Abstract
Volatile organic compounds (VOCs) produced by A. flavus strains were first captured and identified to discern between non-aflatoxigenic and toxigenic phenotypes, and more recently to help with detecting fungal infection, but not with the goal of using VOCs produced by non-aflatoxigenic strains to inhibit growth and/or production of one or more mycotoxins (e.g., aflatoxin and cyclopiazonic acid) by toxigenic aspergilli. In this study, four Aspergillus strains from Louisiana (one non-aflatoxigenic and three toxigenic) were grown on various substrates and had their headspaces captured and analyzed by solid-phase microextraction/gas chromatography/mass spectroscopy (SPME/GC/MS), to find biocontrol and biomarker compounds. Here, we present a collection of nearly 100 fungus-related VOCs, many of which were substrate dependent. Thirty-one were produced across multiple replicates and the rest were observed in a single replicate. At least three VOCs unique to non-aflatoxigenic strain LA1 can be tested for biocontrol properties (e.g., euparone, 4-nonyne), and at least four VOCs unique to toxigenic strains LA2-LA4 can be explored as biomarkers (e.g., 2-heptanone, glycocyamidine) to detect their presence while infecting crops in the field or in storage.
1. Introduction
As a genus, Aspergillus is comprised of many different species of micro-fungi that are efficient biofactories capable of producing myriad primary and secondary metabolites [1,2]. Depending on the metabolite, the impacts on health and economics can be positive or negative. For example, some of these metabolites are beneficial (e.g., citric acid from A. niger) and some are harmful (e.g., aflatoxin from A. flavus). Among the species in this genus that offer the greatest health and economic risks, the most serious is A. flavus, which is a ubiquitous filamentous fungus that has earned notoriety as a pathogen of plants (most of which are important agricultural commodities), humans, and animals (i.e., clinical aspergilloses), as well as a producer of the potent hepatocarcinogenic metabolite, aflatoxin. Metabolites expressed by these fungi can be secreted as extrolites or emitted as volatile organic compounds (VOCs) that serve one or more roles in various ecosystems, such as pathogenicity, self-preservation, and competition [3]. Regarding the genus Aspergillus, studies have explored the impact of A. versicolor metabolites on the growth of Arabidopsis thaliana as a bioindicator of indoor air quality [4]. So far, A. flavus VOCs have been captured to improve detection and species differentiation methods [5,6,7,8,9] and to distinguish between aflatoxigenic and non-aflatoxigenic strains [10,11]. However, studies of their impacts on their preferred plant hosts, or competing microbes (especially other A. flavus strains) are lacking. Consider a weakly pathogenic organism such as A. flavus surrounded by, and competing with, a highly diverse microbial community. It surely produces compounds that are meant to suppress other microbes (i.e., competition), the host (i.e., virulence), and possibly itself (i.e., self-regulation) [12,13,14]. Simultaneously, it may produce compounds that allow for self-improvement (i.e., increased fitness) or mutually beneficial relationships (i.e., symbiosis) with other microbes [12,13,14]. Uncharacterized A. flavus volatiles have been suggested to modify aflatoxin production based on neighbor proximity [15].
Previous studies have demonstrated the variability of VOC production by aflatoxigenic and non-aflatoxigenic Aspergillus strains on various substrates including potato dextrose agar (PDA) [16] and host tissue (cracked corn kernels) [10,11]. In these instances, there were shared VOCs produced by both aflatoxigenic and non-aflatoxigenic A. flavus, and some were unique to each phenotype. Their findings also showed that the production of some compounds was substrate dependent. Although the use of non-aflatoxigenic A. flavus strains as biopesticides is an increasingly popular pre-harvest strategy to mitigate aflatoxin contamination, there is still uncertainty about how they exert control over aflatoxin-producing strains in the field and cause a reduction of aflatoxin levels. Additionally, these biocontrol strains come with their own potential issues, such as meiotic recombination and production of mycotoxins other than aflatoxin [17]. One study from this lab [18] investigated whether any of five VOCs from previous studies [10,11], reportedly unique to either of the two non-aflatoxigenic A. flavus strains examined, had the ability to reduce or prevent growth and production of mycotoxins (aflatoxin and cyclopiazonic acid) in four strains from Louisiana. The non-aflatoxigenic VOCs tested against the Louisiana strains were produced by A. flavus isolates that originated from outside of Louisiana. Northern Regional Research Laboratory (NRRL) strain 5565 was isolated from turkey feed mix prior to the 1980s. It reportedly possesses mycoviruses that cause its non-aflatoxigenic phenotype [19]. NRRL 5918 was isolated from corn in Minnesota in the 1960s, and its non-aflatoxigenic phenotype was suggested to be from broken or missing aflatoxin pathway genes [20]. The four Aspergillus strains subjected to these five unique, non-aflatoxigenic VOCs were from Louisiana corn fields. One strain was non-aflatoxigenic (LA1), while the other three (LA2-LA4) were toxigenic and produced copious quantities of aflatoxin and cyclopiazonic acid (CPA). Although growth was minimally impacted, exposure of the aflatoxigenic strains to three non-aflatoxigenic VOCs from NRRLs 5565 and/or 5918 resulted in significant reductions of aflatoxin, and in two instances resulted in the complete inhibition of CPA [18].
These inhibitory compounds offer an opportunity to reap the benefits of A. flavus biocontrol while circumventing the caveats, thereby mimicking the presence of biocontrol strains without having to use the strains themselves. The ability of LA1 to produce these highly inhibitory compounds was unknown when split-plate studies pairing this strain with each of the toxigenic strains resulted in decreased aflatoxin and/or CPA production (Moore, unpublished data). Because of these observed reductions, it was necessary to assess the VOCs produced by the Louisiana strains; in particular, non-aflatoxigenic LA1, when grown on different substrates. This would allow us to determine if LA1 produced the same three highly effective VOCs as NRRL 5565 and/or 5918. Therefore, this study was the first to screen VOCs from a non-aflatoxigenic A. flavus strain specifically for potential biocontrol compounds. Additionally, it was important to also look for VOCs consistently produced by aflatoxigenic strains (LA2-LA4) that could be used for early detection of fungal infection in susceptible crops. In this study, we used SPME/GC-MS to capture and identify headspace volatiles being produced by these strains while grown individually on four different synthetic media, as well as on cracked corn kernels from each of two genotypes that were either resistant or susceptible to A. flavus.
2. Materials and Methods
2.1. Experimental Conditions
The four Aspergillus strains utilized for this study (LA1-LA4) have been described previously [18,21,22]. Briefly, LA1 is a non-aflatoxigenic and CPA-negative A. flavus strain, exhibiting a mixed morphotype of abundant large (L) and small (S) sclerotia and conidia. It has also been reported as a producer of putative extrolites that reduce aflatoxin and CPA production [23]. LA2 is a highly toxigenic A. flavus strain that exhibits a non-sclerotial morphotype and produces abundant conidia. On yeast extract sucrose (YES) medium, LA2 to produces B aflatoxins at concentrations of around 21,000 ppb and CPA around 1200 ppm [22]. LA3 is also a highly toxigenic A. flavus strain (~54,000 ppb for B aflatoxins and ~16,000 ppm for CPA on YES) that exhibits an abundance of S (small) sclerotia and limited production of conidia. LA4 on YES is a producer of B (~167,000 ppb) and G (~109,000 ppb) aflatoxins, as well as CPA (~5700 ppm), that has been recently reported to share a most recent common ancestor with A. novoparasiticus [18]. LA4 consistently exhibits a non-sclerotial phenotype and produces abundant conidia. Spore suspensions were made for each isolate by growing them on PDA and collecting conidia from 7 d old cultures. Spores were suspended in 1 mL of 0.001% Triton X detergent at a concentration of 1 × 106 spores/mL as used in previous studies involving these strains [18,22,23].
The synthetic media used for this study included corn meal agar (CMA), Czapek’s (CZ) medium, mixed grain agar (MGA), and yeast extract sucrose (YES) medium. For individual assessments on synthetic media, approximately 8 mL of each medium, while in liquid form, was individually pipetted into a 10 mL screw top SPME vial (Restek, Bellefonte, PA, USA) and allowed to solidify at an approximate 45-degree angle on a metal rod in the biosafety cabinet, resulting in a slant for each fungal isolate to colonize. Ten microliters of each fungal isolate’s spore suspension (approximately 10,000 conidia) were inoculated onto an agar slant and the vials were loosely capped with aluminum foil for an incubation period of 1 week at 30 °C [10,11] before being transported to the GC-MS lab for headspace capture. There were three replicate vials prepared for each individual assessment, including blanks which were uninoculated media. The cracked kernel headspace experiments involved an aflatoxin-resistant corn line (MI82; M) and an aflatoxin-susceptible corn line (VA35; V). The corn kernels of both varieties were surface sterilized with a 3 min soak in 5% bleach solution, followed by three washes with sterile water, and then allowed to dry on paper towels in a biosafety cabinet overnight. Approximately three kernels of one corn line were cracked and placed into each vial, which was wet with approximately 5 mL of sterilized water and then inoculated with 10 µL of an individual spore suspension. Blanks for each corn type were also prepared. As with the synthetic media, these vials were capped with foil and incubated at 30 °C, and there were three replicate vials prepared for each assessment. The time points to capture headspace VOCs for the corn involved additional time points: first, 48 h after inoculation (M0 and V0), then after one week of incubation (M1 and V1), and once more at two weeks of incubation (M2 and V2). The 48 h time point was chosen because it takes about 24–48 h for Aspergillus flavus conidiospores to germinate. One week at 30 °C is the typical incubation time for A. flavus studies, hence our use of it for both synthetic and corn-based substrates. The two-week time point was added just to see how much the profile might change as the fungal culture aged.
2.2. Fungal Volatile Procedure
Within minutes of halting incubation, the aluminum foil was replaced with screwcaps containing PTFE-lined silicone septa (Restek, Bellefonte, PA, USA), and volatile organic compounds were analyzed by solid-phase microextraction/gas chromatography/mass spectroscopy (SPME/GC/MS) using a 6890 GC coupled to a 5973 MS controlled by Chemstation (Agilent Technologies, Inc., Santa Clara, CA, USA) and equipped with an MPS2 autosampler (Gerstel, Linthicum, MD, USA). Vials were held at 4 °C in a cooled sample tray until analysis to halt fungal metabolism. Each vial was incubated for 15 min at 65 °C and agitated at 750 rpm with 10 sec on and 1 sec off to move the volatiles into the headspace and increase turbulence. The 1 cm divinylbenzene/carboxen/polydimethylsiloxane (DVB/CAR/PDMS) SPME fiber (MilliporeSigma, Burlington, MA, USA) was then inserted and exposed to the headspace for a 15 min extraction period. The fiber was desorbed for 1 min at 270 °C in the injection port. The column was an Agilent DB-5UI (5% phenyl PDMS, crosslinked) 30 m long, 0.25 mm ID, 0.1 µm film thickness (Agilent Technologies, Inc., Santa Clara, CA, USA). The inlet was operated in pulsed splitless mode, with 50 psi pressure maintained for 1 min. At 1 min, the flow was set at 36 cm/sec and the split valve opened. The oven was set to 35 °C for 1 min, raised to 100 °C at 5°/min, then to 200 °C at 10°/min, then to 270 °C at 25°/min, and held for 3 min. The column was interfaced with the MS through a 280 °C transfer line. The MS was operated in full scan mode, with a threshold of 150, a sampling rate of 2, and a scan range of 33–400 amu resulting in 3.92 scans/sec. In between the injections the fiber was baked at 270 °C in flowing N2 for 8 min to prevent carryover. Data were searched using the Wiley 7th/NIST02 library (Palisade Corporation, Ithaca, NY, USA).
