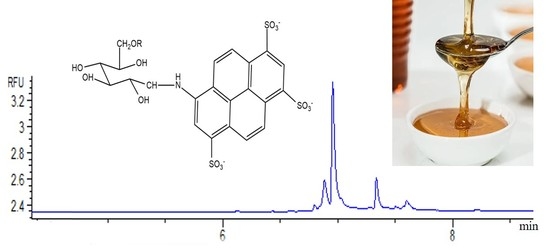
Analysis of Sugars in Honey Samples by Capillary Zone Electrophoresis Using Fluorescence Detection
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Instrumentation
2.3. Sample Preparation for Fluorescence Detection
3. Results and Discussion
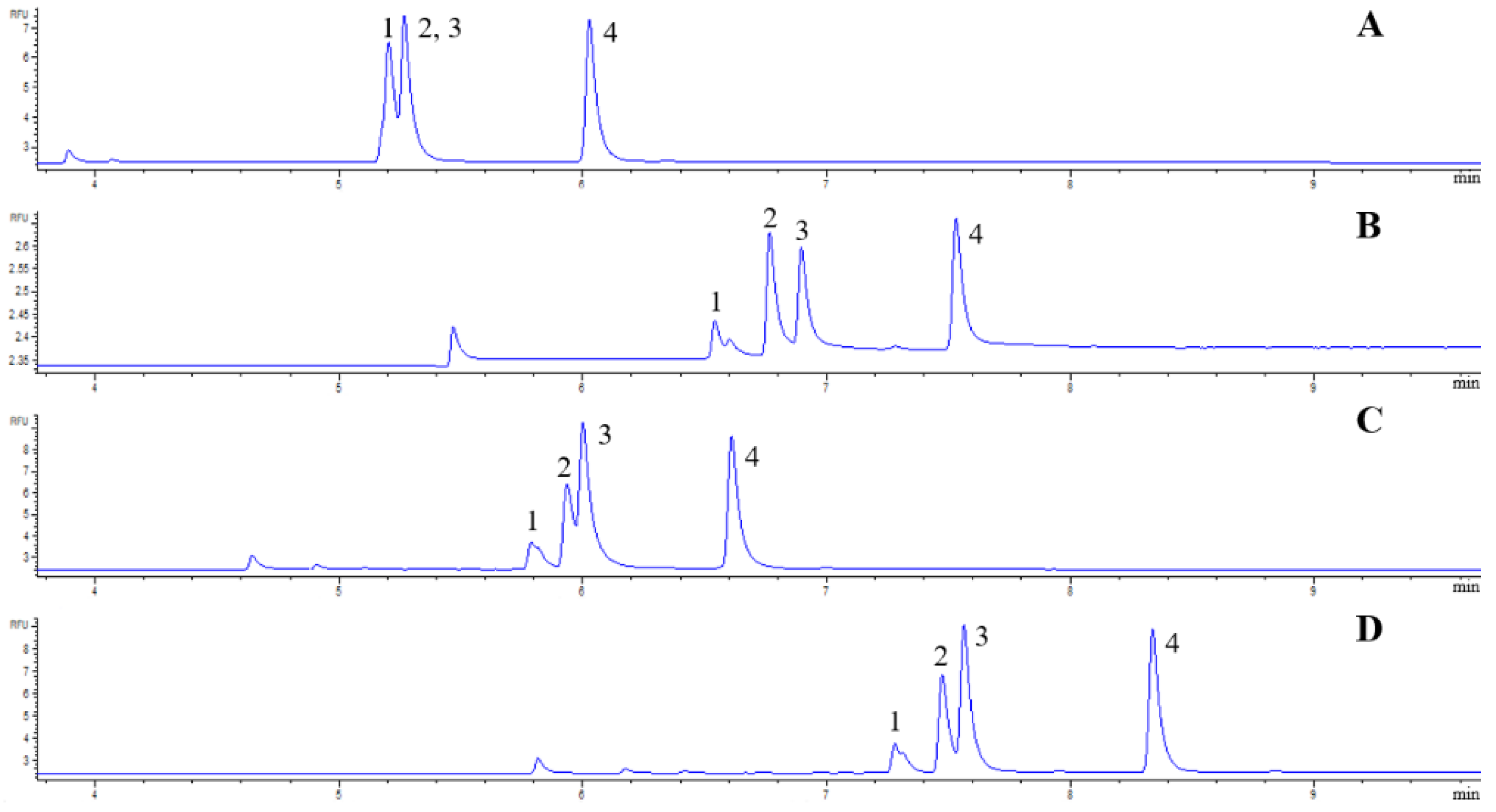
3.1. CZE Separations of APTS-Labeled Carbohydrates
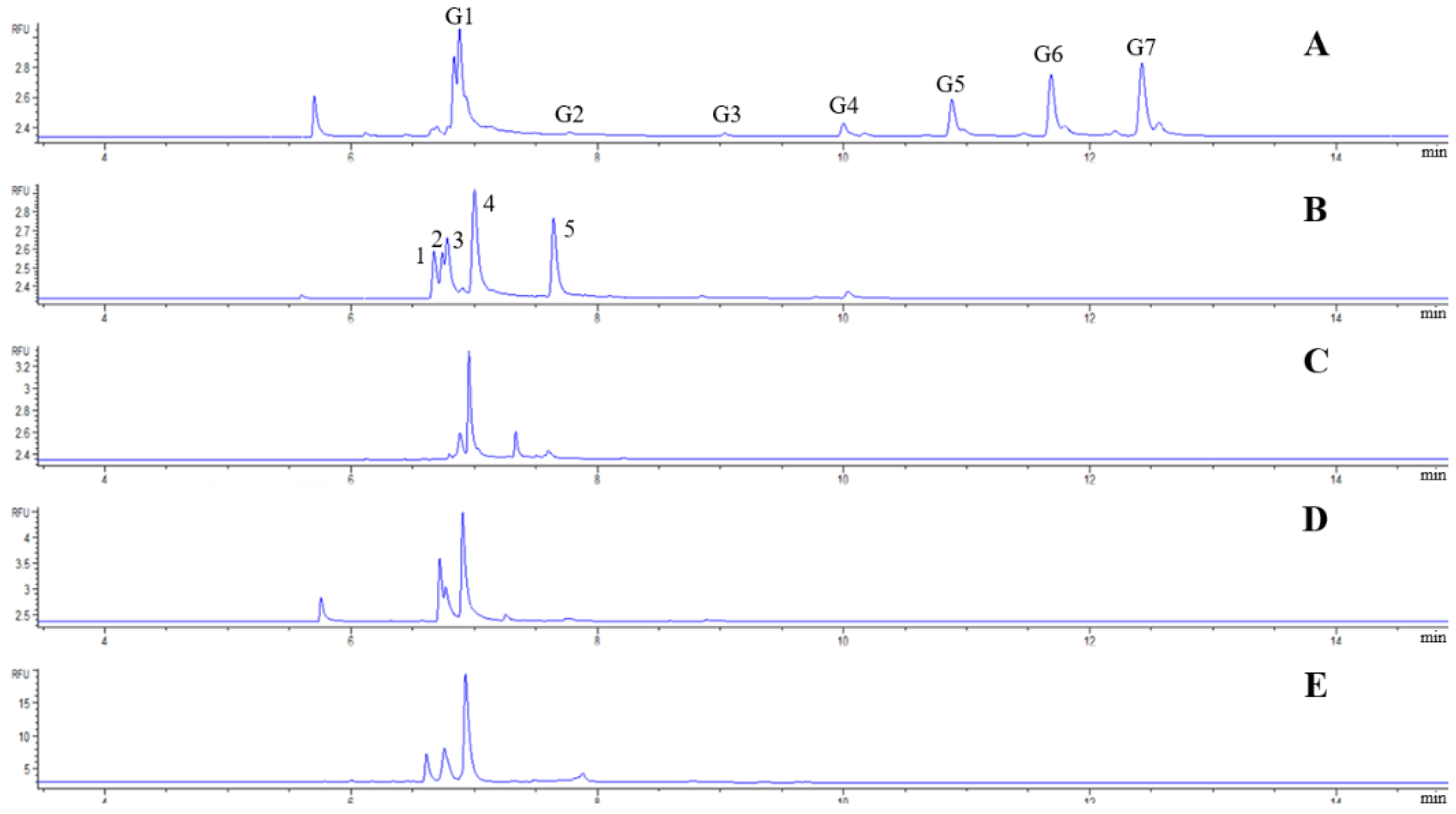
3.2. Analysis of Sugars in Honey Samples
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- Samarghandian, S.; Farkhondeh, T.; Samini, F. Honey and health: A review of recent clinical research. Phcog. Res. 2017, 9, 121–127. [Google Scholar] [PubMed]
- Machado de-Melo, A.A.; Bicudo de Almeida-Muradian, L.; Sancho, M.T.; Pascual-Mate, A. Composition and properties of Apis mellifera honey: A review. J. Apic. Res. 2018, 57, 5–37. [Google Scholar] [CrossRef]
- Asquieri, E.R.; Gomes de Moura, A.S.; Dianiny de Cassia, S.M.; Batista, R.D. Comparison of titulometric and spectrophotometric approaches towards the determination of total soluble and insoluble carbohydrates in foodstuff. Carpathian J. Food Sci. Technol. 2019, 11, 69–79. [Google Scholar]
- BeMiller, J.N. Chapter 10-Carbohydrate Analysis, Food Analysis; Springer: Berlin/Heidelberg, Germany, 2010; pp. 147–177. [Google Scholar]
- Slimestad, R.; Vagen, I.M. Thermal stability of glucose and other sugar aldoses in normal phase high performance liquid chromatography. J. Chromatogr. A 2006, 1118, 281–284. [Google Scholar] [CrossRef]
- Cordella, C.; Militao, J.S.L.T.; Clement, M.C.; Drajnudela, P.; Cabrol-Bass, D. Detection and quantification of honey adulteration via direct incorporation of sugar syrups or bee-feeding: Preliminary study using high-performance anion exchange chromatography with pulsed amperometric detection (HPAEC-PAD) and chemometrics. Anal. Chim. Acta 2005, 531, 239–248. [Google Scholar] [CrossRef]
- Hetricka, E.M.; Kramer, T.T.; Risleya, D.S. Evaluation of a hydrophilic interaction liquid chromatography design space for sugars and sugar alcohols. J. Chromatogr. A 2017, 1489, 65–74. [Google Scholar] [CrossRef]
