Abstract
Genus Salsola (family Amaranthaceae) is one of the most prevailing genera in Saudi Arabia. Although several species were reported for their traditional uses, the majority of Salsola species still need to be phytochemically and biologically explored. The current study presents the GC-MS profiling as well as an in vitro investigation of the bioactivities of the n-hexane extracts from the five Salsola species: Salsola arabica, S. cyclophylla, S. imbricata, S. incanescens and S. villosa. Additionally, the compounds identified in the most active extracts were screened for their interaction with the active sites of cyclooxygenase enzyme isoforms (COX-1 and COX-2). GC-MS analysis of the n-hexane extracts from the five species resulted in the identification of 67 constituents. Oleic acid (75.57%), 1-octadecene (14.46%), cinnamaldehyde α-hexyl (57.15%), octacosyl heptafluorobutyrate (25.36%) and hexadecanoic acid methyl ester (26.15%) represent the major constituents in S. arabica, S. cyclophylla, S. imbricata, S. inscanescence and S. villosa, respectively. Results of bioactivity testing highlighted S. villosa as having the highest anti-oxidant activity (IC50 0.99 ± 0.05 mg/mL), which was closely followed by S. cyclophylla (IC50 1.36 ± 0.06 mg/mL) compared to the IC50 of 0.16 ± 0.01 mg/mL recorded by ascorbic acid. S. villosa was further noted for having the strongest COX-2 inhibitory activity (IC50 4.6 ± 0.13 µg/mL) among the tested extracts followed by S. arabica (IC50 13.1 ± 0.37 µg/mL) and S. cyclophylla (IC50 20.1 ± 0.57 µg/mL). On the other hand, S. imbricata extract displayed the most characteristic inhibition activity against COX-1 (IC50 10.2 ± 0.52 µg/mL), which was non-significant from the standard drug celecoxib. Based upon bioactivity results, the phytoconstituents identified in S. villosa and S. imbricata extracts were investigated for their capability to interact with the active sites of both cyclooxygenase enzyme isoforms adopting molecular docking. Results indicated the possibility to incorporate the compounds to active sites of the enzymes where some of them bind with their polar end into the cavity beyond Arg120 and their aliphatic chain oriented to the catalytically important Tyr385 similar to the natural substrate arachidonic acid, indicating that they could be promising candidates for the future development of selective COX inhibitors.
1. Introduction
Amaranthaceae is a large family that includes around 2000 species classified under 175 genera. Salsola is one of the common genera of the family Amaranthaceae and includes around 120 species. The word Salsola originates from the Latin word salsus, means salty [1]. Salsola species are widespread in the subtropical and moderate areas of Africa, Asia, Europe and North America [2]. In the Qassim region, Kingdom of Saudi Arabia (KSA), the genus Salsola is represented by nine species [3]. Several species are reputed for variable folk medicinal uses, e.g., anti-inflammatory, antihypertensive, anti-ulcer, diuretic, emollient and as purgative. Furthermore, many researchers have stated the anti-inflammatory, anti-oxidant, antinociceptive, antipyretic, antidiabetic and analgesic effects of some Salsola species [1,2,4,5,6]. Different classes of phytoconstituents, e.g., saponins, lignans, alkaloids, flavonoids, sterols and coumarins, were reported in Salsola species [1,2].
Previous reports on Salsola species revealed the identification of saturated fatty acids such as 2,3-dihydroxypropyl palmitate and palmitic acid from S. tetragona. In addition, other unsaturated fatty acids such as oleic, linoleic and linolenic acids had been identified in S. vermiculata and S. tetrandra using UPLC/qTOF-MS analysis [7]. Another group of hydroxylated fatty acids—9,12,13-trihydroxy-10(E)-octadecenoic acid and 9,12,13-trihydroxyoctadeca-10(E),15(Z)-dienoic acid—was detected in the aerial parts of S. tetrandra [8]. In addition, 9,12,13-trihydroxydocosan-10,15,19-trienoic acid was identified in S. inermis [9]. Another tentative identification using UPLC/qTOF-MS analysis implied the presence of hydroxyoctadecatrienoic acid, hydroxyoctadecadienoic acid, hydroxyoctadecenoic acid, trihydroxyoctadecadienoic acid and dihydroxyoctadecenoic acid from S. vermiculata and S. tetrandra [7,10].
Inflammation, pain and fever are immune responses considered as the body’s normal defense mechanism and which are essential for health [11,12]. However, in some circumstances, these responses harm the body and trigger the development of some diseases, e.g., rheumatic arthritis and asthma. Prostaglandins are important inflammatory mediators that are produced from some cell membrane fatty acids via reactions catalyzed by cyclooxygenase enzyme. This enzyme exists in two isoforms known as COX-1 and COX-2 [12]. Under normal conditions, COX-1 is constitutively expressed in cells resulting in the production of protective substances for the stomach and kidney. Accordingly, the suppression of COX-1 results in several side effects. On the other hand, COX-2 is induced by pro-inflammatory agents, such as cytokines, under pathological conditions. So, the levels of COX-2 increase in case of tissue damage or inflammation. Prostanoides produced by COX-2 cause pain, edema and fever. The commonly used non-steroidal anti-inflammatory drugs (NSAIDs) act via the inhibition of COX enzyme. The lack of selectivity of these drugs towards COX-2 isoform causes several side effects, such as gastrointestinal disorders [12].
As natural products represent a continuous and inspiring source of medicinally useful chemical moieties, scientific research targeting the exploration of the bioactivities and phytochemical content of previously unexplored plant species is usually adopted. The Qassim area is rich with plenty of plant species from different plant families. Most of the observed species are inadequately investigated.
