Fabrication of Organic Solvent Nanofiltration Membrane through Interfacial Polymerization Using N-Phenylthioure as Monomer for Dimethyl Sulfoxide Recovery
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials
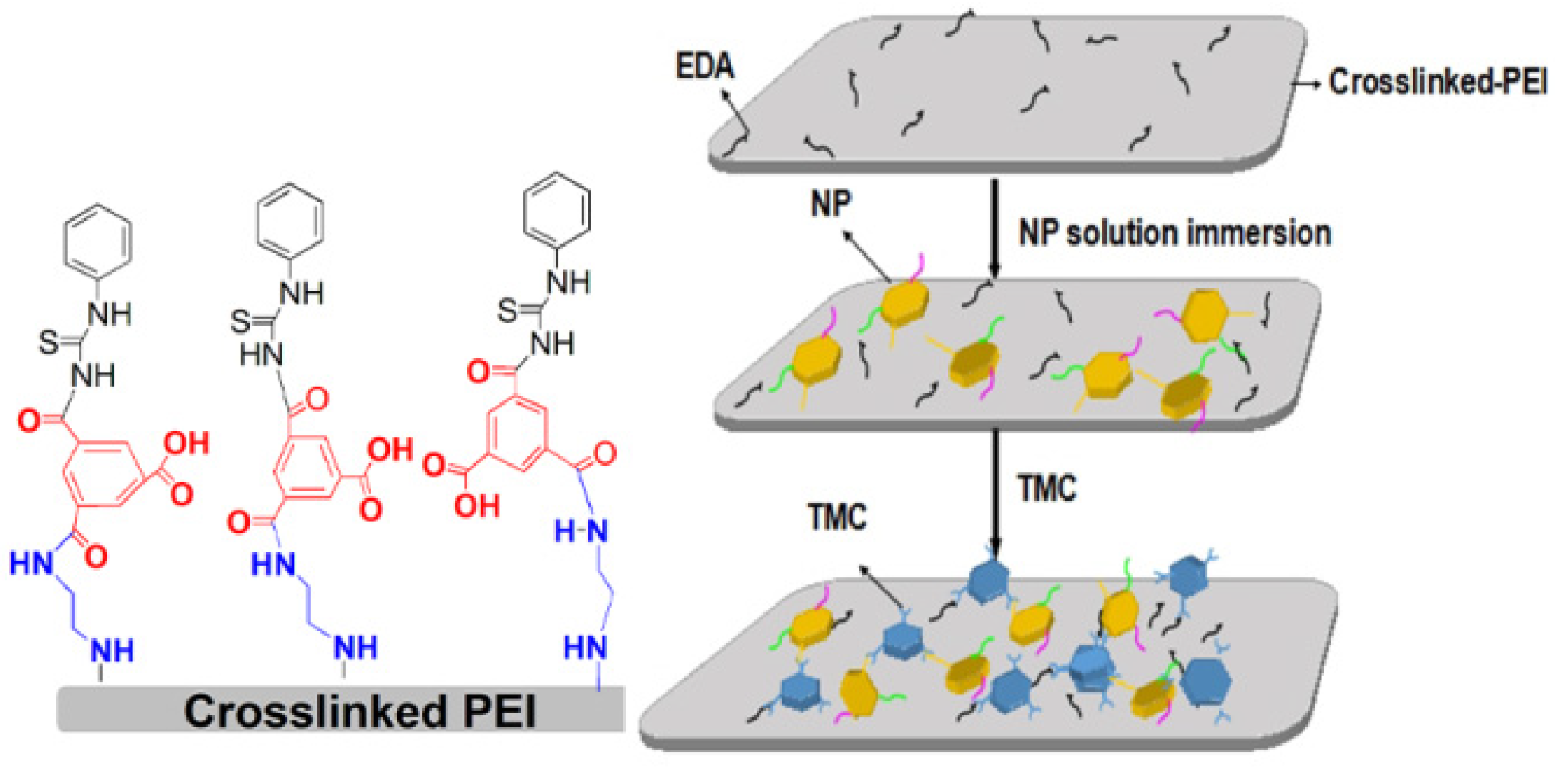
2.2. Membrane Preparation
2.2.1. Polyetherimide Substrate and Crosslinked Membrane
2.2.2. Thin Film Composite Membranes
2.3. Characterization of the Membranes
3. Results
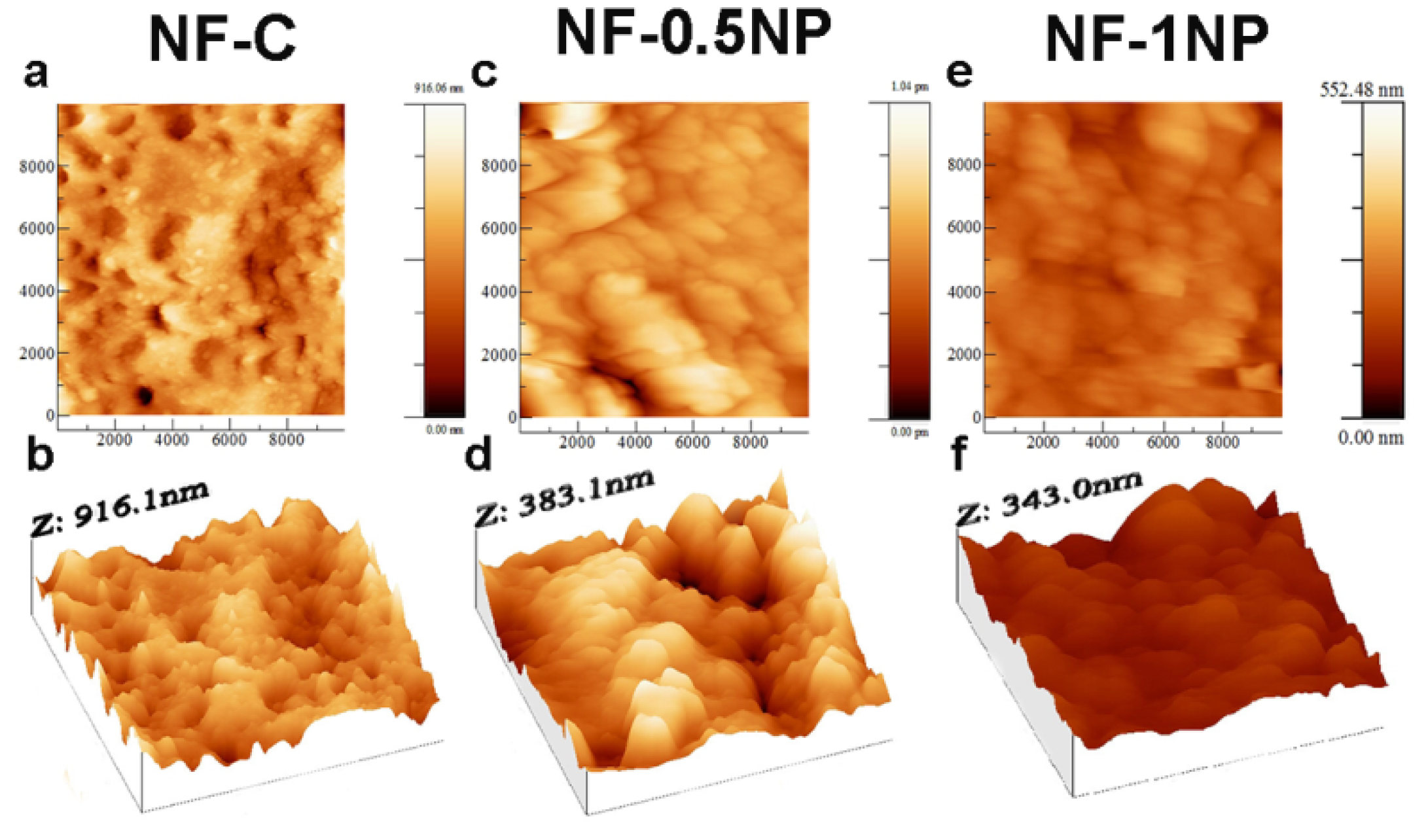
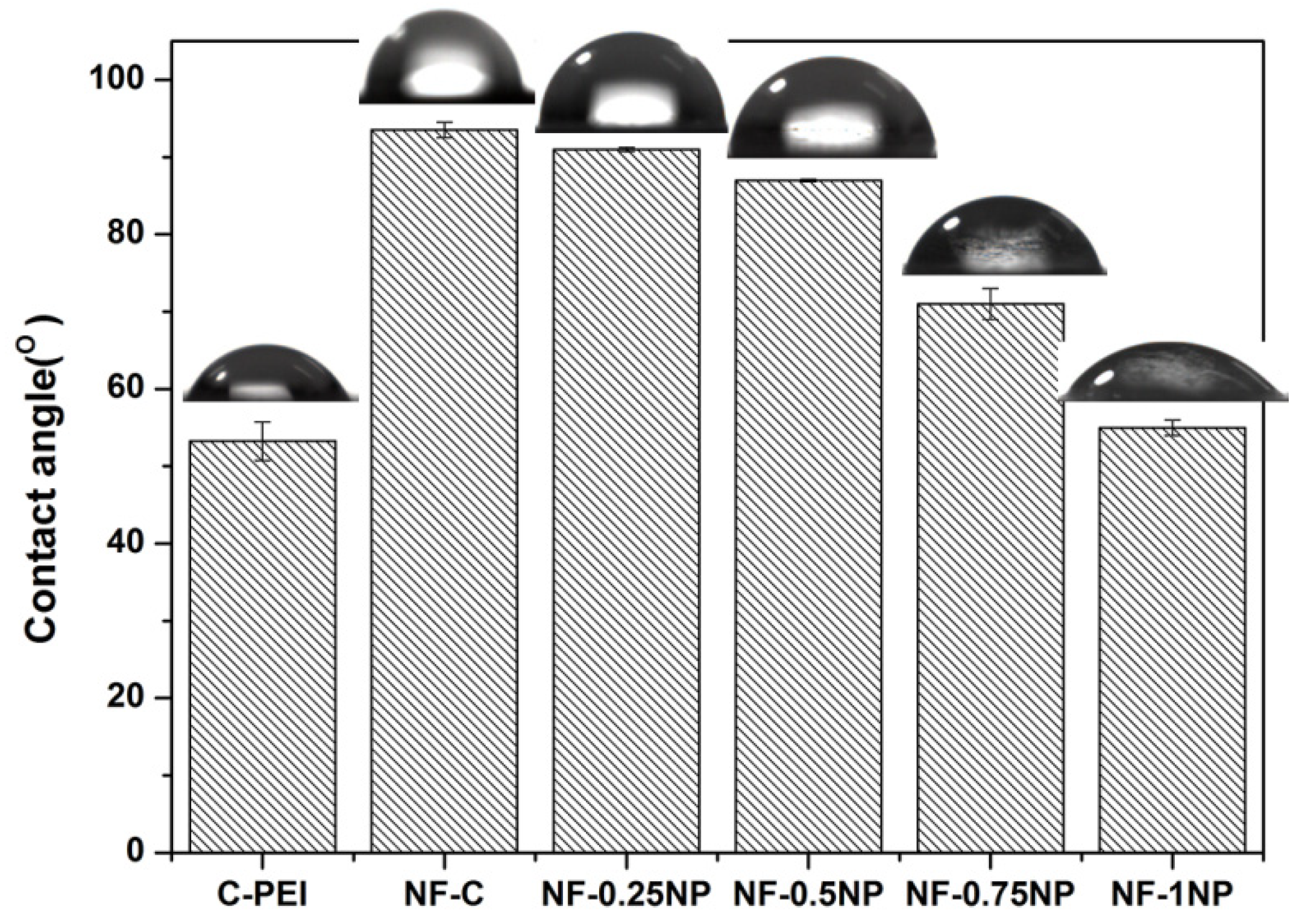
3.1. Morphologies
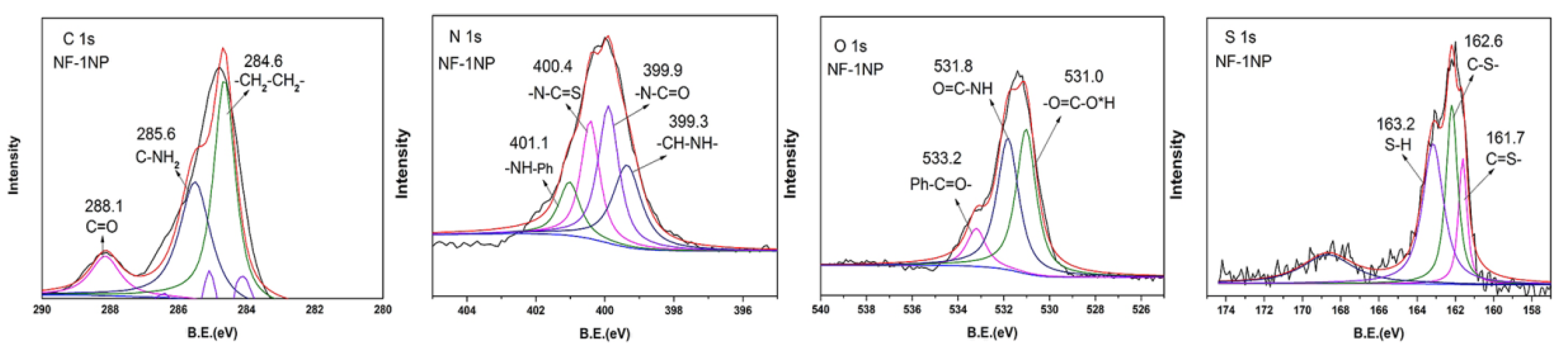
3.2. Chemical Structure Analysis
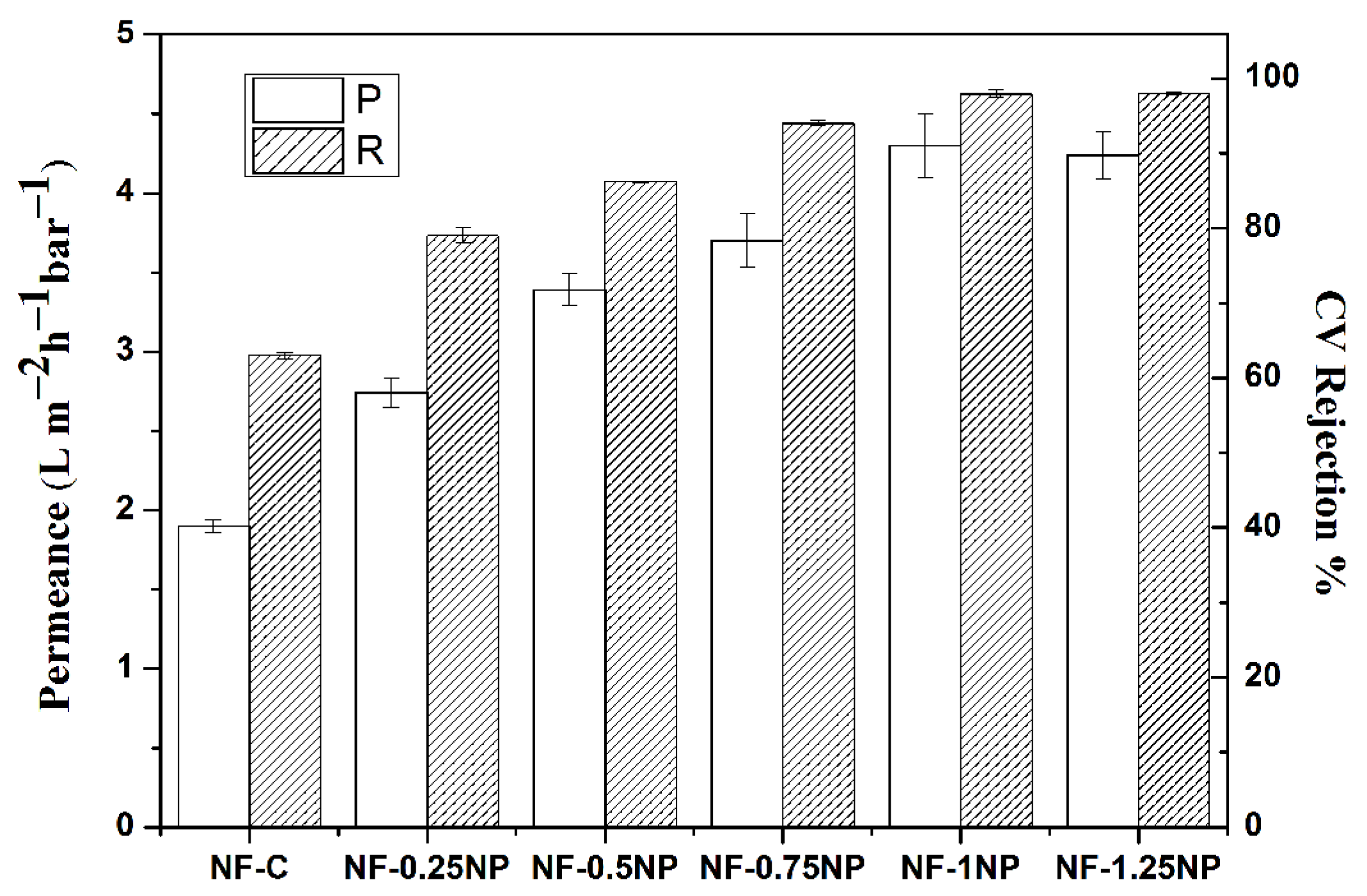
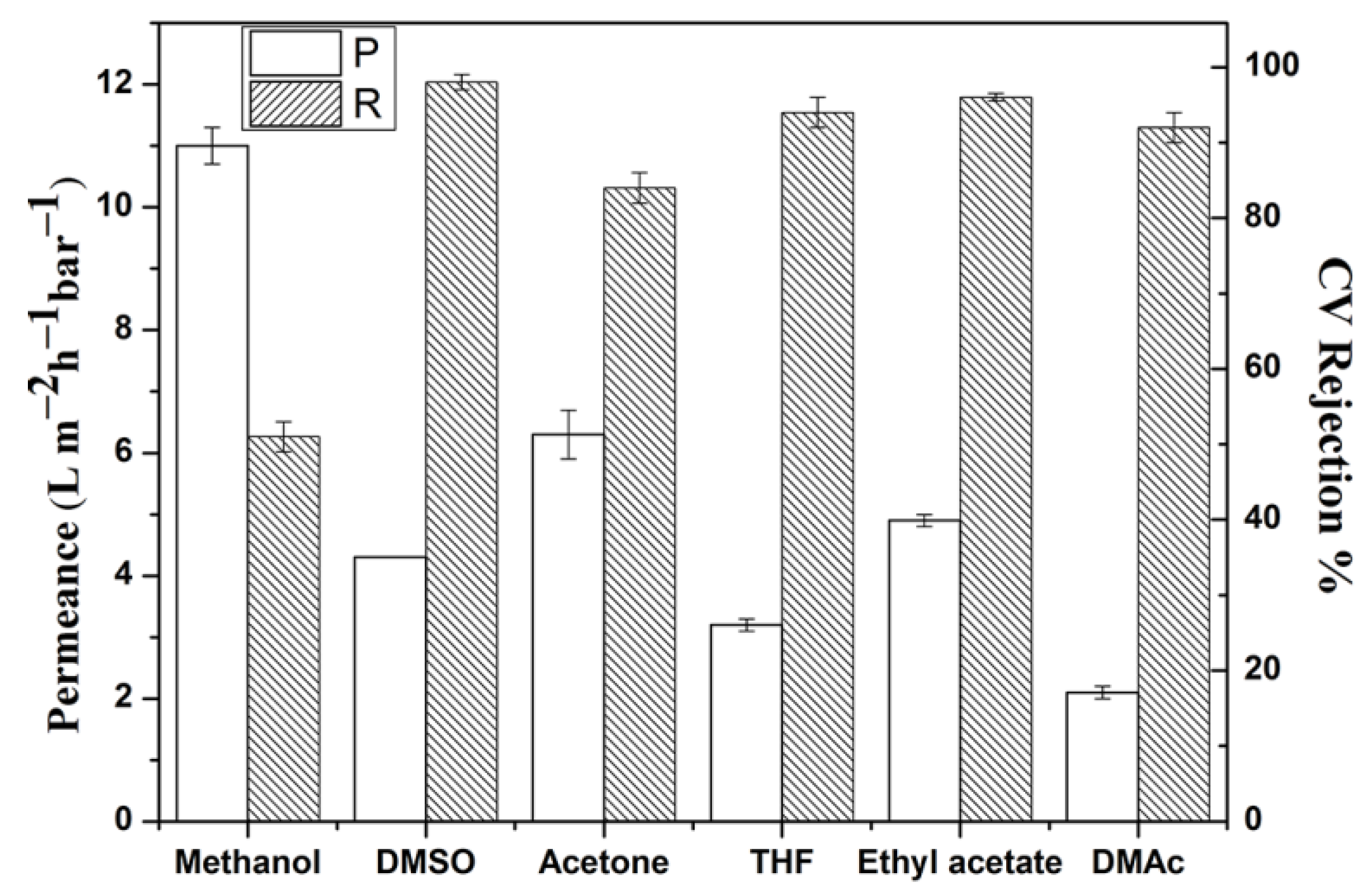
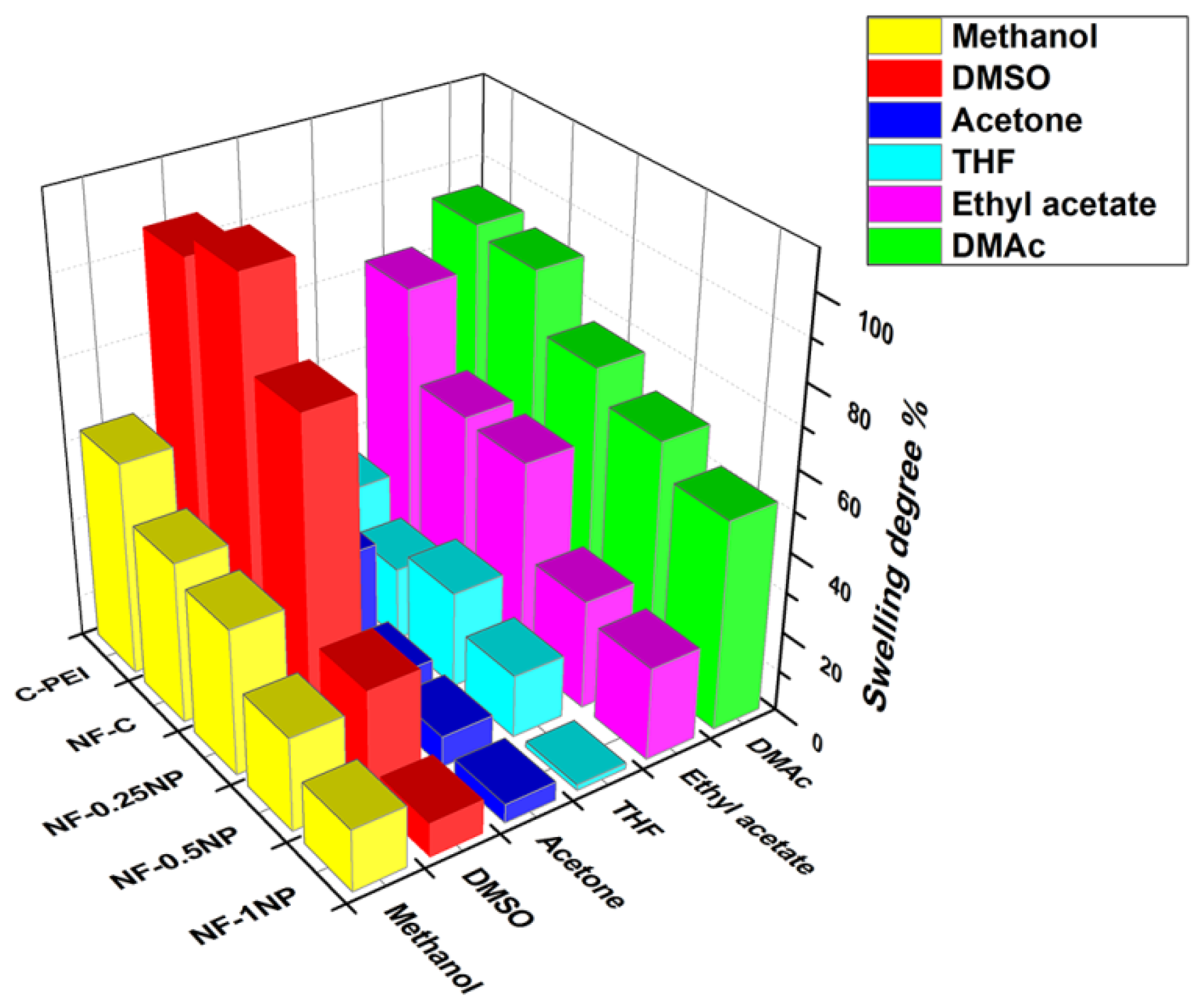
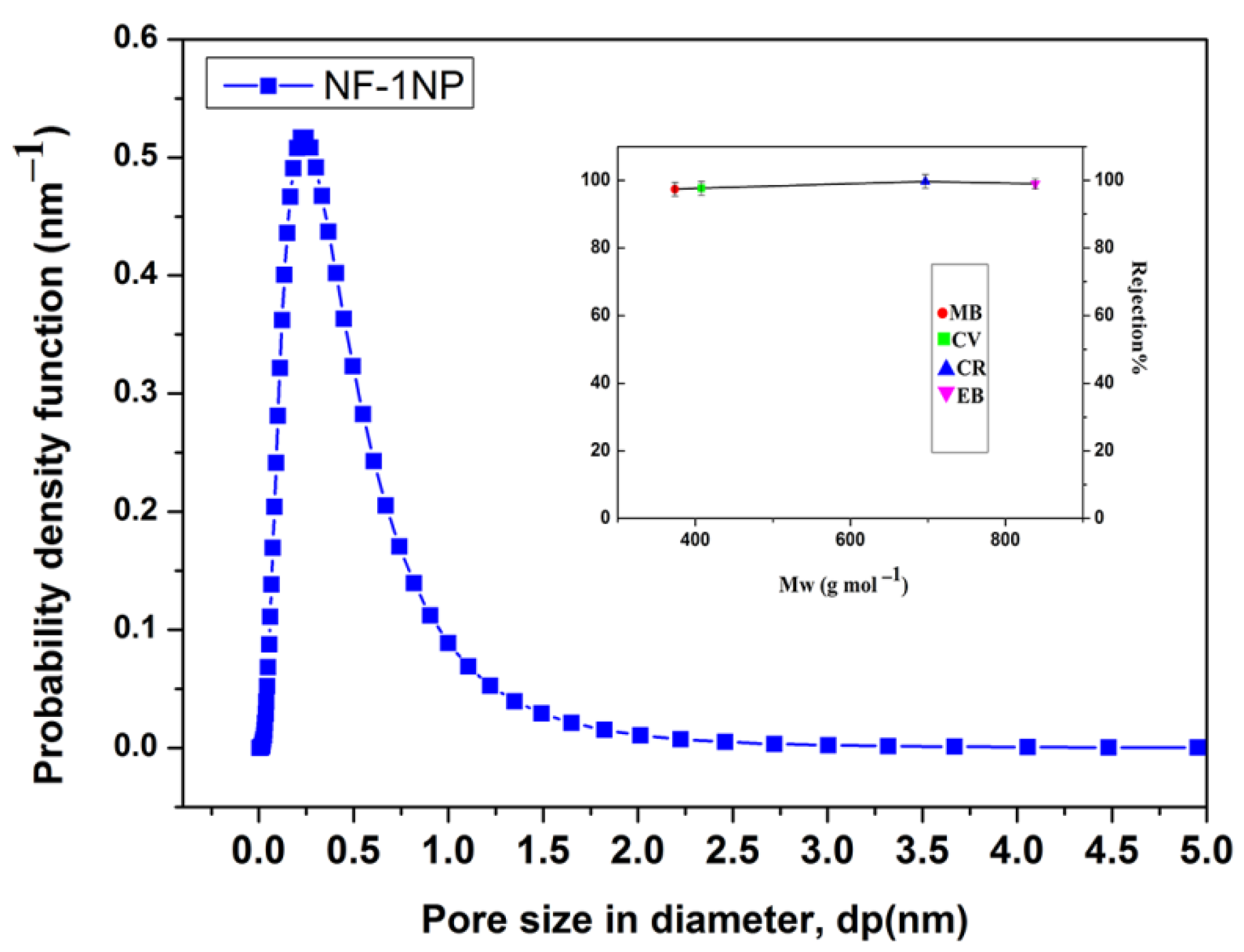
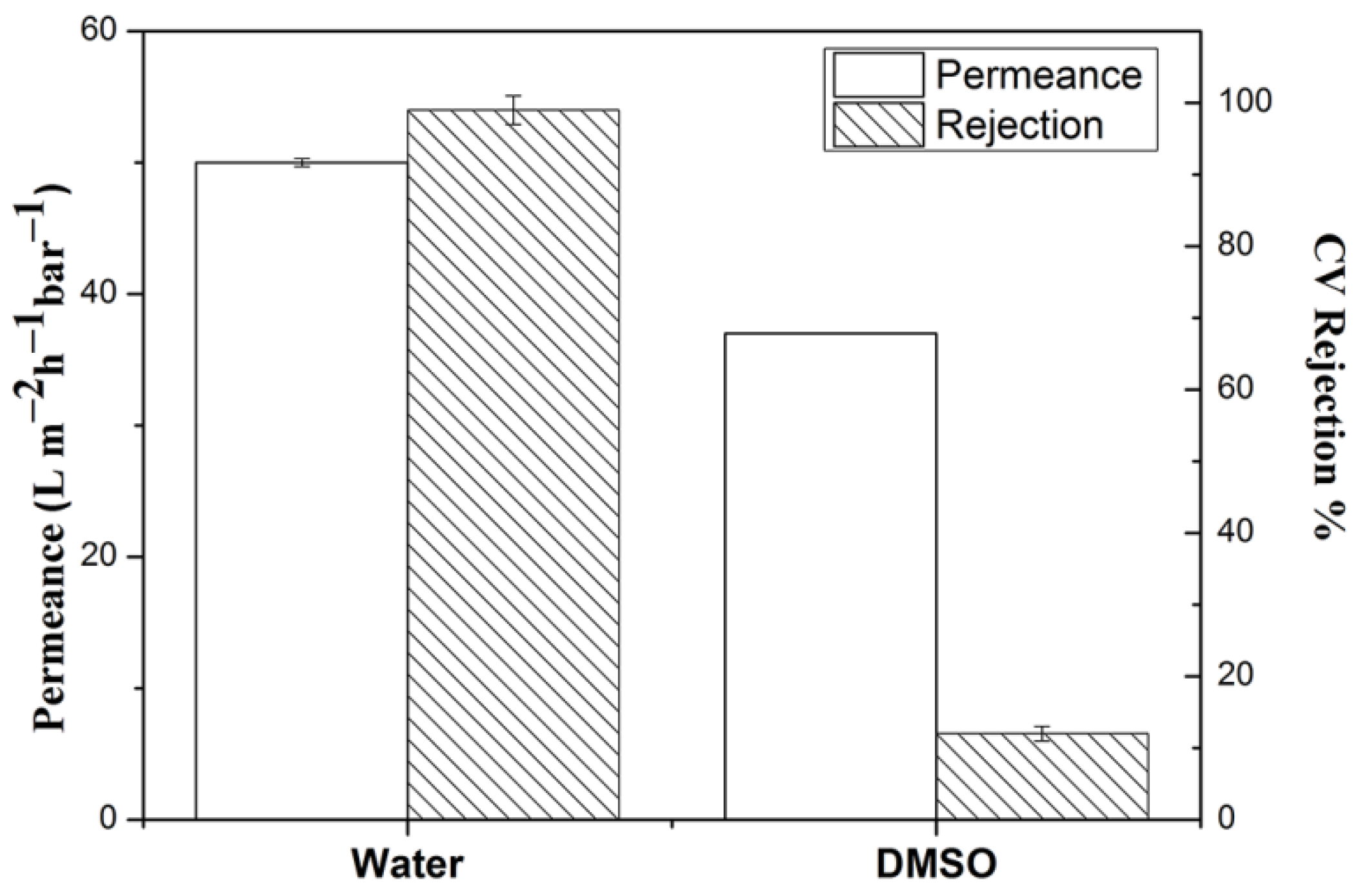
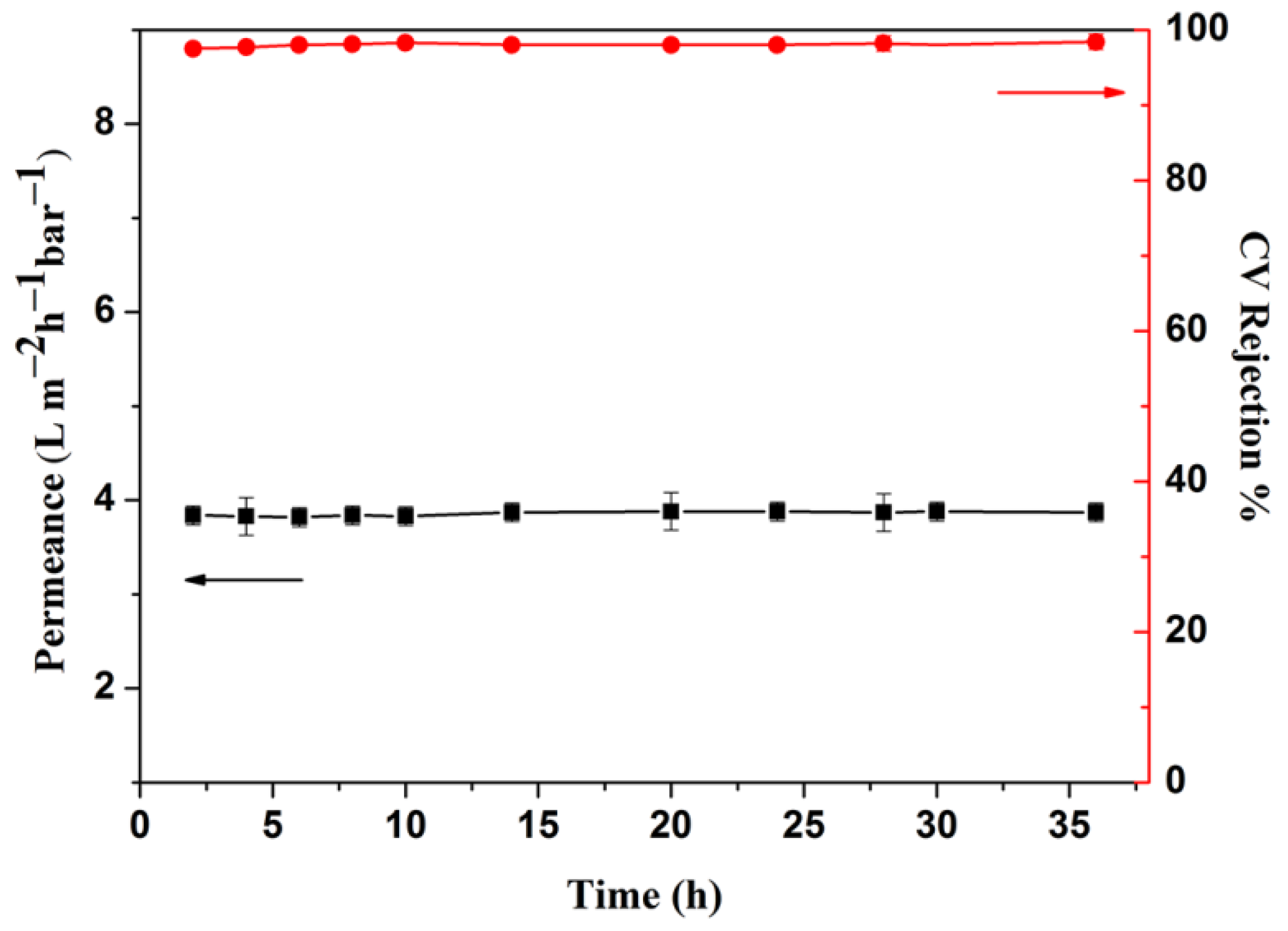
3.3. Membrane Performance
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Shi, G.M.; Feng, Y.; Li, B.; Tham, H.; Lai, J.-Y.; Chung, T.-S. Recent progress of organic solvent nanofiltration membranes. Prog. Polym. Sci. 2021, 123, 101470. [Google Scholar] [CrossRef]
- Scharzec, B.; Holtkötter, J.; Bianga, J.; Dreimann, J.; Vogt, D.; Skiborowski, M. Conceptual study of co-product separation from catalyst-rich recycle streams in thermomorphic multiphase systems by OSN. Chem. Eng. Res. Des. 2020 157, 65–76. [CrossRef]
- Liu, G.; Jin, W. Pervaporation membrane materials: Recent trends and perspectives. J. Membr. Sci. 2021, 636, 119557. [Google Scholar] [CrossRef]
- Hadizade, G.; Binaeian, E.; Emami, M. Preparation and characterization of hexagonal mesoporous silica/polyacrylamide nanocomposite capsule (PAM-HMS) for dye removal from aqueous solutioxns. J. Mol. Liq. 2017, 238, 499–507. [Google Scholar] [CrossRef]
