Abstract
Despite the fact that several analytical methodologies have been reported for the determination of curcumin (CCM) in a wide range of sample matrices, the greener liquid chromatographic approaches to determine CCM are scarce in the literature. Therefore, this research is designed to develop and validate a greener stability-indicating “high-performance liquid chromatography (HPLC)” methodology to determine CCM in an in-house developed nanoemulsion, Curcuma longa L. extract, and commercial tablets. CCM was measured on a Nucleodur (150 mm × 4.6 mm) RP C18 column with 5 µm-sized particles. Ethanol and ethyl acetate (83:17 v/v) made up the greener eluent system, which was pumped at a flow speed of 1.0 mL/min. At a wavelength of 425 nm, CCM was detected. The greener HPLC methodology was linear in the 1–100 µg/mL range, with a determination coefficient of 0.9983. The greener HPLC methodology for CCM estimation was also rapid (Rt = 3.57 min), accurate (%recoveries = 98.90–101.85), precise (%CV = 0.90–1.11), and sensitive (LOD = 0.39 µg/mL and LOQ = 1.17 µg/mL). The AGREE approach predicted the AGREE score of 0.81 for the established HPLC technique, indicating an outstanding greenness profile. The utility of the greener HPLC methodology was demonstrated by determining CCM in the in-house developed nanoemulsion, Curcuma longa extract, and commercial tablets. The % amount of CCM in the in-house developed nanoemulsion, Curcuma longa extract, and commercial tablets was found to be 101.24%, 81.15%, and 78.41%, respectively. The greener HPLC methodology was able to detect its degradation product under various stress conditions, suggesting its stability-indication characteristics. These results suggested that CCM in developed nanoemulsion, plant extract samples, and commercial tablets may be routinely determined using the greener HPLC methodology.
1. Introduction
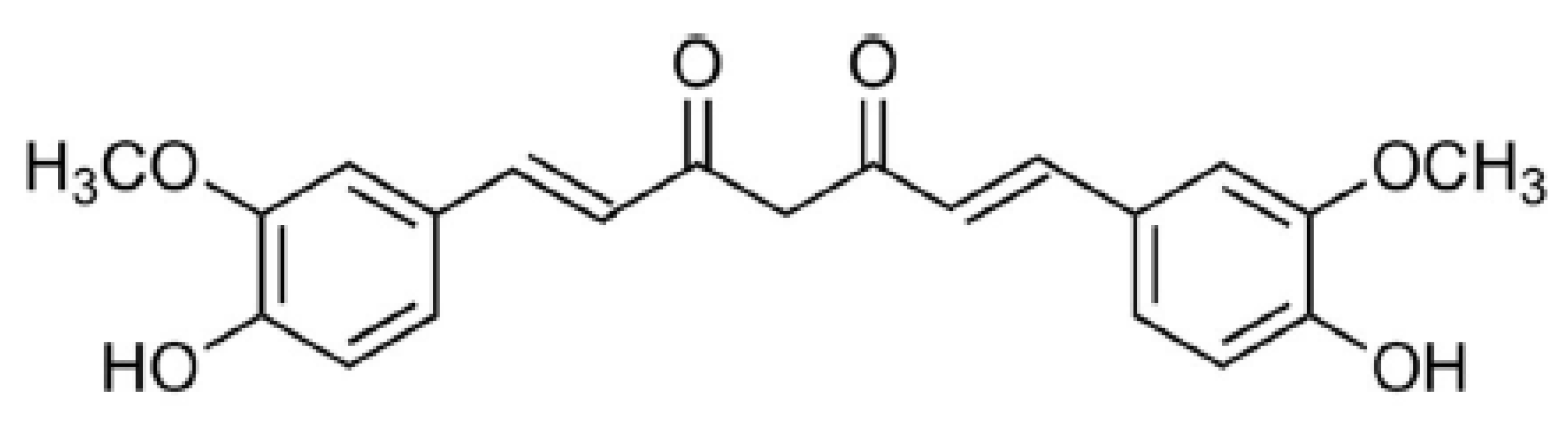
Turmeric is a spice, which is obtained from Curcuma longa L [1]. It is a rich source of phenolic compounds, namely curcuminoids [1,2]. Three main curcuminoids have been reported in C. longa extract: curcumin (CCM) (Figure 1), demethoxycurcumin (DCCM), and bisdemethoxycurcumin (BDCCM) [1,3]. Marketed CCM contains CCM, DCCM, and BDCCM, but its main constituent is CCM [4]. Various therapeutic activities have been reported for C. longa extract, which are mainly due to the presence of curcuminoids [1,4]. CCM is a yellow-colored pigment, which is used for the coloring of various food products [4]. In the literature, the variety of therapeutic activities of CCM are reported, including antioxidant [1,2], anti-inflammatory [5], antimicrobial [6], antibacterial [7], antiviral [8], antiparasitic [9], antimutagenic [10], and antiproliferative activities [11,12,13] etc. As a consequence, the quality control and standardization of CCM in its pharmaceutical products, food products, and herbal extracts are significant due to its variety of therapeutic activities.
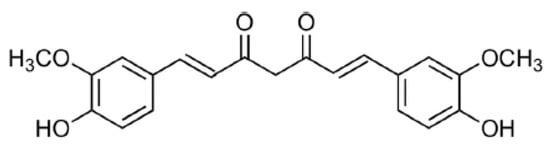
Figure 1.
Molecular structure of curcumin (CCM).
Different analytical techniques have been utilized for the qualitative and quantitative detection of CCM in herbal extracts, commercial pharmaceutical formulations, commercial food products, and biological materials. For the determination of CCM in food products, spices, and herbal extracts, various ultra-violet (UV) spectrometry methods have been reported [14,15,16]. A spectrofluorometric assay has also been reported to determine CCM in a nanoliposomal formulation and mice plasma samples [17]. Fluorescence detection of CCM has also been carried out in the literature [18,19]. Reported spectrometry and fluorescence methods of CCM measurement were less accurate and sensitive than the current method [14,15,16,18,19]. The variety of high-performance liquid chromatographic methods (HPLC) have also been used to determine CCM in food products, pharmaceutical products, and herbal extracts [4,20,21,22,23,24,25,26,27]. However, most of the reported HPLC methods were environmentally toxic and less sensitive than the current HPLC method [4,20,25,26,27]. CCM in food products and herbal extracts has also been identified using liquid chromatography tandem mass-spectrometry (LC-MS) and ultra-performance liquid chromatography tandem mass-spectrometry (UPLC-MS) approaches [28,29,30,31]. Various high-performance thin-layer chromatography (HPTLC) approaches are also reported to determine CCM in herbal extracts and CCM dosage forms [32,33,34,35,36,37]. Some LC-MS and UPLC-MS approaches have also been used to determine CCM in the plasma samples of rat, equine, and human [38,39,40,41]. Carbon nanotube-based composites have also been used to determine CCM in food products [42,43]. Reported LC-MS, UPLC-MS, and HPTLC approaches were also found to be more environmentally toxic than the current HPLC method [28,29,30,31,32,33,34,35,36,37,38,39,40,41,42,43]. Some voltammetry approaches have also been reported to determine CCM in natural food supplements and food spices [44,45,46]. Some electrochemical approaches were also used to determine CCM in its pure form and human blood serum sample [47,48]. Some other techniques, such as the Fourier transform near infrared spectroscopy approach, nanosensor, and solvatochromic approach have also been reported to determine CCM [49,50,51].
The detailed literature survey revealed the wide range of analytical techniques for the determination of CCM in distinct sample matrices. However, the greener/sustainable HPLC approaches of CCM detection are scarce in the literature. Furthermore, no greenness index of any reported HPLC method of CCM analysis has been reported. Several qualitative and quantitative approaches are reported to evaluate the greener characteristics of analytical procedures [52,53,54,55,56]. However, the “analytical GREEnness (AGREE)” metric methodology exclusively considers all twelve green analytical chemistry (GAC) principles for the determination of the greenness profile [54]. As a result, the “AGREE metric methodology” was used for the determination of the greener profile of the present HPLC assay of CCM analysis [54]. Based on all these assumptions, the objective of this research was to design and validate a simple, rapid, and greener HPLC methodology for the determination of CCM in an in-house developed CCM nanoemulsion, C. longa extract, and commercial tablets. The greener HPLC methodology for determining CCM was validated, following the “International Council for Harmonization (ICH)-Q2-R1” procedures [57].
