Abstract
Ethylene glycol dinitrate (EGDN) is a liquid nitrate ester, a secondary explosive. In the past, it was used as an explosive ingredient in dynamite along with nitroglycerine. Due to its various applications, the reliable detection of EGDN in the environment is a key issue for both forensic and environmental applications. In these areas, sensitive and reliable methods for determining the concentration of nitro compounds are needed. Microextraction by packed sorbent (MEPS) is an innovative approach to green technology in the sample preparation field. Compared to conventional solid-phase extraction (SPE), MEPS uses a smaller sample volume and can be easily combined with various chromatographic techniques. An important benefit is the reduction of sorbent amount and up to 100-times repeatable use compared to disposable SPE columns, thus reducing the costs of analysis as well as waste production. Optimal extraction parameters for isolating EGDN from water, e.g., 30 µL of toluene as extraction agent, working in one cycle and in draw/discard mode, were selected. Method validation was performed, obtaining a limit of detection and a limit of quantification of 0.45 pg/μL and 1.34 pg/μL, respectively. Accuracy in terms of recovery rates was evaluated over a wide concentration range, obtaining values from 83.7 to 90.0%. The satisfactory linearity expressed by the coefficient of determination was 0.9914. A matrix factor of −9.3% indicates a weak matrix effect. The application to real environmental water samples and a forensic post-blast wash water sample was realized. EGDN detection in the post-blast samples provides valuable information for forensic technicians.
1. Introduction
Explosives are materials with a great potential energy that can be transformed into stable compounds by rapid decomposition after a sudden impact, electricity, or spark, releasing tremendous sound, heat, blast, and gases [1]. High-energy materials are named explosives, propellants, and fireworks according to their properties and uses [2]. Ethylene glycol dinitrate (EGDN), a liquid nitrate ester also known as nitroglycol, belongs to the secondary explosives with a neutral oxygen balance; therefore, it has a large power index, which makes it a powerful explosive [3]. Secondary explosives are not easily detonated by heat or impact and are generally more powerful than primary explosives. Secondary explosive mixtures dissociate almost immediately into other, more stable, components upon initialization [4]. At present, most explosives are plasticized using inert flexible binders, resulting in formable and easy-to-handle explosive mixtures. Such mixtures are known as plastic explosives and may contain one or more separate explosives combined with a plasticizer. Since plasticizers are non-explosive compounds, their addition reduces the explosive power of the mixture [5]. EGDN is a part of some propellant formulations. EGDN does not actually have many military applications due to its high vapor pressure. It is several times more volatile than nitroglycerin and is used as a gelatinous agent and/or is mixed with nitroglycerin in several types of dynamite to lower its freezing temperature [3].
After an explosion, identifying the original explosive material is an important task for forensic chemists. The misuse of explosives, either to commit a crime or by terrorists, has a significant impact on society. As a result, considerable law-enforcement efforts are focused on identifying the perpetrators. In addition, explosives are present in various compartments of the environment, such as soils or environmental water, either as particles resulting from a detonation or in dissolved form [6]. Explosive substances and their metabolites have harmful effects on flora and fauna and also pose a serious health risk [7]. Pollution and contamination of the environment with explosives occurs during the production or handling of explosives, testing, military training, and activities such as mining. The identification of explosive residues is also widely used in forensic analysis when documenting the culprits, the course and consequences of an incident, or a violation of state law or organizational rules.
A number of modern analytical techniques are available to meet such a task, but many are of limited use if small traces of explosives or explosive residues are present in the sample. In such a case, the application of a sample preconcentration is unavoidable. Due to the variety of samples and low concentrations of analytes, more and more attention is being paid to sample preparation. The most suitable extraction technique should be cheap, fast, simple, and compatible with the separation technique, but above all it should preconcentrate the analytes and remove unwanted interferents.
In terms of conventional techniques, liquid–liquid extraction, solid-phase extraction (SPE), and dispersive solid-phase extraction belong to the most common methods for the extraction [8] of nitro compounds from environmental samples, mainly from water. They are laborious and time-consuming techniques, prone to contamination during the extraction process, and require large amounts of high-purity organic solvent. Nowadays, the development and application of sorption techniques with special design [9] and microextraction techniques [10], such as solid phase microextraction (SPME), dispersive liquid–liquid microextraction (DLLME) and single-drop microextraction (SDME) [11,12], is on the rise. Stir bar sorptive extraction (SBSE) [13] and microextraction with sorbent packed in a syringe (MEPS) for nitroaromatic compounds [14] were applied. Microextraction techniques are generally based on the miniaturization of the basic extraction, in which analytes from large-volume samples are extracted into small-volume extraction phases [15].
MEPS appears to be a promising alternative to SPE [16,17]. The MEPS technique, originally developed by Abdel-Rehim [18], is designed in a syringe format where a small amount of sorbent is packed between the barrel and the needle as a cartridge or inside the syringe as a plug. The syringe contains approximately 1–4 mg of sorbent, which can be used more than 100 times. MEPS is a green sample preparation technique due to the small amount of sorbent used and the small volumes of solvent required. It is a new form of SPE that has been miniaturized to work with sample volumes as small as 10 μL and larger volumes up to 250 μL.
EGDN was chosen because it is increasingly replacing nitroglycerin (NG) in the production of dynamite and is also used as a plasticizer in the production of various explosives. The aim of this paper was to develop an analytical method for the extraction of EGDN from environmental or forensic water samples using the MEPS sample preparation technique followed by gas chromatography (GC) separation and appropriate detection. The target of the work is a detailed study of the relevant extraction and validation parameters. To the best of our knowledge, this is the first time the combination of MEPS-GC with a micro-electron capture detector has been used for EGDN detection in forensic water samples for post-blast residue detection at low concentration levels.
2. Materials and Methods
2.1. Instrumentation
An MEPS syringe (SGE, Melbourne, Australia), a 100 µL gastight syringe body with a sorbent bed (1 mg of C8 and C18 modified silica gel) integrated into the needle, was utilized.
