Multivariate Simultaneous Determination of Some PAHs in Persian Gulf Oil-Contaminated Algae and Water Samples Using Miniaturized Triton X-100-Mediated Fe3O4 Nanoadsorbent and UV-Vis Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Reagents and Samples
2.2. Instrumentation and Software
2.3. Preparations of Fe3O4/TX-100 Nanocomposite
2.4. General Procedure
2.5. Theory of CWT
3. Results
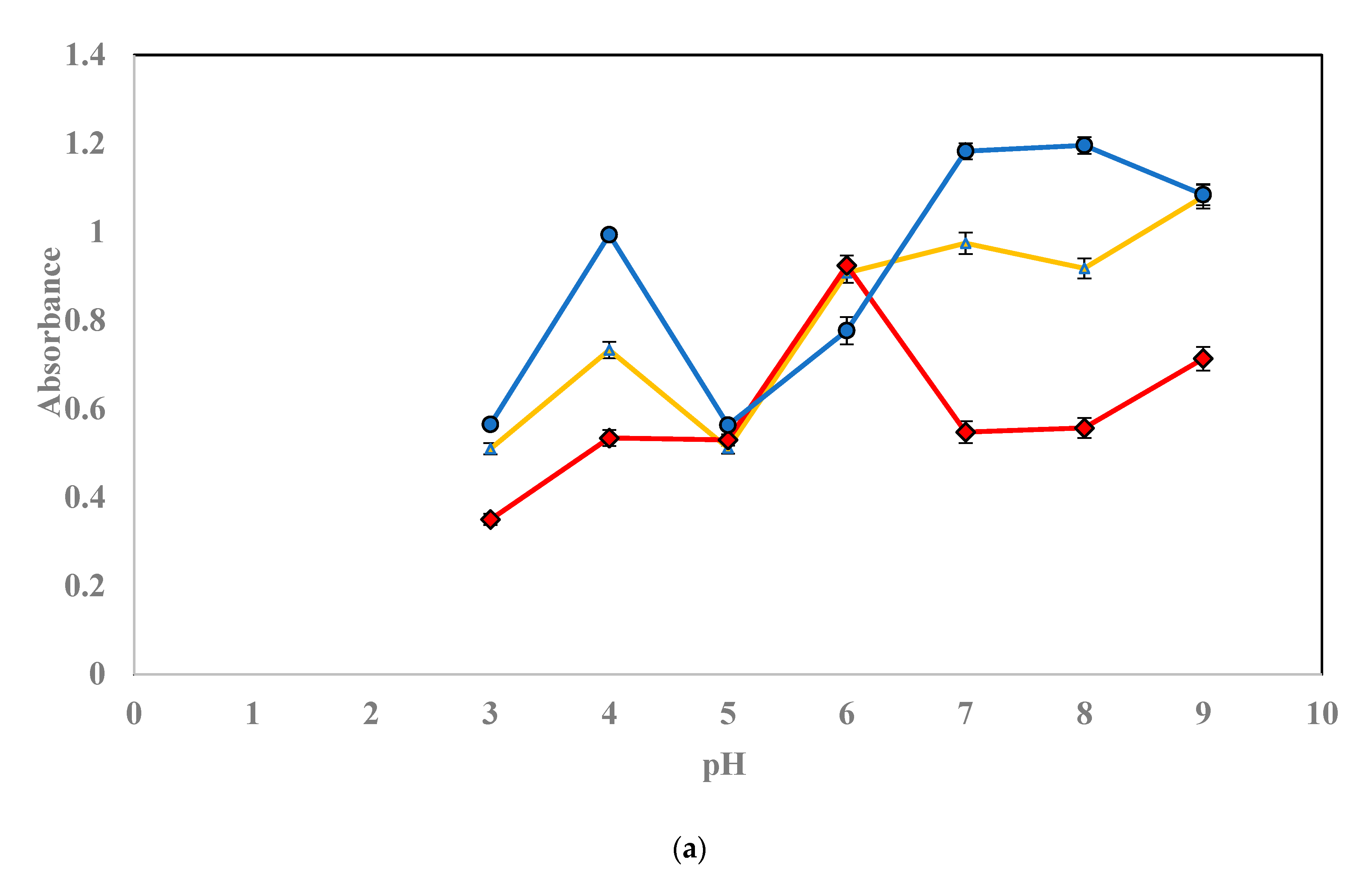
3.1. Optimization of Extraction Conditions
3.1.1. Influence of pH
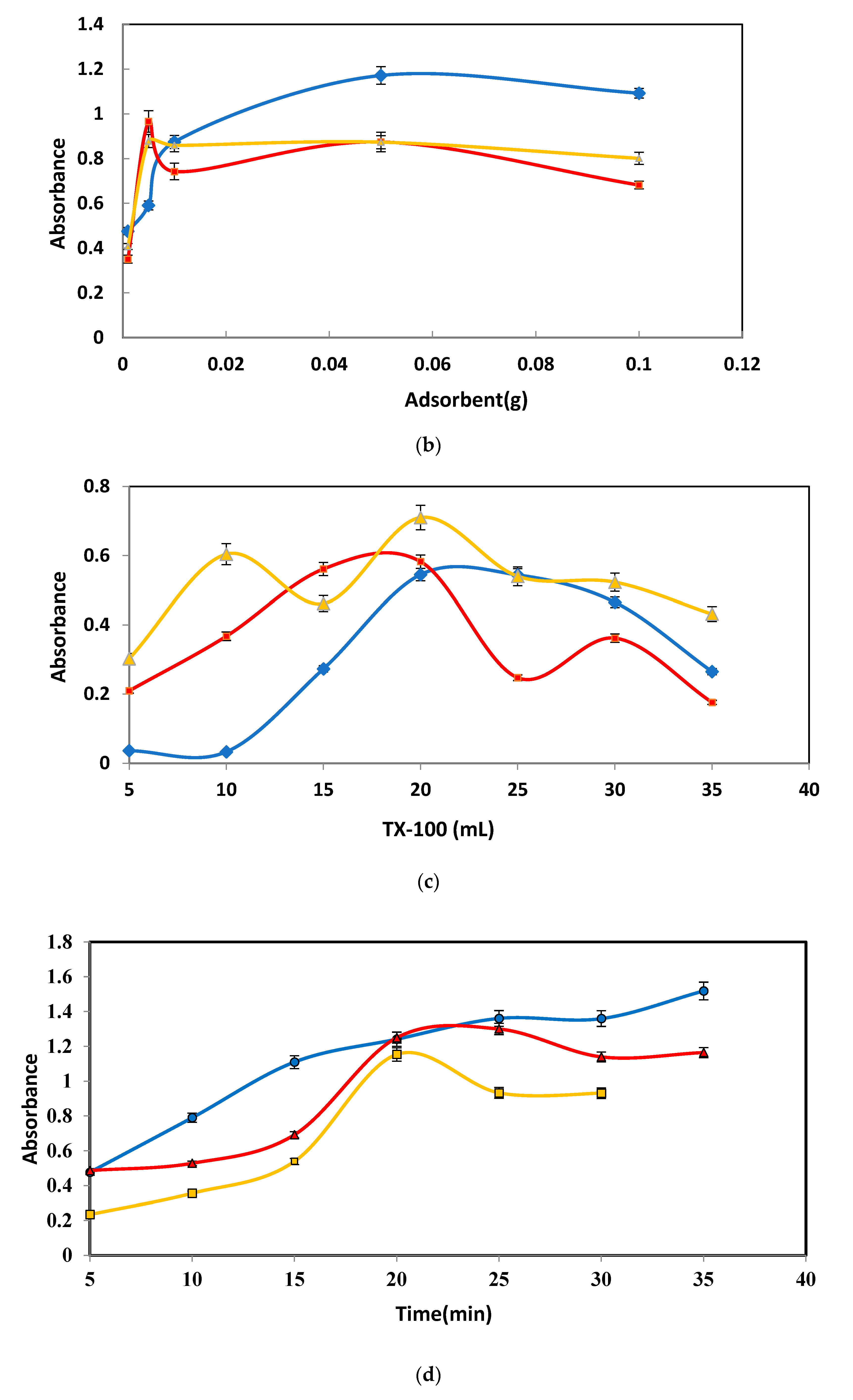
3.1.2. Fe3O4 NPs Sorbent Dose
3.1.3. Influence of TX-100 Concentration
3.1.4. Extraction Time Influence
3.1.5. Desorption Solvent
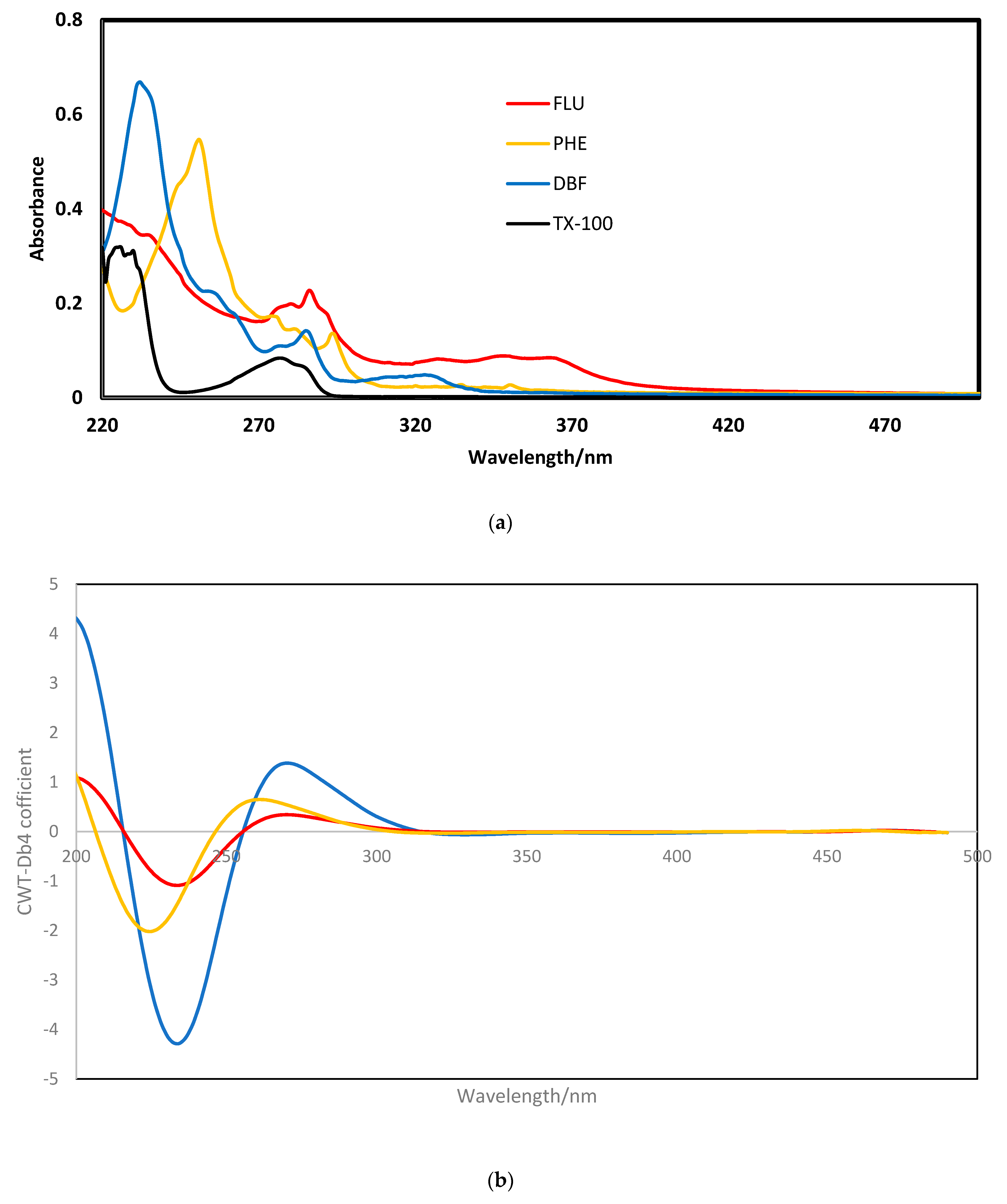
3.2. Primary Study of Nanocomposite Interaction with PAHs
3.2.1. Univariate Calibration
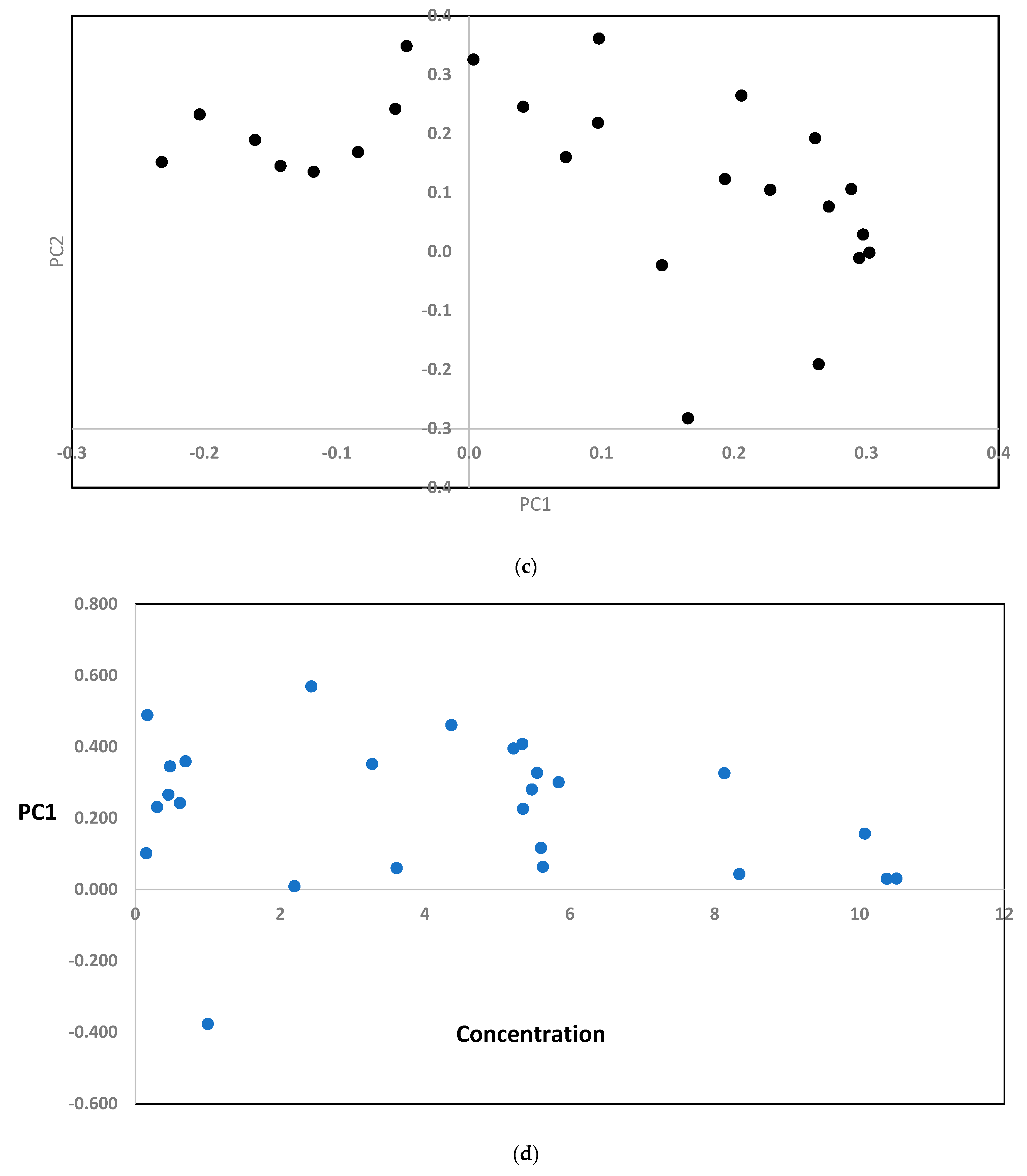
3.2.2. Multivariate Calibration
3.3. Study of Interferences
4. Discussion
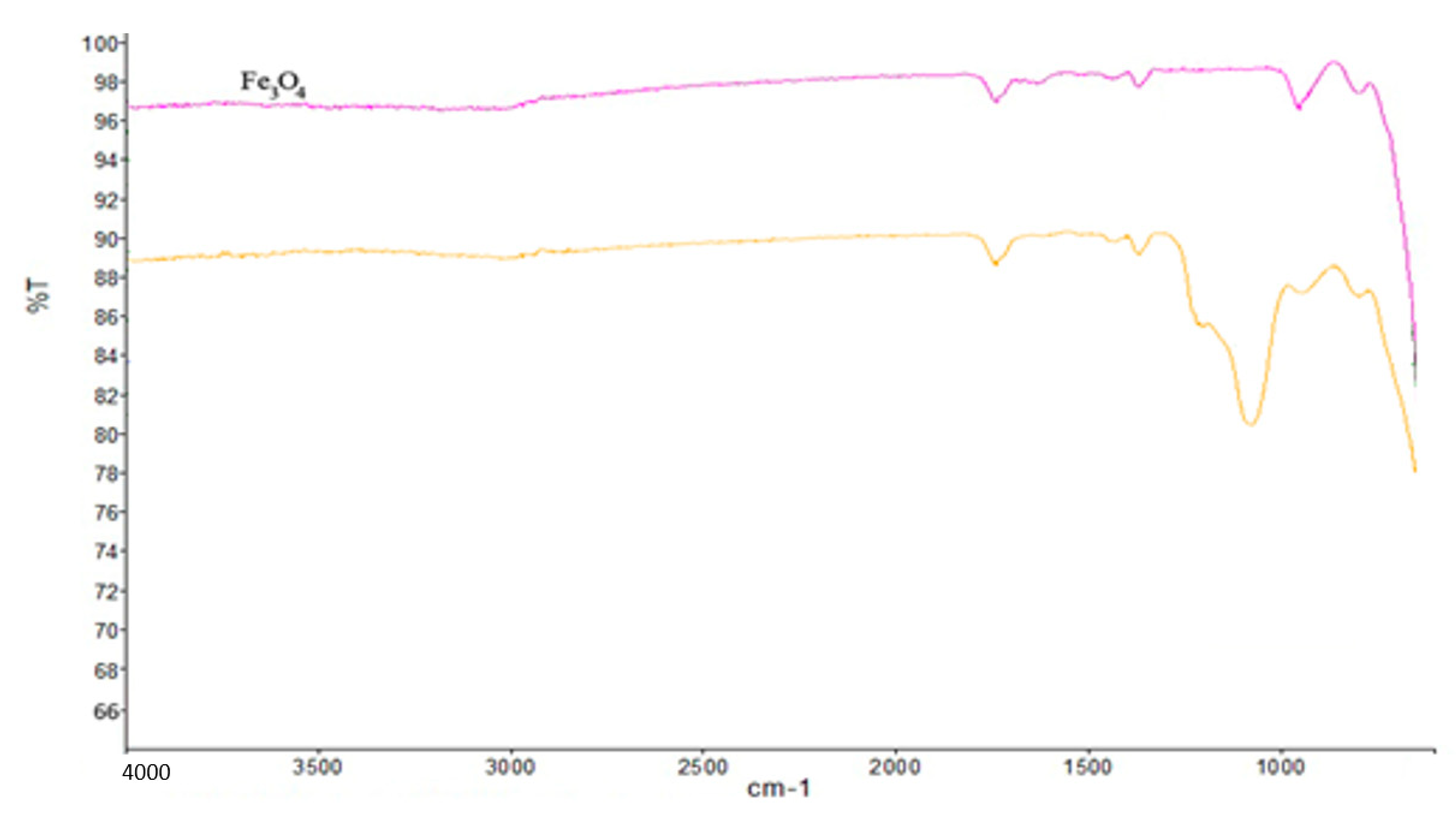
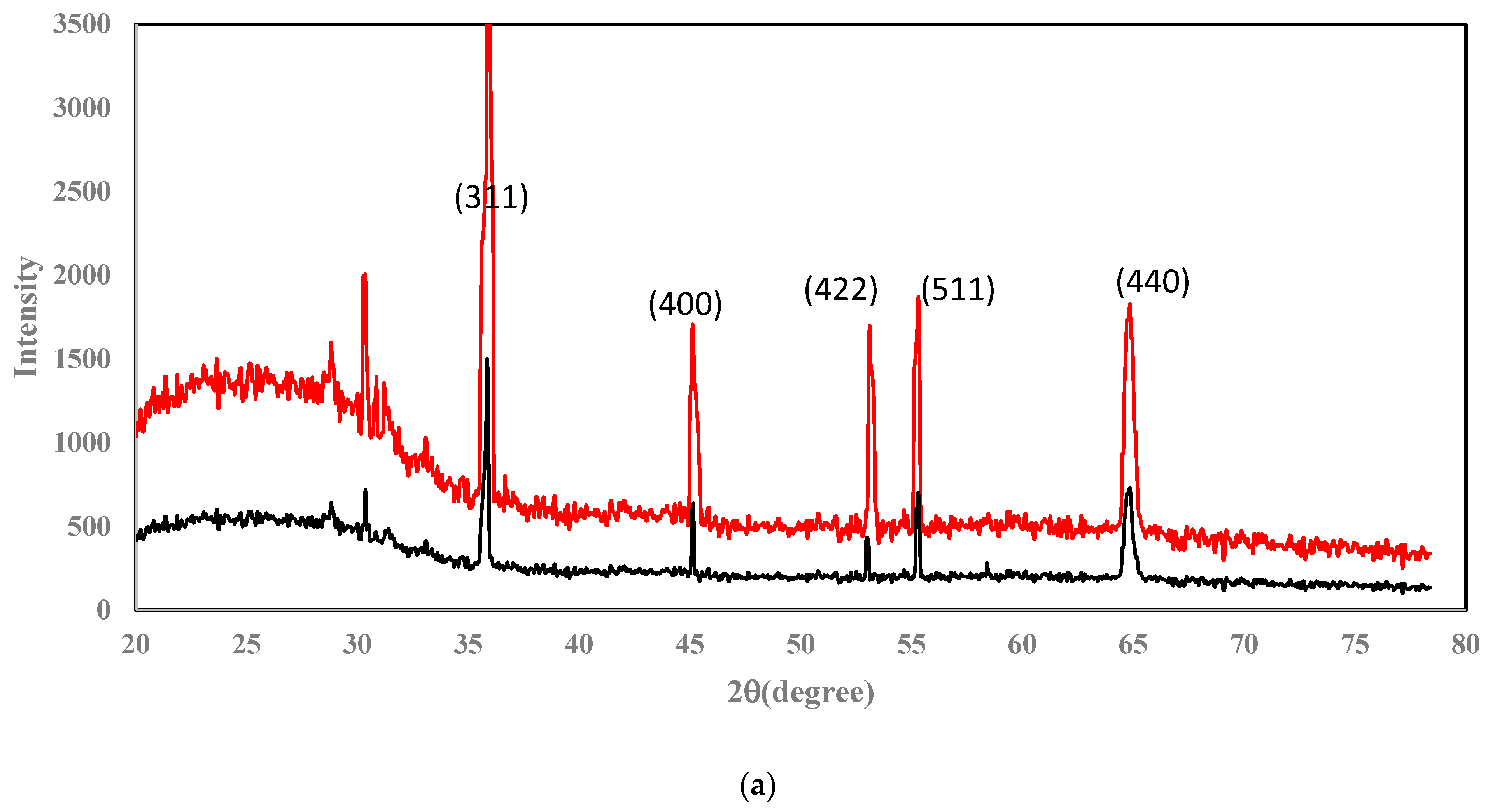
4.1. Characterization of Nanocomposite
4.2. Multivariate Analysis
4.2.1. CWT-FFNNs Analysis
4.2.2. CWT-RBF Analysis
4.3. Analysis of Real Samples
4.4. Comparison of Method with Previous Works
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Wise, S.A.; Sander, L.C.; May, W.E. Determination of polycyclic aromatic hydrocarbons by liquid chromatography. J. Chromatogr. A 1993, 642, 329–349. [Google Scholar] [CrossRef]
- Gazioglu, I.; Zengin, O.S.; Tartaglia, A.; Locatelli, M.; Furton, K.G.; Kabir, A. Determination of polycyclic aromatic hydrocarbons in nutritional supplements by fabric phase sorptive extraction (FPSE) with high-performance liquid chromatography (HPLC) with fluorescence detection. Anal. Lett. 2020, 54, 1683–1696. [Google Scholar] [CrossRef]
- Gazioglu, I.; Kabir, A.; Zengin, O.S.; Tekkeli, E.K.; Furton, K.G.; Tartaglia, A.; Locatelli, M. Development of sol-gel phenyl/methyl/poly(dimethylsiloxane) sorbent coating for fabric phase sorptive extraction and its application in monitoring human exposure to selected polycyclic aromatic hydrocarbons using high performance liquid chromatography-fluorescence detection. J. Chromatogr. B 2021, 1163, 122520. [Google Scholar]
