Extractive Distillation Approach to the Separation of Styrene from Pyrolysis Gasoline Feedstock Coupled with Deep Desulfurization
Abstract
1. Introduction
2. Methods
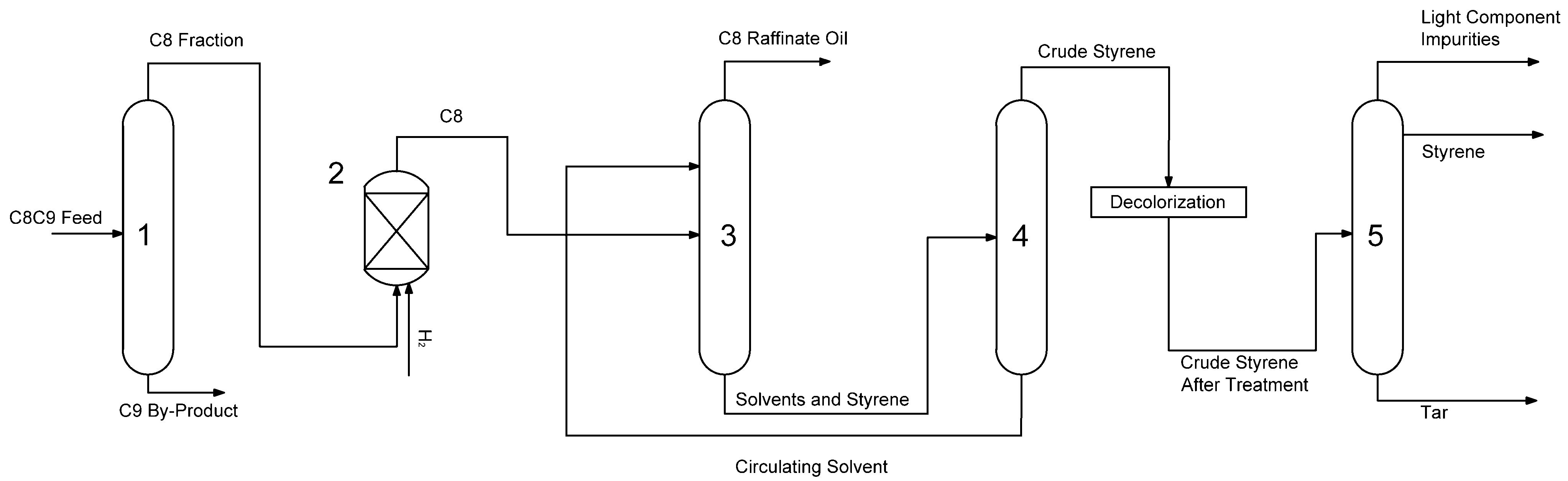
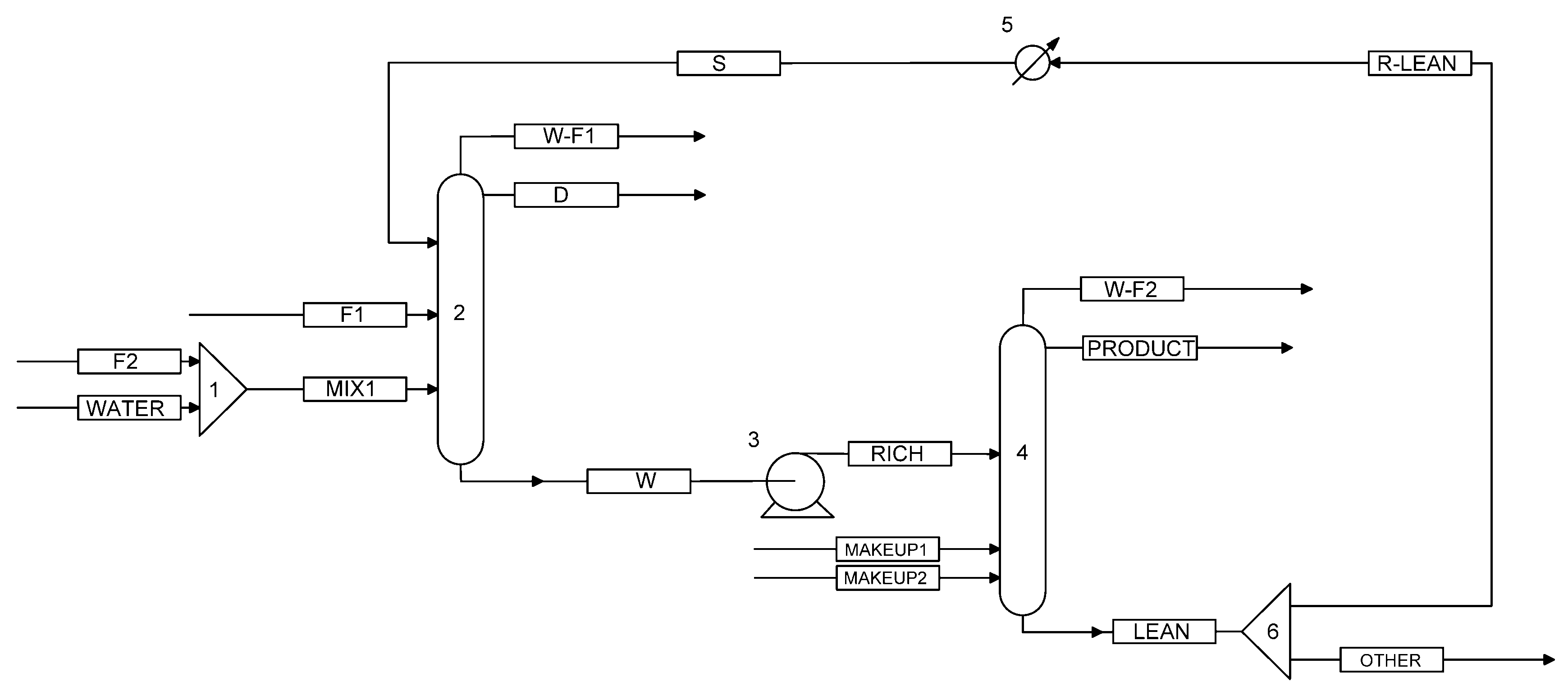
2.1. Process Simulation
2.1.1. Calculation of Activity and Selectivity Coefficients
2.1.2. Construction of the Process Flowchart
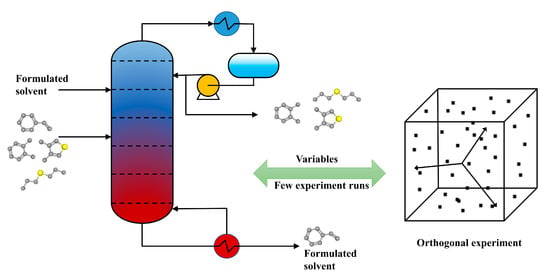
2.2. Orthogonal Experiment
2.3. Linear Regression
3. Results and Discussion
3.1. Selection of Entrainment Agent for Extractive Distillation
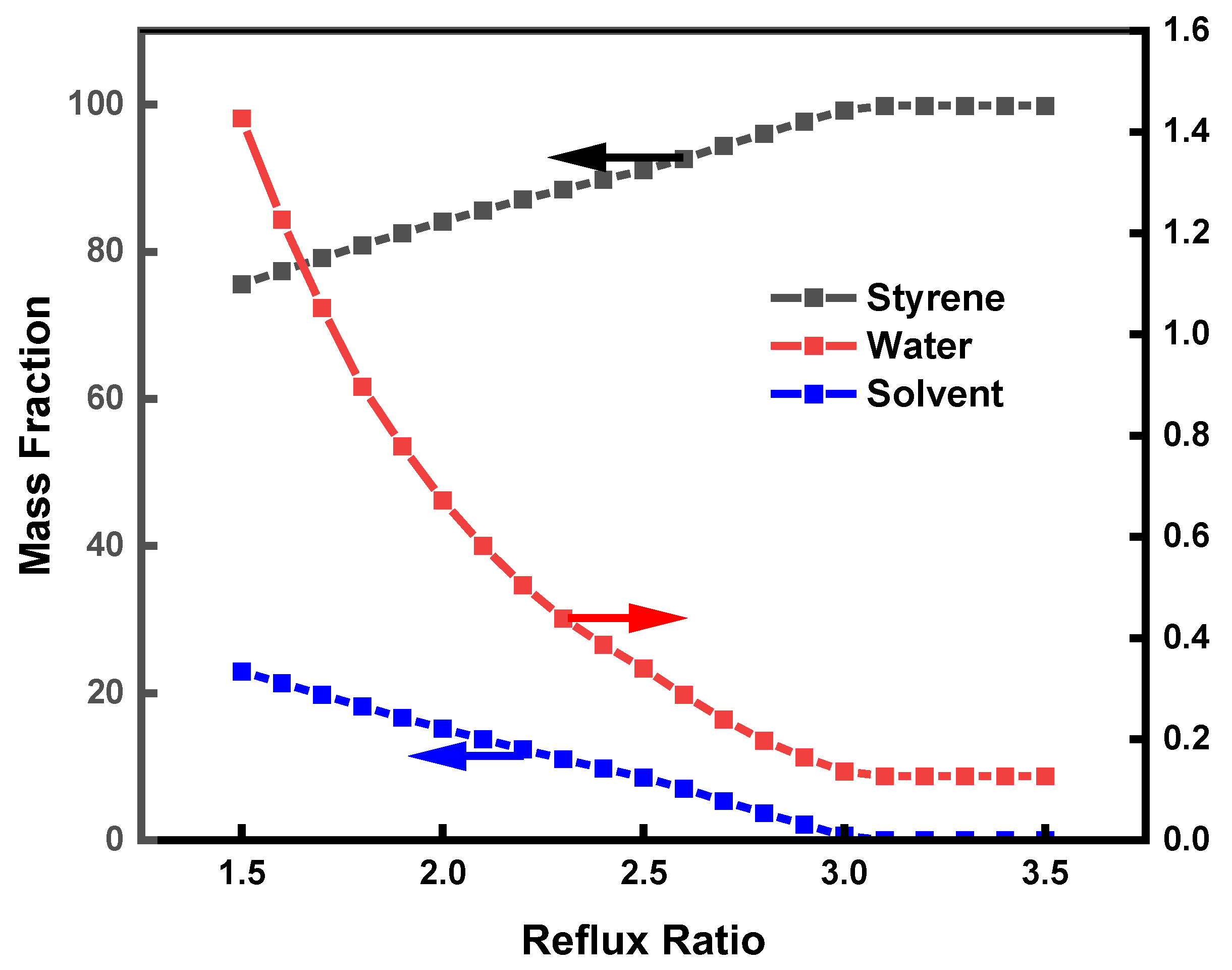
3.2. Process Simulation
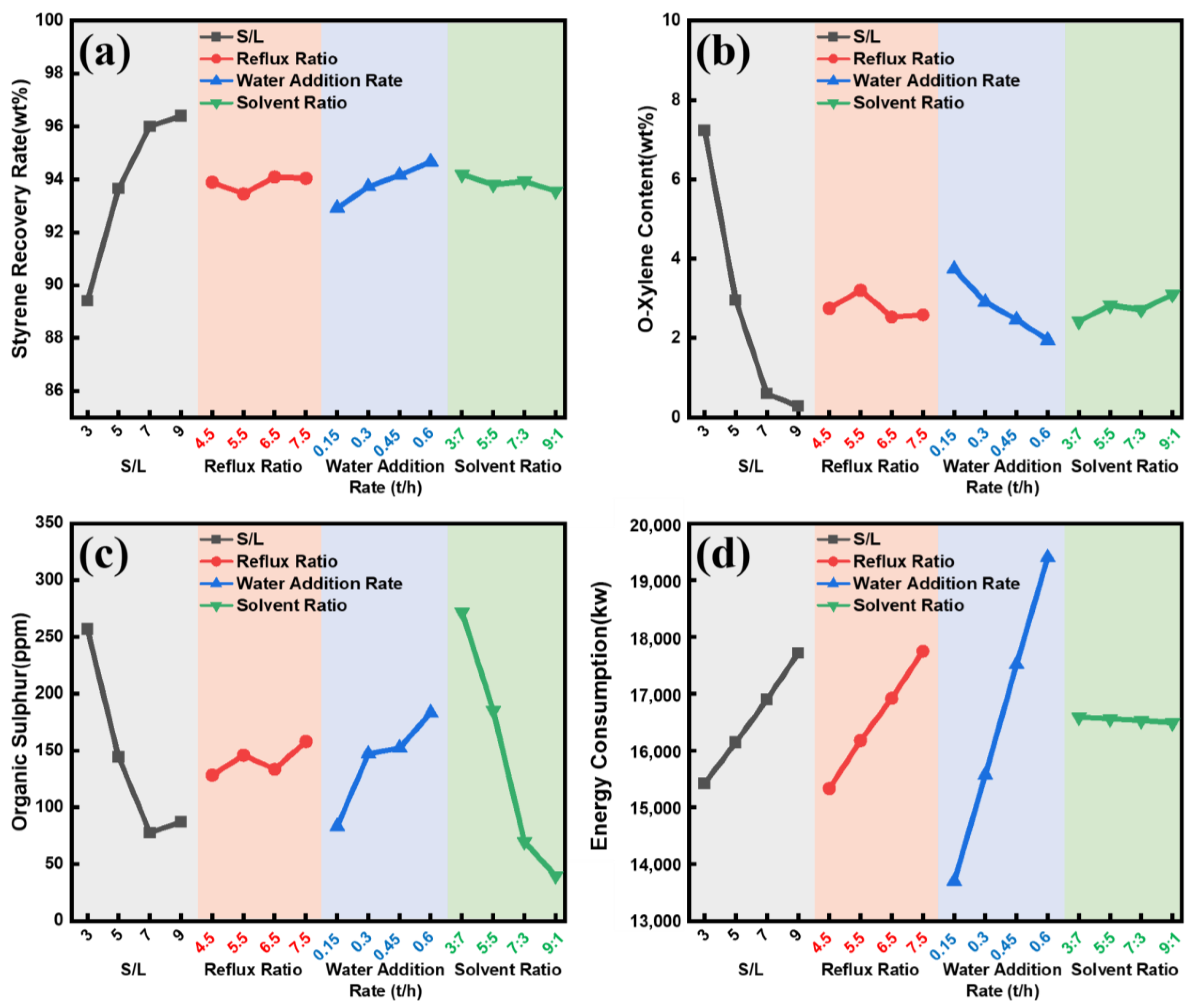
3.3. Extreme Difference Analysis of the Orthogonal Experiment
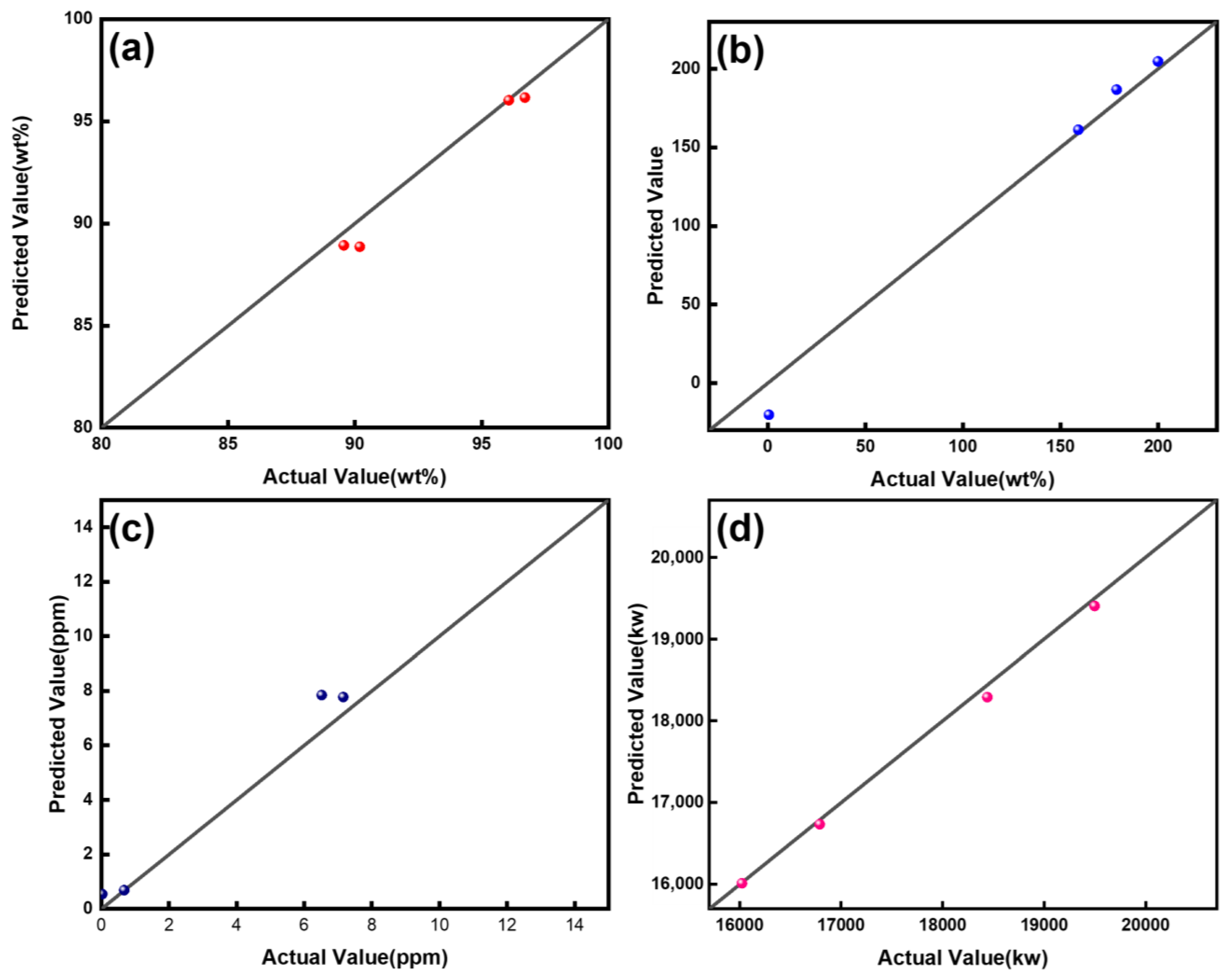
3.4. ANOVA and Linear Regression
3.5. Simulation Optimization of the Process
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Chung, C.; Kim, J.; Sovacool, B.K.; Griffiths, S.; Bazilian, M.; Yang, M. Decarbonizing the chemical industry: A systematic review of sociotechnical systems, technological innovations, and policy options. Energy Res. Soc. Sci. 2023, 96, 102955. [Google Scholar] [CrossRef]
- Randall, G.A. Method of Separating Ethylbenzene from Styrene by Low Pressure Drop Distillation. U.S. Patent 3084108A, 2 April 1963. [Google Scholar]
- Van Tassell, H.M. Separation of Ethylbenzene and Styrene by Low Pressure, High Temperature Distillation. U.S. Patent 3398063A, 20 August 1968. [Google Scholar]
- Welch, V.A. Cascade Reboiling of Ethylbenzene/Styrene Columns. U.S. Patent 6171449B1, 9 January 2001. [Google Scholar]
- Gupta, R.; Uslu, H.; Majumder, S. Production of Styrene from Dehydrogenation of Ethylbenzene. Chem. Eng. Technol. 2022, 45, 817–823. [Google Scholar] [CrossRef]