- Werth, K.; Kaupenjohann, P.; Skiborowski, M. The potential of organic solvent nanofiltration processes for oleochemical industry. Sep. Purif. Technol. 2017, 182, 185–196. [Google Scholar] [CrossRef]
- Zhao, Y.; Tong, T.; Wang, X.; Lin, S.; Reid, E.; Chen, Y. Differentiating Solutes with Precise Nanofiltration for Next Generation Environmental Separations: A Review. Environ. Sci. Technol. 2021, 55, 1359–1376. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.; Guo, Z.; Li, S.; Van der Bruggen, B. Interfacially Polymerized Thin-Film Composite Membranes for Organic Solvent Nanofiltration. Adv. Mater. Interfaces 2020, 8, 2001671. [Google Scholar] [CrossRef]
- Vandezande, P.; Gevers, L.; Vankelecom, I. Solvent resistant nanofiltration: Separating on a molecular level. Chem. Soc. Rev. 2008, 37, 365–405. [Google Scholar] [CrossRef] [PubMed]
- Zhu, P.; Chen, Y.; Wang, L.Y.; Qian, R. A novel approach to separation of waste printed circuit boards using dimethyl sulfoxide. Int. J. Environ. Sci. Technol. 2013, 10, 175–180. [Google Scholar] [CrossRef]
- Feng, W.; Li, J.; Fang, C.; Zhang, L.; Zhu, L. Controllable thermal annealing of polyimide membranes for highly-precise organic solvent nanofiltration. J. Membr. Sci. 2022, 643, 120013. [Google Scholar] [CrossRef]
- Loh, X.X.; Sairam, M.; Bismarck, A.; Steinke, J.; Livingston, A.; Li, K. Crosslinked integrally skinned asymmetric polyaniline membranes for use in organic solvents. J. Membr. Sci. 2009, 326, 635–642. [Google Scholar] [CrossRef]
