Abstract
An automated solid phase extraction (SPE) protocol to determine carbamazepine in human serum has been developed and validated using a simple, rabid and sensitive liquid chromatography-based bio-analytical method. Extraction of carbamazepine was carried out using an on-line SPE tool of a short protein-coated (PC) ODS silica pre-column (PC-ODS-pre-column) and phosphate buffer saline (PBS) with a pH of 7.4 as an extraction solvent. There are two distinct chromatographic modes used by PC-ODS-pre-column. While carbamazepine trapping required reversed-phase liquid chromatography, proteins were extracted from serum samples using PBS by size-exclusion liquid chromatography. Then, carbamazepine was eluted from the PC-ODS-pre-column onto the quantification position using a mixture of methanol-distilled deionized water (50:50, v/v) as an eluent and ODS analytical column. At room temperature (22 ± 1 °C), carbamazepine was completely separated from the co-eluted matrix components and detected at 230 nm. Carbamazepine’s linearity was obtained at concentrations ranging from 50 to 10,000 ng/mL. With good accuracy and precision, carbamazepine recoveries in serum samples ranged from 86.14 to 97.82%. The extraction step was conducted using PBS as a safe and green extraction solvent, making this protocol both cost-effective and ecologically safe.
1. Introduction
Carbamazepine is one of the more established antiepileptic medications [1]. It has been comprehensively used in epilepsy treatment. Recently, it has been regarded as the front-line anticonvulsant that is most usually administered for complex partial seizures. Carbamazepine has a limited therapeutic plasma concentration range. Even slight variations in dosage or blood concentration may cause therapeutic failures that are life-threatening or result in substantial pharmacological side effects that cause chronic or long-lasting impairment or incapacity [2]. Thus, in order to manage patients’ treatment regimens with the use of measured drug concentrations, therapeutic drug monitoring (TDM) of this antiepileptic medication is required. The clinical management of epilepsy patients using carbamazepine frequently uses plasma concentration monitoring. That has led to the continuous need for developing analytical methods to measure carbamazepine in bio-fluid clinical samples for TDM, toxicology purposes and bio-equivalence studies. It is essential to select analytical techniques that are precise, simple, quick, economical, sensitive and environmentally friendly. For the determination of carbamazepine in various matrices, a number of high-performance liquid chromatography (HPLC) methods have been published due to availability of the instruments, analytical procedure simplicity and low operation cost [3,4,5,6,7,8,9,10,11,12,13,14,15,16,17,18]. Moreover, HPLC coupled with mass spectrometry has been used extensively for monitoring of carbamazepine in different matrices [19,20,21,22,23,24,25,26,27].
Unfortunately, due to the complexity of bio-fluid samples, it is difficult to directly analyze serum and plasma by conventional HPLC techniques because the endogenous compounds they contain can interfere with the target molecules or affect the potency and life expectancy of the analytical columns. Consequently, for accurate measurement of carbamazepine concentrations in the serum or plasma samples, it is crucial to develop simple and affordable extraction protocols and straightforward procedures to pre-treat the bio-fluid samples before injecting them into the HPLC system. For biological sample pretreatment, there are three widely used techniques: liquid–liquid extraction (LLE) [10,11,12,13,24,25], off-line solid-phase extraction (SPE) [14,15,16,17,18,19,26], and protein precipitation extraction (PPE) [3,4,5,6,7,8,9,20,21,22,23]. Conventional extraction techniques are typically laborious and time-consuming. They also need suitable internal standards and large amounts of organic solvents.
Modern extraction techniques are being developed and improved by researchers to be more sensitive, accurate and productive than the traditional methodologies. Online or automated sample pre-treatment is crucial to analysis bio-fluid samples for TDM, particularly if the sample is susceptible to degradation when exposed to light, air and humidity, or the concentration of the target molecule is low. The direct injection method enhances the analytical technique’s overall performance by reducing the possibility of human error, conserving samples from loss and contamination and requiring fewer organic solvents, making it an environmentally friendly and unquestionably time-saving technique. These factors all contribute to accurate and precise results. A pre-column made of traditional reversed phase (RP) sorbents has attracted the attention of scientists who want to use automated SPE to remove protein from bio-fluid matrices [28,29]. The precipitation and/or denaturation of some proteinous matrices on the solid supports impose some limitations on the extraction procedures utilized these RP silica sorbents. These drawbacks necessitate the frequent replacement of these SPE pre-columns upon loading with a small number of samples.
While being designed to lose its ability to adsorb matrix proteins, the pre-columns made of RP sorbents coated with proteins have still possessed distinctive RP properties that allowed them to hold small-molecular compounds onto their inner surfaces [30,31]. The distinct features of these pre-columns, which include non-adsorptive outer coat layers toward macro-molecules in the matrix, such as proteins, while allowing the small molecules to bind to the inner surface of the sorbents due to its RP performance, aid in both the removal and enrichment of proteins and analyte, respectively, before they are transferred into the analytical column. Pre-columns such as these are affordable and can lengthen the analytical column’s functional life.
A multi-port switching valve, two columns (the pre- and analytical columns), and two pumps assist in a system manifold by which the online extraction procedure is carried out. In this study, we used ODS protein coated pre-column (ODS-PC-pre-column) as an online SPE tool through its characteristic size exclusion liquid chromatography (SE-LC) mode. At the same time, the analyte is captured by the RP-LC of the same pre-column, while the macromolecules (proteins) are eluted out before switching the valve to the analytical column. This automated system manifold is more rapid, accurate and reliable, as well as eco-friendly, because it saves time, decreases the possibility of using dangerous chemicals, prevents contamination of samples and avoids sample loss.
