Recent Clay-Based Photocatalysts for Wastewater Treatment
Abstract
1. Introduction
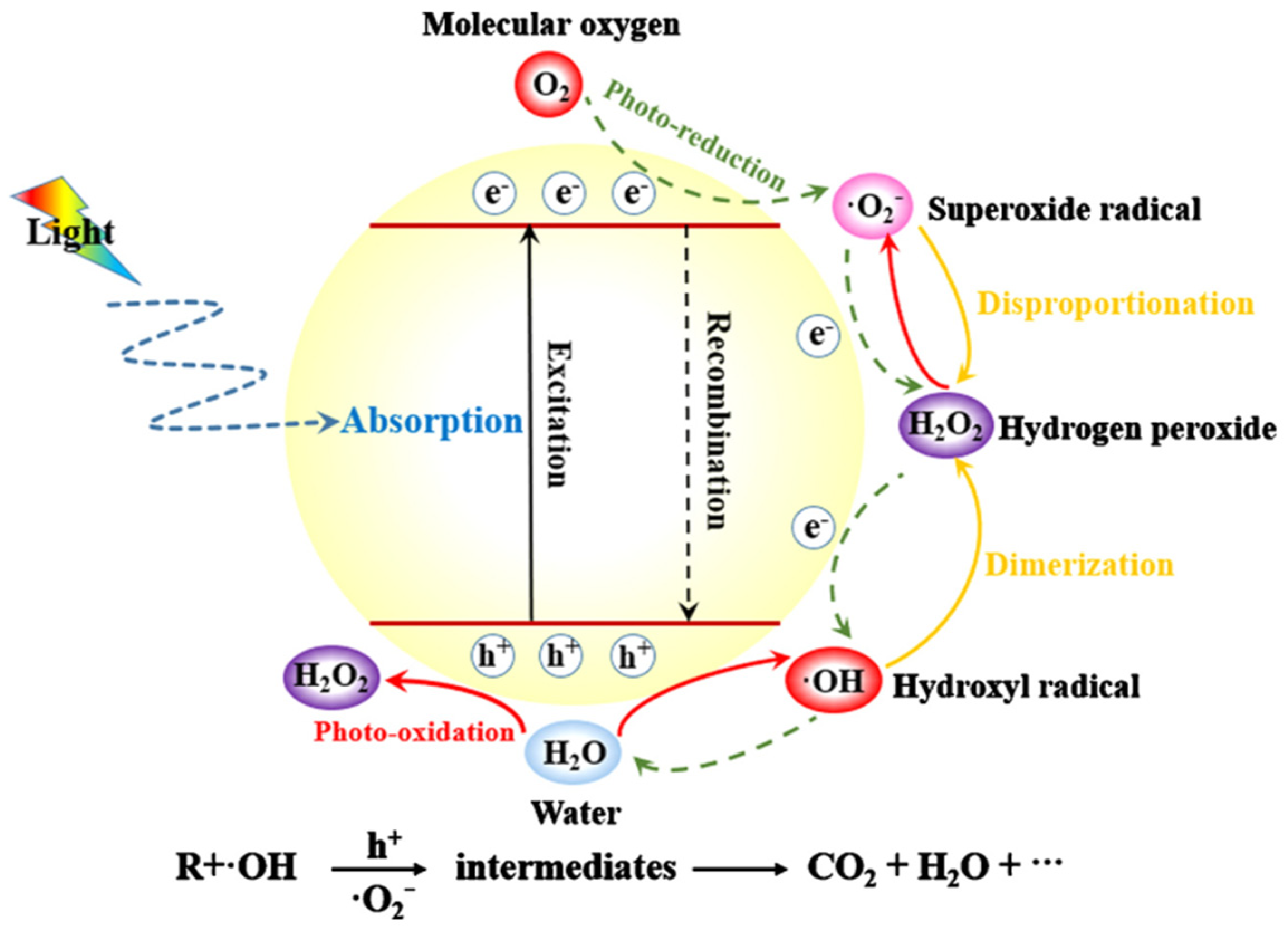
1.1. Photocatalysis and Its Mechanism
1.2. Type and Role of Clay in Photocatalysis
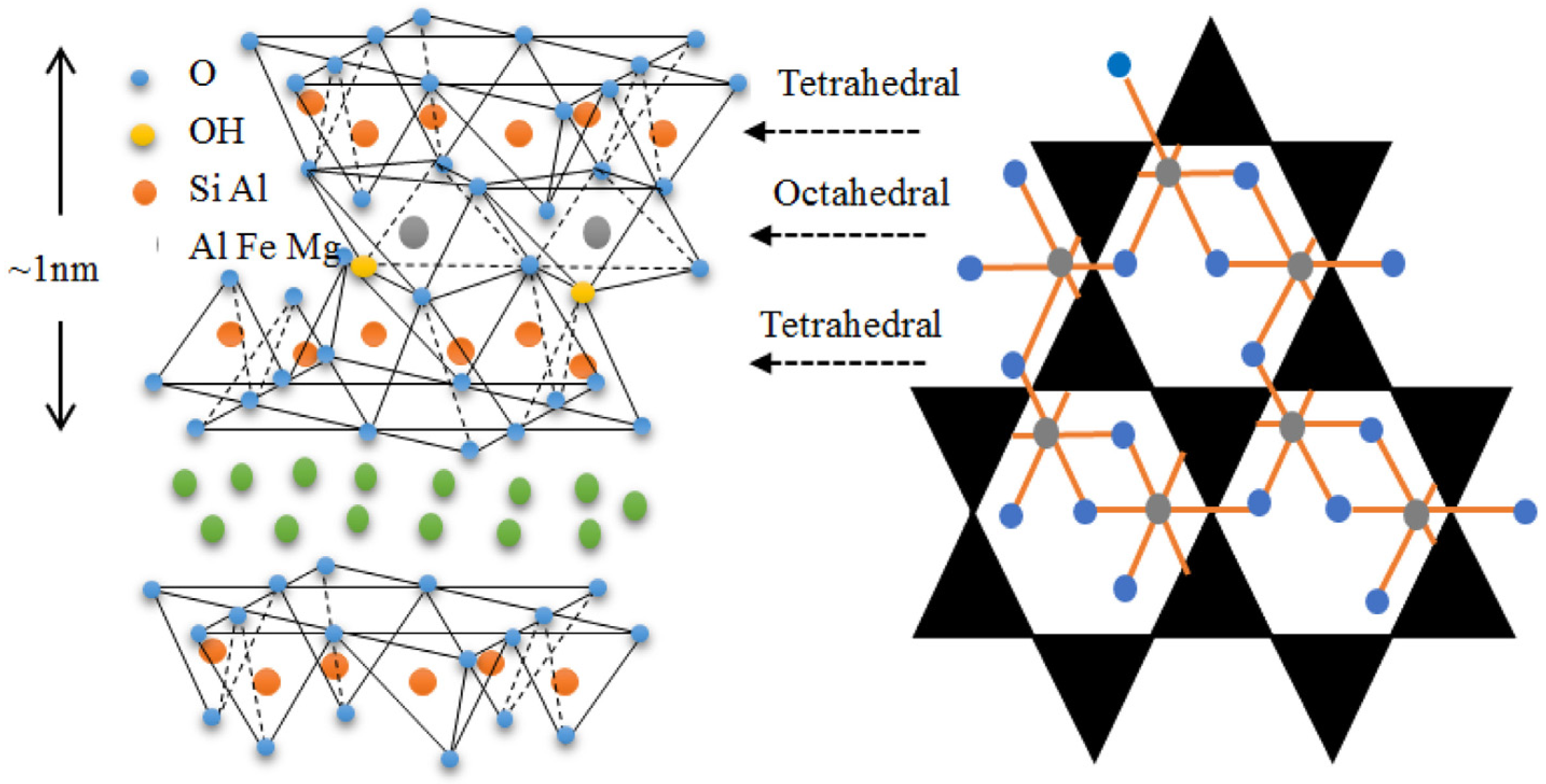
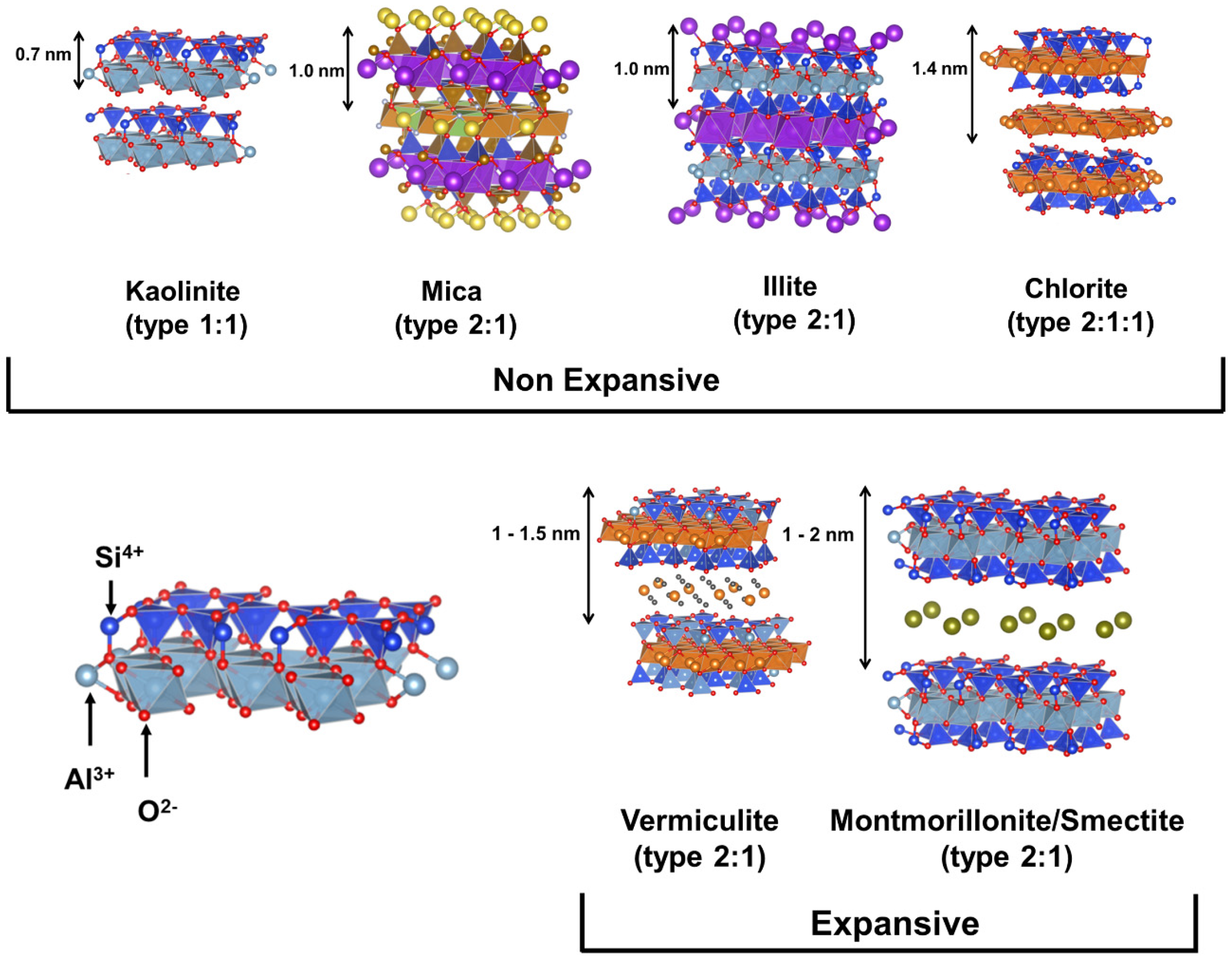
2. Clay-Based Photocatalyst
2.1. Graphitic Carbon Nitride/Clay Composites
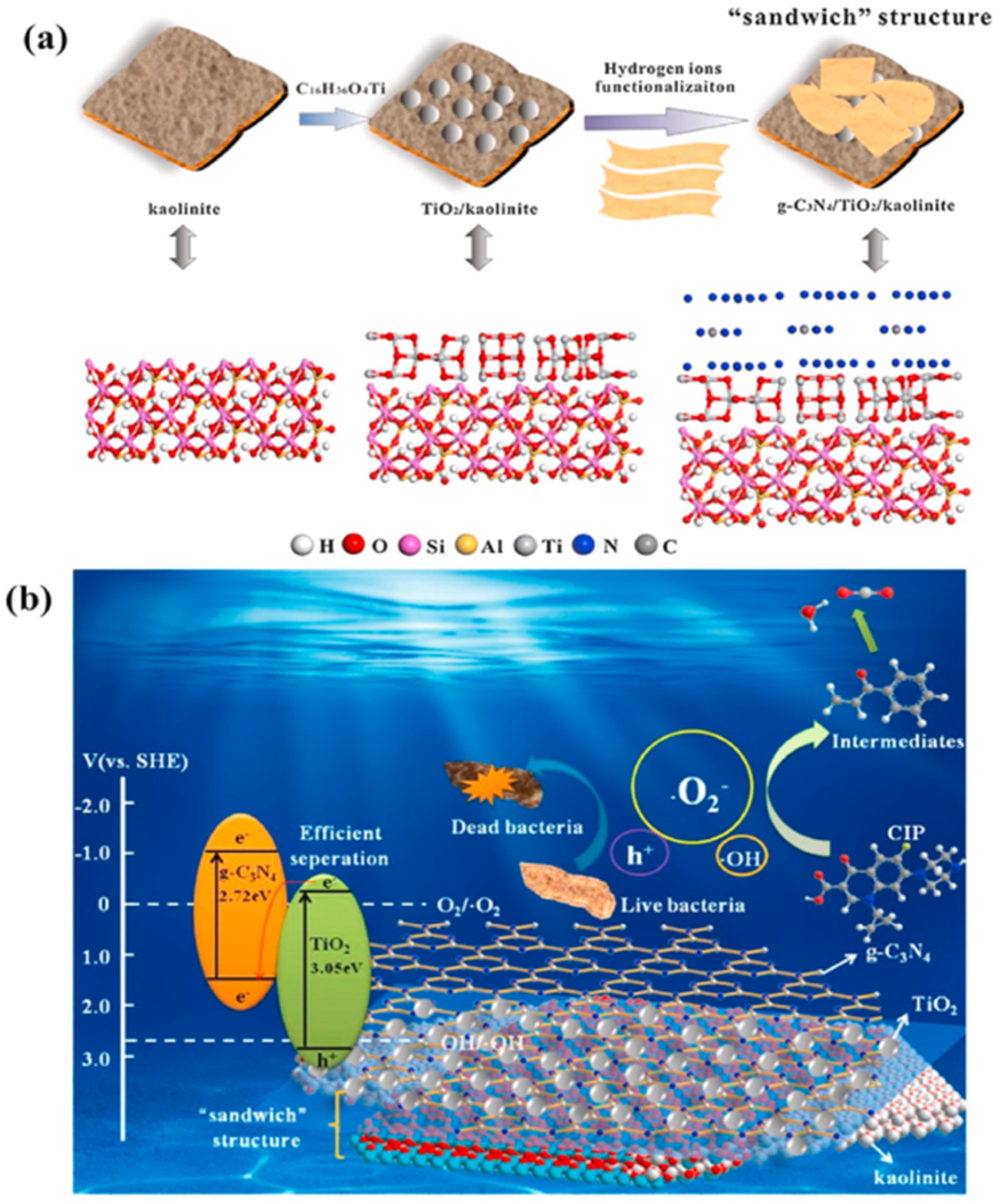
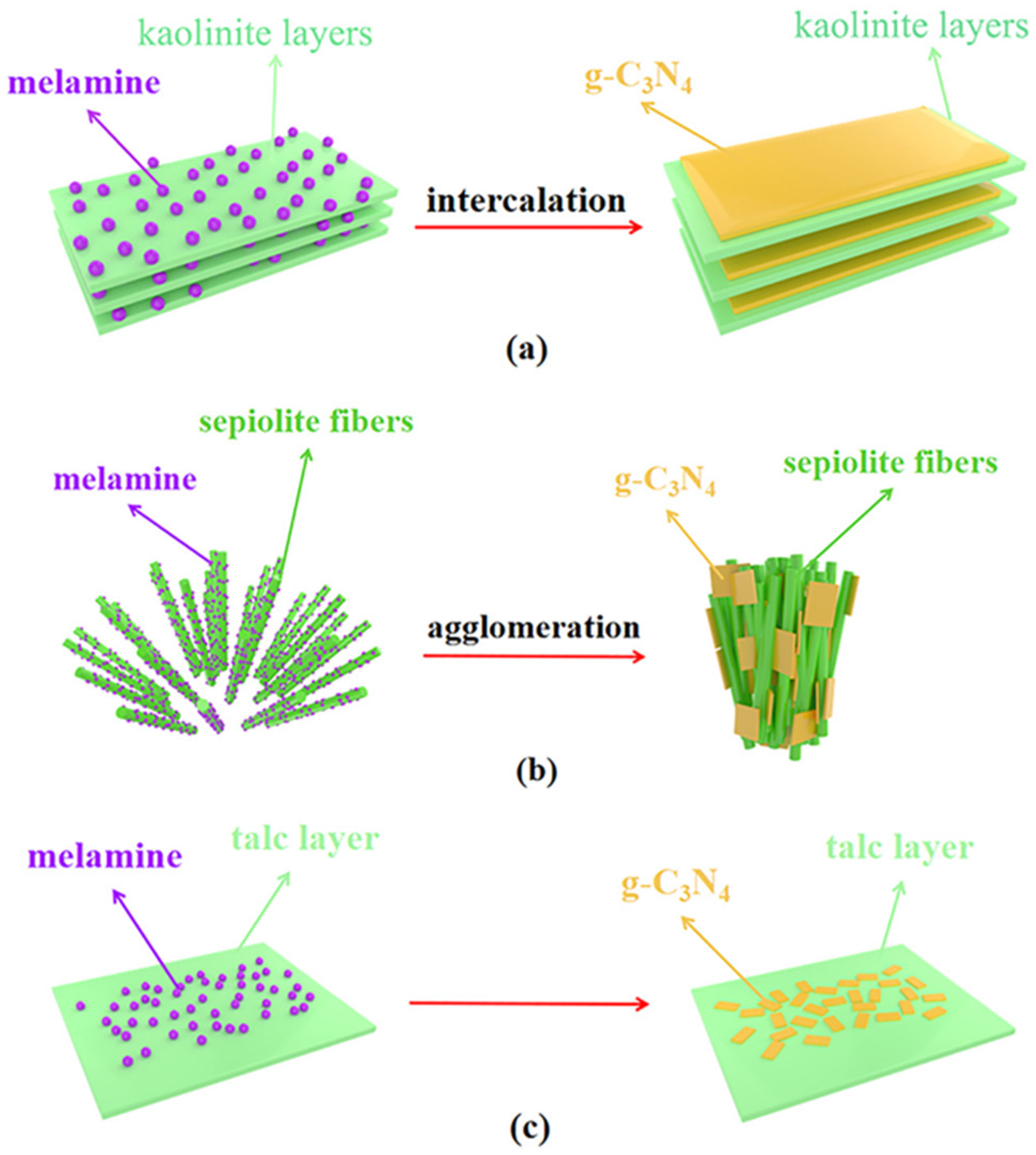
2.1.1. g-C3N4/Kaolinite
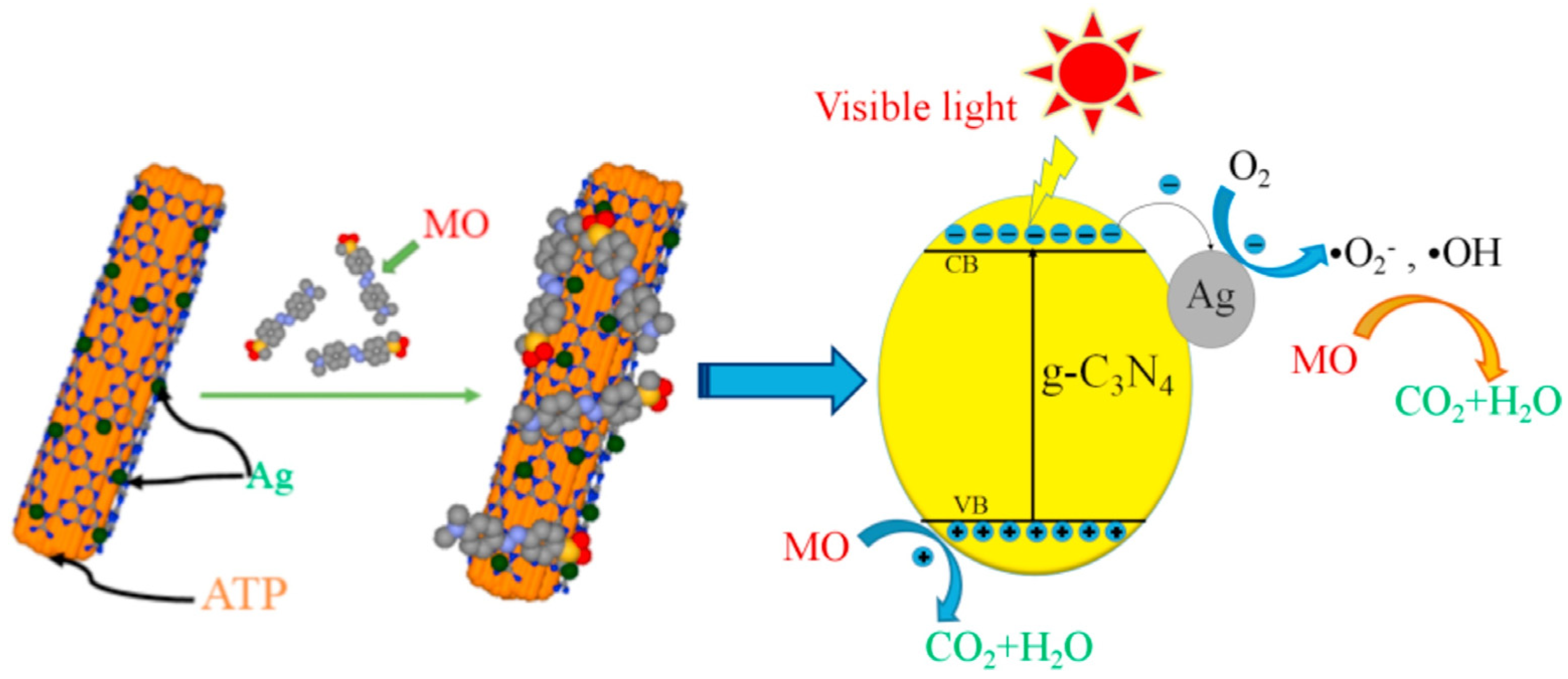
2.1.2. g-C3N4/Attapulgite
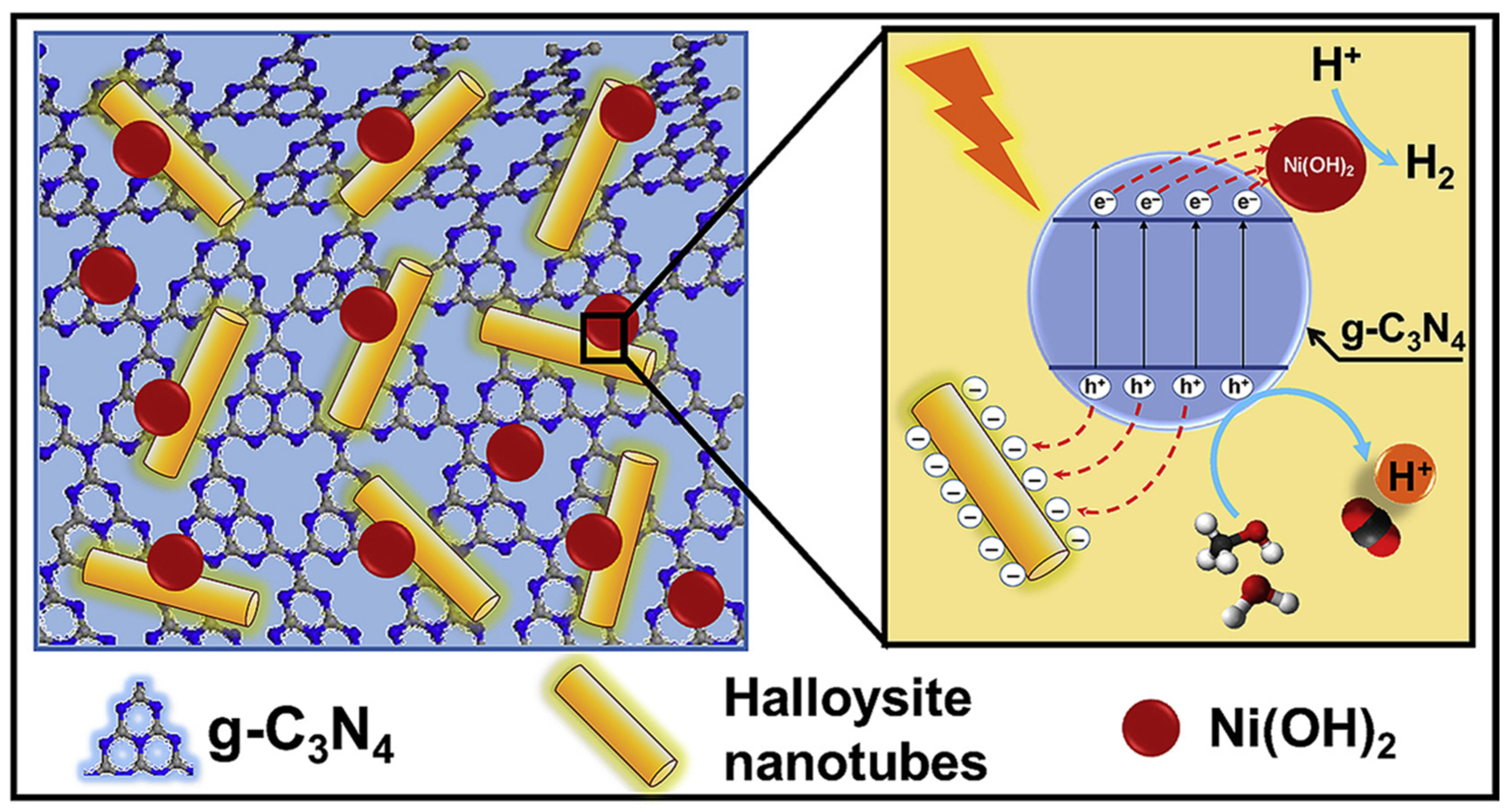
2.1.3. g-C3N4/Halloysite Nanotubes
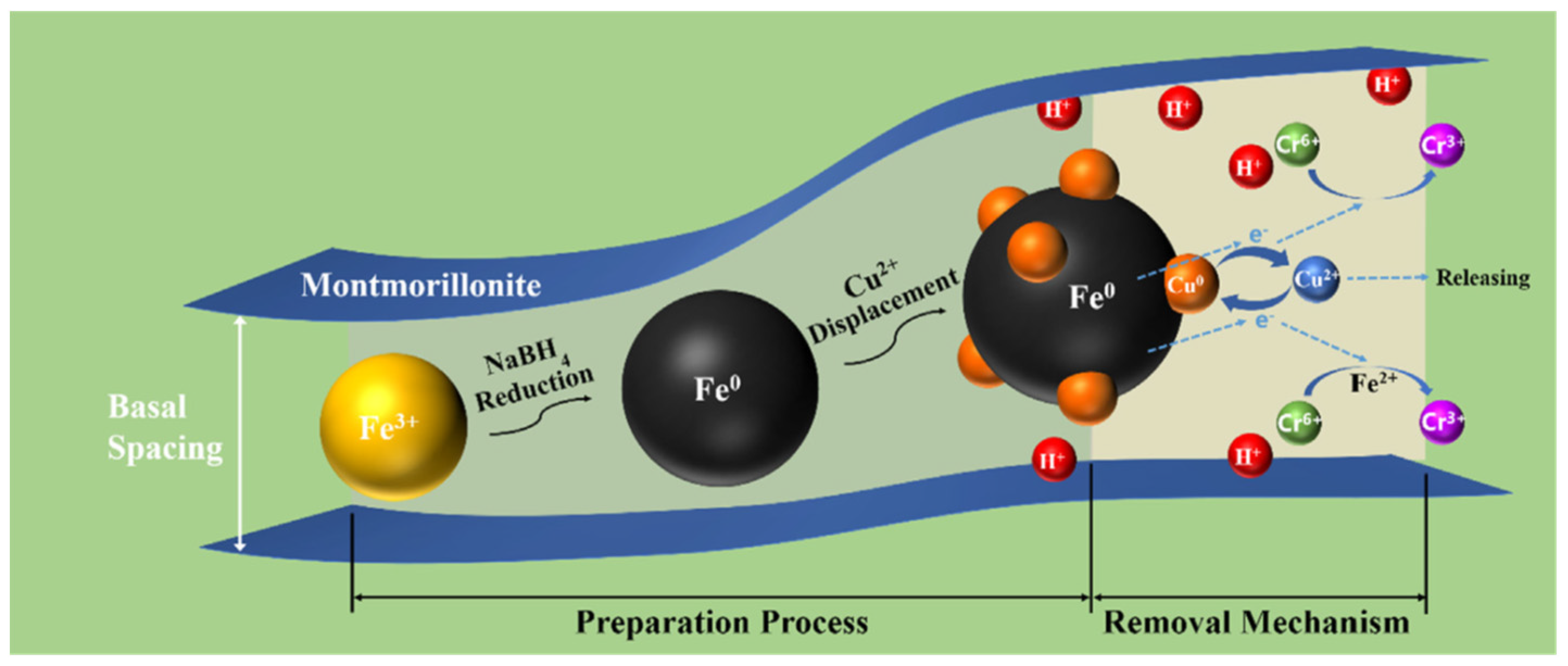
2.1.4. g-C3N4/Montmorillonite
2.1.5. g-C3N4/Rectorite
2.1.6. g-C3N4/Sepiolite
2.1.7. g-C3N4/Vermiculite
2.2. Titanium Dioxide/Clay Composites
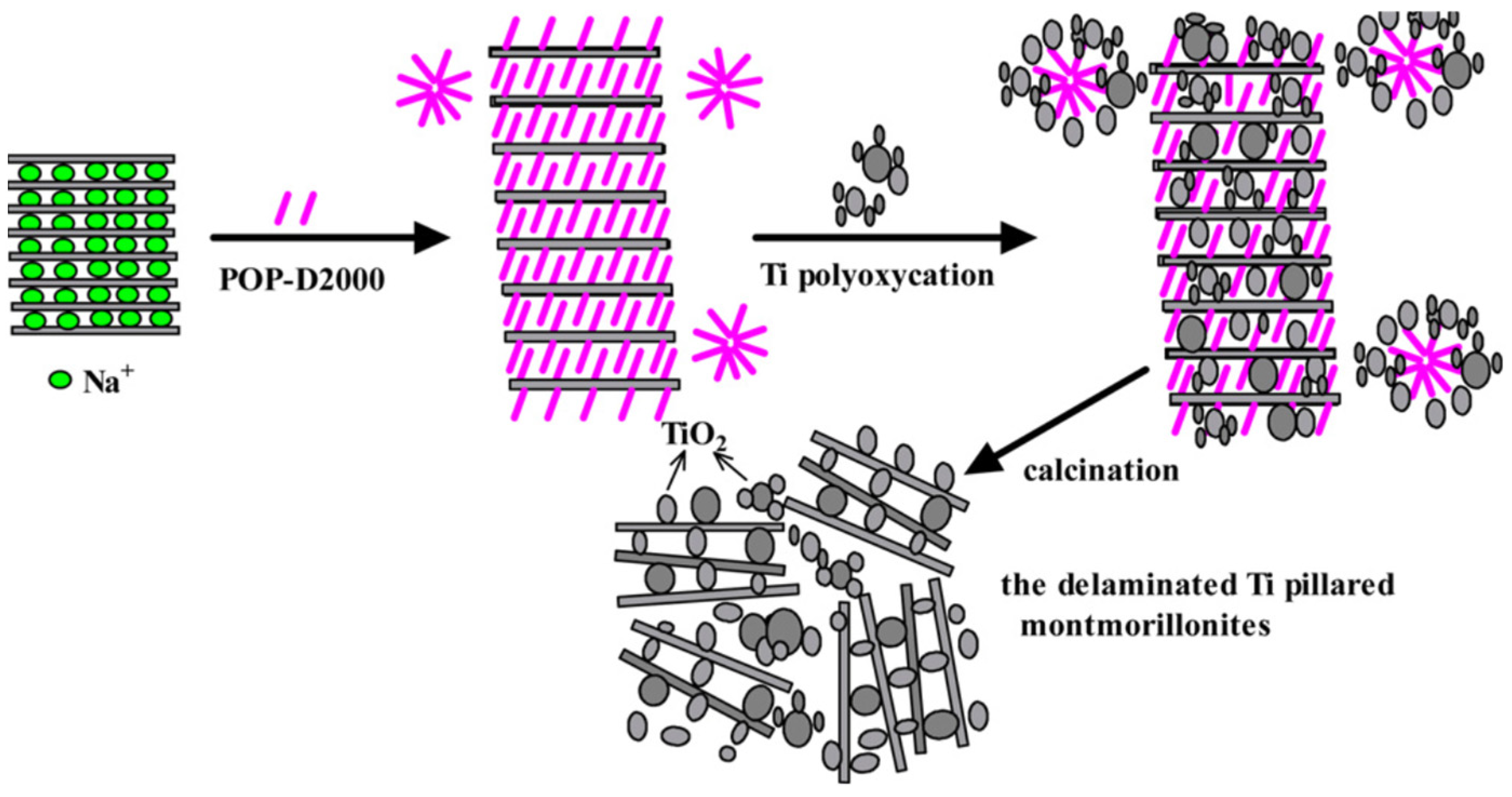
2.2.1. TiO2/Montmorillonite or Smectite
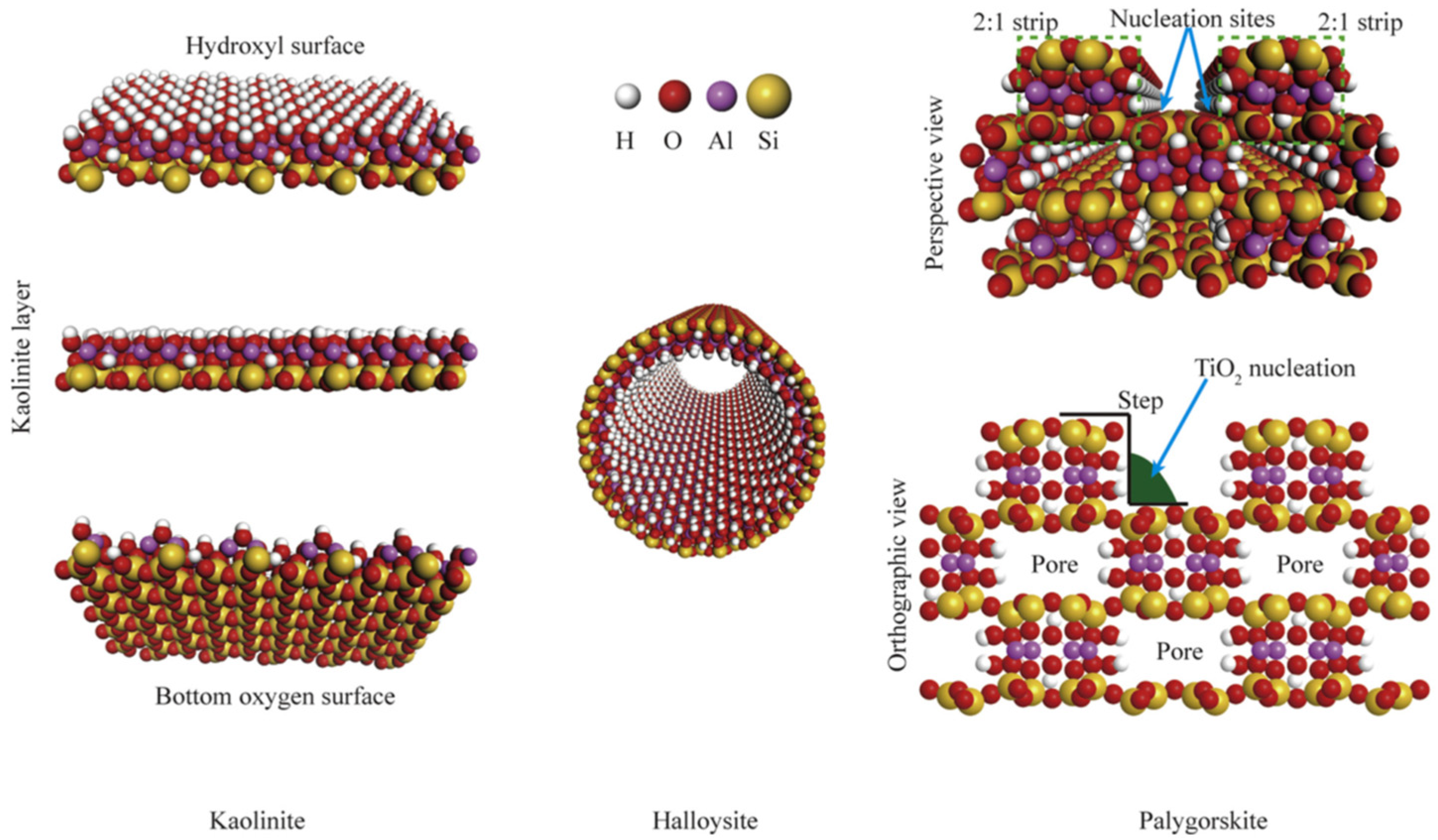
2.2.2. TiO2/Kaolin or Kaolinite
2.2.3. TiO2/Bentonite
2.2.4. TiO2/Zeolite
2.2.5. TiO2/Serpentine
2.2.6. TiO2/Layered Double Hydroxides
2.2.7. TiO2/Sepiolite
2.2.8. TiO2/Romanian
2.2.9. TiO2/Diatomite
2.2.10. TiO2/Anthracite
2.3. Bismuth-Based Photocatalysts/Clay Composites
2.3.1. Bismuth-Based Photocatalysts/Montmorillonite
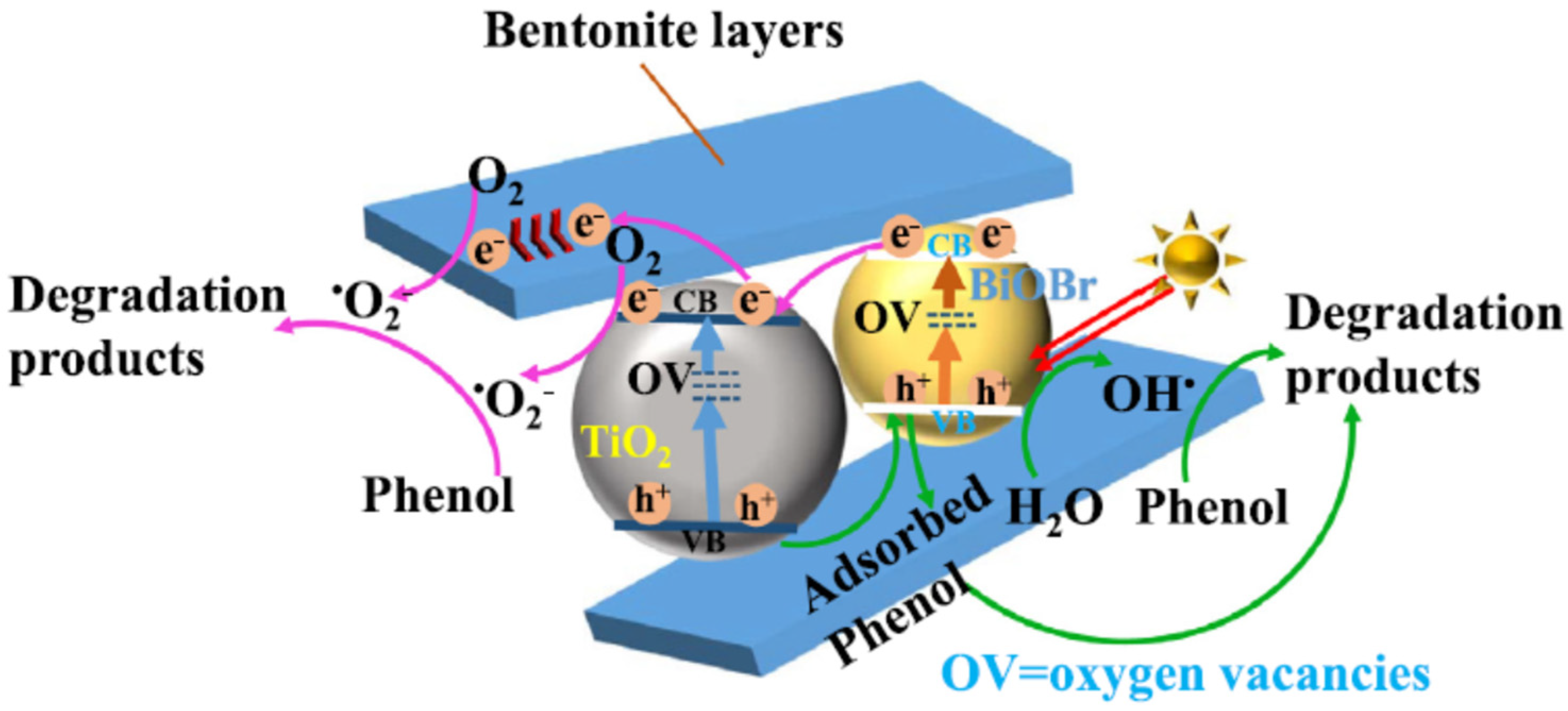
2.3.2. Bismuth-Based Photocatalysts/Bentonite
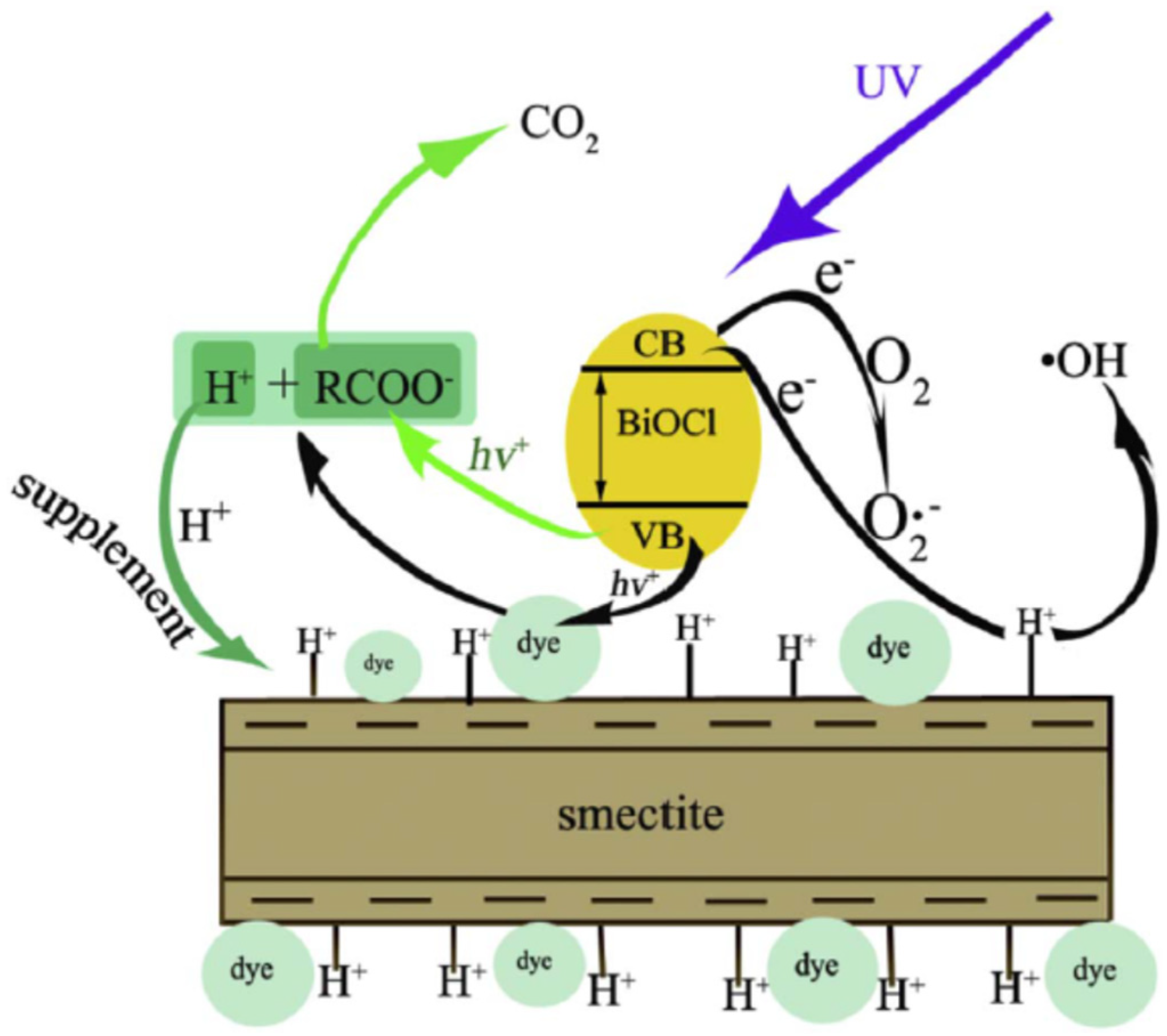
2.3.3. Bismuth-Based Photocatalysts/Sepiolite
2.3.4. Bismuth-Based Photocatalysts/Rectorite
2.3.5. Bismuth-Based Photocatalysts/Saponite
2.3.6. Bismuth-Based Photocatalysts/Palygorskite
2.3.7. Bismuth-Based Photocatalysts/Laponite
2.4. Other Photocatalysts/Clay Composites
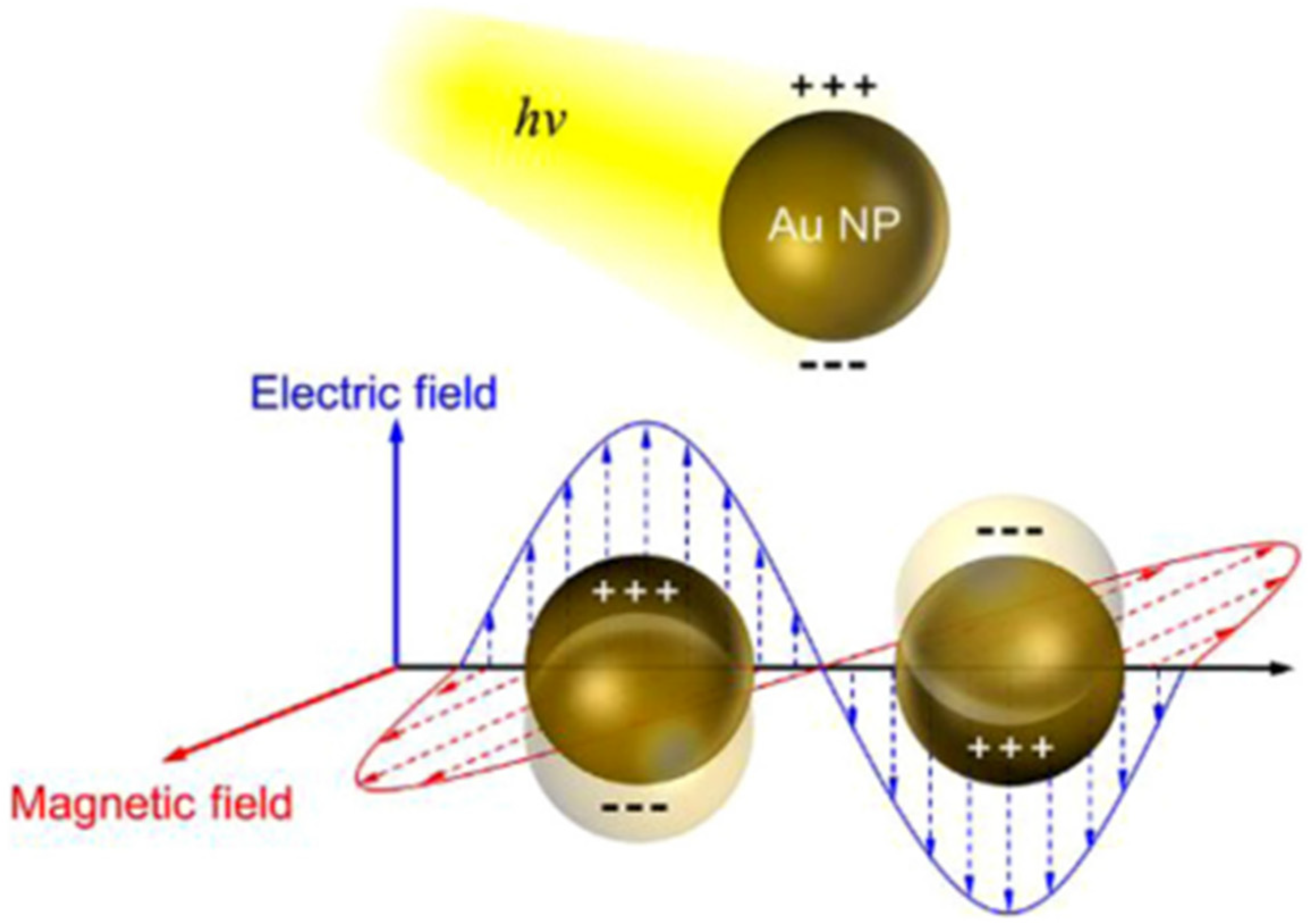
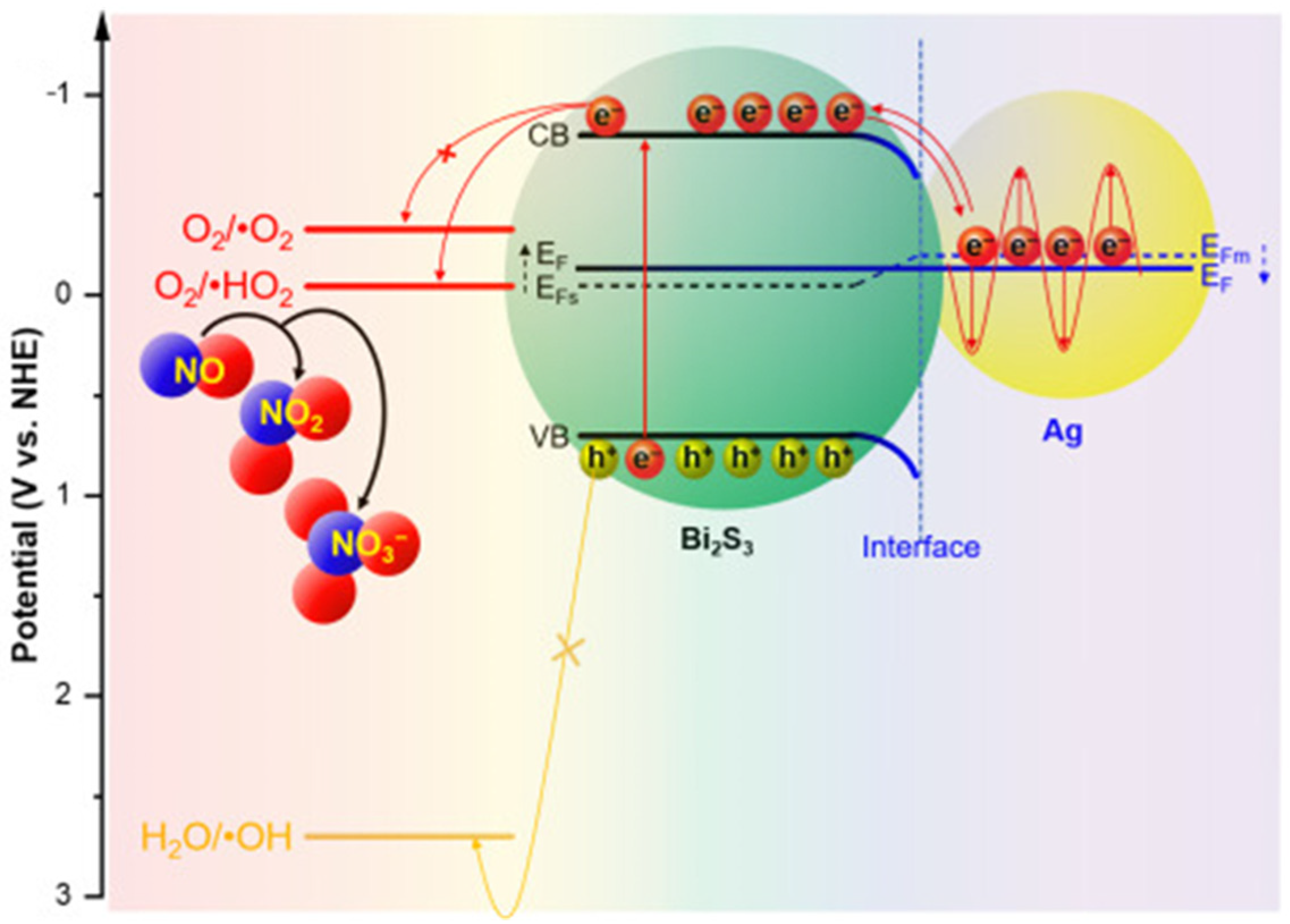
2.4.1. Plasmonic Nanoparticles Incorporated in Clay-Based Photocatalysts
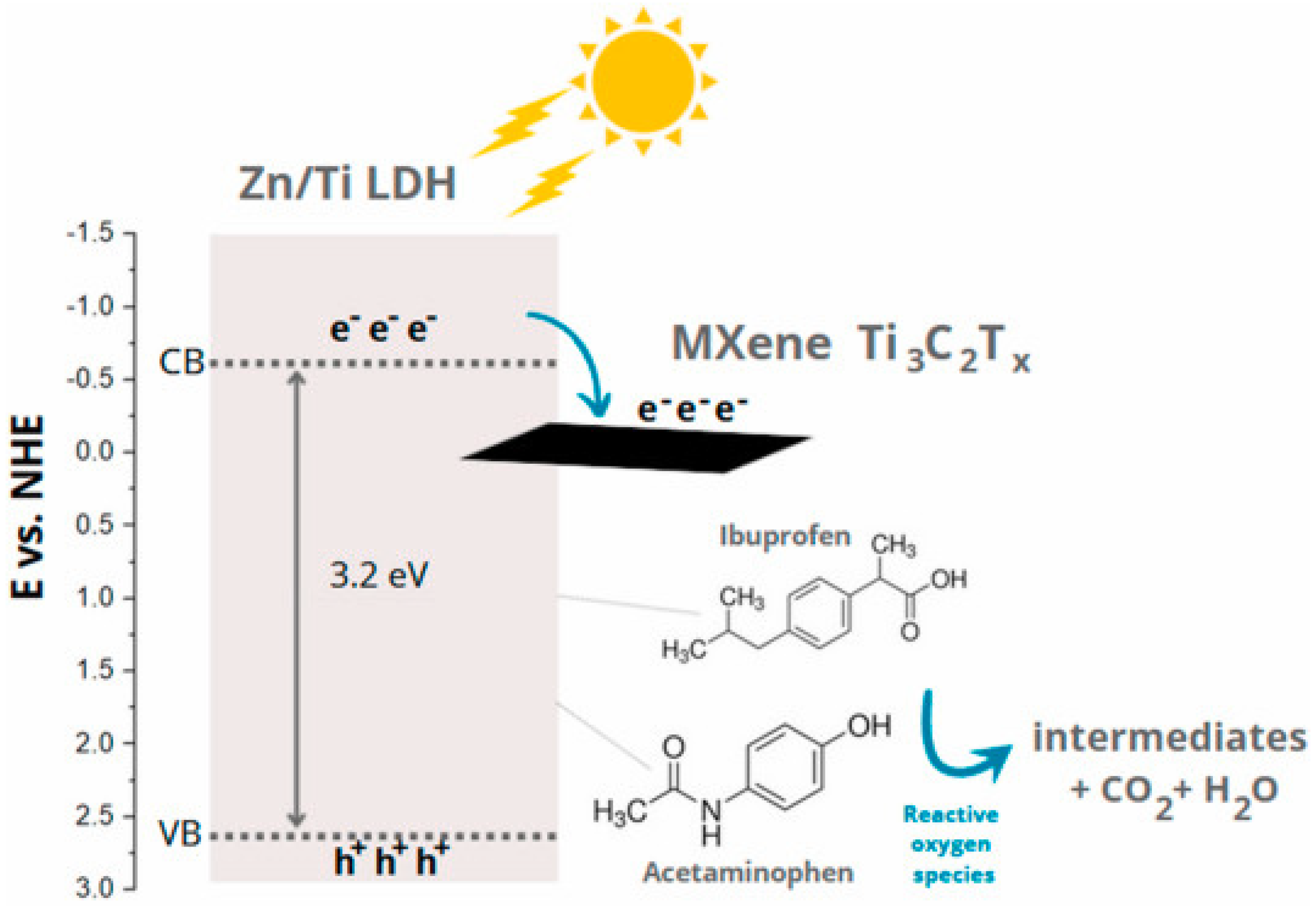
2.4.2. Modification of Clay-Based Photocatalysts with Metal–Organic Frameworks (MOFs), Mxene, Black Phosphorus, and Quantum Dots
3. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Srikhaow, A.; Smith, S.M.; Uraisin, K.; Suttiponparnit, K.; Kongmark, C.; Chuaicham, C. Catalytic remediation of phenol contaminated wastewater using Cu-Zn hydroxide nitrate. RSC Adv. 2016, 6, 36766–36774. [Google Scholar] [CrossRef]
- Tian, Q.; Guo, B.; Chuaicham, C.; Sasaki, K. Mechanism analysis of selenium (VI) immobilization using alkaline-earth metal oxides and ferrous salt. Chemosphere 2020, 248, 126123. [Google Scholar] [CrossRef]
- Prabhu, S.M.; Chuaicham, C.; Park, C.M.; Jeon, B.H.; Sasaki, K. Synthesis and characterization of defective UiO-66 for efficient co-immobilization of arseate and fluoride from single/binary solutions. Environ. Pollut. 2021, 278, 116841. [Google Scholar] [CrossRef]
- Higashimoto, S.; Kurikawa, Y.; Tanabe, Y.; Fukushima, T.; Harada, A.; Murata, M.; Sakata, Y.; Kobayashi, H. Photocatalytic property of WO3 modified with noble metal co-catalysts towards selective hydroxylation of benzene to phenol under visible light irradiation. Appl. Catal. B Environ. 2023, 325, 122289. [Google Scholar] [CrossRef]
- Chen, X.; Wang, Z.; Shen, X.; Zhang, Y.; Lou, Y.; Pan, C.; Zhu, Y.; Xu, J. A plasmonic Z-scheme Ag@AgCl/PDI photocatalyst for the efficient elimination of organic pollutants, antibiotic resistant bacteria and antibiotic resistance genes. Appl. Catal. B Environ. 2023, 324, 122220. [Google Scholar] [CrossRef]
- Ning, L.; Chen, X.; Wang, Z.; Xu, J. High-efficiency pollutant degradation, disinfection and H2O2 production activities of magnetically separable Co-imbedded N-doped carbonaceous framework/supramolecular perylene diimide photocatalyst. Appl. Catal. B Environ. 2023, 324, 122282. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S. Clay supported TiO2 nanoparticles for photocatalytic degradation of environmental pollutants: A review. J. Environ. Chem. Eng. 2018, 6, 6088–6107. [Google Scholar] [CrossRef]
- Balakumar, V.; Manivannan, R.; Chuaicham, C.; Karthikeyan, S.; Sasaki, K. A simple tactic synthesis of hollow porous graphitic carbon nitride with significantly enhanced photocatalytic performance. Chem. Commun. 2021, 57, 6772–6775. [Google Scholar] [CrossRef]
- Chen, X.; Shen, S.; Guo, L.; Mao, S.S. Semiconductor-based Photocatalytic Hydrogen Generation. Chem. Rev. 2010, 110, 6503–6570. [Google Scholar] [CrossRef]
- Ni, M.; Leung, M.K.H.; Leung, D.Y.C.; Sumathy, K. A review and recent developments in photocatalytic water-splitting using TiO2 for hydrogen production. Renew. Sustain. Energy Rev. 2007, 11, 401–425. [Google Scholar] [CrossRef]
- Zhao, X.; Xu, M.; Song, X.; Zhou, W.; Liu, X.; Yan, Y.; Huo, P. Charge separation and transfer activated by covalent bond in UiO-66-NH2/RGO heterostructure for CO2 photoreduction. Chem. Eng. J. 2022, 437, 135210. [Google Scholar] [CrossRef]
- Balu, S.; Chuaicham, C.; Balakumar, V.; Rajendran, S.; Sasaki, K.; Sekar, K.; Maruthapillai, A. Recent development on core-shell photo(electro)catalysts for elimination of organic compounds from pharmaceutical wastewater. Chemosphere 2022, 298, 134311. [Google Scholar] [CrossRef]