Molecular docking analysis is one of the recent important techniques that has accelerated the process of drug design. It is mainly used to illustrate the binding position of the tested molecules towards their protein targets to predict the activity and affinity of these molecules [13,14].
Accordingly, the current study was designed to explore the biological potential as well as the phytochemical content of the non-polar extracts from five Salsola species: S. arabica, S. cyclophylla, S. imbricata, S. inscanescence and S. villosa, prevailing in the Qassim region, KSA. Additionally, the assessment of the potentials of the identified metabolites to interact with the corresponding enzymes in in silico studies has also been performed by docking simulation.
2. Materials and Methods
2.1. Chemicals and Reagents
Human COX-1/COX-2 ELISA Kits were purchased from Cayman Chemical (catalog number 701070/701080), DPPH and Dimethyl sulfoxide (DMSO) from Sigma-Aldrich (St. Louis, MO, USA). Indomethacin and celecoxib were utilized as positive controls in COX-1 and COX-2 inhibitory assays, respectively. Methanol and n-hexane were obtained from the El-Nasr company for chemicals.
2.2. Plant Material and Preparation of the Non-Polar Extracts
The five Salsola species, S. arabica, S. cyclophylla, S. imbricata, S. incanescens and S. villosa, were collected in March 2021 from the Qassim region, KSA, deposited at the College of Pharmacy, Qassim University, and assigned voucher numbers QPP-103, QPP-104, QPP-105, QPP-106 and QPP-107, respectively. Species identities were verified by Ibrahim Aldakhil, botanical expert, Qassim region. The plant samples were cleaned then shade-dried for two weeks. The air-dried samples were finely powdered (100 g each) then separately macerated, at room temperature, in 200 mL n-hexane for 24 h, with frequent shaking, at 100 rpm, using an orbital shaker (Cole-Parmer™ Stuart™ Orbital Shaker, Thermo Scientific, Waltham, MA, USA). The mixtures were separately filtered, and marcs were reextracted with 200 mL n-hexane five times until exhaustion. The solvent was fully evaporated at 30 °C utilizing a rotatory evaporator (R-215, Buchi, Flawil, Switzerland) until dry residue was obtained from each sample.
2.3. Gas Chromatography–Mass Spectroscopy (GC–MS) Analysis
One microliter of the n-hexane extracts of five Salsola species was analyzed by GC-MS using a TRACE GC Ultra Gas Chromatographs (THERMO, Waltham, MA, USA), connected with a thermo-mass spectrometer detector; samples were injected with split mode 1:10 on TR-5 MS column (30 m × 0.32 mm × 0.25 μm). The carrier gas (Helium) was adjusted at a 1 mL/min flow rate, and the oven temperature was programmed from 60 °C with a gradual increase at a rate of 4.0 °C/min until a temperature of 240 °C was reached and maintained for further one minute. Both detector and injector temperatures were adjusted at 210 °C. Mass spectra were obtained using an ionization voltage of 70 eV and spectral range of m/z 40–450. The compounds were identified based on retention indices (relative to n-alkanes C8–C22); mass spectra matching the known compounds were available from the NSIT library database and Wiley spectral library collection.
2.4. Biological Activity
2.4.1. Anti-Inflammatory Study
The anti-inflammatory potential of n-hexane extracts of five Salsola species was evaluated by measuring the ability of the extracts to inhibit COX-1 and COX-2 enzymes. The assay was carried out by using COX-1 and COX-2 inhibitor screening assay kits. The assay depends on the catalytic effect of COX enzymes during the conversion of arachidonic acid to PGH2, which was reduced to PGF2α by SnCl2. The prostanoid product was measured by using enzyme immunoassay (ELISA). The procedure of the assay was conducted according to the instructions recommended by the supplier and previous reports [15,16]. Briefly, the extracts were dissolved in DMSO, and tenfold serial dilution was prepared to yield 1, 0.1, 0.01 and 0.001 µg/mL. In a 96-well plate, 10 μL of COX-1 or COX-2 enzyme, 10 μL of heme, 160 μL of assay buffer and 20 μL of the tested extracts were mixed, shaken in a shaker, and then the mixtures were incubated at 37 °C for 20 min. Afterwards, arachidonic acid (10 µL) was added, and the mixed solutions were shaken and incubated for 2 min at 37 °C. Finally, 30 µL of stannous chloride was added followed by incubation at 37 °C for 15 min to stop the reaction. The PGF2α was measured by ELISA. The two drugs indomethacin and celecoxib were employed as standard drugs.
