Archaeological Evidence for the Dietary Practices and Lifestyle of 18th Century Lisbon, Portugal—Combined Steroidal Biomarker and Microparticle Analysis of the Carbonized Faecal Remains
Abstract
1. Introduction
2. Materials and Methods
2.1. Description of Archaeological Sample
2.2. Microscopy
2.3. ATR-FT-IR
2.4. VP-SEM-EDS
2.5. GC-MS
2.6. Non-Pollen Palynomorphs (NPPs) Extraction
3. Results
3.1. ATR-FT-IR
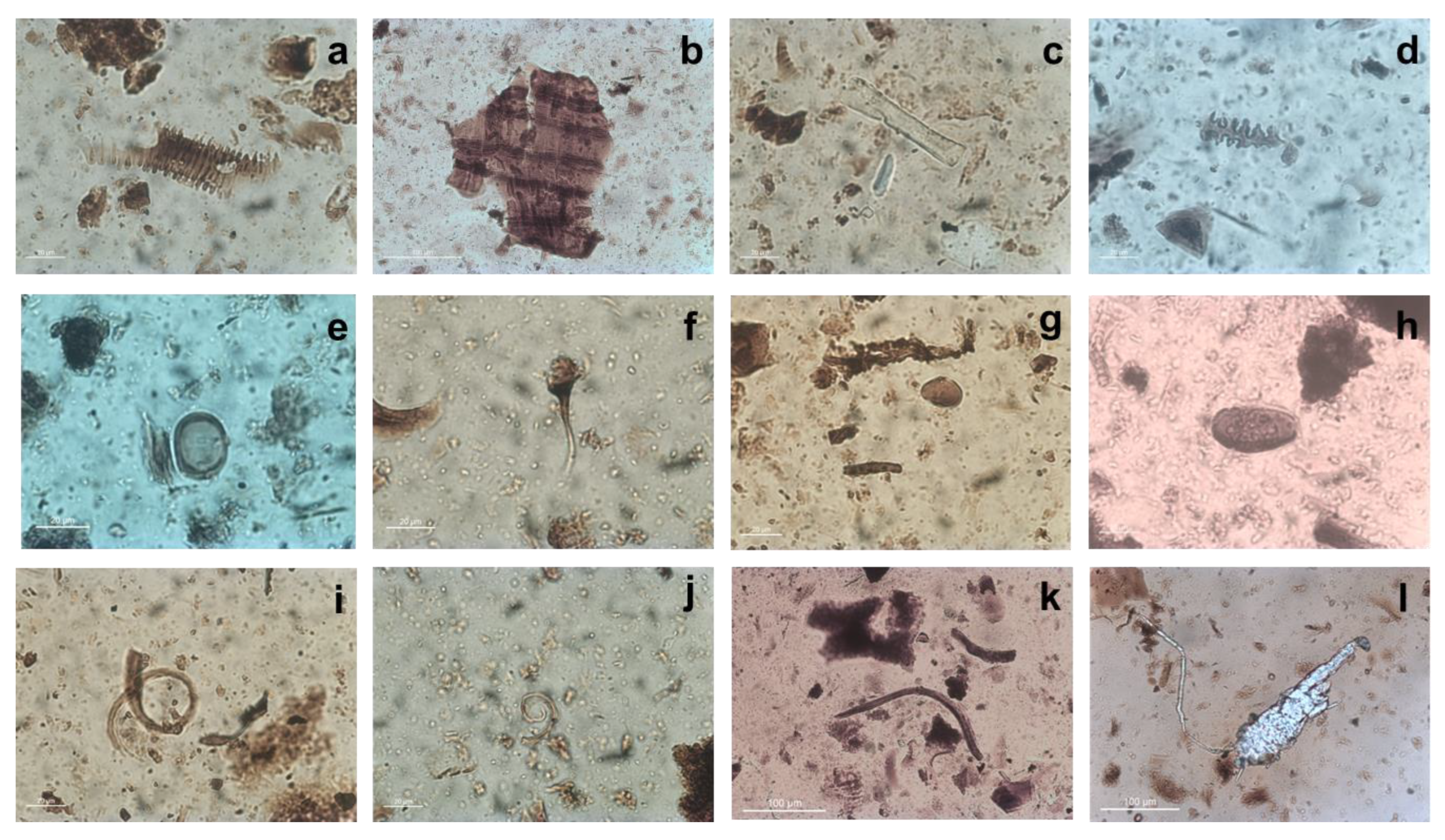
3.2. Microscopy and VP-SEM-EDS
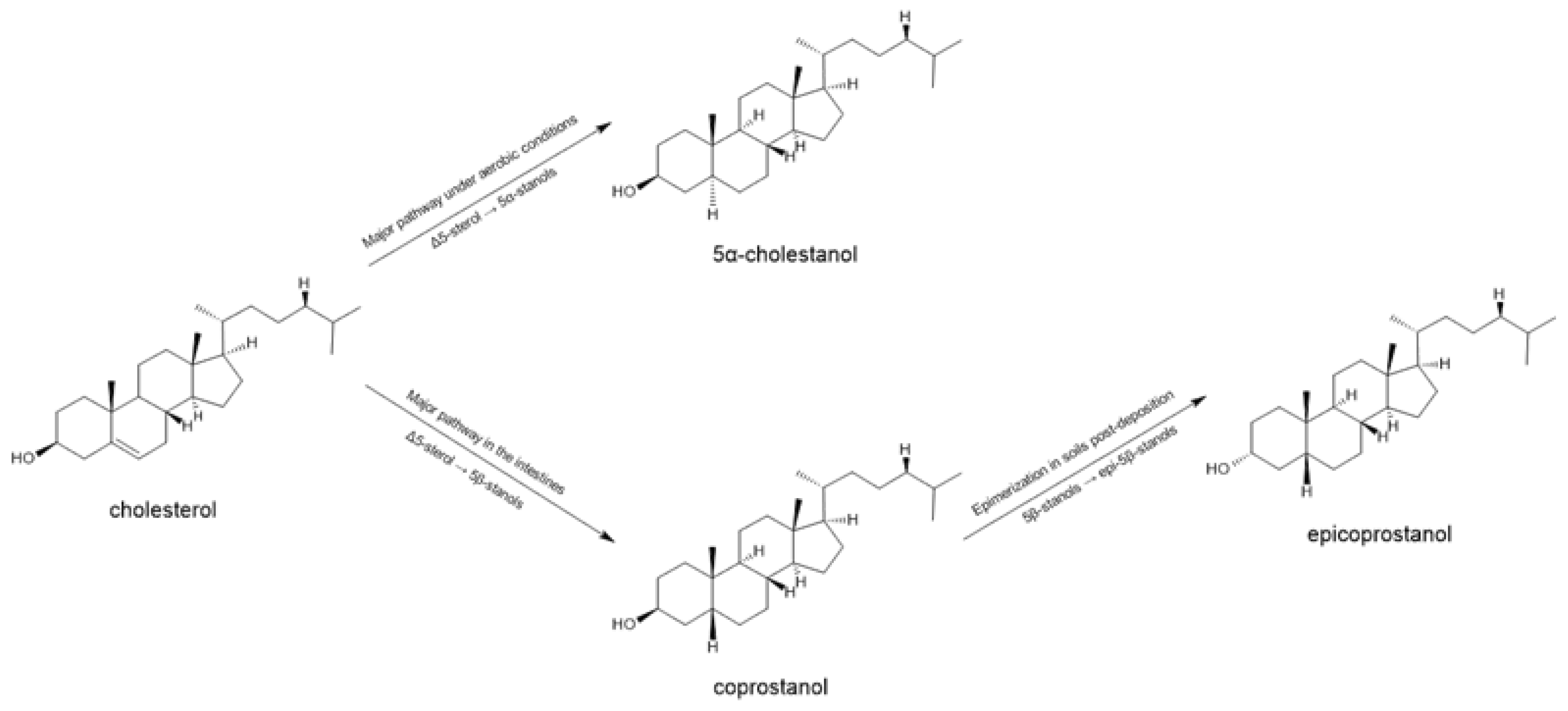
3.3. GC-MS
3.4. Microparticle Analysis
4. Discussion
5. Concluding Remarks
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Oudemans, T.; Boon, J. Molecular archaeology: Analysis of charred (food) remains from prehistoric pottery by pyrolysis—Gas chromatography/mass spectrometry. J. Anal. Appl. Pyrolysis 1991, 20, 197–227. [Google Scholar] [CrossRef]
- Evershed, R.P. Organic Residue Analysis in Archaeology: The Archaeological Biomarker Revolution. Archaeometry 2008, 50, 895–924. [Google Scholar] [CrossRef]
- Craig, O.E.; Steele, V.J.; Fischer, A.; Hartz, S.; Andersen, S.H.; Donohoe, P.; Glykou, A.; Saul, H.; Jones, D.M.; Koch, E.; et al. Ancient lipids reveal continuity in culinary practices across the transition to agriculture in Northern Europe. Proc. Natl. Acad. Sci. USA 2011, 108, 17910–17915. [Google Scholar] [CrossRef]
- Heron, C.; Craig, O.E.; Luquin, A.; Steele, V.J.; Thompson, A.; Piličiauskas, G. Cooking fish and drinking milk? Patterns in pottery use in the southeastern Baltic, 3300–2400 cal BC. J. Archaeol. Sci. 2015, 63, 33–43. [Google Scholar] [CrossRef]
- Carretero, L.G.; Wollstonecroft, M.; Fuller, D.Q. A methodological approach to the study of archaeological cereal meals: A case study at Çatalhöyük East (Turkey). Veg. Hist. Archaeobotany 2017, 26, 415–432. [Google Scholar] [CrossRef] [PubMed]
- Heiss, A.G.; Antolín, F.; Bleicher, N.; Harb, C.; Jacomet, S.; Kühn, M.; Marinova, E.; Stika, H.-P.; Valamoti, S.M. State of the (t)art. Analytical approaches in the investigation of components and production traits of archaeological bread-like objects, applied to two finds from the Neolithic lakeshore settlement Parkhaus Opéra (Zürich, Switzerland). PLoS ONE 2017, 12, e0182401. [Google Scholar] [CrossRef] [PubMed]
- Shoda, S.; Lucquin, A.; Sou, C.I.; Nishida, Y.; Sun, G.; Kitano, H.; Son, J.-H.; Nakamura, S.; Craig, O.E. Molecular and isotopic evidence for the processing of starchy plants in Early Neolithic pottery from China. Sci. Rep. 2018, 8, 1–9. [Google Scholar] [CrossRef]
- Valamoti, S.M.; Marinova, E.; Heiss, A.G.; Hristova, I.; Petridou, C.; Popova, T.; Michou, S.; Papadopoulou, L.; Chrysostomou, P.; Darcque, P.; et al. Prehistoric cereal foods of southeastern Europe: An archaeobotanical exploration. J. Archaeol. Sci. 2019, 104, 97–113. [Google Scholar] [CrossRef]
- Reinhard, K.J.; Bryant, V.M. Coprolite analysis: A biological perspective on archaeology. J. Archaeol. Method Theory 1992, 4, 245–288. [Google Scholar]
- Reinhard, K. Coprolite analysis: The analysis of ancient human feces for dietary data. In Archaeological Method and Theory: An Encyclopedia; Ellispp, L., Ed.; University of Nebraska-Lincoln: Garland, TX, USA, 2000; pp. 124–132. [Google Scholar]
- Hollocher, K.; Hollocher, T.C. Early processes in the fossilization of terrestrial feces to coprolites, and microstructure preservation. NMMNHS 2012, 57, 79–91. [Google Scholar]
- Santiago-Rodriguez, T.M.; Fornaciari, G.; Luciani, S.; Dowd, S.; Toranzos, G.A.; Marota, I.; Cano, R.J. Gut Microbiome of an 11th Century A.D. Pre-Columbian Andean Mummy. PLoS ONE 2015, 10, e0138135. [Google Scholar] [CrossRef]
- Hillman, G.; Wales, S.; McLaren, F.; Evans, J.; Butler, A. Identifying problematic remains of ancient plant foods: A comparison of the role of chemical, histological and morphological criteria. World Archaeol. 1993, 25, 94–121. [Google Scholar] [CrossRef]