- Xiang, Q.; Cheng, B.; Yu, J. Graphene-Based Photocatalysts for Solar-Fuel Generation. Angew. Chem. Int. Ed. 2015, 54, 11350–11366. [Google Scholar] [CrossRef]
- Shearer, C.J.; Cherevan, A.; Eder, D. Application and Future Challenges of Functional Nanocarbon Hybrids. Adv. Mater. 2014, 26, 2295–2318. [Google Scholar] [CrossRef]
- Chuaicham, C.; Sekar, K.; Balakumar, V.; Mittraphab, Y.; Shimizu, K.; Ohtani, B.; Sasaki, K. Fabrication of graphitic carbon nitride/ZnTi-mixed metal oxide heterostructure: Robust photocatalytic decomposition of ciprofloxacin. J. Alloys Compd. 2022, 906, 164294. [Google Scholar] [CrossRef]
- Long, Z.; Li, Q.; Wei, T.; Zhang, G.; Ren, Z. Historical development and prospects of photocatalysts for pollutant removal in water. J. Hazard. Mater. 2020, 395, 122599. [Google Scholar] [CrossRef]
- Tong, H.; Ouyang, S.; Bi, Y.; Umezawa, N.; Oshikiri, M.; Ye, J. Nano-photocatalytic Materials: Possibilities and Challenges. Adv. Mater. 2012, 24, 229–251. [Google Scholar] [CrossRef]
- Chuaicham, C.; Inoue, T.; Balakumar, V.; Tian, Q.; Ohtani, B.; Sasaki, K. Visible light-driven ZnCr double layer oxide photocatalyst composites with fly ashes for the degradation of ciprofloxacin. J. Environ. Chem. Eng. 2022, 10, 106970. [Google Scholar] [CrossRef]
- Okab, A.A.; Alwared, A.I. Photodegradation of tetracycline antibiotic by ternary recyclable Z-scheme g-C3N4/Fe3O4/Bi2WO6/Bi2S3 photocatalyst with improved charge separation efficiency: Characterization and mechanism studies. Environ. Nanotechnol. Monit. Manag. 2023, 19, 100767. [Google Scholar] [CrossRef]
- Mehta, A.; Mishra, A.; Sharma, M.; Singh, S.; Basu, S. Effect of silica/titania ratio on enhanced photooxidation of industrial hazardous materials by microwave treated mesoporous SBA-15/TiO2 nanocomposites. J. Nanoparticle Res. 2016, 18, 209. [Google Scholar] [CrossRef]
- Asiltürk, M.; Şener, Ş. TiO2-activated carbon photocatalysts: Preparation, characterization and photocatalytic activities. Chem. Eng. J. 2012, 180, 354–363. [Google Scholar] [CrossRef]
- Chong, M.N.; Tneu, Z.Y.; Poh, P.E.; Jin, B.; Aryal, R. Synthesis, characterisation and application of TiO2–zeolite nanocomposites for the advanced treatment of industrial dye wastewater. J. Taiwan Inst. Chem. Eng. 2015, 50, 288–296. [Google Scholar] [CrossRef]
- Szczepanik, B. Photocatalytic degradation of organic contaminants over clay-TiO2 nanocomposites: A review. Appl. Clay Sci. 2017, 141, 227–239. [Google Scholar] [CrossRef]
- Liu, J.; Zhang, G. Recent advances in synthesis and applications of clay-based photocatalysts: A review. Phys. Chem. Chem. Phys. 2014, 16, 8178–8192. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Nikolopoulou, A.; Christoforidis, K.C.; Fernández-Garcia, M.; Li, H.; Shu, Y.; Sato, T. Palygorskite–TiO2 nanocomposites: Part 2. photocatalytic activities in decomposing air and organic pollutants. Appl. Clay Sci. 2013, 83–84, 198–202. [Google Scholar] [CrossRef]
- Neeraj, K.; Chandra, M. Basics of Clay Minerals and Their Characteristic Properties. In Clay and Clay Minerals; Gustavo Morari Do, N., Ed.; IntechOpen: Rijeka, Croatia, 2021; Chapter 2. [Google Scholar] [CrossRef]
- Praise, A. Classification of Clay Minerals. In Mineralogy; Miloš, R., Ed.; IntechOpen: Rijeka, Croatia, 2022; Chapter 12. [Google Scholar] [CrossRef]
- Hartati; Prasetyoko, D.; Santoso, M.; Qoniah, I.; Leaw, W.L.; Firda, P.B.D.; Nur, H. A review on synthesis of kaolin-based zeolite and the effect of impurities. J. Chin. Chem. Soc. 2020, 67, 911–936. [Google Scholar] [CrossRef]
- Massaro, M.; Noto, R.; Riela, S. Past, Present and Future Perspectives on Halloysite Clay Minerals. Molecules 2020, 25, 4863. [Google Scholar] [CrossRef]
- Khalifa, A.Z.; Cizer, Ö.; Pontikes, Y.; Heath, A.; Patureau, P.; Bernal, S.A.; Marsh, A.T.M. Advances in alkali-activation of clay minerals. Cem. Concr. Res. 2020, 132, 106050. [Google Scholar] [CrossRef]
- Zou, Y.; Hu, Y.; Shen, Z.; Yao, L.; Tang, D.; Zhang, S.; Wang, S.; Hu, B.; Zhao, G.; Wang, X. Application of aluminosilicate clay mineral-based composites in photocatalysis. J. Environ. Sci. 2022, 115, 190–214. [Google Scholar] [CrossRef]
- Mohamed, A. Effect of potassium saturation on the mineralogical composition and ceramic properties of smectitic clays from the Eastern Desert, Egypt. J. African Earth Sci. 2021, 176, 104139. [Google Scholar] [CrossRef]
- Bourdelle, F. Low-Temperature Chlorite Geothermometry and Related Recent Analytical Advances: A Review. Minerals 2021, 11, 130. [Google Scholar] [CrossRef]
- Franco, J.G.; Ataide, J.A.; Ferreira, A.H.P.; Mazzola, P.G. Lamellar compounds intercalated with anions with solar protection function: A review. J. Drug Deliv. Sci. Technol. 2020, 59, 101869. [Google Scholar] [CrossRef]
- Zubair, M.; Daud, M.; McKay, G.; Shehzad, F.; Al-Harthi, M.A. Recent progress in layered double hydroxides (LDH)-containing hybrids as adsorbents for water remediation. Appl. Clay Sci. 2017, 143, 279–292. [Google Scholar] [CrossRef]