2. Materials and Methods
2.1. Materials
The working standard of CCM was obtained from “E-Merck (Darmstadt, Germany)”. HPLC-grade solvents, such as methanol, ethyl acetate, acetone, and ethanol were provided by “Sigma Aldrich (St. Louis, MO, USA)”. High-pure water was collected from “Milli-Q® water purifier (Millipore, Lyon, France)”. All other chemicals and reagents used were of analytical grades. The fresh rhizomes of Curcuma longa were purchased from the hypermarket in Riyadh, Saudi Arabia. The commercial tablets (having 1000 mg of standardized C. longa extract) were obtained from a pharmacy shop in Riyadh, Saudi Arabia. The nanoemulsion formulation of CCM was developed in the laboratory by the aqueous phase titration method using clove oil (oil phase), Tween-20 (surfactant), Transcutol-HP (cosurfactant), and purified water (aqueous phase).
2.2. Instrumentation and Analytical Conditions
Waters HPLC system, composed of a 1515 isocratic pump, a 717 automatic sampler, quad LC-10A VP pumps, a programmable UV-visible variable wavelength detector, a column oven, an SCL 10AVP system controller, and an inline vacuum degasser, was used to measure CCM at a temperature of 25 ± 1 °C. The Millennium program (Version 32, Waters, Milford, MA, USA) was used to process and evaluate the data. CCM was determined using a Nucleodur (150 mm × 4.6 mm) RP C18 column with 5 µm-sized particles. The mixture of ethanol and ethyl acetate (83:17% v/v) was used as the greener eluent system. The eluent system flowed with a flow speed of 1.0 mL/min. At a wavelength of 425 nm, CCM was detected. The samples (20 µL) were injected into the system using a waters autosampler.
2.3. CCM Calibration Curve
In triplicates (n = 3), the appropriate amount of CCM was dispensed in the eco-friendly eluent system to produce a stock solution with a 200 µg/mL concentration. To obtain the serial dilutions in the required range (1–100 µg/mL) in triplicates (n = 3), the requisite aliquots from the stock solution of CCM (200 µg/mL) were diluted with the greener eluent system. Using the greener HPLC methodology, the chromatographic response for each concentration of CCM was identified. To produce the CCM calibration curve, CCM concentrations were plotted against the measured chromatographic response in triplicates (n =3).
2.4. Sample Preparation for the Determination of CCM in Curcuma Longa Extract
Approximately 10.0 g of fresh rhizomes of C. longa were taken and powdered finely. The fine powder was soaked in 100 mL of ethanol-water mixture (50:50 v/v) and ultrasonicated for about 60 min. The temperature was kept constant at 25 °C. The supernatant was recovered and filtered using nylon filter paper. The solvents were evaporated using a rotary vacuum evaporator at 40 °C. The obtained sample was used to determine CCM in C. longa extract using the greener HPLC approach.
2.5. Analytical Method Development
As the eluent systems, different combinations of greener solvents were examined to develop a trustworthy stability-indicating greener HPLC assay for the detection of CCM in an in-house developed nanoemulsion, C. longa extract, and commercial tablets. The greener solvent compositions of methanol-water, ethanol-water, acetone-water, methanol-ethanol, ethyl acetate-methanol, ethyl acetate-ethanol, ethyl acetate-acetone, acetone-ethanol, and acetone-methanol were among the numerous greener solvents that were examined. Various aspects were taken into account when determining the best greener eluent system, including the affordability, greenness/toxicity profile, the assay’s sensitivity, the analysis duration, the chromatographic parameters, and the solvents’ compatibilities with one another. As a consequence, various greener solvent compositions were examined as the eluent system in combined forms. Finally, the most trustworthy eluent system for future investigation was discovered to be a 83:17 volume-to-volume blend of ethanol and ethyl acetate.
2.6. Validation Parameters
Following ICH-Q2-R1 procedures, the greener HPLC technique for the measurement of CCM was verified for various parameters [57]. By drawing the linearity graphs, the linearity of the greener HPLC methodology was examined in the 1–100 μg/mL range. CCM solutions that had just been prepared were added to the HPLC system in triplicates (n = 3), and the chromatographic response was recorded. The CCM calibration curve was derived by plotting the CCM concentration vs. chromatographic response.
The system appropriateness parameters for the greener HPLC technology were derived using a number of chromatographic characteristics, including resolution (Rs), selectivity factor (α), tailing factor (As), capacity factor (k), and theoretical plates number (N) [58,59].
The intra-day and inter-day accuracy of the greener HPLC technology was assessed using a standard addition/spiking method in terms of % recovery [57]. To investigate intra-day accuracy at three different quality control (QC) levels, the pre-analyzed QC level of CCM (10 µg/mL) was spiked with an extra 50, 100, 150% of CCM solution to obtain low QC (LQC = 15 µg/mL), middle QC (MQC = 20 µg/mL), and high QC (HQC = 25 µg/mL). The obtained LQC, MQC, and HQC of CCM were analyzed on the same day in three replicates (n = 3) to measure intra-day accuracy. On three distinct days, three replicates (n = 3) of CCM’s LQC, MQC, and HQC levels were obtained by the spiking method and used to examine inter-day accuracy. The percentage recovery, percentage coefficient of variance (%CV), and standard error were computed at each QC level.
The greener HPLC methodology’s precision was examined using intra-day and inter-day variations. On the same day, three replicates (n = 3) of the LQC, MQC, and HQC levels of CCM were used to assess the intra-day precision. At the LQC, MQC, and HQC of the CCM on three distinct days, inter-day precision was assessed in three replicates (n = 3).
To examine the influence of intentional chromatographic alterations on CCM measurement, the robustness of the greener HPLC methodology was evaluated. The CCM MQC (20 µg/mL) was chosen for the robustness assessment. By altering the greener eluent’s composition, flow speed, and detecting wavelength, robustness was assessed. The initial ethanol: ethyl acetate (83:17 v/v) eluent system was altered to ethanol: ethyl acetate (85:15 v/v) and ethanol: ethyl acetate (81:19 v/v) for the robustness assessment, and the differences in chromatographic response were recorded. For the purpose of evaluating robustness, the original flow speed of 1 mL/min was changed to flow rates of 1.15 mL/min and 0.85 mL/min, and the changes in chromatographic response were noted. For the robustness assessment, the initial detection wavelength (425 nm) was changed to detection wavelengths of 430 nm and 420 nm, and the differences in chromatographic response were recorded.
The standard deviation approach was used to evaluate the sensitivity of the greener HPLC methodology in terms of “limit of detection (LOD) and limit of quantitation (LOQ)” [57]. The standard deviation of the blank sample (without CCM) was computed following the injection of the blank sample into the HPLC apparatus three times (n = 3). After that, established techniques that have been documented in the literature were used to calculate the LOD and LOQ values for CCM [57,58].
The solution stability of CCM in stock solution and nanoemulsion was performed at the MQC level (20 µg/mL) at two distinct temperatures, namely, the bench temperature (25 ± 0.5 °C) and refrigeration temperature (4 ± 0.5 °C). These studies were carried out for the period of 72 h. The MQC concentration of CCM was freshly produced in a greener eluent system. The freshly prepared nanoemulsion was also diluted with the greener eluent system to obtain the MQC level of CCM. Both solutions were stored at 25 ± 0.5 °C and 4 ± 0.5 °C for 72 h and the decomposition of CCM was evaluated by measuring the rest of CCM after storage.
2.7. Forced Degradation/Selectivity Studies
Forced degradation studies under a variety of stress conditions, including acidic (HCl) stress, basic (NaOH) stress, oxidative (H2O2) stress, thermal stress, and photolytic stress conditions, were conducted in order to evaluate the selectivity and stability-indicating property of the greener HPLC methodology [56,60]. The degradation studies were performed in mild conditions, as recommended by ICH [57]. The 40 μg/mL of CCM was freshly prepared into the eluent system for acid and base-induced degradation. By mixing 4 mL of 1 M HCl and 4 mL of 1 M NaOH into an aliquot (1 mL) of this solution, acid and base hydrolysis were applied. For the determination of CCM in the presence of its acid- and base-degradation products, respectively, these mixtures were refluxed for 48 h at 60 °C before being evaluated using the greener HPLC approach [60].
The 40 μg/mL of CCM was freshly produced and introduced into the eluent system for oxidative degradation testing. This solution was oxidatively degraded by adding 4 mL of 30% H2O2 to an aliquot (1 mL) of it. For the detection of CCM in the presence of its oxidative-degradation products, this mixture was refluxed for 48 h at 60 °C before being evaluated using the greener HPLC approach [60].
The 40 μg/mL concentration of CCM (1.0 mL) was diluted with eluent system to produce a total volume of 5.0 mL. This solution was then subjected to a hot air oven for 48 h at 60 °C for thermal degradation tests. It was then assessed utilizing the ecofriendly HPLC technology for the detection of CCM in the presence of its thermal-degradation products [60].