- Faulkner, C.T. Prehistoric Diet and Parasitic Infection in Tennessee: Evidence from the Analysis of Desiccated Human Paleofeces. Am. Antiq. 1991, 56, 687–700. [Google Scholar] [CrossRef]
- Karpinski, E.; Mead, J.I.; Poinar, H.N. Molecular identification of paleofeces from Bechan Cave, southeastern Utah, USA. Quat. Int. 2017, 443, 140–146. [Google Scholar] [CrossRef]
- Battillo, J. Farmers who forage: Interpreting paleofecal evidence of wild resource use by early corn farmers in the North American Southwest. Archaeol. Anthr. Sci. 2019, 11, 5999–6016. [Google Scholar] [CrossRef]
- Borry, M.; Cordova, B.; Perri, A.; Wibowo, M.; Honap, T.P.; Ko, J.; Yu, J.; Britton, K.; Girdland-Flink, L.; Power, R.C.; et al. CoproID predicts the source of coprolites and paleofeces using microbiome composition and host DNA content. Peer J. 2020, 8, e9001. [Google Scholar] [CrossRef] [PubMed]
- Hagan, R.W.; Hofman, C.A.; Hübner, A.; Reinhard, K.; Schnorr, S.; Lewis, C.M.; Sankaranarayanan, K.; Warinner, C.G. Comparison of extraction methods for recovering ancient microbial DNA from paleofeces. Am. J. Phys. Anthr. 2019, 171, 275–284. [Google Scholar] [CrossRef]
- Sonderman, E.M.; Dozier, C.A.; Smith, M.F. Analysis of a coprolite from Conejo Shelter, Texas: Potential ritualistic viperous snake consumption. J. Archaeol. Sci. Rep. 2019, 25, 85–93. [Google Scholar] [CrossRef]
- Tolar, T.; Galik, A. A Study of Dog Coprolite from Late Neolithic Pile-Dwelling Site in Slovenia. Archaeol. Discov. 2019, 7, 20–29. [Google Scholar] [CrossRef]
- Taylor, A.; Hutson, J.M.; Bryant, V.M.; Jenkins, D.L. Dietary items in Early to Late Holocene human coprolites from Paisley Caves, Oregon, USA. Palynology 2019, 44, 12–23. [Google Scholar] [CrossRef]
- Romaniuk, A.A.; Panciroli, E.; Buckley, M.; Chowdhury, M.P.; Willars, C.; Herman, J.S.; Troalen, L.G.; Shepherd, A.N.; Clarke, D.V.; Sheridan, A.; et al. Combined visual and biochemical analyses confirm depositor and diet for Neolithic coprolites from Skara Brae. Archaeol. Anthr. Sci. 2020, 12, 1–15. [Google Scholar] [CrossRef]
- Zhang, Y.; Zhang, D.; Yang, Y.; Wu, X. Pollen and lipid analysis of coprolites from Yuhuicun and Houtieying, China: Implications for human habitats and diets. J. Archaeol. Sci. Rep. 2019, 29, 102135. [Google Scholar] [CrossRef]
- Sistiaga, A.; Berna, F.; Laursen, R.; Goldberg, P. Steroidal biomarker analysis of a 14,000 years old putative human coprolite from Paisley Cave, Oregon. J. Archaeol. Sci. 2014, 41, 813–817. [Google Scholar] [CrossRef]
- Hjulström, B.; Isaksson, S. Identification of activity area signatures in a reconstructed Iron Age house by combining element and lipid analyses of sediments. J. Archaeol. Sci. 2009, 36, 174–183. [Google Scholar] [CrossRef]
- Baeten, J.; Marinova, E.; De Laet, V.; Degryse, P.; De Vos, D.; Waelkens, M. Faecal biomarker and archaeobotanical analyses of sediments from a public latrine shed new light on ruralisation in Sagalassos, Turkey. J. Archaeol. Sci. 2011, 39, 1143–1159. [Google Scholar] [CrossRef]
- Sistiaga, A.; Mallol, C.; Galván, B.; Summons, R.E. The Neanderthal Meal: A New Perspective Using Faecal Biomarkers. PLoS ONE 2014, 9, e101045. [Google Scholar] [CrossRef]
- Mackay, H.; Davies, K.L.; Robertson, J.; Roy, L.; Bull, I.D.; Whitehouse, N.J.; Crone, A.; Cavers, G.; McCormick, F.; Brown, A.G.; et al. Characterising life in settlements and structures: Incorporating faecal lipid biomarkers within a multiproxy case study of a wetland village. J. Archaeol. Sci. 2020, 121, 105202. [Google Scholar] [CrossRef]
- Shillito, L.-M.; Bull, I.D.; Matthews, W.; Almond, M.J.; Williams, J.M.; Evershed, R.P. Biomolecular and micromorphological analysis of suspected faecal deposits at Neolithic Çatalhöyük, Turkey. J. Archaeol. Sci. 2011, 38, 1869–1877. [Google Scholar] [CrossRef]