- Das, T.K.; Ganguly, S.; Bhawal, P.; Remanan, S.; Mondal, S.; Das, N.C. Mussel inspired green synthesis of silver nanoparticles-decorated halloysite nanotube using dopamine: Characterization and evaluation of its catalytic activity. Appl. Nanosci. 2018, 8, 173–186. [Google Scholar] [CrossRef]
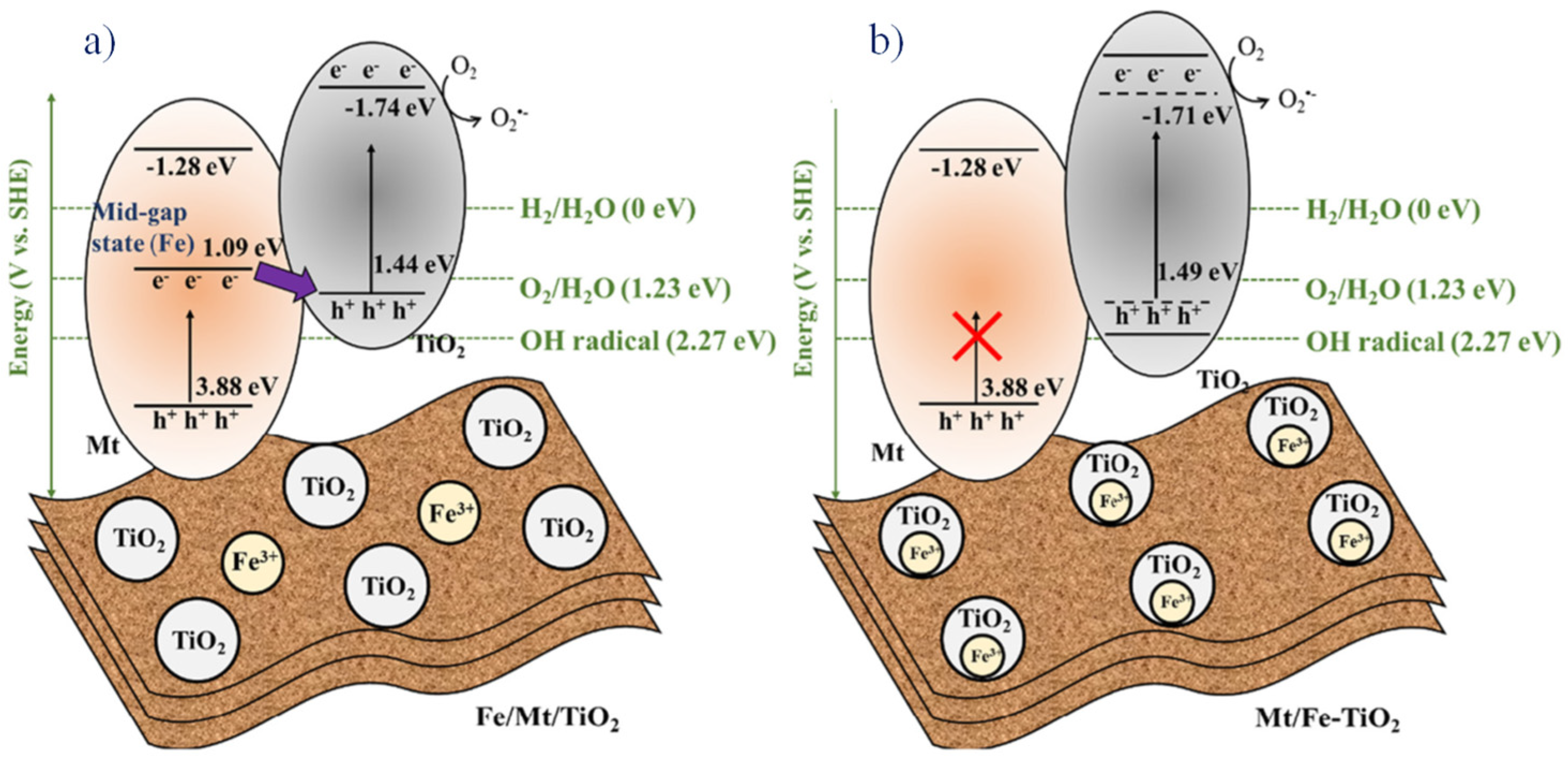
- Chuaicham, C.; Xiong, Y.; Sekar, K.; Chen, W.; Zhang, L.; Ohtani, B.; Dabo, I.; Sasaki, K. A promising Zn-Ti layered double hydroxide/Fe-bearing montmorillonite composite as an efficient photocatalyst for Cr(VI) reduction: Insight into the role of Fe impurity in montmorillonite. Appl. Surf. Sci. 2021, 546, 148835. [Google Scholar] [CrossRef]
- Dong, Y.; Liu, Z.; Chen, L. Removal of Zn(II) from aqueous solution by natural halloysite nanotubes. J. Radioanal. Nucl. Chem. 2012, 292, 435–443. [Google Scholar] [CrossRef]
- Mogyorósi, K.; Farkas, A.; Dékány, I.; Ilisz, I.; Dombi, A. TiO2-Based Photocatalytic Degradation of 2-Chlorophenol Adsorbed on Hydrophobic Clay. Environ. Sci. Technol. 2002, 36, 3618–3624. [Google Scholar] [CrossRef]
- Kameshima, Y.; Tamura, Y.; Nakajima, A.; Okada, K. Preparation and properties of TiO2/montmorillonite composites. Appl. Clay Sci. 2009, 45, 20–23. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Nikolopoulou, A.; Tsolis-Katagas, P.; Panagiotaras, D.; Kacandes, H.G.; Zhang, P.; Yin, S.; Sato, T.; Katsuki, H. Palygorskite- and Halloysite-TiO2 nanocomposites: Synthesis and photocatalytic activity. Appl. Clay Sci. 2010, 50, 118–124. [Google Scholar] [CrossRef]
- Papoulis, D.; Komarneni, S.; Panagiotaras, D.; Stathatos, E.; Toli, D.; Christoforidis, K.C.; Fernández-García, M.; Li, H.; Yin, S.; Sato, T.; et al. Halloysite–TiO2 nanocomposites: Synthesis, characterization and photocatalytic activity. Appl. Catal. B Environ. 2013, 132–133, 416–422. [Google Scholar] [CrossRef]
- Rhouta, B.; Bouna, L.; Maury, F.; Senocq, F.; Lafont, M.C.; Jada, A.; Amjoud, M.; Daoudi, L. Surfactant-modifications of Na+–beidellite for the preparation of TiO2–Bd supported photocatalysts: II—Physico-chemical characterization and photocatalytic properties. Appl. Clay Sci. 2015, 115, 266–274. [Google Scholar] [CrossRef]
- Khumchoo, N.; Khaorapapong, N.; Ontam, A.; Intachai, S.; Ogawa, M. Efficient Photodegradation of Organics in Acidic Solution by ZnO–Smectite Hybrids. Eur. J. Inorg. Chem. 2016, 2016, 3157–3162. [Google Scholar] [CrossRef]
- Paiva, J.P.; Santos, B.A.M.C.; Kibwila, D.M.; Gonçalves, T.C.W.; Pinto, A.V.; Rodrigues, C.R.; Leitão, A.C.; Cabral, L.M.; Pádula, M.D. Titanium Dioxide–Montmorillonite Nanocomposite as Photoprotective Agent Against Ultraviolet B Radiation-Induced Mutagenesis in Saccharomyces cerevisiae: A Potential Candidate for Safer Sunscreens. J. Pharm. Sci. 2014, 103, 2539–2545. [Google Scholar] [CrossRef]
- Tahir, M.; Amin, N.S. Photocatalytic reduction of carbon dioxide with water vapors over montmorillonite modified TiO2 nanocomposites. Appl. Catal. B Environ. 2013, 142–143, 512–522. [Google Scholar] [CrossRef]
- Jeong, E.; Lim, J.W.; Seo, K.-W.; Lee, Y.-S. Effects of physicochemical treatments of illite on the thermo-mechanical properties and thermal stability of illite/epoxy composites. J. Ind. Eng. Chem. 2011, 17, 77–82. [Google Scholar] [CrossRef]
- Zhuang, G.; Zhang, Z.; Guo, J.; Liao, L.; Zhao, J. A new ball milling method to produce organo-montmorillonite from anionic and nonionic surfactants. Appl. Clay Sci. 2015, 104, 18–26. [Google Scholar] [CrossRef]
- Li, Z.; Chen, M.; Ai, Z.; Wu, L.; Zhang, Q. Mechanochemical synthesis of CdS/MgAl LDH-precursor as improved visible-light driven photocatalyst for organic dye. Appl. Clay Sci. 2018, 163, 265–272. [Google Scholar] [CrossRef]
- Xue, B.; Yang, K.; Wang, X.; Chi, Q.; Jiang, Y. The role of potassium chlorate on expansion of dickite layers and the preparation of a novel TiO2 impregnated dickite photocatalyst using expanded dickite as carrier. RSC Adv. 2016, 6, 9803–9811. [Google Scholar] [CrossRef]
- Zhu, T.T.; Zhou, C.H.; Kabwe, F.B.; Wu, Q.Q.; Li, C.S.; Zhang, J.R. Exfoliation of montmorillonite and related properties of clay/polymer nanocomposites. Appl. Clay Sci. 2019, 169, 48–66. [Google Scholar] [CrossRef]
- Ong, W.-J.; Tan, L.-L.; Ng, Y.H.; Yong, S.-T.; Chai, S.-P. Graphitic Carbon Nitride (g-C3N4)-Based Photocatalysts for Artificial Photosynthesis and Environmental Remediation: Are We a Step Closer To Achieving Sustainability? Chem. Rev. 2016, 116, 7159–7329. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Basu, S.; Shetti, N.P.; Reddy, K.R.; Aminabhavi, T.M. Graphitic carbon nitride (g–C3N4)–based metal-free photocatalysts for water splitting: A review. Carbon 2019, 149, 693–721. [Google Scholar] [CrossRef]
- Wen, J.; Xie, J.; Chen, X.; Li, X. A review on g-C3N4-based photocatalysts. Appl. Surf. Sci. 2017, 391, 72–123. [Google Scholar] [CrossRef]
- Fu, J.; Yu, J.; Jiang, C.; Cheng, B. g-C3N4-Based Heterostructured Photocatalysts. Adv. Energy Mater. 2018, 8, 1701503. [Google Scholar] [CrossRef]