- Weiβ, K.; Alt, M. Determination of single sugars, including inulin, in plants and feed materials by high-performance liquid chromatography and refraction index detection. Fermentation 2017, 3, 36. [Google Scholar]
- Al-Sanea, M.M.; Gamal, M. Critical analytical review: Rare and recent applications of refractive index detector in HPLC chromatographic drug analysis. Microchem. J. 2022, 178, 107339. [Google Scholar] [CrossRef]
- Jalaludin, I.; Kim, J. Comparison of ultraviolet and refractive index detections in the HPLC analysis of sugars. Food Chem. 2021, 365, 130514. [Google Scholar] [CrossRef]
- Suzuki, S.; Honda, S. A tabulated review of capillary electrophoresis of carbohydrates. Electrophoresis 1998, 19, 2539–2560. [Google Scholar] [CrossRef]
- Rassi, Z.E. Recent developments in capillary electrophoresis and capillary electrochromatography of carbohydrate species. Electrophoresis 1999, 20, 3134–3144. [Google Scholar] [CrossRef]
- Lu, G.; Crihfield, C.L.; Gattu, S.; Veltri, L.M.; Holland, L.A. Capillary electrophoresis separations of glycans. Chem. Rev. 2018, 118, 7867–7885. [Google Scholar] [CrossRef] [PubMed]
- Arentoft, A.M.; Michaelsen, S.; Sorensen, H. Determination of oligosaccharides by capillary zone electrophoresis. J. Chromatogr. A 1993, 652, 517–524. [Google Scholar] [CrossRef]
- Honda, S. Separation of neutral carbohydrates by capillary electrophoresis. J. Chromatogr. A 1996, 720, 337–351. [Google Scholar] [CrossRef]
- Mantovani, V.; Galeotti, F.; Maccari, F.; Volpi, N. Recent advances in capillary electrophoresis separation of monosaccharides, oligosaccharides, and polysaccharides. Electrophoresis 2018, 39, 179–189. [Google Scholar] [CrossRef]
- Soga, T.; Serwe, M. Determination of carbohydrates in food samples by capillary electrophoresis with indirect UV detection. Food Chem. 2000, 69, 339–344. [Google Scholar] [CrossRef]
