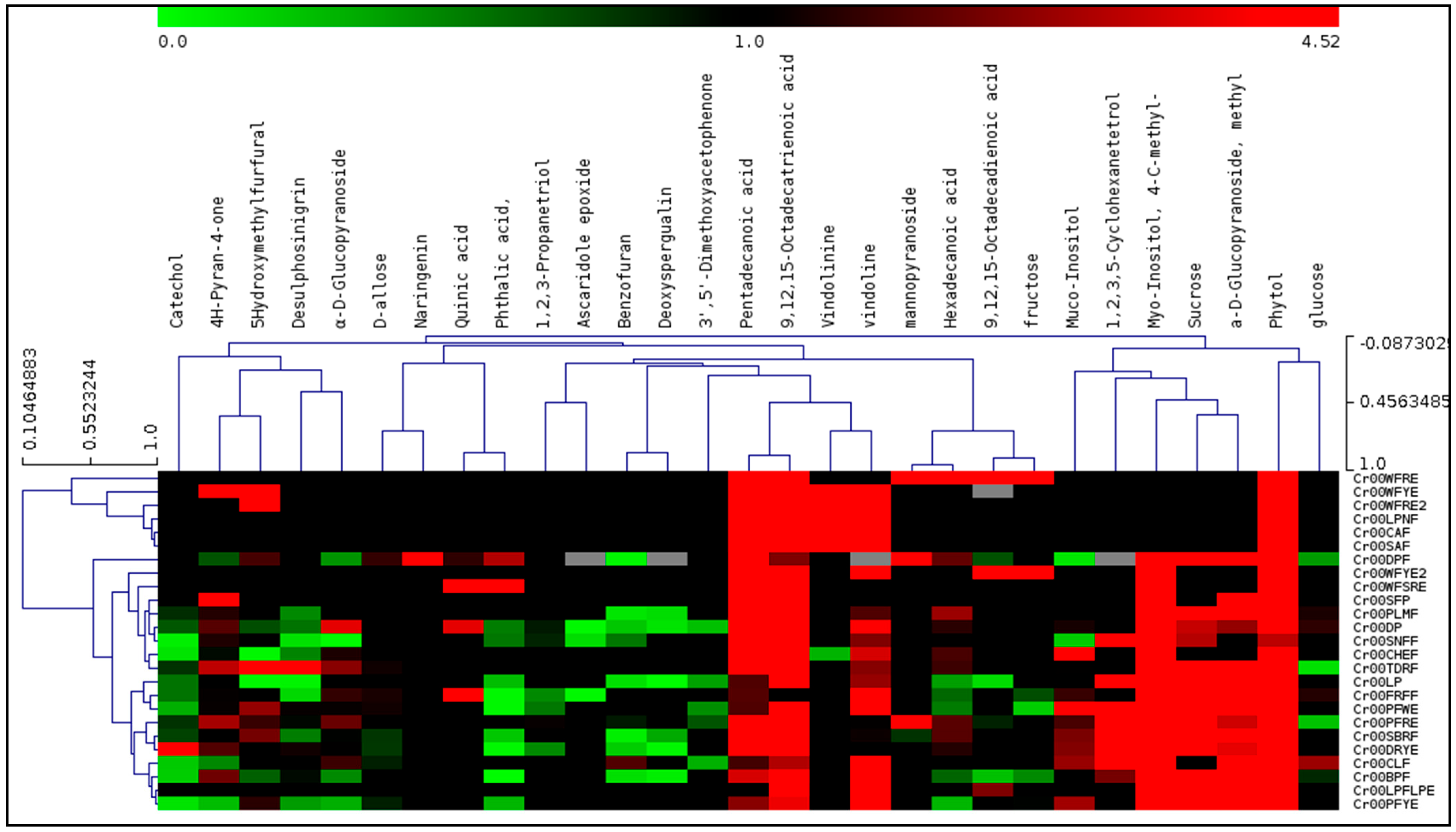
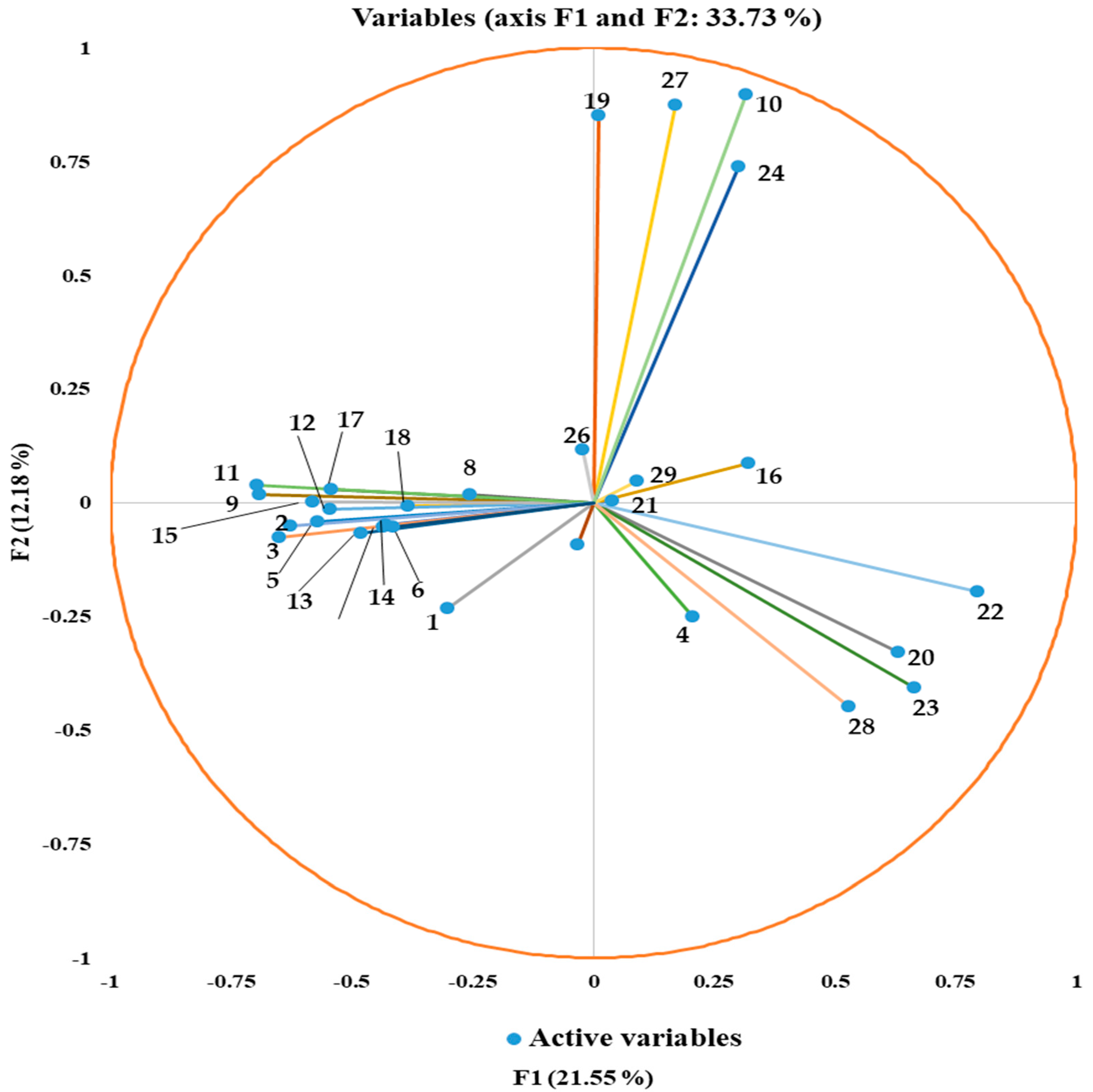
3.2. Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA) of GC–MS
4. Discussion
4.1. Gas Chromatography–Mass Spectrometry (GC–MS) Analysis
4.2. GC-MS Data Used for Principal Component Analysis (PCA) and Hierarchical Cluster Analysis (HCA)
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Tang, J.; Dunshea, F.R.; Suleria, H.A. LC-ESI-QTOF/MS characterization of phenolic compounds from medicinal plants (hops and juniper berries) and their antioxidant activity. Foods 2019, 9, 7. [Google Scholar] [CrossRef] [PubMed]
- Rani, J. Ethanobotanical survey and traditional uses of medicinal plants in Jind district of Haryana India. Plant Arch. 2019, 19, 1241–1247. [Google Scholar]
- Alsarhan, A.; Sultana, M.; Khatib, A.A.; Kadir, M.R.A. Review on some Malaysian traditional medicinal plants with therapeutic properties. J. Basic Appl. Sci. 2014, 10, 149–159. [Google Scholar] [CrossRef]
- Mahomoodally, M.F. Traditional medicines in Africa: An appraisal of ten potent African medicinal plants. Evid.-Based Complement. 2013, 2013, 617459. [Google Scholar] [CrossRef]
- Alam, M.M.; Emon, N.U.; Alam, S.; Rudra, S.; Akhter, N.; Mamun, M.M.; Ganguly, A. Assessment of pharmacological activities of Lygodium Microphyllum Cav. leaves in the management of pain, inflammation, pyrexia, diarrhea, and helminths: In vivo, in vitro and in silico approaches. Biomed. Pharmacother. 2021, 139, 111644. [Google Scholar] [CrossRef]
- Kapoor, M.; Rani, J.; Kumar, A.; Ghosh, S. Phytochemical screening for antimicrobial and antioxidant activity of periwinkle. Indian J. Hortic. 2021, 4, 428–435. [Google Scholar] [CrossRef]