For photolytic degradation investigations, a 1.0 mL aliquot of 40 μg/mL concentration was diluted with the eluent system to obtain the total volume of 5.0 mL. This solution was then subjected to a UV chamber at 254 nm for 48 h. Then, CCM was determined using the ecofriendly HPLC methodology while the photolytic-degradation products were present [60].
2.8. Greenness Measurement
The greenness profile for the ecofriendly HPLC methodology was determined using the “AGREE metric approach” [54]. The AGREE scores (0.0–1.0) were derived using the “AGREE: The Analytical Greenness Calculator (version 0.5, Gdansk University of Technology, Gdansk, Poland, 2020)”.
2.9. Application of Greener HPLC Methodology in Determination of CCM in In-House Developed Nanoemulsion
In the lab, a CCM nanoemulsion was created and evaluated. One mL of an in-house developed nanoemulsion containing 20 mg/mL of CCM was appropriately diluted with the eluent system to produce 100 mL of stock solution in order to determine the CCM content. Following a suitable dilution with the eluent system and a 15-min sonication of this solution, the CCM content was determined using the ecofriendly HPLC approach. The potential for interference from components of the formulation that are nanoemulsions was also investigated.
2.10. Application of Greener HPLC Methodology in Determination of CCM in Curcuma Longa Extract
An amount of 1 mL of freshly prepared Curcuma longa extract was appropriately diluted with the eluent system to create 50 mL of stock solution in order to measure the CCM content. Following a suitable dilution with the eluent system and a 15-min sonication of this solution, the CCM content was determined using the ecofriendly HPLC approach.
2.11. Application of Greener HPLC Methodology in Determination of CCM in Commercial Tablets
The average mass of ten marketed tablets—each having 1000 mg of C. longa standardized extract—was determined. Ten tablets were crushed using a glass pestle and mortar to obtain fine powder. An amount of 100 mL of the eluent system was combined with a portion of the powder containing an average weight of the tablet. Then, 1 mL of this solution was added to 50 mL of the eluent system. To eliminate any insoluble excipient, the obtained solution of the commercial tablet was filtered using Whatman filter paper (No. 41) and sonicated at 25 °C for 25 min. Using the eco-friendly HPLC approach, the amount of CCM in commercial tablets was determined.
3. Results and Discussion
3.1. Analytical Method Development
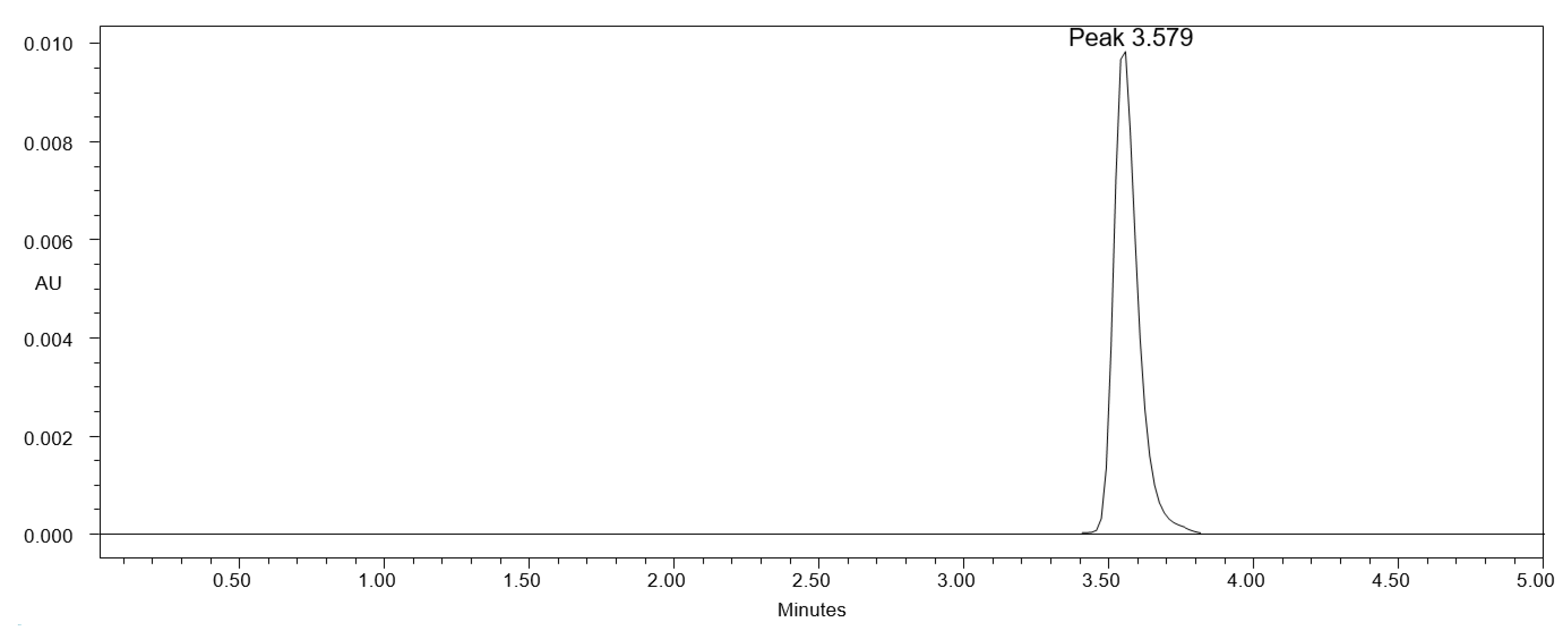
Table 1 provides a summary of the measured chromatographic characteristics and the composition of distinct greener eluent systems. The use of methanol-water, ethanol-water, and acetone-water in different compositions during the analytical method development step resulted in a subpar chromatographic response of CCM, which was exhibiting higher As values (As > 2.0) with low N values (<2000). Furthermore, the use of methanol-ethanol, methanol-acetone, and methanol-ethyl acetate in distinct compositions caused CCM to have a poor chromatographic response, as well as increased As values (As > 1.20) and low N values (<3000). Further, the use of ethanol-acetone and ethyl acetate-acetone was also examined as the greener eluent systems. With high As values (As > 1.80) and low N values (<2500), the chromatographic response of CCM was once more subpar. However, a well-resolved and intact CCM chromatographic peak with good As values and greater N values was shown by the binary mixture of ethanol and ethyl acetate in distinct composition. The binary mixture of ethanol and ethyl acetate (83:17 v/v) gave the best chromatographic response and reliable retention time (Rt), as well as As and N values, among the various ethanol and ethyl acetate mixes examined (Figure 2). As a consequence, the binary combination of ethanol and ethyl acetate (83:17 v/v) was selected as the final greener eluent system for measuring CCM with an appropriate As (1.09) and N (5081), quick analysis (Rt = 3.57 min), and a good analysis period (5 min). The finally used solvents, such as ethanol and ethyl acetate, are non-toxic and environmentally safe [61,62]. Both solvents have been extensively studied as green solvents in the literature [58,59,60,61,62]. As a result, these solvents were used in this study to create a greener HPLC approach to determine CCM.

Table 1.
The optimization of greener eluent systems and measured analytical responses for standard curcumin (CCM) (mean ± SD, n = 3).
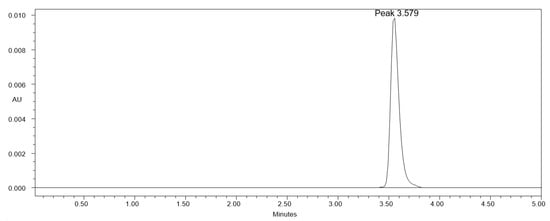
Figure 2.
A greener high-performance liquid chromatography (HPLC) chromatogram of CCM in solution derived using ethanol: ethyl acetate (83:17 v/v) greener eluent system.
3.2. Validation Parameters
Numerous validation parameters for the greener HPLC methodology were assessed following ICH-Q2-R1 procedures [57]. The linearity graphs were produced using freshly prepared CCM samples (1–100 µg/mL). The outcomes of a linear regression analysis of the CCM calibration curve are summarized in Table 2. The linear calibration curve for CCM was between 1 and 100 µg/mL. According to estimates, the calibration curve’s determination coefficient (R2) and regression coefficient (R) values are 0.9983 and 0.9991, respectively, suggesting a good relation between CCM concentrations vs. the measured response. These data demonstrated the efficiency of the greener HPLC methodology for determining CCM.

Table 2.
Linear regression data for the calibration curve of CCM for the greener high-performance liquid chromatography (HPLC) methodology (mean ± SD, n = 3).
The system suitability parameters for the greener HPLC methodology were estimated using the Rs, α, As, k, and N and results are summarized in Table 3. The greener HPLC methodology’s values for Rs, peak symmetry, α, As, k, and N were found to be satisfactory and trustworthy for determining CCM.