2. Materials and Methods
2.1. Chemicals and Reagents
Pure standard carbamazepine, kindly provided by Egyptian Drug Authority (EDA) Administration Center (Cairo, Egypt), was used and certified to contain 99.8%. Sodium chloride, sodium hydroxide, potassium chloride, potassium dihydrogen phosphate, disodium hydrogen phosphate, ethylene diamine tetra-acetic acid disodium salt (Sigma-Aldrich, Inc., St. Louis, MO, USA), acetic acid (Riedel-de Haën Laboratory Chemicals, Seelze, Germany) and phosphoric acid (BDH Laboratory Supplies Poole, Dorset, UK) were analytical grade. Methanol used was HPLC grade (Sigma-Aldrich, Darmstadt, Germany). The ODS sorbent was obtained from Waters Associates (Milford, CT, USA) and used for preparation of PC-pre-column manually in our Laboratory. Distilled deionized water was used for the preparation of all reagents and solutions. Drug-free serum samples were obtained from VACSERA (Cairo, Egypt).
2.2. Instruments
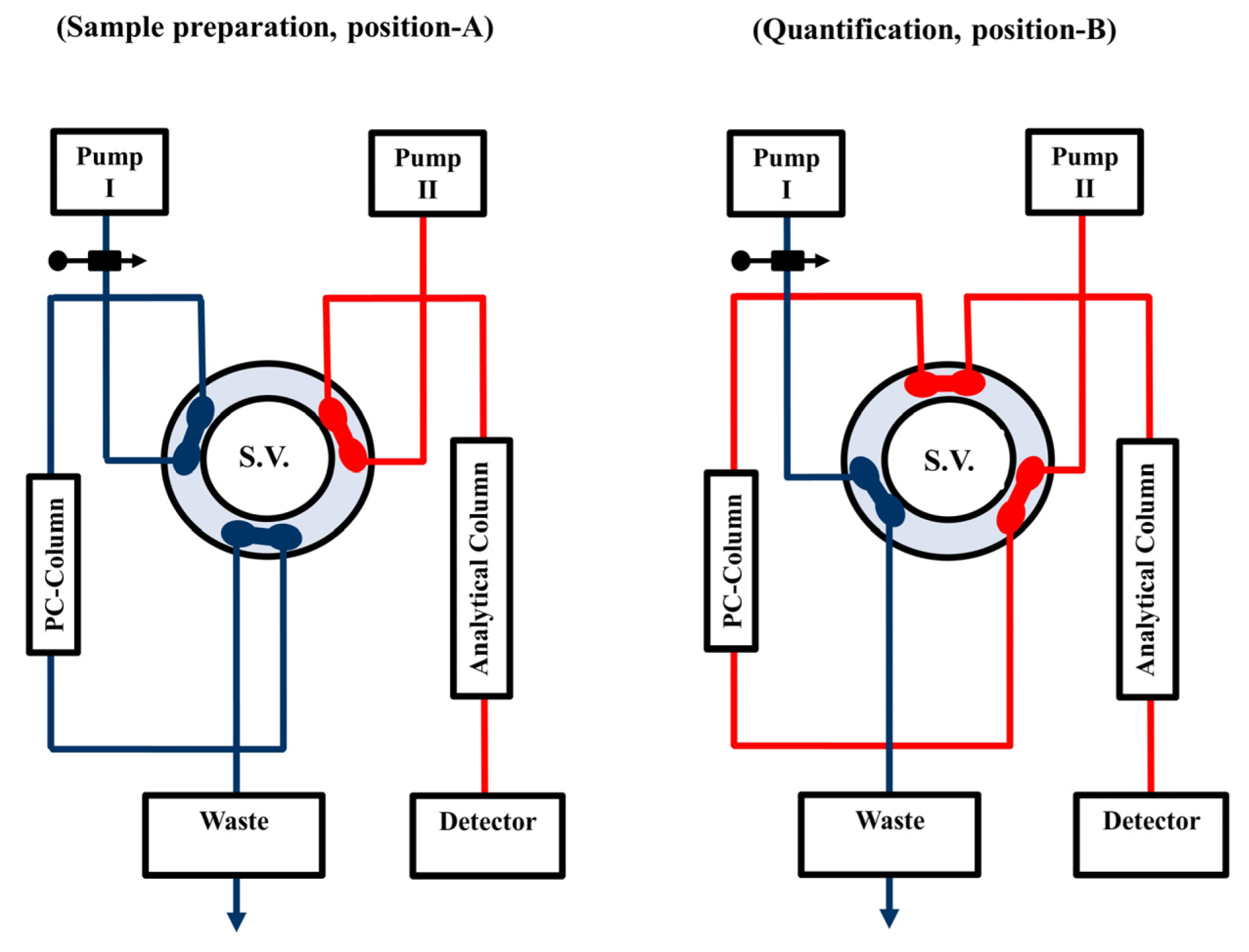
Figure 1 represents a back flushed (BF) direct sample injection (DSI) bio-analytical HPLC-based manifold (BA-HPLC) with UV detection (BF-DSI-BA-HPLC-UV). This manifold comprises two pumps (Agilent 1100 Series Iso pump G1310A, Agilent Technologies, Santa Clara, CA, USA); one is used to load serum samples onto the PC-pre-column (laboratory prepared) of ODS silica (PC-ODS-pre-column) (20 mm × 4.6 mm i.d., 20 µm particle size) [32] in the extraction position, and the other is used to flush carbamazepine from the PC-ODS-pre-column to the analytical column of ZORBAX Eclipse XDB-C18 (15 cm × 0.46 cm i.d. (5 µm particle size)) in a BF mode for the quantification purpose. An injector, model 7725i (with a 100-µL loop), and a flow switching high-pressure valve, model 7000, (Rheodyne, Berkeley, CA, USA) were utilized to load samples and facilitate the flushing of carbamazepine from the extraction position onto the quantification position at a flow rate of 1 mL/min. To monitor carbamazepine at 230 nm, a model Agilent 1100 Series VWD G1314A UV/Vis detector was employed. Software from Agilent, LC ChemStation, was used for data collecting. All experiments were conducted at (21 ± 1 °C).