- Lombardo, U.; Szabo, K.; Capriles, J.; May, J.-H.; Amelung, W.; Hutterer, R.; Lehndorff, E.; Plotzki, A.; Veit, H. Early and Middle Holocene Hunter-Gatherer Occupations in Western Amazonia: The Hidden Shell Middens. PLoS ONE 2013, 8, e72746. [Google Scholar] [CrossRef]
- Zocatelli, R.; Lavrieux, M.; Guillemot, T.; Chassiot, L.; Le Milbeau, C.; Jacob, J. Fecal biomarker imprints as indicators of past human land uses: Source distinction and preservation potential in archaeological and natural archives. J. Archaeol. Sci. 2017, 81, 79–89. [Google Scholar] [CrossRef]
- Lin, D.S.; Connor, W. Fecal steroids of the coprolite of a Greenland Eskimo mummy, AD 1475: A clue to dietary sterol intake. Am. J. Clin. Nutr. 2001, 74, 44–49. [Google Scholar] [CrossRef] [PubMed]
- Nielsen, N.H.; Henriksen, P.S.; Mortensen, M.F.; Enevold, R.; Mortensen, M.N.; Scavenius, C.; Enghild, J.J. The last meal of Tollund Man: New analyses of his gut content. Antiquity 2021, 95, 1195–1212. [Google Scholar] [CrossRef]
- Prost, K.; Birk, J.J.; Lehndorff, E.; Gerlach, R.; Amelung, W. Steroid Biomarkers Revisited—Improved Source Identification of Faecal Remains in Archaeological Soil Material. PLoS ONE 2017, 12, e0164882. [Google Scholar] [CrossRef]
- Bull, I.D.; Simpson, I.A.; van Bergen, P.F.; Evershed, R.P. Muck ‘n’ molecules: Organic geochemical methods for detecting ancient manuring. Antiquity 1999, 73, 86–96. [Google Scholar] [CrossRef]
- Bull, I.; Evershed, R.P.; Betancourt, P.P. An organic geochemical investigation of the practice of manuring at a Minoan site on Pseira Island, Crete. Geoarchaeology 2001, 16, 223–242. [Google Scholar] [CrossRef]
- Bull, I.; Elhmmali, M.M.; Roberts, D.J.; Evershed, R.P. The Application of Steroidal Biomarkers to Track the Abandonment of a Roman Wastewater Course at the Agora (Athens, Greece)*. Archaeometry 2003, 45, 149–161. [Google Scholar] [CrossRef]
- Prost, K.; Bradel, P.L.; Lehndorff, E.; Amelung, W. Steroid dissipation and formation in the course of farmyard manure composting. Org. Geochem. 2018, 118, 47–57. [Google Scholar] [CrossRef]
- Kaiser, J.; Lerch, M. Sedimentary faecal lipids as indicators of Baltic Sea sewage pollution and population growth since 1860 AD. Environ. Res. 2021, 204, 112305. [Google Scholar] [CrossRef]
- Lin, D.S.; Connor, W.; Napton, L.K.; Heizer, R.F. The steroids of 2000-year-old human coprolites. J. Lipid Res. 1978, 19, 215–221. [Google Scholar] [CrossRef] [PubMed]
- Evershed, R.P.; Connolly, R.C. Post-Mortem Transformations of Sterols in Bog Body Tissues. J. Archaeol. Sci. 1994, 21, 577–583. [Google Scholar] [CrossRef]
- Shillito, L.-M.; Whelton, H.L.; Blong, J.C.; Jenkins, D.L.; Connolly, T.J.; Bull, I.D. Pre-Clovis occupation of the Americas identified by human fecal biomarkers in coprolites from Paisley Caves, Oregon. Sci. Adv. 2020, 6, eaba6404. [Google Scholar] [CrossRef] [PubMed]
- Bull, I.D.; Lockheart, M.J.; Elhmmali, M.M.; Roberts, D.J.; Evershed, R.P. The origin of faeces by means of biomarker detection. Environ. Int. 2002, 27, 647–654. [Google Scholar] [CrossRef] [PubMed]
- Leeming, R.; Ball, A.; Ashbolt, N.; Nichols, P. Using faecal sterols from humans and animals to distinguish faecal pollution in receiving waters. Water Res. 1996, 30, 2893–2900. [Google Scholar] [CrossRef]
- Harrault, L.; Milek, K.; Jardé, E.; Jeanneau, L.; Derrien, M.; Anderson, D.G. Faecal biomarkers can distinguish specific mammalian species in modern and past environments. PLoS ONE 2019, 14, e0211119. [Google Scholar] [CrossRef]
- Shillito, L.-M.; Blong, J.C.; Green, E.J.; van Asperen, E.N. The what, how and why of archaeological coprolite analysis. Earth-Sci. Rev. 2020, 207, 103196. [Google Scholar] [CrossRef]
- Pereira, A.S. The Opportunity of a Disaster: The Economic Impact of the 1755 Lisbon Earthquake. J. Econ. Hist. 2009, 69, 466–499. [Google Scholar] [CrossRef]