Table 3.
Optimized chromatographic peak parameters to determine CCM for the greener HPLC methodology (mean ± SD, n = 3).
The percent recovery at three distinct QC levels was used to evaluate the intra-day and inter-day accuracy of the greener HPLC methodology. The results are summarized in Table 4. At three distinct QC levels, the intra-day and inter-day percent recoveries of CCM were determined to be 99.84–101.85 and 98.90–101.44 percent, respectively. High percent recoveries for the greener HPLC methodology for determining CCM point to its accuracy.

Table 4.
Intra-day and inter-day accuracy data of CCM for the greener HPLC methodology (mean ± SD; n = 3).
The results of the intra-day and inter-day precisions are included in Table 5 and are expressed in %CV. For CCM, the intraday precision percent CVs were observed to range from 0.86 to 0.94%. Contrarily, the %CVs for inter-day precision ranged between 0.96 and 1.17%. Low %CVs in the greener HPLC methodology for determining CCM indicated its precision.

Table 5.
Intra-day and inter-day precision data of CCM for the greener HPLC approach (mean ± SD; n = 3).
Table 6 summarizes the outcomes of the robustness evaluation for the MQC level of CCM. When evaluating robustness by altering the composition of the eluent system, the %CV and Rt were found to be 1.02–1.28% and 3.55–3.59 min, respectively. The %CV and Rt were calculated to be 1.16–1.18% and 3.23–3.81 min, respectively, in the scenario of a robustness assessment when the flow speed was altered. The %CV and Rt were found to be 1.14–1.22% and 3.54–3.60 min, respectively, in the scenario of the robustness assessment by altering the detecting wavelength. Low CVs and minimal Rt value variation in the greener HPLC methodology for detecting CCM point to its robustness.

Table 6.
Robustness data of CCM at MQC (20 µg/mL) for the greener HPLC methodology (mean ± SD; n = 3).
The results of analyzing the sensitivity of the ecofriendly HPLC methodology in terms of “LOD and LOQ” are presented in Table 2. According to calculations, the “LOD and LOQ” using the ecofriendly HPLC approach are 0.39 ± 0.03 µg/mL and 1.17 ± 0.09 µg/mL, respectively. These results indicated that the ecofriendly HPLC technology would be sensitive enough to identify and measure CCM in a wide range of concentrations.
The stability of CCM in stock solution and nanoemulsion formulation at two distinct temperatures was also determined. The results of stability determination at two distinct temperatures are presented in Table 7. The CCM degradation was determined by measuring the rest of CCM concentration after storage. The CCM decomposition was very low when stored for 72 h at 25 ± 0.5 °C and at 4 ± 0.5 °C when the peak areas of the stored CCM solution and nanoemulsion were compared to those derived using a freshly prepared CCM solution and CCM nanoemulsion. The precision of the CCM stock solution and nanoemulsion in terms of %CV was measured to be 0.71–0.80% and 0.74–0.79%, respectively, at two distinct temperatures. In addition, the % recovery of the CCM stock solution and nanoemulsion was determined to be 98.45–99.85% and 100.70–100.85%, respectively, at two distinct temperatures. CCM was found to be sufficiently stable in stock solution and nanoemulsion formulation at 25 and 4 °C based on these results.

Table 7.
Stability data of CCM in stock solution and nanoemulsion formulation at MCQ level at two different temperatures (mean ± SD; n = 3).
3.3. Forced Degradation and Selectivity Studies
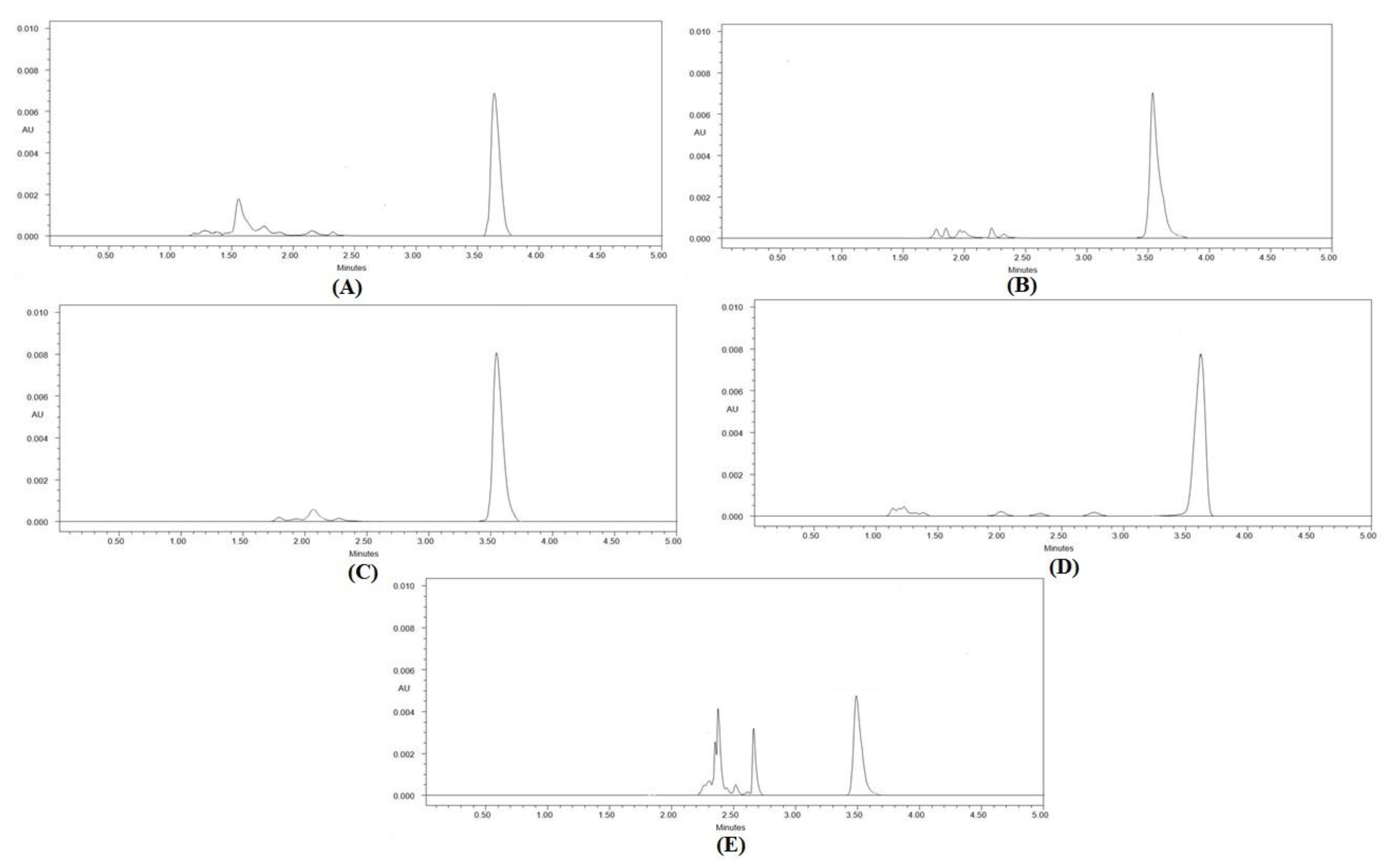
By subjecting the 40 µg/mL concentration of CCM to various stress conditions, the selectivity and stability-indicating properties for the greener HPLC methodology were assessed. Figure 3 and Table 8 provide an overview of the outcomes of selectivity under various stress circumstances using the ecofriendly HPLC approach.
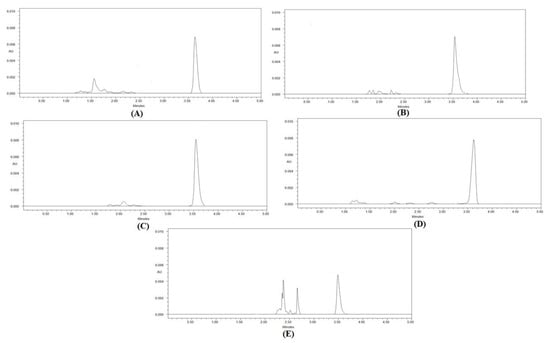
Figure 3.
Greener HPLC chromatograms of CCM recorded under (A) acid-induced degradation, (B) base-induced degradation, (C) oxidative degradation, (D) thermal degradation, and (E) photolytic degradation of CCM.

Table 8.
Results of forced-degradation studies of CCM at 40 µg/mL concentration under various stress tests for the greener HPLC assay (mean ± SD; n = 3).
