Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage
Abstract
1. Introduction
2. Experiments
2.1. Materials
2.2. Characterization
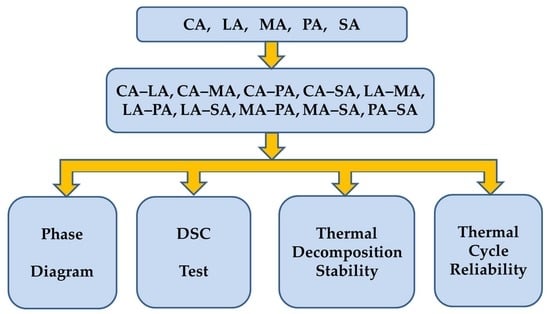
2.3. Preparation of the Acid Binary Eutectic Mixtures
3. Results and Discussion
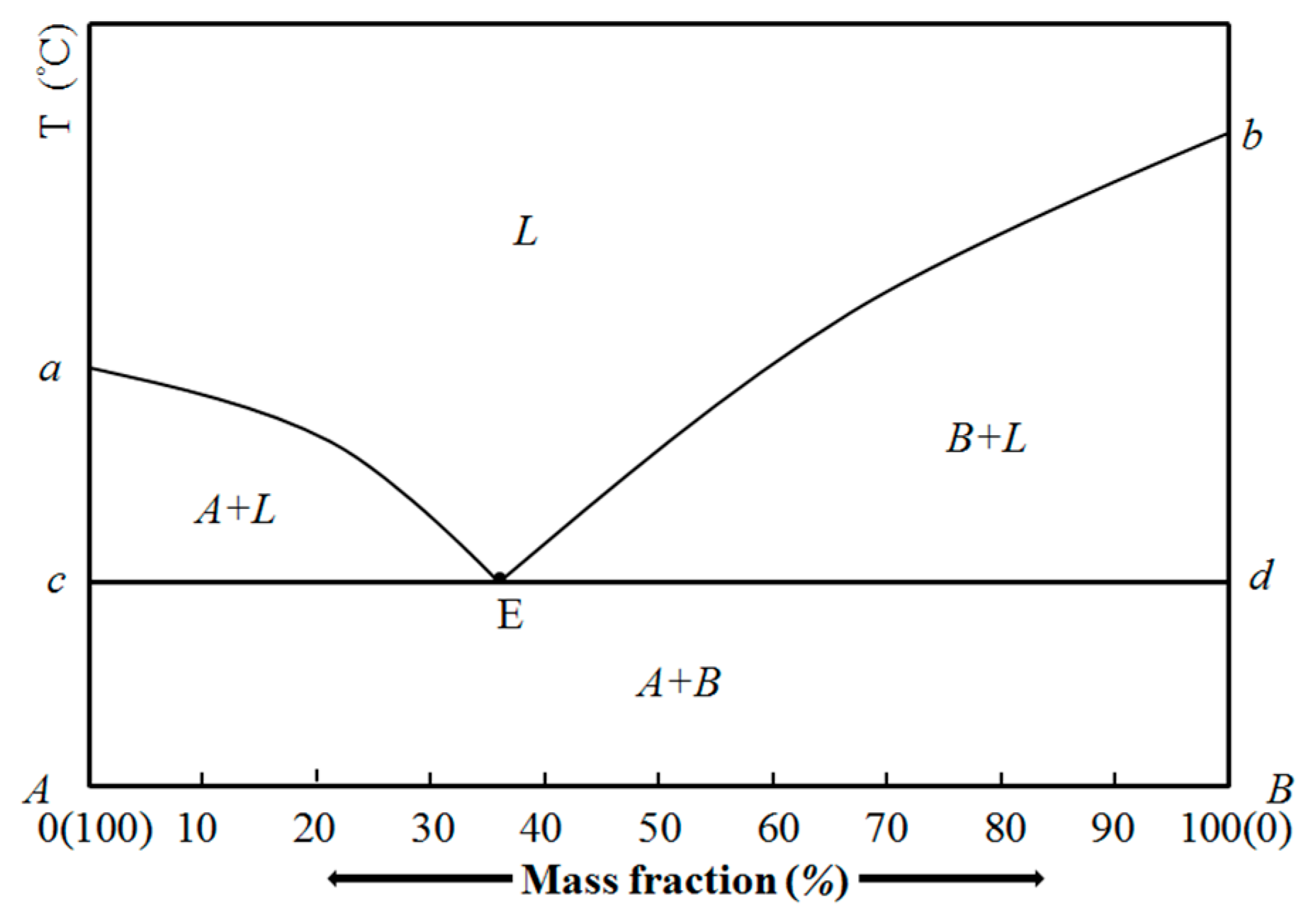
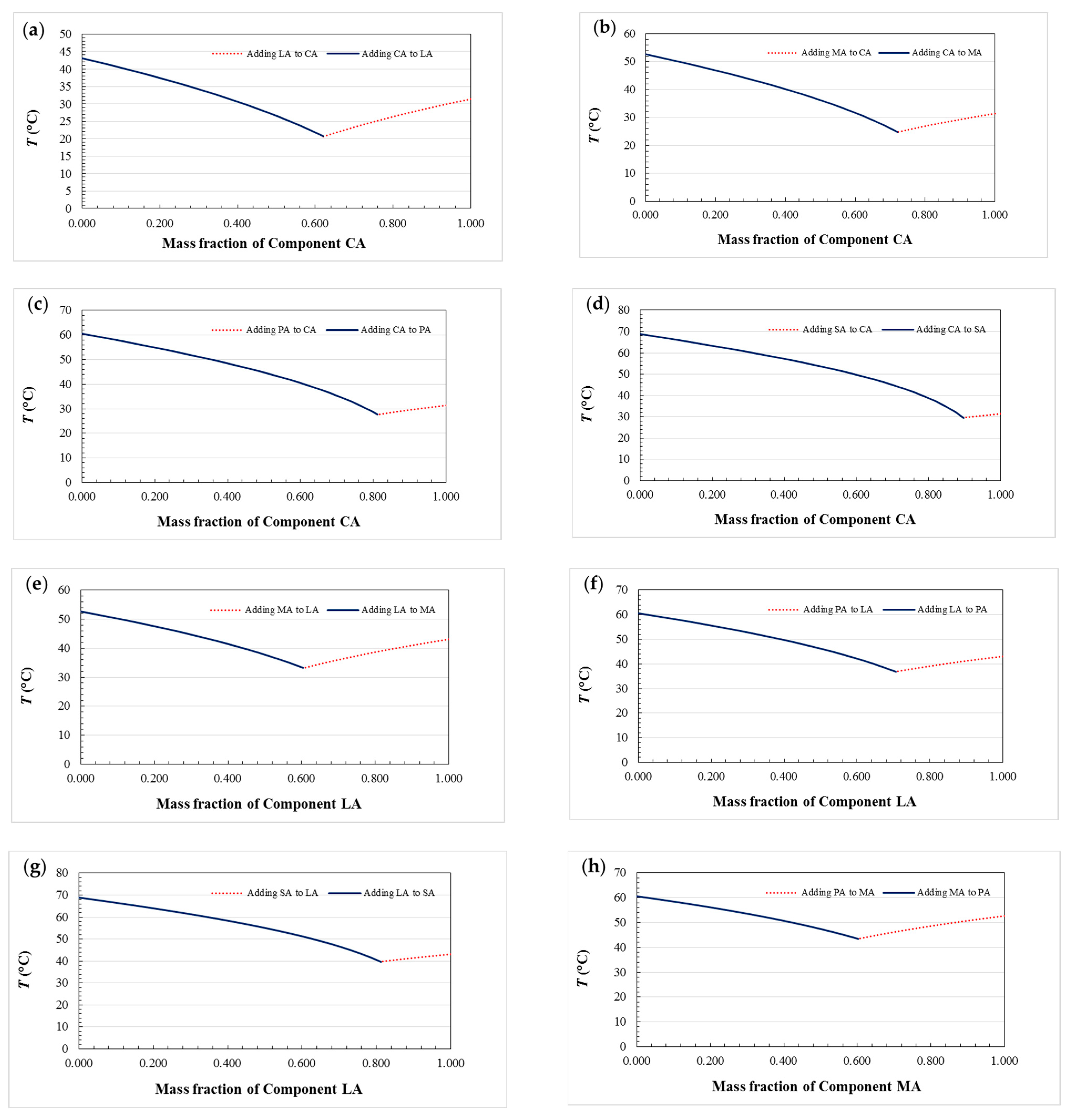
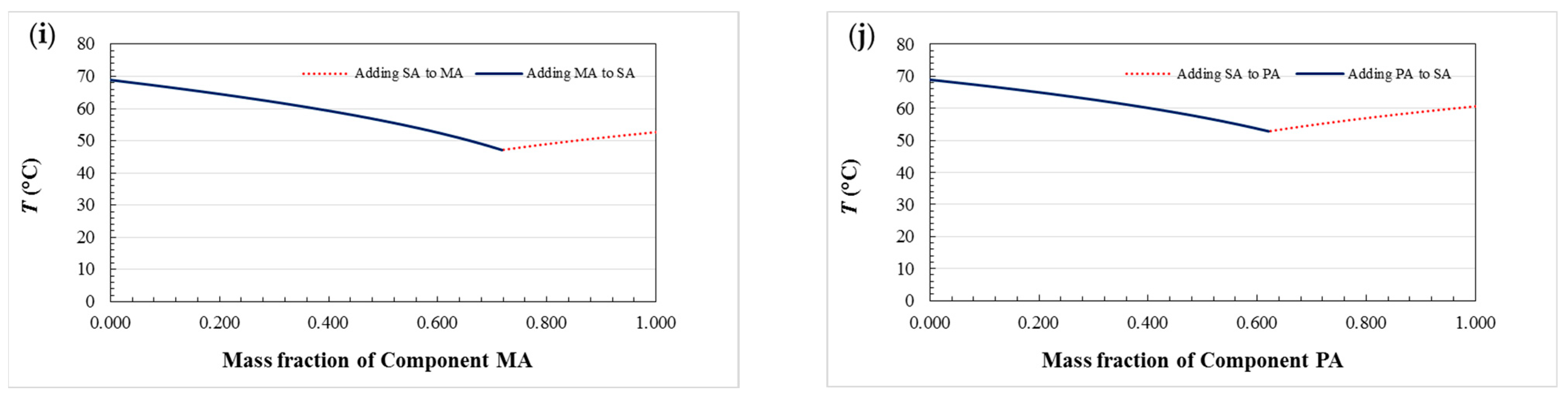
3.1. Phase Diagram of Fatty Acid Binary Eutectic Mixtures
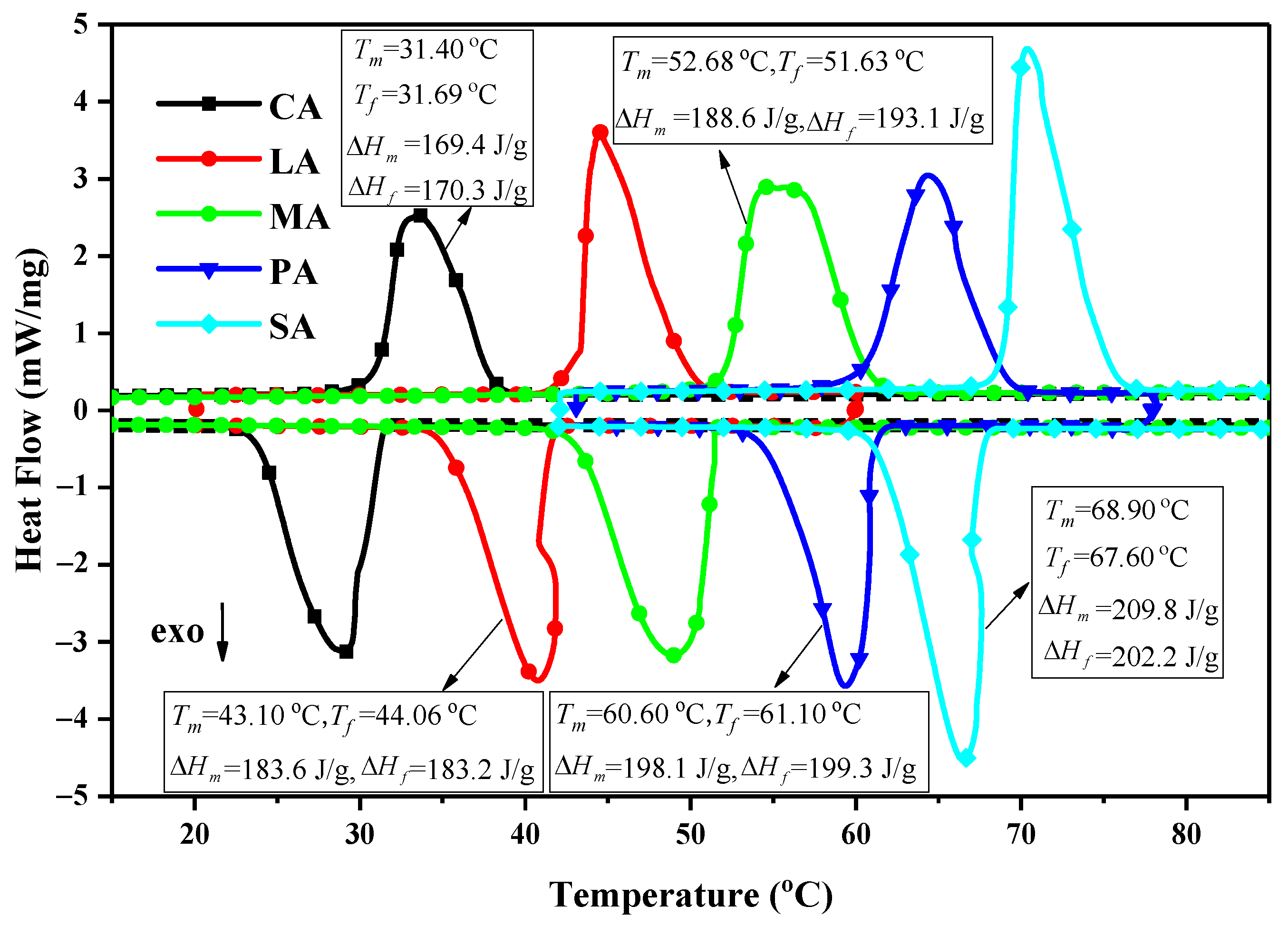
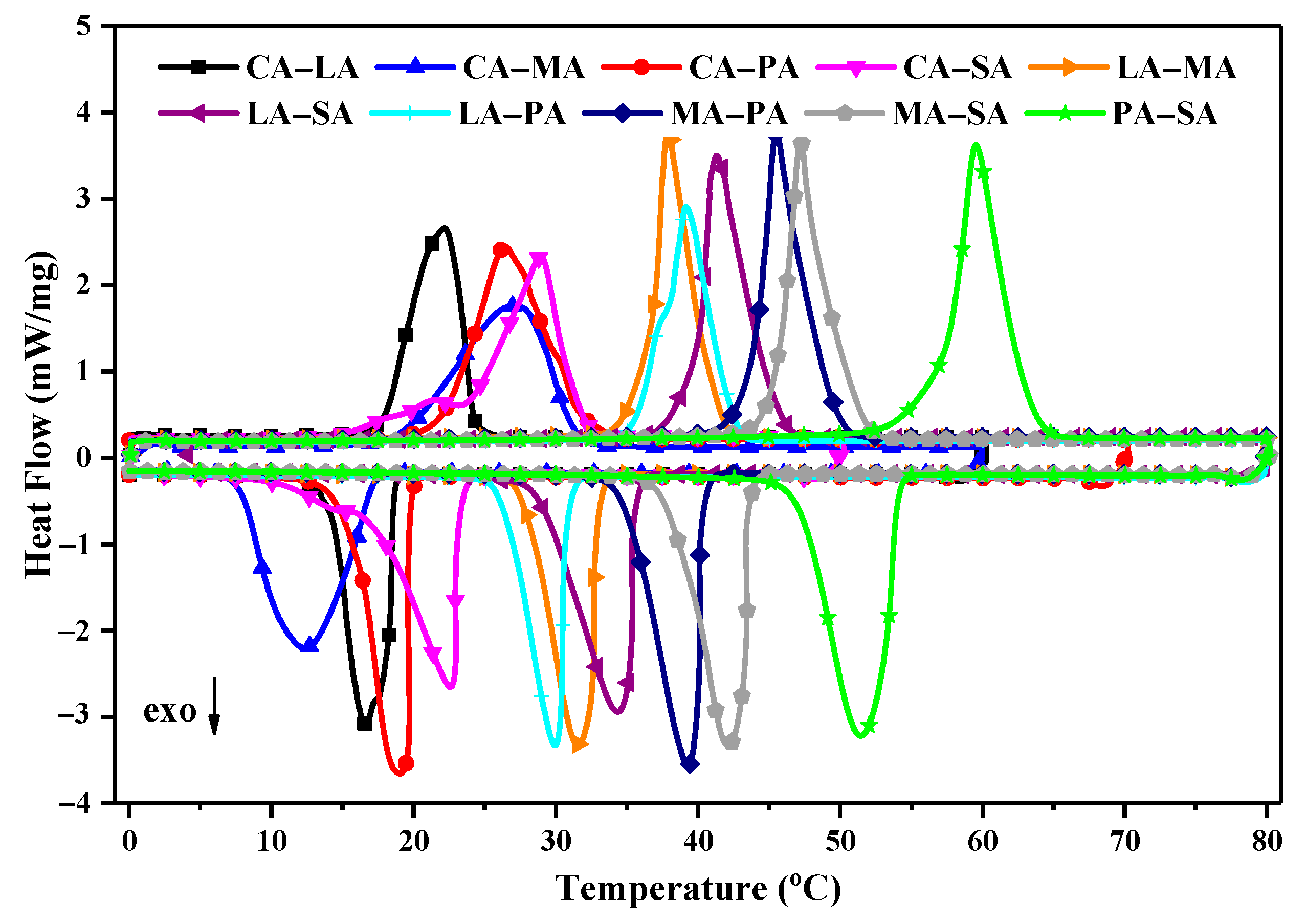
3.2. DSC Analysis of Fatty Acids Binary Eutectic Mixture
3.3. Thermal Stability Analysis of Fatty Acids Binary Eutectic Mixture
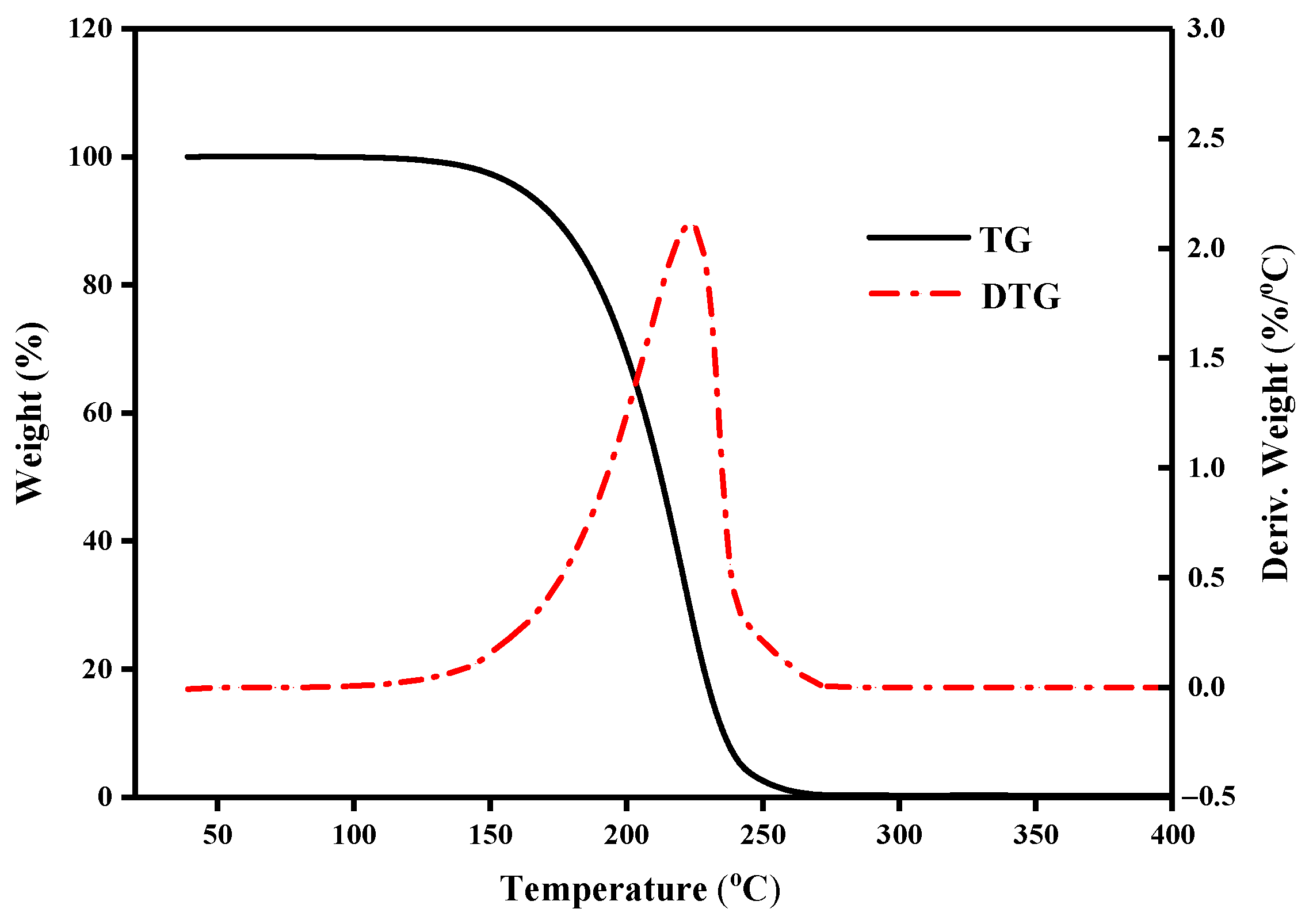
3.3.1. Thermal Decomposition Stability
3.3.2. Thermal Cycle Reliability
4. Conclusions
- (1)
- The theoretical eutectic points of ten fatty acid binary eutectic mixtures are calculated, and the phase diagrams of ten fatty acids binary eutectic compounds are drawn. There are some differences compared with the experimental values, which may be caused by impurities in the pure fatty acid used and experimental errors.
- (2)
- The phase change temperature of these fatty acid binary eutectic mixtures is between 17.7 °C and 57.1 °C, and the phase change latent heat is between 145.2 J/g and 193.0 J/g, which has the suitable temperature and larger phase change latent heat.
- (3)
- The fatty acid binary eutectic mixtures have good thermal stability in the temperature range below 100 °C, the change of phase transition temperature and latent heat with the number of thermal cycles is small, and they have good long-term cycle thermal reliability.
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Liu, C.; Song, Y.; Xu, Z.; Zhao, J.; Rao, Z. Highly efficient thermal energy storage enabled by a hierarchical structured hypercrosslinked polymer/expanded graphite composite. Int. J. Heat Mass Transf. 2019, 148, 119068. [Google Scholar] [CrossRef]