- Santos, A.; Correia, M.; Loureiro, C.; Fernandes, P.; da Costa, N.M. The historical reconstruction of the 1755 earthquake and tsunami in downtown Lisbon, Portugal. J. Mar. Sci. Eng. 2019, 7, 208. [Google Scholar] [CrossRef]
- Fonseca, J.F.B.D. A Reassessment of the Magnitude of the 1755 Lisbon Earthquake. Bull. Seism. Soc. Am. 2020, 110, 1–17. [Google Scholar] [CrossRef]
- Silva, P.G.; Elez, J.; Pérez-López, R.; Giner-Robles, J.L.; Gómez-Diego, P.V.; Roquero, E.; Rodríguez-Pascua, M.; Bardají, T. The AD 1755 Lisbon Earthquake-Tsunami: Seismic source modelling from the analysis of ESI-07 environmental data. Quat. Int. 2021, in press. [Google Scholar] [CrossRef]
- Henriques, J.P.; Filipe, V.G. Sob os escombros da Casa. In O Dia Em Que a Casa Foi Abaixo; Henriques, J.P., Filipe, V.G., Eds.; Câmara Municipal de Lisboa: Lisbon, Portugal, 2020; pp. 9–13. [Google Scholar]
- Rubio, L.; Costa, M.; Barrulas, P.; Lores, M.; Garcia-Jares, C.; Barrocas-Dias, C. Understanding the chemical and mineralogical composition of commercial henna and jagua tattoos and dyes—A multi-analytical approach. Anal. Bioanal. Chem. 2022, 414, 6233–6246. [Google Scholar] [CrossRef]
- Evershed, R.P.; Heron, C.; Goad, L.J. Analysis of organic residues of archaeological origin by high-temperature gas chromatography and gas chromatography-mass spectrometry. Analyst 1990, 115, 1339–1342. [Google Scholar] [CrossRef]
- Moore, P.D.; Webb, J.A.; Collinson, M.E. Pollen Analysis; Blackwell Scientific Publications: Oxford, UK, 1991; p. 216. [Google Scholar]
- Magri, D.; Di Rita, F. Archaeopalynological Preparation Techniques. In Plant Microtechniques and Protocols; Yeung, E.C.T., Stasolla, C., Sumner, M.J., Huang, B.Q., Eds.; Springer International Publishing: Berlin/Heidelberg, Germany, 2015; pp. 495–506. [Google Scholar]
- Reinhard, K.J.; Confalonieri, U.E.; Herrmann, B.; Ferreira, L.F.; de Araujo, A.J. Recovery of parasite remains from coprolites and latrines: Aspects of paleoparasitological technique. Anthropol. Fac. Publ. 1986, 29. [Google Scholar]
- Warnock, P.J.; Reinhard, K.J. Methods for extracting pollen and parasite eggs from latrine soils. J. Archaeol. Sci. 1992, 19, 261–264. [Google Scholar] [CrossRef]
- Albert, R.M.; Ruíz, J.A.; Sans, A. PhytCore ODB: A new tool to improve efficiency in the management and exchange of information on phytoliths. J. Archaeol. Sci. 2016, 68, 98–105. [Google Scholar] [CrossRef]
- Shumilovskikh, L.S.; Shumilovskikh, E.S.; Schlütz, F.; van Geel, B. NPP-ID: Non-Pollen Palynomorph Image Database as a research and educational platform. Veg. Hist. Archaeobotany 2021, 31, 323–328. [Google Scholar] [CrossRef]
- Ouatmane, A.; Provenzano, M.; Hafidi, M.; Senesi, N. Compost Maturity Assessment Using Calorimetry, Spectroscopy and Chemical Analysis. Compos. Sci. Util. 2000, 8, 124–134. [Google Scholar] [CrossRef]
- Larkin, P.J. Infrared and Raman Spectroscopy. In Principles and Spectral Interpretation; Larkin, P.J., Ed.; Elsevier: Amsterdam, The Netherlands, 2011; p. 228. [Google Scholar]
- Derrick, M.R.; Stulik, D.; Landry, J.M. Infrared Spectroscopy in Conservation Science; The Getty Conservation Institute: Los Angeles, CA, USA, 1999; p. 235. [Google Scholar]
- Gur-Arieh, S.; Mintz, E.; Boaretto, E.; Shahack-Gross, R. An ethnoarchaeological study of cooking installations in rural Uzbekistan: Development of a new method for identification of fuel sources. J. Archaeol. Sci. 2013, 40, 4331–4347. [Google Scholar] [CrossRef]
- Lang, Q.; Zhang, B.; Liu, Z.; Jiao, W.; Xia, Y.; Chen, Z.; Li, D.; Ma, J.; Gai, C. Properties of hydrochars derived from swine manure by CaO assisted hydrothermal carbonization. J. Environ. Manag. 2018, 233, 440–446. [Google Scholar] [CrossRef]