A gas chromatograph HP 6890N (Agilent Technologies, Avondale, PA, USA) was equipped with a micro-electron capture detector. A capillary chromatographic column CP-Sil 8 CB (15 m × 0.15 mm I.D. × 0.15 μm film thickness) with 5% phenyl-polydimethylsiloxane stationary phase and connected with 1 m non-polar pre-column was used. The split/splitless injector operating in the splitless mode was maintained at 170 °C. The flow of carrier gas (Hydrogen) was kept constant at 1.1 mL/min. A splitless time of 1 min was set in the case of peak confirmation.
The measurement of pH was realized by a pH-meter, type pH/Ion 510 (Eutech Instruments, Vernon Hills, IL, USA). Sartorius Analytic MC1 scales (Sartorius, Göttingen, Germany) were used for weighing reference material.
2.2. Chemicals and Materials
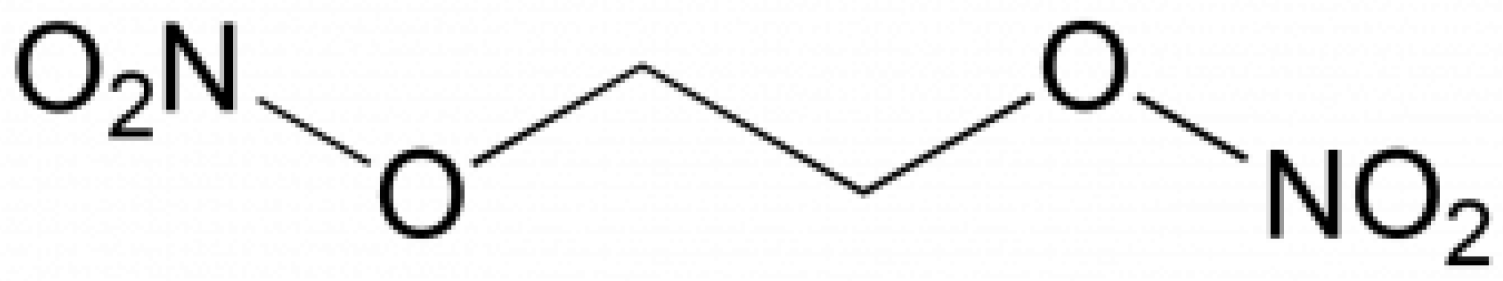
Ethylene glycol dinitrate (also known as nitroglycol, molecular formula C2H4N2O6) is a colorless oily liquid with a boiling point of 114 °C. The purity of the standard was higher than 97.5%. The structure formula is shown in Figure 1.
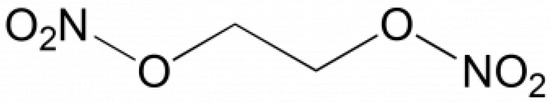
Figure 1.
Formula of ethylene glycol dinitrate.
The following solvents were utilized: Acetone with purity ≥ 99.80% (Centralchem, Bratislava, Slovak Republic), Acetonitrile with purity ≥ 99.95% (VWR Chemicals, Radnor, PA, USA), Ethyl Acetate with purity ≥ 99.80% (Merck KGaA, Darmstadt, Germany), Methanol with purity ≥ 99.8% (VWR Chemicals, Radnor, PA, USA) and Toluene with purity ≥ 99.90% (Merck KGaA, Darmstadt, Germany).
A stock solution of 1 mg/mL standard was prepared in methanol and kept refrigerated at −18 °C. The working solutions were prepared by diluting the stock solution in a ratio of 1:100 v/v in methanol and kept at 4 °C. The variability of the working solutions was prepared by dilution in methanol or in toluene.
Hydrochloric acid (HCl) (37%) and sodium hydroxide (NaOH) for pH balancing were obtained from Centralchem, Bratislava, Slovak Republic.
Real environmental water samples were obtained from the Danube River and the post-blast residues wash water sample was processed by the Forensic Institute, Bratislava.
2.3. Methods and Procedures
2.3.1. Procedure MEPS
The extraction procedure took place in the following consecutive steps: sorbent conditioning, analyte enrichment, elution and washing [19].
First, the MEPS cartridge was conditioned with 50 μL MeOH, then 50 μL deionized water in five replicates. The next step was sample loading (including EGDN enrichment) by spiked model water sample or real water sample. A volume of 50 μL in the mode draw-discard was selected. In the next step, the sorbent bed was dried by aspiring 100 μL of air five times. Elution of the trapped analyte was performed by toluene at a volume of 30 μL. Washing of the sorbent bed was performed with MeOH and deionized water. A portion of 1 μL of extract was then analyzed by the GC method.
2.3.2. Real Samples Treatment
Explosive material provided by the Pyrotechnic Department of the Forensic Institute was detonated in a military special-purpose facility in Stupava, Slovakia. Aluminum foil was placed at a distance of 1 m from the epicenter of the explosion as an underlay base for sampling. After the explosion, residues of energetic material were collected and washed with a small volume of water in the laboratory. Aluminum foil was placed into the washing water and stirred for ten minutes in a closed container. A polypropylene syringe filter with 0.45 μm pore size (Whatman) was used to filter the washing water. Subsequently, the resulting wash water sample was processed using the MEPS technique described above (Section 2.3.1).
2.3.3. GC Analyses
The volume of the injected solution was 1 µL. The GC oven temperature program was the following: initial temperature was set at 80 °C for 1 min, increased by a gradient of 15 °C/min up to 130 °C and held for 1 min; then increased by a gradient of 7 °C/min up to 160 °C and kept for 1 min; and then increased by a gradient of 45 °C/min to 250 °C and held for 1 min. The temperature of the micro-ECD (electron capture detector) was maintained at 250 °C and the flow of make-up gas (Nitrogen) was 60 mL/min. For GC-MS, the electron ionization was performed at 70 eV. A 3 min solvent delay was set. The mass range (m/z 40–400) was recorded in full scan mode. The peak identification of targets was based on the retention times and full scan spectra of the standard. The selected ion monitoring (SIM) mode was employed for the quantification of analytes.