- Jiang, H.; Li, Y.; Wang, D.; Hong, X.; Liang, B. Recent Advances in Heteroatom Doped Graphitic Carbon Nitride (g-C3N4) and g-C3N4/Metal Oxide Composite Photocatalysts. Curr. Org. Chem. 2020, 24, 673–693. [Google Scholar] [CrossRef]
- Wu, J.; Li, N.; Zhang, X.-H.; Fang, H.-B.; Zheng, Y.-Z.; Tao, X. Heteroatoms binary-doped hierarchical porous g-C3N4 nanobelts for remarkably enhanced visible-light-driven hydrogen evolution. Appl. Catal. B Environ. 2018, 226, 61–70. [Google Scholar] [CrossRef]
- Humayun, M.; Ullah, H.; Shu, L.; Ao, X.; Tahir, A.A.; Wang, C.; Luo, W. Plasmon Assisted Highly Efficient Visible Light Catalytic CO2 Reduction Over the Noble Metal Decorated Sr-Incorporated g-C3N4. Nano-Micro Lett. 2021, 13, 209. [Google Scholar] [CrossRef]
- Humayun, M.; Fu, Q.; Zheng, Z.; Li, H.; Luo, W. Improved visible-light catalytic activities of novel Au/P-doped g-C3N4 photocatalyst for solar fuel production and mechanism. Appl. Catal. A Gen. 2018, 568, 139–147. [Google Scholar] [CrossRef]
- He, C.; Zhang, J.H.; Zhang, W.X.; Li, T.T. Type-II InSe/g-C3N4 Heterostructure as a High-Efficiency Oxygen Evolution Reaction Catalyst for Photoelectrochemical Water Splitting. J. Phys. Chem. Lett. 2019, 10, 3122–3128. [Google Scholar] [CrossRef]
- Zhao, X.; You, Y.; Huang, S.; Wu, Y.; Ma, Y.; Zhang, G.; Zhang, Z. Z-scheme photocatalytic production of hydrogen peroxide over Bi4O5Br2/g-C3N4 heterostructure under visible light. Appl. Catal. B Environ. 2020, 278, 119251. [Google Scholar] [CrossRef]
- Yang, Y.; Yang, F.; Li, Z.; Zhang, N.; Hao, S. Z-scheme g-C3N4/C/S-g-C3N4 heterostructural nanotube with enhanced porous structure and visible light driven photocatalysis. Microporous Mesoporous Mater. 2021, 314, 110891. [Google Scholar] [CrossRef]
- Ma, W.; Wang, N.; Guo, Y.; Yang, L.; Lv, M.; Tang, X.; Li, S. Enhanced photoreduction CO2 activity on g-C3N4: By synergistic effect of nitrogen defective-enriched and porous structure, and mechanism insights. Chem. Eng. J. 2020, 388, 124288. [Google Scholar] [CrossRef]
- Chuaicham, C.; Pawar, R.; Sasaki, K. Dye-sensitized Photocatalyst of Sepiolite for Organic Dye Degradation. Catalysts 2019, 9, 235. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Yang, S.; He, X.; Zheng, S.; Sun, Z.; Dionysiou, D.D. A review of clay based photocatalysts: Role of phyllosilicate mineral in interfacial assembly, microstructure control and performance regulation. Chemosphere 2021, 273, 129723. [Google Scholar] [CrossRef]
- Qin, W.-L.; Lv, H.; Xia, T.; Ye, Y.; Chen, X.-G.; Lyu, S.-S. TiO2 Intercalated Talc Nanocomposite: Preparation, Characterization, and Its Photocatalytic Performance. J. Nanosci. Nanotechnol. 2017, 17, 6558–6565. [Google Scholar] [CrossRef]
- Sun, Z.; Yao, G.; Zhang, X.; Zheng, S.; Frost, R.L. Enhanced visible-light photocatalytic activity of kaolinite/g-C3N4 composite synthesized via mechanochemical treatment. Appl. Clay Sci. 2016, 129, 7–14. [Google Scholar] [CrossRef]
- Sun, Z.; Yuan, F.; Li, X.; Li, C.; Xu, J.; Wang, B. Fabrication of Novel Cyanuric Acid Modified g-C3N4/Kaolinite Composite with Enhanced Visible Light-Driven Photocatalytic Activity. Minerals 2018, 8, 437. [Google Scholar]
- Li, C.; Sun, Z.; Zhang, W.; Yu, C.; Zheng, S. Highly efficient g-C3N4/TiO2/kaolinite composite with novel three-dimensional structure and enhanced visible light responding ability towards ciprofloxacin and S. aureus. Appl. Catal. B Environ. 2018, 220, 272–282. [Google Scholar] [CrossRef]
- Cao, Z.; Jia, Y.; Wang, Q.; Cheng, H. High-efficiency photo-Fenton Fe/g-C3N4/kaolinite catalyst for tetracycline hydrochloride degradation. Appl. Clay Sci. 2021, 212, 106213. [Google Scholar] [CrossRef]
- Wang, X.; Zhao, Z.; Shu, Z.; Chen, Y.; Zhou, J.; Li, T.; Wang, W.; Tan, Y.; Sun, N. One-pot synthesis of metakaolin/g-C3N4 composite for improved visible-light photocatalytic H2 evolution. Appl. Clay Sci. 2018, 166, 80–87. [Google Scholar] [CrossRef]
- Zhu, Z.; Yu, Y.; Dong, H.; Liu, Z.; Li, C.; Huo, P.; Yan, Y. Intercalation Effect of Attapulgite in g-C3N4 Modified with Fe3O4 Quantum Dots To Enhance Photocatalytic Activity for Removing 2-Mercaptobenzothiazole under Visible Light. ACS Sustain. Chem. Eng. 2017, 5, 10614–10623. [Google Scholar] [CrossRef]
- Xie, H.; Zhang, J.; Wang, D.; Liu, J.; Wang, L.; Xiao, H. Construction of three-dimensional g-C3N4/attapulgite hybrids for Cd(II) adsorption and the reutilization of waste adsorbent. Appl. Surf. Sci. 2020, 504, 144456. [Google Scholar] [CrossRef]
- Xu, Y.; Zhang, L.; Chen, J.; Fu, Y.; Li, Q.; Yin, J.; Cheng, Z.; Kan, W.; Zhao, P.; Zhong, H.; et al. Synthesis of nano-Ag-assisted attapulgite/g-C3N4 composites with superior visible light photocatalytic performance. Mater. Chem. Phys. 2019, 221, 447–456. [Google Scholar] [CrossRef]
- Li, J.; Zhou, M.; Ye, Z.; Wang, H.; Ma, C.; Huo, P.; Yan, Y. Enhanced photocatalytic activity of g-C3N4–ZnO/HNT composite heterostructure photocatalysts for degradation of tetracycline under visible light irradiation. RSC Adv. 2015, 5, 91177–91189. [Google Scholar] [CrossRef]