- Souayfane, F.; Fardoun, F.; Biwole, P.H. Phase change materials (PCM) for cooling applications in buildings: A review. Energy Build. 2016, 129, 396–431. [Google Scholar] [CrossRef]
- Da Cunha, J.P.; Eames, P. Thermal energy storage for low and medium temperature applications using phase change materials—A review. Appl. Energy 2016, 177, 227–238. [Google Scholar] [CrossRef]
- Jamekhorshid, A.; Sadrameli, S.M.; Farid, M. A review of microencapsulation methods of phase change materials (PCMs) as a thermal energy storage (TES) medium. Renew. Sustain. Energy Rev. 2014, 31, 531–542. [Google Scholar] [CrossRef]
- Yuan, Y.; Yuan, Y.; Zhang, N.; Du, Y.; Cao, X. Preparation and thermal characterization of capric–myristic–palmitic acid/expanded graphite composite as phase change material for energy storage. Mater. Lett. 2014, 125, 154–157. [Google Scholar] [CrossRef]
- Fei, H.; Wang, L.; He, Q.; Du, W.; Gu, Q.; Pan, Y. Preparation and Properties of a Composite Phase Change Energy Storage Gypsum Board Based on Capric Acid-Paraffin/Expanded Graphite. ACS Omega 2021, 6, 6144–6152. [Google Scholar] [CrossRef]
- Liu, C.; Yuan, Y.; Zhang, N.; Cao, X.; Yang, X. A novel PCM of lauric–myristic–stearic acid/expanded graphite composite for thermal energy storage. Mate. Lett. 2014, 120, 43–46. [Google Scholar] [CrossRef]
- Horibe, A.; Jang, H.; Haruki, N.; Sano, Y.; Kanbara, H.; Takahashi, K. Melting and solidification heat transfer characteristics of phase change material in a latent heat storage vessel: Effect of perforated partition plate. Int. J. Heat Mass Transf. 2015, 82, 259–266. [Google Scholar] [CrossRef]
- Liu, F.; Zhu, J.; Liu, J.; Ma, B.; Zhou, W.; Li, R. Preparation and properties of capric-stearic acid/White Carbon Black composite for thermal storage in building envelope. Energy Build. 2018, 158, 1781–1789. [Google Scholar] [CrossRef]