- Farrokhzadeh, S.; Razmi, H. Use of chicken feet yellow membrane as a biosorbent in miniaturized solid phase extraction for determination of polycyclic aromatic hydrocarbons in several real samples. Microchem. J. 2018, 142, 403–410. [Google Scholar] [CrossRef]
- Azizi, A.; Shahhoseini, F.; Bottaro, C.S. Magnetic molecularly imprinted polymers prepared by reversible addition fragmentation chain transfer polymerization for dispersive solid phase extraction of polycyclic aromatic hydrocarbons in water. J. Chromatogr. A 2020, 1610, 460534. [Google Scholar] [CrossRef]
- Speltini, A.; Scalabrini, A.; Maraschi, F.; Sturini, M.; Profumo, A. Newest applications of molecularly imprinted polymers for extraction of contaminants from environmental and food matrices: A review. Anal. Chim. Acta 2017, 974, 1–26. [Google Scholar] [CrossRef]
- Carasek, E.; Morés, L.; Merib, J. Basic principles, recent trends and future directions of microextraction techniques for the analysis of aqueous environmental samples. Trends Environ. Anal. Chem. 2018, 19, e00060. [Google Scholar] [CrossRef]
- Kiss, G.; Varga-Puchony, Z.; Hlavay, J. Determination of polycyclic aromatic hydrocarbons in precipitation using solid-phase extraction and column liquid chromatography. J. Chromatogr. A 1996, 725, 261–272. [Google Scholar] [CrossRef]
- Doong, R.-A.; Chang, S.-M.; Sun, Y.-C. Solid-phase microextraction for determining the distribution of sixteen US Environmental Protection Agency polycyclic aromatic hydrocarbons in water samples. J. Chromatogr. A 2000, 879, 177–188. [Google Scholar] [CrossRef]
- Akvan, N.; Azimi, G.; Parastar, H. Chemometric assisted determination of 16 PAHs in water samples by ultrasonic assisted emulsification microextraction followed by fast high-performance liquid chromatography with diode array detector. Microchem. J. 2019, 150, 104056. [Google Scholar] [CrossRef]