VOCs found in the headspace of a particular fungal strain and medium were excluded if they were also detected in the blank headspace as they might not be purely fungal in origin. VOCs were considered legitimately fungal if they (1) were not detected in the blank, and (2) had peak area over 10 million plus a match quality over 70. Headspace compounds with no CAS number for authentication were also excluded. When pure compounds were available, co-eluted VOCs that had the same match quality, two different VOCs that shared the same retention time, or VOCs with a peak area over 100 million (but poor match quality) were authenticated using readily available standards. Fungal VOC profiles for each strain and substrate were illustrated as percentages of total peak area in pie charts using Microsoft Excel. Any statistical analysis conducted for this study involved GraphPad Prism 9.1.0 (GraphPad Software, Boston, MA, USA).
3. Results
3.1. Headspace Profiles on Synthetic Media
There was a multitude of VOCs found in the headspace profiles of our four Louisiana Aspergillus strains as they grew on synthetic media. Compounds captured in the blank headspaces are listed in Table S1. Table 1 lists the VOCs considered legitimately fungal, both shared and unique, for more than one replicate of each strain on each synthetic medium. Some singleton VOCs, only detected in one replicate vial, were recorded because they had peak areas over 10 million and match qualities of at least 70 (Table S2). These singletons were grouped in all figures as “Others”. The VOCs inherently present in YES blanks, and observed during fungal growth, included benzaldehyde, benzeneacetaldehyde, hexadecanoic acid, and tetradecanoic acid. Benzaldehyde and hexadecanoic acid were also detected in the headspaces of blanks and fungal strains with MGA and CZ media. The CMA headspaces for both blanks and fungi included hexadecenoic acid and nonanal. The VOCs also present in the blanks were not included as percentages contributing to the total headspace profiles shown in the figures.

Table 1.
Headspace VOC profiles for multiple Aspergillus strains while growing on different synthetic media for one week.
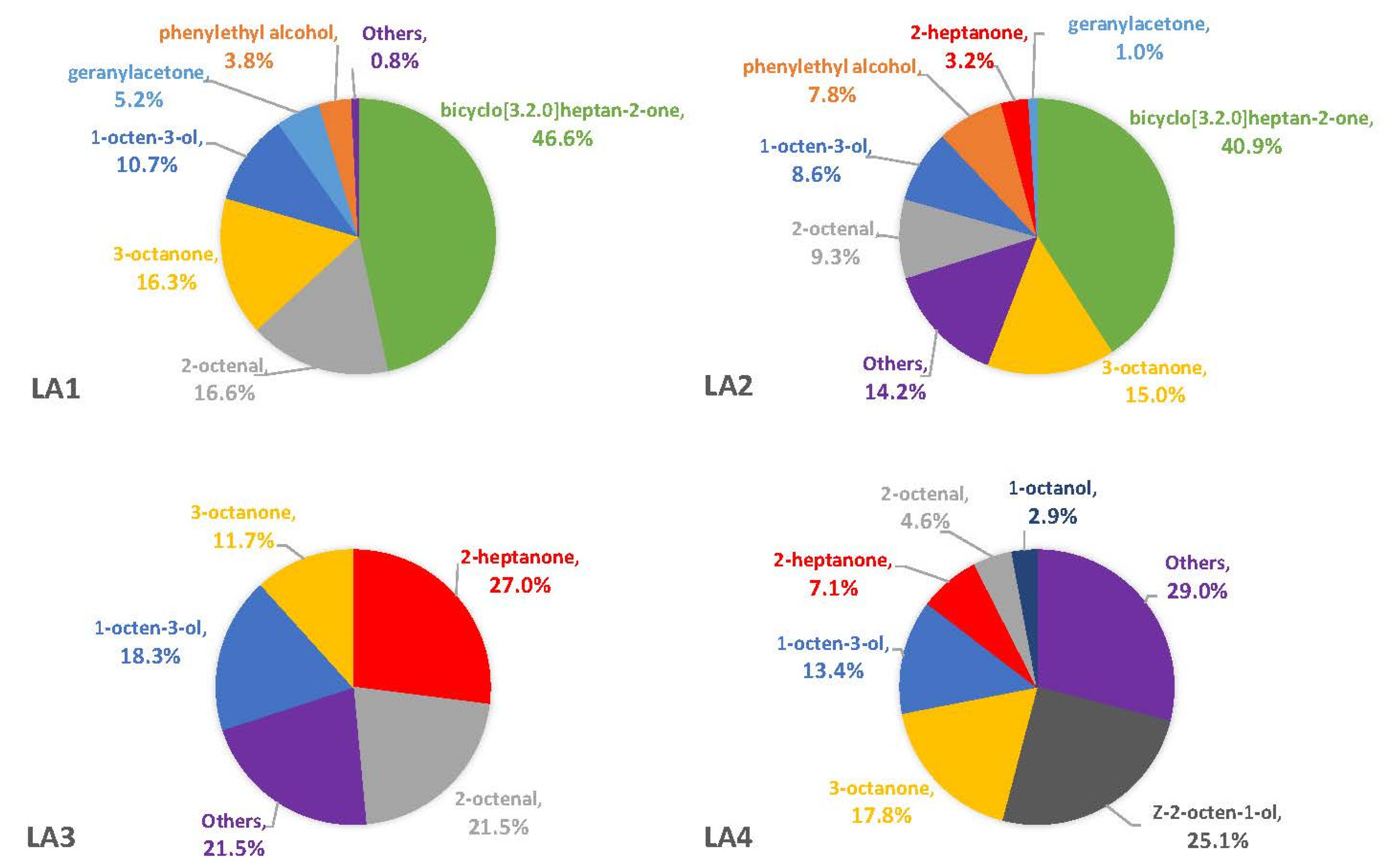
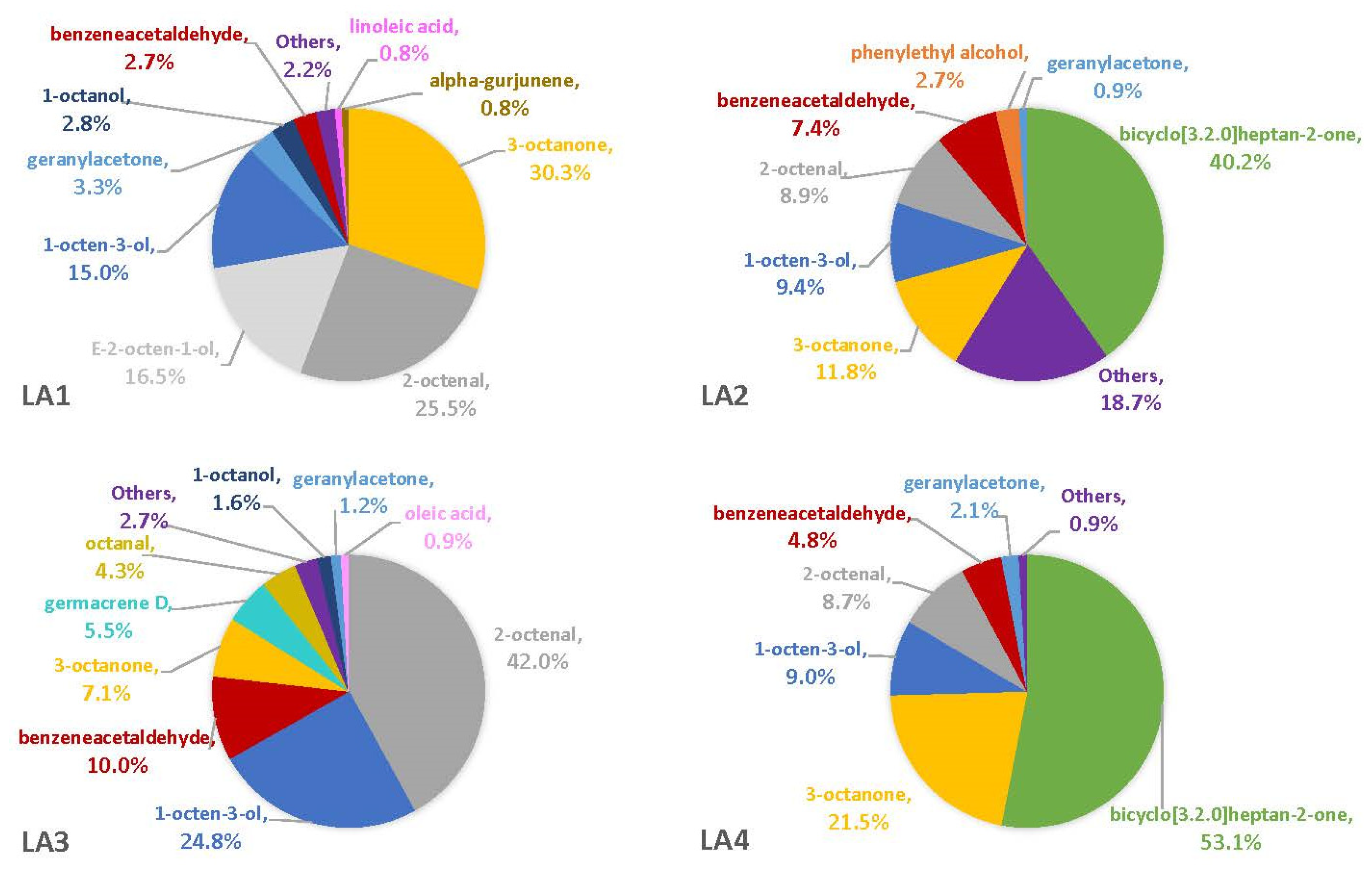
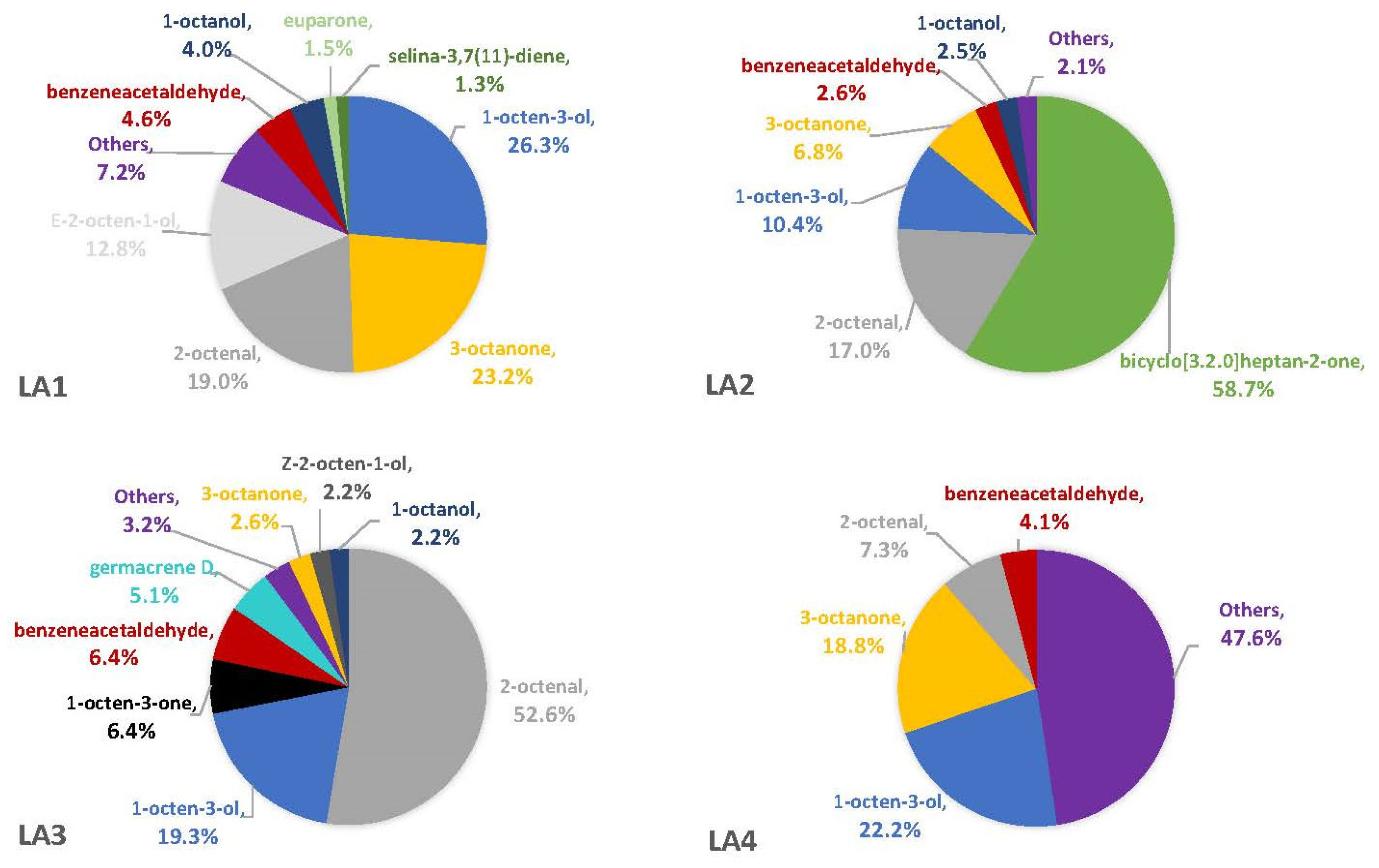
The percentages of VOCs given throughout the Results and Discussion sections are based solely on the VOCs that were not also observed in the blanks. This is because there is no certainty that the fungus was producing the compound on a substrate if it was also observed in the associated blank. On three of the four synthetic media, the fungal VOCs consistently produced by all strains were 1-octen-3-ol, 3-octanone, and 2-octenal. These compounds comprised 33–52% of the headspaces for each of the four fungal strains on YES (Figure 1), 29–74% on MGA (Figure 2), and 34–75% on CZ (Figure 3). On CMA, 1-octen-3-ol was produced only by LA2 (Figure 4). The one compound (out of the three) produced by LA1 at the highest concentration alternated based on the growth medium. For example, this strain produced 2-octenal at the highest concentration on YES, 3-octanone highest on MGA, and 1-octen-3-ol highest on CZ. These three compounds were not produced by LA1 on CMA. 3-octanone was produced at the highest concentrations for LA2 and LA4 on both YES and MGA, while half of the LA2 profile on CMA is 1-octen-3-ol. LA3 was consistent in the production of its dominant VOC, 2-octenal, across YES, MGA, and CZ media, but not with CMA. One other VOC, bicyclo[3.2.0]heptan-2-one, was found to contribute greatly to the headspace profiles of multiple strains. It contributed around 40% to each of the LA1 and LA2 headspaces on YES, around 50% to the LA2 and LA4 headspaces on MGA, and its production on CZ was nearly 68% for LA2. It was detected as a singleton (found only with one replicate) in some instances but never with CMA. For example, it was the only singleton VOC for LA4 on YES, contributing to 29% of the headspace profile based mainly on its large peak area (354,948,052). Strain LA3 did not produce this compound on any synthetic medium. Fungal growth on CMA resulted in the lowest number of detectable fungal VOCs. LA2’s headspace on this medium included only four VOCs that could be considered as putatively fungal (not also observed with the blanks), while LA1 (n = 3), LA3 (n = 0) and LA4 (n = 1) had even fewer VOCs present. Two-way ANOVA analysis was conducted for these top four VOCs, assessing the significance of differences for mean peak areas of each VOC across all four strains (Figure S1). Statistical significance was only found with YES (based on VOC and LA strain; p ≤ 0.0067) and CZ (based solely on LA strain; p = 0.0033). No significance was found with the MGA medium, and no comparisons could be made with CMA.
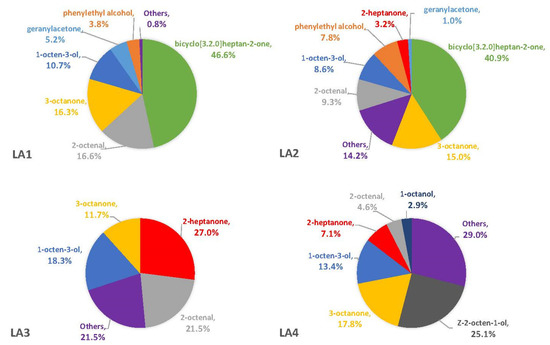
Figure 1.
Average percentages (over three replicates) of VOCs contributing to total headspace profile of each Aspergillus strain while growing on YES medium.
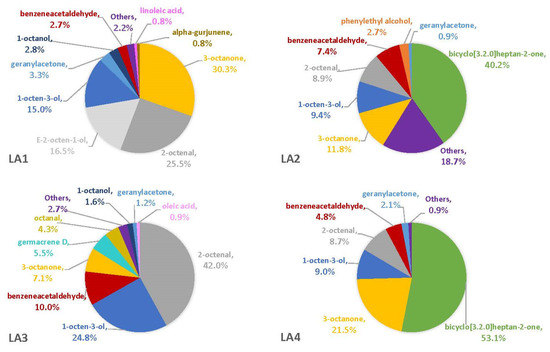
Figure 2.
Average percentages (over three replicates) of VOCs contributing to total headspace profile of each Aspergillus strain while growing on MGA medium.