- Choudhury, F.K.; Pandey, P.; Meitei, R.; Cardona, D.; Gujar, A.C.; Shulaev, V. GC-MS/MS Profiling of Plant Metabolites. Methods Mol. Biol. 2022, 2396, 101–115. [Google Scholar]
- Falcao, M.A.; Scopel, R.; Almeida, R.N.; Santo, A.T.D.E.; Franceschini, G.; Garcez, J.J.; Vargas, R.M.; Cassel, E. Supercritical fluid extraction of vinblastine from Catharanthus roseus. J. Supercrit. Fluids 2017, 129, 9–15. [Google Scholar] [CrossRef]
- Leonti, M.; Stafford, G.I.; Cero, M.D.; Cabras, S.; Castellanos, M.E.; Casu, L.; Weckerle, C.S. Reverse ethnopharmacology and drug discovery. J. Ethnopharmacol. 2017, 198, 417–431. [Google Scholar] [CrossRef]
- Nagaraja, S.K.; Nayaka, S.; Kumar, R.S. Phytochemical Analysis, GC–MS Profiling, and In Vitro Evaluation of Biological Applications of Different Solvent Extracts of Leonotis nepetifolia (L.) R.Br. Flower Buds. Appl. Biochem. Biotechnol. 2023, 195, 1197–1215. [Google Scholar] [CrossRef]
- Sobhy, S.; Al-Askar, A.A.; Bakhiet, E.K.; Elsharkawy, M.M.; Arishi, A.A.; Behiry, S.I.; Abdelkhalek, A. Phytochemical Characterization and Antifungal Efficacy of Camphor (Cinnamomum camphora L.) Extract against Phytopathogenic Fungi. Separations 2023, 10, 189. [Google Scholar] [CrossRef]
- Ali, A.; Javaid, A.; Shoaib, A. GC-MS analysis and antifungal activity of methanolic root extract of Chenopodium album against Sclerotium rolfsii. Planta Daninha 2017, 35, e017164713. [Google Scholar] [CrossRef]
- Haron, F.K.; Shah, M.D.; Yong, Y.S.; Tan, J.K.; Lal, M.T.M.; Venmathi Maran, B.A. Antiparasitic potential of methanol extract of brown alga Sargassum polycystum (Phaeophyceae) and its LC-MS/MS metabolite profiling. Diversity 2022, 14, 796. [Google Scholar] [CrossRef]
- Sharma, M. Vascular Flora of Punjab and Chandigarh; Om Publications: Noida, India, 2021; Volume 1. [Google Scholar]