2.4.2. Anti-Oxidant Activity
The scavenging potentials of the tested extracts were assessed using 2,2-diphenyl-1-picryl-hydrazyl (DPPH), which is a free radical model. The anti-oxidant activity of n-hexane extracts of the five Salsola species was estimated according to the following method [17] using ascorbic acid as positive control. An aliquot of 300 µL of samples as well as ascorbic acid solutions (100–1000 µg/mL) were mixed with 3.0 mL of DPPH solution (60 µg/mL, methanol). The mixture was shaken and then allowed to stand for 30 min in the dark at ambient temperature. Then, the absorbance was measured at 517 nm. Control was prepared using methanol (300 µL) instead of the sample. The free radical scavenging activity was calculated using the following equation:
Scavenging effect (%) = [absorbance of control − absorbance of sample/absorbance of control)] × 100
2.5. Molecular Docking
Docking simulation was performed adopting the Molecular Operating Environment, MOE (version 2015.10) software. The structures of human COX-2 (PDB: 5IKV) [18] and ovine COX-1 (PDB: 5WBE) [19] enzymes were acquired from the Protein Data Bank https://www.rcsb.org/structure accessed on 20 November 2022. The enzyme structures were prepared by deleting unwanted chains and ligands, adding missing hydrogens, adding missing amino acids, and fixing their potential. The binding site was determined based upon the PLB (Propensity for Ligand Binding) score using the Site Finder module, which computes the plausible binding sites from the 3D atomic coordinates of the enzyme; then, dummy atoms were assigned, with the location of the co-crystallized ligand used as a reference to explore which of the predicted sites was more likely for the docking process. Inceptive validation of docking was conducted, which included the re-docking of ligands that were formerly co-crystallized with the receptors (flufenamic acid for human COX-2 “5IKV” and mofezolac for ovine COX-1 “5WBE”) performed using the Amber10: EHT force field, London ΔG scoring function for the placement of poses, and the GBVI/WSA ΔG scoring function for poses refinement to detect the binding energy score, amino acid interactions and relative mean square deviation (rmsd). The rmsd factor was 1.1 Å for flufenamic acid in 5IKV and 0.9 Å for mofezolac in 5WBE. The structures of tested compounds were constructed by the MOE Builder module, 3D protonated, partially charged and energy minimized using force field Amber10: EHT, and then saved in the .moe format; ligand databases were prepared and stored as mdb (molecular database) files. MOE uses three successive algorithm methods for effective energy minimization: SD (Steepest Descent), CG (Conjugate Gradient) and TN (Truncated Newton). Following the validation of the docking process, the ligand databases were docked into the active sites of the prepared enzymes using parameters that were used in the re-docking process. For each docked compound, 30 docked poses were chosen, followed by refinement into the best 5 docked poses. Analysis of the binding site interactions with the studied compounds has been performed. The most promising structures regarding binding affinity were selected for detailed investigation.
2.6. Statistical Analysis
In this paper, the experiments were performed in triplicate, and data were expressed as means ± standard errors of the mean (SEM). Analysis was performed utilizing Graph Pad Prism 6 software (San Diego, CA, USA). An ANOVA test followed by a Tukey–Kramer post ANOVA test was used to compare the results from different extracts and standard drugs, and p values ˂ 0.05 were considered statistically significant.
3. Results
3.1. GC-MS Analysis of the n-hexane Extracts
GC-MS analysis of n-hexane extracts of the 5 Salsola species resulted in the identification of 67 compounds (Table 1) as follows: 28 compounds detected in S. cyclophylla, 22 from S. villosa, 18 in S. inscanescence, 13 in S. imbricata and 8 from S. arabica. Among the detected constituents, oleic acid (51) was the most prevailing compound (75.57%) in S. arabica, and cinnamaldehyde α-hexyl- (20, 57.15%) was the major detected compound in S. imbricata. Meanwhile, octacosyl heptafluorobutyrate (58, 25.36%), octacosanol (57, 19.30%) and 1-heptacosanol (56, 12.58%) were the major constituents in S. inscanescens, whereas 1-octadecene (25, 14.46%) and 1-hexadecanol (11, 13.66%) were the major detected constituents in S. cyclophylla extract.

Table 1.
GC-MS data of the n-hexane extracts of different Salsola species.
3.2. Anti-Oxidant Activity
The anti-oxidant activity of the n-hexane extracts from the five Salsola species was evaluated using DPPH method. The IC50 values of the five tested species were calculated, and the results illustrated in (Figure 1) show S. villosa to be the most active extract with an (IC50 of 0.99 ± 0.05 mg/mL) followed by S. cyclophylla (IC50 1.36 ± 0.06 mg/mL), S. imbricata (IC50 2.01 ± 0.05 mg/mL), S. inscanescense (IC50 3.29 ± 0.20 mg/mL) and S. arabica (IC50 3.70 ± 0.08 mg/mL).
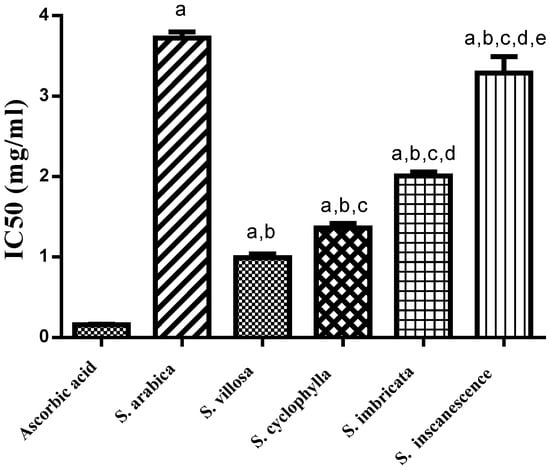
Figure 1.
Anti-oxidant activity of n-hexane extracts of the five Salsola species using DPPH. Results were presented as mean ± SEM, n = 3, a Significantly different from ascorbic acid, b significantly different from S. arabica, c significantly different from S. villosa, d significantly different from S. cyclophylla, e significantly different from S. imbricata at p < 0.05.
3.3. Anti-Inflammatory Activity
The anti-inflammatory potential of the n-hexane extracts from the five Salsola species was evaluated by measuring their ability to inhibit COX-1 and COX-2 enzymes. The results listed in (Table 2) highlight the characteristic activity of S. imbricata against COX-1 enzyme (IC50 10.2 ± 0.52 µg/mL), which was non-significantly different from the standard drug celecoxib (IC50 13.9 ± 0.70 µg/mL). The evaluation of COX-2 inhibitory activity indicated S. villosa extract as the most active COX-2 inhibitor among tested extracts, with an IC50 of 4.6 ± 0.13 µg/mL. Interestingly, S. villosa extract displayed selective COX-2 inhibition with a selectivity index (SI of 10), comparable to that of the standard drug celecoxib (SI = 16.6) and indomethacin (SI = 0.18).