- Matthias, M.; Cédric, V.; Marloes, T.; Guy, K.; Vankelecom, I. Crosslinked PVDF-membranes for Solvent Resistant Nanofiltration. J. Membr. Sci. 2018, 566, 223–230. [Google Scholar]
- Chisca, S.; Duong, P.; Emwas, A.; Sougrat, R.; Nunes, S. Crosslinked copolyazoles with a zwitterionic structure for organic solvent resistant membranes. Polym. Chem. 2015, 6, 543–554. [Google Scholar] [CrossRef]
- Wang, X.; Wang, N.; Li, X.; An, Q.-F. A review of nano-confined composite membranes fabricated inside the porous support. Adv. Membr. 2021, 1, 100005. [Google Scholar] [CrossRef]
- Zhu, J.; Yuan, S.; Wang, J.; Zhang, Y.; Tian, M.; Van der Bruggen, B. Microporous organic polymer-based membranes for ultrafast molecular separations. Prog. Polym. Sci. 2020, 110, 101308. [Google Scholar] [CrossRef]
- Feng, Y.; Weber, M.; Maletzko, C.; Chung, T. Fabrication of organic solvent nanofiltration membranes via facile bioinspired one-step modification. Chem. Eng. Sci. 2019, 198, 74–84. [Google Scholar] [CrossRef]
- Zhang, H.; Mao, H.; Wang, J.; Ding, R.; Du, Z.; Liu, J.; Cao, S. Mineralization-inspired preparation of composite membranes with polyethyleneimine–nanoparticle hybrid active layer for solvent resistant nanofiltration. J. Membr. Sci. 2014, 470, 70–79. [Google Scholar] [CrossRef]
- Peyravi, M.; Jahanshahi, M.; Rahimpour, A.; Javadi, A.; Hajavi, S. Novel thin film nanocomposite membranes incorporated with functionalized TiO2 nanoparticles for organic solvent nanofiltration. Chem. Eng. J. 2014, 241, 155–166. [Google Scholar] [CrossRef]