- Popp, P.; Bauer, C.; Wennrich, L. Application of stir bar sorptive extraction in combination with column liquid chromatography for the determination of polycyclic aromatic hydrocarbons in water samples. Anal. Chim. Acta 2001, 436, 1–9. [Google Scholar] [CrossRef]
- Hassan, A.A.; Tanimu, A.; Ganiyu, S.A.; Yaagoob, I.Y.; Alhooshani, K. Selective removal of Cd(II), As(III), Pb(II) and Cr(III) ions from water resources using novel 2-anthracene ammonium-based magnetic ionic liquids. Arab. J. Chem. 2022, 15, 104136. [Google Scholar] [CrossRef]
- Hassan, A.A.; Sajid, M.; Al Ghafly, H.; Alhooshani, K. Ionic liquid-based membrane-protected micro-solid-phase extraction of organochlorine pesticides in environmental water samples. Microchem. J. 2020, 158, 105295. [Google Scholar] [CrossRef]
- Cañas, A.; Richter, P.; Escandar, G.M. Chemometrics-assisted excitation–emission fluorescence spectroscopy on nylon-attached rotating disks. Simultaneous determination of polycyclic aromatic hydrocarbons in the presence of interferences. Anal. Chim. Acta 2014, 852, 105–111. [Google Scholar] [CrossRef] [PubMed]
- Ferrer, R.; Beltrán, J.; Guiteras, J. Use of cloud point extraction methodology for the determination of PAHs priority pollutants in water samples by high-performance liquid chromatography with fluorescence detection and wavelength programming. Anal. Chim. Acta 1996, 330, 199–206. [Google Scholar] [CrossRef]
- Santos, P.M.; del Nogal Sánchez, M.; Pavón, J.L.P.; Cordero, B.M. Quantitative and qualitative analysis of polycyclic aromatic hydrocarbons in urine samples using a non-separative method based on mass spectrometry. Talanta 2018, 181, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Yan, Y.; Fan, J.; Shen, K.; Cao, Y.; Kang, X.; Zhu, H. Sampling and concentration of particulate matter bound polycyclic aromatic hydrocarbons (PAHs) basing on polystyrene nanofibers followed a determination by gas chromatography-mass spectrometry. Microchem. J. 2022, 178, 107295. [Google Scholar] [CrossRef]
