Study on Selective Adsorption Behavior and Mechanism of Quartz and Magnesite with a New Biodegradable Collector
Abstract
:1. Introduction
2. Materials and Methods
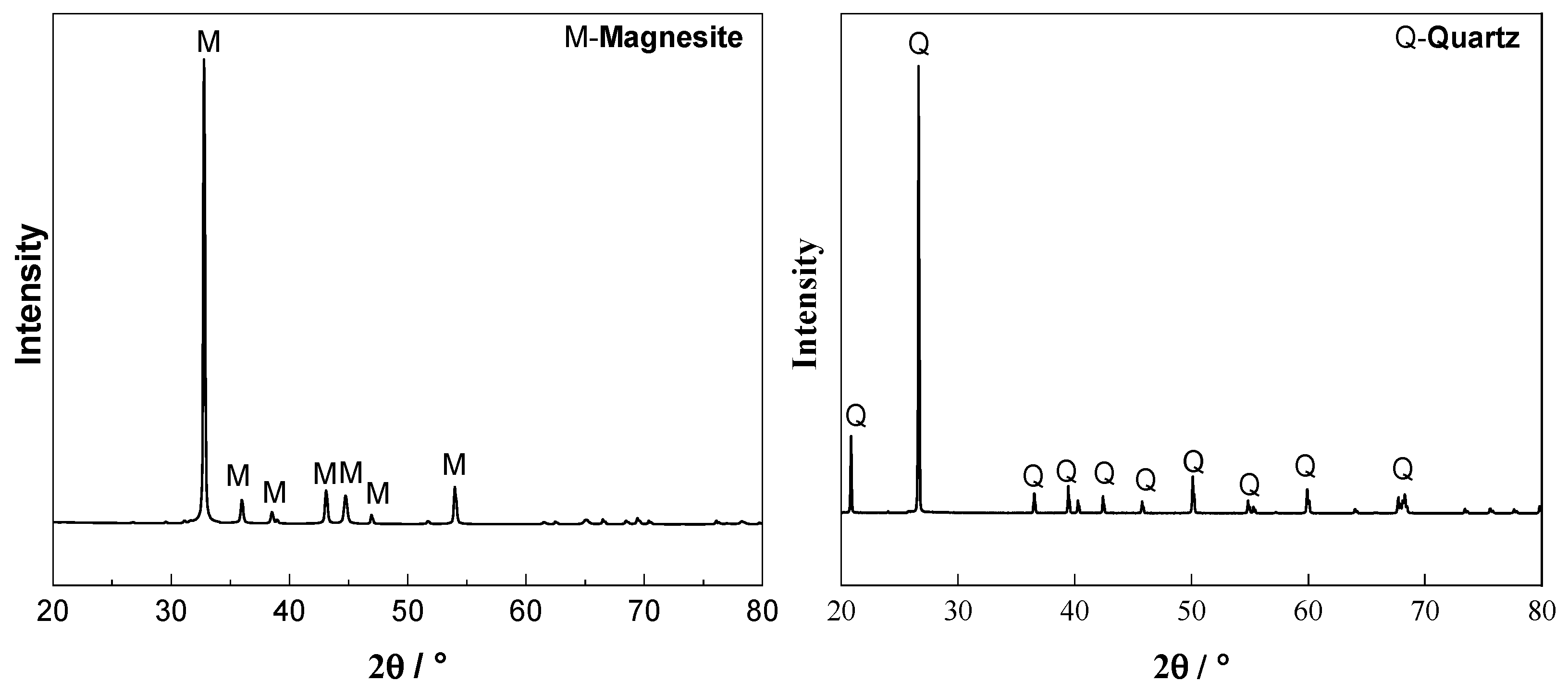
2.1. Materials
2.2. Methods
2.2.1. Flotation Experiments
2.2.2. Contact Angle Measurements
2.2.3. AFM Analysis
2.2.4. FTIR Analysis
2.2.5. XPS Analysis
3. Results and Discussion
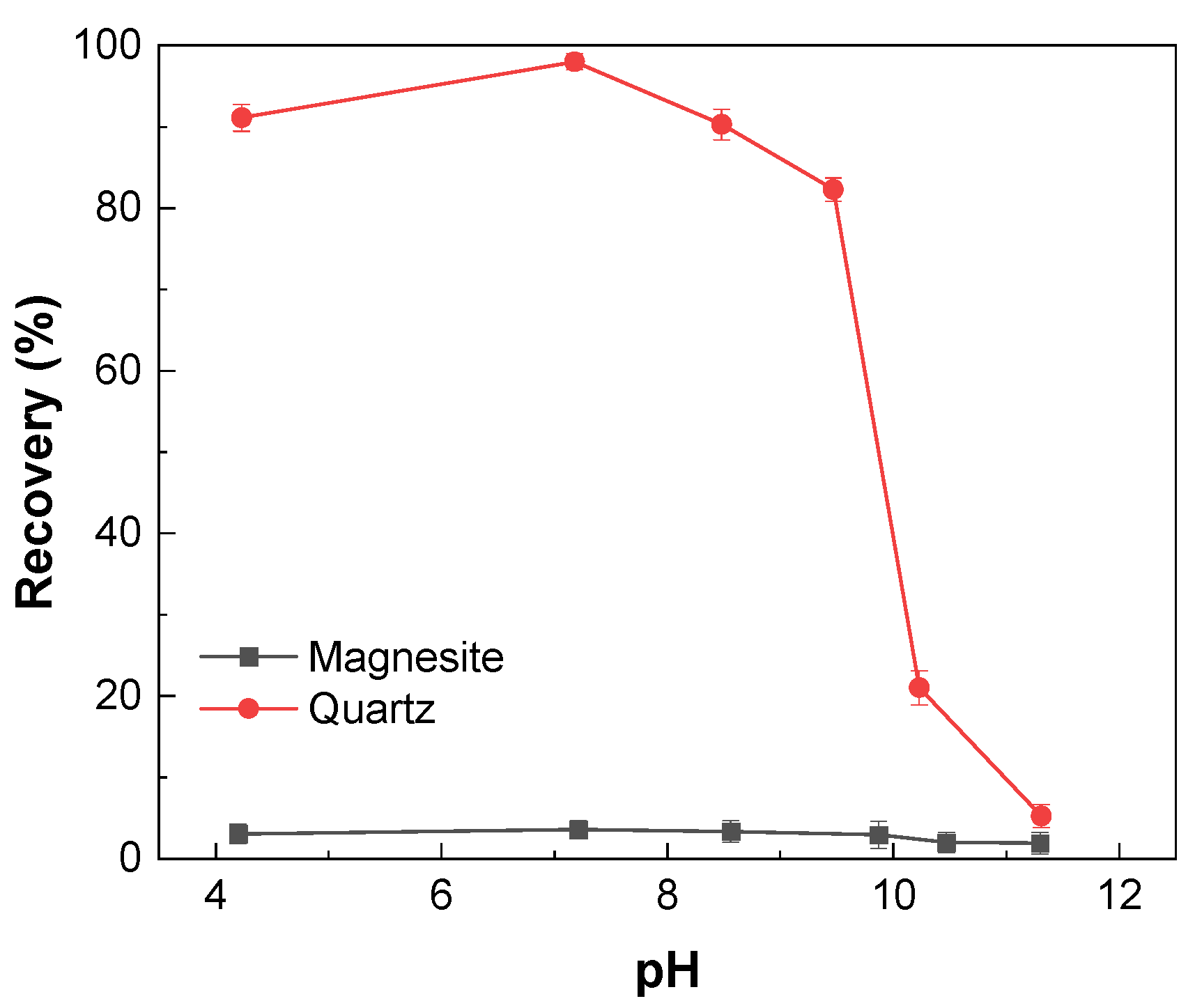
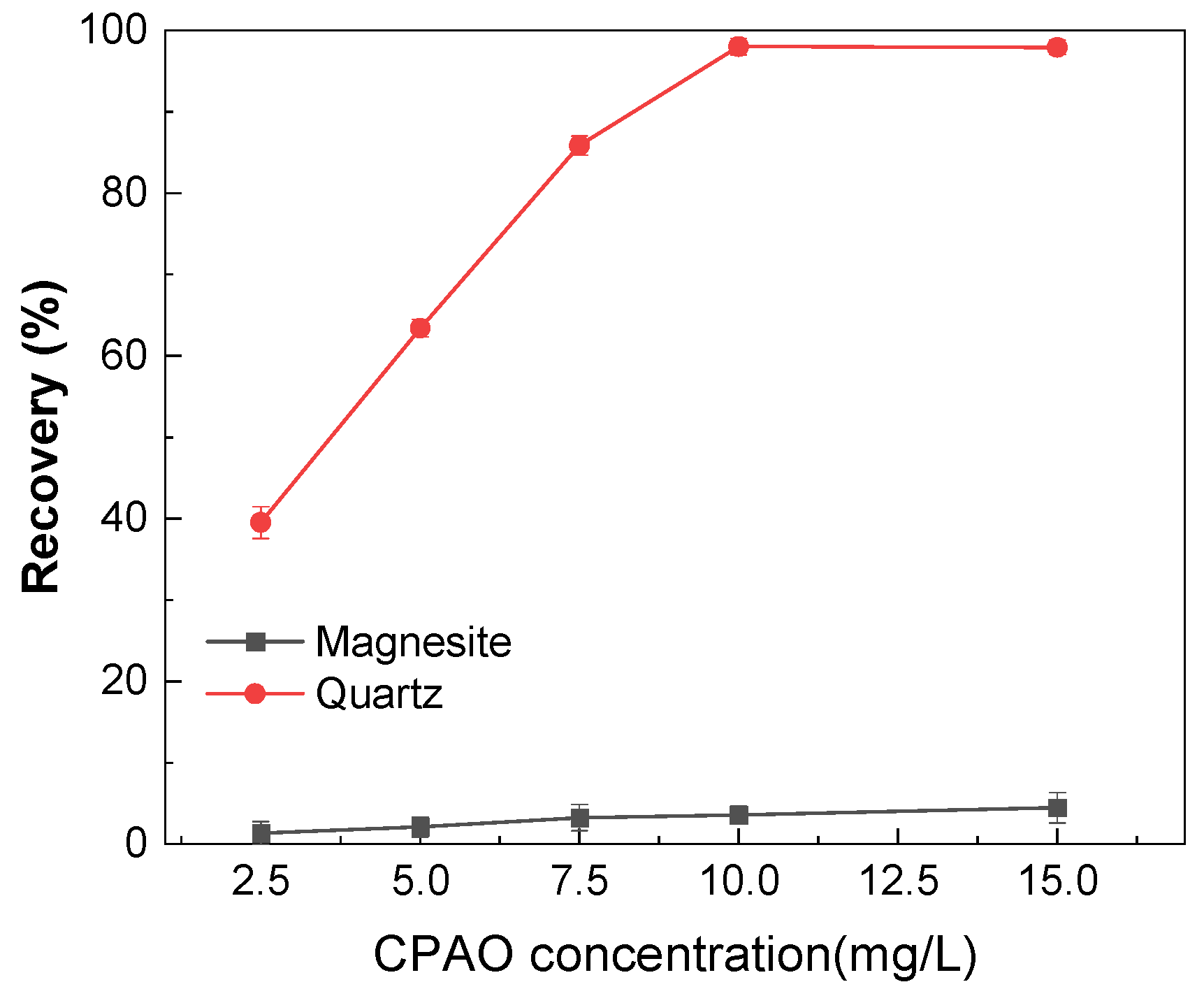
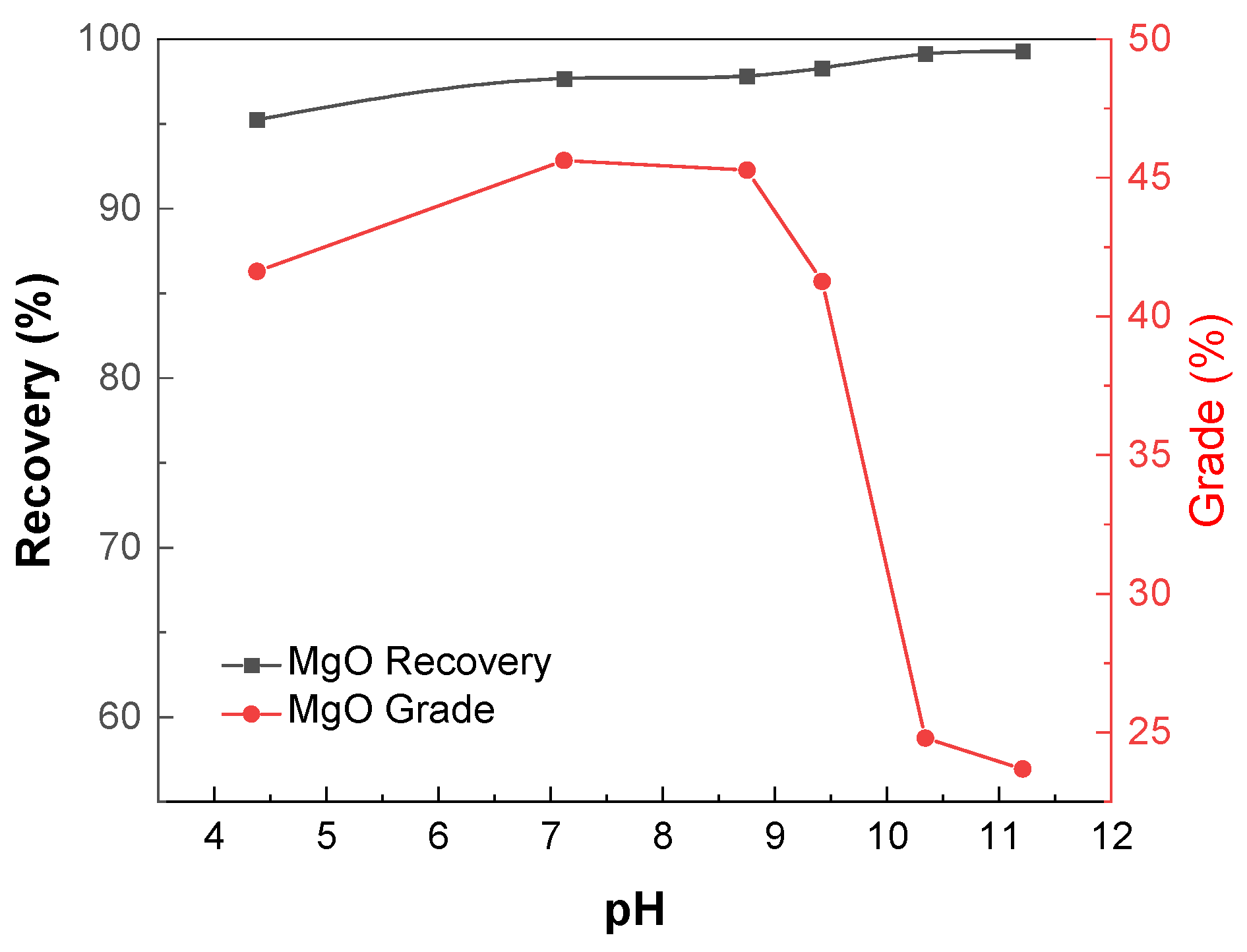
3.1. Micro-Flotation Experiments of Single Minerals
3.2. Micro-Flotation Experiments of Artificially Mixed Minerals
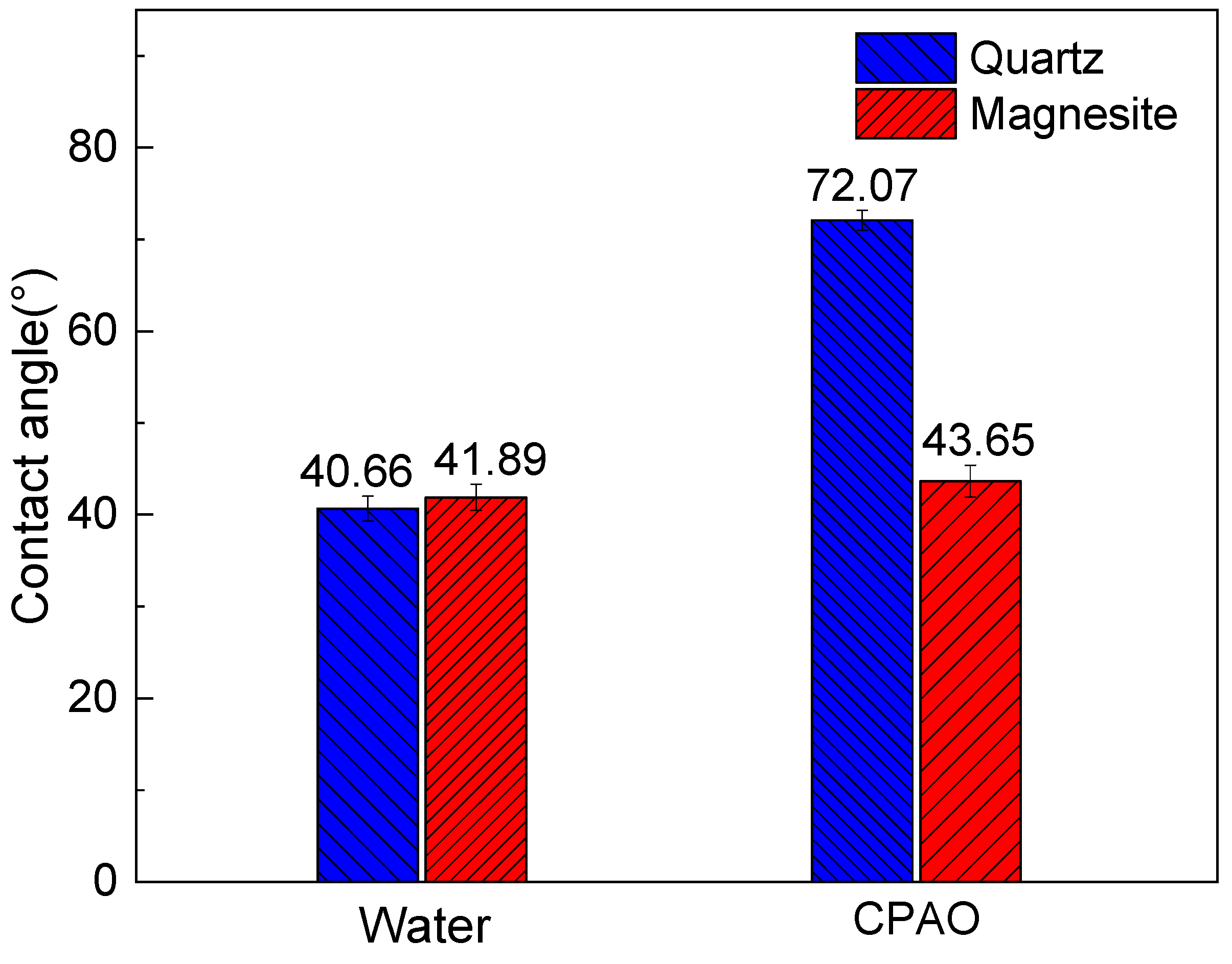
3.3. Contact Angle Measurements
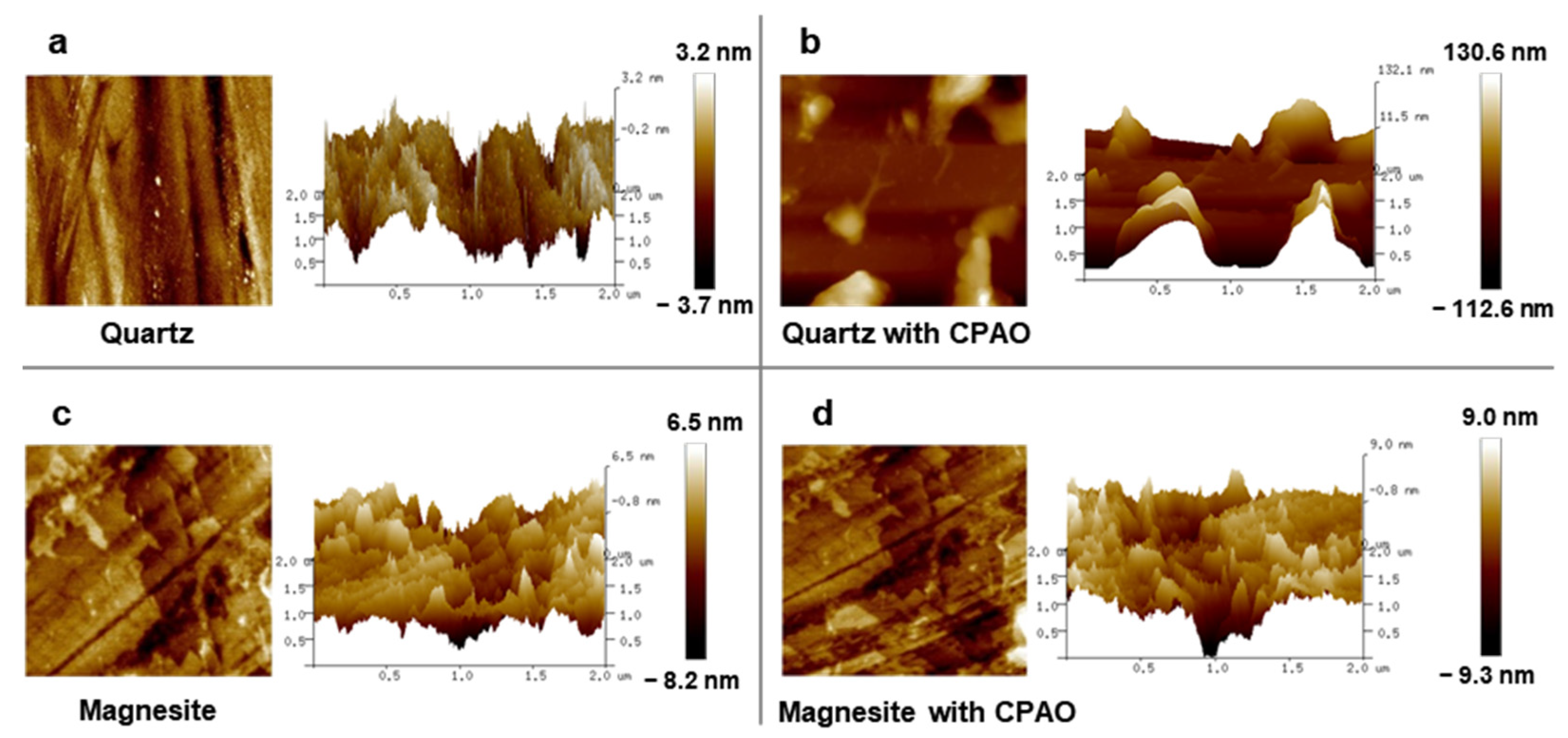