- Farahani, M.D.A.; Hua, D.; Chung, T.-S. Cross-linked mixed matrix membranes (MMMs) consisting of amine-functionalized multi-walled carbon nanotubes and P84 polyimide for organic solvent nanofiltration (OSN) with enhanced flux. J. Membr. Sci. 2018, 548, 319–331. [Google Scholar] [CrossRef]
- Alduraiei, F.; Kumar, S.; Liu, J.; Nunes, S.; Szekely, G. Rapid fabrication of fluorinated covalent organic polymer membranes for organic solvent nanofiltration. J. Membr. Sci. 2022, 648, 120345. [Google Scholar] [CrossRef]
- Huang, Y.; Li, S.-L.; Fu, Z.; Gong, G.; Hu, Y. Preparation of microporous organic solvent nanofiltration (OSN) composite membrane from a novel tris-phenol monomer. Sep. Purif. Technol. 2022, 301, 121985. [Google Scholar] [CrossRef]
- Li, S.; Yang, F.; Liu, S.; Li, H.; Su, B.; Han, L.; Gao, X.; Gao, C. Effective regulating interfacial polymerization process of OSN membrane via in-situ constructed nano-porous interlayer of 2D TpHz covalent organic frameworks. J. Membr. Sci. 2023, 665, 121101. [Google Scholar] [CrossRef]
- Lin, G.-S.; Yang, J.; Mou, C.-Y.; Tung, K.-L. Realizing ultrathin silica membranes with straight-through channels for high-performance organic solvent nanofiltration (OSN). J. Membr. Sci. 2021, 627, 119224. [Google Scholar] [CrossRef]
- Shi, G.M.; Chung, T.-S. Teflon AF2400/polyethylene membranes for organic solvent nanofiltration (OSN). J. Membr. Sci. 2020, 602, 117972. [Google Scholar] [CrossRef]
- Gao, Z.; Shi, G.; Cui, Y.; Chung, T.-S. Organic solvent nanofiltration (OSN) membranes made from plasma grafting of polyethylene glycol on cross-linked polyimide ultrafiltration substrates. J. Membr. Sci. 2018, 565, 169–178. [Google Scholar] [CrossRef]