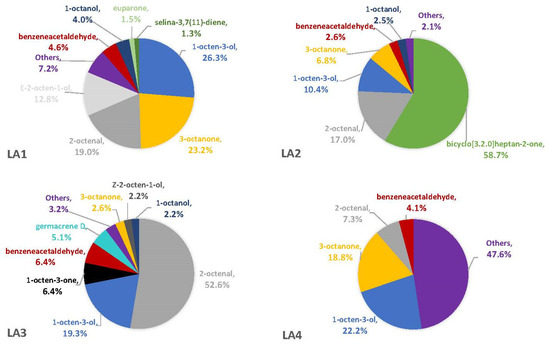
Figure 3.
Average percentages (over three replicates) of VOCs contributing to total headspace profile of each Aspergillus strain while growing on CZ medium.
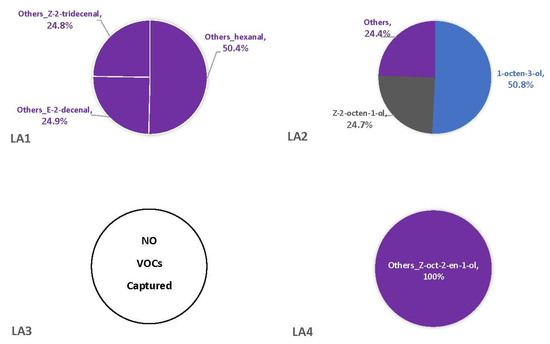
Figure 4.
Average percentages (over three replicates) of VOCs contributing to total headspace profile of each Aspergillus strain while growing on CMA medium.
Regarding total VOCs present on any given medium, LA2 produced the most while growing on YES (n = 11), MGA (n = 15), and CMA (n = 4). LA1 and LA3 were comparable in their total VOC production on both YES, MGA, and CZ, but on CMA there were no VOCs detected with LA3. LA4 produced a maximum of nine total VOCs on CZ. There were several other VOCs that were produced by all four fungal strains, and they were substrate dependent. Benzeneacetaldehyde was produced by all strains on MGA and CZ. The compound geranylacetone was produced by each strain at low quantities (<4%) on MGA. A VOC known as 2-heptanone was produced solely by the three aflatoxigenic strains (LA2-LA4), but it was only detected with YES as the substrate. Another VOC that was only captured with the aflatoxigenic strains was cis-2-octen-1-ol. This compound was observed with every medium but was not consistently produced on a particular medium by every aflatoxigenic strain. The numbers of wholly unique VOCs (Table 1) also varied and were substrate dependent, with MGA resulting in the greatest number of unique VOCs to be produced by any fungal strain (n = 5). CZ medium was the only other substrate associated with a unique compound. There were four unique VOCs present with LA1 growth on MGA and CZ, and three were present in the LA3 headspace on MGA and CZ. There were no unique VOCs associated with LA2 or LA4 on synthetic media.
Supplemental Figures S2–S6 display the headspace profiles in a different way, showing the impacts of the four synthetic media on each strain to indicate changes based on the environment. For LA1 (Figures S2 and S3), there was much overlap in VOCs being produced for YES, MGA, and CZ media. A consistent increase in the numbers of singleton VOCs produced for each respective medium was observed, with CMA resulting in 100% of LA1’s profile including only three singleton compounds that were not observed with any other medium. A similar amount of overlap was evident for LA2 profiles (Figure S4). Despite the different nutrients provided by the YES, MGA, and CZ media, LA2 produced a large amount of bicyclo[3.2.0]heptan-2-one on each medium. This VOC was not produced by LA2 on CMA, however, for which the profile was greatly diminished. The LA3 profile changed the most with each substrate (Figure S5), whereby no VOCs could be captured on CMA, a moderate number was observed with YES, and both the MGA and CZ media promoted highly diverse profiles. What was most apparent was the consistently high production of 1-octen-3-ol and 2-octenal on YES, MGA, and CZ media. LA4 was very much affected by substrate, having large portions of each profile comprised of singleton VOCs (Figure S6). More than half of its profile on MGA consisted of bicyclo[3.2.0]heptan-2-one. Not evident in the figures, this compound was also produced at relatively high abundance with YES and CZ media, although as singleton VOCs with large peak areas (Table S2). With CMA, the only fungus-related VOC captured was cis-2-octen-1-ol, which was produced with multiple replicates of this strain on YES and as a singleton with very large peak area on CZ.
3.2. Headspace Profiles on Susceptible and Resistant Corn Genotypes
The headspace profiles of each fungal strain captured while growing on cracked corn kernels of either the susceptible (VA35) or resistant (MI82) corn genotypes were vastly different from those associated with synthetic media. Table 2 lists the shared and unique fungal VOC profiles considered putatively fungal (not also observed with the blanks) at each time point, and their blank VOC profiles are listed in Table S3. The VOCs inherently present in VA35 blanks, and observed during all fungal growth, after 48 h (V0) included hexanal and 2-pentylfuran. The additional production of 2,3-butanediol and nonanal were detected with blanks at this same time point. After one week (V1), none of these compounds were detected with VA35 blanks. Instead, 2-methylbutylamine and 2-nonanone were captured as blank singletons, while 2-pentylfuran and hexanal were captured also with fungal strains. At two weeks (V2), the following compounds were detected in multiple replicates of the blanks: 2-pentylfuran, 2,4-pentadienal, and 5-isopropyl-2,3-dimethylpyrazine. The VOCs associated with multiple replicates of MI82 blanks and fungi after 48 h (M0) included 2-pentylfuran, 2,4-pentadienal, and hexanal. At the one-week time point (M1), the VOCs detected in both blanks and fungal headspaces included 2-pentylfuran and 2,4-pentadienal. 2,4-pentadienal, hexanal, and benzeneacetaldehyde were captured after two weeks of incubation (M2).

Table 2.
Headspace VOC profiles for multiple Aspergillus strains while growing on two different corn varieties over two weeks.