3.4. AFM Analysis
3.5. FTIR Analysis
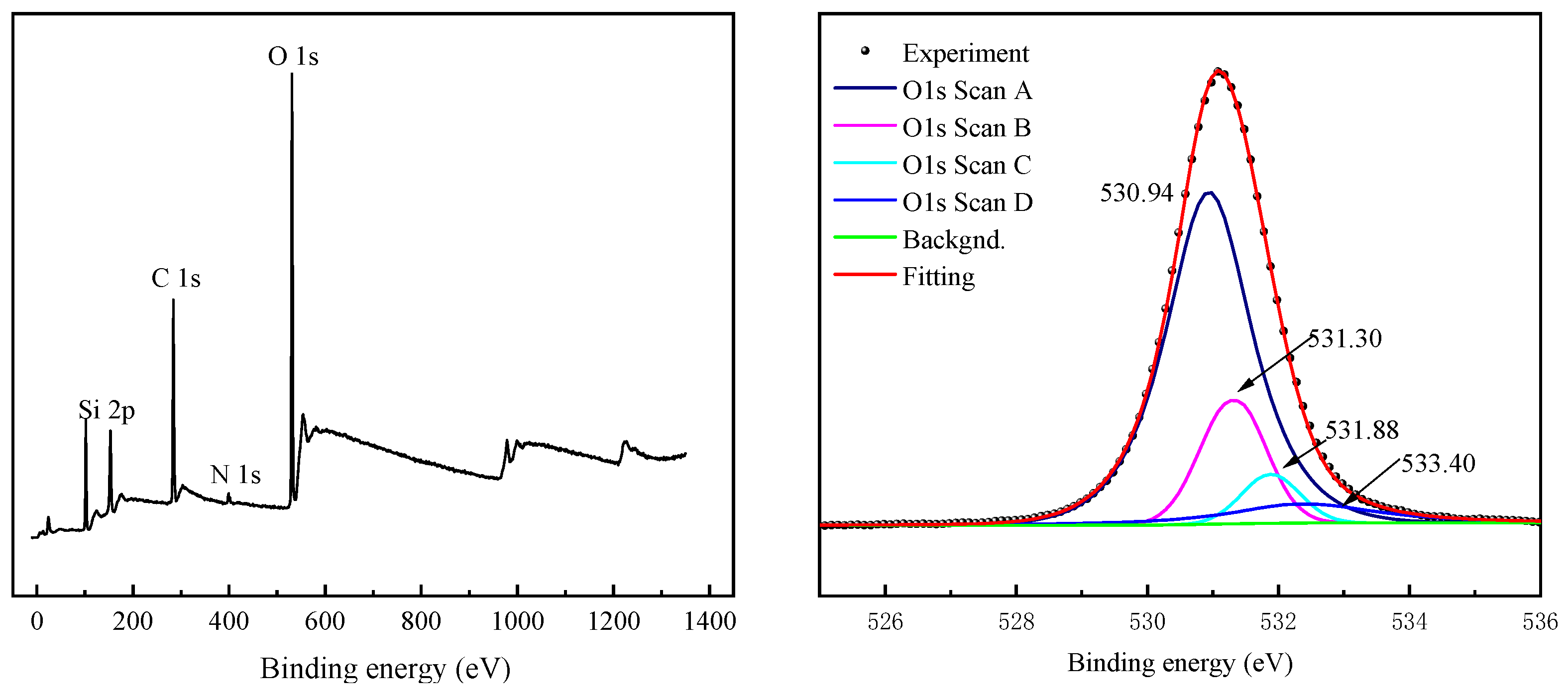
3.6. XPS Analysis
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhu, Y.; Yang, L.; Hu, X.; Zhang, X.; Zheng, G. Flotation separation of quartz from magnesite using carboxymethyl cellulose as depressant. Trans. Nonferrous Met. Soc. China 2022, 32, 1623–1637. [Google Scholar] [CrossRef]
- Prasad, S.V.S.; Prasad, S.B.; Verma, K.; Mishra, R.K.; Kumar, V.; Singh, S. The role and significance of Magnesium in modern day research—A review. J. Magnes. Alloy 2022, 10, 1–61. [Google Scholar] [CrossRef]
- Liu, W.; Liu, W.; Dai, S.; Yang, T.; Li, Z.; Fang, P. Enhancing the purity of magnesite ore powder using an ethanolamine-based