- Sun, H.; Zhang, J.; Li, K.; Wang, H.; Zhu, X. Efficient Oxidative Dehydrogenation of Ethylbenzene over K/CeO2 with Exceptional Styrene Yield. Catalysts 2023, 13, 781. [Google Scholar] [CrossRef]
- Dai, X.; Cao, T.; Lu, X.; Bai, Y.; Qi, W. Tailored Pd/C bifunctional catalysts for styrene production under an ethylbenzene oxidative dehydrogenation assisted direct dehydrogenation scheme. Appl. Catal. B Environ. 2023, 324, 122205. [Google Scholar] [CrossRef]
- Gary, J.H.; Handwerk, G.E.; Kaiser, M.J. Petroleum Refining: Technology and Economics, 5th ed.; CRC Press: Kiev, Ukraine, 2007. [Google Scholar]
- Busca, G. Production of Gasolines and Monocyclic Aromatic Hydrocarbons: From Fossil Raw Materials to Green Processes. Energies 2021, 14, 4061. [Google Scholar] [CrossRef]
- Jongmans, M.T.G.; Schuur, B.; de Haan, A.B. Ionic Liquid Screening for Ethylbenzene/Styrene Separation by Extractive Distillation. Ind. Eng. Chem. Res. 2011, 50, 10800–10810. [Google Scholar] [CrossRef]
- Sendich, E. Planning and Urban Design Standards; John Wiley & Sons: Hoboken, NJ, USA, 2006. [Google Scholar]
- Jongmans, M.T.G.; Hermens, E.; Raijmakers, M.; Maassen, J.I.W.; Schuur, B.; de Haan, A.B. Conceptual process design of extractive distillation processes for ethylbenzene/styrene separation. Chem. Eng. Res. Des. 2012, 90, 2086–2100. [Google Scholar] [CrossRef]
- Jie, K.; Liu, M.; Zhou, Y.; Little, M.A.; Bonakala, S.; Chong, S.Y.; Stephenson, A.; Chen, L.; Huang, F.; Cooper, A.I. Styrene Purification by Guest-Induced Restructuring of Pillar[6]arene. J. Am. Chem. Soc. 2017, 139, 2908–2911. [Google Scholar] [CrossRef]
- Dey, A.; Chand, S.; Maity, B.; Bhatt, P.M.; Ghosh, M.; Cavallo, L.; Eddaoudi, M.; Khashab, N.M. Adsorptive Molecular Sieving of Styrene over Ethylbenzene by Trianglimine Crystals. J. Am. Chem. Soc. 2021, 143, 4090–4094. [Google Scholar] [CrossRef]
