Catalytic Ozonation for Pulp and Paper Mill Wastewater Treatment: COD Reduction and Organic Matter Degradation Mechanism
Abstract
1. Introduction
2. Materials and Methods
2.1. Materials and Natural Wastewater
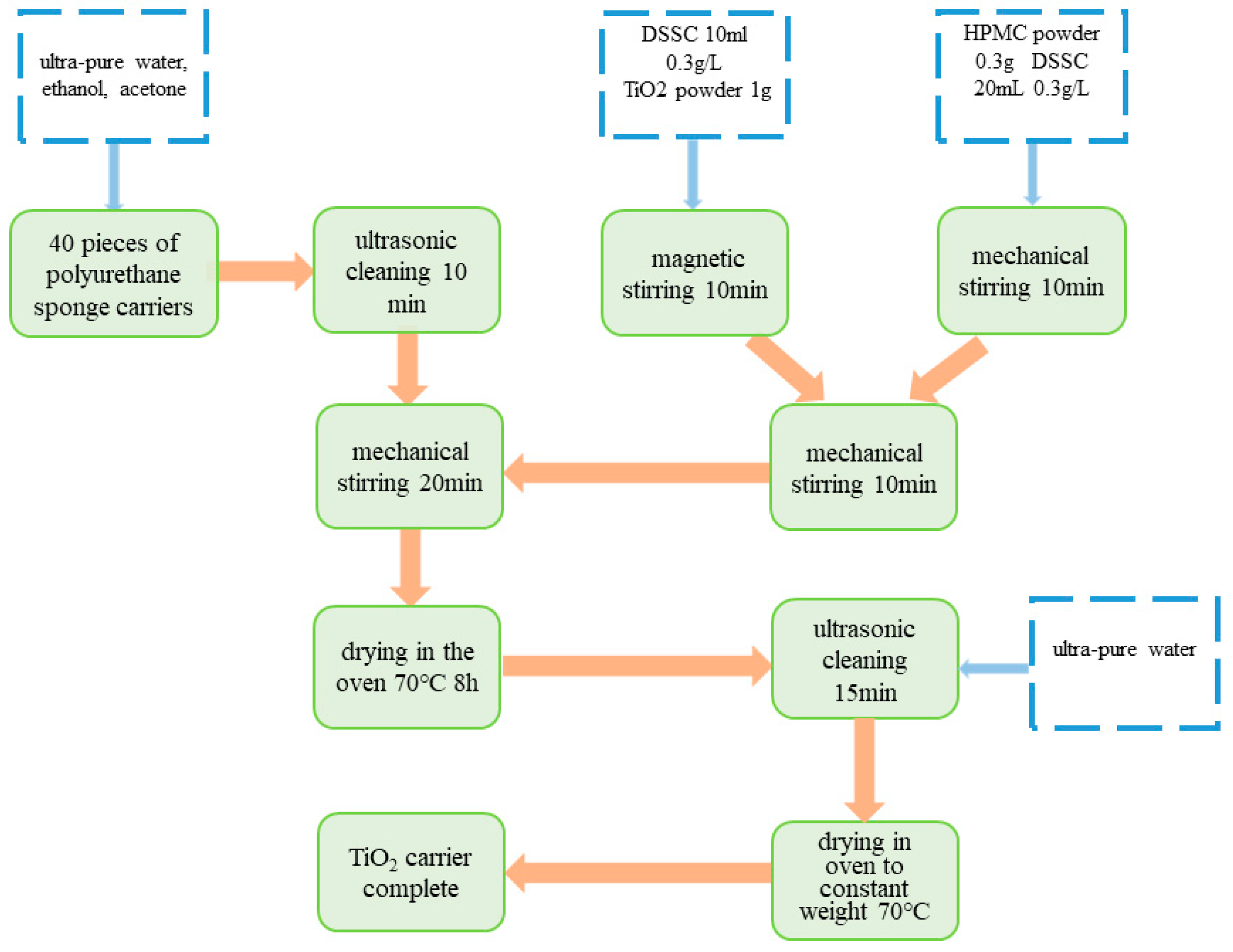
2.2. Sponge Cubes and Titanium Dioxide Coating Procedure
2.3. Experimental Procedure
2.4. Analysis Methods
3. Results and Discussion
3.1. Exploration of Optimum Process Conditions
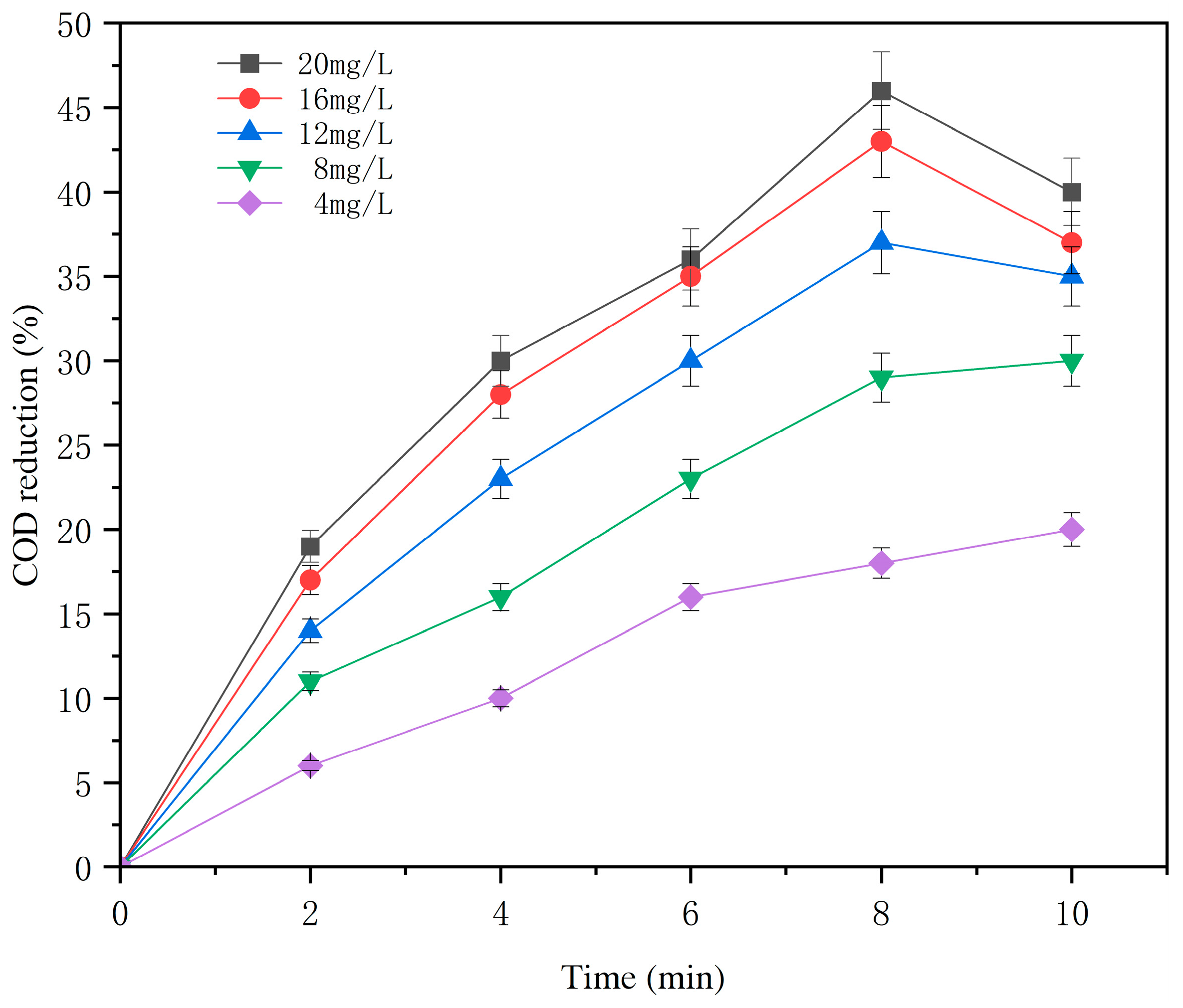
3.1.1. Effect of Ozone Concentration and Time
3.1.2. Effect of pH
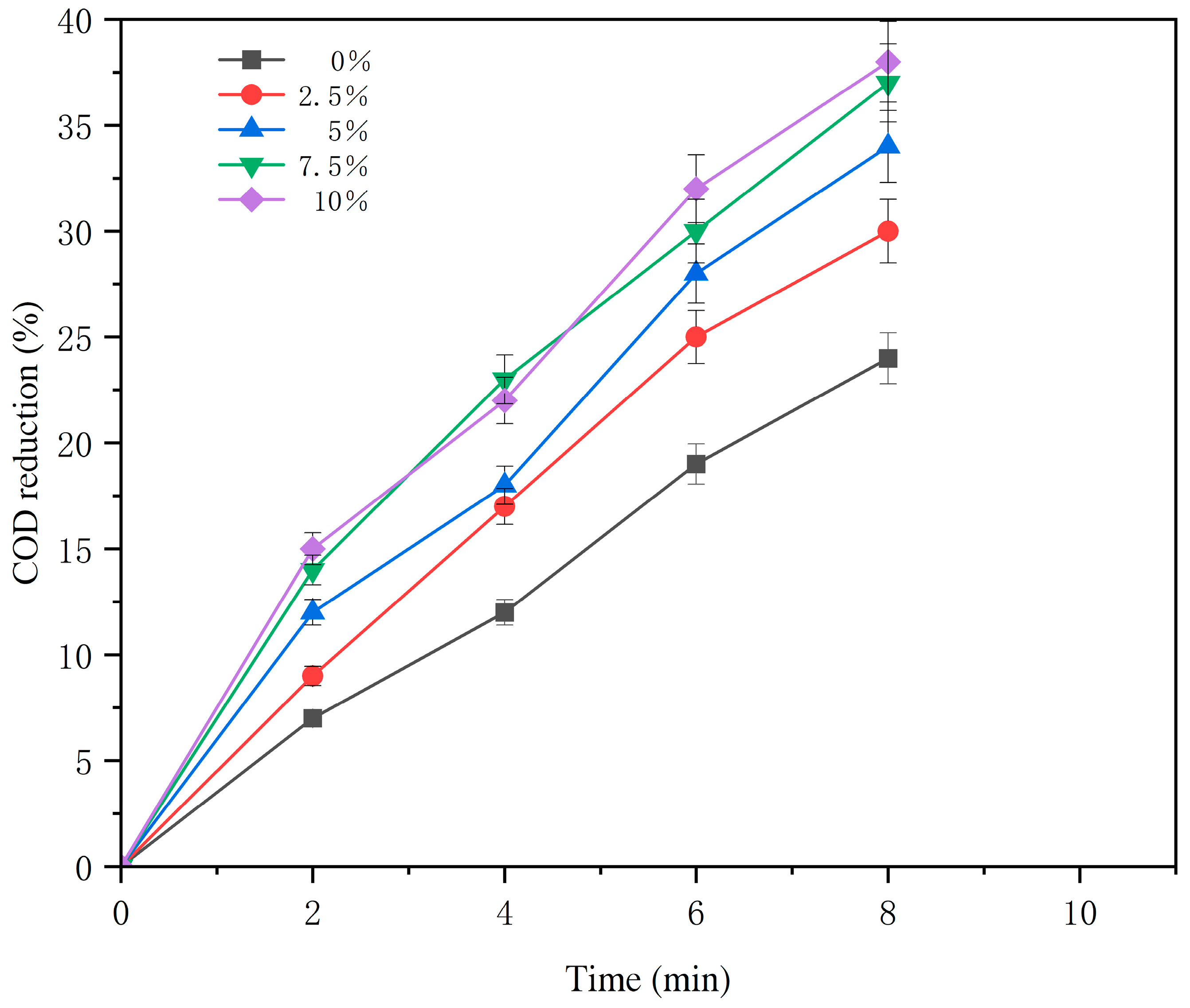
3.1.3. Effect of the Catalyst Filling Ratio
3.2. Kinetic Study
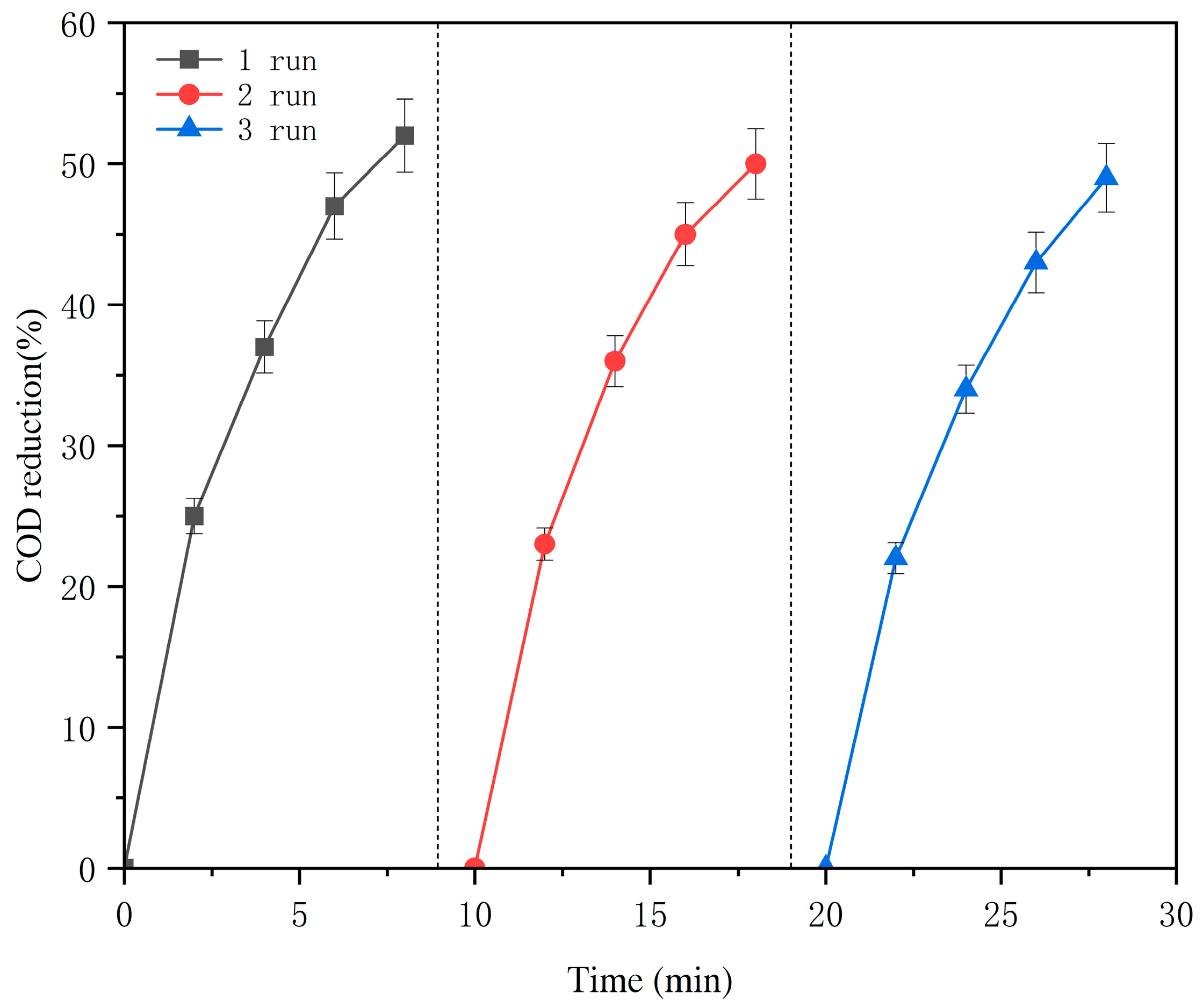
3.3. Reusability Test
3.4. Water Quality Indicators under Optimal Process Conditions
3.5. FT-IR Analysis of Effluent Water Quality in Different Systems
3.6. GC-MS Analysis of Pulp and Paper Mill Wastewater before and after Degradation by Different Systems
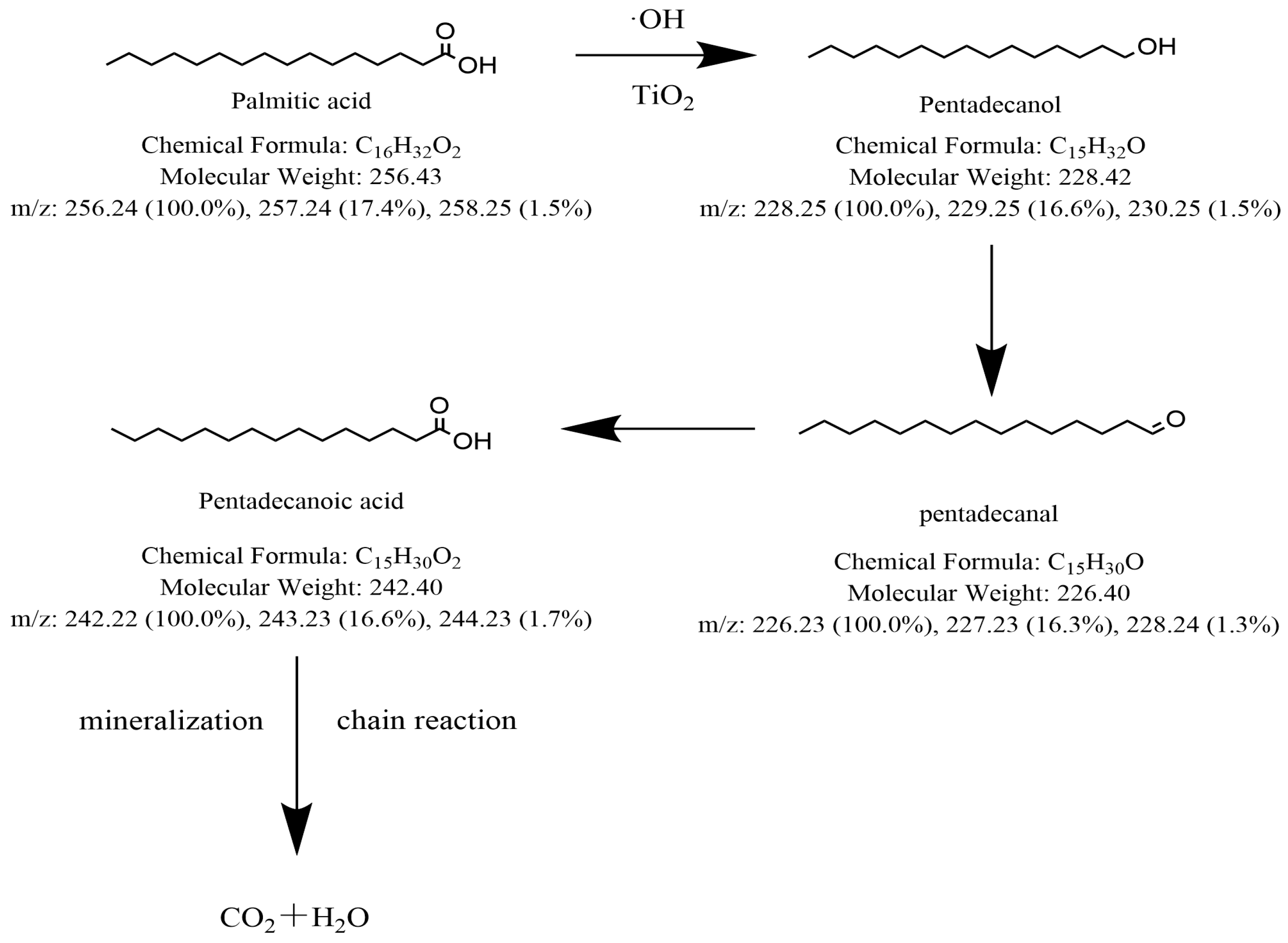
3.7. Analysis of Degradation Mechanism of Two Kinds of DCSs
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Kumar, A.; Srivastava, N.K.; Gera, P. Removal of color from pulp and paper mill wastewater-methods and techniques—A review. J. Environ. Manag. 2021, 298, 113527. [Google Scholar] [CrossRef] [PubMed]
- Toczyowska-Mamińska, R. Limits and perspectives of pulp and paper industry wastewater treatment—A review—ScienceDirect. Renew. Sustain. Energy Rev. 2017, 78, 764–772. [Google Scholar] [CrossRef]
- Zhang, C.H. Analytical Methods for the Dissolved and Colloidal Substances in Wastepaper Pulp-Water System. Shanghai Pap. Mak. 2006, 37, 52–56. [Google Scholar]
- Liang, J.; He, Y.; Zhu, J.; Du, W. Accumulation of dissolved and colloidal substances in water recycled during papermaking. Chem. Eng. J. 2011, 168, 604–609. [Google Scholar]