2.3.4. Analytical Method Validation
Fortified model samples were analyzed for validation purposes. The linear range of the method analyzing model water samples was fortified at a concentration in the interval from 0.0015 ng/μL to 0.01 ng/L. The lowest calibration level (LCL) was determined according to the analyte response. Each extraction was repeated six times. Recovery studies were performed in six replicates at six fortification levels (0.0015, 0.002, 0.004, 0.006, 0.008, 0.01 ng/μL). Precision was calculated as the relative standard deviation (RSD) expressed as intra-day precision (six replicates the same analyzing sequence) and inter-day precision (replicates in various days in the range of 1 month). Limits of detection (LODs) and the limits of quantifications (LOQs), using a method based on the standard deviation of the response and the slope, were calculated for EGDN in a neat solvent as well as for the fortified model water samples.
3. Results and Discussion
When using the MEPS technique, the following parameters have a significant impact on the effectiveness of the method: type of sorbent, sample volume, number of extraction steps, type and volume of elution solvent, ionic strength, and sample pH.
3.1. Selection of Extraction Conditions
Analytes are retained on the sorbent material through various forms of interactions (hydrophobic, polar and ionic), and a critical parameter in MEPS treatment is the selection of the appropriate type of sorbent. The choice of sorbent will determine the extraction efficiency (e.g., selectivity and affinity), because it depends on the strength of the interaction between the target analytes and the sorbent used in MEPS [20]. At the beginning of the study, two commercially available sorbents, namely C8- and C18-modified silica gels, were tested. C18 sorbent was chosen as a suitable type of sorbent, using which the largest peak areas were achieved. Further experiments were carried out using the C18 sorbent cartridge.
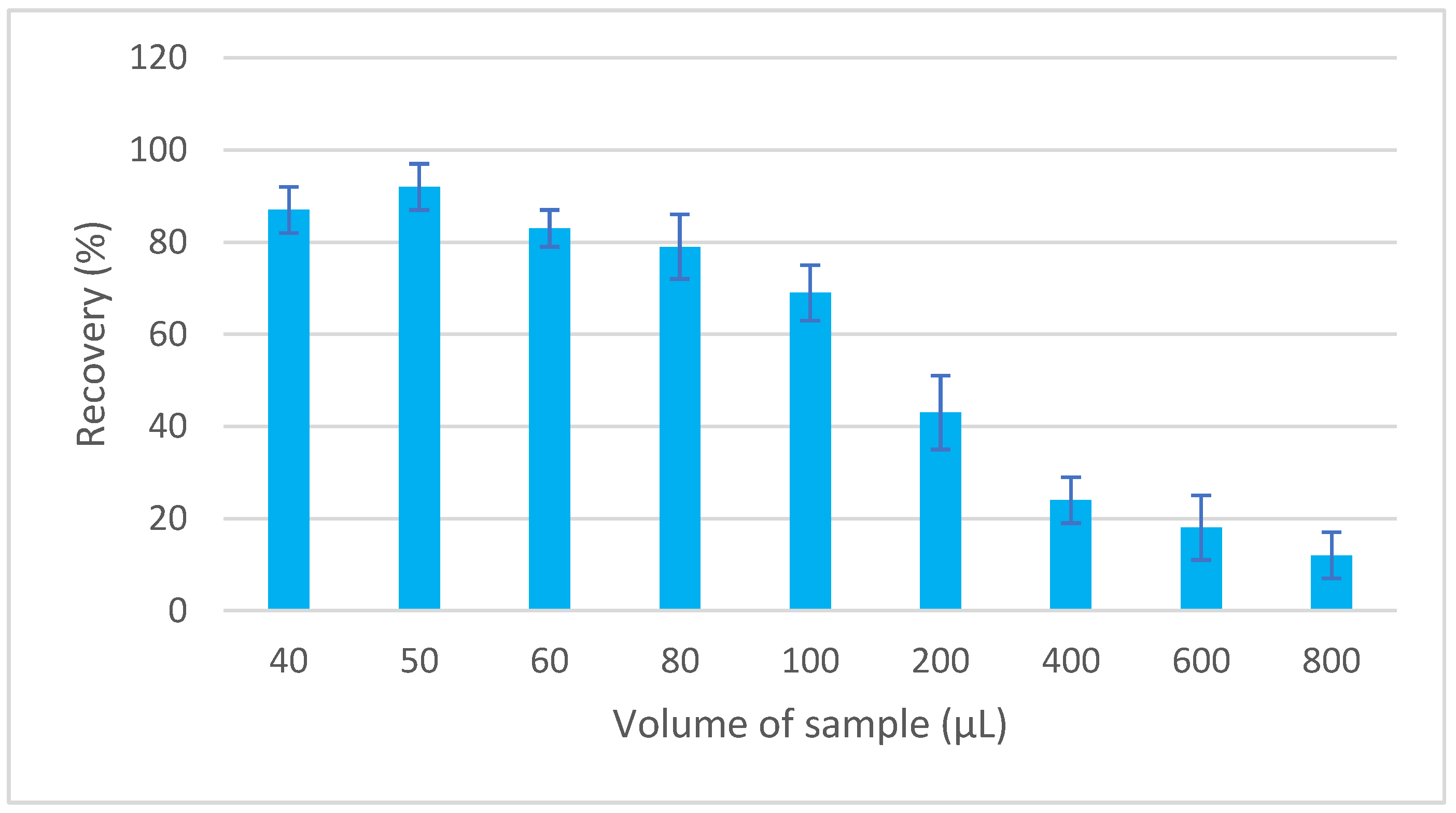
MEPS is a miniaturized form of SPE that has been designed to handle both small (10 μL) and large (>1000 μL) sample volumes. The sample flow through the sorbent can be in the range from 10 to 20 μL/s to achieve optimal interaction between the analyte and the sorbent [21]. To determine the appropriate volume of the aspirated sample, volumes in the range of 40–800 µL were tested. The concentration of EGDN in the sample was kept constant while the volume of the aspirated sample was varied. The sorbed EGDN was eluted with a volume of 30 μL of toluene, and a portion of 1 μL of the extract was injected for GC analysis. A volume of 50 µL provided the optimal recovery (Figure 2).
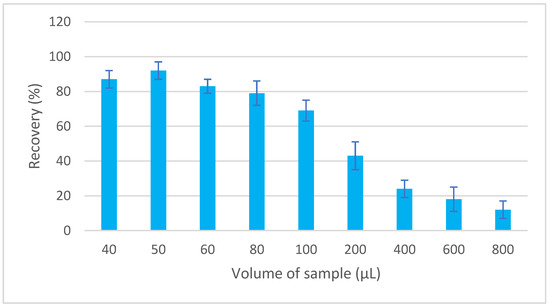
Figure 2.