- Ding, Y.; Dey, A.; Alimi, L.O.; Bhatt, P.M.; Du, J.; Maaliki, C.; Eddaoudi, M.; Jacquemin, J.; Khashab, N.M. Optimizing Host–Guest Selectivity for Ethylbenzene Capture Toward Superior Styrene Purification. Chem. Mater. 2022, 34, 197–202. [Google Scholar] [CrossRef]
- Torres-Knoop, A.; Heinen, J.; Krishna, R.; Dubbeldam, D. Entropic Separation of Styrene/Ethylbenzene Mixtures by Exploitation of Subtle Differences in Molecular Configurations in Ordered Crystalline Nanoporous Adsorbents. Langmuir 2015, 31, 3771–3778. [Google Scholar] [CrossRef] [PubMed]
- Niu, X.; Lv, W.; Sun, Y.; Dai, H.; Chen, H.; Chen, X. In situ fabrication of 3D COF-300 in a capillary for separation of aromatic compounds by open-tubular capillary electrochromatography. Microchim. Acta 2020, 187, 233. [Google Scholar] [CrossRef] [PubMed]
- Larriba, M.; de Riva, J.; Navarro, P.; Moreno, D.; Delgado-Mellado, N.; García, J.; Ferro, V.R.; Rodríguez, F.; Palomar, J. COSMO-based/Aspen Plus process simulation of the aromatic extraction from pyrolysis gasoline using the {[4empy][NTf2] + [emim][DCA]} ionic liquid mixture. Sep. Purif. Technol. 2018, 190, 211–227. [Google Scholar] [CrossRef]
- Navarro, P.; de Dios-García, I.; Larriba, M.; Delgado-Mellado, N.; Ayuso, M.; Moreno, D.; Palomar, J.; García, J.; Rodríguez, F. Dearomatization of pyrolysis gasoline by extractive distillation with 1-ethyl-3-methylimidazolium tricyanomethanide. Fuel Process. Technol. 2019, 195, 106156. [Google Scholar] [CrossRef]
- Karpińska, M.; Domańska, U.; Wlazło, M. Separation of ethylbenzene/styrene systems using ionic liquids in ternary LLE. J. Chem. Thermodyn. 2016, 103, 423–431. [Google Scholar] [CrossRef]
- Karpińska, M.; Wlazło, M.; Domańska, U. Investigation on the ethylbenzene/styrene separation efficiency with ionic liquids in liquid–liquid extraction. Chem. Eng. Res. Des. 2017, 128, 214–220. [Google Scholar] [CrossRef]