The forced degradation investigations’ chromatograms showed well-separated CCM peaks, along with a few other peaks of degradation products (Figure 3). In total, 87.85% of CCM was preserved under acid stress degradations, while 13.15 percent was degraded (Table 8). As a result, it was discovered that CCM was adequately stable under acidic degradations. The Rt value for CCM breakdown under acid stress was somewhat off (Rt = 3.53 min) (Figure 3A). In total, 84.02% of CCM was still present at base-stress degradations, while 15.98% was degraded (Table 8). As a result, it was discovered that CCM was adequately stable under alkali degradations. Additionally, a small shift was made to the Rt value of CCM during base-stress degradation (Rt = 3.56 min) (Figure 3B). Only 3.15% of CCM was found to be destroyed under oxidative-stress degradations, leaving 96.85% of the original material intact. As a result, it was discovered that CCM was adequately stable under oxidative stress degradation. The Rt value of CCM during oxidative stress degradation was not altered (Rt = 3.57 min) (Figure 3C). Under thermal degradation, 99.92% of CCM remained and only a negligible amount (0.08%) was degraded. Hence, CCM was found to be highly stable under thermal degradation. The Rt value of CCM under thermal degradation was not shifted (Rt = 3.57 min) (Figure 3D). Under photolytic degradation, 45.60% of CCM remained and 54.40% was degraded. As a result, CCM was found to be highly unstable under photolytic degradation. The Rt value of CCM under photolytic degradation was not shifted (Rt = 3.57 min) (Figure 3E). Overall, the maximum degradation of CCM was found under photolytic degradation. The degradation patterns of CCM were found to be identical with those reported previously in the literature [23]. Since the greener HPLC methodology was able to detect CCM in the presence of its degradation products, it can be considered as a stability-indicating one. Overall, these results indicated the selectivity and stability-indicating characteristics of the greener HPLC methodology.
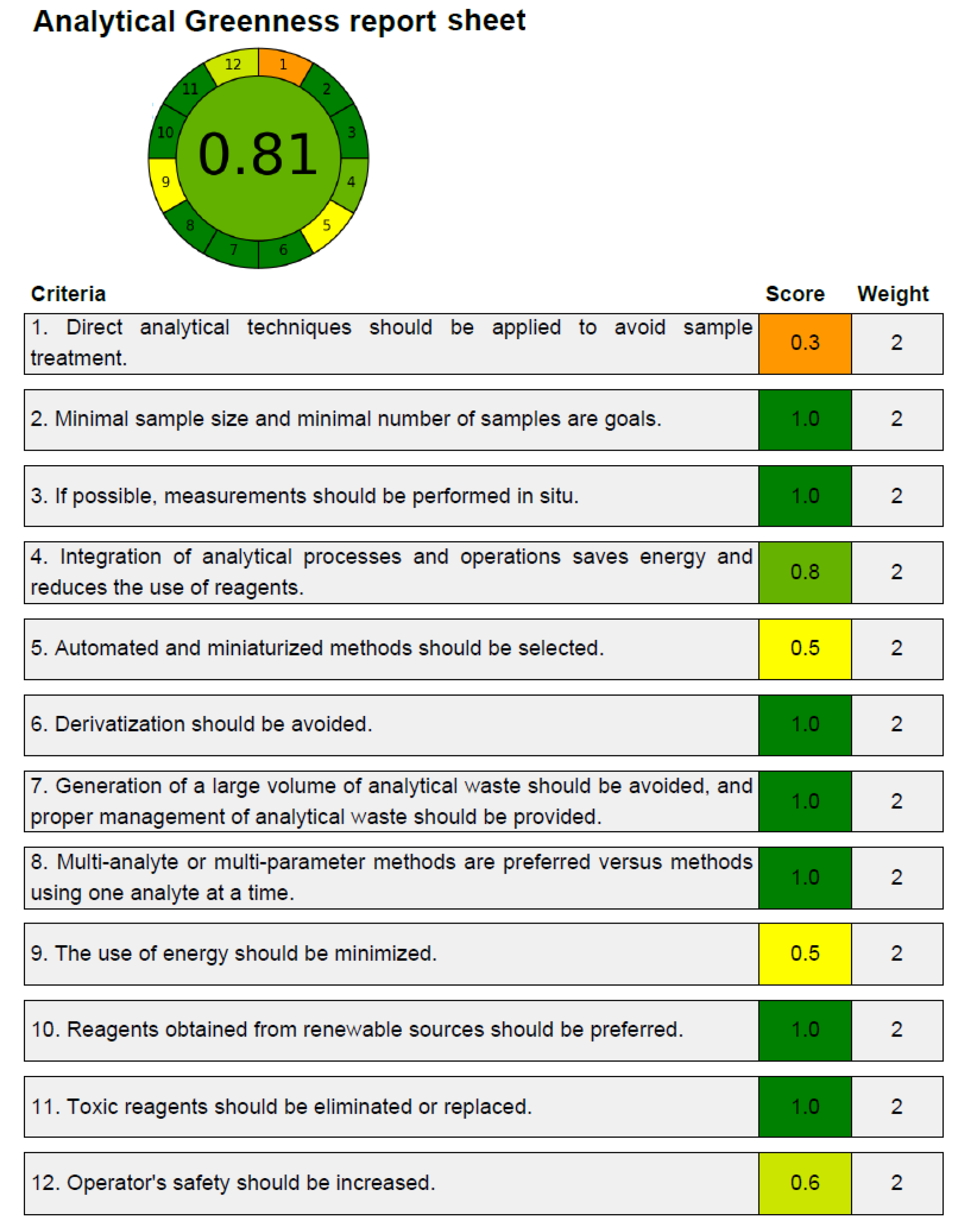
3.4. Greenness Measurement
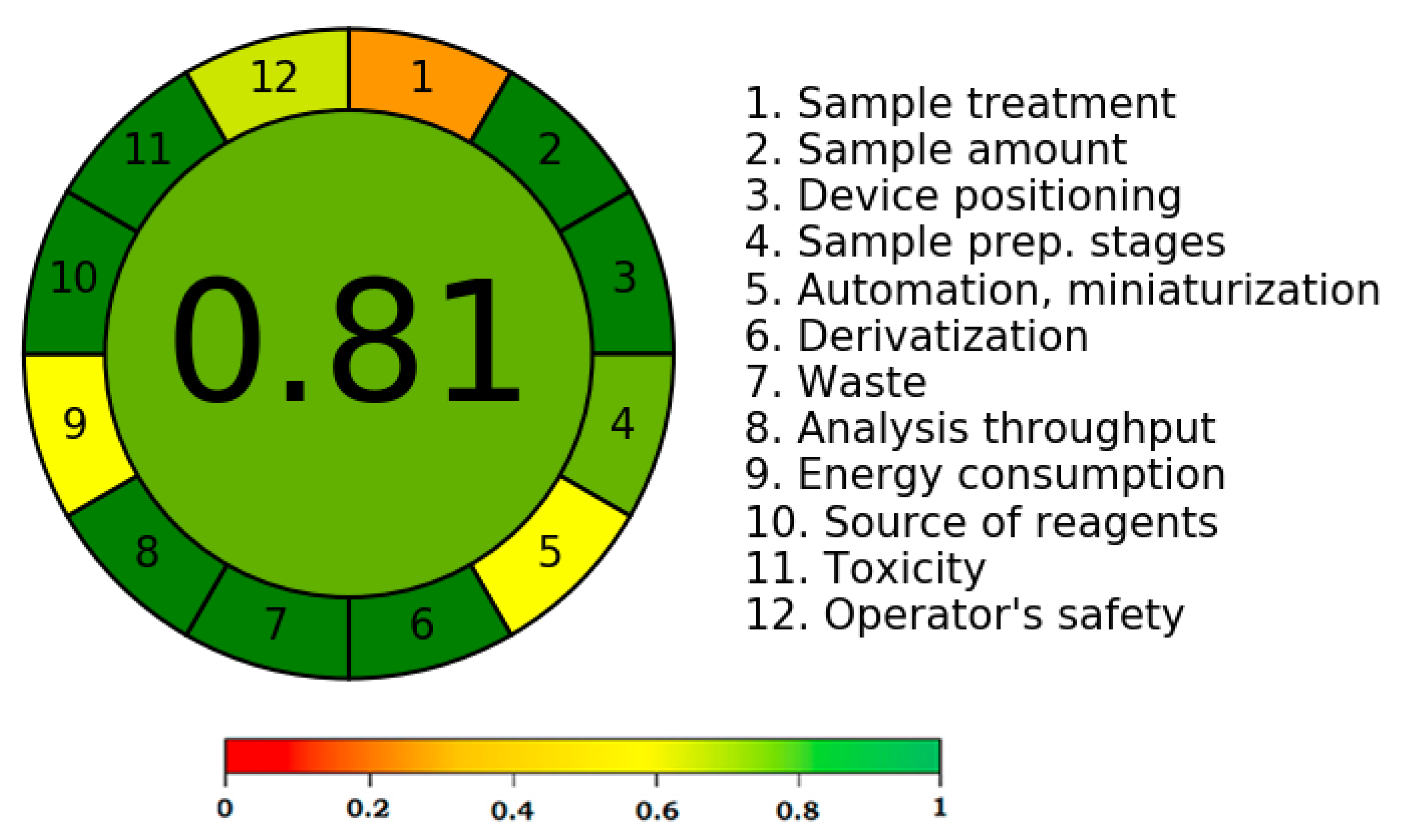
A number of analytical techniques are used to assess the greenness characteristics of analytical procedures [52,53,54,55,56]. Only the AGREE approach takes into account all twelve GAC principles while evaluating the analytical approaches’ greenness [54]. As a result, the new HPLC methodology’s greenness properties were identified utilizing the AGREE approach. The predicted overall AGREE score employing the twelve distinct GAC principles is summarized in Figure 4. Figure 5 includes the AGREE report sheet and AGREE score for each GAC concept. The different AGREE scores for each criteria of GAC were assigned by the AGREE calculator. The assigned scores ranged from 0.0 to 1.0. The established HPLC methodology’s overall AGREE score was 0.81, indicating that it possesses exceptional greenness properties for the measurement of CCM. The AGREE score for the reported analytical methods of CCM measurement has not been determined in the literature. However, the AGREE score for the HPLC assay of some other drugs has been reported recently [58,59]. The AGREE score for the HPLC assay of the emtricitabine measurement has been reported to be 0.72 using the AGREE calculator [58]. Similarly, the AGREE score for the HPLC assay of the doxorubicin measurement has been reported to be 0.79 using the AGREE calculator [59]. The recorded AGREE score for the HPLC assay of the CCM measurement was better than those reported for the measurement of emtricitabine and doxorubicin [58,59]. Overall, the greenness profile of the current HPLC assay was outstanding.