- Kakehia, K.; Funakuboa, T.; Suzukia, S.; Odaa, Y.; Kitada, Y. 3-Aminobenzamide and 3-aminobenzoic acid, tags for capillary electrophoresis of complex carbohydrates with laser-induced fluorescent detection. J. Chromatogr. A 1999, 863, 205–218. [Google Scholar] [CrossRef]
- Carvalho, A.Z.; da Silva, J.A.F.; do Lago, C.L. Determination of mono- and disaccharides by capillary electrophoresis with contactless conductivity detection. Electrophoresis 2003, 24, 2138–2143. [Google Scholar] [CrossRef]
- Campa, C.; Coslovi, A.; Flamigni, A.; Rossi, M. Overview on advances in capillary electrophoresis-mass spectrometry of carbohydrates: A tabulated review. Electrophoresis 2006, 27, 2027–2050. [Google Scholar] [CrossRef]
- Starr, C.M.; Masada, R.I.; Hague, C.; Skop, E.; Klock, J.C. Fluorophore-assisted carbohydrate electrophoresis in the separation, analysis, and sequencing of carbohydrates. J. Chromatogr A 1996, 720, 295–321. [Google Scholar] [CrossRef]
- Paulus, A.; Klockow, A. Detection of carbohydrates in capillary electrophoresis. J. Chromatogr. A 1996, 720, 353–376. [Google Scholar] [CrossRef] [PubMed]
- Guttman, A. Analysis of monosaccharide composition by capillary electrophoresis. J. Chromatogr. A 1997, 763, 271–277. [Google Scholar] [CrossRef] [PubMed]
- Olajos, M.; Hajos, P.; Guenther, K.; Bonn, G.K.; Guttman, A. Sample preparation for the analysis of complex carbohydrates by multicapillary gel electrophoresis with light emitting diode induced fluorescence detection. Anal. Chem. 2008, 80, 4241–4246. [Google Scholar] [CrossRef] [PubMed]
- Shaheen, R.; Senn, J.P. Quantification of polysaccharides in water using capillary electrophoresis. Int. J. Environ. Anal. Chem. 2005, 85, 177–198. [Google Scholar] [CrossRef]
- Rizelio, V.M.; Tenfen, L.; Silveira, R.; Valdemiro, L.; Gonzaga, L.V.; Costa, A.C.O.; Fett, R. Development of a fast capillary electrophoresis method for determination of carbohydrates in honey samples. Talanta 2012, 93, 62–66. [Google Scholar] [CrossRef]