- Rezaee, M.; Assadi, Y.; Hosseini, M.-R.M.; Aghaee, E.; Ahmadi, F.; Berijani, S. Determination of organic compounds in water using dispersive liquid–liquid microextraction. J. Chromatogr. A 2006, 1116, 1–9. [Google Scholar] [CrossRef]
- Ghasemi, J.B.; Zolfonoun, E. Simultaneous spectrophotometric determination of trace amount of polycyclic aromatic hydrocarbons in water samples after magnetic solid-phase extraction by using projection pursuit regression. Environ. Monit. Assess. 2013, 185, 2297–2305. [Google Scholar] [CrossRef]
- Rezaei, A.; Abbasi Tarighat, M.; Mohammadi, K. Characterization and theoretical studies of synthesized (E)-2((3-methylbenzilidene)amino) phenol complexes for the fabrication of novel electrochemical sensor for determination of Pb2+ and Hg2+ ions. J. Mater. Sci. Mater. Electron. 2019, 30, 13347–13359. [Google Scholar] [CrossRef]
- Wierucka, M.; Biziuk, M. Application of magnetic nanoparticles for magnetic solid-phase extraction in preparing biological, environmental and food samples. TrAC Trends Anal. Chem. 2014, 59, 50–58. [Google Scholar] [CrossRef]
- Cherkashina, K.; Voznesenskiy, M.; Osmolovskaya, O.; Vakh, C.; Bulatov, A. Effect of surfactant coating of Fe3O4 nanoparticles on magnetic dispersive micro-solid phase extraction of tetracyclines from human serum. Talanta 2020, 214, 120861. [Google Scholar] [CrossRef] [PubMed]
- Passarella, S.; Guerriero, E.; Quici, L.; Ianiri, G.; Cerasa, M.; Notardonato, I.; Protano, C.; Vitali, M.; Russo, M.V.; De Cristofaro, A. PAHs presence and source apportionment in honey samples: Fingerprint identification of rural and urban contamination by means of chemometric approach. Food Chem. 2022, 382, 132361. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Aziz, O.; El Kosasy, A.; Okeil, S.E.-S. Comparative study for determination of some polycyclic aromatic hydrocarbons ‘PAHs’ by a new spectrophotometric method and multivariate calibration coupled with dispersive liquid–liquid extraction. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2014, 133, 119–129. [Google Scholar] [CrossRef]