- Li, W.; Lou, L.; Hai, Y.; Fu, C.; Zhang, J. Polyamide thin film composite membrane using mixed amines of thiourea and m-phenylenediamine. RSC Adv. 2015, 5, 54125–54132. [Google Scholar] [CrossRef]
- Zhou, A.; Shi, C.; He, X.; Fu, Y.; Anjum, A.; Zhang, J.; Li, W. Polyarylester nanofiltration membrane prepared from monomers of vanillic alcohol and trimesoyl chloride. Sep. Purif. Technol. 2018, 193, 58–68. [Google Scholar] [CrossRef]
- Li, W.; Bian, C.; Fu, C.; Zhou, A.; Shi, C.; Zhang, J. A poly(amide-co-ester) nanofiltration membrane using monomers of glucose and trimesoyl chloride. J. Membr. Sci. 2016, 504, 185–195. [Google Scholar] [CrossRef]
- Tarboush, B.; Rana, D.; Matsuura, T.; Arafat, H.; Narbaitz, R. Preparation of thin-film-composite polyamide membranes for desalination using novel hydrophilic surface modifying macromolecules. J. Membr. Sci. 2008, 325, 166–175. [Google Scholar] [CrossRef]
- Zhou, A.; Li, L.; Li, M.; Chen, Q. Fabrication of Poly(amide-co-ester) Solvent Resistant Nanofiltration Membrane from P-nitrophenol and Trimethyl Chloride via Interfacial Polymerization. Separations 2022, 9, 28. [Google Scholar] [CrossRef]
- Lin, S.; Dence, C. Methods in Lignin Chemistry; Springer: Berlin/Heidelberg, Germany; New York, NY, USA, 1992. [Google Scholar]
- Ahmad, A.; Ooi, B. Properties–performance of thin film composites membrane: Study on trimesoyl chloride content and polymerization time. J. Membr. Sci. 2005, 255, 67–77. [Google Scholar] [CrossRef]
- Lu, T.-D.; Chen, B.-Z.; Wang, J.; Jia, T.-Z.; Cao, X.-L.; Wang, Y.; Xing, W.; Lau, C.; Sun, S.-P. Electrospun nanofiber substrates that enhance polar solvent separation from organic compounds in thin-film composites. J. Mater. Chem. A 2018, 6, 15047–15056. [Google Scholar] [CrossRef]