Dependence of recovery of EGDN on sample volume.
A suitable elution agent should be an organic solvent such as methanol, isopropanol, acetonitrile or toluene, single or mixed with acidic or basic additives, to have the ability to displace the entire amount of analyte from the sorbent bed in small volumes (optimally at about 20–50 μL). The appropriate type of elution solvent has a significant impact because the sorbed analytes on the MEPS bed must be eluted with an elution efficiency close to 100%. A constant mass of analytes was sorbed to select the eluent while varying the type of solvent to determine their desorption effectivity. The volume of elution solvent was kept constant, the sorbent was washed with 30 μL of solvent, and then re-extraction was carried out twice with the same volume of solvent to check for carryover. The following solvents were tested: toluene, methanol, acetonitrile, ethyl acetate and acetone. Acetone and acetonitrile provided recoveries lower than 30%, while ethyl acetate, methanol and toluene were able to recover EGDN at 72, 80, and 86%, respectively. Toluene was selected for further experiments. A portion of 30 μL was applied in single, double, and triple elution cycles (thus total volumes of 30, 60 and 90 μL were applied). A toluene volume of 30 μL was found to be a sufficient volume for efficient desorption.
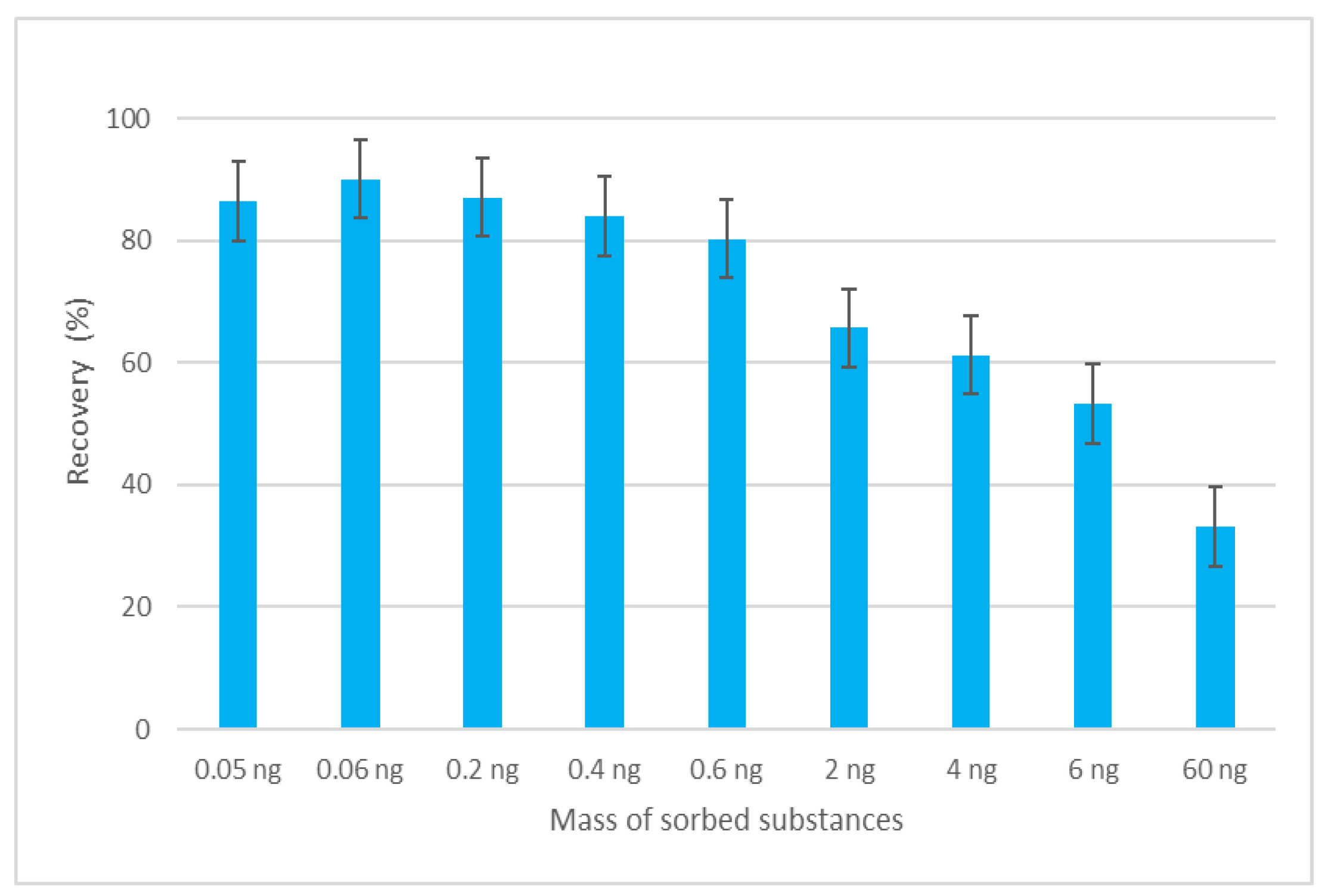
The efficiency of the MEPS sorbent was evaluated in terms of its sorption capacity. The sorption capacity of the sorbent represents the amount of analyte that can be isolated from the sample, based on the amount of sorbent material used. The amount of sorbent in the MEPS sorbent bed is very small compared to the SPE cartridge (1–4 mg versus 50–500 mg), which means that the amount of sorbed analytes per sorbent can be a critical point in finding suitable parameters for efficient extraction [19]. To determine the maximum mass of sorbed substances, analyte masses in the range of 0.05 ng to 60 ng at a constant sample volume were tested. The dependence of the recovery on the mass of sorbed analyte is depicted in Figure 3. The data show that when the mass of 0.6 ng was reached, the recovery of the analytes began to fall below an acceptable level, and it is assumed that above this value, the capacity of the sorbent was exceeded.