- Bortolato, S.A.; Arancibia, J.A.; Escandar, G.M. Chemometrics-assisted excitation—Emission fluorescence spectroscopy on nylon membranes. Simultaneous determination of benzo [a] pyrene and dibenz [a, h] anthracene at parts-per-trillion levels in the presence of the remaining EPA PAH priority pollutants as interferences. Anal. Chem. 2008, 80, 8276–8286. [Google Scholar]
- Bravo, M.A.; Valverde, B.; Toledo-Neira, C. Evaluation of three-way fluorescence data-based for simultaneous determination of polycyclic aromatic hydrocarbons in tea infusion samples at sub-ppb levels by second-order multivariate calibration. Microchem. J. 2019, 151, 104208. [Google Scholar] [CrossRef]
- Tarighat, M.A.; Nabavi, M.; Mohammadizadeh, M.R. Chemometrics-assisted spectrophotometric method for simultaneous determination of Pb2+ and Cu2+ ions in different foodstuffs, soil and water samples using 2-benzylspiro [isoindoline-1,5′-oxazolidine]-2′,3,4′-trione using continuous wavelet transformation and partial least squares–Calculation of pKf of complexes with rank annihilation factor analysis. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2015, 145, 54–62. [Google Scholar]
- Tarighat, M.A.; Hasaninejad, A.; Abdi, G. Chemometrics-Enhanced Micelle-Mediated Extraction Spectrophotometric Method for Simultaneous Determination of Cu2+ and Zn2+ in Medicinal Plant, Rice and Water Samples Using Continuous Wavelet Transform. Food Anal. Methods 2016, 9, 1928–1938. [Google Scholar] [CrossRef]
- Tarighat, M.A. Orthogonal projection approach and continuous wavelet transform-feed forward neural networks for simultaneous spectrophotometric determination of some heavy metals in diet samples. Food Chem. 2016, 192, 548–556. [Google Scholar] [CrossRef]