- Song, C.; Zheng, H.; Shan, S.; Wu, S.; Wang, H.; Christie, P. Low-Temperature Hydrothermal Carbonization of Fresh Pig Manure: Effects of Temperature on Characteristics of Hydrochars. J. Environ. Eng. 2019, 145, 04019029. [Google Scholar] [CrossRef]
- Rehman, I.; Bonfield, W. Characterization of hydroxyapatite and carbonated apatite by photo acoustic FTIR spectroscopy. J. Mater. Sci. Mater. Med. 1997, 8, 1–4. [Google Scholar] [CrossRef]
- Lebon, M.; Reiche, I.; Gallet, X.; Bellot-Gurlet, L.; Zazzo, A. Rapid Quantification of Bone Collagen Content by ATR-FTIR Spectroscopy. Radiocarbon 2016, 58, 131–145. [Google Scholar] [CrossRef]
- Shillito, L.M.; Almond, M.J.; Wicks, K.; Marshall, L.-J.R.; Matthews, W. The use of FT-IR as a screening technique for organic residue analysis of archaeological samples. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 72, 120–125. [Google Scholar] [CrossRef] [PubMed]
- Goldberg, P.; Berna, F.; Macphail, R. Comment on “DNA from pre-Clovis human coprolites in Oregon, North America”. Science 2009, 325, 148. [Google Scholar] [CrossRef] [PubMed]
- Réveillé, V.; Mansuy, L.; Jardé, É.; Garnier-Sillam, É. Characterisation of sewage sludge-derived organic matter: Lipids and humic acids. Org. Geochem. 2003, 34, 615–627. [Google Scholar] [CrossRef]
- Fry, G.F. Analysis of Prehistoric Coprolites from Utah. Anthropol. Pap. 1977, 97, 45. [Google Scholar]
- Larkin, N.R.; Alexander, J.; Lewis, M.D. Using Experimental Studies of Recent Faecal Material to Examine Hyaena Coprolites from the West Runton Freshwater Bed, Norfolk, U.K. J. Archaeol. Sci. 2000, 27, 19–31. [Google Scholar] [CrossRef]
- Sperança, M.A.; de Aquino, F.W.B.; Fernandes, M.A.; Lopez-Castillo, A.; Carneiro, R.L.; Pereira-Filho, E.R. Application of Laser-Induced Breakdown Spectroscopy and Hyperspectral Images for Direct Evaluation of Chemical Elemental Profiles of Coprolites. Geostand. Geoanalytical Res. 2016, 41, 273–282. [Google Scholar] [CrossRef]
- Shillito, L.-M.; Almond, M.J.; Nicholson, J.; Pantos, M.; Matthews, W. Rapid characterisation of archaeological midden components using FT-IR spectroscopy, SEM–EDX and micro-XRD. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2009, 73, 133–139. [Google Scholar] [CrossRef] [PubMed]
- Canti, M. An Investigation of Microscopic Calcareous Spherulites from Herbivore Dungs. J. Archaeol. Sci. 1997, 24, 219–231. [Google Scholar] [CrossRef]
- Pesquero, M.D.; Salesa, M.J.; Espílez, E.; Mampel, L.; Siliceo, G.; Alcalá, L. An exceptionally rich hyaena coprolites concentration in the Late Miocene mammal fossil site of La Roma 2 (Teruel, Spain): Taphonomical and palaeoenvironmental inferences. Palaeogeogr. Palaeoclim. Palaeoecol. 2011, 311, 30–37. [Google Scholar] [CrossRef]
- Eglinton, G.; Gonzalez, A.; Hamilton, R.; Raphael, R. Hydrocarbon constituents of the wax coatings of plant leaves: A taxonomic survey. Phytochemistry 1962, 1, 89–102. [Google Scholar] [CrossRef]
- Evershed, R.P.; Dudd, S.N.; Copley, M.S.; Berstan, R.; Stott, A.W.; Mottram, H.; Buckley, S.A.; Crossman, Z. Chemistry of Archaeological Animal Fats. Accounts Chem. Res. 2002, 35, 660–668. [Google Scholar] [CrossRef] [PubMed]
- Eerkens, J.W. Gc-Ms Analysis and Fatty Acid Ratios of Archaeological Potsherds From The Western Great Basin Of North America*. Archaeometry 2005, 47, 83–102. [Google Scholar] [CrossRef]
- Khorkova, A.N.; Danilov, D.A.; Kiseleva, D.V.; Dubyagina, E.V. Fatty acid composition of organic residue on bronze age pottery (Bozshakol, Kazakhstan) by GC–MS after acid methanolysis. AIP Conf. Proc. 2020, 2313, 050055. [Google Scholar] [CrossRef]
- Stern, B.; Heron, C.; Serpico, M.; Bourriau, J. A Comparison of Methods for Establishing Fatty Acid Concentration Gradients Across Potsherds: A Case Study Using Late Bronze Age Canaanite Amphorae. Archaeometry 2000, 42, 399–414. [Google Scholar] [CrossRef]