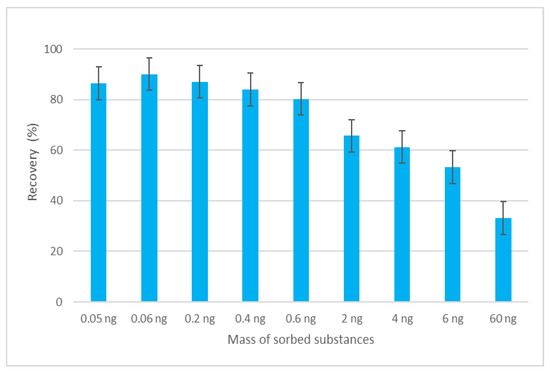
Figure 3.
Dependence of recovery of EGDN on the mass of sorbed substances.
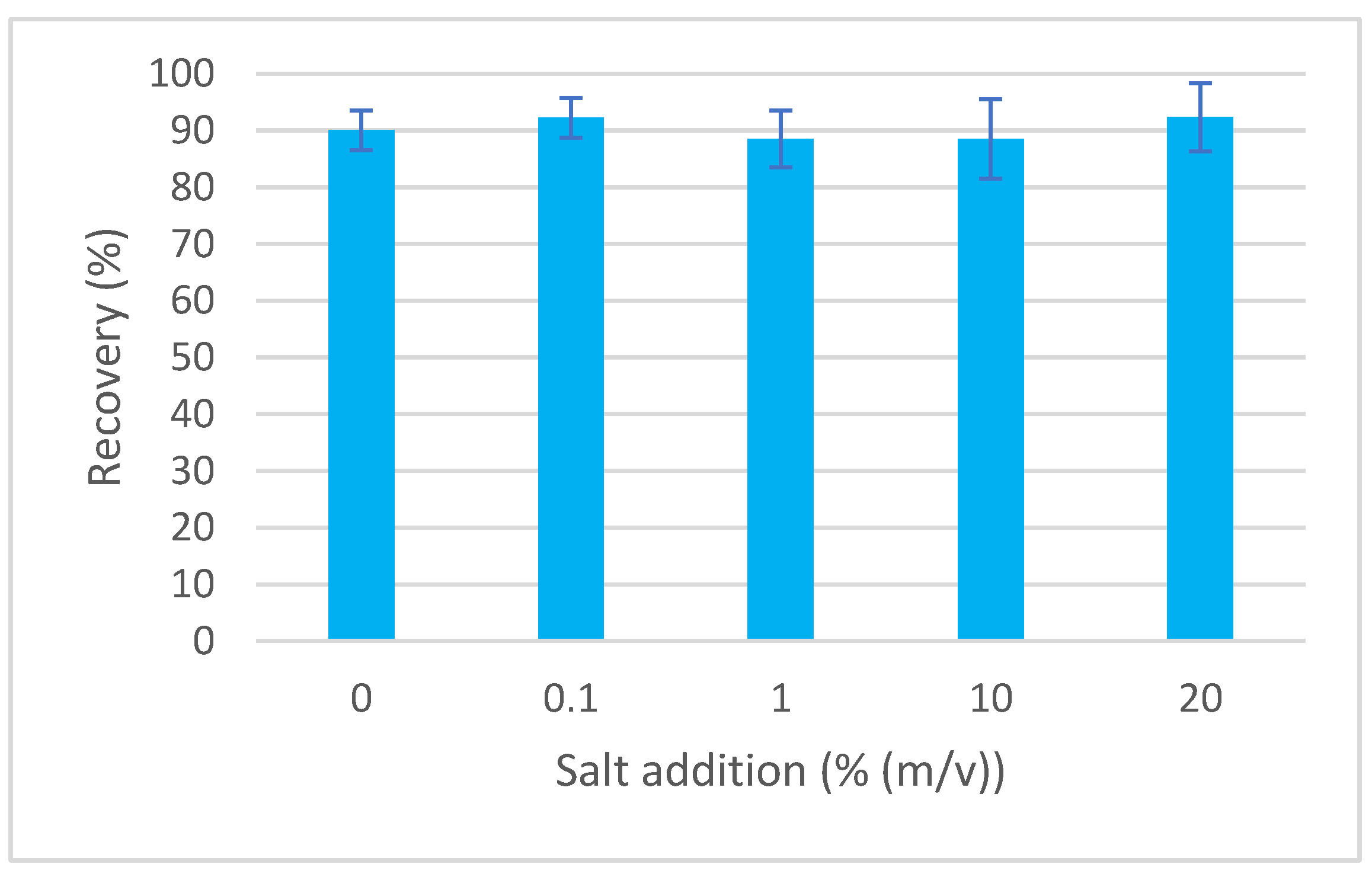
The efficiency of extraction techniques can be affected by changing ionic strength. The addition of salt can cover the remaining charged sites on the surface of the sorbent, thereby minimizing secondary interactions. Salt (NaCl) is usually added in the range of 0–20% (Figure 4). Adding NaCl modifies the solvation ability of water and polar compounds can be extracted more easily [16]. The variability of recovery for various additions of NaCl (in the range of 0–20%) was between 88.5% and 92.3%, thus the influence was concluded as not significant, and no addition of salt was performed for the developed method.
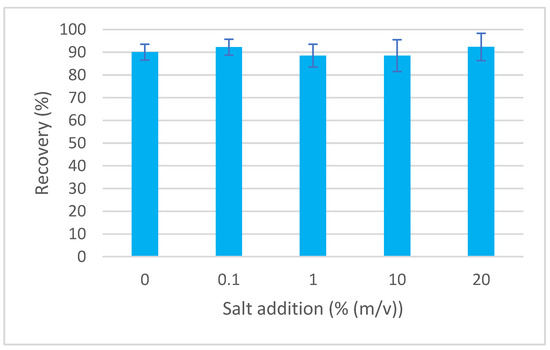
Figure 4.
Dependence of recovery of EGDN on ionic strength.