- Tarighat, M.A.; Afkhami, A. Simultaneous spectrophotometric determination of Cu(II), Co(II) and Ni(II) using ratio spectra-continuous wavelet transformation in some food and environmental samples. J. Braz. Chem. Soc. 2012, 23, 1312–1319. [Google Scholar] [CrossRef]
- Afkhami, A.; Madrakian, T.; Abbasi-Tarighat, M. Simultaneous determination of calcium, magnesium and zinc in different foodstuffs and pharmaceutical samples with continuous wavelet transforms. Food Chem. 2008, 109, 660–669. [Google Scholar] [CrossRef]
- Afkhami, A.; Abbasi-Tarighat, M.; Bahram, M. Artificial neural networks for determination of enantiomeric composition of α-phenylglycine using UV spectra of cyclodextrin host–guest complexes: Comparison of feed-forward and radial basis function networks. Talanta 2008, 75, 91–98. [Google Scholar] [CrossRef] [PubMed]
- Tarighat, M.A.; Ghorghosheh, F.H.; Abdi, G. Fe3O4@SiO2-Ag nanocomposite colorimetric sensor for determination of arginine and ascorbic acid based on synthesized small size AgNPs by cystoseria algae extract. Mater. Sci. Eng. B 2022, 283, 115855. [Google Scholar] [CrossRef]
- Jafari, A.; Tahani, K.; Dastan, D.; Asgary, S.; Shi, Z.; Yin, X.-T.; Zhou, W.-D.; Garmestani, H.; Ţălu, Ş. Ion implantation of copper oxide thin films; statistical and experimental results. Surf. Interfaces 2020, 18, 100463. [Google Scholar] [CrossRef]
- Fathinezhad, M.; AbbasiTarighat, M.; Dastan, D. Chemometrics heavy metal content clusters using electrochemical data of modified carbon paste electrode. Environ. Nanotechnol. Monit. Manag. 2020, 14, 100307. [Google Scholar] [CrossRef]
- Mas, S.; de Juan, A.; Tauler, R.; Olivieri, A.C.; Escandar, G.M. Application of chemometric methods to environmental analysis of organic pollutants: A review. Talanta 2010, 80, 1052–1067. [Google Scholar] [CrossRef]
- Grossmann, A.; Morlet, J. Decomposition of Hardy functions into square integrable wavelets of constant shape. SIAM J. Math. Anal. 1984, 15, 723–736. [Google Scholar] [CrossRef]