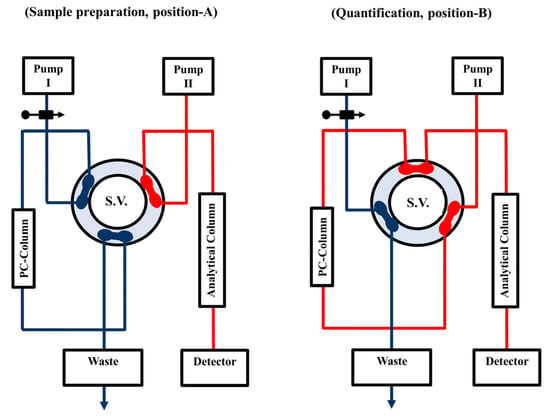
Figure 1.
Scheme of the BF-DSI-BA-HPLC-UV methodology in the BF elution mode. Position (A) shows the system manifold in the sample preparation step, ready for loading, washing, trapping and pre-concentration: HPLC circulation (separation and quantification) is isolated from extraction side. Position (B) displays the quantification step, ready for separation and measurement: the PC-ODS-pre-column is connected with an ODS analytical column in a BF elution mode via a 6-ports high pressure switching valve (S.V.).
2.3. Mobile Phase
Two different elution systems were employed in the BF-DSI-BA-HPLC-UV method. One of them is composed of phosphate buffer saline (PBS) (pH 7.4) (mobile phase 1, MI) to move serum sample into the PC-ODS-pre-column for the extraction of proteins and trapping of carbamazepine. The other mobile phase consists of a mixture of methanol and distilled deionized water in the ratio of 50 to 50 (v/v) (mobile phase 2, MII). MII functioned as an analytical mobile phase that aided in the separation and quantification processes. Both MI and MII were freshly prepared on the day of use, filtered by 0.45 µm regenerated cellulose membrane filters, and degassed over 5 min by ultrasonication.
2.4. BF-DSI-BA-HPLC-UV
The main steps of the BF-DSI-BA-HPLC-UV method are shown in Figure 1. Prior to sample loading, pump I brings MI onto the PC-ODS-pre-column for equilibration as a first part of the extraction setup. In the meanwhile, MII by pump II equilibrates the analytical ODS column. The serum sample, in a 100-µL aliquot, is then placed into the sample loop and transferred to the PC-ODS-pre-column by MI. During this step, MI is flushed down the PC-ODS-pre-column at a rate of 1 mL/min for two minutes. During this flushing period, large protein fragments and other poorly retained endogenous matrix components could be eliminated. The switching valve is then switched to the quantification setting. The analytical column is coupled in a back-flushed mode to the PC-ODS-pre-column. Thus, MII can transfer the clean sample fraction containing carbamazepine from the PC-ODS-pre-column onto the analytical column at a flow rate of 1 mL/min. After a minute, the switching valve is repositioned to its original set-up, and while the analytical column separates and quantifies carbamazepine, the PC-ODS-pre-column is eluted with MI to retrieve its original condition for the new sample analysis.
2.5. Calibration Standards and Quality Control Samples
In order to prepare a stock carbamazepine solution (10 mg/mL), the accurately weighed amount of analyte was dissolved in the appropriate volume of methanol. Seven standards with a concentration range of 0.5–100 µg/mL were prepared by serially diluting appropriate volumes of carbamazepine stock solution with methanol. Various carbamazepine concentrations, ranging from 50 to 10,000 ng/mL, were achieved by diluting each standard into a drug-free human serum in a ratio of 1 to 9. Serum calibration standards were kept frozen (−20 °C) until analysis. Before the chromatographic run, frozen serum standards after being thawed at room temperature (20 ± 1 °C) were centrifuged at 5000 rpm for 5 min. BF-DSI-BA-HPLC-UV technique was used to measure carbamazepine in the clear supernatant after filtration through 0.45 µm membrane filter tip.
Carbamazepine quality control (QC) samples were prepared using the same particular procedure for preparation of serum calibration standards to obtain the 50, 500, 5000 and 8000 ng/mL QC samples.
2.6. Recovery, Precision, and Accuracy
The recovery of carbamazepine from the serum sample was assessed by comparing the concentration of the analyte determined from the spiked sample to that indicated to be present as follows:
Recovery (%) = [mean measured concentration/spiked concentration] × 100
The BF-DSI-BA-HPLC-UV method’s precision and accuracy were calculated by comparing five replicates of each QC sample to the matrix-matched standard curve. Precision was calculated as the relative standard deviation (RSD %) of the mean concentrations obtained from one day (intra-day validation) and on five separate days (inter-day validation), while the percentage of relative error (RE%) of the mean found concentrations for intra- and inter-day validation was used to describe the accuracy as follows:
RE (%) = [(mean measured concentration-spiked concentration)/spiked concentration] × 100
3. Results
HPLC-UV methods are the most commonly used and widely popular techniques. The availability of the instruments, analytical procedure simplicity and low operation cost make this technique attractive. Therefore, this study describes a combination of SPE with HPLC-UV for the automated extraction and pre-concentration as well as quantification of carbamazepine at very low concentration levels in bio-fluid samples.
3.1. Chromatographic Conditions
The selectivity of the on-line extraction protocol could be explained through exploiting the advantage of the PC-ODS-pre-column dual characteristics for SE-LC and RP-LC modes. Protein matrix contents are removed easily from the packing materials when the bio-fluid sample is passed through the PC-ODS-pre-column via SE-LC, whereas carbamazepine can be kept on the same pre-column by means of RP-LC mode. Therefore, MI should be able to manage carbamazepine retention behavior while also eliminating serum macromolecular proteins. Furthermore, MI should be well-suited with proteinous matrices to prevent protein accumulation and subsequent clogging of the PC-ODS-pre-column inlet. For loading and trapping of carbamazepine on the PC-ODS-pre-column, PBS (pH 7.4) was the exemplary mobile phase (MI). While carbamazepine was still retained by RP-LC mode of PC-ODS-pre-column, most of the serum matrix proteins were extracted by SE-LC mode with the aid of MI. Then, carbamazepine was moved to a (ZORBAX Eclipse XDB-C18) analytical column via a switching valve. In this stage, the MII back-flushed the trapped carbamazepine from the PC-ODS-pre-column into the analytical column. To prevent interferences caused by the endogenous serum components, MII content was adjusted to obtain sufficient selectivity of the BF-DSI-BA-HPLC-UV methodology to separate carbamazepine from the co-eluted components. The effect of methanol content as the main factor affecting the retention behavior of carbamazepine was investigated, and a mixture of methanol and distilled deionized water (50:50, v/v) was applied as an optimal condition to separate carbamazepine from matrix components of serum samples.