- Steele, V.J.; Stern, B.; Stott, A.W. Olive oil or lard?: Distinguishing plant oils from animal fats in the archeological record of the eastern Mediterranean using gas chromatography/combustion/isotope ratio mass spectrometry. Rapid Commun. Mass Spectrom. 2010, 24, 3478–3484. [Google Scholar] [CrossRef]
- Férézou, J.; Gouffier, E.; Coste, T.; Chevallier, F. Daily Elimination of Fecal Neutral Sterols by Humans. Digestion 1978, 18, 201–212. [Google Scholar] [CrossRef] [PubMed]
- Furtula, V.; Liu, J.; Chambers, P.; Osachoff, H.; Kennedy, C.; Harkness, J. Sewage Treatment Plants Efficiencies in Removal of Sterols and Sterol Ratios as Indicators of Fecal Contamination Sources. Water Air Soil Pollut. 2011, 223, 1017–1031. [Google Scholar] [CrossRef]
- Shah, V.G.; Dunstan, R.H.; Geary, P.M.; Coombes, P.; Roberts, T.K.; Von Nagy-Felsobuki, E. Evaluating potential applications of faecal sterols in distinguishing sources of faecal contamination from mixed faecal samples. Water Res. 2007, 41, 3691–3700. [Google Scholar] [CrossRef] [PubMed]
- Marques, A.C.; Henriques, J.P. O Dia em Que a Casa Foi Abaixo; Henriques, J.P., Filipe, V.G., Eds.; Câmara Municipal de Lisboa: Lisbon, Portugal, 2020; pp. 56–57. [Google Scholar]
- van Geel, B. Non-Pollen Palynomorphs. In Tracking Environmental Change Using Lake Sediments, Terrestrial, Algal and Silicaceous Indicators; Smol, J.P., Birks, H.J.B., Last, W.M., Eds.; Springer: Berlin/Heidelberg, Germany, 2001; pp. 99–119. [Google Scholar] [CrossRef]
- Miola, A. Tools for Non-Pollen Palynomorphs (NPPs) analysis: A list of Quaternary NPP types and reference literature in English language (1972–2011). Rev. Palaeobot. Palynol. 2012, 186, 142–161. [Google Scholar] [CrossRef]
- Rosen, A.M. Phytolith Analysis. In Encyclopedia of Archaeology; Pearsall, D., Ed.; Academic Press: Cambridge, MA, USA, 2008; pp. 1818–1822. [Google Scholar]
- International Committee for Phytolith Taxonomy (ICPT). International Code for Phytolith Nomenclature (ICPN) 2.0. Ann. Bot. 2019, 124, 189–199. [Google Scholar] [CrossRef] [PubMed]
- Pals, J.; Van Geel, B.; Delfos, A. Paleoecological studies in the Klokkeweel bog near hoogkarspel (prov. of Noord-Holland). Rev. Palaeobot. Palynol. 1980, 30, 371–418. [Google Scholar] [CrossRef]
- Égüez, N.; Corso, M.D.; Wieckowska-Lüth, M.; Delpino, C.; Tarantini, M.; Biagetti, S. A pilot geo-ethnoarchaeological study of dung deposits from pastoral rock shelters in the Monti Sibillini (central Italy). Archaeol. Anthr. Sci. 2020, 12, 1–19. [Google Scholar] [CrossRef]
- Brinkkemper, O.; van Haaster, H. Eggs of intestinal parasites whipworm (Trichuris) and mawworm (Ascaris): Non-pollen palynomorphs in archaeological samples. Rev. Palaeobot. Palynol. 2012, 186, 16–21. [Google Scholar] [CrossRef]
- Anastasiou, E.; Papathanasiou, A.; Schepartz, L.A.; Mitchell, P.D. Infectious disease in the ancient Aegean: Intestinal parasitic worms in the Neolithic to Roman Period inhabitants of Kea, Greece. J. Archaeol. Sci. Rep. 2018, 17, 860–864. [Google Scholar] [CrossRef]
- Reinhard, K.J.; Bryant Jr, V.M. Pathoecology and the future of coprolite studies in bioarchaeology. Pap. Nat. Resour. 2008, 43, 199–216. [Google Scholar]
- Evershed, R.P.; Bethell, P.; Reynolds, P.; Walsh, N. 5β-Stigmastanol and Related 5β-Stanols as Biomarkers of Manuring: Analysis of Modern Experimental Material and Assessment of the Archaeological Potential. J. Archaeol. Sci. 1997, 24, 485–495. [Google Scholar] [CrossRef]
- Schroeter, N.; Lauterbach, S.; Stebich, M.; Kalanke, J.; Mingram, J.; Yildiz, C.; Schouten, S.; Gleixner, G. Biomolecular Evidence of Early Human Occupation of a High-Altitude Site in Western Central Asia During the Holocene. Front. Earth Sci. 2020, 8, 20. [Google Scholar] [CrossRef]
- Lerch, M.; Bromm, T.; Geitner, C.; Haas, J.N.; Schäfer, D.; Glaser, B.; Zech, M. Human and livestock faecal biomarkers at the prehistorical encampment site of Ullafelsen in the Fotsch Valley, Stubai Alps, Austria—Potential and limitations. Biogeosciences 2022, 19, 1135–1150. [Google Scholar] [CrossRef]