- Zhang, S.; Niu, H.; Hu, Z.; Cai, Y.; Shi, Y. Preparation of carbon coated Fe3O4 nanoparticles and their application for solid-phase extraction of polycyclic aromatic hydrocarbons from environmental water samples. J. Chromatogr. A 2010, 1217, 4757–4764. [Google Scholar] [CrossRef]
- Galán-Cano, F.; del Carmen Alcudia-León, M.; Lucena, R.; Cárdenas, S.; Valcárcel, M. Ionic liquid coated magnetic nanoparticles for the gas chromatography/mass spectrometric determination of polycyclic aromatic hydrocarbons in waters. J. Chromatogr. A 2013, 1300, 134–140. [Google Scholar] [CrossRef]

| Diverse Species | Analyte | ||
|---|---|---|---|
| PHE | FLU | DBF | |
| Naphthalene | 300 | 150 | 300 |
| Pyrene | 150> | 150 | 300> |
| Methylanthracene | 150 | 150 | 150 |
| Parameter | PAHs | ||
|---|---|---|---|
| PHE | FLU | DBF | |
| Hidden layer | 1 | 1 | 1 |
| Output nodes | 4 | 4 | 4 |
| Learning rate | 0.1 | 0.1 | 0.05 |
| Momentum | 5 | 0.1 | 0.1 |
| Hidden layer transfer function | Tansig | Tansig | Tansig |
| Output layer transfer function | Tansig | Tansig | Tansig |
| Epoch | 100 | 100 | 100 |
| Sample | Proposed Method | HPLC | Student’s t-Test | Critical Value | ||||||
|---|---|---|---|---|---|---|---|---|---|---|
| FLU | PHE | DBF | FLU | PHE | DBF | FLU | PHE | DBF | ||
| Water | 13.5 ± 0.58 | 20.2 ± 1.0 | 23.1 ± 1.1 | 12 ± 0.94 | 18 ± 0.86 | 20.5 ± 2.8 | 1.92 | 2.4 | 1.2 | 3.75 |
| Water | 52.5 ± 1.2 | 105.8 ± 2.8 | BDL | 55.0 ± 1.8 | 103.1 ± 0.52 | - | −1.63 | 1.34 | - | |
| Algae | 52 ± 0.52 | 79.5 ± 2.5 | BDL | 54.0 ± 0.64 | 81.2 ± 1.2 | BDL | −3.43 | −0.86 | - | |
| Algae | 97.0 ± 1.8 | 93.0 ± 1.05 | BDL | 97.5 ± 2.5 | 91.3 ± 0.05 | BDL | −0.23 | 2.29 | - | |
| Algae | 99.5 ± 1.1 | 102.5 ± 2.05 | 10.2 ± 0.5 | 102.5 ± 0.94 | 105.5 ± 2.9 | 10.8 ± 0.5 | −2.9 | −1.31 | 0.6 | |
| Method | Detection | Concentration (ng mL−1) | Detection Limit (ng mL−1) | |||||
|---|---|---|---|---|---|---|---|---|
| DBF | PHE | FLU | DBF | PHE | FLU | Reference | ||