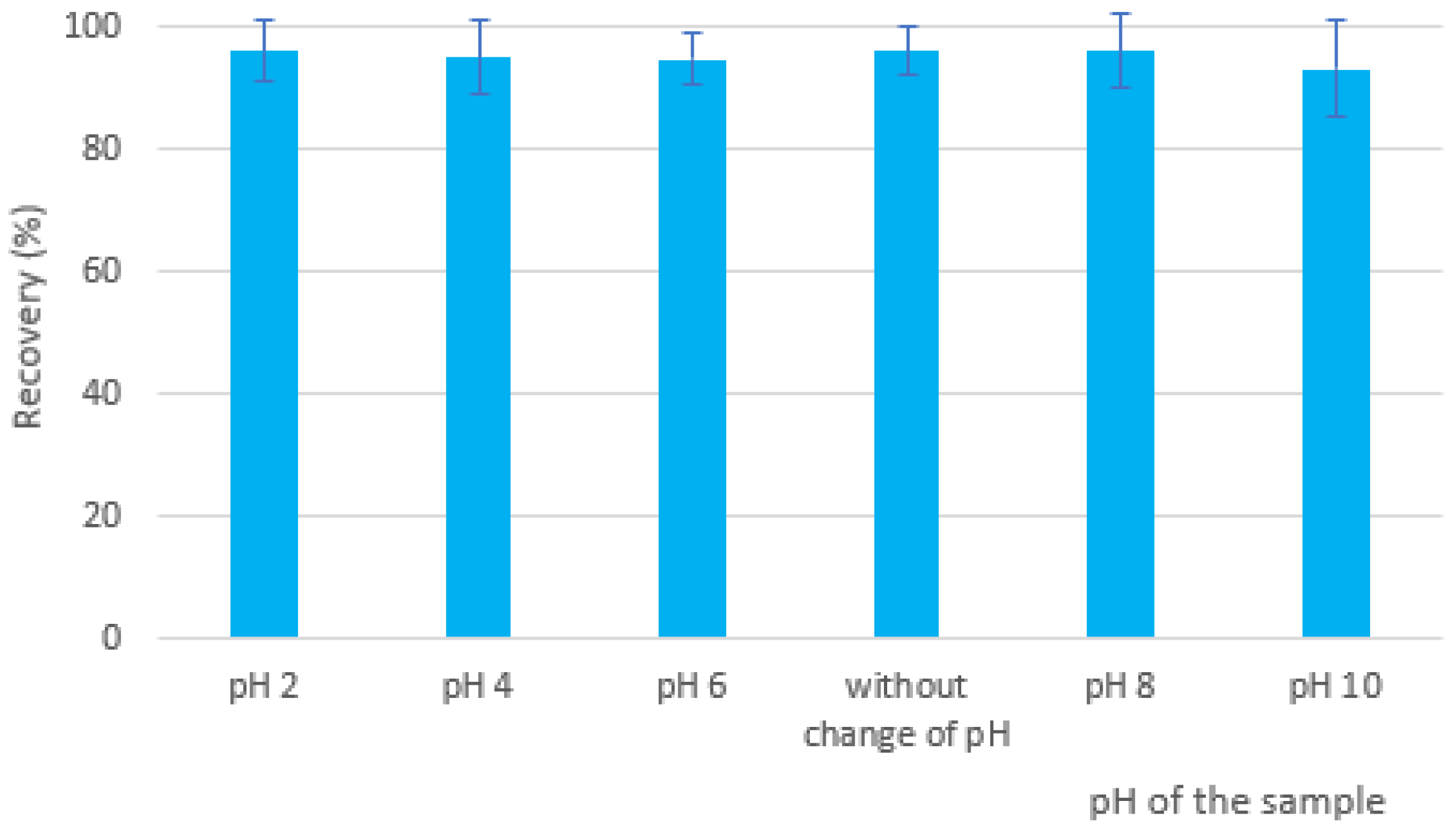
The model water sample fortified by EGDN was extracted at different pH values: pH 2, pH 4 and pH 6 (adjusted with 0.1 M HCl), pH 8 and pH 10 (adjusted with 0.1 M NaOH), as well as the sample without modification of pH, were analyzed. No significant change in the EGDN recovery was observed (Figure 5) after the addition of acidic or basic modifiers, suggesting that their extraction was not affected by the change in the sample pH. The best recovery results were recorded at unchanged pH and pH 2 and 8. A slight decrease in recovery was noticed at pH 10. Since we do not expect real samples with a pH higher than 8, the final analytical method was performed without the addition of acidic or basic modifiers.
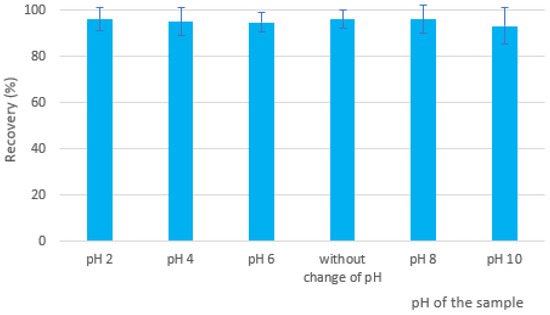
Figure 5.
Dependence of recovery of EGDN on pH change.
The long-term stability of the MEPS cell with the sorbent was tested and it was shown that the sorbent can be used up to 130 times without reducing the efficiency of the sorbent. This proves the high economic benefit of this method in comparison to solid-phase extraction, which uses new cartridges with sorbent for each single analysis.
3.2. Validation of the Method
To confirm the suitability of the method for determining the target analyte, the validation of the analytical method was carried out using a wide range of experimental results. Several parameters, such as the linear dynamic range of the method, limit of detection (LOD), limit of quantification (LOQ), and linearity and precision of the method (which was evaluated as the intra-day precision and inter-day precision), were obtained during method validation.
Accuracy was evaluated in terms of recovery at six concentration levels. Recoveries were calculated by comparing the peak areas obtained after MEPS extraction to the peak areas obtained by analyzing the standard mixture at the same concentration level. The obtained data are summarized in Table 1. The recovery data ranged from 83.7% to 90.0%. Inter-day and intra-day precision were recorded in the range of 0.92% to 4.7% and the range 1.3% to 5.3%, respectively.

Table 1.
Recoveries of EGDN at various concentration levels with corresponding relative standard deviation of intra-day and inter-day precision.
A lowest calibration level (LCL) of 0.0015 ng/μL was determined. Linearity was studied for a water sample fortified with the EGDN standard and extracted by the MEPS technology as well as for a standard of EGDN dissolved in a neat solvent. Based on the LCL of EGDN, a calibration dependence was constructed at concentration levels ranging from LCL to 0.1 ng/μL. The data are shown in Table 2.