- Meindersma, G.W.; Hansmeier, A.R.; de Haan, A.B. Ionic Liquids for Aromatics Extraction. Present Status and Future Outlook. Ind. Eng. Chem. Res. 2010, 49, 7530–7540. [Google Scholar] [CrossRef]
- Torres Cantero, C.A.; Lopez Lopez, G.; Alvarado, V.M. Control structures evaluation for a salt extractive distillation pilot plant: Application to bio-ethanol dehydration. Energies 2017, 10, 1276. [Google Scholar] [CrossRef]
- Rumbo Morales, J.Y.; Perez Vidal, A.F.; Ortiz Torres, G. Adsorption and separation of the H2O/H2SO4 and H2O/C2H5OH mixtures: A simulated and experimental study. Processes 2020, 8, 290. [Google Scholar] [CrossRef]
- Torres Cantero, C.A.; Pérez Zúñiga, R.; Martínez García, M. Design and control applied to an extractive distillation column with salt for the production of bioethanol. Processes 2022, 10, 1792. [Google Scholar] [CrossRef]
- Hadj-Kali, M.K.; El Blidi, L.; Mulyono, S.; Wazeer, I.; Ali, E.; Rallapalli, J. Deep Eutectic Solvents for the Separation of Toluene/1-Hexene via Liquid–Liquid Extraction. Separations 2022, 9, 369. [Google Scholar] [CrossRef]
- Vega, A.; Díez, F.; Esteban, R.; Coca, J. Solvent Selection for Cyclohexane−Cyclohexene−Benzene Separation by Extractive Distillation Using Non-Steady-State Gas Chromatography. Ind. Eng. Chem. Res. 1997, 36, 803–807. [Google Scholar] [CrossRef]
- Lei, Z.; Dai, C.; Chen, B.; Ding, Z. Special Distillation Processes; Elsevier: Amsterdam, The Netherlands, 2021. [Google Scholar]
- Chen, B.; Lei, Z.; Li, Q.; Li, C. Application of CAMD in separating hydrocarbons by extractive distillation. AIChE J. 2005, 51, 3114–3121. [Google Scholar] [CrossRef]
- Cretoiu, L.; Xu, S.; Gentry, J.; Kumar, S. Pyrolysis Value Upgrade with GT-Styrene. In Proceedings of the ERTC Petrochemical Conference Sofitel Rive Gauche Hotel, Paris, France, 3–5 March 2003. [Google Scholar]