- eFIG (2006 onwards). Efloraofindia—An Online Database of Indian Plants Developed by the Members of Efloraofindia Google Group (eFIG). Available online: https://sites.google.com/site/efloraofindia/ (accessed on 25 July 2016).
- Kupchan, S.M.; Tsou, G. Tumor inhibitors: A new antileukemic simaroubolid from Brucea antidysenterica. J. Organomet. Chem. 1973, 38, 178–179. [Google Scholar] [CrossRef]
- Wagenen, B.C.V.; Larsen, R.; Cardellina, J.H.; Ran-Dazzo, D.; Lidert, Z.C.; Swithenbank, C. Ulosantoin, a potent insecticide from the sponge Ulosaruetzleri. J. Organomet. Chem. 1993, 58, 335–337. [Google Scholar]
- Kapoor, M.; Rani, J. Qualitative and quantitative analysis of phytochemicals by GC-MS and antioxidant activity of Catharanthus roseus (L.) G. Don. J. Pharm. Phytochem. 2019, 8, 378–385. [Google Scholar]
- Suganya, R.; Thangaraj, M. Isolation and characterization of leaf extract of Derris trifoliate. Int. J. ChemTech Res. 2014, 6, 4115–4122. [Google Scholar]
- Mohammed, G.J.; Al-Jassani, M.J.; Hameed, I.H. Antibacterial, Antifungal Activity and Chemical analysis of Punica grantanum (Pomegranate peel) using GC-MS and FTIR spectroscopy. Int. J. Pharm. Phytochem. Res. 2016, 8, 480–494. [Google Scholar]
- Hiller, K.; Hangebrauk, J.; Jager, C.; Spura, J.; Schreiber, K.; Schomburg, D. MetaboliteDetector: Comprehensive analysis tool for targeted and non-targeted GC/MS based metabolome analysis. Anal. Chem. 2009, 81, 3429–3439. [Google Scholar] [CrossRef]
- Mallard, W.G.; Linstrom, P.J.; NIST Chemistry Web Book (Eds.) NIST Standard Reference Database, National Institute of Standards and Technology. 2008. Available online: https://webbook.nist.gov (accessed on 5 November 2019).