Figure 4.
Analytical GREEnness (AGREE) score for the established HPLC assay of CCM determination predicted using AGREE calculator.
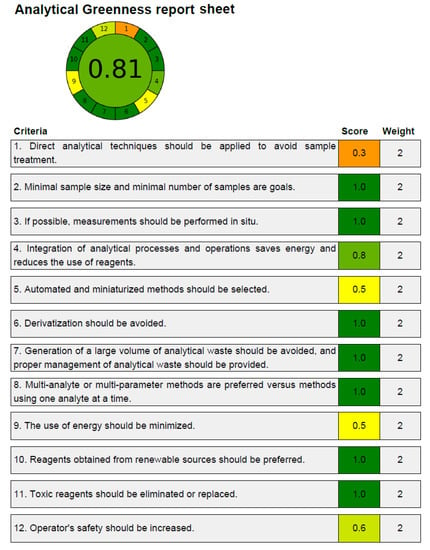
Figure 5.
AGREE scale sheet presenting AGREE scale for 12 distinct components of GAC for the established HPLC methodology of CCM determination recorded using AGREE calculator.
3.5. Determination of CCM in In-House Developed Nanoemulsion, Curcuma Longa Extract, and Commercial Tablets
The stability-indicating greener HPLC methodology for CCM determination was shown to be efficient, rapid, and sensitive. This technique was therefore applied to ascertain CCM in an in-house developed nanoemulsion, Curcuma longa extract, and commercial tablets. The CCM percentage assay was 101.24 ± 0.72% in the in-house developed nanoemulsion. The amount of CCM was determined to be 81.15 ± 0.64% in Curcuma longa extract. The amount of CCM in commercial tablets (containing 1000 mg of standardized C. longa extract) was found to be 78.41 ± 0.58%. The assay of CCM in ordinary emulsion formulation using the HPLC method has been reported to be 99.45% [23], which was similar to the present method of CCM assay in nanoemulsion formulation. The amount of CCM in six different commercial C. longa extracts using the HPLC method has been reported in the range of 69.82 to 86.79% [26]. The amount of CCM in C. longa extract using the present HPLC method was superior to most of the reported extracts [26]. Using another HPLC method, the amount of CCM in C. longa extract has been reported as 30.76 mg/g (equivalent to 3.06%) [27], which was inferior to the present HPLC method. These findings indicated that the greener HPLC methodology would work well for figuring out CCM in its laboratory developed formulations, commercially available products, and distinct plant extracts.
4. Conclusions
The CCM in an in-house developed nanoemulsion, Curcuma longa extract, and commercial tablets has been identified and verified using a quick, sensitive, stability-indicating, and greener HPLC approach. The stability-indicating greener HPLC methodology was validated according to ICH-Q2-R1 procedures. For detecting CCM, the greener HPLC approach is more efficient, accurate, precise, stability-indicating, robust, sensitive, and selective. The greener HPLC methodology was found to be suitable for the determination of CCM in the in-house developed nanoemulsion, Curcuma longa extract, and commercial tablets. The AGREE evaluation suggested outstanding greenness characteristics of the established HPLC methodology. Because of its selectivity and stability-indicating properties, the greener HPLC method was able to identify CCM, even in the presence of its degradation products. Based on these findings, it is possible to effectively determine CCM in a variety of sample matrices using the stability-indicating greener HPLC methodology. In future, further studies can be carried out to determine CCM in the complex matrices of biological samples and CCM pharmacokinetic evaluation.
Author Contributions
Conceptualization, S.A. and F.S.; methodology, N.H., M.M.G. and S.M.B.A.; software, N.H., S.A.A. and F.S.; validation, S.A. and P.A.; formal analysis, S.A.A. and P.A.; investigation, F.S. and N.H.; resources, S.A.; data curation, P.A.; writing—original draft preparation, F.S.; writing—review and editing, N.H., S.A. and P.A.; visualization, S.A.; supervision, F.S. and S.A.; project administration, F.S. and S.A.; funding acquisition, S.A. and M.M.G. All authors have read and agreed to the published version of the manuscript.
Funding
This study is supported via funding from Prince Sattam Bin Abdulaziz University project number (PSAU/2023/R/1444). The APC was funded by PSAU.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Acknowledgments
The authors are grateful to Prince Sattam Bin Abdulaziz University for supporting this work via project number (PSAU/2023/R/1444). The authors are also grateful to AlMaarefa University for their generous support.
Conflicts of Interest
The authors declare no conflict of interest.