3.2. Switching Valve Timing
Using a short PC-ODS-pre-column (2 cm × 0.46 cm i.d.), online SPE of proteins from serum samples could be achieved rapidly and effectively. The particle size of the packed ODS sorbents was approximately 20 µm. To clean up and trap carbamazepine, the PC-ODS-pre-column must be flushed with solvent-free mobile phases. As a result, a sufficient volume of untreated serum samples can be injected onto this pre-column without causing denaturation and protein precipitation. It was found that two minutes of MI flowing at 1 mL/min were adequate to extract macromolecular serum proteins effectively and trap as well as enrich carbamazepine onto PC-pre-column’s head in the extraction set-up. Furthermore, rapid transferring time (the duration of time required to transfer the clean fraction containing carbamazepine from the PC-ODS-pre-column to the analytical column) with MII is necessary to thoroughly transfer the analyte to the quantification position and prevent further peak broadening and dispersion. In less than a minute, carbamazepine was completely eluted from the PC-ODS-pre-column to the analytical column in BF mode while using 50% methanol in MII. For complete extraction, trapping and pre-concentration, the configuration of the system manifold is maintained in its original position for two minutes next to sample injection. After that, the manifold was rotated to the quantification set-up for a period of one minute before returning to the extraction set-up to prepare the PC-ODS-pre-column for the next sample rum.
3.3. Back-Flush Elution Mode
To separate the analyte from the complex serum matrix and reduce the interferences of the endogenous components, it is important to transfer the clear fraction containing carbamazepine from the extraction step to the quantification position. Thus, the clean fraction, following online SPE, must be compatible with the stated RP-HPLC post-separation at the quantification position. This is a critical requirement for the current approach. Since PC-pre-column is packed with ODS-silica materials, SPE can also be carried out in the RP mode. As a result, BF elution mode by MII is an effective way to overcome the long flushing time required by on-through elution mode for direct chromatographic measurement of carbamazepine in untreated human serum. In all cases, carbamazepine was trapped on the PC-ODS-pre-column’s head, while the interfering matrix proteins were eliminated by elution with MI (Figure 2). The analyte was then online transferred in a BF mode to the analytical ODS column by MII for the final separation and measurement.
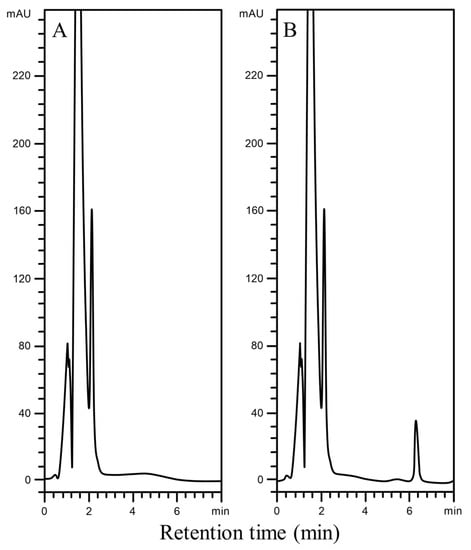
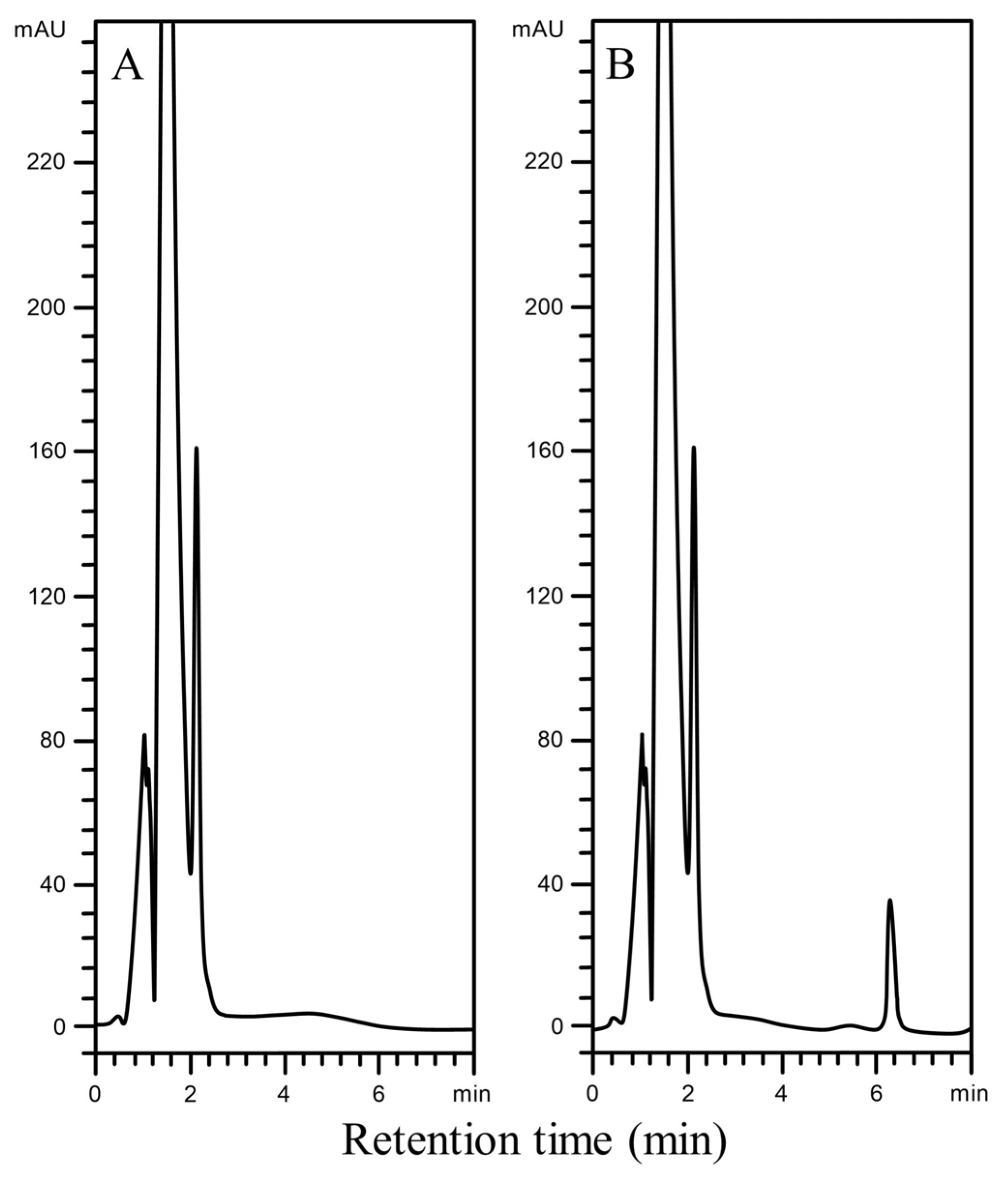
Figure 2.