- Mozafari, A.A.; Vafaee, Y.; Shahyad, M. Phytochemical composition and in vitro antioxidant potential of Cynodon dactylon leaf and rhizome extracts as affected by drying methods and temperatures. J. Food Sci. Technol. 2018, 55, 2220–2229. [Google Scholar] [CrossRef]
- Karpagasundari, C.; Kulothungan, S. Analysis of bioactive compounds in Physalis minima leaves using GC MS, HPLC, UV-VIS and FTIR techniques. J. Pharm. Phytochem. 2014, 3, 196–201. [Google Scholar]
- Qi, P.; Jiang, J.; Qi, H.; Zhang, W. Synthesis and antiproliferative activity of new polyoxo 2-Benzyl-2,3-dihydrobenzofurans and their related compounds. Lett. Drug Des. Discov. 2013, 10, 886–894. [Google Scholar] [CrossRef]
- Mohammad, H.; Prabhu, K.; Rao, M.R.K.; Sundaram, R.L.; Shil, S.; Vijayalakshmi, N. The GC MS studies of one Ayurvedic medicine, Amritarishtam. Res. J. Pharm. Technol. 2019, 12, 351–356. [Google Scholar] [CrossRef]
- Vijayakumar, A.; Duraipandiyan, V.; Jeyaraj, B.; Agastian, P.; Raj, M.K.; Ignacimuthu, S. Phytochemical analysis and in vitro antimicrobial activity of Illicium griffithii Hook. f. & Thoms extracts. Asian Pac. J. Trop Dis. 2012, 2, 190–199. [Google Scholar]
- Savych, A.; Duchenko, M.; Shepeta, Y.; Davidenko, A.; Polonets, O. Analysis of carbohydrates content in the plant components of antidiabetic herbal mixture by GC-MS. Pharmacia 2021, 68, 721–730. [Google Scholar] [CrossRef]
- Efferth, T.; Olbrich, A.; Sauerbrey, A.; Ross, D.D.; Gebhart, E.; Neugebauer, M. Activity of ascaridol from the anthelmintic herb Chenopodium anthelminticum L. against sensitive and multidrug-resistant tumor cells. Anticancer Res. 2002, 22, 4221–4224. [Google Scholar]
- Hadi, I.; Hussein, H.M. Antimicrobial Activity and spectral chemical analysis of methanolic leaves extract of Adiantum Capillus-Veneris using GC-MS and FT-IR spectroscopy. Int. J. Pharm. Phytochem. Res. 2016, 8, 369–385. [Google Scholar]
- Hadi, M.Y.; Mohammed, G.J.; Hameed, I.H. Analysis of bioactive chemical compounds of Nigella sativa using gas chromatography-mass spectrometry. J. Pharmacogn. Phytother. 2016, 8, 8–24. [Google Scholar]
- Savych, A.; Bilyk, O.; Vaschuk, V.; Humeniuk, I. Analysis of inulin and fructans in Taraxacum officinale L. roots as the main inulin-containing component of antidiabetic herbal mixture. Pharmacia 2021, 68, 527–532. [Google Scholar] [CrossRef]
- James-Okoro, P.P.O.; Iheagwam, F.N.; Sholeye, M.I.; Umoren, I.A.; Adetuyi, B.O.; Ogundipe, A.E.; Braimah, A.A.; Adekunbi, T.S.; Ogunlana, O.E.; Ogunlana, O.O. Phytochemical and in vitro antioxidant assessment of Yoyo bitters. World News Nat. Sci. 2021, 37, 1–17. [Google Scholar]
- Kamal, S.A.; Hamza, L.F.; Hameed, I.H. Antibacterial activity of secondary metabolites isolated from Alternaria alternate. Afr. J. Biotechnol. 2015, 14, 2972–2994. [Google Scholar]
- Alghamdi, S.S.; Khan, M.A.; El-Harty, E.H.; Ammar, M.H.; Farooq, M.; Migdadi, H.M. Comparative phytochemical profiling of different soybean (Glycine max (L.) Merr) genotypes using GC–MS. Saudi J. Biol. Sci. 2018, 25, 15–21. [Google Scholar] [CrossRef]
- Sarumathy, K.; Rajan, M.D.; Vijay, T.; Jayakanthi, J. Evaluation of phytoconstituents, nephro-protective and antioxidant activities of Clitoria ternatea. J. Appl. Pharm. Sci. 2011, 1, 164–172. [Google Scholar]