Table 2.
Validation parameters.
The prepared calibration solutions were analyzed in six replicates. The calibration curve was given as y = a * x + b, where a is the slope of the calibration curve and b represents the intercept. In the evaluation of linearity, the coefficient of determination, R2, was calculated. LODs were calculated using the standard deviation of the response (sa) and the slope (b) of the calibration curve according to following equation:
LOD = (3.3 * sa)/b
The LOQ was calculated as follows:
LOQ = (10 * sa)/b
The matrix effect (ME) represents the influence of sample components on the analyte signal, which can be either positive or negative [22]. Matrix effects are evaluated by means of matrix factors (MF). Matrix factors are calculated according to the following equation:
where bM and bs are slopes of the calibration curves of standards and extracts of fortified water samples, respectively. This is a method of calculating MF using a calibration curve, when the slope of the calibration curve of the mixture of standards in the solvent (bs) and the slope of the calibration curve of the extract of the water sample (bM) are compared. The matrix effect is considered significant if the MF exceeds the value of ±20%. The matrix factor at the value of −9.7% indicates only a weak matrix effect without the need for correction of the calibration and quantification process.
3.3. Applicability of the Developed Method
Considering the applicability of the developed analytical method, the method was applied to determine EGDN in two various types of water sample—environmental and forensic. The river water samples were acquired in Slovakia. Three samples from various sampling points of the Danube River were analyzed. No levels above the limit of detection were found (Table 3).

Table 3.
Applicability of the developed method.
A forensic real sample of explosives commonly used for civil and construction purposes was tested for trace amounts of EGDN after an explosion. The analysis of the post-blast residues of energetic material after the explosion (Ecodanubit) was realized according to the procedure described above. EGDN was detected at levels higher than LOD, providing the forensic technician with useful information to investigate the forensic problem. Confirmation by mass spectrometric detection is recommended for the identification of compounds in unknown samples. When using the full scan mode to detect unknown samples, the minimum detection limit will not be as low. Further experiments would be required to quantify the analyte.
3.4. Comparison of the MEPS Method with Other Available Extraction Methods
Conventional techniques such as liquid–liquid extraction and SPE are still widely used techniques in various fields of chemistry. However, in recent years, some of these techniques have been modernized, in which their most pressing shortcomings have been re-addressed. Studies in this area have also led to the development of new, faster and more efficient extraction and concentration techniques, such as solid phase microextraction (SPME), stir bar sorptive extraction (SBSE), single-drop microextraction (SDME) and microextraction by packed sorbent (MEPS) [16]. Miniaturized analytical techniques have gained attention due to their many special properties. Among the many advantages, the use of a small volume of solvent, commonly in microliters or solventless arrangement, the low volumes of the required sample, and an increase in the sensitivity of the analysis are the most important benefits [23]. Dramatically reduced waste production as well as lower labor-intensive and tedious operations are other important benefits of microextraction techniques. Variability in selectivity designing by appropriate specific sorbent selection (fibers, multilayer fibers/stir bars, thin films) or emerged green solvents are attracting attention for broad application areas.
One of the most significant advantages of MEPS is the fact that the same sorbent syringe can be reused 40–60 times for complicated matrices and more than 100 times for other samples such as water. This technique is not time-consuming compared to solid-phase microextraction (SPME) and solid phase extraction (SPE). Another advantage of MEPS is the small amount of sorbent (1–4 mg); therefore, only a relatively small volume of solvent is required to elute the analytes from the sorbent in accordance with a subsequent analytical technique such as chromatography. One of the disadvantages of the MEPS technique is the low variability of available commercial sorbents. Another disadvantage is the strong dependence of the analyte recovery on the continuous movement of the piston, which determines the flow of the sample through the syringe with the sorbent bed. It should be emphasized that microextraction techniques, as well as MEPS, are also limited by the rapid saturation of the sorbent [16,21]. For the determination of low levels of organic pollutants, microextraction techniques are fast, cheap, and green alternatives to conventional alternatives.
4. Conclusions
The release of nitro compounds into the environment and the subsequent contamination of the ecosystem, soil, groundwater, and surface water represents significant environmental risks. From a forensic point of view, the detection of EGDN might be an important result for the comprehensive work of forensic technicians, who can predict the original energetic material used for the detonation. This work was focused on determining the appropriate extraction parameters of a fast, efficient, and solvent-minimalized analytical method based on microextraction by packed sorbent followed by gas chromatography with appropriate detection. As part of the method validation, linearity, limit of detection (LOD) and limit of quantification (LOQ) were evaluated. The accuracy of the method in terms of recovery at various concentration levels was determined and the precision was expressed using relative standard deviation (RSD). Matrix effects were discussed, showing an acceptable impact of the matrix on the calibration process. The applicability of the proposed method to real-life samples was demonstrated in fields of environmental as well as forensic origin. Work with real samples showed the possible limitations of the study in matrix interferences, and a detailed study of interferences in the huge variability of environmental and forensic samples will follow in the future.
Author Contributions
Conceptualization, S.H.; methodology, S.H., T.L. and T.P.; validation, S.H. and P.U.; investigation, T.P.; T.L. and P.U.; resources, S.H.; writing—original draft preparation, S.H.; writing—review and editing, P.U., T.P. and S.H.; project administration, S.H.; funding acquisition, S.H. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the Slovak Research and Development Agency under the Contract No. APVV-19-0149. The work was supported by the Scientific Grant Agency of the Ministry of Education of the Slovak Republic (VEGA project No. 1/0412/20).
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest. The funders had no role in the design of the study; in the collection, analyses, or interpretation of data; in the writing of the manuscript; or in the decision to publish the results.