- Elarem, R.; Alqahtani, T.; Mellouli, S.; Aich, W.; Ben Khedher, N.; Kolsi, L.; Jemni, A. Numerical study of an Evacuated Tube Solar Collector incorporating a Nano-PCM as a latent heat storage system. Case Stud. Therm. Eng. 2021, 24, 100859. [Google Scholar] [CrossRef]
- Aydin, D.; Casey, S.P.; Riffat, S. The latest advancements on thermochemical heat storage systems. Renew. Sustain. Energy Rev. 2015, 41, 356–367. [Google Scholar] [CrossRef]
- Sun, N.; Xiao, Z. Synthesis and Performances of Phase Change Materials Microcapsules with a Polymer/BN/TiO2 Hybrid Shell for Thermal Energy Storage. Energy Fuel 2017, 31, 10186–10195. [Google Scholar] [CrossRef]
- Nirwan, A.; Kumar, R.; Mondal, B.; Kumar, J.; Bera, A.; Kumar, R. Thermal performance assessment of lauric acid and palmitic acid based multi-transformation phase change material and exfoliated graphite composites. Energy Sources Part A Recovery Util. Environ. Eff. 2020, 9, 1–13. [Google Scholar] [CrossRef]
- Nazir, H.; Batool, M.; Ali, M.; Kannan, A.M. Fatty acids based eutectic phase change system for thermal energy storage applications. Appl. Therm. Eng. 2018, 142, 466–475. [Google Scholar] [CrossRef]
- Pielichowska, K.; Pielichowski, K. Phase change materials for thermal energy storage. Prog. Mater Sci. 2014, 65, 67–123. [Google Scholar] [CrossRef]
- Kenisarin, M.M. Thermophysical properties of some organic phase change materials for latent heat storage. A review. Sol. Energy 2014, 107, 553–575. [Google Scholar] [CrossRef]
- Zhou, D.; Yuan, J.; Zhou, Y.; Liu, Y. Preparation and Properties of Capric–Myristic Acid/Expanded Graphite Composite Phase Change Materials for Latent Heat Thermal Energy Storage. Energies 2020, 13, 2462. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A. Ternary mixture of fatty acids as phase change materials for thermal energy storage applications. Energy Rep. 2016, 2, 274–279. [Google Scholar] [CrossRef]