- Blanco, M.A.; Negro, C.; Gaspar, I.; Tijero, J. Slime problems in the paper and board industry. Appl. Microbiol. Biotechnol. 1996, 46, 203–208. [Google Scholar] [CrossRef]
- Rekhate, C.V.; Srivastava, J.K. Recent advances in ozone-based advanced oxidation processes for treatment of wastewater—A review—ScienceDirect. Chem. Eng. J. Adv. 2020, 3, 100031. [Google Scholar] [CrossRef]
- Sousa, M.R.S.; Lora-Garcia, J.; López-Pérez, M.-F. Modelling approach to an ultrafiltration process for the removal of dissolved and colloidal substances from treated wastewater for reuse in recycled paper manufacturing. J. Water Process Eng. 2018, 21, 96–106. [Google Scholar] [CrossRef]
- Jepsen, K.L.; Bram, M.V.; Pedersen, S.; Yang, Z. Membrane Fouling for Produced Water Treatment: A Review Study From a Process Control Perspective. Water 2018, 10, 847. [Google Scholar] [CrossRef]
- Liu, S.; Yang, D.M.; Fang, J.; Gao, S.; Tan, T. Mechanism of Advanced Oxidation Processes and Their Application Progress in Papermaking Effluent Treatment. Trans. China Pulp Pap. 2010, 25, 56–62. [Google Scholar]
- Jin, X.; Wu, C.; Fu, L.; Tian, X.; Wang, P.; Zhou, Y.; Zuo, J. Development, dilemma and potential strategies for the application of nanocatalysts in wastewater catalytic ozonation: A review. J. Environ. Sci. 2023, 124, 330–349. [Google Scholar] [CrossRef]
- He, S.; Li, J.; Xu, J.; Mo, L.; Zhu, L.; Luan, P.; Zeng, J. Heterogeneous catalytic ozonation of paper-making wastewater with α-Fe2O3/γ-Al2O3 as a catalyst for increased TOC and color removals. Desalin. Water Treat. 2017, 95, 192–199. [Google Scholar] [CrossRef]
- Zhuang, H.; Guo, J.; Hong, X. Advanced treatment of paper-making wastewater using catalytic ozonation with waste rice straw-derived activated carbon-supported manganese oxides as a novel and efficient catalyst. Pol. J. Environ. Stud. 2018, 27, 451–457. [Google Scholar] [CrossRef]