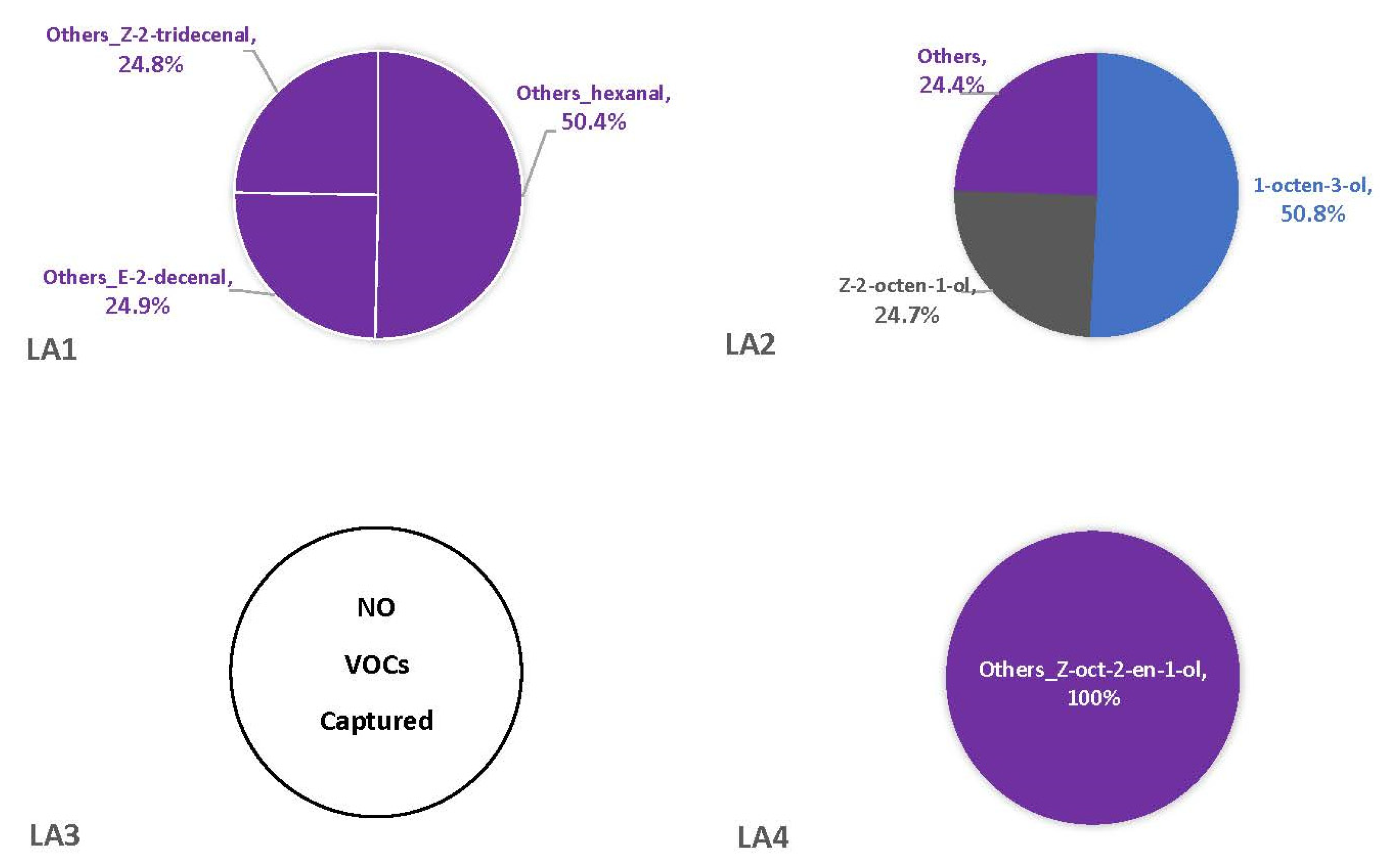
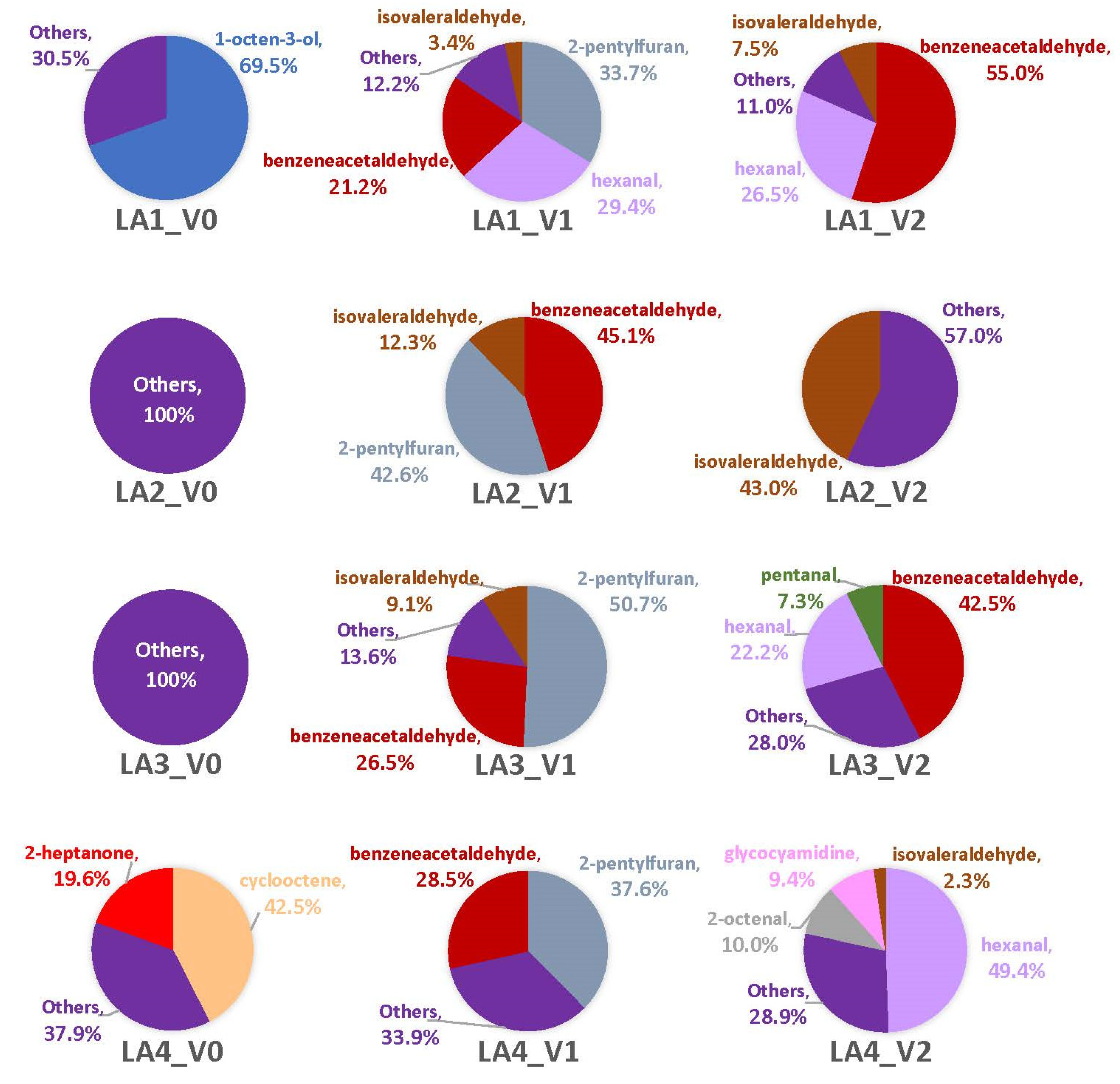
There were three shared compounds captured in the headspaces of all four strains across multiple replicates: isovaleraldehyde, 2-pentylfuran, and benzeneacetaldehyde (Table 2). However, only 2-pentylfuran and benzeneacetaldehyde were produced by all four strains on one corn type and time point (V1). Isovaleraldehyde was produced on both corn types, but inconsistently observed across strains and time points. Unlike with synthetic media, fewer fungal VOCs were observed in the headspaces of two or more replicates when grown on either of the corn types (Table 2). Most were singleton VOCs only detected in one replicate vial (Tables S4 and S5) resulting in about half of headspace profiles being dominated by these “Others”. Comparatively, there were fewer instances of singleton VOCs dominating the headspaces of LA1-LA4 on VA35 (Figure 5) than on MI82 (Figure 6). Overall, the V1 and V2 time points had 22 VOCs present across all strains, while time point V0 had 15. Among the strains, LA1 had 19 VOCs across all time points, with the others having 18 (LA4), 15 (LA3), and 7 (LA2). Some of the VOCs associated with each strain were singletons, at times comprising the majority (or the entirety) of the strain’s headspace profile. For example, the headspaces of LA2 and LA3 were comprised solely (100%) of singleton VOCs, which were identified and listed in Table S4. Additionally, some of these singleton VOCs were unique.
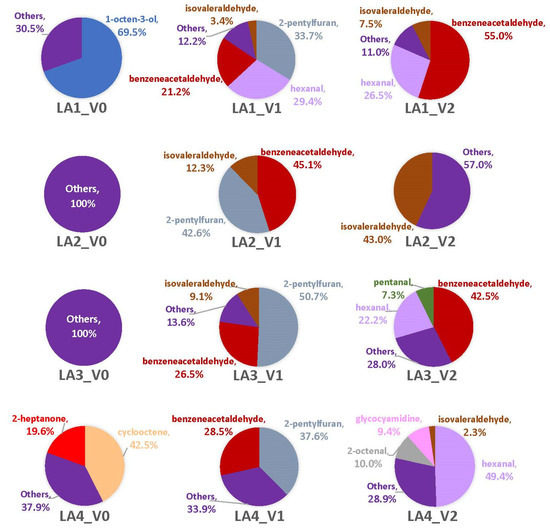
Figure 5.
Average percentages (over three replicates) of VOCs contributing to total headspace profile of each Aspergillus strain while growing on cracked kernels of VA35, an A. flavus-susceptible variety of corn, at each of three sampling time points (48 h, one week, and two weeks).
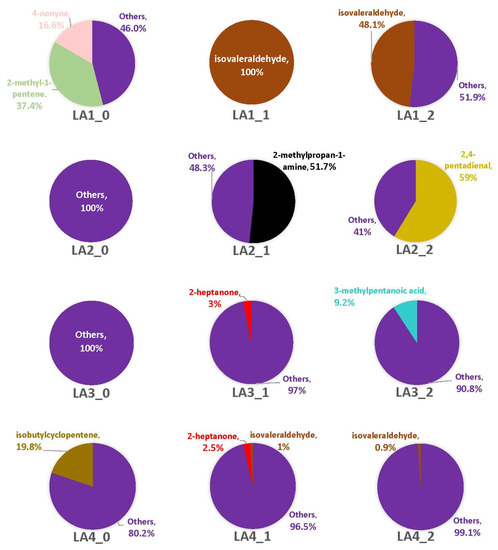
Figure 6.
Average percentages (over three replicates) of VOCs contributing to total headspace profile of each Aspergillus strain while growing on cracked kernels of MI82, an A. flavus-resistant variety of corn, at each of three time points (48 h, one week, and two weeks).
The combination of 2-pentylfuran and benzeneacetaldehyde contributed the most to each strain’s headspace (by a range of 56%-87%) at V1, with the presence of other compounds like isovaleraldehyde (LA1-LA3) in the headspace, as well as singleton VOCs (Figure 6; Table 2 and Table S4). By V2, benzeneacetaldehyde was a major component of the headspaces of LA1 (55%) and LA3 (42.5%) but was absent from LA2 and LA4. Isovaleraldehyde was 43% of the LA2 headspace (most of its headspace was comprised of two singleton VOCs), while hexanal comprised nearly half of LA4’s (49.6%). LA4 had the highest number of VOCs present at V2 with nine; of which, five were singletons and three of those were unique to LA4. Each of the other strains at this time point had two singleton VOCs contributing to their headspace profiles. Only V1 had a consistency of the top three VOCs being produced across most or all strains. Two-way ANOVA of time point V1 revealed statistical significance only for differences among the means of the VOCs (p = 0.0016; Figure S7).