- Jaksic, J.; Ostojic, S.; Micic, D.; Tokic Vujosevic, Z.; Milovanovic, J.; Karkalic, R.E.; O’Connor, K.T.; Kenny, S.; Casey, W.; Nikodinovic-Runic, J.; et al. Thermal properties of 3-hydroxy fatty acids and their binary mixtures as phase change energy storage materials. Int. J. Energy Res. 2019, 44, 1294–1302. [Google Scholar] [CrossRef]
- Majó, M.; Sánchez, R.; Barcelona, P.; García, J.; Fernández, A.I.; Barreneche, C. Degradation of Fatty Acid Phase-Change Materials (PCM): New Approach for Its Characterization. Molecules 2021, 26, 982. [Google Scholar] [CrossRef]
- Jebasingh, E.B.; Arasu, V.A. Characterisation and stability analysis of eutectic fatty acid as a low cost cold energy storage phase change material. J. Energy Storage 2020, 31, 101708. [Google Scholar] [CrossRef]
- Kahwaji, S.; Johnson, M.B.; Kheirabadi, A.C.; Groulx, D.; White, M.A. Fatty acids and related phase change materials for reliable thermal energy storage at moderate temperatures. Sol. Energy Mater. Sol. Cells 2017, 167, 109–120. [Google Scholar] [CrossRef]
- Kant, K.; Shukla, A.; Sharma, A. Performance evaluation of fatty acids as phase change material for thermal energy storage. J. Energy Storage 2016, 6, 153–162. [Google Scholar] [CrossRef]
- Sarı, A. Thermal reliability test of some fatty acids as PCMs used for solar thermal latent heat storage applications. Energy Convers. Manag. 2003, 44, 2277–2287. [Google Scholar] [CrossRef]
- Sarı, A. Eutectic mixtures of some fatty acids for low temperature solar heating applications: Thermal properties and thermal reliability. Appl. Therm. Eng. 2005, 25, 2100–2107. [Google Scholar] [CrossRef]
- Sarı, A.; Hekimoğlu, G.; Tyagi, V.V. Low cost and eco-friendly wood fiber-based composite phase change material: Development, characterization and lab-scale thermoregulation performance for thermal energy storage. Energy 2020, 195, 116983. [Google Scholar] [CrossRef]