Table 2.
Results of anti-inflammatory and antioxidant activity of n-hexane extracts of different Salsola species.
3.4. Molecular Docking
To characterize the inhibitory activity of S. villosa and S. imbricata extracts against COX-2 and COX-1 enzymes, respectively, molecular docking of the identified metabolites in each extract was accomplished using reported crystal structures of COX-2 and COX-1 as macromolecular models; the results are summarized in Table 3, where metabolites 17, 30, 32, 43, 44 and 49 exhibited the highest binding affinities to COX-2 with free binding energies from −8.966 to −8.166 kcal/mol compared to that of the co-crystallized ligand flufenamic acid (−6.9 kcal/mol), while metabolites 66, 17, and 46 exhibited the highest binding scores with COX-1 (−8.817, −8.658, and −8.160 kcal/mol, respectively) compared to the co-crystallized ligand mofezolac (−8.662 kcal/mol).

Table 3.
Binding scores of S. villosa and S. imbricata identified metabolites docked into active sites of COX-2 and COX-1 enzymes, respectively.
4. Discussion
The phytochemical content of the n-hexane extracts from the five tested Salsola species was explored by adopting GC-MS analysis. The results in (Table 1) indicate the prevalence of oleic acid (51, 75.57%) in S. arabica species. Oleic acid is a non-essential monounsaturated fatty acid that was reported to have a beneficial role as an anti-inflammatory agent in autoimmune diseases and protective effects in breast cancer [20]. The most prevailing compounds detected in S. cyclophylla were identified as the hydrocarbon 1-octadecene (25, 14.46%) followed by the alcoholic hydrocarbon 1-hexadecanol (11, 13.66%), benzoic acid pentadecyl ester (34, 11.31%) and 2,4-di-tert-butylphenol (10, 10.41%). Cinnamaldehyde, α-hexyl- (20) was the most prevailing compound in S. imbricata extract (57.15%), which seemed to be responsible for its characteristic odor. It is an aromatic volatile compound used as a flavor and fragrance compound found in chamomile oil, which is considered as one of its common sources [21]. S. inscanescens displayed octacosyl heptafluorobutyrate (58) as the major detected compound (25.36%) in addition to the three alcohols octacosanol (57, 19.3%), 1-heptacosanol (56, 12.58%) and 1-dotriacontanol (55, 6.30%). Another notable finding was the predominance of the three fatty acid methyl esters 8-exadecenoic acid methyl ester (38, 26.15%), 9,12-octadecadienoic acid (Z,Z)-, methyl ester (43, 17.81%) and 9-octadecenoic acid (Z)- methyl ester (44, 16.34%) in S. villosa. Interestingly, this is the first report of the GC-MS profiling of the n-hexane extracts from the five mentioned Salsola species.
The anti-oxidant activity of the lipophilic extracts from the five Salosala species was evaluated by the DPPH method, which depends on the disappearance of the characteristic violet color of DPPH upon mixing with a solution of an anti-oxidant agent [22]. The current results (Figure 1) noted the n-hexane fraction of S. villosa as the most active with an (IC50 of 0.99 ± 0.05 mg/mL), followed by S. cyclophylla (IC50 1.36 ± 0.06 mg/mL) and S. imbricata (IC50 2.01 ± 0.05 mg/mL). The observed anti-oxidant activity of S. villosa might be attributable to its high content of the three fatty acids hexadecanoic acid methyl ester, 9,12-octadecadienoic acid (Z,Z)- and 9-octadecenoic acid (Z)- methyl ester, which were previously acknowledged for their anti-oxidant activity [23]. On the other hand, S. cyclophylla extract contained 2,4-di-tert-butylphenol, which is a phenolic compound previously stated to be an anti-oxidant agent [24]. Furthermore, the fatty acid methyl ester hexadecanoic acid methyl ester detected in appreciable concentrations in S. cyclophylla, S. imbricata, S. incanescens and S. villosa was previously stated to be anti-oxidant compound [23].
Meanwhile, several Salsola species were previously investigated for anti-inflammatory activity [4,5]. The anti-inflammatory potential of the total extract as well as the isolated compound of S. grandis was stated using an in vivo model [6]. Similarly, the crude extract as well as the compounds isolated from S. imbricata, collected from Egypt, were reported as potent anti-inflammatory drugs using the NO production inhibitory model [1]. One more study investigated the anti-inflammatory potential of hydroalcoholic extract of S. imbricata growing in Pakistan using a carrageenan-induced paw edema model [25]. Iannuzzi et al. compared the anti-inflammatory potential of the wild and cultivated species of S. soda using three human recombinant enzymes, and the results indicated that S. soda could stop the pathological conditions related to the inflammatory process [2]. In 2021, a recent study reported the significant anti-inflammatory activity of the hydro-alcoholic extract of S. cyclophylla using an in vivo model [5]. In light of those previous reports, the current study was devoted to assessing the anti-inflammatory potential of the lipophilic extracts of five Salsola species by measuring their ability to inhibit COX-1 and COX-2 enzymes. The results listed in (Table 2) indicate the n-hexane extract of S. villosa as the most active COX-2 inhibitor among the tested extracts, with an IC50 of 4.6 µg/mL. S. arabica came in the second position with an (IC50 of 13.1 µg/mL). GC-MS analysis of the lipophilic extract of S. villosa indicated the richness of this extract with fatty acid methyl esters, especially hexadecanoic acid methyl ester, 9,12-octadecadienoic acid- methyl ester and 9-octadecenoic acid-methyl ester. Interestingly, hexadecanoic acid was previously reported to exhibit anti-inflammatory properties through the inhibition of phospholipase A2 [26]. In addition 9,12-octadecadienoic acid-methyl ester was reported to have anti-inflammatory and hepatoprotective activities [27,28], while 9-octadecenoic acid was previously acknowledged for anti-oxidant and anticancer activity [29]. All these records might imply the characteristic COX-2 inhibitory activity of this extract when compared to other tested Salsola species.