Representative chromatograms for the determination of carbamazepine in serum by BF-DSI-BA-HPLC-UV method: (A) drug-free human serum; (B) drug-free human serum spiked with 200 ng/mL carbamazepine.
3.4. Breakthrough Study and Loading Capacity of PC-ODS-Pre-Column
For the breakthrough study, it was important to keep in mind that for PC-ODS-pre-column to function at its best performance, carbamazepine retention time should be high during SE-LC for macromolecular serum proteins and low for the BF elution of analyte to the quantification step. This was accomplished by the preparation of PC-pre-column from silica sorbents (ODS) with RP characteristics necessary to successfully trap the analyte. The breakthrough study was conducted by loading QC samples containing carbamazepine at 50 and 8000 ng/mL and more MI was passed before rotating the six-port switching valve to the quantification position. After flushing the PC-ODS-pre-column with MI for 10 min, the recovery of carbamazepine was varied from 86.14 to 97.82%, which sufficiently demonstrated that the analyte was considerably trapped during the on-line chromatographic extraction of macromolecular serum proteins.
The loading capacity of the PC-ODS-pre-column was examined by injecting different volumes of QC sample containing carbamazepine at 8000 ng/mL and flushing them with 2 mL of MI. Peak areas were shown to be proportional to the total amount of carbamazepine present in volumes ranging from 100 to 200 µL, with recovery percentages exceeding 97%. These results indicate that the loading capacity of the PC-ODS-pre-column is sufficient for complete trapping of carbamazepine at concentrations greater than 10 μg/mL. However, an aliquot of 100-μL was adequate for more carbamazepine samples to be analyzed using the same PC-ODS-pre-column.
3.5. PC-ODS-Pre-Column Lifetime
The efficiency of the PC-ODS-pre-column to cleanup proteins and trap carbamazepine from untreated serum samples was evaluated as a function of the injected volumes. It was revealed that the PC-ODS-column could be loaded with adequate extraction performance by at least 250 times with aliquot of 100-µL serum sample. Increasing the volumes above this amount gradually increased the column-back pressure until certain levels were reached at which an inadequate extraction recovery was obtained. Therefore, replacing the PC-ODS-pre-column regularly is advised whenever excessive back pressure is observed.
3.6. Method Validation
3.6.1. Linearity, Detection and Quantification Limits
In order to account for the necessary practical range in accordance with the therapeutic concentrations of carbamazepine after administration of tablet dosage form, the calibration standards were prepared by spiking drug-free human serum samples with carbamazepine concentrations ranged from 50 to 10,000 ng/mL. Plotting the measured peak areas (N = 5) by the BF-DSI-BA-HPLC-UV technique against the corresponding carbamazepine concentrations enabled the construction of a seven-point calibration curve, and it was linear in the necessary concentration range (50–10,000 ng/mL) for carbamazepine with a correlation coefficient (r2) of greater than 0.999 from a 100-µL serum aliquot. The lowest level of carbamazepine that can be clearly recognized above the baseline signal is known as the detection limit (LOD), which is stated to be 3 times the signal-to-noise ratio and was found to be 12 ng/mL, while the quantification limit (LOQ), which is roughly 10 times the signal-to-noise ratio and represents the lowest concentration that can be quantitatively measured with accuracy and precision (within 20%), was found to be 42 ng/mL. Table 1 shows the characteristic parameters of the carbamazepine standard curve.

Table 1.
Characteristic parameters for the regression equation of the carbamazepine in human serum by the BF-DSI-BA-HPLC-UV method.
3.6.2. Recovery, Precision, and Accuracy
The recovery for the proposed BF-DSI-BA-HPLC-UV methodology was evaluated by comparing the measured concentrations of carbamazepine spiked in drug-free human samples to the corresponding nominal concentrations. The linear regression equation of the matrix matched calibration standards was used to calculate the analyte concentration. With respect to the automated SPE performance, the developed BF-DSI-BA-HPLC-UV technique delivered high extraction recovery ranging from 86.14 to 97.82%. In order to determine the precision and accuracy, five duplicates of spiked healthy serum samples were examined within one day and over the course of five days to calculate the intra-day and inter-day validation results, respectively. The intra-day precision and accuracy were adequate, with RSD (%) being in the range 7.98–3.32% and with mean RE (%) ranging from −13.86 to −2.18%, while the inter-day validation results of RSD (%) and RE (%) were ranged from 8.55 to 3.97% and −14.45 to −2.98%, respectively. Table 2 provides a summary of the method validation results.

Table 2.
Precision and accuracy validation of the BF-DSI-BA-HPLC-UV method for analysis of carbamazepine in human serum.