- Hojamberdiev, M.; Khan, M.M.; Kadirova, Z.; Kawashima, K.; Yubuta, K.; Teshima, K.; Riedel, R.; Hasegawa, M. Synergistic effect of g-C3N4, Ni(OH)2 and halloysite in nanocomposite photocatalyst on efficient photocatalytic hydrogen generation. Renew. Energy 2019, 138, 434–444. [Google Scholar] [CrossRef]
- Li, C.; Sun, Z.; Huang, W.; Zheng, S. Facile synthesis of g-C3N4/montmorillonite composite with enhanced visible light photodegradation of rhodamine B and tetracycline. J. Taiwan Inst. Chem. Eng. 2016, 66, 363–371. [Google Scholar] [CrossRef]
- Xu, J.; Qi, Y.; Wang, W.; Wang, L. Montmorillonite-hybridized g-C3N4 composite modified by NiCoP cocatalyst for efficient visible-light-driven photocatalytic hydrogen evolution by dye-sensitization. Int. J. Hydrogen Energy 2019, 44, 4114–4122. [Google Scholar] [CrossRef]
- Wan, X.; Khan, M.A.; Wang, F.; Xia, M.; Lei, W.; Zhu, S.; Fu, C.; Ding, Y. Facile synthesis of protonated g-C3N4 and acid-activated montmorillonite composite with efficient adsorption capacity for PO43− and Pb(II). Chem. Eng. Res. Des. 2019, 152, 95–105. [Google Scholar] [CrossRef]
- Sun, Z.; Zhang, X.; Zhu, R.; Dong, X.; Xu, J.; Wang, B. Facile Synthesis of Visible Light-Induced g-C3N4/Rectorite Composite for Efficient Photodegradation of Ciprofloxacin. Materials 2018, 11, 2452. [Google Scholar]
- Zhu, L.; Zhou, P.-J.; Chen, C. g-C3N4/rectorite as a highly efficient catalyst for peroxymonosulfate activation to remove organic contaminants in water. J. Environ. Chem. Eng. 2022, 10, 107168. [Google Scholar] [CrossRef]
- Chuaicham, C.; Pawar, R.R.; Karthikeyan, S.; Ohtani, B.; Sasaki, K. Fabrication and characterization of ternary sepiolite/g-C3N4/Pd composites for improvement of photocatalytic degradation of ciprofloxacin under visible light irradiation. J. Colloid Interface Sci. 2020, 577, 397–405. [Google Scholar] [CrossRef]
- Fan, E.; Hu, F.; Miao, W.; Xu, H.; Shao, G.; Liu, W.; Li, M.; Wang, H.; Lu, H.; Zhang, R. Preparation of g-C3N4/vermiculite composite with improved visible light photocatalytic activity. Appl. Clay Sci. 2020, 197, 105789. [Google Scholar] [CrossRef]
- Chen, Y.; Wen, L.; Chen, J.; Luo, H.A.; Liu, J. In situ growth of g-C3N4 on clay minerals of kaolinite, sepiolite, and talc for enhanced solar photocatalytic energy conversion. Appl. Clay Sci. 2022, 216, 106337. [Google Scholar] [CrossRef]
- Bakbolat, B.; Daulbayev, C.; Sultanov, F.; Beissenov, R.; Umirzakov, A.; Mereke, A.; Bekbaev, A.; Chuprakov, I. Recent Developments of TiO2-Based Photocatalysis in the Hydrogen Evolution and Photodegradation: A Review. Nanomaterials 2020, 10, 1790. [Google Scholar] [CrossRef]
- Guo, Q.; Zhou, C.; Ma, Z.; Yang, X. Fundamentals of TiO2 Photocatalysis: Concepts, Mechanisms, and Challenges. Adv. Mater. 2019, 31, e1901997. [Google Scholar] [CrossRef]
- Wang, Y.; Gong, Y.; Lin, N.; Yu, L.; Du, B.; Zhang, X. Enhanced removal of Cr (VI) from aqueous solution by stabilized nanoscale zero valent iron and copper bimetal intercalated montmorillonite. J. Colloid Interface Sci. 2022, 606, 941–952. [Google Scholar] [CrossRef]
- Chen, D.; Zhu, Q.; Zhou, F.; Deng, X.; Li, F. Synthesis and photocatalytic performances of the TiO2 pillared montmorillonite. J. Hazard. Mater. 2012, 235, 186–193. [Google Scholar]
- Zhang, L.; Chuaicham, C.; Balakumar, V.; Sekar, K.; Ohtani, B.; Sasaki, K. Determination of the roles of FeIII in the interface between titanium dioxide and montmorillonite in FeIII-doped montmorillonite/titanium dioxide composites as photocatalysts. Appl. Clay Sci. 2022, 227, 106577. [Google Scholar] [CrossRef]
- Belessi, V.; Lambropoulou, D.; Konstantinou, I.; Katsoulidis, A.; Pomonis, P.; Petridis, D.; Albanis, T. Structure and photocatalytic performance of TiO2/clay nanocomposites for the degradation of dimethachlor. Appl. Catal. B Environ. 2007, 73, 292–299. [Google Scholar] [CrossRef]
- Mahy, J.G.; Tsaffo Mbognou, M.H.; Léonard, C.; Fagel, N.; Woumfo, E.D.; Lambert, S.D. Natural Clay Modified with ZnO/TiO2 to Enhance Pollutant Removal from Water. Catalysts 2022, 12, 148. [Google Scholar] [CrossRef]
- Shirzad-Siboni, M.; Farrokhi, M.; Darvishi Cheshmeh Soltani, R.; Khataee, A.; Tajassosi, S. Photocatalytic reduction of hexavalent chromium over ZnO nanorods immobilized on kaolin. Ind. Eng. Chem. Res. 2014, 53, 1079–1087. [Google Scholar] [CrossRef]
- Li, C.; Zhu, N.; Dong, X.; Zhang, X.; Chen, T.; Zheng, S.; Sun, Z. Tuning and controlling photocatalytic performance of TiO2/kaolinite composite towards ciprofloxacin: Role of 0D/2D structural assembly. Adv. Powder Technol. 2020, 31, 1241–1252. [Google Scholar] [CrossRef]
- Li, X.; Peng, K.; Chen, H.; Wang, Z. TiO2 nanoparticles assembled on kaolinites with different morphologies for efficient photocatalytic performance. Sci. Rep. 2018, 8, 11663. [Google Scholar]
- Kutláková, K.M.; Tokarský, J.; Kovář, P.; Vojtěšková, S.; Kovářová, A.; Smetana, B.; Kukutschová, J.; Čapková, P.; Matějka, V. Preparation and characterization of photoactive composite kaolinite/TiO2. J. Hazard. Mater. 2011, 188, 212–220. [Google Scholar]