References
- El-Hack, M.E.A.; El-Saadony, M.T.; Swelum, A.A.; Arif, M.; Ghanima, M.M.A.; Shukry, M.; Noreldin, A.; Taha, A.E.; El-Tarabily, K.A. Curcumin, the active substance of turmeric: Its effects on health and ways to improve its bioavailability. J. Sci. Food Agric. 2021, 101, 5747–5762. [Google Scholar] [CrossRef] [PubMed]
- Reeta, V.; Kalia, S. Turmeric: A review of its effects on human health. J. Med. Plant Stud. 2022, 10, 61–63. [Google Scholar]
- Fabianowska-Majewska, K.; Kaufman-Szymczyk, A.; Szymanska-Kolba, A.; Jakubik, J.; Majeski, G. Curcumin from turmeric rhizome: A potential modulator of DNA methylation machinery in breast cancer inhibition. Nutrients 2021, 13, 332. [Google Scholar] [CrossRef] [PubMed]
- Jayaprakasha, G.K.; Rao, L.J.M.; Sakariah, K.K. Improved HPLC method for the determination of curcumin, demethoxycurcumin, and bisdemethoxycurcumin. J. Agric. Food Chem. 2002, 50, 3668–3672. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Wang, Y.; Cai, N.; Xu, T.; He, F. Anti-inflammatory effects of curcumin in acute lung injury: In vivo and in vitro experimental model studies. Int. Immunopharmacol. 2021, 96, E107600. [Google Scholar] [CrossRef]
- Trigo-Gutierrez, J.K.; Vega-Chacon, Y.; Soares, A.B.; de Oliveira Mima, E.G. Antimicrobial activity inflammatory of curcumin in nanoformulations: A comprehensive review. Int. J. Mol. Sci. 2021, 22, 7130. [Google Scholar] [CrossRef]
- Negahdari, R.; Ghavimi, M.A.; Barzegar, A.; Memar, M.Y.; Balazadeh, L.; Bohlouli, S.; Sharifi, S.; Dizaj, S.M. Antibacterial effects of nanocurcumin inside the implant fixture: An in vitro study. Clin. Exp. Dental Res. 2021, 7, 163–169. [Google Scholar] [CrossRef]
- Ardebili, A.; Pouriayevali, M.H.; Aleshikh, S.; Zahani, M.; Ajorloo, M.; Izanloo, A.; Siyadatpanah, A.; Nikoo, H.R.; Wilairatana, P.; Coutinho, H.D.M. Antiviral therapeutic potential of curcumin: An update. Molecules 2021, 26, 6994. [Google Scholar] [CrossRef]
- Urosevic, M.; Nikolic, L.; Gajic, I.; Nikolic, V.; Dinic, A.; Miljkovic, V. Curcumin: Biological activities and modern pharmaceuticals. Antibiotics 2022, 11, 135. [Google Scholar] [CrossRef]
- Eremina, N.V.; Zhanataev, A.K.; Durnev, A.D. Induced cell death as a possible pathway of antimutagenic action. Bull. Exp. Biol. Med. 2021, 171, 1–14. [Google Scholar] [CrossRef]
- Morshidi, K.; Borran, S.; Ebrahimi, M.S.; Khooy, M.J.M.; Seyedi, Z.S.; Amiri, A.; Abbasi-Kolli, M.; Fallah, M.; Khan, H.; Sahebkar, A.; et al. Therapeutic effect of curcumin in gastrointestinal cancers: A comprehensive review. Phytother. Res. 2021, 35, 3834–3897. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Yang, X.; Lin, J.; Song, F.; Shao, Y. Low curcumin concentration enhances the anticancer effects of 5-fluorouracil against colorectal cancer. Phytomedicine 2021, 85, E153547. [Google Scholar] [CrossRef] [PubMed]
- Arena, A.; Romeo, M.A.; Benedetti, R.; Masuelli, L.; Bei, R.; Montani, M.S.G.; Cirone, M. New insights into curcumin- resveratrol-mediated anticancer effects. Pharmaceuticals 2021, 14, 1068. [Google Scholar] [CrossRef]
- Unsal, Y.E.; Tuzen, M.; Soylak, M. Ultrasound-assisted ionic liquid-dispersive liquid–liquid of curcumin in food samples microextraction and its spectrophotometric determination. J. AOAC Int. 2019, 102, 217–221. [Google Scholar] [CrossRef] [PubMed]
- Altunay, N.; Unal, Y.; Elik, A. Towards green analysis of curcumin from tea, honey and spices: Extraction by deep eutectic solvent assisted emulsification liquid-liquid microextraction method based on response surface design. Food Addit. Cont. Part A. 2020, 37, 869–881. [Google Scholar] [CrossRef] [PubMed]
- Gurmen, K.; Sahin, U.; Yilmaz, E.; Soylak, M.; Sahan, S. Determination of curcumin in food with homogenous liquid-phase microextraction preconcentration and spectrophotometric determination. Anal. Lett. 2023, 56, 807–815. [Google Scholar] [CrossRef]
- Karimi, M.; Mashreghi, M.; Saremi, S.S.; Jaafari, M.R. Spectrofluorometric method development and validation for the determination of curcumin in nanoliposomes and plasma. J. Fluoresc. 2020, 30, 1113–1119. [Google Scholar] [CrossRef]
- Yang, R.; Mu, W.-Y.; Chen, Q.-Y. Urazole-Au nanocluster as a novel fluorescence probe for curcumin determination and mitochondria imaging. Food Anal. Methods 2019, 12, 1805–1812. [Google Scholar] [CrossRef]
- Yu, C.; Zhuang, Q.; Cui, H.; Li, Y.; Ding, Y.; Lin, J.; Duan, Y. A fluorescent “turn-off” probe for the determination of curcumin using upconvert luminescent carbon dots. J. Fluoresc. 2020, 30, 1469–1476. [Google Scholar] [CrossRef]
- Naidu, M.M.; Shyamala, B.N.; Manjunatha, J.R.; Sulochanamma, G.; Srinivas, P. Simple HPLC method for resolution of curcuminoids with antioxidant potential. J. Food Sci. 2009, 74, C312–C318. [Google Scholar] [CrossRef]
- Jangle, R.D.; Thorat, B.N. Reversed-phase high-performance liquid chromatography method for analysis of curcuminoids and curcuminoid-loaded liposome formulation. Indian J. Pharm. Sci. 2013, 75, 60–66. [Google Scholar] [PubMed]
- Ali, I.; Haque, A.; Saleem, K. Separation and identification of curcuminoids in turmeric powder by HPLC using phenyl column. Anal. Methods 2014, 6, 2526–2536. [Google Scholar] [CrossRef]
- Syed, H.K.; Bin Liew, K.; Loh, G.O.K.; Peh, K.K. Stability indicating HPLC–UV method for detection of curcumin in Curcuma longa extract and emulsion formulation. Food Chem. 2015, 170, 321–326. [Google Scholar] [CrossRef]
- Hwang, K.-W.; Son, D.; Jo, H.-W.; Kim, C.H.; Seong, K.C.; Moon, J.-K. Levels of curcuminoid and essential oil compositions in turmerics (Curcuma longa L.) grown in Korea. Appl. Biol. Chem. 2016, 59, 209–215. [Google Scholar] [CrossRef]
- Osorio-Tobon, J.F.; Carvalho, P.I.N.; Barbero, G.F.; Nogueira, G.C.; Rostango, M.A.; Meireles, M.A.D.A. Fast analysis of curcuminoids from turmeric (Curcuma longa L.) by high-performance liquid chromatography using a fused-core column. Food Chem. 2016, 200, 167–174. [Google Scholar] [CrossRef]
- Peram, M.R.; Jalalpure, S.S.; Joshi, S.A.; Palkar, M.B.; Diwan, P.V. Single robust RP-HPLC analytical method for quantification of curcuminoids in commercial turmeric products, Ayurvedic medicines, and nanovesicular systems. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 487–498. [Google Scholar] [CrossRef]
- Erpina, E.; Rafi, M.; Darusman, L.K.; Vitasari, A.; Putra, B.R.; Rohaeti, E. Simultaneous quantification of curcuminoids and xanthorrhizol in Curcuma xanthorrhiza by high-performance liquid chromatography. J. Liq. Chromatogr. Relat. Technol. 2017, 40, 635–639. [Google Scholar] [CrossRef]
- Jiang, H.; Somogyi, A.; Jacobsen, N.E.; Timmermann, B.N.; Gang, D.R. Analysis of curcuminoids by positive and negative electrospray ionization and tandem mass spectrometry. Rapid Commun. Mass Spectrom. 2006, 20, 1001–1012. [Google Scholar] [CrossRef]
- Jiang, H.; Timmermann, B.N.; Gang, D.R. Use of liquid chromatography–electrospray ionization tandem mass spectrometry to identify diarylheptanoids in turmeric (Curcuma longa L.) rhizome. J. Chromatogr. A. 2006, 1111, 21–31. [Google Scholar] [CrossRef]
- Avula, B.; Wang, Y.-H.; Khan, I.A. Quantitative determination of curcuminoids from the roots of Curcuma longa, Curcuma species and dietary supplements using an UPLC-UV-MS method. J. Chromatogr. Sep. Tech. 2012, 3, E1000120. [Google Scholar] [CrossRef]
- Cheng, J.; Weijun, K.; Yun, L.; Jiabo, W.; Haitao, W.; Qingmiao, L.; Xiaohe, X. Development and validation of UPLC method for quality control of Curcuma longa Linn.: Fast simultaneous quantitation of three curcuminoids. J. Pharm. Biomed. Anal. 2010, 53, 43–49. [Google Scholar] [CrossRef] [PubMed]
- Ansari, M.J.; Ahmad, S.; Kohli, K.; Ali, J.; Khar, R.K. Stability-indicating HPTLC determination of curcumin in bulk drug and pharmaceutical formulations. J. Pharm. Biomed. Anal. 2005, 39, 132–138. [Google Scholar] [CrossRef] [PubMed]