- De los Santos Ramos, W.; Poznyak, T.; Chairez, I. Remediation of lignin and its derivatives from pulp and paper industry wastewater by the combination of chemical precipitation and ozonation. J. Hazard. Mater. 2009, 169, 428–434. [Google Scholar] [CrossRef]
- Yan, Z.; Zhu, J.; Hua, X.; Liang, D.; Zhang, L. Catalytic ozonation for the degradation of polyvinyl alcohol in aqueous solution using catalyst based on copper and manganese. J. Clean. Prod. 2020, 272, 122856. [Google Scholar] [CrossRef]
- Yang, Y.; Ma, J.; Qin, Q.; Zhai, X. Degradation of nitrobenzene by nano-TiO2 catalyzed ozonation. J. Mol. Catal. A Chem. 2007, 267, 41–48. [Google Scholar] [CrossRef]
- Wang, S.J.; Ma, J.; Yang, Y.X.; Liang, J. Degradation and Transformation of Organic Compounds in Songhua River Water by Catalytic Ozonation in the Presence of TiO2/Zeolite. Ozone Sci. Eng. 2011, 33, 236–242. [Google Scholar] [CrossRef]
- Zhao, T.Y.; Cheng, H.; Liang, Y.N.; Xiong, J.H.; Zhu, H.X.; Wang, S.F.; Liang, J.X.; Chen, G.N. Preparation of TiO2/Sponge Composite for Photocatalytic Degradation of 2,4,6-Trichlorophenol. Water Air Soil Pollut. 2020, 231, 412. [Google Scholar] [CrossRef]
- Liang, Y.; Jiao, C.; Pan, L.; Zhao, T.; Zhou, Q. Degradation of chlorine dioxide bleaching wastewater and response of bacterial community in the intimately coupled system of visible-light photocatalysis and biodegradation. Environ. Res. 2021, 195, 110840. [Google Scholar] [CrossRef] [PubMed]
- Li, Y.P.; Gorenflo, A.; Frimmel, F.H. Ozonation and biodegradability of lignin in water. J. Beijing Inst. Technol. 2002, 11, 290–294. [Google Scholar]