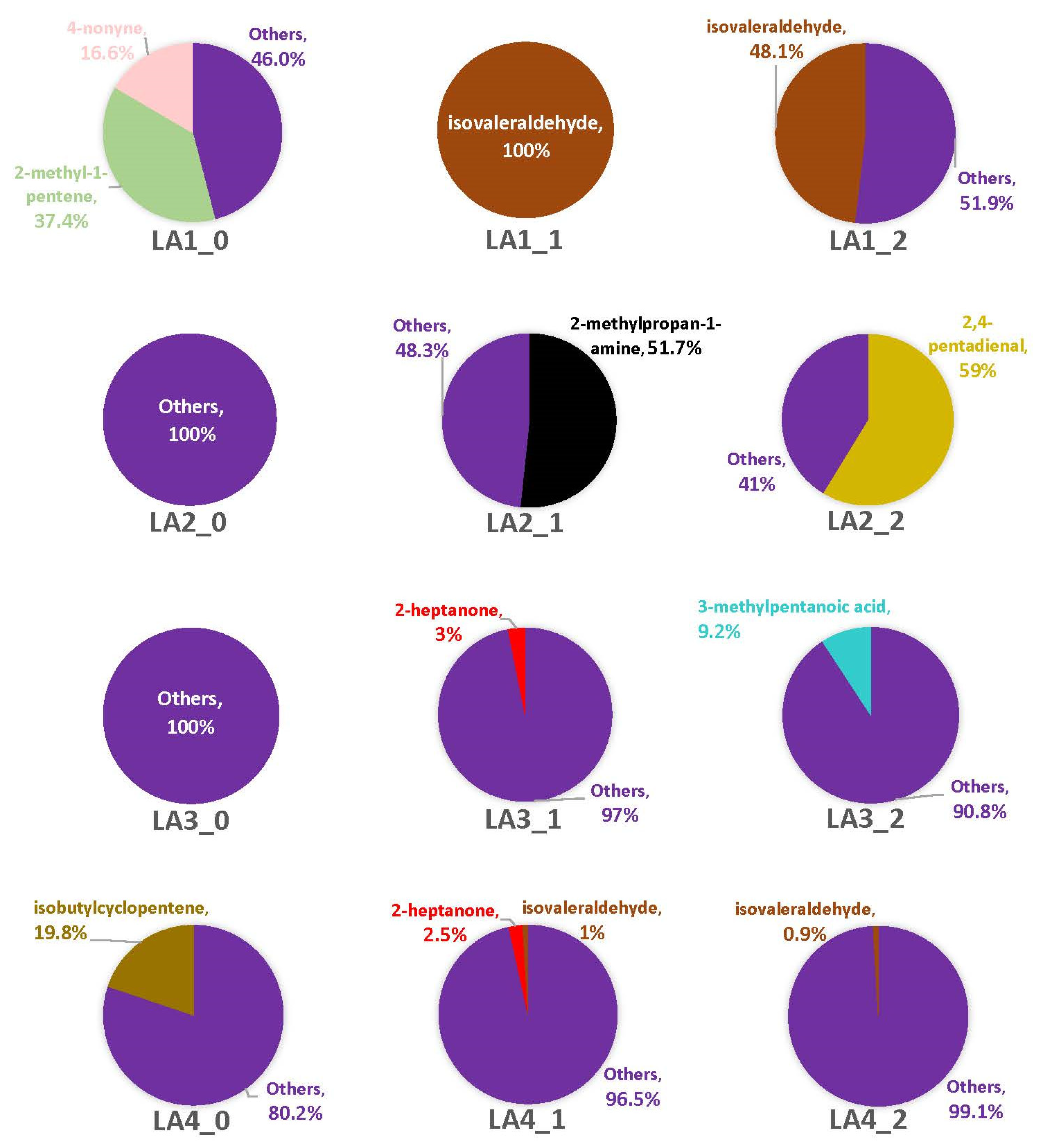
Overall, the M0 time point included 11 VOCs across all strains, while M1 had 25 VOCs and M2 had 27. Among the strains, LA2 had seven VOCs across all time points, with the others having nine (LA1), 19 (LA3), and 28 (LA4). There were far more headspace profiles dominated by singleton VOCs with MI82 than observed with VA35 (Figure 6; Table 2 and Table S5). However, at least one strain produced at least one VOC across multiple replicates for at least one time point. For example, a little more than half of the LA1 headspace at M0 contained 2-methyl-1-pentene and 4-nonyne, and the remaining profile included two singleton VOCs that were both unique. Both LA2 and LA3 were again comprised solely of singleton VOCs. Unlike with VA35, however, on MI82 there was only one fungal VOC present in each strain’s headspace. About 20% of LA4 headspace was isobutylcyclopentene at M0. The remaining 80% was comprised of four singleton VOCs (Table S5).
With the M1 time point, LA1’s headspace across all replicates was completely comprised of isovaleraldehyde, with no singleton VOCs present. The headspace of LA2 did contain a majority (nearly 52%) of 2-methylpropan-1-amine, as well as two singleton VOCs (Table S5). Three percent of LA3’s headspace included 2-heptanone, while the rest of the headspace was comprised of seven singleton VOCs. LA4 had the highest number of VOCs for this time point (n = 13), of which a small percentage (3%) of its headspace included 2-heptanone and isovaleraldehyde across multiple replicates. The remaining profile VOCs were singletons. At M2, about 48% of the LA1 headspace was from isovaleraldehyde and the rest of the headspace was comprised of three singletons. 2,4-pentadienal comprised 59% of LA2’s overall headspace, with two singleton VOCs. Most of the LA3 headspace at M2 was comprised of singleton VOCs, but one compound, 3-methylpentanoic acid, was observed across multiple replicates. Of the nine singleton VOCs captured in its headspace, five were unique to LA3. LA4 had less than 1% of its headspace profile comprised of isovaleraldehyde, while singletons comprised the remaining 99%. Four of the nine singleton VOCs were unique to LA4. The profiles of all sampling time points with this corn genotype were too diverse to make any statistical correlations.
Supplemental Figure S8 displays the headspace profiles of LA1 for both corn types, showing the impacts of each genotype across the three sampling time points. The headspace profiles for LA1 were vastly different based solely on the corn genotype being colonized. With VA35, there was a shift in the dominant VOC(s) and a decrease in the number singletons captured (Table S4). Profiles associated with MI82 showed about half of LA1’s headspace comprised of singleton VOCs (Table S5) for the first and last time points, two at M0 and three at M2, but the M1 headspace being only isovaleraldehyde. When comparing the profiles of each toxigenic strain by corn genotype (Figure S9), it was obvious that there were many instances of singleton VOCs (Tables S4 and S5) dominating their headspaces. Additionally, each toxigenic strain produced more singleton VOCs on the MI82 genotype. The only instance when the LA2 headspace lacked singleton VOCs was at V1, and the two dominant VOCs (2-pentylfuran and benzeneacetaldehyde) were not produced at any other time point with VA35 or with MI82. Every profile captured for LA3 had singleton VOCs, and the fewest were captured with VA35 at the latter two time points (V1 and V2). About a third of the LA4 profiles with VA35 were singleton compounds (Table S4), and over 80% of its MI82 headspace, at every time point, was comprised of singleton VOCs (Table S5).
4. Discussion
4.1. Differences in Synthetic Media May Explain Observed Diversity of A. flavus VOCs
Although there were many VOCs shared among the headspaces of our examined fungal strains, different strains of A. flavus (and even non-flavus strains) can have VOC profiles as variable as the substrates on which they grow. Each of the synthetic media used was distinct in the nutritive properties offered to the fungi. The use of MGA as a substrate resulted in the production of 30 headspace VOCs (14 of them unique for this study), CZ resulted in the production of 23 (7 of them unique), YES resulted in the production of 17 (3 of them unique) and CMA only 8 (3 of them unique). MGA contains multi-grain cereal with a mixture of wheat, oat, rice, and barley. These are four different grains that could each facilitate a different headspace profile associated with A. flavus infection, and they were all combined into a single substrate. The CZ medium contained a mixture of many different micronutrients, so perhaps the relatively high number of fungal VOCs produced with this substrate related to the chemical diversity present within the medium. The YES medium contained yeast extract, which includes the components of yeast cells (without the cell walls). It is unknown if the profile diversity associated with YES resulted solely from the nutrients provided by yeast cell contents. CMA, containing corn nutrients from the inclusion of store-bought corn meal, contributed the least to the headspace profiles of our fungi.
Our finding that two of the top four VOCs captured on synthetic media (1-octen-3-ol and 3-octanone) supports a previous report about their dominance in the headspace profiles of several fungal genera, including Aspergillus, Penicillium, and Fusarium [24]. Reports suggest these compounds act as attractants to some insects while also deterring others, which could benefit A. flavus [25]. A literature search offered other possible roles for these compounds in A. flavus. One study identified 1-octen-3-ol as a self-inhibiting compound in filamentous fungi that is produced, when the niche is approaching an overabundance of conidiospores, to halt their germination [26]. It also has been shown to repel the corn pest Sitophilus zeamais, as well as inhibit growth and fumonisin B1 production by Fusarium verticillioides [24]. The production of 3-octanone has been linked with fungal growth and sporulation for Aspergillus and Penicillium [27]. However, despite the colonization of corn kernels by all four strains, 3-octanone was not readily found in our corn-based headspaces. This could be based on the timing of headspace sampling. In previous studies from this lab, 3-octanone has shown itself to be an effective inhibitor of aflatoxin and CPA production but not of fungal growth [18]. The other two dominant VOCs captured with synthetic media in this study were 2-octenal and bicyclo[3.2.0]heptan-2-one. A 2020 study showed that 2-octenal offered anti-growth activity against several plant pathogenic fungi [28]. Bicyclo[3.2.0]heptan-2-one has been reported to have antimicrobial properties, specifically against pathogenic bacteria [29]. Despite the overall contribution of this VOC to headspace profiles on synthetic media, it was never captured in the headspace of LA3. The most obvious difference between LA3 and the other strains examined is that LA3 produced very few conidia, so perhaps bicyclo[3.2.0]heptan-2-one production correlates with conidium production. Further research is necessary to confirm or refute this. Based on their associated properties, all four of the dominant VOCs benefit A. flavus, either in its self-regulation or its competitiveness. Future studies will use the same experimental protocol and parameters from this study to capture and compare the VOCs observed here to those produced on the same synthetic media with each of four Aspergillus strains (one non-aflatoxigenic and three aflatoxigenic) from Arizona, Georgia, and Mississippi.
4.2. VOCs Associated with Corn Genotypes May Not Be Solely from A. flavus
In comparing the headspace profiles of A. flavus-susceptible (VA35) and -resistant (MI82) corn genotypes, we observed greater profile diversity associated with MI82. Additionally, both corn types offered greater headspace diversity than any of the synthetic media. Including singletons, 41 were captured while the fungal strains grew on the resistant genotype compared to the 34 captured on the susceptible genotype. Twenty-eight of the MI82 VOCs, and seventeen of the VA35 VOCs, were unique to each corn genotype. Given that MI82 is supposed to be resistant to A. flavus fungi, one might expect less growth of the fungus and, subsequently, fewer fungal VOCs being emitted. It is possible that the fungus employs a greater number of VOCs to attempt survival and successful colonization of these resistant varieties of corn. Alternatively, the corn-based VOCs may not be solely fungal in origin, being produced/released by the host tissue as it is being colonized by fungi, supporting a greater number of plant-based volatiles from fungal infection of the MI82-resistant genotype. VOCs emitted from uninfected corn kernels had already been assessed with our blanks, so any VOCs from fungal infection (and not captured in the blanks) could be either fungal or plant+fungal. The lack of reproducibility across replicates that resulted in the capture of so many singleton VOCs may indicate they require additional scrutiny. The inclusion of additional replicates could also help with this.