- Zhang, Y.; Gan, H.; Zhang, G. A novel mixed-phase TiO2/kaolinite composites and their photocatalytic activity for degradation of organic contaminants. Chem. Eng. J. 2011, 172, 936–943. [Google Scholar]
- Wu, A.; Wang, D.; Wei, C.; Zhang, X.; Liu, Z.; Feng, P.; Ou, X.; Qiang, Y.; Garcia, H.; Niu, J. A comparative photocatalytic study of TiO2 loaded on three natural clays with different morphologies. Appl. Clay Sci. 2019, 183, 105352. [Google Scholar] [CrossRef]
- Djellabi, R.; Fouzi Ghorab, M.; Bianchi, C.L.; Cerrato, G.; Morandi, S. Removal of crystal violet and hexavalent chromium using TiO2-bentonite under sunlight: Effect of TiO2 content. J. Chem. Eng. Process. Technol. 2016, 7, 1000276. [Google Scholar]
- Yang, C.; Zhu, Y.; Wang, J.; Li, Z.; Su, X.; Niu, C. Hydrothermal synthesis of TiO2–WO3–bentonite composites: Conventional versus ultrasonic pretreatments and their adsorption of methylene blue. Appl. Clay Sci. 2015, 105, 243–251. [Google Scholar] [CrossRef]
- Dallabona, I.D.; Mathias, Á.L.; Jorge, R.M.M. A new green floating photocatalyst with Brazilian bentonite into TiO2/alginate beads for dye removal. Colloids Surf. A Physicochem. Eng. Asp. 2021, 627, 127159. [Google Scholar] [CrossRef]
- Sun, Q.; Hu, X.; Zheng, S.; Sun, Z.; Liu, S.; Li, H. Influence of calcination temperature on the structural, adsorption and photocatalytic properties of TiO2 nanoparticles supported on natural zeolite. Powder Technol. 2015, 274, 88–97. [Google Scholar]
- Sun, Z.; Zheng, L.; Zheng, S.; Frost, R.L. Preparation and characterization of TiO2/acid leached serpentinite tailings composites and their photocatalytic reduction of Chromium (VI). J. Colloid Interface Sci. 2013, 404, 102–109. [Google Scholar] [CrossRef]
- Yang, Y.; Yan, L.; Li, J.; Li, J.; Yan, T.; Sun, M.; Pei, Z. Synergistic adsorption and photocatalytic reduction of Cr (VI) using Zn-Al-layered double hydroxide and TiO2 composites. Appl. Surf. Sci. 2019, 492, 487–496. [Google Scholar]
- Li, F.; Dai, Y.; Gong, M.; Yu, T.; Chen, X. Synthesis, characterization of magnetic-sepiolite supported with TiO2, and the photocatalytic performance over Cr (VI) and 2,4-dichlorophenol co-existed wastewater. J. Alloys Compd. 2015, 638, 435–442. [Google Scholar] [CrossRef]
- Dvininov, E.; Popovici, E.; Pode, R.; Cocheci, L.; Barvinschi, P.; Nica, V. Synthesis and characterization of TiO2-pillared Romanian clay and their application for azoic dyes photodegradation. J. Hazard. Mater. 2009, 167, 1050–1056. [Google Scholar] [CrossRef]
- Sun, Z.; Hu, Z.; Yan, Y.; Zheng, S. Effect of preparation conditions on the characteristics and photocatalytic activity of TiO2/purified diatomite composite photocatalysts. Appl. Surf. Sci. 2014, 314, 251–259. [Google Scholar]
- Ramadan, H.; Ali, R.A.; Mobarak, M.; Badawi, M.; Selim, A.Q.; Mohamed, E.A.; Bonilla-Petriciolet, A.; Seliem, M.K. One-step fabrication of a new outstanding rutile TiO2 nanoparticles/anthracite adsorbent: Modeling and physicochemical interpretations for malachite green removal. Chem. Eng. J. 2021, 426, 131890. [Google Scholar]
- Savun-Hekimoğlu, B.; Eren, Z.; Ince, N.H. Photocatalytic Destruction of Caffeine on Sepiolite-Supported TiO2 Nanocomposite. Sustainability 2020, 12, 10314. [Google Scholar] [CrossRef]
- Zhang, Y.; Miao, B.; Chen, Q.; Bai, Z.; Cao, Y.; Davaa, B. Synthesis, Structure, and Photocatalytic Activity of TiO2-Montmorillonite Composites. Catalysts 2022, 12, 486. [Google Scholar] [CrossRef]
- Sreekala, S.V.; Vayalveettil, A.; Kochu, J.K.; Ramakrishnan, R.T.; Pillai, H.P.S. Bentonite-titanium dioxide functional nanocomposites suitable for wastewater treatment: An integrated photocatalyst-adsorbent system. New J. Chem. 2022, 46, 4772–4783. [Google Scholar] [CrossRef]
- Keller, W.D.; Matlack, K. The pH of clay suspensions in the field and laboratory, and methods of measurement of their pH. Appl. Clay Sci. 1990, 5, 123–133. [Google Scholar] [CrossRef]
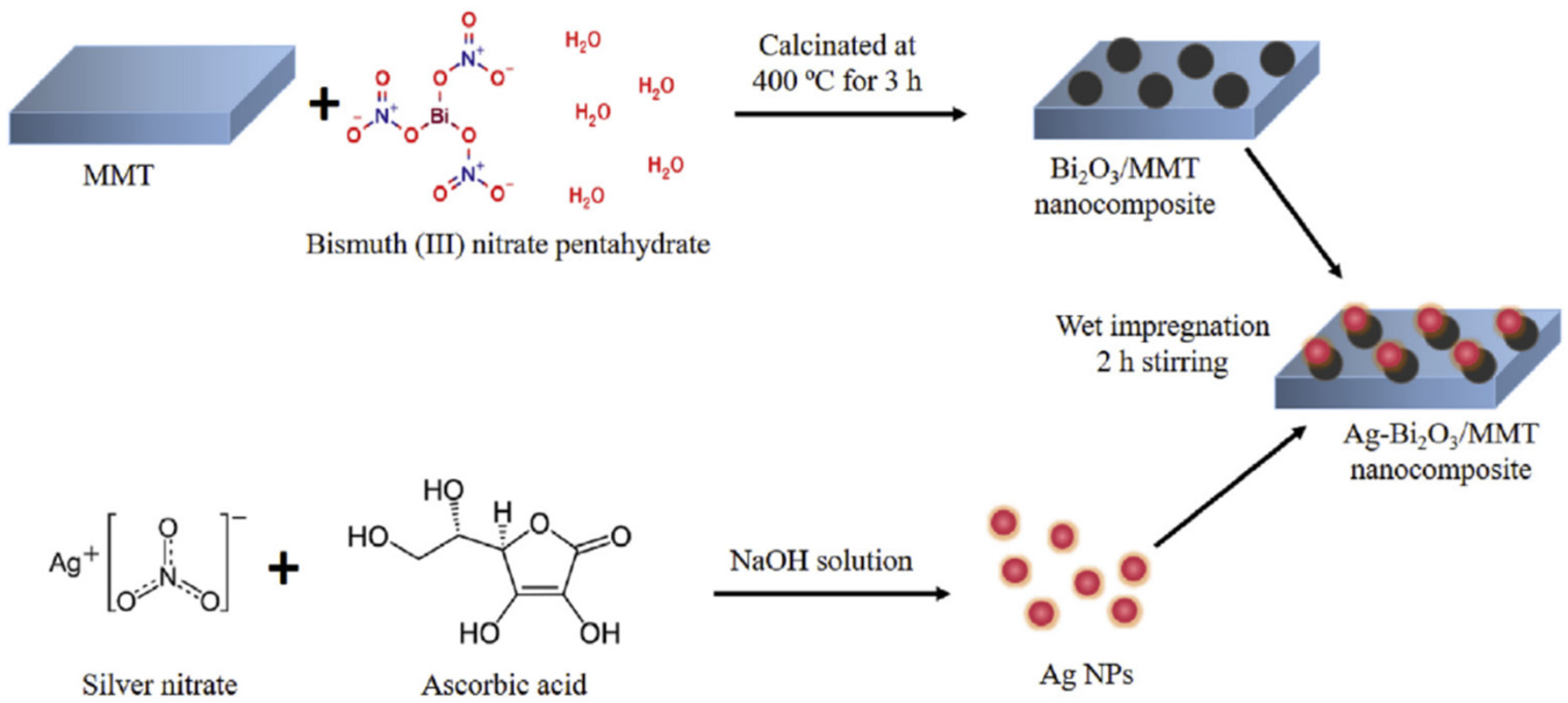
- Tun, P.P.; Wang, J.; Khaing, T.T.; Wu, X.; Zhang, G. Fabrication of functionalized plasmonic Ag loaded Bi2O3/montmorillonite nanocomposites for efficient photocatalytic removal of antibiotics and organic dyes. J. Alloys Compd. 2020, 818, 152836. [Google Scholar] [CrossRef]
- Xu, C.; Wu, H.; Gu, F.L. Efficient adsorption and photocatalytic degradation of Rhodamine B under visible light irradiation over BiOBr/montmorillonite composites. J. Hazard. Mater. 2014, 275, 185–192. [Google Scholar] [CrossRef]
- Naing, H.H.; Li, Y.; Ghasemi, J.B.; Wang, J.; Zhang, G. Enhanced visible-light-driven photocatalysis of in-situ reduced of bismuth on BiOCl nanosheets and montmorillonite loading: Synergistic effect and mechanism insight. Chemosphere 2022, 304, 135354. [Google Scholar] [CrossRef]
- Xu, C.; Gu, F.L.; Wu, H. BiOCl-montmorillonite as a photocatalyst for highly efficient removal of Rhodamine B and Orange G: Importance of the acidity and dissolved oxygen. Appl. Clay Sci. 2017, 147, 28–35. [Google Scholar] [CrossRef]