- Wang, X.; Xia, J.; Ding, S.; Zhang, S.; Ding, J. Removing organic matters from reverse osmosis concentrate using advanced oxidation-biological activated carbon process combined with Fe3+/humus-reducing bacteria. Ecotoxicol. Environ. Saf. 2020, 203, 110945. [Google Scholar] [CrossRef] [PubMed]
- Catalkaya, E.C.; Kargi, F. Color, TOC and AOX removals from pulp mill effluent by advanced oxidation processes: A comparative study. J. Hazard. Mater. 2007, 139, 244–253. [Google Scholar] [CrossRef] [PubMed]
- Bijan, L.; Mohseni, M. Using ozone to reduce recalcitrant compounds and to enhance biodegradability of pulp and paper effluents. Water Sci. Technol. 2004, 50, 173–182. [Google Scholar] [CrossRef]
- Naydenov, A.; Mehandjiev, D. Complete oxidation of benzene on manganese dioxide by ozone. Appl. Catal. A Gen. 1993, 97, 17–22. [Google Scholar] [CrossRef]
- Nakhate, P.H.; Gadipelly, C.R.; Joshi, N.T.; Marathe, K.V. Engineering aspects of catalytic ozonation for purification of real textile industry wastewater at the pilot scale. J. Ind. Eng. Chem. 2019, 69, 77–89. [Google Scholar] [CrossRef]
- Nie, S.; Wang, S.; Qin, C.; Yao, S.; Li, K. Removal of hexenuronic acid by xylanase to reduce adsorbable organic halides formation in chlorine dioxide bleaching of bagasse pulp. Bioresour. Technol. 2015, 196, 413–417. [Google Scholar] [CrossRef]
- Haq, I.; Kalamdhad, A.S.; Pandey, A. Genotoxicity evaluation of paper industry wastewater prior and post-treatment with laccase producing Pseudomonas putida MTCC 7525. J. Clean. Prod. 2022, 342, 130981. [Google Scholar] [CrossRef]
- Sonkar, M.; Kumar, M.; Dutt, D.; Kumar, V. Treatment of pulp and paper mill effluent by a novel bacterium Bacillus sp. IITRDVM-5 through a sequential batch process. Biocatal. Agric. Biotechnol. 2019, 20, 101232. [Google Scholar]