- Wei-Wei, P.; Wen-Cheng, T.; Long-Sheng, T.; Zhuo, Y.; Ming, Z. Development and Application of Styrene Recovery Process from Pyrolysis Gasoline. Pet. Process. Petrochem. 2021, 52, 163–169. [Google Scholar]
- Ayuso, M.; Navarro, P.; Moya, C. Extractive distillation with ionic liquids to separate benzene, toluene, and xylene from pyrolysis gasoline: Process design and techno-economic comparison with the morphylane process. Ind. Eng. Chem. Res. 2022, 61, 2511–2523. [Google Scholar] [CrossRef]
- Fredenslund, A. Vapor-Liquid Equilibria Using UNIFAC: A Group-Contribution Method; Elsevier: Amsterdam, The Netherlands, 2012. [Google Scholar]
- Fredenslund, A. UNIFAC and related group-contribution models for phase equilibria. Fluid Phase Equilibria 1989, 52, 135–150. [Google Scholar] [CrossRef]
- Alessi, P.; Kikic, I.; Fredenslund, A.; Rasmussen, P. UNIFAC and infinite dilution activity coefficients. Can. J. Chem. Eng. 1982, 60, 300–304. [Google Scholar] [CrossRef]
- Dong, S.; Sun, X.; Wang, L.; Li, Y.; Zhao, W.; Xia, L.; Xiang, S. Prediction, Application, and Mechanism Exploration of Liquid–Liquid Equilibrium Data in the Extraction of Aromatics Using Sulfolane. Processes 2023, 11, 1228. [Google Scholar] [CrossRef]
- Fredenslund, A.; Gmehling, J.; Michelsen, M.L.; Rasmussen, P.; Prausnitz, J.M. Computerized Design of Multicomponent Distillation Columns Using the UNIFAC Group Contribution Method for Calculation of Activity Coefficients. Ind. Eng. Chem. Process Des. Dev. 1977, 16, 450–462. [Google Scholar] [CrossRef]
- Gmehling, J. Group contribution methods for the estimation of activity coefficients. Fluid Phase Equilibria 1986, 30, 119–134. [Google Scholar] [CrossRef]
- Lei, Z.; Li, C.; Chen, B. Extractive Distillation: A Review. Sep. Purif. Rev. 2003, 32, 121–213. [Google Scholar] [CrossRef]