The four dominant, fungal-related VOCs captured with synthetic media were not among the dominant VOCs captured with the infected-corn headspaces. Additionally, the top VOCs for each corn variety were not consistently observed, varying with each time point and fungal strain. Only the one-week time point for VA35 offered consistency of VOCs captured across all the strains, which included 2-pentylfuran and benzeneacetaldehyde. Reportedly, 2-pentylfuran has plant growth-promoting potential as evidenced through studies involving its production by the bacterium Bacillus megaterium [30]; however, it is unknown if this bacterium existed within our surface-sterilized VA35 corn samples. In relation to plants, 2-pentylfuran has been reported to be produced by soybean plants with antifungal activity against A. flavus [31]. It also has been associated with multiple species of Aspergillus as a biomarker for the detection of A. flavus infection of corn [9], aspergillosis infection in humans [32], as well as for assessing the presence of Aspergillus in building materials [6]. A 2008 study associated this compound with non-aflatoxigenic A. flavus [33], which is contradictory to this study since 2-pentylfuran was present in the headspaces of all four strains. It must be stated that 2-pentylfuran was present in the MI82 headspaces at all three time points, as well as VA35 blank headspaces at time points V0 and V2, but not at V1. Therefore, its detection in V1 headspaces for all four strains may indicate it was not produced solely by fungi. Benzeneacetaldehyde has been reported in the volatile profiles of Chinese teas [34], however, whether the volatile is inherently produced by the tea plant or the myriad fermentation-related microbes in the brick teas, is unclear. Aspergillus fungi do colonize the tea bricks during the fermentation of Chinese teas such as Fu Brick and Pu’er [35]. Benzeneacetaldehyde also has been reported to contribute to the odor and flavor profile of koji fermentation, which involves Aspergillus oryzae [36]. Benzeneacetaldehyde was present in the headspaces of MI82 blanks at all time points; however, it was only detected as a singleton VOC in the VA35 blank headspace at V2. This suggests it may be of fungal origin from the strains growing on VA35. Other compounds present with multiple replicates, strains, and/or time points on VA35 included hexanal (LA1, LA3, LA4) and isovaleraldehyde (LA1-LA4). Hexanal has reported antimicrobial properties [37], even negatively impacting A. flavus at the cellular and metabolic levels [38]. If A. flavus strains are inherently able to produce this compound, then there is support for its purpose in this fungus to be for self-regulation and/or competitiveness against other microbes. The literature relating isovaleraldehyde with fungi is limited. A study from 1981 suggested that this compound is an attractant to zoospores of Phytophthora palmivora [39], so its purpose in A. flavus is unclear. For MI82, the VOCs isovaleraldehyde (LA1 and LA4) and 2-heptanone (LA3 and LA4) were present with multiple replicates, strains, and/or time points. Some bacterial species, as well as multiple filamentous fungi, especially those from the Aspergillus and Penicillium genera, produce 2-heptanone, which has proven antibacterial, antifungal, and anti-nematode activity [40,41]. Therefore, its purpose in A. flavus remains to be determined. Future studies will use the same experimental protocol and parameters from this study to capture and compare headspace VOCs from these resistant and susceptible corn kernels when infected with each of four Aspergillus strains (one non-aflatoxigenic and three aflatoxigenic) from Arizona, Georgia, and Mississippi. As well, headspace VOCs will be captured and compared involving other corn genotypes (resistant and susceptible) that have been infected with an aflatoxigenic A. flavus strain via pin-bar inoculation of cobs as they mature on plants in the greenhouse.
4.3. LA1 Produces VOCs That Could Be Tested for Biocontrol Properties
Uncharacterized extrolites [23] and VOCs (unpublished data) from LA1 have been shown to reduce aflatoxin and CPA levels in vitro. However, LA1 would not make a good biocontrol strain because it produces the tremorgenic mycotoxin aflatrem in concentrations of the tens-of-thousands ppb [22]. Feeding studies have shown that aflatrem causes neurological deterioration in mice [42,43], so it would not be safe for consumers to ingest high quantities of aflatrem, even though the aflatoxin levels are greatly reduced. The potential ingestion of other potent mycotoxins is one important consideration that should be given to any potential biocontrol strain before it is applied to a crop at high concentrations. Five VOCs reportedly unique to non-aflatoxigenic A. flavus [10,11] that we previously tested for their impact on toxigenic strains [18] were 2-methyl-1-butanol, trans-2-methyl-2-butenal, 2,3-dihydrofuran, 3-octanone, and decane. Of those five, we only captured 3-octanone, and it was found in the headspaces of all four strains. LA1 did not produce any of the other four “non-aflatoxigenic” VOCs on synthetic media or corn kernels. We captured other headspace VOCs, unique to LA1, that could be tested for their potential as inhibitive to the growth and/or mycotoxin production of aspergilli. Four LA1-specific VOCs were captured from multiple replicates on synthetic media (Table 1), as well as five singleton VOCs (Table S3). One of the four Table 1 compounds, α-gurjunene, was produced on MGA. It was also produced as a singleton VOC on YES. This compound has been associated with toxigenic A. flavus in other studies [8,16,44] and should therefore be ruled out as unique or underscoring biocontrol efficacy by non-aflatoxigenic strains such as LA1. Linoleic acid (also produced on MGA) has been linked to increased sporulation and sclerotium production in A. flavus [45], while also serving as an antifungal shown to inhibit the growth of other plant pathogenic fungi [46]. Therefore, if this VOC positively correlates with conidium and sclerotium production by toxigenic strains, and toxin concentrations are enhanced in sclerotia, then this compound might pose a risk and should not be used as a biocontrol VOC. Another compound from LA1, also produced on MGA, was selina-3,7(11)-diene. Although not previously associated with A. flavus, this compound is a component of hemp oil with proven antimicrobial activity [47]. Studies involving euparone, an LA1-specific compound produced on CZ, are lacking and therefore its purpose in A. flavus is also unknown. Regarding the LA1-specific VOCs captured on either corn type (Table 2), only one (4-nonyne) was present with multiple replicates. It was produced on both corn types at the first time point (V0, M0) although it was a singleton VOC on VA35 at V0. A literature search did not reveal any previous associations of 4-nonyne with A. flavus. A literature search involving the singleton VOCs associated with LA1 while colonizing each substrate was also conducted; however, in lieu of reporting them here, the compounds and their relative citations are included in Table S6.
Our synthetic media and corn genotype studies offered 14 LA1 compounds to test for potential biocontrol properties. Only three were associated with multiple replicates of LA1 (Table 3; Figure S10), although the singleton VOCs in Table S6 may still have value for testing as biocontrol compounds. Investigations involving these compounds will depend on their availability and cost. Future in vitro studies will assess the gene-level impacts of highly inhibitory VOCs against aflatoxigenic Aspergillus strains. These inhibitory VOCs could then be tested as sprays or fumigants in growth chambers and greenhouse experiments. A separate study from this lab is identifying the extrolites produced by LA1 that could also be tested for biocontrol properties.

Table 3.
LA1-specific VOCs with potential to be investigated as biocontrol compounds.
4.4. VOCs Exist That Could Be Used to Screen for the Presence of Aflatoxigenic Aspergilli
Across all the substrates used for this study, there are many that could be tested further as biomarkers for early detection of aflatoxigenic fungi, especially if the VOCs unique to each aflatoxigenic strain are included. For this study, however, we will focus solely on those VOCs that were shared among multiple aflatoxigenic strains. One potential biomarker VOC could be 2-heptanone, which has been mentioned as a potential biomarker for the detection of A. flavus in buildings [6], as well as infected corn [9]. We observed 2-heptanone being produced on synthetic media and cracked corn kernels by the three aflatoxigenic strains. LA2 produced this compound on YES but did not produce it on either corn type. Despite only being observed with aflatoxigenic strains on YES, 2-heptanone was detected in a single replicate of LA1 when growing on VA35 (V1). 2-heptanone was not detected with the other two LA1_V1 replicates, nor was it detected with any other LA1 samples on synthetic media or corn. The reason for its presence in the LA1 headspace is unclear but could be the result of an aflatoxigenic fungal contaminant in that single LA1_V1 vial. Another compound only observed with aflatoxigenic isolates on synthetic media (also present with this single replicate of LA1_V1) was cis-2-octen-1-ol, which supports the likelihood of contamination of this replicate. This putatively aflatoxigenic VOC was present only with strains LA2-LA4 on synthetic media and was not present in any of the cracked corn samples. It has been associated with the A. flavus volatile profile as the reason for its musty odor [48], but the aflatoxigenicity of the examined A. flavus strain was not reported. It was associated with an aflatoxigenic A. flavus strain in one of the De Lucca et al. studies [10]. Another compound associated with multiple aflatoxigenic strains while growing on synthetic media was 1-octen-3-one (LA2 and LA3) (Table 1 and Table S3). Found with multiple replicates of LA3 on CZ and one replicate of LA2 on CZ, 1-octen-3-one has been reported to be a potential biomarker to detect the presence of ochratoxigenic A. carbonarius during storage of grapes and raisins [49]. Additionally, it has been shown to inhibit seed germination of Arabidopsis thaliana [50]. On both corn types, glycocyamidine was present, but only with LA3 and LA4 (Table 2 and Table S5). It was produced with all replicates of LA4 at V2 and a single replicate of LA3 at M2. No reports linking glycoyamidine with A. flavus could be found, therefore the reason for its presence in the headspace profile of this fungus is unknown. Many singleton VOCs were observed with aflatoxigenic strains on both corn types. A literature search was conducted on them, and citations associated with these compounds (if found) were noted in Table S7.
After excluding those VOCs that might not be fungal in origin or that associated with non-aflatoxigenic A. flavus, our synthetic media and corn genotype studies offered four compounds to further investigate as potential biomarkers to detect the presence of aflatoxigenic fungi (Table 4; Figure S11). The number of potential biomarkers increases if we include potential VOCs that were singletons or only associated with a single aflatoxigenic strain. As stated with potential biocontrol VOCs, investigations involving these compounds as potential biomarkers will depend on their availability and cost. Future biomarker work will involve the creation of a VOC library or database listing compounds associated with infection by aflatoxin-producing fungi, which would benefit researchers and developers of technologies for VOC detection (i.e., an e-nose device). Technology that could be trained to detect one or more of these compounds in the field, early in the growing season, would benefit growers and consumers.

Table 4.
VOCs to be investigated as potential biomarkers for aflatoxigenic fungi.
4.5. Comparisons with Other A. flavus VOC Studies Reveal a True Diversity of Compounds
Li and coworkers [9] suggested other VOCs they associated with corn infection by an aflatoxin-producing strain, including 2-octenal, benzeneacetaldehyde, trans-2-heptenal, and 2-pentylfuran. In our study, 2-octenal and benzeneacetaldehyde were found in the headspaces of all four strains, including non-aflatoxigenic LA1, on synthetic media and corn. The Li et al. study did not include a non-aflatoxigenic strain to ensure the observed VOCs were solely from aflatoxigenic A. flavus. The VOC 2-pentylfuran was detected in the blank headspaces of both corn types, as well as headspaces with fungal growth, so we did not consider it truly fungal for this study. In fact, 2-pentylfuran was not detected in the headspaces of any fungal strain we examined on synthetic media, suggesting it was host-related and may be a compound that corn produces during A. flavus infection. The only other compound from the Li study that our findings support as a potential biomarker for infection by aflatoxigenic fungi is trans-2-heptenal. We captured this VOC on corn in the headspaces of LA3 (V0 and V1) and LA4 (M2). In comparison to the other studies that assessed VOC profiles for A. flavus-infected crops, we did not capture the same VOCs reported in the Börjesson et al. study [5], but the compounds they reported were based on the infection of wheat and oats. 2-heptanone was associated with A. flavus in the Gao et al. study [6], however, it was only detected at low concentrations on gypsum board and not with the synthetic medium they used (malt extract agar). Additionally, compounds such as 1-octen-3-ol and 3-octanone were not associated with A. flavus in their study, which conflicted with our findings. Eleven of the fifty-two VOCs reported by Sun et al. [7] being produced on corn meal substrate (without agar) were also observed in this study. However, some of the VOCs they associated only with non-aflatoxigenic A. flavus NRRL 21882 did not correlate with our findings, such as 1-octen-3-ol, which was captured in the headspaces of all four Louisiana strains on multiple substrates. A subsequent study by Sun et al. [8] listed over 40 VOCs produced by A. flavus on 4 different synthetic media, including CMA and CZ, and the only compounds that were also observed in our study were α-gurjunene and germacrene D. We captured α-gurjunene with YES and MGA (only with LA1) and germacrene D with MGA and CZ (only with LA3). Regarding the first De Lucca et al. study [10], 3-octanone (on PDA) was the only VOC (out of 24) that was also captured in our study. Ten VOCs of the one hundred and eighteen De Lucca and co-workers captured on corn kernels [11] were also produced by our Louisiana strains. It must be stated that the A. flavus strains used in the studies above were not the same strains used in this study, so our findings support that headspace diversity is likely based not only on the substrate but also on the strain utilizing the substrate and the timing of headspace capture [11].