- Almijbilee, M.; Wu, X.; Zhou, A.; Zheng, X.; Cao, X.; Li, W. Polyetheramide organic solvent nanofiltration membrane prepared via an interfacial assembly and polymerization procedure. Sep. Purif. Technol. 2020, 234, 116033. [Google Scholar] [CrossRef]
- Wang, Y.; Gu, J.; Zhou, A.; Kong, A.; Almijbilee, M.A.; Zheng, X.; Zhang, J.; Li, W. Poly[acrylate-co-amide] network composite via photo polymerization for organic solvent nanofiltration separation. Sep. Purif. Technol. 2020, 246, 116855. [Google Scholar] [CrossRef]
- Zheng, X.; Zhou, A.; Wang, Y.; He, X.; Zhao, S.; Zhang, J.; Li, W. Modulating hydrophobicity of composite polyamide membranes to enhance the organic solvent nanofiltration. Sep. Purif. Technol. 2019, 223, 211–223. [Google Scholar] [CrossRef]
- Zhou, A.; Wang, Y.; Cheng, D.; Li, M.; Wang, L. Effective interfacially polymerized polyarylester solvent resistant nanofiltration membrane from liquefied walnut shell. Korean J. Chem. Eng. 2022, 39, 1566–1575. [Google Scholar] [CrossRef]
- Michaels, A.S. Analysis and Prediction of Sieving Curves for Ultrafiltration Membranes: A Universal Correlation? Sep. Sci. Technol. 1980, 15, 1305–1322. [Google Scholar] [CrossRef]
- Singh, S.; Khulbe, K.; Matsuura, T.; Ramamurthy, P. Membrane characterization by solute transport and atomic force microscopy. J. Membr. Sci. 1988, 142, 111–127. [Google Scholar] [CrossRef]
- Blumenschein, S.; Kätzel, U. An heuristic-based selection process for organic solvent nanofiltration membranes. Sep. Purif. Technol. 2017, 183, 83–95. [Google Scholar] [CrossRef]