collector: Insights from experiment and theory. J. Mol. Liq. 2018, 268, 215–222. [Google Scholar] [CrossRef]
- Chen, X.; Liu, W.; Peng, X.; Sun, W. Effect and mechanism of depressant amino trimethylene phosphonic acid on flotation separation of magnesite and dolomite. Conserv. Util. Miner. Resour. 2022, 42, 91–99. [Google Scholar] [CrossRef]
- Yang, Y.; Xiong, X.; Chen, J.; Peng, X.; Chen, D.; Pan, F. Research advances in magnesium and magnesium alloys worldwide in 2020. J. Magnes. Alloy 2021, 9, 705–747. [Google Scholar] [CrossRef]
- Kalaitzidou, K.; Pagona, Ε.; Zouboulis, A.; Mitrakas, M. Exploitation of the fine rejected run of mine (ROM 0–4 mm) material to produce refractories in combination with the mining by-products of magnesite mine. Mater. Chem. Phys. 2022, 292, 126743. [Google Scholar] [CrossRef]
- Mao, Y.; Liu, W.; Chen, X.; Wang, Z.; Liu, W.; Sun, W.; Shen, Y. The role of sodium tripolyphosphate in wet grinding process of magnesite. Colloids Surf. A Physicochem. Eng. Asp. 2023, 668, 131449. [Google Scholar] [CrossRef]
- Wonyen, D.G.; Kromah, V.; Gibson, B.; Nah, S.; Chelgani, S.C. A Review of Flotation Separation of Mg Carbonates (Dolomite and Magnesite). Minerals 2018, 8, 354. [Google Scholar] [CrossRef]
- Yang, B.; Yin, W.; Yao, J.; Zhu, Z.; Sun, H.; Chen, K.; Cao, S. Selective collection and differential adsorption of pentaethoxylated laurylamine for the flotation recovery of magnesite from quartz. Colloids Surf. A Physicochem. Eng. Asp. 2021, 625, 126991. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Liu, W.; Tong, K.; Shen, Y.; Zhao, S.; Zhou, S. Efficient separation of magnesite and quartz using eco-friendly Dimethylaminopropyl lauramide experimental and mechanistic studies. Miner. Eng. 2022, 188, 107814. [Google Scholar] [CrossRef]
- Zhang, W.; Liu, S.; Ren, Q.; Tu, R.; Qiu, F.; Xu, S.; Sun, W.; Tian, M. Proposing a novel factor influencing flotation collector abilities to collect minerals by comparing two cationic collectors N,N-bis(2-hydroxy-3-chloropropyl) dodecylamine and dodecylamine. Appl. Surf. Sci. 2023, 640, 158284. [Google Scholar] [CrossRef]
- Sun, W.; Liu, W.; Dai, S.; Yang, T.; Duan, H.; Liu, W. Effect of Tween 80 on flotation separation of magnesite and dolomite using NaOL as the collector. J. Mol. Liq. 2020, 315, 113712. [Google Scholar] [CrossRef]
- Sun, H.; Yin, W.; Yang, B.; Chen, K.; Sheng, Q. Efficiently separating magnesite from quartz using N-hexadecyltrimethylammonium chloride as a collector via reverse flotation. Miner. Eng. 2021, 166, 106899. [Google Scholar] [CrossRef]
- Wang, X.; Liu, W.; Duan, H.; Fang, P.; Zhang, N.; Zhou, X. Study on quantitative structure-biodegradability relationships of amine collectors by GFA-ANN method. J. Hazard. Mater. 2021, 415, 125628. [Google Scholar] [CrossRef]