5. Conclusions
This study demonstrated that A. flavus volatile profiles vary greatly and are dependent on several factors, including the type of fungal strain, the substrate, and the timing of headspace capture and analysis. A total of about 130 VOCs were captured, either from substrate blanks, from single replicates, or across multiple replicates. Ninety-eight could be considered truly fungal or derived from fungal colonization of the substrate. Among these, most were unique to a particular strain. Many more VOCs were present in the analyses but were excluded because of small peak areas and poor match qualities. LA1 offered a total of 14 VOCs to investigate further as potential biocontrol compounds (unique to a non-aflatoxigenic strain with proven inhibitory potential), although 11 of them were singletons that could be artifacts given that they were not replicated. The three toxigenic strains offered sixty-seven potential biomarker compounds (unique to 1 or more toxigenic strains representing different sclerotial morphotypes and species within the genus), although only 13 were produced in multiple replicates. Of those, only four were produced by multiple toxigenic strains. The authors hope this information can further the improvement of the A. flavus biocontrol strategy and facilitate early detection of aflatoxin-contaminating fungi in food and feed crops.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10030157/s1, Table S1: Headspace VOCs captured with non-inoculated synthetic media (blanks); Table S2: Singleton VOCs (“Others”) captured only once for each set of replicates on synthetic media; Table S3: Headspace VOCs captured with non-inoculated corn kernels (blanks); Table S4: Singleton VOCs (“Others”) captured only once for each set of replicates on VA35 corn; Table S5: Singleton VOCs (“Others”) captured only once for each set of replicates on MI82 corn; Table S6: LA1-specific singleton VOCs that may have biocontrol properties; Table S7. LA2-LA4 singleton VOCs that may serve as biomarkers of infection by multiple aflatoxigenic fungi; Figure S1: Results of 2-way ANOVA involving the top four VOCs produced by examined strains on each synthetic medium; Figure S2: Average percentages (over three replicates) of VOCs contributing to total headspace profile of LA1 with each synthetic medium; Figure S3: Total Ion Chromatograms showing LA1 headspace profiles with each synthetic medium; Figure S4: Average percentages (over three replicates) of VOCs contributing to total headspace profile of LA2 with each synthetic medium; Figure S5: Average percentages (over three replicates) of VOCs contributing to total headspace profile of LA3 with each synthetic medium; Figure S6: Average percentages (over three replicates) of VOCs contributing to total headspace profile of LA4 with each synthetic medium; Figure S7: Results of 2-way ANOVA involving the top three VOCs produced by examined strains on VA35 corn at the one-week time point; Figure S8: Average percentages (over three replicates) of VOCs contributing to total headspace profile of LA1 while growing on cracked kernels of VA35 (top row) and MI82 (bottom row) at each of three sampling time points; Figure S9: Average percentages (over three replicates) of VOCs contributing to total headspace profiles of toxigenic strains LA2-LA4 while growing on cracked kernels of VA35 (top row) and MI82 (bottom row) at each of three sampling time points; Figure S10: Structural formulas for the LA1-specific VOCs to be tested for potential biocontrol properties; Figure S11: Structural formulas for the VOCs present in LA2-LA4 to be investigated as potential biomarkers for the presence of crop infection by aflatoxigenic fungi. [51,52,53,54,55,56,57,58,59,60,61,62,63,64,65,66] are cited in the supplementary materials.
Author Contributions
Conceptualization, G.G.M.; methodology, G.G.M. and S.W.L.; validation, S.W.L.; formal analysis, S.W.L.; writing—original draft preparation, G.G.M. and S.W.L.; writing—review and editing, G.G.M. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The authors would like to acknowledge Casey Grimm, Matthew Lebar, and Michael Santiago for acting as internal reviewers prior to submission of this manuscript for official review.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Andersen, M.R. Elucidation of primary metabolic pathways in Aspergillus species: Orphaned research in characterizing orphan genes. Brief. Funct. Genom. 2014, 13, 451–455. [Google Scholar] [CrossRef][Green Version]
- Frisvad, J.C. Taxonomy, chemodiversity, and chemoconsistency of Aspergillus, Penicillium, and Talaromyces species. Front. Microbiol. 2015, 5, e773. [Google Scholar] [CrossRef]
- Hung, R.; Lee, S.; Bennett, J.W. Fungal volatile organic compounds and their role in ecosystems. Appl. Microbiol. Biotechnol. 2015, 99, 3395–3405. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.; Hung, R.; Yin, G.; Klich, M.A.; Grimm, C.; Bennett, J.W. Arabidopsis thaliana as bioindicator of fungal VOCs in indoor air. Mycobiology 2016, 44, 162–170. [Google Scholar] [CrossRef]
- Börjesson, T.; Stöllman, U.; Schnürer, J. Volatile metabolites produced by six fungal species compared with other indicators of fungal growth on cereal grains. Appl. Environ. Microbiol. 1992, 58, 2599–2605. [Google Scholar] [CrossRef]
- Gao, P.; Korley, F.; Martin, J.; Chen, B.T. Determination of unique microbial volatile organic compounds produced by five Aspergillus species commonly found in problem buildings. AIHA J. 2002, 63, 135–140. [Google Scholar] [CrossRef]
- Sun, D.; Wood-Jones, A.; Wang, W.; Vanlangenberg, C.; Jones, D.; Gower, J.; Simmons, P.; Baird, R.; Mlsna, T. Monitoring MVOC profiles over time from isolates of Aspergillus flavus using SPME GC-MS. J. Agric. Chem. Environ. 2014, 3, 48–63. [Google Scholar] [CrossRef]
- Sun, D.; She, J.; Gower, J.L.; Stokes, C.E.; Windham, G.L.; Baird, R.E.; Mlsna, T.E. Effects of growth parameters on the analysis of Aspergillus flavus volatile metabolites. Separations 2016, 3, 13. [Google Scholar] [CrossRef]
- Li, H.; Kang, X.; Wang, S.; Mo, H.; Xu, D.; Zhou, W.; Hu, L. Early detection and monitoring for Aspergillus flavus contamination in maize kernels. Food Control 2020, 121, e107636. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Boué, S.M.; Carter-Wientjes, C.H.; Bland, J.M.; Bhatnagar, D.; Cleveland, T.E. Volatile profiles of toxigenic and non-toxigenic Aspergillus flavus using SPME for solid phase extraction. Ann. Agric. Environ. Med. 2010, 17, 301–308. [Google Scholar]
- De Lucca, A.J.; Boué, S.M.; Carter-Wientjes, C.H.; Bhatnagar, D. Volatile profiles and aflatoxin production by toxigenic and non-toxigenic isolates of Aspergillus flavus grown on sterile and non-sterile cracked corn. Ann. Agric. Environ. Med. 2012, 19, 91–98. [Google Scholar] [PubMed]
- Cary, J.W.; Gilbert, M.K.; Lebar, M.D.; Majumdar, R.; Calvo, A.M. Aspergillus flavus secondary metabolites: More than just aflatoxins. Food Saf. 2018, 6, 7–32. [Google Scholar] [CrossRef]
- Keller, N.P. Fungal secondary metabolism: Regulation, function and drug discovery. Nat. Rev. Microbiol. 2019, 17, 167–180. [Google Scholar] [CrossRef]
- Macheleidt, J.; Mattern, D.J.; Fischer, J.; Netzker, T.; Weber, J.; Schroeckh, V.; Valiante, V.; Brakhage, A.A. Regulation and role of fungal secondary metabolites. Annu. Rev. Genet. 2016, 50, 371–392. [Google Scholar] [CrossRef]
- Sweany, R.R.; Damann, K.E., Jr. Influence of neighboring clonal-colonies on aflatoxin production by Aspergillus flavus. Front. Microbiol. 2020, 10, 3038. [Google Scholar] [CrossRef]
- Zeringue, H.J.; Bhatnagar, D.; Cleveland, T.E. C15H24 volatile compounds unique to aflatoxigenic strains of Aspergillus flavus. Appl. Environ. Microbiol. 1993, 59, 2264–2270. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G. Practical considerations will ensure the continued success of pre-harvest biocontrol using non-aflatoxigenic Aspergillus flavus strains. Crit. Rev. Food Sci. Nutr. 2022, 62, 4208–4225. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. The potential role of fungal volatile organic compounds in Aspergillus flavus biocontrol efficacy. Biol. Control 2021, 160, e104686. [Google Scholar] [CrossRef]
- Schmidt, F.R.; Lemke, P.A.; Esser, K. Viral influences on aflatoxin formation by Aspergillus flavus. Appl. Microbiol. Biotechnol. 1986, 24, 248–252. [Google Scholar] [CrossRef]
- Cotty, P.J.; Bhatnagar, D. Variability among atoxigenic Aspergillus flavus strains in ability to prevent aflatoxin contamination and production of aflatoxin biosynthetic pathway enzymes. Appl. Environ. Microbiol. 1994, 60, 2248–2251. [Google Scholar] [CrossRef]
- Sweany, R.R.; Damann, K.E., Jr.; Kaller, M.D. Comparison of soil and corn kernel Aspergillus flavus populations: Evidence for niche specialization. Phytopathology 2011, 101, 952–959. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. Cumulative effects of non-aflatoxigenic Aspergillus flavus volatile organic compounds to abate toxin production by mycotoxigenic aspergilli. Toxins 2022, 14, 340. [Google Scholar] [CrossRef] [PubMed]
- Moore, G.G.; Lebar, M.D.; Carter-Wientjes, C.H. The role of extrolites secreted by nonaflatoxigenic Aspergillus flavus in biocontrol efficacy. J. Appl. Microbiol. 2019, 126, 1257–1264. [Google Scholar] [CrossRef] [PubMed]
- Herrera, J.M.; Pizzolitto, R.P.; Zunino, M.P.; Dambolena, J.S.; Zygadlo, J.A. Effect of fungal volatile organic compounds on a fungus and an insect that damage stored maize. J. Stored Prod. Res. 2015, 62, 74–80. [Google Scholar] [CrossRef]
- Combet, E.; Eastwood, D.C.; Burton, K.S.; Henderson, J. Eight-carbon volatiles in mushrooms and fungi: Properties, analysis, and biosynthesis. Mycoscience 2006, 47, 317–326. [Google Scholar] [CrossRef]