- Van der Bruggen, B.; Jansen, J.; Figoli, A.; Geens, J.; Van Baelen, D.; Drioli, E.; Vandecasteele, C. Determination of parameters affecting transport in polymeric membranes: Parallels between pervaporation and nanofiltration. J. Phys. Chem. B 2004, 108, 13273–13279. [Google Scholar] [CrossRef]
- Li, Y.; Xue, J.; Zhang, X.; Cao, B.; Li, P. Formation of Macrovoid-Free PMDA-MDA Polyimide Membranes Using a Gelation/Non-Solvent-Induced Phase Separation Method for Organic Solvent Nanofiltration. Ind. Eng. Chem. Res. 2019, 58, 6712–6720. [Google Scholar] [CrossRef]
- Xing, D.Y.; Chan, S.; Chung, T.-S. The ionic liquid [EMIM]OAc as a solvent to fabricate stable polybenzimidazole membranes for organic solvent nanofiltration. Green Chem. 2014, 16, 1383–1392. [Google Scholar] [CrossRef]
- Aburabie, J.; Emwas, A.-H.; Peinemann, K.-V. Silane-Crosslinked Asymmetric Polythiosemicarbazide Membranes for Organic Solvent Nanofiltration. Macromol. Mater. Eng. 2019, 304, 1800551. [Google Scholar] [CrossRef]
- Sun, S.-P.; Chung, T.-S.; Lu, K.-J.; Chan, S.-Y. Enhancement of flux and solvent stability of Matrimid®thin-film composite membranes for organic solvent nanofiltration. AIChE J. 2014, 60, 3623–3633. [Google Scholar] [CrossRef]
- Aburabie, J.; Neelakanda, P.; Karunakaran, M.; Peinemann, K.-V. Thin-film composite crosslinked polythiosemicarbazide membranes for organic solvent nanofiltration (OSN). React. Funct. Polym. 2015, 86, 225–232. [Google Scholar] [CrossRef]



| Membrane | Element Content (Atom.%) | |||
|---|---|---|---|---|
| C | N | O | S | |
| C-PEI | 76.59 | 8.45 | 14.75 | 0.21 |
| NF-C | 69.9 | 12.51 | 17.3 | 0.29 |
| NF-1NP | 71.20 | 12.57 | 14.71 | 1.52 |