- Ke, H. Phase diagrams, eutectic mass ratios and thermal energy storage properties of multiple fatty acid eutectics as novel solid-liquid phase change materials for storage and retrieval of thermal energy. Appl. Therm. Eng. 2017, 113, 1319–1331. [Google Scholar] [CrossRef]
- Yang, L.; Cao, X.; Zhang, N.; Xiang, B.; Zhang, Z.; Qian, B. Thermal reliability of typical fatty acids as phase change materials based on 10,000 accelerated thermal cycles. Sustain. Cities Soc. 2019, 46, 101380. [Google Scholar] [CrossRef]
- Zhou, D.; Zhou, Y.; Liu, Y.; Luo, X.; Yuan, J. Preparation and Performance of Capric-Myristic Acid Binary Eutectic Mixtures for Latent Heat Thermal Energy Storages. J. Nanomater. 2019, 2019, 2094767. [Google Scholar] [CrossRef]
- Mailhé, C.; Duquesne, M.; Palomo del Barrio, E.; Azaiez, M.; Achchaq, F. Phase Diagrams of Fatty Acids as Biosourced Phase Change Materials for Thermal Energy Storage. Appl. Sci. 2019, 9, 1067. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Prediction of the properties of eutectic fatty acid phase change materials. Thermochim. Acta 2018, 660, 94–100. [Google Scholar] [CrossRef]
- Kahwaji, S.; White, M.A. Data supporting the prediction of the properties of eutectic organic phase change materials. Data Brief 2018, 17, 724–730. [Google Scholar] [CrossRef] [PubMed]
- Singh, R.P.; Kaushik, S.C.; Rakshit, D. Performance evaluation of charging process in a cascade latent heat storage system (C-LHSS) based on heat flux DSC results. Int. J. Therm. Sci. 2020, 151, 106274. [Google Scholar] [CrossRef]
- Cai, Y.; Ke, H.; Lin, L.; Fei, X.; Wei, Q.; Song, L.; Hu, Y.; Fong, H. Preparation, morphology and thermal properties of electrospun fatty acid eutectics/polyethylene terephthalate form-stable phase change ultrafine composite fibers for thermal energy storage. Energy Convers. Manag. 2012, 64, 245–255. [Google Scholar] [CrossRef]
- Yuan, Y.; Tao, W.; Cao, X.; Bai, L. Theoretic Prediction of Melting Temperature and Latent Heat for a Fatty Acid Eutectic Mixture. J. Chem. Eng. Data 2011, 56, 2889–2891. [Google Scholar] [CrossRef]