Similarly, the observed mild COX-2 inhibitory activity of S. arabica extract could be attributable to its most abundant fatty acid, oleic acid (75.57%), which was demonstrated to induce beneficial anti-inflammatory effects in autoimmune diseases [30,31] and a protective effect in breast cancer as well as the improvement of immune system function [32]. To the best of our knowledge, this is the first report identifying the phytoconstituents of the non-polar extracts of the five tested Salsola species as well as the in vitro evaluation of their anti-oxidant and anti-inflammatory potential.
Molecular Docking
The two cyclooxygenase enzymes are homologous to a great extent, as they exhibit 61% sequence identity; upon comparing the active site residues only, they achieve 78% sequence identity [33]. COX enzymes are homodimers consisting of firmly linked monomers, each including three domains: epidermal growth factor domain, membrane binding domain and the catalytic domain that incorporates the cyclooxygenase active site [34]. Previous crystallographic investigations elucidated that COX enzymes incorporate extended a hydrophobic channel from membrane binding domain to the active site. The entrance of the channel is wide and then narrows into a stricture formed by three critical residues, Arg120, Tyr355 and Glu524, where they comprise a hydrogen-bonding network [19,34].
Although the active sites of both enzymes are similar, in COX-2, an extra side pocket surrounded by Val523 (instead of Ile523 in COX-1) is present above Arg120 and Tyr355, leading to the augmentation of the available space in the active site; also, Arg513 is reserved for COX-2 (His513 in COX-1) [35]. Docking simulation of the identified compounds in a non-polar extract of S. villosa into the active site of COX-2 enzyme revealed that among the identified metabolites, the series of acetylenic and olefinic fatty acid methyl esters (metabolites 17, 30, 32, 43, 44, and 49) exhibited the highest binding affinities, where in both metabolites 17 and 43, the carboxylate moiety is positioned near Arg120 and Tyr355 and forms a salt bridge with the conserved Arg120 in a manner resembling the substrate arachidonic acid and most carboxylate-containing NSAIDs [19], and both metabolites are bound to the active site through several hydrophobic contacts (Figure 2a and Figure 3a,b). In metabolites 30 and 44, the aliphatic chain is wedged into the side pocket that is bordered by Val523 and forms many hydrophobic interactions with active site residues (Figure 2b and Figure 3c,d). On the other hand, metabolite 49 is positioned where the carboxylate moiety interacts through the formation of two hydrogen bonds with the catalytically important Tyr385 and one hydrogen bond with Ser530 (Figure 2c and Figure 3e), while metabolite 32’s carboxylate moiety forms a hydrogen bond with Tyr355, and its aliphatic end extends above the helix including Ser530 and Gly533 (Figure 2d and Figure 3f).
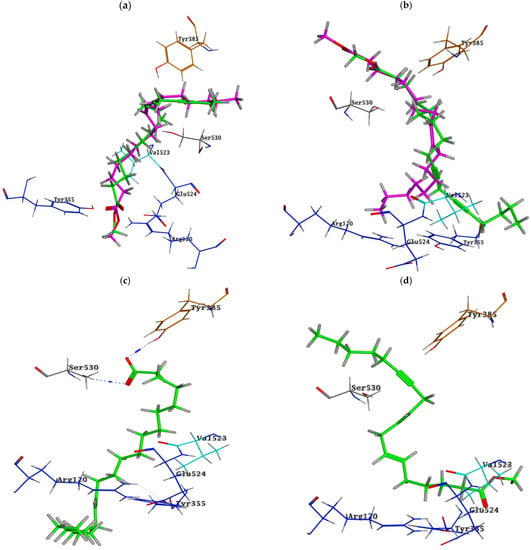
Figure 2.
Binding poses of (a) metabolites 17 “green” and 43 “magenta”, (b) metabolites 30 “green” and 44 “magenta”, (c) metabolite 49 and (d) metabolite 32 into COX-2 active site; ligands are depicted in the stick model. The residues are shown in labelled wire models.
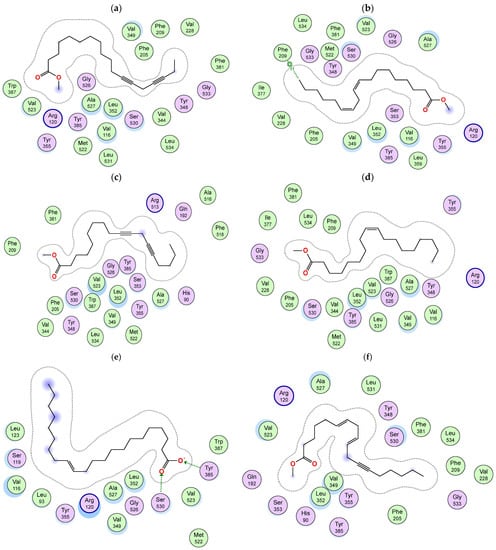
Figure 3.