- Veluchamy, C.; Kalamdhad, A.S. Enhancement of hydrolysis of lignocellulose waste pulp and paper mill sludge through different heating processes on thermal pretreatment. J. Clean. Prod. 2017, 168, 219–226. [Google Scholar] [CrossRef]
- Lin, Z.; Li, J.; Shen, W.; Corriou, J.-P.; Chen, X.; Xi, H. Different photocatalytic levels of organics in papermaking wastewater by flocculation-photocatalysis and SBR-photocatalysis: Degradation and GC–MS experiments, adsorption and photocatalysis simulations. Chem. Eng. J. 2021, 412, 128715. [Google Scholar] [CrossRef]
- Ledea-Lozano, O.E.; Fernández-García, L.; Gil-Ibarra, D.; Tena, N.; Salas, J.J. Characterization of different ozonized sunflower oils I. Chemical changes during ozonization. Grasas Aceites 2019, 70, 329. [Google Scholar] [CrossRef]
- Díaz Gómez, M.F.; Ledea Lozano, O.E.; Gómez Regüeiferio, M.; Garcés Mancheño, R.; Alaiz Barragán, M.S.; Martínez Force, E. Comparative study of the ozonation of genetically modified and not modified sunflower oils. Química Nova 2009, 32, 2467–2472. [Google Scholar] [CrossRef]
- Cox, H.H.J.; Deshusses, M.A. Biological waste air treatment in biotrickling filters. Curr. Opin. Biotechnol. 1998, 9, 256–262. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.; Shi, J.L.; von Gunten, U.; McCurry, D.L. Ozonation of organic compounds in water and wastewater: A critical review. Water Res. 2022, 213, 118053. [Google Scholar] [CrossRef] [PubMed]




| COD mg/L | BOD5 mg/L | NH3-N mg/L | TN mg/L | TP mg/L | Chromaticity | SS mg/L | |
|---|---|---|---|---|---|---|---|
| Wastewater | 953 | 254 | 14.23 | 13.55 | 0.61 | 3841 | 2439 |
| COD mg/L | B/C | NH3-N mg/L | TN mg/L | TN mg/L | Chromaticity | SS mg/L | |
|---|---|---|---|---|---|---|---|
| Wastewater | 953 | 0.26 | 24.23 | 13.55 | 0.61 | 3841 | 2439.4 |
| Ozone oxidation | 715 | 0.33 | 23.05 | 12.31 | 0.63 | 184 | 982.2 |