- Xu, T.; Zhu, R.; Zhu, J.; Liang, X.; Liu, Y.; Xu, Y.; He, H. BiVO4/Fe/Mt composite for visible-light-driven degradation of acid red 18. Appl. Clay Sci. 2016, 129, 27–34. [Google Scholar] [CrossRef]
- Landge, V.K.; Sonawane, S.H.; Sivakumar, M.; Sonawane, S.S.; Babu, G.U.B.; Boczkaj, G. S-scheme heterojunction Bi2O3-ZnO/Bentonite clay composite with enhanced photocatalytic performance. Sustain. Energy Technol. Assess. 2021, 45, 101194. [Google Scholar] [CrossRef]
- Dlamini, M.C.; Dlamini, M.L.; Mente, P.; Tlhaole, B.; Erasmus, R.; Maubane-Nkadimeng, M.S.; Moma, J.A. Photocatalytic abatement of phenol on amorphous TiO2-BiOBr-bentonite heterostructures under visible light irradiation. J. Ind. Eng. Chem. 2022, 111, 419–436. [Google Scholar] [CrossRef]
- Vaizoğullar, A.İ. Synthesis, characterization, and photocatalytic activities of novel bentonite-supported BiOCl–ZnO heterojunction (BiOCl–ZnO–Bentonite) under UV light. Asia Pac. J. Chem. Eng. 2018, 13, e2154. [Google Scholar] [CrossRef]
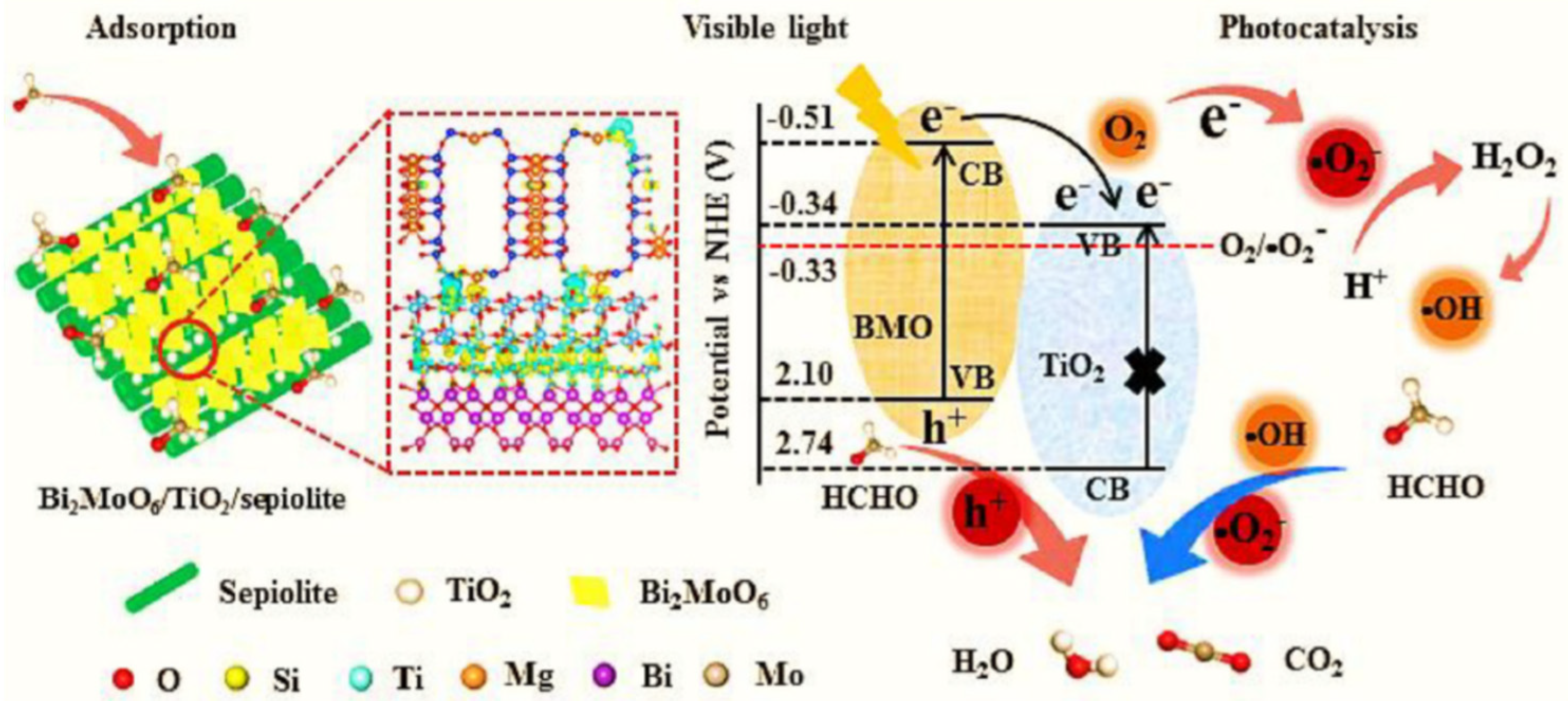
- Hu, X.; Li, C.; Song, J.; Zheng, S.; Sun, Z. Hierarchical assembly of visible-light-driven Bi2MoO6/TiO2/sepiolite composite for effective formaldehyde removal. Appl. Clay Sci. 2022, 227, 106590. [Google Scholar] [CrossRef]
- Naing, H.H.; Wang, K.; Li, Y.; Mishra, A.K.; Zhang, G. Sepiolite supported BiVO4 nanocomposites for efficient photocatalytic degradation of organic pollutants: Insight into the interface effect towards separation of photogenerated charges. Sci. Total Environ. 2020, 722, 137825. [Google Scholar] [CrossRef]
- Wang, J.; Fan, J.; Li, J.; Wu, X.; Zhang, G. Ultrasound assisted synthesis of Bi2NbO5F/rectorite composite and its photocatalytic mechanism insights. Ultrason. Sonochemistry 2018, 48, 404–411. [Google Scholar] [CrossRef]
- Chuaicham, C.; Sekar, K.; Balakumar, V.; Uchida, J.; Katsurao, T.; Sakabe, H.; Ohtani, B.; Sasaki, K. Efficient photocatalytic degradation of emerging ciprofloxacin under visible light irradiation using BiOBr/carbon quantum dot/saponite composite. Environ. Res. 2022, 212, 113635. [Google Scholar] [CrossRef]
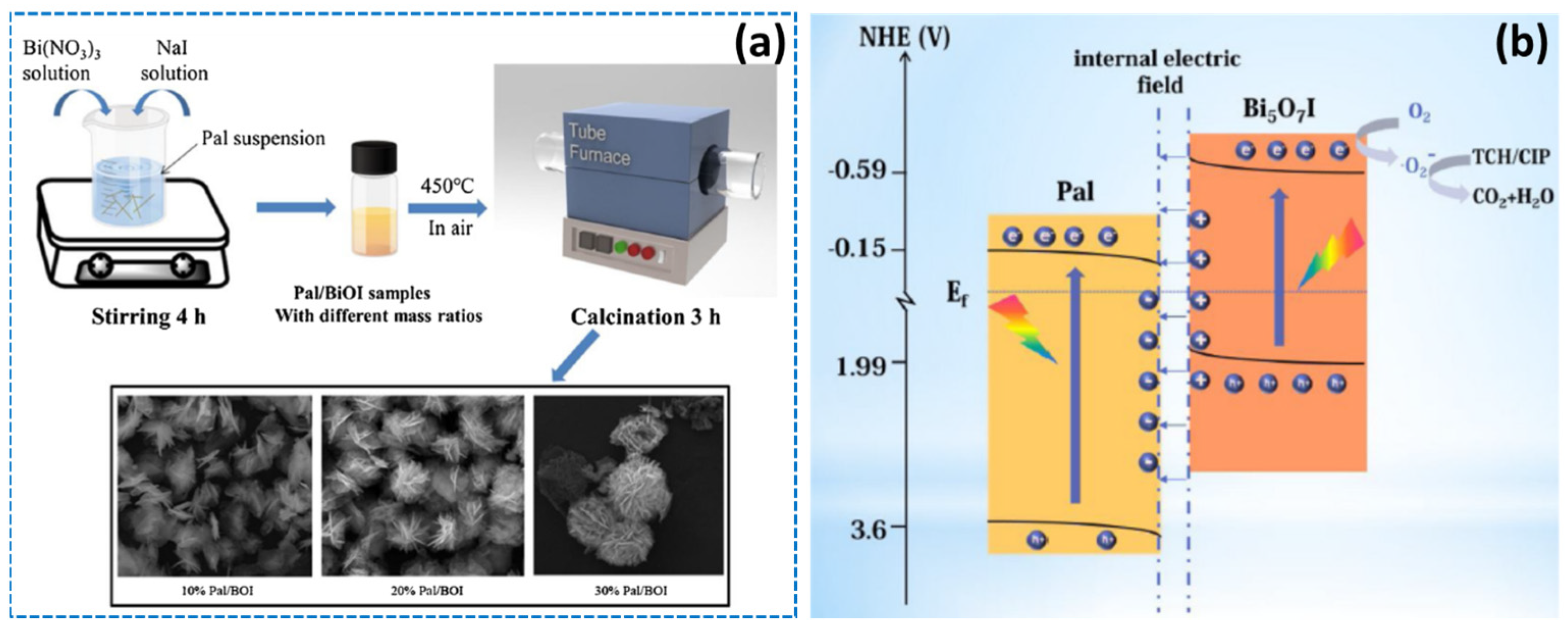
- Luo, Y.; Wang, K.; Hu, T.; Liu, X. Controlled synthesis of palygorskite/Bi5O7I hybrid microspheres with high efficient photodegradation of antibiotics. Colloids Surf. A Physicochem. Eng. Asp. 2021, 616, 126225. [Google Scholar] [CrossRef]
- Liu, J.; Jatav, S.; Wessel, P.; Hill, E.H. Templating Unidirectional Bismuth Oxyiodide Crystal Growth with Layered Silicates for Enhanced Photocatalysis. J. Phys. Chem. C 2022, 126, 4975–4983. [Google Scholar] [CrossRef]
- Tun, P.; Wang, K.; Naing, H.; Wang, J.; Zhang, G. Facile preparation of visible-light-responsive kaolin-supported Ag@AgBr composites and their enhanced photocatalytic properties. Appl. Clay Sci. 2019, 175, 76–85. [Google Scholar] [CrossRef]
- Li, Z.; Zhang, Q.; Liu, X.; Wu, L.; Hu, H.; Zhao, Y. One-step mechanochemical synthesis of plasmonic Ag/Zn–Al LDH with excellent photocatalytic activity. J. Mater. Sci. 2018, 53, 12795–12806. [Google Scholar] [CrossRef]
- Pham, M.-T.; Hussain, A.; Bui, D.-P.; Nguyen, T.-M.T.; You, S.-J.; Wang, Y.-F. Surface plasmon resonance enhanced photocatalysis of Ag nanoparticles-decorated Bi2S3 nanorods for NO degradation. Environ. Technol. Innov. 2021, 23, 101755. [Google Scholar] [CrossRef]
- Mishra, A.; Mehta, A.; Kainth, S.; Basu, S. Effect of different plasmonic metals on photocatalytic degradation of volatile organic compounds (VOCs) by bentonite/M-TiO2 nanocomposites under UV/visible light. Appl. Clay Sci. 2018, 153, 144–153. [Google Scholar] [CrossRef]
- Praneeth, N.V.S.; Paria, S. Clay-supported anisotropic Au-modified N,S-doped TiO2 nanoparticles for enhanced photocatalytic dye degradation and esterification reactions. New J. Chem. 2020, 44, 2619–2629. [Google Scholar] [CrossRef]
- Kaur, H.; Singh, S.; Pal, B. Effect of plasmonic metal (Cu, Ag, and Au) loading over the physicochemical and photocatalytic properties of Mg-Al LDH towards degradation of tetracycline under LED light. Appl. Surf. Sci. 2023, 609, 155455. [Google Scholar] [CrossRef]
- Tonda, S.; Jo, W.-K. Plasmonic Ag nanoparticles decorated NiAl-layered double hydroxide/graphitic carbon nitride nanocomposites for efficient visible-light-driven photocatalytic removal of aqueous organic pollutants. Catal. Today 2018, 315, 213–222. [Google Scholar] [CrossRef]
- Shabib, F.; Fazaeli, R.; Aliyan, H.; Richeson, D. Hierarchical mesoporous plasmonic Pd-Fe3O4/NiFe-LDH composites: Characterization, and kinetic study of a photodegradation catalyst for aqueous metoclopramide. Environ. Technol. Innov. 2022, 27, 102515. [Google Scholar] [CrossRef]
- Pawar, R.R.; Chuaicham, C.; Sekar, K.; Rajendran, S.; Sasaki, K. Synthesis, characterization, and application of MOF@clay composite as a visible light-driven photocatalyst for Rhodamine B degradation. Chemosphere 2022, 291, 132922. [Google Scholar] [CrossRef]