- Asprion, N. Modeling, Simulation, and Optimization 4.0 for a Distillation Column. Chem. Ing. Tech. 2020, 92, 879–889. [Google Scholar] [CrossRef]
- Choi, Y.J.; Cho, K.W.; Cho, B.W.; Yeo, Y.-K. Optimization of the Sulfolane Extraction Plant Based on Modeling and Simulation. Ind. Eng. Chem. Res. 2002, 41, 5504–5509. [Google Scholar] [CrossRef]
- Liang, Y.-Z.; Fang, K.-T.; Xu, Q.-S. Uniform design and its applications in chemistry and chemical engineering. Chemom. Intell. Lab. Syst. 2001, 58, 43–57. [Google Scholar] [CrossRef]
- Lundstedt, T.; Seifert, E.; Abramo, L.; Thelin, B.; Nyström, Å.; Pettersen, J.; Bergman, R. Experimental design and optimization. Chemom. Intell. Lab. Syst. 1998, 42, 3–40. [Google Scholar] [CrossRef]
- Fang, K.; Liu, M.-Q.; Qin, H.; Zhou, Y.-D. Theory and Application of Uniform Experimental Designs; Springer: Berlin/Heidelberg, Germany, 2018; Volume 221. [Google Scholar]
- Leardi, R. Experimental design in chemistry: A tutorial. Anal. Chim. Acta 2009, 652, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Gunst, R.F.; Mason, R.L. Fractional factorial design. WIREs Comput. Stat. 2009, 1, 234–244. [Google Scholar] [CrossRef]
- Darani, N.S.; Behbahani, R.M.; Shahebrahimi, Y.; Asadi, A.; Mohammadi, A.H. Simulation and Optimization of the Acid Gas Absorption Process by an Aqueous Diethanolamine Solution in a Natural Gas Sweetening Unit. ACS Omega 2021, 6, 12072–12080. [Google Scholar] [CrossRef]
- Su, X.; Yan, X.; Tsai, C.-L. Linear regression. WIREs Comput. Stat. 2012, 4, 275–294. [Google Scholar] [CrossRef]
- Weisberg, S. Applied Linear Regression; John Wiley & Sons: Hoboken, NJ, USA, 2005; Volume 528. [Google Scholar]

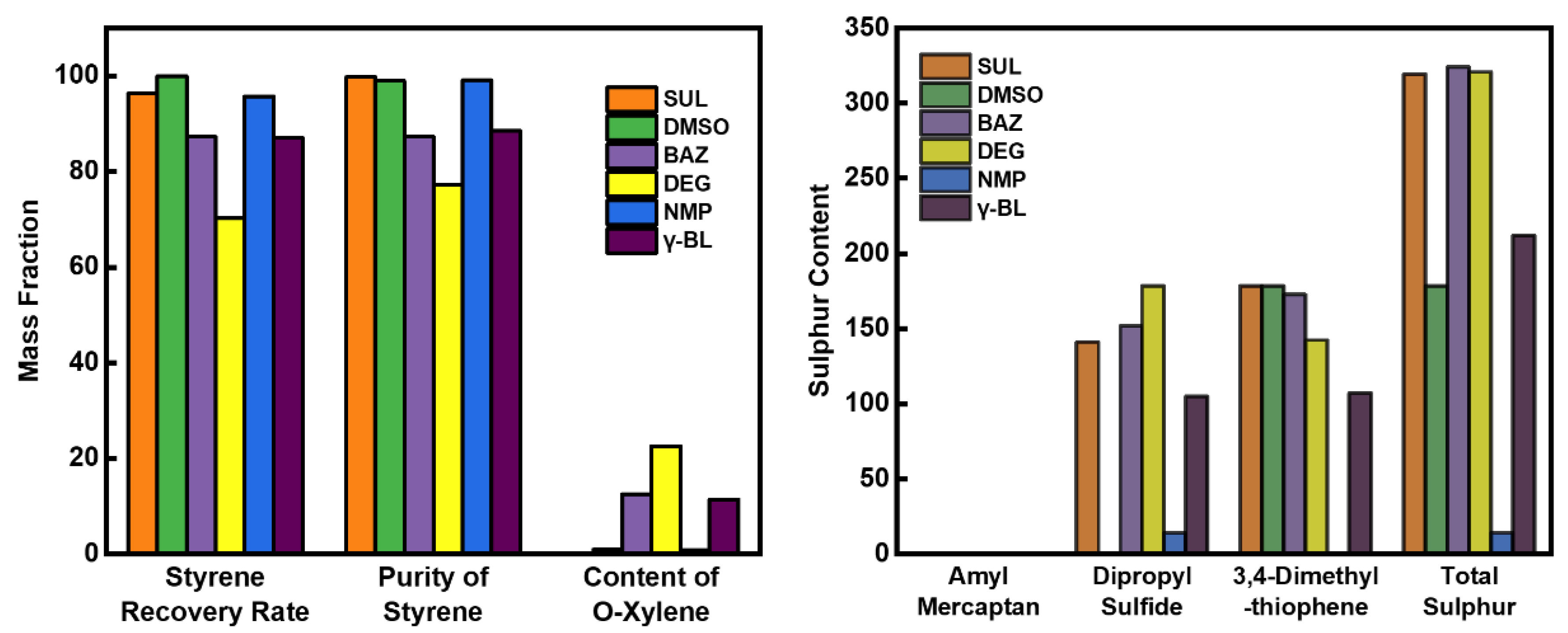
| Extractant | Molecular Structure | Ligand Atom | Functional Group | Strength of Polarity | Molecular Weight | Density, kg·m−3 | Boiling Point, °C | Melting Point, °C | Viscosity, mPa·s | Toxicity |