| Ozone-catalyzed oxidation | 453 | 0.37 | 25.03 | 11.97 | 0.67 | 153 | 849.4 |
| RT | Organic Compound | Relative Content (%) | |||
|---|---|---|---|---|---|
| Wastewater | Ozonation | Catalytic Ozonation | Pure TiO2/O3 | ||
| 4.344 | 2-Methyl-2-pentenoic acid | 0.11 | - | - | - |
| 6.502 | 2-Aminoanthracene | - | 0.06 | 0.18 | 0.12 |
| 6.634 | Benzaldehyde | - | 0.12 | - | 0.06 |
| 6.934 | 2,2-Diethoxyacetophenone | - | 0.72 | 0.6 | 0.52 |
| 7.741 | 2-Ethylhexanol | - | 0.34 | 0.55 | 0.42 |
| 8.145 | Nineteen alkane | 0.13 | - | - | - |
| 8.154 | Dodecane | - | 0.05 | 0.13 | 0.07 |
| 8.241 | Octadecane | - | 0.72 | 0.03 | 0.34 |
| 8.379 | Acetophenone | - | - | 0.03 | - |
| 8.867 | 3,6-Dimethyldecane | - | - | 0.05 | - |
| 8.911 | Nonyl aldehyde | - | 0.82 | - | 0.12 |
| 9.133 | Phenethyl alcohol | 0.02 | - | - | - |
| 9.416 | p-Ethylbenzoic acid | 0.01 | - | - | - |
| 9.68 | Diethoxymethane | - | 0.1 | 0.13 | 0.11 |
| 9.968 | 3,4-Dimethylaniline | - | - | 0.03 | 0.01 |
| 10.103 | Myristyl alcohol | - | - | - | 0.27 |
| 10.218 | Dodecene | 0.13 | - | - | - |
| 10.44 | 1,13-Tetradecanediene | 0.01 | - | - | - |
| 10.52 | Styrene | - | - | - | 0.61 |
| 10.687 | 2,4-Dimethylbenzaldehyde | 0.08 | - | - | - |
| 11.267 | 2-Ethylhexylhexyl ester | 0.01 | - | - | - |
| 11.4 | Nonanoic acid | - | 0.05 | 0.13 | 0.27 |
| 11.506 | Pentadecane | - | 0.13 | 0.12 | 0.11 |
| 12.324 | Myristic aldehyde | - | - | - | 0.02 |
| 12.582 | 1,3-Diisocyanato-2-methylbenzene | - | - | 0.22 | 0.13 |
| 13.107 | Tetradecane | 0.03 | 0.12 | 0.08 | 0.09 |
| 13.252 | Tetrahydro-alpha-naphthol | - | - | 0.07 | 0.01 |
| 13.366 | 3-Isopropylaniline | 0.04 | - | - | - |
| 13.905 | Dimethyl phthalate | 0.04 | - | - | - |
| 14.621 | 3,5-Di-tert-butylphenol | 0.77 | 1.89 | 2.56 | 2.04 |
| 14.765 | 4-Tetradecyl methoxyacetate | - | 0.12 | 0.05 | 0.07 |