- Huang, X.; Alva, G.; Liu, L.; Fang, G. Preparation, characterization and thermal properties of fatty acid eutectics/bentonite/expanded graphite composites as novel form–stable thermal energy storage materials. Sol. Energy Mater. Sol. Cells 2017, 166, 157–166. [Google Scholar] [CrossRef]
- Zhang, Y.; Su, Y.; Ge, X. Prediction of the Melting Temperature and the Fusion Heat of (Quasi-) Eutectic PCM. J. China Univ. Sci. Technol. 1995, 25, 474–478. (In Chinese) [Google Scholar]

| Fatty Acid | Formula | Temperature of Phase Change /°C | Latent Heat of Phase Change /J·g−1 | Density /kg·(m3)−1 | Specific Heat /kJ·(kg·°C)−1 | Thermal Conductivity /W·(m·K)−1 | ||
|---|---|---|---|---|---|---|---|---|
| Solid | Liquid | Solid | Liquid | |||||
| CA | C10H20O2 | 30.1–32 | 149.1–155.5 | 1004 | 878 | 1.9 | 2.1 | 0.153 |
| LA | C12H24O2 | 42.4–44 | 174.9–186.4 | 1007 | 862 | 1.7 | 2.3 | 0.147 |
| MA | C14H28O2 | 52.2–58 | 180.5–188.6 | 990 | 861 | 1.7 | 2.4 | 0.150 |
| PA | C16H32O2 | 58.9–64 | 185.4–212.1 | 989 | 850 | 1.9 | 2.8 | 0.162 |
| SA | C18H36O2 | 68.54–70.8 | 201.8–222.8 | 965 | 848 | 1.6 | 2.2 | 0.172 |
| Binary Eutectic Mixtures | Theoretical Eutectic Mass Ratio | Tm (°C) | ΔHm (J/g) |
|---|---|---|---|
| CA–LA | 62.0/38.0 | 20.61 | 181.0 |
| CA–MA | 72.2/27.8 | 24.81 | 173.0 |
| CA–PA | 81.1/18.9 | 27.61 | 175.0 |
| CA–SA | 89.7/10.3 | 29.63 | 172.0 |
| LA–MA | 60.4/39.6 | 33.16 | 187.0 |
| LA–PA | 70.7/29.3 | 36.87 | 191.0 |
| LA–SA | 81.2/18.8 | 39.73 | 188.0 |
| MA–PA | 60.3/39.7 | 43.48 | 191.0 |
| MA–SA | 71.8/28.2 | 47.15 | 192.0 |
| PA–SA | 62.1/37.9 | 52.79 | 202.0 |
| Binary Eutectic Mixtures | Theoretical Eutectic Mass Ratio | Experimental Eutectic Mass Ratio | Absolute Error (%) | Relative Error (%) |
|---|---|---|---|---|
| CA–LA | 62.0/38.0 | 60.5/39.5 | 1.5/1.5 | 2.42/3.95 |
| CA–MA | 72.2/27.8 | 72.0/28.0 | 0.2/0.2 | 0.28/0.72 |