- Jadhav, V.; Kalase, V.; Patil, P. GC-MS analysis of bioactive compounds in methanolic extract of Holigarna grahamii (wight) Kurz. IJHM 2014, 35, 35–39. [Google Scholar]
- Rani, S.; Singh, V.; Sharma, M.K.; Sisodia, R. GC–MS based metabolite profiling of medicinal plant-Catharanthus roseus under cadmium stress. Plant Physiol. Rep. 2021, 26, 491–502. [Google Scholar] [CrossRef]
- Lee, W.; Woo, E.R.; Lee, D.G. Phytol has antibacterial property by inducing oxidative stress response in Pseudomonas aeruginosa. Free Radic. Res. 2016, 50, 1309–1318. [Google Scholar] [CrossRef]
- Rao, M.R.K.; Ravi, A.; Narayanan, S.; Prabhu, K.; Kalaiselvi, V.S.; Dinakar, S.; Kotteeswaran, N. Antioxidant study and GC MS analysis of an ayurvedic medicine ‘Talisapatradi Choornam’. Int. J. Pharm. Sci. Rev. Res. 2016, 36, 158–166. [Google Scholar]
- Baeshen, N.A.; Almulaiky, Y.Q.; Afifi, M.; Al-Farga, A.; Ali, H.A.; Baeshen, N.N.; Baeshen, M.N. GC-MS Analysis of Bioactive Compounds Extracted from Plant Rhazya stricta Using Various Sol-vents. Plants 2023, 12, 960. [Google Scholar] [CrossRef]
- Varadharajan, R.; Rajalingam, D.; Palani, S. GCMS/MS analysis and cardioprotective potential of Cucumis callosus on doxorubicin induced cardiotoxicity in rats. Int. J. Pharm. Pharm. Sci. 2016, 8, 239–245. [Google Scholar] [CrossRef]
- Sahukari, R.; Punabaka, J.; Bhasha, S.; Ganjikunta, V.S.; Kondeti Ramudu, S.; Kesireddy, S.R.; Korivi, M. Phytochemical profile, free radical scavenging and anti-inflammatory properties of Acalypha Indica root extract: Evidence from in vitro and in vivo studies. Molecules 2021, 26, 6251. [Google Scholar] [CrossRef]
- Beulah, G.G.; Soris, P.T.; Mohan, V.R. GC-MS determination of bioactive compounds of Dendrophthoe falcata (LF) Ettingsh: An epiphytic plant. Int. J. Health Sci. Res. 2018, 8, 261–269. [Google Scholar]
- Hussein, H.M. Analysis of trace heavy metals and volatile chemical compounds of Lepidium sativum using atomic absorption spectroscopy, gas chromatography-mass spectrometric and fourier-transform infrared spectroscopy. Res. J. Pharm. Biol. Chem. Sci. 2016, 7, 2529–2555. [Google Scholar]
- Rajeswari, G.; Murugan, M.; Mohan, V.R. GC-MS analysis of bioactive components of Hugonia mystax L. bark (Linaceae). J. Pharm. Biomed. Sci. 2013, 29, 818–824. [Google Scholar]
- Dhull, S.B.; Punia, S. Essential fatty acids: Introduction. In Essential Fatty Acids; CRC Press: Boca Raton, FL, USA, 2020; pp. 1–18. [Google Scholar]
- Starlin, T.; Prabha, P.S.; Thayakumar, B.K.A.; Gopalakrishnan, V.K. Screening and GC-MS profiling of ethanolic extract of Tylophora pauciflora. J. Biomed. Inform. 2019, 15, 425–429. [Google Scholar] [CrossRef]
- Hameed, I.H.; Hussein, H.J.; Kareem, M.A.; Hamad, N.S. Identification of five newly described bioactive chemical compounds in methanolic extract of Mentha viridis by using gas chromatography-mass spectrometry (GC-MS). J. Pharm. Phyther. 2015, 7, 107–125. [Google Scholar]
- Archana, D.; Dixitha, M.; Santhy, M. Antioxidant and anti-clastogenic potential of Piper longum L. Int. J. Appl. Pharm. 2015, 7, 11–14. [Google Scholar]