Two-dimensional diagram of the binding interactions of (a) metabolite 17, (b) metabolite 43, (c) metabolite 30, (d) metabolite 44, (e) metabolite 49 and (f) metabolite 32 with the active site residues of COX-2 enzyme. Magenta circles indicate polar amino acids and green circles indicate nonpolar amino acids.
Docking of S. imbricata identified metabolites in the active site of COX-1 and pointed out metabolites 66, 17 and 46, which exhibited the highest binding scores; these compounds could undertake poses allowing their hydrophobic chains to be positioned and pointed to the small channel of the COX-1 active site where the binding pocket exhibits a hydrophobic nature in this area, and hence these aliphatic chains simulate the chain of arachidonic acid: the COX-1 substrate. The aliphatic chain of metabolite 66 is located at the hydrophobic entry of the binding site in the proximity of Arg120, Tyr355 and Glu524, and on the other hand, the phenyl ring inside the pocket is stabilized by an arene–hydrogen bond with Ser353 (Figure 4a and Figure 5a). Both metabolites 17 and 46 are bound into the active site of COX-1 in an inverted binding mode (compared to COX-2), with the carboxylate moiety positioned towards the catalytic Tyr385 and Ser530 and the aliphatic end extended through the constricted gate formed by Arg120, Tyr355, and Glu524 (Figure 4b and Figure 5b,c).
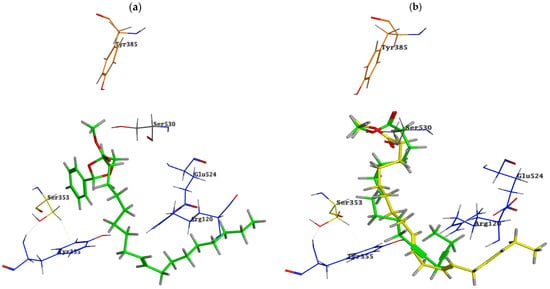
Figure 4.
Binding poses of (a) metabolite 66 “green” and (b) metabolites 17 “green” and 46 “yellow” into the COX-1 active site; ligands are depicted with a stick model. The residues are shown in labelled wire models.
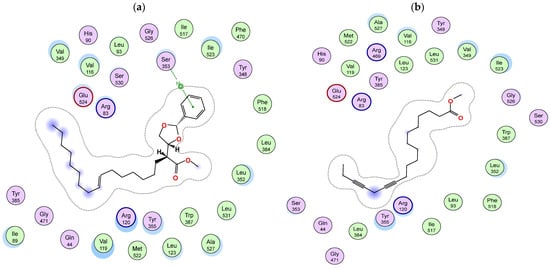
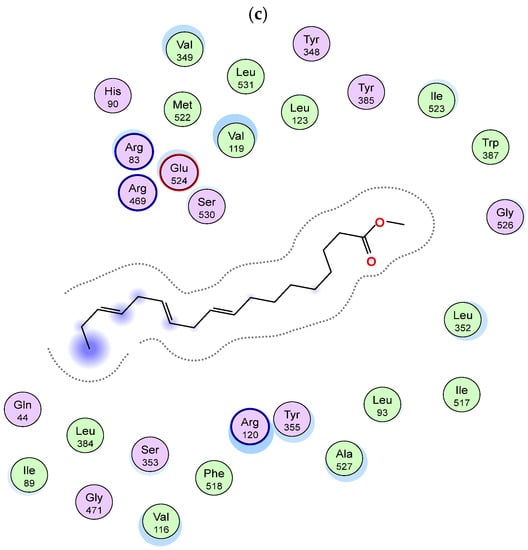
Figure 5.
Two-dimensional diagram of the binding interactions of (a) metabolite 66, (b) metabolite 17 and (c) metabolite 46 with the active site residues of COX-1 enzyme. Magenta circles indicate polar amino acids and green circles indicate nonpolar amino acids.
5. Conclusions
GC-MS analysis of lipophilic constituents in the five Salsola species led to the identification of 67 metabolites. Interestingly, the highest percentage among the identified compounds was recorded for oleic acid in S. arabica extract. Furthermore, cinnamaldehyde, α-hexyl- predominated in S. imbricata extract and was suggested to be responsible for its characteristic odor. Notably, S. villosa extract achieved the most characteristic anti-oxidant and COX-2 inhibitory activities among all the tested extracts. Docking simulation of its identified metabolites revealed that some of these metabolites may compete with natural substrates for COX-2 enzyme. The presented results suggested S. villosa n-hexane extract to be a specific COX-2 blocker with remarkable anti-oxidant activity, and its metabolites could be promising candidates for the future development of selective COX-2 inhibitors.
Author Contributions
Conceptualization, E.A. (Elham Amin); investigation, E.A. (Elham Amin), E.A. (Ebtihaj Almutairi), M.A. and F.A.; methodology, E.I.A.M., A.E. and M.H.A.H.; software, M.S.A.-B.; writing—original draft preparation, E.A. (Elham Amin), A.E. and M.H.A.H.; writing—review and editing, M.S.A.-B., E.I.A.M., A.E. and M.H.A.H.; supervision, E.A. (Elham Amin). All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Data Availability Statement
Not applicable.
Acknowledgments
The researchers would like to thank the Deanship of Scientific Research, Qassim University, for funding the publication of this project.
Conflicts of Interest
The authors declare no conflict of interest in this work.