- Pathania, V.; Gupta, A.P.; Singh, B. Improved HPTLC method for determination of curcuminoids from Curcuma longa. J. Liq. Chromatogr. Relat. Technol. 2006, 29, 877–887. [Google Scholar] [CrossRef]
- Green, C.E.; Hibbert, S.L.; Bailey-Shaw, Y.A.; Williams, L.A.D.; Mitchell, S.; Garraway, E. Extraction, processing, and storage effects on curcuminoids and oleoresin yields from Curcuma longa L. grown in Jamaica. J. Agric. Food Chem. 2008, 56, 3664–3670. [Google Scholar] [CrossRef]
- Paramasivam, M.; Poi, R.; Banerjee, H.; Bandyopadhyay, A. High-performance thin layer chromatographic method for quantitative determination of curcuminoids in Curcuma longa germplasm. Food Chem. 2009, 113, 640–644. [Google Scholar] [CrossRef]
- Taha, M.N.; Krawinkel, M.B.; Morlock, G.E. High-performance thin-layer chromatography linked with (bio)assays and mass spectrometry—A suited method for discovery and quantification of bioactive components? Exemplarily shown for turmeric and milk thistle extracts. J. Chromatogr. A. 2015, 1394, 137–147. [Google Scholar] [CrossRef]
- Baghel, U.S.; Nagar, A.S.; Pannu, M.S.; Singh, D.; Yadav, R. HPLC and HPTLC methods for simultaneous estimation of quercetin and curcumin in polyherbal formulation. Indian J. Pharm. Sci. 2017, 79, 197–203. [Google Scholar] [CrossRef]
- Chen, W.; Fan-Havard, P.; Yee, L.D.; Cao, Y.; Stoner, G.D.; Chan, K.K.; Liu, Z. A liquid chromatography–tandem mass spectrometric method for quantification of curcumin-O-glucuronide and curcumin in human plasma. J. Chromatogr. B. 2012, 900, 89–93. [Google Scholar] [CrossRef]
- Kunati, S.R.; Yang, S.; William, B.M.; Xu, Y. An LC–MS/MS method for simultaneous determination of curcumin, curcumin glucuronide and curcumin sulfate in a phase II clinical trial. J. Pharm. Biomed. Anal. 2018, 156, 189–198. [Google Scholar] [CrossRef]
- Liu, Y.; Siard, M.; Adams, A.; Keowen, M.L.; Miller, T.K.; Garza, F., Jr.; Andrews, F.M.; Seeram, N.P. Simultaneous quantification of free curcuminoids and their metabolites in equine plasma by LC-ESI–MS/MS. J. Pharm. Biomed. Anal. 2018, 154, 31–39. [Google Scholar] [CrossRef]
- Yu, W.; Wen, D.; Cai, D.; Zheng, J.; Gan, H.; Jiang, F.; Liu, X.; Lao, B.; Yu, W.; Guan, Y.; et al. Simultaneous determination of curcumin, tetrahydrocurcumin, quercetin, and paeoniflorin by UHPLC-MS/MS in rat plasma and its application to a pharmacokinetic study. J. Pharm. Biomed. Anal. 2019, 172, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Hua, Q.; Gao, L.; Raoa, S.-Q.; Yanga, Z.-Q.; Li, T.; Gong, X. Nitrogen and chlorine dual-doped carbon nanodots for determination of curcumin in food matrix via inner filter effect. Food Chem. 2019, 280, 195–202. [Google Scholar] [CrossRef] [PubMed]
- Deng, P.; Wei, Y.; Li, W.; Shi, S.; Zhou, C.; Li, J.; Yao, L.; Ding, J.; He, Q. A novel platform based on MnO2 nanoparticles and carboxylated multi-walled carbon nanotubes composite for accurate and rapid determination of curcumin in commercial food products. J. Food Compos. Anal. 2023, 115, E104940. [Google Scholar] [CrossRef]
- Raril, C.; Manjunatha, J.G.; Tigari, G. Low-cost voltammetric sensor based on ananionic surfactant modified carbon nanocomposite material for the rapid determination of curcumin in natural food supplement. Inst. Sci. Technol. 2020, 48, 561–582. [Google Scholar] [CrossRef]
- Burc, M.; Gungor, O.; Duran, S.T. Voltammetric determination of curcumin in spices using platinum electrode electrochemically modified with poly(vanillin-co-caffeic acid). Anal. Bioanal. Electrochem. 2020, 12, 625–643. [Google Scholar]
- Pushpanjali, P.A.; Manjunatha, J.G.; Amrutha, B.M.; Hareesha, N. Development of carbon nanotube-based polymer-modified electrochemical sensor for the voltammetric study of Curcumin. Mater. Res. Innov. 2021, 25, 412–420. [Google Scholar] [CrossRef]
- Tigari, G.; Manjunatha, J.G. Poly(glutamine) film-coated carbon nanotube paste electrode for the determination of curcumin with vanillin: An electroanalytical approach. Mon. Chem. 2020, 151, 1681–1688. [Google Scholar] [CrossRef]
- Rahimnejad, M.; Zokhtareh, R.; Moghadamnia, A.A.; Asghary, M. An electrochemical sensor based on reduced grapheme oxide modified carbon paste electrode for curcumin determination in human blood serum. Purtug. Electrochem. Acta 2020, 38, 29–42. [Google Scholar] [CrossRef]
- Thangavel, K.; Dhivya, K. Determination of curcumin, starch and moisture content in turmeric by Fourier transform near infrared spectroscopy (FT-NIR). Eng. Agric. Environ. Food 2019, 12, 264–269. [Google Scholar] [CrossRef]
- Gong, X.; Wang, H.; Liu, Y.; Hu, Q.; Gao, Y.; Yang, Z.; Shuang, S.; Dong, C. A di-functional and label-free carbon-based chem-nanosensor for real-time monitoring of pH fluctuation and quantitative determining of curcumin. Anal. Chim. Acta 2019, 1057, 132–144. [Google Scholar] [CrossRef]
- Lizonova, D.; Brejchova, A.; Kralova, E.; Hanus, J.; Stepanek, F. Solvatochromic shift enables intracellular fate determination of curcumin nanocrystals. Partic. Partic. Sys. Character. 2022, 39, E2200115. [Google Scholar] [CrossRef]
- Abdelrahman, M.M.; Abdelwahab, N.S.; Hegazy, M.A.; Fares, M.Y.; El-Sayed, G.M. Determination of the abused intravenously administered madness drops (tropicamide) by liquid chromatography in rat plasma; an application to pharmacokinetic study and greenness profile assessment. Microchem. J. 2020, 159, E105582. [Google Scholar] [CrossRef]
- Duan, X.; Liu, X.; Dong, Y.; Yang, J.; Zhang, J.; He, S.; Yang, F.; Wang, Z.; Dong, Y. A green HPLC method for determination of nine sulfonamides in milk and beef, and its greenness assessment with analytical eco-scale and greenness profile. J. AOAC Int. 2020, 103, 1181–1189. [Google Scholar] [CrossRef] [PubMed]
- Pena-Pereira, F.; Wojnowski, W.; Tobiszewski, M. AGREE-Analytical GREEnness metric approach and software. Anal. Chem. 2020, 92, 10076–10082. [Google Scholar] [CrossRef]
- Alam, P.; Salem-Bekhit, M.M.; Al-Joufi, F.A.; Alqarni, M.H.; Shakeel, F. Quantitative analysis of cabozantinib in pharmaceutical dosage forms using green RP-HPTLC and green NP-HPTLC methods: A comparative evaluation. Sus. Chem. Pharm. 2021, 21, E100413. [Google Scholar] [CrossRef]
- Foudah, A.I.; Shakeel, F.; Alqarni, M.H.; Alam, P. A rapid and sensitive stability-indicating green RP-HPTLC method for the quantitation of flibanserin compared to green NP-HPTLC method: Validation studies and greenness assessment. Microchem. J. 2021, 164, E105960. [Google Scholar] [CrossRef]
- ICH. International Conference on Harmonization (ICH), Q2 (R1): Validation of Analytical Procedures—Text and Methodology. ICH: Geneva, Switzerland, 2005. [Google Scholar]
- Haq, N.; Alshehri, S.; Alam, P.; Ghoneim, M.M.; Hasan, Z.; Shakeel, F. Green analytical chemistry approach for the determination of emtricitabine in human plasma, formulations, and solubility study samples. Sus. Chem. Pharm. 2022, 26, E100648. [Google Scholar] [CrossRef]
- Haq, N.; Alanazi, F.K.; Samem-Bekhit, M.M.; Rabea, S.; Alam, P.; Alsarra, I.A.; Shakeel, F. Greenness estimation of chromatographic assay for the determination of anthracycline-based antitumor drug in bacterial ghost matrix of Salmonella typhimurium. Sus. Chem. Pharm. 2022, 26, E100642. [Google Scholar] [CrossRef]
- Haq, N.; Iqbal, M.; Alanazi, F.K.; Alsarra, I.A.; Shakeel, F. Applying green analytical chemistry for rapid analysis of drugs: Adding health to pharmaceutical industry. Arabian J. Chem. 2017, 10, S777–S785. [Google Scholar] [CrossRef]
- Hackl, K.; Kunz, W. Some aspects of green solvents. Comp. Rend. Chim. 2018, 21, 572–580. [Google Scholar] [CrossRef]
- Welton, T. Solvents and sustainable chemistry. Proc. Math Phys. Eng. Sci. 2015, 471, E2015052. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).