| Membrane | Permeance | Dyes | Mw | Rejection | Ref. |
|---|---|---|---|---|---|
| PMDA-MDA | 6.3 | RB | 1017.64 | 89.5% | [42] |
| Polybenzimidazole | 0.31 | Sudan IV | 380.00 | 40% | [43] |
| Polythiosemicarbazide | 2.2 | Direct Red | 1373.00 | 100% | [44] |
| DAP-TMC | 4.5 | RB | 1017.64 | 91% | [46] |
| PIP-TMC | 1.0 | Fast Green FCF | 808.85 | 90% | [33] |
| NF-1NP | 4.30 | CV | 407.99 | 97.8% | This work |
| NF-1.25NP | 4.24 | CV | 407.99 | 98% |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, A.; Hu, G.; Guo, K.; Zhang, M.; Liu, X. Fabrication of Organic Solvent Nanofiltration Membrane through Interfacial Polymerization Using N-Phenylthioure as Monomer for Dimethyl Sulfoxide Recovery. Separations 2023, 10, 179. https://doi.org/10.3390/separations10030179
Zhou A, Hu G, Guo K, Zhang M, Liu X. Fabrication of Organic Solvent Nanofiltration Membrane through Interfacial Polymerization Using N-Phenylthioure as Monomer for Dimethyl Sulfoxide Recovery. Separations. 2023; 10(3):179. https://doi.org/10.3390/separations10030179
Chicago/Turabian StyleZhou, Ayang, Guangle Hu, Keying Guo, Mengnan Zhang, and Xiangnan Liu. 2023. "Fabrication of Organic Solvent Nanofiltration Membrane through Interfacial Polymerization Using N-Phenylthioure as Monomer for Dimethyl Sulfoxide Recovery" Separations 10, no. 3: 179. https://doi.org/10.3390/separations10030179
APA StyleZhou, A., Hu, G., Guo, K., Zhang, M., & Liu, X. (2023). Fabrication of Organic Solvent Nanofiltration Membrane through Interfacial Polymerization Using N-Phenylthioure as Monomer for Dimethyl Sulfoxide Recovery. Separations, 10(3), 179. https://doi.org/10.3390/separations10030179