| CA–PA | 81.1/18.9 | 82.8/17.2 | 1.7/1.7 | 2.10/8.99 |
| CA–SA | 89.7/10.3 | 88.3/11.7 | 1.4/1.4 | 1.56/13.59 |
| LA–MA | 60.4/39.6 | 60.0/40.0 | 0.4/0.4 | 0.66/1.01 |
| LA–PA | 70.7/29.3 | 69.8/30.2 | 0.9/0.9 | 1.27/3.07 |
| LA–SA | 81.2/18.8 | 80.5/19.5 | 0.7/0.7 | 0.86/3.72 |
| MA–PA | 60.3/39.7 | 60.8/39.2 | 0.5/0.5 | 0.83/1.26 |
| MA–SA | 71.8/28.2 | 71.5/28.5 | 0.3/0.3 | 0.42/1.06 |
| PA–SA | 62.1/37.9 | 63.5/36.5 | 1.4/1.4 | 2.25/3.69 |
| Binary Eutectic Mixtures | Melting | Freezing | ||
|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tf (°C) | ΔHf (J/g) | |
| CA–LA | 17.70 | 155.2 | 18.60 | 142.2 |
| CA–MA | 19.43 | 150.9 | 18.35 | 149.2 |
| CA–PA | 22.10 | 165.6 | 20.00 | 159.7 |
| CA–SA | 23.40 | 150.5 | 23.40 | 148.0 |
| LA–MA | 36.10 | 158.9 | 32.80 | 149.7 |
| LA–PA | 37.80 | 164.0 | 34.60 | 154.8 |
| LA–SA | 39.90 | 167.1 | 35.60 | 158.3 |
| MA–PA | 44.10 | 182.2 | 40.40 | 171.9 |
| MA–SA | 44.80 | 186.9 | 43.70 | 184.4 |
| PA–SA | 57.10 | 193.0 | 53.70 | 188.6 |
| Binary Eutectic Mixtures | Experimental Value | Theoretical Value | Error | |||
|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tf (°C) | ΔHf (J/g) | Tm (°C) | ΔHm (J/g) | |
| CA–LA | 17.70 | 155.2 | 20.61 | 181.0 | 2.91 | 25.8 |
| CA–MA | 19.43 | 150.9 | 24.81 | 173.0 | 5.38 | 22.1 |
| CA–PA | 22.10 | 165.6 | 27.61 | 175.0 | 5.51 | 9.4 |
| CA–SA | 23.40 | 150.5 | 29.63 | 172.0 | 6.23 | 21.5 |
| LA–MA | 36.10 | 158.9 | 33.16 | 187.0 | −2.94 | 28.1 |
| LA–PA | 37.80 | 164.0 | 36.87 | 191.0 | −0.93 | 27.0 |
| LA–SA | 39.90 | 167.1 | 39.73 | 188.0 | −0.17 | 20.9 |
| MA–PA | 44.10 | 182.2 | 43.48 | 191.0 | −0.62 | 8.8 |
| MA–SA | 44.80 | 186.9 | 47.15 | 192.0 | 2.35 | 5.1 |
| PA–SA | 57.10 | 193.0 | 52.79 | 202.0 | −4.31 | 9.0 |
| Binary Eutectic Mixtures | Initial Weight Loss Temperature (°C) | Epitaxial Initiation Temperature (°C) | Maximum Temperature of Weight Loss Rate (°C) |
|---|---|---|---|