|---|---|---|---|---|---|---|---|---|---|---|
| Sulfolane (SUL) | 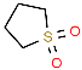 | S | S=O | Strong | 120.17 | 1.261 | 287.1 | 27.4 | 10.286 | None |
| Dimethyl sulfoxide (DMSO) |  | S | S=O | Strong | 78.13 | 1.100 | 190.7 | 18.4 | 1.987 | Slight |
| Benzylamine (BAZ) | 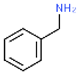 | N | -NH2 | Strong | 107.15 | 0.981 | 184.5 | −30.0 | 1.599 | Moderate |
| Diethylene glycol (DEG) |  | O | -O-, -OH | Strong | 106.12 | 1.118 | 245.0 | −10.0 | 35.700 | Slight |
| N-Methylpyrrolidone (NMP) |  | N | C=O | Strong | 99.13 | 1.028 | 204.0 | −24.0 | 1.650 | Low |
| 1,4-Butyrolactone (γ-BL) | 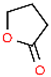 | O | -O-, C=O | Strong | 86.09 | 1.120 | 204.0 | −45.0 | 1.700 | Low |
| Water | — | O | -OH | Strong | 18.02 | 1.000 | 100.0 | 0.0 | 2.980 | None |
| Level | Solent/Oil Ratio | Reflux Ratio | Water Addition Rate | Solent Ratio |
|---|---|---|---|---|
| A | B | C | D | |
| 1 | 3 | 4.5 | 1500 | 3:7 |
| 2 | 5 | 5.0 | 3000 | 5:5 |
| 3 | 7 | 5.5 | 4500 | 7:3 |
| 4 | 9 | 6.0 | 6000 | 9:1 |
| Items | Industrial Data | Simulation Results | Relative Error % |
|---|---|---|---|
| Styrene recovery rate (wt%) | 96.43 | 96.41 | 0.02 |
| Styrene purity (wt%) | 99.78 | 99.80 | 0.02 |
| O-Xylene content (wt%) | 0.07 | 0.09 | 18.60 |
| Organic sulfur content (ppm) | 297.469 | 319.33 | 6.85 |
| Factors | S-S | DOF | F | Significance |
|---|---|---|---|---|
| A | 123.422 | 3 | 86.97815 | ** |
| B | 1.016 | 3 | 0.715997 | |
| C | 6.631 | 3 | 4.673009 | |
| D | 0.811 | 3 | 0.571529 | |
| Error | 1.419 | 3 | ||
| Total | 133.299 | 15 |
| y1 | y2 | y3 | y4 | |
|---|---|---|---|---|
| A | ** | ** | ** | |
| B | ** | |||
| C | ** | |||
| D | * |
| Non-Standardized Coefficient | t | p | VIF | ||
|---|---|---|---|---|---|
| B | Standard Error | ||||