| Carbon-coated Fe3O4 nanoparticle | HPLC | - | 0.01–1 | 0.01–0.1 | - | 0.2 | 0.6 | [38] |
| Ultrasonic-assisted emulsification microextraction–diode array detector | HPLC-DAD | - | 0.5–15 | 0.25–15 | 9.41 | 11.13 | [9] | |
| Magnetic solid-phase extraction–projection pursuit regression | Spectrophotometric | - | - | 3–40 | - | - | 0.65 | [19] |
| Ionic-liquid-coated magnetic nanoparticles | GC/MS | - | - | - | 0.06 | 0.04 | [39] | |
| Miniaturized Fe3O4/TX-100 solid-phase extraction | Spectrophotometric | 48–5000 | 2.4–250 | 50–3750 | 12.5 | 0.58 | 9.5 | Proposed method |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Tarighat, M.A.; Behroozi, A.; Abdi, G.; Proestos, C. Multivariate Simultaneous Determination of Some PAHs in Persian Gulf Oil-Contaminated Algae and Water Samples Using Miniaturized Triton X-100-Mediated Fe3O4 Nanoadsorbent and UV-Vis Detection. Separations 2023, 10, 334. https://doi.org/10.3390/separations10060334
Tarighat MA, Behroozi A, Abdi G, Proestos C. Multivariate Simultaneous Determination of Some PAHs in Persian Gulf Oil-Contaminated Algae and Water Samples Using Miniaturized Triton X-100-Mediated Fe3O4 Nanoadsorbent and UV-Vis Detection. Separations. 2023; 10(6):334. https://doi.org/10.3390/separations10060334
Chicago/Turabian StyleTarighat, Maryam Abbasi, Ameneh Behroozi, Gholamreza Abdi, and Charalampos Proestos. 2023. "Multivariate Simultaneous Determination of Some PAHs in Persian Gulf Oil-Contaminated Algae and Water Samples Using Miniaturized Triton X-100-Mediated Fe3O4 Nanoadsorbent and UV-Vis Detection" Separations 10, no. 6: 334. https://doi.org/10.3390/separations10060334
APA StyleTarighat, M. A., Behroozi, A., Abdi, G., & Proestos, C. (2023). Multivariate Simultaneous Determination of Some PAHs in Persian Gulf Oil-Contaminated Algae and Water Samples Using Miniaturized Triton X-100-Mediated Fe3O4 Nanoadsorbent and UV-Vis Detection. Separations, 10(6), 334. https://doi.org/10.3390/separations10060334