- Karthikeyan, A.V.P.; Sudan, I. GC-MS profile of in-vivo and in-vitro shoots of Cleome gynandra L. Int. J. Pharm. Pharm. Sci. 2017, 9, 21–26. [Google Scholar]
- Kumar, P.P.; Kumaravel, P.; Lalitha, C. Screening of antioxidant activity, total phenolics and GC-MS study of Vitex negundo. Afr. J. Biomed. Res. 2010, 4, 191–195. [Google Scholar]
- Al-Mawla, A.H.; Abu-Serag, N.A. Determination of bioactive compounds in leaves of Urospermum picroides (L.) Desf. Plant Arch. 2019, 19, 1299–1308. [Google Scholar]
- Nishanthini, A.; Mohan, V.R.; Jeeva, S. Phytochemical, FT-IR, and GC-MS analysis of stem and leaf of Tiliacora acuminata (lan.) hook f and Thomas (menispermaceae). Int. J. Pharm. Sci. Res. 2014, 5, 3977–3986. [Google Scholar]
- Teoh, W.Y.; Yong, Y.S.; Razali, F.N.; Stephenie, S.; Shah, M.D.; Tan, J.K.; Gnanaraj, C.; Mohd, E.N. LC-MS/MS and GC-MS Analysis for the Identification of Bioactive Metabolites Responsible for the Antioxidant and Antibacterial Activities of Lygodium microphyllum (Cav.) R. Br. Separations 2023, 10, 215. [Google Scholar] [CrossRef]
- Ghosh, G.; Panda, P.; Rath, M.P.; Sharma, T.A.; Das, D. GC-MS analysis of bioactive compounds in the methanol extract of Clerodendrum viscosum leaves. Pharmacogn. Res. 2015, 7, 110–113. [Google Scholar]


| Sr. No. | Accession No. | PUN No. | District | Latitude | Longitude | Altitude (m) |
|---|---|---|---|---|---|---|
| 1 | Cr00PFRE | 62713 | Bhiwani, Haryana | 30.30° N | 74.60° E | 219 m |
| 2 | Cr00LPFLPE | 62714 | Patiala, Punjab | 30.36° N | 76.45° E | 250 m |
| 3 | Cr00DPF | 62715 | Narwana, Haryana | 29.60° N | 76.11° E | 232 m |
| 4 | Cr00WFYE | 62716 | Hissar, Haryana | 29.15° N | 75.72° E | 216 m |
| 5 | Cr00WFRE | 62717 | Jind, Haryana | 29.32° N | 76.30° E | 219 m |
| 6 | Cr00WFRE2 | 62718 | Rohtak, Haryana | 28.89° N | 76.59° E | 220 m |
| 7 | Cr00WFSRE | 62719 | Delhi | 28.64° N | 77.09° E | 216 m |
| 8 | Cr00WFYE2 | 62720 | Patra, Punjab | 29.20° N | 76.19° E | 250 m |
| 9 | Cr00DP | 62721 | Rewari, Haryana | 28.20° N | 76.62° E | 244 m |
| 10 | Cr00LP | 62722 | Bhiwani, Haryana | 30.30° N | 74.60° E | 219 m |
| 11 | Cr00BPF | 62723 | Panchkula, Haryana | 30.69° N | 76.85° E | 217 m |
| 12 | Cr00SFP | 62724 | Bhiwani, Haryana | 30.30° N | 74.60° E | 219 m |
| 13 | Cr00CAF | 62725 | Chandigarh | 30.71° N | 76.78° E | 321 m |
| 14 | Cr00LPNF | 62726 | Kaithal, Haryana | 29.79° N | 76.41° E | 248 m |
| 15 | Cr00SBRF | 62727 | Devigarh, Punjab | 24.77° N | 73.74° E | 251 m |
| 16 | Cr00PLMF | 62728 | Patiala, Punjab | 30.36° N | 76.45° E | 250 m |
| 17 | Cr00CHEF | 62729 | Samana, Punjab | 30.15° N | 76.19° E | 250 m |
| 18 | Cr00SNFF | 62730 | Bhiwani, Haryana | 30.30° N | 74.60° E | 219 m |
| 19 | Cr00CLF | 62731 | Chandigarh | 30.71° N | 76.78° E | 321 m |
| 20 | Cr00SAF | 62732 | Hansi, Haryana | 29.10° N | 75.96° E | 219 m |
| 21 | Cr00TDRF | 62733 | Rajpura, Punjab | 30.47° N | 76.59° E | 250 m |
| 22 | Cr00DRYE | 62734 | Jind, Haryana | 29.32° N | 76.30° E | 219 m |
| 23 | Cr00FRFF | 62735 | Tosham, Haryana | 28.87° N | 75.89° E | 219 m |
| 24 | Cr00PFWE | 62736 | Patiala, Punjab | 30.36° N | 76.45° E | 250 m |
| 25 | Cr00PFYE | 62737 | Patiala, Punjab | 30.36° N | 76.45° E | 250 m |