| 14.888 | Tetradecane | 0.05 | - | - | - |
| 15.178 | Dodecanoic acid | - | - | 0.03 | 0.52 |
| 15.386 | Dihexadecane | 0.04 | - | - | - |
| 15.603 | Diethyl phthalate | 0.03 | - | - | - |
| 15.746 | Octadecyl chloride | 0.04 | - | - | - |
| 15.918 | stilbene | 2.09 | - | - | - |
| 15.996 | 4-Methyloctane | 0.05 | - | - | - |
| 16.156 | Dodecylbenzene | 0.07 | - | - | - |
| 16.428 | Triallyl isocyanurate | 0.1 | - | - | - |
| 16.701 | Heptadecane | 0.09 | - | - | - |
| 16.841 | n-hexadecane | 0.11 | - | - | - |
| 16.849 | Tridecane | 0.25 | 0.19 | 0.14 | 0.17 |
| 16.931 | 4-Methyldodecane | 0.11 | - | - | - |
| 17.141 | 5-Phenyldodecane | 0.07 | - | - | - |
| 17.271 | Eighteen methyl carbonate | 0.07 | - | - | - |
| 17.318 | Octadecane | - | - | 0.12 | 0.14 |
| 17.477 | 2,4,6-Trimethyl-N-(2,4,6-trimethylphenyl)-aniline | 0.12 | - | - | - |
| 17.574 | 3,5-Di-tert-butyl-4-hydroxybenzaldehyde | 0.17 | - | - | - |
| 17.854 | n-octacosane | 0.45 | 0.32 | 0.12 | 0.22 |
| 18.15 | 2-Methyl-benzo[f]quinoline | - | - | 0.13 | - |
| 18.213 | 7,11-Hexadecadienyl acetate | 0.64 | - | - | - |
| 18.453 | Oleic acid amide | 6.58 | - | - | - |
| 18.581 | Dimethyl phthalate | - | - | 0.11 | 0.09 |
| 18.841 | 2-Pentadecanone | 2.46 | 1.35 | 0.21 | 0.65 |
| 19.082 | Eicosane | - | 0.21 | 0.37 | 0.32 |
| 19.186 | Stearamide | 0.19 | - | - | - |
| 19.448 | Palmitic acid | 5.67 | 2.21 | - | 0.09 |
| 19.502 | Dibutyl phthalate | 0.24 | - | - | - |
| 19.726 | Ethyl oleate | 0.48 | - | - | - |
| 19.856 | N-Pentadecanenitrile | - | - | 0.05 | - |
| 20.092 | 2,2′-Methylenebis(4-methyl-6-tert-butylphenol) | 1.45 | - | - | - |
| 20.607 | 1-21 Alcohol | 0.59 | - | - | - |
| 20.871 | N,N-Dimethyldodecanamide | 0.84 | - | - | - |
| 21.096 | 9,12-Octadecadienoic acid (Z,Z)- | - | 1.82 | 2.11 | 1.76 |