- Dominguez, M.A.; Jacksen, J.; Emmer, A.; Centurion, M.E. Capillary electrophoresis method for the simultaneous determination of carbohydrates and proline in honey samples. Microchem. J. 2016, 129, 1–4. [Google Scholar] [CrossRef]
- Lua, Y.; Hub, Y.; Wangc, T.; Yanga, X.; Zhaoc, Y. Rapid determination and quantitation of compositional carbohydrates to identify honey by capillary zone electrophoresis. J. Food 2017, 15, 531–537. [Google Scholar] [CrossRef]
- Liang, P.; Sun, M.; He, P.; Zhang, L.; Chen, G. Determination of carbohydrates in honey and milk by capillary electrophoresis in combination with graphene–cobalt microsphere hybrid paste electrodes. Food Chem. 2016, 190, 64–70. [Google Scholar] [CrossRef]
- Chen, F.T.; Dobashi, T.S.; Evangelista, R.A. Quantitative analysis of sugar constituents of glycoproteins by capillary electrophoresis. Glycobiology 1998, 8, 1045–1052. [Google Scholar] [CrossRef]
- Volpi, N. Capillary Electrophoresis of Carbohydrates, from Monosaccharides to Complex Polysaccharides; Springer: Berlin/Heidelberg, Germany, 2011. [Google Scholar]
- Horvath, J.; Dolnik, V. Polymer wall coatings for capillary electrophoresis. Electrophoresis 2001, 22, 644–655. [Google Scholar] [CrossRef]
- Hamm, M.; Wang, Y.; Rustandi, R.R. Characterization of N-Linked glycosylation in a monoclonal antibody produced in NS0 cells using capillary electrophoresis with laser-induced fluorescence detection. Pharmaceuticals 2013, 6, 393–406. [Google Scholar] [CrossRef] [PubMed]
- Bunz, S.C.; Rapp, E.; Neususs, C. Capillary electrophoresis/mass spectrometry of APTS-labeled glycans for the identification of unknown glycan species in capillary electrophoresis/laser-induced fluorescence systems. Anal. Chem. 2013, 85, 10218–10224. [Google Scholar] [CrossRef] [PubMed]
- Manikis, I.; Thrasivoulou, A. The relation of physicochemical characteristics of honey and the crystallization sensitive parameters. Apiacta 2001, 36, 106–112. [Google Scholar]