| CA–LA | 110.8 | 169.5 | 199.3 |
| CA–MA | 110.7 | 164.2 | 202.4 |
| CA–PA | 110.1 | 161.1 | 198.4 |
| CA–SA | 107.5 | 158.3 | 186.6 |
| LA–MA | 140.1 | 198.1 | 232.2 |
| LA–PA | 134.4 | 187.7 | 225.4 |
| LA–SA | 134.8 | 191.3 | 222.6 |
| MA–PA | 157.4 | 210.6 | 245.2 |
| MA–SA | 157.9 | 214.9 | 247.3 |
| PA–SA | 165.7 | 225.5 | 258.5 |
| Binary Eutectic Mixtures | Before Thermal Cycling | After Thermal Cycling | Difference | |||
|---|---|---|---|---|---|---|
| Tm (°C) | ΔHm (J/g) | Tm (°C) | ΔHm (J/g) | Tm (°C) | ΔHm (%) | |
| CA–LA | 17.70 | 155.2 | 17.90 | 150.2 | 0.20 | −3.2 |
| CA–MA | 19.43 | 150.9 | 20.51 | 145.3 | 1.08 | −3.7 |
| CA–PA | 22.10 | 165.6 | 21.10 | 160.3 | −1.00 | −3.2 |
| CA–SA | 23.40 | 150.5 | 24.50 | 145.3 | 1.10 | −3.5 |
| LA–MA | 36.10 | 158.9 | 35.40 | 151.4 | −0.70 | −4.7 |
| LA–PA | 37.80 | 164.0 | 35.20 | 152.3 | −2.60 | −7.1 |
| LA–SA | 39.90 | 167.1 | 38.50 | 162.3 | −1.40 | −2.9 |
| MA–PA | 44.10 | 182.2 | 44.30 | 176.2 | 0.20 | −3.3 |
| MA–SA | 44.80 | 186.9 | 43.10 | 180.2 | −1.70 | −3.6 |
| PA–SA | 57.10 | 193.0 | 55.60 | 186.5 | −1.50 | −3.4 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, D.; Xiao, S.; Xiao, X.; Liu, Y. Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage. Separations 2023, 10, 49. https://doi.org/10.3390/separations10010049
Zhou D, Xiao S, Xiao X, Liu Y. Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage. Separations. 2023; 10(1):49. https://doi.org/10.3390/separations10010049
Chicago/Turabian StyleZhou, Dongyi, Shuaizhe Xiao, Xianghua Xiao, and Yicai Liu. 2023. "Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage" Separations 10, no. 1: 49. https://doi.org/10.3390/separations10010049
APA StyleZhou, D., Xiao, S., Xiao, X., & Liu, Y. (2023). Preparation, Phase Diagrams and Characterization of Fatty Acids Binary Eutectic Mixtures for Latent Heat Thermal Energy Storage. Separations, 10(1), 49. https://doi.org/10.3390/separations10010049