3.6.3. Selectivity and Specificity
By comparing five different samples of blank serum and blank serum spiked with carbamazepine (8000 ng/mL), the selectivity of the current method was evaluated. The high selectivity of the BF-DSI-BA-HPLC-UV methodology is demonstrated by the lack of endogenous matrix interferences at or close to the carbamazepine detected peak (Figure 2A,B).
3.7. Stability Studies
To guarantee the validity and reliability of the BF-DSI-BA-HPLC-UV method’s results, it was crucial to test the biological sample’s stability while handling and storing. Stability studies were conducted on two sets of QC samples at low (50 ng/mL) and high (8000 ng/mL) concentration levels of carbamazepine by exposing both of them to three freezing and thawing cycles, long-term stability for four weeks at −20 °C, and short-term stability for 8 h at room temperature and twenty-four hours at 5 °C. The concentration of carbamazepine after each storage period was correlated with the same spiked serum concentration that was recently prepared. The mean peak areas of the freshly prepared serum samples and those after one, two, and three freeze-thaw cycles did not chromatographically change. Furthermore, the serum samples did not exhibit any discernible chromatographic alterations while kept in the refrigerator (5 °C) and at−20 °C for 24 h and 4 weeks, respectively. Processed serum samples exhibited adequate stability at room temperature after standing for at least 8 h.
3.8. Robustness
Experimental parameters such as the methanol content and flow rates of MII were deliberately changed and the resolution between carbamazepine and the co-eluted endogenous components from serum matrix was assessed to ascertain the robustness of BF-DSI-BA-HPLC-UV system manifold. The chromatographic resolution and retention times were unaffected by a ±2% change in methanol strength and flow rate, illustrating adequate method robustness.
3.9. Features of the PC-ODS-Pre-Column and BF-DSI-BA-HPLC-UV Methodology Regarding Their Performance, Economic Perspective and Green Evaluation
The procedure of sample preparation has the biggest influence on how well the quantitative analysis turned out. In recent years, it has become discouraged to use organic solvents and other ingredients that are harmful to both the environment and human health. While maintaining the effectiveness of the analytical process, numerous attempts have also been made to decrease solvent consumption and run time. As a result, researchers used to pay more attention to the online SPE technique because it raised the quality of the results; improved operational efficiency, time-saving and cost reduction; and increased the instrument’s productivity. Short columns for on-line SPE packed with conventional silica-bonded RP materials were made by some researchers and employed to clean up endogenous proteins in biological samples while assessing various drugs. These columns, unfortunately, displayed certain limits caused by the precipitation and denaturation of some matrix’s proteins on the solid supports, which result in clogging and an increase in back pressure. Therefore, conventional RP columns should be replaced often while loading fewer samples. Contrarily, pre-columns such as the PC-ODS-pre-column were designed to address this issue by losing the capacity to adsorb matrix’s proteins while showing unique RP properties to capture and retain relatively small molecular analytes. The most notable characteristic of the PC-ODS-pre-column is the immobilized protein layers, which are present at the outer surfaces of the RP (ODS) silica sorbents. These layers allowed macromolecular proteins to be eluted outside the column by ZE-LC while allowing the internee of small molecular analytes into the porous spherical particles of the ODS by RP-LC with the assistance of MI. Additionally, the PC-ODS-pre-column’s loading capacity is sufficient to tolerate large serum volume to permit the trapping and enrichment of carbamazepine completely. Given the effectiveness of HPLC-UV, an attempt was made to develop a simple, accurate, precise, rapid, and cost-effective BF-DSI-BA-HPLC-UV technique for measuring carbamazepine in human serum. The benefits of using ODS-PC-pre-column lie in the automation as well as an environmentally friendly extraction protocol. Regarding the financial side, the PC-ODS-pre-column is made manually in our laboratory using a simple and affordable technique [32], which reduces the overall cost and duration of the entire procedure. The online extraction protocol was conducted by incorporating an extra pump, a small pre-column, and a high pressure switching valve into a conventional HPLC instrumentation. Regarding the BF-DSI-BA-HPLC-UV methodology, it was shown to be superior to the traditional pre-treatment procedures because it resulted in excellent recoveries (86.14–97.82%) with high levels of accuracy and precision. Table 3 lists the calibration ranges, LOQs, sample matrices and sample preparation procedures for various published HPLC techniques used for carbamazepine analysis. As shown from this table, the proposed BF-DSI-BA-HPLC-UV method was more sensitive than the other HPLC-UV [4,5,6,7,8,10,11,12,14,17,18]. Although two HPLC-UV methods [13,16] showed higher sensitivity than the BF-DSI-BA-HPLC-UV technique, sample preparation involving many steps prior to analysis, such as LLE, evaporation and reconstitution, could affect the system performance in sample throughput and introduce errors, in addition to the use of more hazardous organic solvents. Moreover, the BF-DSI-BA-HPLC-UV has comparable results with most of the HPLC/MS techniques [20,21,22,23]. Internal standards, which were additional steps required by traditional HPLC procedures are no longer necessary in the current study because the extraction process is being automated. In addition, it is more simple to employ an HPLC with a low-cost UV detection system rather than expensive instruments such as a mass spectrophotometer to monitor carbamazepine in human serum. Finally, the fact that PBS (MI) is a safe and green solvent for automated SPE is the main reason why the BF-DSI-BA-HPLC-UV method is considered as a green extraction approach. Thus, BF-DSI-BA-HPLC-UV technique was beneficial for TDM because it allowed for the determination of low levels of carbamazepine concentrations in human serum.

Table 3.
Comparison of the calibration ranges, LOQs, sample matrices and sample preparation procedures with other chromatographic methods.