References
- Osman, S.M.; El Kashak, W.A.; Wink, M.; El Raey, M.A. New isorhamnetin derivatives from Salsola imbricata Forssk. leaves with distinct anti-inflammatory activity. Pharmacogn. Mag. 2016, 12, S47. [Google Scholar] [CrossRef]
- Iannuzzi, A.M.; Moschini, R.; De Leo, M.; Pineschi, C.; Balestri, F.; Cappiello, M.; Braca, A.; Del-Corso, A. Chemical profile and nutraceutical features of Salsola soda (agretti): Anti-inflammatory and antidiabetic potential of its flavonoids. Food Biosci. 2020, 37, 100713. [Google Scholar] [CrossRef]
- Abdalla, W.E.; El Ghazali, G.E.; Al-Soqeer, A.R.A. A Checklist to the Family Chenopodiaceae in Qassim Region, Saudi Arabia. J. Agric. Vet. Sci. 2016, 8, 2. [Google Scholar] [CrossRef]
- Janbaz, K.; Aslam, N.; Imran, I.; Jabeen, Q. Evaluation of anti-inflammatory, analgesic and antipyretic activities of Salsola imbricata forssk in rats. JAPS J. Anim. Plant Sci. 2021, 31, 862–867. [Google Scholar] [CrossRef]
- Mohammed, H.A.; Al-Omar, M.S.; Mohammed, S.A.; Alhowail, A.H.; Eldeeb, H.M.; Sajid, M.S.; Abd-Elmoniem, E.M.; Alghulayqeh, O.A.; Kandil, Y.I.; Khan, R.A. Phytochemical Analysis, Pharmacological and Safety Evaluations of Halophytic Plant, Salsola cyclophylla. Molecules 2021, 26, 2384. [Google Scholar] [CrossRef]
- Küçükboyacı, N.; Küpeli Akkol, E.; Suntarİhsan Çalış, İ.; Çalış, İ. In vivo Anti-Inflammatory and Antinociceptive Activities of the Extracts and Chemical Constituents of an Endemic Turkish Plant, Salsola grandis. Rec. Nat. Prod. 2016, 10, 369–379. [Google Scholar]
- Rasheed, D.M.; El Zalabani, S.M.; Koheil, M.A.; El-Hefnawy, H.M.; Farag, M.A. Metabolite profiling driven analysis of Salsola species and their anti-acetylcholinesterase potential. Nat. Prod. Res. 2013, 27, 2320–2327. [Google Scholar] [CrossRef]
- Oueslati, M.H.; Ben Jannet, H.; Mighri, Z.; Chriaa, J.; Abreu, P.M. Phytochemical constituents from Salsola tetrandra. J. Nat. Prod. 2006, 69, 1366–1369. [Google Scholar] [CrossRef]
- Elsharabasy, F.S.; Hosney, A.M. Chemical constituents from the aerial parts of Salsola inermis. Egypt. Pharm. J. 2013, 12, 90. [Google Scholar] [CrossRef]
- ElNaggar, M.H.; Eldehna, W.M.; Abourehab, M.A.; Abdel Bar, F.M. The old world salsola as a source of valuable secondary metabolites endowed with diverse pharmacological activities: A review. J. Enzym. Inhib. Med. Chem. 2022, 37, 2036–2062. [Google Scholar] [CrossRef]
- Recio, C.M.; Andujar, I.; Rios, L.J. Anti-inflammatory agents from plants: Progress and potential. Curr. Med. Chem. 2012, 19, 2088–2103. [Google Scholar] [CrossRef]
- Taylor, J.; Van Staden, J.; Jäger, A. COX-1 and COX-2 inhibitory activity in extracts prepared from Eucomis species, with further reference to extracts from E. autumnalis autumnalis. S. Afr. J. Bot. 2002, 68, 80–85. [Google Scholar] [CrossRef]
- Taghizadeh, M.S.; Niazi, A.; Moghadam, A.; Afsharifar, A. Experimental, molecular docking and molecular dynamic studies of natural products targeting overexpressed receptors in breast cancer. PLoS ONE 2022, 17, e0267961. [Google Scholar] [CrossRef]
- Sanghani, H.; Ganatra, S.; Pande, R. Molecular—Docking studies of potent anticancer agent. J. Comput. Sci. Syst. Biol. 2012, 5, 12–15. [Google Scholar] [CrossRef]
- Karim, N.; Khan, I.; Khan, W.; Khan, I.; Khan, A.; Halim, S.A.; Khan, H.; Hussain, J.; Al-Harrasi, A. Anti-nociceptive and anti-inflammatory activities of asparacosin a involve selective cyclooxygenase 2 and inflammatory cytokines inhibition: An in-vitro, in-vivo, and in-silico approach. Front. Immunol. 2019, 10, 581. [Google Scholar] [CrossRef]
- Amin, E.; Elwekeel, A.; Alshariedh, N.F.; Abdel-Bakky, M.S.; Hassan, M.H. GC-MS Analysis and Bioactivities of the Essential Oil of Suaeda aegyptiaca. Separations 2022, 9, 439. [Google Scholar] [CrossRef]
- Bakar, M.F.A.; Mohamed, M.; Rahmat, A.; Fry, J. Phytochemicals and antioxidant activity of different parts of bambangan (Mangifera pajang) and tarap (Artocarpus odoratissimus). Food Chem. 2009, 113, 479–483. [Google Scholar] [CrossRef]
- Orlando, B.J.; Malkowski, M.G. Substrate-selective inhibition of cyclooxygeanse-2 by fenamic acid derivatives is dependent on peroxide tone. J. Biol. Chem. 2016, 291, 15069–15081. [Google Scholar] [CrossRef]