- Gamal, M.; Naguib, I.A.; Panda, D.S.; Abdallah, F. Comparative study of four greenness assessment tools for selection of greenest analytical method for assay of hyoscine N-butyl bromide. Anal. Methods 2021, 13, 369–380. [Google Scholar] [CrossRef]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE—Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Plotka-Wasylka, J.; Wojnowsk, W. Complementary green analytical procedure index (ComplexGAPI) and software. Green Chem. 2021, 23, 8657–8665. [Google Scholar] [CrossRef]


| Glucose | Fructose | ||
|---|---|---|---|
| Regression equation | 0.078x − 0.2736 | 0.044x − 11.39 | |
| Correlation coefficient | 0.9988 | 0.9916 | |
| LOD (ng/mL) | 8.89 | 8.76 | |
| LOQ (ng/mL) | 29.6 | 29.2 | |
| Range (ng/mL) | 30–1000 | 30–1000 | |
| RSD %, (N = 5) | time | 0.496 | 0.509 |
| area | 2.48 | 2.82 | |
| Theoretical plate number | 110,140 | 215,657 | |
| Concentration (m/m%) | |||
|---|---|---|---|
| Phacelia honey | Acacia honey | Honeydew honey | |
| Glucose | 37.8 ± 1.5 | 26.7 ± 1.1 | 19 ± 0.76 |
| Fructose | 32.7 ± 1.3 | 43.2 ± 1.7 | 27.1 ± 1 |
| Fructose/Glucose | 0.86 | 1.61 | 1.42 |
| n = 3 | |||
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Andrasi, M.; Gyemant, G.; Sajtos, Z.; Nagy, C. Analysis of Sugars in Honey Samples by Capillary Zone Electrophoresis Using Fluorescence Detection. Separations 2023, 10, 150. https://doi.org/10.3390/separations10030150
Andrasi M, Gyemant G, Sajtos Z, Nagy C. Analysis of Sugars in Honey Samples by Capillary Zone Electrophoresis Using Fluorescence Detection. Separations. 2023; 10(3):150. https://doi.org/10.3390/separations10030150
Chicago/Turabian StyleAndrasi, Melinda, Gyongyi Gyemant, Zsofi Sajtos, and Cynthia Nagy. 2023. "Analysis of Sugars in Honey Samples by Capillary Zone Electrophoresis Using Fluorescence Detection" Separations 10, no. 3: 150. https://doi.org/10.3390/separations10030150
APA StyleAndrasi, M., Gyemant, G., Sajtos, Z., & Nagy, C. (2023). Analysis of Sugars in Honey Samples by Capillary Zone Electrophoresis Using Fluorescence Detection. Separations, 10(3), 150. https://doi.org/10.3390/separations10030150