- Bhuvaneswari, K.; Palanisamy, G.; Pazhanivel, T.; Maiyalagan, T.; Shanmugam, P.; Grace, A.N. In-situ development of metal organic frameworks assisted ZnMgAl layered triple hydroxide 2D/2D hybrid as an efficient photocatalyst for organic dye degradation. Chemosphere 2021, 270, 128616. [Google Scholar] [CrossRef]
- Liu, S.; Jiang, X.; Waterhouse, G.I.N.; Zhang, Z.-M.; Yu, L.-m. Construction of Z-scheme Titanium-MOF/plasmonic silver nanoparticle/NiFe layered double hydroxide photocatalysts with enhanced dye and antibiotic degradation activity under visible light. Sep. Purif. Technol. 2021, 278, 119525. [Google Scholar] [CrossRef]
- Wang, T.; Yang, Y.; Deng, Q.; Zhang, X.; Xiong, L.; Tang, Z.; Li, P.; Yin, N.; Sun, A.; Chen, D.; et al. In-situ construction of 3D marigold-like CoAl-LDH/Ti3C2 heterosystem collaborating with 2D/2D interface for efficient photodegradation of multiple antibiotics. Appl. Surf. Sci. 2021, 569, 151084. [Google Scholar] [CrossRef]
- Ma, Y.; Xu, D.; Chen, W.; Tang, Y.; Wang, X.; Li, L.; Wang, J. Oxygen-vacancy-embedded 2D/2D NiFe-LDH/MXene Schottky heterojunction for boosted photodegradation of norfloxacin. Appl. Surf. Sci. 2022, 572, 151432. [Google Scholar] [CrossRef]
- Grzegórska, A.; Wysocka, I.; Głuchowski, P.; Ryl, J.; Karczewski, J.; Zielińska-Jurek, A. Novel composite of Zn/Ti-layered double hydroxide coupled with MXene for the efficient photocatalytic degradation of pharmaceuticals. Chemosphere 2022, 308, 136191. [Google Scholar] [CrossRef]
- Yang, J.; Jing, R.; Wang, P.; Liang, D.; Huang, H.; Xia, C.; Zhang, Q.; Liu, A.; Meng, Z.; Liu, Y. Black phosphorus nanosheets and ZnAl-LDH nanocomposite as environmental-friendly photocatalysts for the degradation of Methylene blue under visible light irradiation. Appl. Clay Sci. 2021, 200, 105902. [Google Scholar] [CrossRef]
- Amani-Ghadim, A.R.; Khodam, F.; Seyed Dorraji, M.S. ZnS quantum dot intercalated layered double hydroxide semiconductors for solar water splitting and organic pollutant degradation. J. Mater. Chem. A 2019, 7, 11408–11422. [Google Scholar] [CrossRef]
- Khodam, F.; Amani-Ghadim, A.R.; Ashan, N.N.; Sareshkeh, A.T.; Bayat, F.; Gholinejad, M.; Dorraji, M.S.S. CdTe quantum dots incorporated in CoNiAl layered double hydroxide interlayer spaces as a highly efficient visible light driven photocatalyst for degradation of an azo dye and Bisphenol A. J. Alloys Compd. 2022, 898, 162768. [Google Scholar] [CrossRef]
- Ganguly, S.; Das, P.; Das, T.K.; Ghosh, S.; Das, S.; Bose, M.; Mondal, M.; Das, A.K.; Das, N.C. Acoustic cavitation assisted destratified clay tactoid reinforced in situ elastomer-mimetic semi-IPN hydrogel for catalytic and bactericidal application. Ultrason. Sonochemistry 2020, 60, 104797. [Google Scholar] [CrossRef]
| Pollutant | Photocatalyst | Clay | Efficiency | Time | Ref. |
|---|---|---|---|---|---|
| 2-mercaptobenzothiazole | Fe3O4@g-C3N4/ATP | attapulgite | 90% | 90 min | [72] |
| ciprofoxacin | Sepiolite/g-C3N4 | sepiolite | 90% | 60 min | [82] |
| methylene blue | TiO2/montmorillonite | montmorillonite | 98% | 90 min | [88] |
| Cr(VI) | TiO2/kaolin | kaolin | 98% | 120 min | [92] |
| 2,4-dichlorophenol | TiO2/sepiolite | sepiolite | 90% | 120 min | [104] |
| Cr(VI) | TiO2/bentonite | bentonite | 100% | 180 min | [98] |
| tetracycline | Ag-loaded Bi2O3/montmorillonite | montmorillonite | 100% | 45 min | [112] |
| rhodamine B | BiOBr/Na-montmorillonite | montmorillonite | 100% | 60 min | [113] |
| rhodamine B | BiOCl/montmorillonite | montmorillonite | 100% | 30 min | [115] |
| formaldehyde | Bi2MoO6/TiO2/sepiolite | sepiolite | 100% | 195 min | [120] |
| methylene blue | BiVO4/sepiolite | sepiolite | 90% | 150 min | [121] |
| ciprofoxacin | BiOBr/saponite/CQD | saponite | 100% | 5 min | [123] |
| ciprofoxacin | Bi5O7I/palygorskite | palygorskite | 100% | 80 min | [124] |
| rhodamine B | Pd-Fe3O4/NiFe-LDH | NiFe-LDH | 96% | 30 min | [133] |
| rhodamine B | MOFs@sepiolite | sepiolite | 88% | 120 min | [134] |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chuaicham, C.; Trakulmututa, J.; Shu, K.; Shenoy, S.; Srikhaow, A.; Zhang, L.; Mohan, S.; Sekar, K.; Sasaki, K. Recent Clay-Based Photocatalysts for Wastewater Treatment. Separations 2023, 10, 77. https://doi.org/10.3390/separations10020077
Chuaicham C, Trakulmututa J, Shu K, Shenoy S, Srikhaow A, Zhang L, Mohan S, Sekar K, Sasaki K. Recent Clay-Based Photocatalysts for Wastewater Treatment. Separations. 2023; 10(2):77. https://doi.org/10.3390/separations10020077
Chicago/Turabian StyleChuaicham, Chitiphon, Jirawat Trakulmututa, Kaiqian Shu, Sulakshana Shenoy, Assadawoot Srikhaow, Li Zhang, Sathya Mohan, Karthikeyan Sekar, and Keiko Sasaki. 2023. "Recent Clay-Based Photocatalysts for Wastewater Treatment" Separations 10, no. 2: 77. https://doi.org/10.3390/separations10020077
APA StyleChuaicham, C., Trakulmututa, J., Shu, K., Shenoy, S., Srikhaow, A., Zhang, L., Mohan, S., Sekar, K., & Sasaki, K. (2023). Recent Clay-Based Photocatalysts for Wastewater Treatment. Separations, 10(2), 77. https://doi.org/10.3390/separations10020077