References
- Anilanmert, B.; Cengiz, S. New Techniques and Methods in Explosive Analysis. Applications of Modern Mass Spectrometry, 1st ed.; Atta-ur-Rahman, F.R.S., Choudhary, M.I., Musharraf, S.G., Eds.; Bentham Books Imprint: Singapore, 2020; Volume 1, pp. 128–202. [Google Scholar]
- Naik, V.; Patil, K.C. High Energy Materials. A Brief History and Chemistry of Fireworks and Rocketry. Resonance 2015, 20, 431–444. [Google Scholar] [CrossRef]
- Fettaka, H.; Llefebvre, M. Ethylene Glycol Dinitrate (EGDN): From Commercial Precursors, Physicochemical and Detonation Characterization. Cent. Europ. J. Energetic Mater. 2015, 12, 287–305. [Google Scholar]
- Akhavan, J. Classification of explosives materials. In Chemistry of Explosives, 1st ed.; Akhavan, J., Ed.; The Royal Society of Chemistry: London, UK, 2007; pp. 21–48. [Google Scholar]
- Zapata, F.; García-Ruiz, C. Chemical Classification of Explosives. Crit. Rev. Anal. Chem. 2020, 51, 656–673. [Google Scholar] [CrossRef] [PubMed]
- Rylott, E.L.; Bruce, N.C. Right on target: Using plants and microbes to remediate explosives. Int. J. Phytoremed. 2019, 21, 1051–1064. [Google Scholar] [CrossRef] [PubMed]
- Frankovská, J.; Kordík, J.; Slaninka, I.; Jurkovič, Ľ.; Greif, V.; Šottník, P.; Dananaj, I.; Mikita, S.; Dercová, K.; Jánová, V. Atlas Sanačných Metód A Environtentálnych Záťaží, 1st ed.; Štátny Geologický Ústav Dionýza Štúra: Bratislava, Slovakia, 2010; pp. 53–54. ISBN 978-80-89343-39-3. Available online: https://www.minzp.sk/files/sekcia-geologie-prirodnych-zdrojov/atlas-sanacnych-metod-environmentalnych-zatazi.pdf (accessed on 20 November 2022).
- Zarei, A.R.; Nedaei, M.; Ghorbanian, S.A. Ferrofluid of magnetic clay and menthol based deep eutectic solvent: Application in directly suspended droplet microextraction for enrichment of some emerging contaminant explosives in water and soil samples. J. Chromatogr. A. 2018, 1553, 32–42. [Google Scholar] [CrossRef] [PubMed]
- Rosendo, L.M.; Brinca, A.T.; Pires, B.; Catarro, G.; Rosado, T.; Guiné, R.P.F.; Araújo, A.R.T.S.; Anjos, O.; Gallardo, E. Miniaturized Solid Phase Extraction Techniques Applied to Natural Products. Processes 2023, 11, 243. [Google Scholar] [CrossRef]
- Yılmaz, E.; Garipcan, B.; Patra, H.K.; Uzun, L. Molecular Imprinting Applications in Forensic Science. Sensors 2017, 17, 691. [Google Scholar] [CrossRef] [PubMed]
- Sarfraz-Yazdi, A.; Es´Haghi, Z. Comparison of Hollow Fiber and Single-Drop Liquid-Phase Microextraction Techniques for HPLC Determination of Aniline Derivatives in Water. Chromatographia 2006, 63, 563–569. [Google Scholar] [CrossRef]
- Suchana, S.; Passeport, E. Optimization of a solid-phase microextraction technique for chloro and nitro- substituted aromatic compounds using design of experiments. J. Chromatogr. A. 2020, 1621, 461083. [Google Scholar] [CrossRef] [PubMed]
- Galmiche, M.; Colin, A.; Clavos, M.C.; Pallez, C.; Rosin, C.; Dauchy, X. Determination of nitroaromatic explosive residues in water by stir bar sorptive extraction-gas chromatography-tandem mass spectrometry. Anal. Bioanal. Chem. 2020, 413, 159–169. [Google Scholar] [CrossRef] [PubMed]
- Dhingra, G.; Bansal, P.; Dhingra, N.; Rani, S.; Malik, K.A. Development of a microextraction by packed sorbent with gas chromatography-mass spectrometry method for quantification of nitroexplosives in aqueous and fluidic biological samples. J. Sep. Sci. 2017, 41, 639–647. [Google Scholar] [CrossRef] [PubMed]
- Jalilli, V.; Barkhordari, A.; Ghiasvand, A. A comprehensive look at solid-phase microextraction technique: A review of reviews. Microchem. J. 2019, 152, 104319. [Google Scholar] [CrossRef]
- Páleníková, A.; Hrouzková, S. Microextraction in packed syringe: Solvent-minimized sample preparation technique. Monatsh. Chem. 2014, 145, 537–549. [Google Scholar] [CrossRef]
- Moein, M.M.; Said, R.; Abdel-Rehim, M. Microextraction by packed sorbent. Bioanalysis 2015, 7, 2155–2161. [Google Scholar] [CrossRef] [PubMed]
- Abdel-Rehim, M. New trend in sample preparation: On-line microextraction in packed syringe for liquid and gas chromatography applications I. Determination of local anaesthetics in human plasma samples using gas chromatography-mass spectrometry. J. Chromatogr. B 2004, 801, 317–321. [Google Scholar] [CrossRef] [PubMed]
- Szarka, A.; Hrouzková, S.; Deszatová, T.; Zichová, S. Microextraction in packed syringe coupled with GC-MS for the determination of pesticides in environmental water samples. Fres. Environ. Bull. 2017, 26, 2665–2672. [Google Scholar]
- Pereira, J.; Gonçalves, J.; Alves, V.; Câmara, J.S. Microextraction using packed sorbent as an effective and high-throughput sample extraction technique: Recent applications and future trends. Sample Prep. 2013, 1, 38–53. [Google Scholar] [CrossRef]
- Abdel-Rehim, M. Microextraction by packed sorbent (MEPS): A tutorial. Anal. Chim. Acta 2011, 701, 119–128. [Google Scholar] [CrossRef] [PubMed]
- Pizzutti, I.R.; Vela, G.M.E.; de Kok, A.; Scholten, J.M.; Dias, J.V.; Cardoso, C.D.; Concenço, G.; Vivian, R. Determination of paraquat and diquat: LC-MS method optimization and validation. Food Chem. 2016, 209, 248–255. [Google Scholar] [CrossRef] [PubMed]
- Silva, C.; Cavaco, C.; Prestrelo, R.; Pereira, J.; Câmara, J.S. Microextraction by Packed Sorbent (MEPS) and Solid-Phase Microextraction (SPME) as Sample Preparation Procedures for the Metabolomic Profiling of Urine. Metabolites 2014, 4, 71–97. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).