- Ledger, M.L.; Anastasiou, E.; Shillito, L.-M.; Mackay, H.; Bull, I.; Haddow, S.; Knüsel, C.J.; Mitchell, P.D. Parasite infection at the early farming community of Çatalhöyük. Antiquity 2019, 93, 573–587. [Google Scholar] [CrossRef]
- Zatoń, M.; Niedźwiedzki, G.; Marynowski, L.; Benzerara, K.; Pott, C.; Cosmidis, J.; Krzykawski, T.; Filipiak, P. Coprolites of Late Triassic carnivorous vertebrates from Poland: An integrative approach. Palaeogeogr. Palaeoclim. Palaeoecol. 2015, 430, 21–46. [Google Scholar] [CrossRef]
- Veiga, P.; Juste, C.; Lepercq, P.; Saunier, K.; Béguet, F.; Gérard, P. Correlation between faecal microbial community structure and cholesterol-to-coprostanol conversion in the human gut. FEMS Microbiol. Lett. 2005, 242, 81–86. [Google Scholar] [CrossRef]
- Juste, C.; Gérard, P. Cholesterol-to-Coprostanol Conversion by the Gut Microbiota: What We Know, Suspect, and Ignore. Microorganisms 2021, 9, 1881. [Google Scholar] [CrossRef] [PubMed]
- Cuevas-Tena, M.; Alegria, A.; Lagarda, M.J.; Venema, K. Impact of plant sterols enrichment dose on gut microbiota from lean and obese subjects using TIM-2 in vitro fermentation model. J. Funct. Foods 2019, 54, 164–174. [Google Scholar] [CrossRef]
- Grimalt, J.O.; Fernandez, P.; Bayona, J.M.; Albaiges, J. Assessment of fecal sterols and ketones as indicators of urban sewage inputs to coastal waters. Environ. Sci. Technol. 1990, 24, 357–363. [Google Scholar] [CrossRef]
- Manhita, A.; Martins, S.; da Silva, M.G.; Lopes, M.D.C.; Dias, C.B. Transporting Olive Oil in Roman Times: Chromatographic Analysis of Dressel 20 Amphorae from Pax Julia Civitas, Lusitania. Chromatographia 2020, 83, 1055–1064. [Google Scholar] [CrossRef]
- McAfee, A.J.; McSorley, E.M.; Cuskelly, G.J.; Moss, B.W.; Wallace, J.M.W.; Bonham, M.P.; Fearon, A.M. Red meat consumption: An overview of the risks and benefits. Meat Sci. 2010, 84, 1–13. [Google Scholar] [CrossRef]
- Pozio, E. Foodborne nematodes. In Foodborne Parasites in the Food Supply Web; Gajadhar, A.A., Ed.; Woodhead Publishing: Sawston, UK, 2015; pp. 165–199. [Google Scholar]
- Di Berardino, S.E. Water and sanitation management in medieval Portugal. Water Supply 2017, 18, 630–637. [Google Scholar] [CrossRef]
- Teixeira, A.; Silva, R.B.D. The Water Supply and Sewage Networks in Sixteenth Century Lisbon: Drawing the Renaissance City. In The History of Water Management in the Iberian Peninsula; Springer: Berlin/Heidelberg, Germany, 2020; pp. 3–24. [Google Scholar] [CrossRef]
- Silva, R.B.D. Higiene e Saneamento. In O Dia em Que a Casa Foi Abaixo; Henriques, J.P., Filipe, V.G., Eds.; Câmara Municipal de Lisboa: Lisbon, Portugal, 2020; pp. 53–54. [Google Scholar]
- López-Bravo, C.; López, J.P.; Adell, E.M. The management of water heritage in Portuguese cities: Recent regeneration projects in Évora, Lisbon, Braga and Guimarães. Front. Arch. Res. 2021, 11, 73–88. [Google Scholar] [CrossRef]
- Hall, A.; Hewitt, G.; Tuffrey, V.; De Silva, N. A review and meta-analysis of the impact of intestinal worms on child growth and nutrition. Matern. Child Nutr. 2008, 4, 118–236. [Google Scholar] [CrossRef]
- Moreira, A. Um tratado de medicina inédito do século XVIII: Estudo comparativo com fonte impressa e aspetos de variação na língua do Minho. Ph.D. Thesis, Universidade do Minho, Braga, Portugal, 2016. [Google Scholar]
- Batata, M.H.; Rodrigues, A.P.; Botelho, A.; Baixinho, C.L. Opções terapêuticas do século XVIII. O caso do banho na Medicina Lusitana. Escola Superior de Enfermagem de Lisboa (ESEL). In Aprender História de enfermagem: Um processo de descoberta, Ferreira, Ó. et al., Org; Escola Superior de Enfermagem de Lisboa (ESEL); Lisboa, Portugal, 2018; pp. 31–50. [Google Scholar]