4. Conclusions
This current research established an automated bio-analytical HPLC-UV method for the routine determination of carbamazepine in human serum samples. The use of a PC-ODS-pre-column, which is produced manually in our lab, is essential to the efficiency of this on-line SPE extraction protocol. Via on-line elution with MI, PC-ODS pre-column aids in cleaning the endogenous protein molecules from serum matrix while trapping carbamazepine (a small molecule) within its inner surface, which is beneficial for the PC-pre-column selectivity. This straightforward automated approach reduces the need for any step prior to injection, which, in turn, leads to considerable improvements throughout the entire process. Firstly, it does away with the need for sample preparation with potentially hazardous organic solvents, making this process both cost-effective and ecologically safe. Secondly, there is no need to apply an internal standard due to automated extraction and the absence of interference between the analyte peak and the peaks of the endogenous matrix components. Most importantly, there is no need to analyze biological samples using costly techniques such as LC/MS, especially for routine analysis and therapeutic drug monitoring, since the present method can be used to determine carbamazepine in biological samples at its therapeutic concentration levels. That is because the recovery of carbamazepine ranged from 86.14 to 97.82%; this approach was shown to be reliable, sensitive, selective, accurate and durable for measuring other pharmaceuticals at their therapeutic concentrations for further clinical and pharmacokinetic studies.
Author Contributions
H.S.: sample analysis, investigation, validation and writing—original draft. H.E. and M.M.: English editing, investigation and validation. A.A. and Y.A.: methodology, sample analysis and results interpretation. G.H. and S.E.: conceptualization and supervision. A.S.: investigation, results interpretation, validation and writing—original draft. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Not applicable.
Data Availability Statement
Not applicable.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Olling, M.; Mensinga, T.T.; Barends, D.M.; Groen, C.; Lake, O.A.; Meulenbent, J. Bioavailability of Carbamazepine from Four Different Products and the Occurrence of Side Effects. Biopharm. Drug Dispos. 1999, 20, 19–28. [Google Scholar] [CrossRef]
- Prakash, S.; Rathore, C.; Rana, K.; Patel, H. Antiepileptic Drugs and Serotonin Syndrome- A Systematic Review of Case Series and Case Reports. Seizure 2021, 91, 117–131. [Google Scholar] [CrossRef] [PubMed]
- Datar, P.A. Quantitative Bioanalytical and Analytical Method Development of Dibenzazepine Derivative, Carbamazepine: A Review. J. Pharm. Anal. 2015, 5, 213–222. [Google Scholar] [CrossRef] [PubMed]
- Queiroz, M.E.C.; Carrilho, E.; Carvalho, D.; Lanças, F.M. Comparison of High-Resolution Gas Chromatography and High-Performance Liquid Chromatography for Simultaneous Determination of Lamotrigine and Carbamazepine in Plasma. Chromatographia 2001, 53, 485–489. [Google Scholar] [CrossRef]
- Yoshida, T.; Imai, K.; Motohashi, S.; Hamano, S.I.; Sato, M. Simultaneous Determination of Zonisamide, Carbamazepine and Carbamazepine-10,11-Epoxide in Infant Serum by High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 2006, 41, 1386–1390. [Google Scholar] [CrossRef]
- Fedorova, G.A.; Baram, G.I.; Grachev, M.A.; Aleksandrov, Y.A.; Tyuleneva, G.N.; Starodubtsev, A.V. Application of Micro-Column HPLC to the Determination of Phenobarbital and Carbamazepine in Human Blood Serum. Chromatographia 2001, 53, 495–497. [Google Scholar] [CrossRef]
- Ezzeldin, E.; Shahat, A.A.; Basudan, O.A. Development and Validation of an HPLC Method for the Determination of Carbamazepine in Human Plasma. Life Sci. J. 2013, 10, 2159–2163. [Google Scholar]
- Levert, H.; Odou, P.; Robert, H. Simultaneous Determination of Four Antiepileptic Drugs in Serum by High-Performance Liquid Chromatography. Biomed. Chromatogr. 2002, 24, 19–24. [Google Scholar] [CrossRef]
- Tuchila, C.; Baconi, D.L.; Pirvu, C.D.; Balalau, D.O.; Vlasceanu, A.M.; Stan, M.; Balalau, C. Therapeutic Drug Monitoring and Methods of Quantitation for Carbamazepine. J. Mind Med. Sci. 2017, 4, 101–114. [Google Scholar] [CrossRef]
- Ateş, Z.; Özden, T.; Özilhan, S.; Toptan, S. Simultaneous Determination of Carbamazepine and its Active Metabolite Carbamazepine-10,11-Epoxide in Human Plasma by UPLC. Chromatographia 2007, 66, 123–127. [Google Scholar] [CrossRef]
- Behbahani, M.; Najafi, F.; Bagheri, S.; Bojdi, M.; Salarian, M.; Bagheri, A. Application of Surfactant Assisted Dispersive Liquid–Liquid Microextraction as an Efficient Sample Treatment Technique for Preconcentration and Trace Detection of Zonisamide and Carbamazepine in Urine and Plasma Samples. J. Chromatogr. A 2013, 1308, 25–31. [Google Scholar] [CrossRef] [PubMed]
- Greiner-sosanko, E.; Lower, D.R.; Virji, M.A.; Krasowski, M.D. Simultaneous Determination of Lamotrigine, Zonisamide, and Carbamazepine in Human Plasma by High-Performance Liquid Chromatography. Biomed. Chromatogr. 2007, 228, 225–228. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.K.; Ban, E.; Woo, J.S.; Kim, C. Analysis of Carbamazepine and its Active Metabolite, Carbamazepine-10, 11-Epoxide, in Human Plasma Using High-Performance Liquid Chromatography. Anal. Bioanal. Chem. 2006, 386, 1931–1936. [Google Scholar] [CrossRef] [PubMed]