| Sr. No. | RT | Name of the Compound | Molecular Formula | MW | Peak Area (%) | Pharmacological Actions | Relative % Amount of Compounds |
|---|---|---|---|---|---|---|---|
| 1 | 5.75 | 4H-Pyran-4-one | C6H8O4 | 144 | 0.63 | Antioxidant, Antimicrobial, Anti-inflammatory [23] | 0.90 |
| 2 | 6.39 | Catechol/Resorcinol | C6H6O2 | 110.1 | 0.06 | Dermatological/Acne treatment [24] | 0.08 |
| 3 | 6.53 | Benzofuran, 2,3-dihydro | C8H8O | 120.1 | 0.61 | Antioxidant, Analgesic, Antimutagenic [25] | 0.87 |
| 4 | 6.67 | 5Hydroxymethylfurfural | C3H6O3 | 126 | 30.86 | Antioxidant, Antiproliferative activity [26] | 1.2 |
| 5 | 6.77 | 1,2,3-Propanetriol, 1-acetate/acetin | C5H10O4 | 134.1 | 0.84 | Antimicrobial [27] | 0.01 |
| 6 | 7.03 | L-Glucose | C6H12O6 | 180.1 | 1.17 | Sweetening agents [28] | 0.20 |
| 7 | 7.44 | Ascaridole epoxide | C10H16O3 | 184 | 0.14 | Anticarcinogenic [29] | 0.0 |
| 8 | 8.01 | Deoxyspergualin | C17H37N7O3 | 387.5 | 0.11 | Treatment of autoimmune disease [30] | 0.24 |
| 9 | 8.73 | Sucrose | C12H22O11 | 342.2 | 3.14 | Hypercholesterolemic, Preservative [31] | 4.52 |
| 10 | 8.77 | D-fructose | C6H12O6 | 180.156 | 3.36 | Hypercholesterolemic, Preservative [32] | 0.093 |
| 11 | 9.02 | D-allose | C6H12O6 | 180.156 | 0.86 | Antioxidant [33] | 0.24 |
| 12 | 9.07 | Desulphosinigrin | C10H17NO6S | 279.3 | 0.06 | Antioxidant [34] | 0.07 |
| 13 | 9.38 | 3′,5′-Dimethoxyacetophenone | C10H12O3 | 180.2 | 0.17 | Antioxidant [35] | 7.37 |
| 14 | 9.83 | α-D-Glucopyranoside, O-α-Dglucopyranosyl-(1.fwdarw.3)-ß-d-fru | C18H32O16 | 504 | 0.06 | Antidiabetic and antitumour activity [31] | 0.07 |
| 15 | 10.05 | 1,2,3,5-Cyclohexanetetrol+ | C6H12O4 | 148.1 | 5.12 | Poly-Hydroxy compound, Analgesic, Anesthetic, Antioxidant, Antiseptic, Antibacterial, Antiviral, Cancer preventive [36] | 9.24 |
| 16 | 10.07 | Quinic acid | C7H12O6 | 192.167 | 3.73 | Antioxidant [37] | 43.88 |
| 17 | 10.94 | Myo-Inositol, 4-C-methyl- | C7H14O6 | 194.1 | 30.45 | Antioxidant and Antimicrobial [33] | 3.43 |
| 18 | 11.90 | Muco-Inositol | C6H12O6 | 180.1 | 2.38 | Chemopreventive [33] | 1.40 |
| 19 | 12.29 | Hexadecanoic acid, methyl ester | C17H34O2 | 270.4 | 0.97 | Palmitic acid methyl ester Antioxidant, Hypocholesterolemic, Nematicide, Pesticide, Antiandrogenic flavor, Hemolytic,5-Alpha reductase inhibitor [38] | 15.00 |
| 20 | 12.64 | Pentadecanoic acid | C15H30O2 | 242.4 | 10.41 | Antimicrobial, Antioxidant, Anticancer | 3.47 |
| 21 | 13.38 | Phytol | C20H40O | 296 | 17.17 | Hypocholesterolemic, Antimicrobial, Anticancer, Diuretic, Anti-inflammatory [39] | 3.89 |
| 22 | 14.32 | 9,12,15-Octadecatrienoic acid, methyl ester | C19H32O2 | 292.2 | 2.70 | Anticancer, Nematicides, Antocoronary [38] | 1.75 |
| 23 | 16.08 | 2,20-Cycloaspidospermidine-3-carboxylic acid, Vindolinine | C21H24N2O2 | 336 | 4.95 | Anticancer [38] | 0.05 |
| 24 | 16.73 | 9,12,15-Octadecatrienoic acid, | C27H52O4Si2 | 496.2 | 0.04 | Anti-inflammation [38] | 0.24 |
| 25 | 19.77 | Octadecane, 3-ethyl-5-(2-ethylbutyl)- | C26H54 | 366 | 0.01 | Antimicrobial and antifungal [40,41] | 0.41 |
| 26 | 20.57 | Aspidospermidine-3-carboxylic acidvindoline | C22H28N2O5 | 400 | 12.09 | Alkaloids [41] Anticancer | 0.15 |