- Cingolani, G.; Panella, A.; Perrone, M.G.; Vitale, P.; Di Mauro, G.; Fortuna, C.G.; Armen, R.S.; Ferorelli, S.; Smith, W.L.; Scilimati, A. Structural basis for selective inhibition of Cyclooxygenase-1 (COX-1) by diarylisoxazoles mofezolac and 3-(5-chlorofuran-2-yl)-5-methyl-4-phenylisoxazole (P6). Eur. J. Med. Chem. 2017, 138, 661–668. [Google Scholar] [CrossRef]
- Sales-Campos, H.; Reis de Souza, P.; Crema Peghini, B.; Santana da Silva, J.; Ribeiro Cardoso, C. An overview of the modulatory effects of oleic acid in health and disease. Mini-Rev. Med. Chem. 2013, 13, 201–210. [Google Scholar] [CrossRef]
- ZURE, C.V.; PINJARI, R.V. Development and Validation of 2D GC-FID Method for Quantitative Analysis of cis-and trans-Hexyl Cinnamic Aldehyde and its Major Impurity 2-Hexyl-2-decenal. Asian J. Chem. 2018, 30, 1088–1092. [Google Scholar] [CrossRef]
- Molyneux, P. The use of the stable free radical diphenylpicrylhydrazyl (DPPH) for estimating antioxidant activity. Songklanakarin J. Sci. Technol. 2004, 26, 211–219. [Google Scholar]
- Pinto, M.E.; Araujo, S.G.; Morais, M.I.; Sá, N.P.; Lima, C.M.; Rosa, C.A.; Siqueira, E.P.; Johann, S.; Lima, L.A. Antifungal and antioxidant activity of fatty acid methyl esters from vegetable oils. An. Acad. Bras. Cienc. 2017, 89, 1671–1681. [Google Scholar] [CrossRef]
- Vahdati, S.N.; Lashkari, A.; Navasatli, S.A.; Ardestani, S.K.; Safavi, M. Butylated hydroxyl-toluene, 2, 4-Di-tert-butylphenol, and phytol of Chlorella sp. protect the PC12 cell line against H2O2-induced neurotoxicity. Biomed. Pharmacother. 2022, 145, 112415. [Google Scholar] [CrossRef]
- Javed, F.; Jabeen, Q. Salsola imbricata Forssk. ameliorates acetic acid-induced inflammatory bowel disease by modulating dysregulated antioxidant enzyme system and cytokine signaling pathways in mice. Asian Pac. J. Trop. Biomed. 2021, 11, 527. [Google Scholar] [CrossRef]
- Aparna, V.; Dileep, K.V.; Mandal, P.K.; Karthe, P.; Sadasivan, C.; Haridas, M. Anti-inflammatory property of n-hexadecanoic acid: Structural evidence and kinetic assessment. Chem. Biol. Drug Des. 2012, 80, 434–439. [Google Scholar] [CrossRef]
- Sermakkani, M.; Thangapandian, V. GC-MS analysis of Cassia italica leaf methanol extract. Asian J. Pharm. Clin. Res. 2012, 5, 90–94. [Google Scholar]
- Ha, Y.L.; Storkson, J.; Pariza, M.W. Inhibition of benzo (a) pyrene-induced mouse forestomach neoplasia by conjugated dienoic derivatives of linoleic acid. Cancer Res. 1990, 50, 1097–1101. [Google Scholar] [PubMed]
- Wilsy, J.I.; Beschi, D.A.; Appavoo, M.R.; Wilsy, J.I. GC-MS analysis, collected from Kavalkinaru area, Tirunelveli District, Tamil Nadu, India. Eur. J. Mol. Clin. 2021, 7, 4287–4292. [Google Scholar]
- Linos, A.; Kaklamanis, E.; Kontomerkos, A.; Koumantaki, Y.; Gazi, S.; Vaiopoulos, G.; Tsokos, G.; Kaklamanis, P. The effect of olive oil and fish consumption on rheumatoid arthritis-a case control study. Scand. J. Rheumatol. 1991, 20, 419–426. [Google Scholar] [CrossRef]
- Kremer, J.M.; Lawrence, D.A.; Jubiz, W.; Digiacomo, R.; Rynes, R.; Bartholomew, L.E.; Sherman, M. Dietary fish oil and olive oil supplementation in patients with rheumatoid arthritis clinical and immunologic effects. Arthritis Rheum. Off. J. Am. Coll. Rheumatol. 1990, 33, 810–820. [Google Scholar] [CrossRef] [PubMed]
- Lipworth, L.; Martı́nez, M.a.E.; Angell, J.; Hsieh, C.-C.; Trichopoulos, D. Olive oil and human cancer: An assessment of the evidence. PrevMed 1997, 26, 181–190. [Google Scholar] [CrossRef] [PubMed]
- Llorens, O.; Perez, J.J.; Palomer, A.; Mauleon, D. Differential binding mode of diverse cyclooxygenase inhibitors. J. Mol. Graph. Model. 2002, 20, 359–371. [Google Scholar] [CrossRef] [PubMed]
- Marnett, L.J.; Rowlinson, S.W.; Goodwin, D.C.; Kalgutkar, A.S.; Lanzo, C.A. Arachidonic Acid Oxygenation by COX-1 and COX-2: Mechanisms of Catalysis and Inhibition* 210. J. Biol. Chem. 1999, 274, 22903–22906. [Google Scholar] [CrossRef]
- Limongelli, V.; Bonomi, M.; Marinelli, L.; Gervasio, F.L.; Cavalli, A.; Novellino, E.; Parrinello, M. Molecular basis of cyclooxygenase enzymes (COXs) selective inhibition. Proc. Natl. Acad. Sci. USA 2010, 107, 5411–5416. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).