- Chitarra, G.S.; Abee, T.; Rombouts, F.M.; Posthumus, M.A.; Dijksterhuis, J. Germination of Penicillium paneum conidia is regulated by 1-octen-3-ol, a volatile self-inhibitor. Appl. Environ. Microbiol. 2004, 70, 2823–2829. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, T.; Kimura, T.; Tanaka, H.; Kaneko, S.; Ichii, S.; Kiuchi, M.; Suzuki, T. Analysis of volatile metabolites emitted by soil-derived fungi using head space solid-phase microextraction/gas chromatog-raphy/mass spectrometry: I. Aspergillus fumigatus, Aspergillus nidulans, Fusarium solani and Penicillium paneum. Surf. Interface Anal. 2012, 44, 694–698. [Google Scholar] [CrossRef]
- Liarzi, O.; Benichis, M.; Gamliel, A.; Ezra, D. trans-2-Octenal, a single compound of a fungal origin, controls Sclerotium rolfsii, both in vitro and in soil. Pest Manag. Sci. 2020, 76, 2068–2071. [Google Scholar] [CrossRef]
- Das, M.; Prakash, H.S.; Nalini, M.S. Antibacterial metabolites from Bipolaris specifera, an endophytic fungus from the endemic medicinal plant, Zingiber nimmonii (J. Graham) Dalzell. 3 Biotech 2020, 10, e317. [Google Scholar] [CrossRef]
- Zou, C.; Li, Z.; Yu, D. Bacillus megaterium strain XTBG34 promotes plant growth by producing 2-pentylfuran. J. Microbiol. 2010, 48, 460–466. [Google Scholar] [CrossRef]
- Boué, S.M.; Shih, B.Y.; Carter-Wientjes, C.H.; Cleveland, T.E. Effect of soybean lipoxygenase on volatile generation and inhibition of Aspergillus flavus mycelial growth. J. Agric. Food Chem. 2005, 53, 4778–4783. [Google Scholar] [CrossRef]
- Syhre, M.; Scotter, J.M.; Chambers, S.T. Investigation into the production of 2-Pentylfuran by Aspergillus fumigatus and other respiratory pathogens in vitro and human breath samples. Med. Mycol. 2008, 46, 209–215. [Google Scholar] [CrossRef]
- Jurjevic, Z.; Rains, G.C.; Wilson, D.M.; Lewis, W.J. Volatile metabolites associated with one aflatoxigenic and one nontoxigenic Aspergillus flavus strain grown on two different substrates. Phytopathol. Mediterr. 2008, 47, 266–271. [Google Scholar] [CrossRef]
- Shi, J.; Zhu, Y.; Zhang, Y.; Lin, Z.; Lv, H.P. Volatile composition of Fu-brick tea and Pu-erh tea analyzed by comprehensive two-dimensional gas chromatography-time-of-flight mass spectrometry. LWT 2019, 103, 27–33. [Google Scholar] [CrossRef]
- Xiang, M.; Chu, J.; Cai, W.; Ma, H.; Zhu, W.; Zhang, X.; Ren, J.; Xiao, L.; Liu, D.; Liu, X. Microbial succession and interactions during the manufacture of Fu Brick Tea. Front. Microbiol. 2022, 13, e892437. [Google Scholar] [CrossRef]
- Feng, Y.; Cui, C.; Zhao, H.; Gao, X.; Zhao, M.; Sun, W. Effect of koji fermentation on generation of volatile compounds in soy sauce production. Int. J. Food Sci. Technol. 2013, 48, 609–619. [Google Scholar] [CrossRef]
- Shi, C.; Jash, A.; Lim, L.T. Activated release of hexanal and salicylaldehyde from imidazolidine precursors encapsulated in electrospun ethylcellulose-poly(ethylene oxide) fibers. SN Appl. Sci. 2021, 3, 385. [Google Scholar] [CrossRef]
- Li, S.-F.; Zhang, S.-B.; Lv, Y.-Y.; Zhai, H.-C.; Li, N.; Hu, Y.-S.; Cai, J.-P. Metabolomic analyses revealed multifaceted effects of hexanal on Aspergillus flavus growth. Appl. Microbiol. Biotechnol. 2021, 105, 3745–3757. [Google Scholar] [CrossRef]
- Cameron, J.N.; Carlile, M.J. Binding of isovaleraldehyde, an attractant, to zoospores of the fungus Phytophthora palmivora in relation to zoospore chemotaxis. J. Cell Sci. 1981, 49, 273–281. [Google Scholar] [CrossRef]
- Gehrig, R.; Knight, S. Formation of 2-heptanone from caprylic acid by spores of various filamentous fungi. Nature 1961, 192, 1185. [Google Scholar] [CrossRef]
- Popova, A.A.; Koksharova, O.A.; Lipasova, V.A.; Zaitseva, J.V.; Katkova-Zhukotskaya, O.A.; Eremina, S.I.; Mironov, A.S.; Chernin, L.S.; Khmel, I.A. Inhibitory and toxic effects of volatiles emitted by strains of Pseudomonas and Serratia on growth and survival of selected microorganisms, Caenorhabditis elegans, and Drosophila melanogaster. BioMed Res. Int. 2014, 2014, 125704. [Google Scholar] [CrossRef]
- Gallagher, R.T.; Wilson, B.J. Aflatrem, the tremorgenic mycotoxin from Aspergillus flavus. Mycopathologia 1979, 66, 183–185. [Google Scholar] [CrossRef]
- Valdes, J.J.; Cameron, J.E.; Cole, R.J. Aflatrem: A tremorgenic mycotoxin with acute neurotoxic effects. Environ. Health Perspect. 1985, 62, 459–463. [Google Scholar] [CrossRef]
- Josselin, L.; De Clerck, C.; De Boevre, M.; Moretti, A.; Jijakli, M.H.; Soyeurt, H.; Fauconnier, M.-L. Volatile organic compounds emitted by Aspergillus flavus strains producing or not aflatoxin B1. Toxins 2021, 13, 705. [Google Scholar] [CrossRef]
- Calvo, A.M.; Hinze, L.L.; Gardner, H.W.; Keller, N.P. Sporogenic effect of polyunsaturated fatty acids on development of Aspergillus spp. Appl. Environ. Microbiol. 1999, 65, 3668–3673. [Google Scholar] [CrossRef]
- Walters, D.; Raynor, L.; Mitchell, A.; Walker, A.; Walker, K. Antifungal activities of four fatty acids against plant pathogenic fungi. Mycopathologia 2004, 157, 87–90. [Google Scholar] [CrossRef]
- Menghini, L.; Ferrante, C.; Carradori, S.; D’Antonio, M.; Orlando, G.; Cairone, F.; Cesa, S.; Filippi, A.; Fraschetti, C.; Zengin, G.; et al. Chemical and bioinformatics analyses of the anti-leishmanial and anti-oxidant activities of hemp essential oil. Biomolecules 2021, 11, 272. [Google Scholar] [CrossRef]
- Kaminśki, E.; Libbey, L.M.; Stawicki, S.; Wasowicz, E. Identification of the predominant volatile compounds produced by Aspergillus flavus. Appl. Microbiol. 1972, 24, 721–726. [Google Scholar] [CrossRef]
- Cheng, Z.; Li, M.; Marriott, P.J.; Zhang, X.; Wang, S.; Li, J.; Ma, L. Chemometric analysis of the volatile compounds generated by Aspergillus carbonarius strains isolated from grapes and dried vine fruits. Toxins 2018, 10, 71. [Google Scholar] [CrossRef]
- Inamdar, A.A.; Morath, S.; Bennett, J.W. Fungal volatile organic compounds: More than just a funky smell? Annu. Rev. Microbiol. 2020, 74, 101–116. [Google Scholar] [CrossRef]
- Karbin, S.; Rad, A.B.; Arouiee, H.; Jafarnia, S. Antifungal activities of the essential oils on post-harvest disease agent Aspergillus flavus. Adv. Environ. Biol. 2009, 3, 219–225. [Google Scholar]
- Cleveland, T.E.; Carter-Wientjes, C.H.; De Lucca, A.J.; Boué, S.M. Effect of soybean volatile compounds on Aspergillus flavus growth and aflatoxin production. J. Food Sci. 2009, 74, H83–H87. [Google Scholar] [CrossRef] [PubMed]
- Bisignano, G.; Laganà, M.G.; Trombetta, D.; Arena, S.; Nostro, A.; Uccella, N.; Mazzanti, G.; Saija, A. In vitro antibacterial activity of some aliphatic aldehydes from Olea europaea L. FEMS Microbiol. Lett. 2001, 198, 9–13. [Google Scholar] [CrossRef] [PubMed]
- Ntalli, N.; Skourti, A.; Nika, E.P.; Boukouvala, M.C.; Kavallieratos, N.G. Five natural compounds of botanical origin as wheat protectants against adults and larvae of Tenebrio molitor L. and Trogoderma granarium Everts. Environ. Sci. Pollut. Res. 2021, 28, 42763–42775. [Google Scholar] [CrossRef]
- Caboni, P.; Ntalli, N.G.; Aissani, N.; Cavoski, I.; Angioni, A. Nematicidal activity of (E,E)-2,4-decadienal and (E)-2-decenal from Ailanthus altissima against Meloidogyne javanica. J. Agric. Food Chem. 2012, 60, 1146–1151. [Google Scholar] [CrossRef]
- Kramer, R.; Abraham, W.R. Volatile sesquiterpenes from fungi: What are they good for? Phytochem. Rev. 2012, 11, 15–37. [Google Scholar] [CrossRef]
- Li, X.-D.; Li, X.; Li, X.-M.; Yin, X.-L.; Wang, B.-G. Antimicrobial bisabolane-type sesquiterpenoids from the deep-sea sediment-derived fungus Aspergillus versicolor SD-330. Nat. Prod. Res. 2021, 35, 4265–4271. [Google Scholar] [CrossRef]
- Yeo, S.K.; Ali, A.Y.; Hayward, O.A.; Turnham, D.; Jackson, T.; Bowen, I.D.; Clarkson, R. β-Bisabolene, a sesquiterpene from the essential oil extract of Opoponax (Commiphora guidottii), exhibits cytotoxicity in breast cancer cell lines. Phytother. Res. 2016, 30, 418–425. [Google Scholar] [CrossRef]
- Zellagui, A.; Gherraf, N.; Rhouati, S. Chemical composition and antibacterial activity of the essential oils of Ferula vesceritensis Coss et Dur. leaves, endemic in Algeria. Org. Med. Chem. Lett. 2012, 2, e31. [Google Scholar] [CrossRef]
- Becker, E.M.; Herrfurth, C.; Irmisch, S.; Köllner, T.G.; Feussner, I.; Karlovsky, P.; Splivallo, R. Infection of corn ears by Fusarium spp. induces the emission of volatile sesquiterpenes. J. Agric. Food Chem. 2014, 62, 5226–5236. [Google Scholar] [CrossRef]
- Rodrigues, A.C.B.; Bomfim, L.M.; Neves, S.P.; Menezes, L.R.; Dias, R.B.; Soares, M.B.; Prata, A.P.N.; Rocha, C.A.G.; Costa, E.V.; Bezerra, D.P. Antitumor properties of the essential oil from the leaves of Duguetia gardneriana. Planta Med. 2015, 81, 798–803. [Google Scholar] [CrossRef]
- Li, S.F.; Zhang, S.B.; Lv, Y.Y.; Zhai, H.C.; Hu, Y.S.; Cai, J.P. Heptanal inhibits the growth of Aspergillus flavus through disturbance of plasma membrane integrity, mitochondrial function and antioxidant enzyme activity. LWT 2022, 154, e112655. [Google Scholar] [CrossRef]
- Gardini, F.; Lanciotti, R.; Guerzoni, M.E. Effect of trans-2-hexenal on the growth of Aspergillus flavus in relation to its concentration, temperature and water activity. Lett. Appl. Microbiol. 2001, 33, 50–55. [Google Scholar] [CrossRef]
- De Lucca, A.J.; Carter-Wientjes, C.H.; Boué, S.; Bhatnagar, D. Volatile trans-2-hexenal, a soybean aldehyde, inhibits Aspergillus flavus growth and aflatoxin production in corn. J. Food Sci. 2011, 76, M381–M386. [Google Scholar] [CrossRef]
- Todokoro, T.; Negoro, H.; Kotaka, A.; Hata, Y.; Ishida, H. Aspergillus oryzae FaeA is responsible for the release of ferulic acid, a precursor of off-odor 4-vinylguaiacol in sake brewing. J. Biosci. Bioeng. 2022, 133, 140–145. [Google Scholar] [CrossRef]
- Upadhyay, P.; Singh, N.K.; Tupe, R.; Odenath, A.; Lali, A. Biotransformation of corn bran derived ferulic acid to vanillic acid using engineered Pseudomonas putida KT2440. Prep. Biochem. Biotechnol. 2020, 50, 341–348. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).