| Constant | 84.738 | 3.229 | 26.243 | <0.01 | - |
| A | 1.166 | 0.140 | 8.326 | <0.01 | 1 |
| B | 0.220 | 0.560 | 0.392 | 0.703 | 1 |
| C | 0 | 0 | 2.037 | 0.066 | 1 |
| D | −0.175 | 0.280 | −0.626 | 0.544 | 1 |
| R2 | 0.871 | ||||
| F | F (4,7) = 17.758, p = 0.001 | ||||
| Items | SUL | Mixed Solvent |
|---|---|---|
| Styrene recovery (wt%) | 96.41 | 96.48 |
| Styrene purity (wt%) | 99.78 | 99.84 |
| O-Xylene content (wt%) | 0.09 | 0.02 |
| Organic sulfur content (ppm) | 319.33 | 6.98 |
| Energy consumption (kW) | 16,658.6 | 18,709.09 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Guo, G.; Liu, C.; Chen, Y.; Zhao, Q.; Gao, W.; Wang, H.; Yang, F.; Shen, B.; Wu, D.; Sun, H. Extractive Distillation Approach to the Separation of Styrene from Pyrolysis Gasoline Feedstock Coupled with Deep Desulfurization. Separations 2023, 10, 341. https://doi.org/10.3390/separations10060341
Guo G, Liu C, Chen Y, Zhao Q, Gao W, Wang H, Yang F, Shen B, Wu D, Sun H. Extractive Distillation Approach to the Separation of Styrene from Pyrolysis Gasoline Feedstock Coupled with Deep Desulfurization. Separations. 2023; 10(6):341. https://doi.org/10.3390/separations10060341
Chicago/Turabian StyleGuo, Guanchu, Chuanlei Liu, Yuxiang Chen, Qiyue Zhao, Weikang Gao, Hao Wang, Fengjing Yang, Benxian Shen, Di Wu, and Hui Sun. 2023. "Extractive Distillation Approach to the Separation of Styrene from Pyrolysis Gasoline Feedstock Coupled with Deep Desulfurization" Separations 10, no. 6: 341. https://doi.org/10.3390/separations10060341
APA StyleGuo, G., Liu, C., Chen, Y., Zhao, Q., Gao, W., Wang, H., Yang, F., Shen, B., Wu, D., & Sun, H. (2023). Extractive Distillation Approach to the Separation of Styrene from Pyrolysis Gasoline Feedstock Coupled with Deep Desulfurization. Separations, 10(6), 341. https://doi.org/10.3390/separations10060341