- Patil, K.M.; Bodhankar, S.L. Simultaneous Determination of Lamotrigine, Phenobarbitone, Carbamazepine and Phenytoin in Human Serum by High-Performance Liquid Chromatography. J. Pharm. Biomed. Anal. 2005, 39, 181–186. [Google Scholar] [CrossRef]
- Zhou, Z.; Jiang, J.-Q. Simultaneous Detection of Sulfamethoxazole, Diclofenac, Carbamazepine, and Bezafibrate by Solid Phase Extraction and High Performance Liquid Chromatography with Diode Array Detection. J. Appl. Spectrosc. 2014, 81, 273–278. [Google Scholar] [CrossRef]
- Shimoyama, R.; Ohkubo, T.; Sugawara, K. Monitoring of Carbamazepine and Carbamazepine 10, 11-Epoxide in Breast Milk and Plasma by High-Performance Liquid Chromatography. Ann. Clin. Biochem. 2000, 37, 210–215. [Google Scholar] [CrossRef]
- Vermeij, T.A.C.; Edelbroek, P.M. Robust Isocratic High Performance Liquid Chromatographic Method for Simultaneous Determination of Seven Antiepileptic Drugs Including Lamotrigine, Oxcarbazepine and Zonisamide in Serum after Solid-Phase Extraction. J. Chromatogr. B 2007, 857, 40–46. [Google Scholar] [CrossRef]
- Mandrioli, R.; Albani, F.; Casamenti, G.; Sabbioni, C.; Raggi, M.A. Simultaneous High-Performance Liquid Chromatography Determination of Carbamazepine and Five of its Metabolites in Plasma of Epileptic Patients. J. Chromatogr. B 2001, 762, 109–116. [Google Scholar] [CrossRef]
- Subramanian, M.; Birnbaum, A.K.; Remmel, R.P. High-Speed Simultaneous Determination of Nine Antiepileptic Drugs Using Liquid Chromatography–Mass Spectrometry. Ther. Drug Monit. 2008, 30, 347–356. [Google Scholar] [CrossRef]
- Yin, L.; Wang, T.; Zhang, Y.; Zhao, X.; Yang, Y.; Gu, J. Simultaneous Determination of Ten Antiepileptic Drugs in Human Plasma by Liquid Chromatography and Tandem Mass Spectrometry with Positive/Negative Ion-Switching Electrospray Ionization and its Application in Therapeutic Drug Monitoring. J. Sep. Sci. 2016, 39, 964–972. [Google Scholar] [CrossRef]
- Rodina, T.A.; Mel’nikov, E.S.; Sokolov, A.V.; Prokof’ev, A.B.; Arkhipov, V.V.; Aksenov, A.A.; Pozdnyakov, D.L. Rapid HPLC-MS/MS Determination of Carbamazepine and Carbamazepine-10,11-Epoxide. Pharm. Chem. J. 2016, 50, 419–423. [Google Scholar] [CrossRef]
- Breton, H.; Cociglio, M.; Bressolle, F.; Peyriere, H.; Blayac, J.P.; Hillaire-Buys, D. Liquid Chromatography–Electrospray Mass Spectrometry Determination of Carbamazepine, Oxcarbazepine and Eight of Their Metabolites in Human Plasma. J. Chromatogr. B 2005, 828, 80–90. [Google Scholar] [CrossRef] [PubMed]
- Taibon, J.; Schmid, R.; Lucha, S.; Pongratz, S.; Tarasov, K.; Seger, C.; Timm, C.; Thiele, R.; Herlan, J.M.; Kobold, U. An LC-MS/MS Based Candidate Reference Method for the Quantification of Carbamazepine in Human Serum. Clin. Chim. Acta 2017, 472, 35–40. [Google Scholar] [CrossRef] [PubMed]
- Lajeunesse, A.; Vernouillet, G.; Eullaffroy, P.; Gagnon, C.; Sauvé, S. Determination of Carbamazepine in Aquatic Organisms by Liquid–Liquid Extraction and Liquid Chromatography-Tandem Mass Spectrometry. J. Environ. Monit. 2009, 11, 723–725. [Google Scholar] [CrossRef]
- Rooyen, G.F.V.; Badenhorst, D.; Swart, K.J.; Hundt, H.K.L.; Scanes, T.; Hundt, A.F. Determination of Carbamazepine and Carbamazepine 10,11-Epoxide in Human Plasma by Tandem Liquid Chromatography–Mass Spectrometry with Electrospray Ionisation. J. Chromatogr. B 2002, 769, 1–7. [Google Scholar] [CrossRef]
- Qu, L.; Fan, Y.; Wang, W.; Ma, K.; Zheng Yin, Z. Development, Validation and Clinical Application of an Online-SPE-LC-HRMS/MS for Simultaneous Quantification of Phenobarbital, Phenytoin, Carbamazepine, and its Active Metabolite Carbamazepine 10,11-Epoxide. Talanta 2016, 158, 77–88. [Google Scholar] [CrossRef]
- Miao, X.; Metcalfe, C.D. Determination of Carbamazepine and its Metabolites in Aqueous Samples Using Liquid Chromatography-Electrospray Tandem Mass Spectrometry. Anal. Chem. 2003, 75, 3731–3738. [Google Scholar] [CrossRef]
- Martin, P.D.; Jones, G.R.; Stringer, F.; Wilson, I.D. Comparison of Normal and Reversed-Phase Solid Phase Extraction Methods for Extraction of β-Blockers from Plasma Using Molecularly Imprinted Polymers. Analyst 2003, 128, 345–350. [Google Scholar] [CrossRef]
- Liang, Y.; Zhou, I. Recent Advances of Online Coupling of Sample Preparation Techniques with Ultrahigh Performance Liquid Chromatography and Supercritical Fluid Chromatography. J. Sep. Sci. 2019, 42, 226–242. [Google Scholar] [CrossRef]
- Emara, S.; Masujima, T.; Zarad, W.; Kamal, M.; Fouad, M.; El-bagary, R. An Eco-Friendly Direct Injection HPLC Method for Methyldopa Determination in Serum by Mixed-Mode Chromatography Using a Single Protein-Coated Column. J. Chromatogr. Sci. 2015, 53, 1353–1360. [Google Scholar] [CrossRef]
- Emara, S.; Saleh, G.; Fathy, M.; Bakr, M.A. Chromatographic Assay and Pharmacokinetic Studies of Propofol in Human Serum. Biomed. Chromatogr. 1999, 13, 299–303. [Google Scholar] [CrossRef]
- Emara, S.; El-Gindy, A.; Mesbah, M.K.; Hadad, G.M. Direct Injection Liquid Chromatographic Technique for Simultaneous Determination of Two Antihistaminic Drugs and Their Main Metabolites in Serum. J. AOAC Int. 2007, 90, 384–390. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).