| 27 | 22.69 | Condyfolan, 14,19-didehydro-12-methoxy-, (14E)- | C19H24N2O | 322.4 | 0.29 | Antimicrobial [42] | 0.78 |
| 28 | 22.76 | Aspidospermidine-3-carboxylic acid, | C22H28N8O5 | 400.4 | 0.11 | Anticancer [43] | 0.19 |
| 29 | 22.97 | Phthalic acid, di(oct-3-yl) ester | C24H38O4 | 390.5 | 0.54 | Antimicrobial and Antifouling [44] | 0.05 |
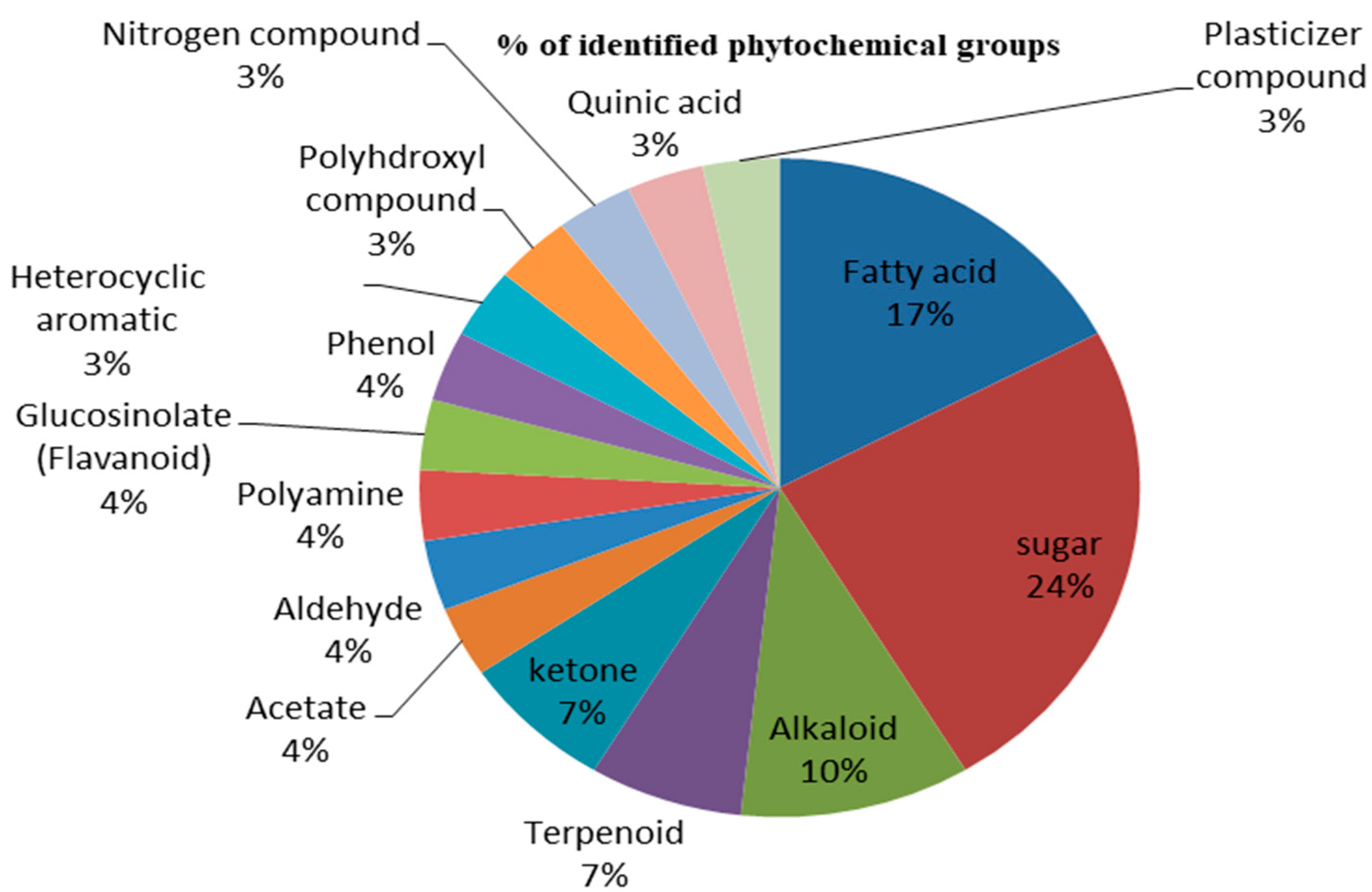
| Sr. No. | Name of Compounds | Nature of Compounds |
|---|---|---|
| 1. | Hexadecanoic acid, methyl ester | Fatty acid |
| 2. | Pentadecanoic acid | |
| 3. | 9,12,15-Octadecatrienoic acid, | |
| 4. | Octadecane, 3-ethyl-5-(2-ethylbutyl)- | |
| 5. | 9,12,15-Octadecatrienoic acid, methyl ester | |
| 6. | L-Glucose | Sugar |
| 7. | Sucrose; D-Glucose-6-O (sugar), α-D-galactopyranosyl | |
| 8. | D-fructose | |
| 9. | D-allose | |
| 10. | Muco-Inositol | |
| 11. | α-D-Glucopyranoside, O-α-Dglucopyranosyl-(1.fwdarw.3)-ß-d-fru | |
| 12. | Myo-Inositol, 4-C-methyl- | |
| 13. | 2,20-Cycloaspidospermidine-3-carboxylic acidVindolinine | Alkaloid |
| 14. | Aspidospermidine-3-carboxylic acid, | |
| 15. | Aspidospermidine-3-carboxylic acidvindoline | |
| 16. | Ascaridole epoxide | Monoterpenoid |
| 17. | Phytol | Terpenoid (diterpenoid) |
| 18. | 3′,5′-Dimethoxyacetophenone | Ketone |
| 19. | 4H-Pyran-4-one/Maltol | Ketone |
| 20. | 1,2,3-Propanetriol, 1-acetate/acetin | Acetate |
| 21. | 5Hydroxymethylfurfural | Aldehyde |
| 22. | Deoxyspergualin | Polyamine |
| 23. | Desulphosinigrin | Glucosinolate (Flavanoid) |
| 24. | Catechol/Resorcinol | Phenol |
| 25. | Benzofuran, 2,3-dihydro | Heterocyclic aromatic |
| 26. | 1,2,3,5-Cyclohexanetetrol+ | Polyhdroxyl compound |
| 27. | Condyfolan, 14,19-didehydro-12-methoxy-, (14E)- | Nitrogen compound |
| 28. | (1R,3R,4R,5R)-(-)-Quinic acid | Quinic acid |
| 29. | Phthalic acid, di(oct-3-yl) ester | Plasticizer compound |
| F1 | F2 | F3 | F4 | F5 | |
|---|---|---|---|---|---|
| Eigenvalue | 6.250 | 3.533 | 2.887 | 2.342 | 2.077 |
| Variability (%) | 21.550 | 12.181 | 9.955 | 8.076 | 7.162 |
| Cumulative (%) | 21.550 | 33.732 | 43.687 | 51.762 | 58.924 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rani, J.; Kapoor, M.; Dhull, S.B.; Goksen, G.; Jurić, S. Identification and Assessment of Therapeutic Phytoconstituents of Catharanthus roseus through GC-MS Analysis. Separations 2023, 10, 340. https://doi.org/10.3390/separations10060340
Rani J, Kapoor M, Dhull SB, Goksen G, Jurić S. Identification and Assessment of Therapeutic Phytoconstituents of Catharanthus roseus through GC-MS Analysis. Separations. 2023; 10(6):340. https://doi.org/10.3390/separations10060340
Chicago/Turabian StyleRani, Jyoti, Manish Kapoor, Sanju Bala Dhull, Gulden Goksen, and Slaven Jurić. 2023. "Identification and Assessment of Therapeutic Phytoconstituents of Catharanthus roseus through GC-MS Analysis" Separations 10, no. 6: 340. https://doi.org/10.3390/separations10060340
APA StyleRani, J., Kapoor, M., Dhull, S. B., Goksen, G., & Jurić, S. (2023). Identification and Assessment of Therapeutic Phytoconstituents of Catharanthus roseus through GC-MS Analysis. Separations, 10(6), 340. https://doi.org/10.3390/separations10060340