| 21.133 | Oleic acid | 4.17 | 2.32 | 1.73 | 2.11 |
| 21.311 | Pentadecanoic acid | 0.5 | - | - | - |
| 21.448 | Stearyl Nitrile | 2.15 | - | - | - |
| 21.615 | 1-Chlorononane | 1.22 | 0.54 | 0.14 | 0.22 |
| 21.696 | (5E)-5-Octadecene | 1.07 | - | - | - |
| 21.703 | Cyclohexadecane | - | - | 0.33 | 0.14 |
| 22.147 | N,N-Dimethyloctanamide | - | 0.25 | 0.34 | 0.17 |
| 23.188 | Oleic acid amide | 1.45 | - | - | - |
| 23.51 | Ditetradecane | - | - | 0.4 | 0.43 |
| 23.548 | Eruvic acid amide | 7.51 | 3.25 | 0.64 | 1.23 |
| 23.655 | 6-Ethyl-3-octanol | 2.34 | - | - | - |
| 24.699 | Nonyl hexanoate | 1.1 | 0.64 | - | 0.12 |
| 24.724 | Dihexadecanoic acid | - | 1.15 | 1.64 | 3.22 |
| 26.219 | Nineteen alkane | - | 0.32 | 0.48 | 0.34 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhou, C.; Zhang, J.; Cai, Y.; Xiong, J. Catalytic Ozonation for Pulp and Paper Mill Wastewater Treatment: COD Reduction and Organic Matter Degradation Mechanism. Separations 2023, 10, 148. https://doi.org/10.3390/separations10030148
Zhou C, Zhang J, Cai Y, Xiong J. Catalytic Ozonation for Pulp and Paper Mill Wastewater Treatment: COD Reduction and Organic Matter Degradation Mechanism. Separations. 2023; 10(3):148. https://doi.org/10.3390/separations10030148
Chicago/Turabian StyleZhou, Chenxu, Jiaming Zhang, Yuxuan Cai, and Jianhua Xiong. 2023. "Catalytic Ozonation for Pulp and Paper Mill Wastewater Treatment: COD Reduction and Organic Matter Degradation Mechanism" Separations 10, no. 3: 148. https://doi.org/10.3390/separations10030148
APA StyleZhou, C., Zhang, J., Cai, Y., & Xiong, J. (2023). Catalytic Ozonation for Pulp and Paper Mill Wastewater Treatment: COD Reduction and Organic Matter Degradation Mechanism. Separations, 10(3), 148. https://doi.org/10.3390/separations10030148