- Kumm, K.J.; Reinhard, K.J.; Piombino-Mascali, D.; Araujo, A. Archaeoparasitological investigation of a mummy from Sicily (18th–19th century AD). Anthropologie 2010, 48, 177–184. [Google Scholar]
- Allen, S.D.; Almond, M.J.; Bell, M.G.; Hollins, P.; Marks, S.; Mortimore, J.L. Infrared spectroscopy of the mineralogy of coprolites from Brean Down: Evidence of past human activities and animal husbandry. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2002, 58, 959–965. [Google Scholar] [CrossRef] [PubMed]




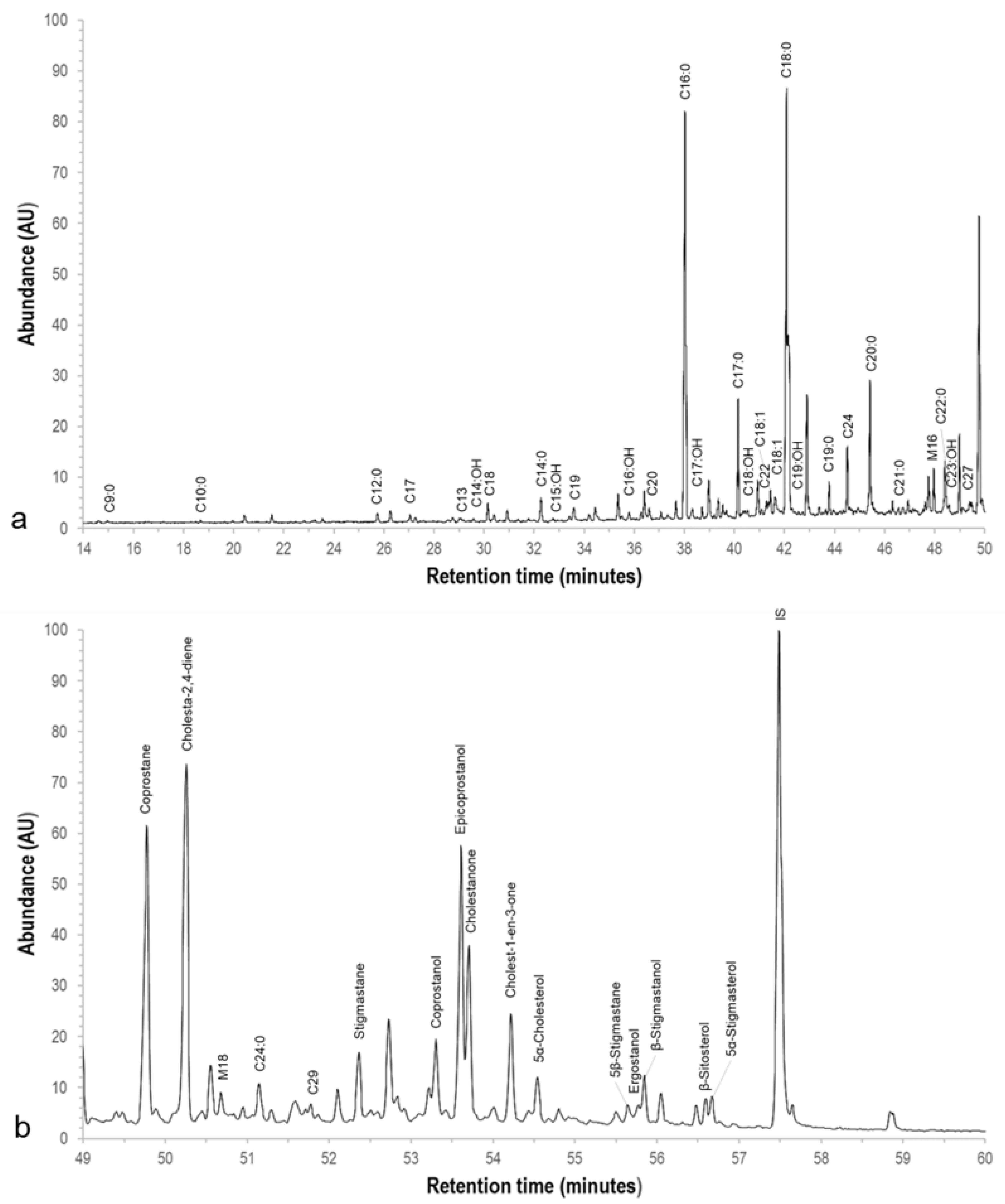
| Compound | Retention Time | Compound | Retention Time | ||
|---|---|---|---|---|---|
| (Minutes) | (Minutes) | ||||
| Steroids | Coprostane | 49.758 | Fatty acids | Pelargonic acid (C9:0) | 14.966 |
| Cholesta-2,4-diene | 50.258 | Capric acid (C10:0) | 18.675 | ||
| Stigmastane | 52.362 | Lauric acid (C12:0) | 25.733 | ||
| Coprostanol | 53.38 | Tridecylic acid (C13:0) | 29.078 | ||
| Epicoprostanol | 53.61 | Myristic acid (C14:0) | 32.279 | ||
| Cholestanone | 53.706 | Palmitic acid (C16:0) | 38.024 | ||
| Cholest-1-en-3-one | 54.221 | Margaric acid (C17:0) | 40.148 | ||
| 5α-Cholesterol | 54.542 | Octadecenoic acid isomer (C18:1) | 41.442 | ||
| 5β-Stigmastane | 55.642 | Octadecenoic acid isomer (C18:1) | 41.608 | ||
| Ergostanol | 55.775 | Stearic acid (C18:0) | 42.142 | ||
| β-Stigmastanol | 55.842 | Nonadecylic acid (C19:0) | 43.783 | ||
| β-Sitosterol | 56.583 | Arachidic acid (C20:0) | 45.415 | ||
| 5α-Stigmasterol | 56.675 | Heneicosylic acid (C21:0) | 46.932 | ||
| Alcohols | Myristyl alcohol (C14:OH) | 29.583 | Behenic acid (C22:0) | 48.407 | |
| Pentadecyl alcohol (C15:OH) | 32.79 | Lignoceric acid (C24:0) | 51.143 | ||
| Palmityl alcohol (C16:OH) | 35.792 | Alkanes | Heptadecane (C17) | 27.042 | |
| Heptadecyl alcohol (C17:OH) | 38.327 | Octadecane (C18) | 30.408 | ||
| Stearyl alcohol (C18:OH) | 40.425 | Nonadecane (C19) | 33.56 | ||
| Nonadecyl alcohol (C19:OH) | 42.325 | Eicosane (C20) | 36.59 | ||
| Arachidyl alcohol (C20:OH) | 45.608 | Docosane (C22) | 41.075 | ||
| Tricosyl alcohol (C23:OH) | 48.567 | Tetracosane (C24) | 44.608 | ||
| Acylglycerols | Monopalmitin | 47.96 | Heptacosane (C27) | 49.142 | |
| Monostearin | 50.676 | Nonacosane (C29) | 51.858 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fundurulic, A.; Manhita, A.; Filipe, V.G.; Henriques, J.P.; Marques, A.; Celant, A.; Magri, D.; Barrocas Dias, C. Archaeological Evidence for the Dietary Practices and Lifestyle of 18th Century Lisbon, Portugal—Combined Steroidal Biomarker and Microparticle Analysis of the Carbonized Faecal Remains. Separations 2023, 10, 85. https://doi.org/10.3390/separations10020085
Fundurulic A, Manhita A, Filipe VG, Henriques JP, Marques A, Celant A, Magri D, Barrocas Dias C. Archaeological Evidence for the Dietary Practices and Lifestyle of 18th Century Lisbon, Portugal—Combined Steroidal Biomarker and Microparticle Analysis of the Carbonized Faecal Remains. Separations. 2023; 10(2):85. https://doi.org/10.3390/separations10020085
Chicago/Turabian StyleFundurulic, Ana, Ana Manhita, Vanessa Galiza Filipe, José Pedro Henriques, António Marques, Alessandra Celant, Donatella Magri, and Cristina Barrocas Dias. 2023. "Archaeological Evidence for the Dietary Practices and Lifestyle of 18th Century Lisbon, Portugal—Combined Steroidal Biomarker and Microparticle Analysis of the Carbonized Faecal Remains" Separations 10, no. 2: 85. https://doi.org/10.3390/separations10020085
APA StyleFundurulic, A., Manhita, A., Filipe, V. G., Henriques, J. P., Marques, A., Celant, A., Magri, D., & Barrocas Dias, C. (2023). Archaeological Evidence for the Dietary Practices and Lifestyle of 18th Century Lisbon, Portugal—Combined Steroidal Biomarker and Microparticle Analysis of the Carbonized Faecal Remains. Separations, 10(2), 85. https://doi.org/10.3390/separations10020085