- Zhao, P.; Liu, W.; Liu, W.; Shen, Y.; Cui, B.; Zhao, Q. Novel low-foam viscous cationic collector 2-[2-(Tetradecylamino)ethoxy]ethanol: Design, synthesis, and flotation performance study to quartz. Sep. Purif. Technol. 2023, 307, 122633. [Google Scholar] [CrossRef]
- Sun, H.; Yin, W. Selective flotation separation of magnesite from quartz by palmitoyl trimethylammonium chloride. Sep. Purif. Technol. 2022, 295, 121201. [Google Scholar] [CrossRef]
- Brezáni, I.; Škvarla, J.; Sisol, M. Reverse froth flotation of magnesite ore by using (12-4-12) cationic gemini surfactant. Miner. Eng. 2017, 110, 65–68. [Google Scholar] [CrossRef]
- Liu, W.; Peng, X.; Liu, W.; Wang, X.; Zhao, Q.; Wang, B. Effect mechanism of the iso-propanol substituent on amine collectors in the flotation of quartz and magnesite. Powder Technol. 2020, 360, 1117–1125. [Google Scholar] [CrossRef]
- Zhao, J.; Dai, C.; Fang, J.; Feng, X.; Yan, L.; Zhao, M. Surface properties and adsorption behavior of cocamidopropyl dimethyl amine oxide under high temperature and high salinity conditions. Colloids Surf. A Physicochem. Eng. Asp. 2014, 450, 93–98. [Google Scholar] [CrossRef]
- Lopez-Chavez, E.; Garcia-Quiroz, A.; Gonzalez-Garcia, G.; Orozco-Duran, G.E.; Zamudio-Rivera, L.S.; Martinez-Magadan, J.M.; Buenrostro-Gonzalez, E.; Hernandez-Altamirano, R. Quantum chemical characterization of zwitterionic structures: Supramolecular complexes for modifying the wettability of oil–water–limestone system. J. Mol. Graph. Modell. 2014, 51, 128–136. [Google Scholar] [CrossRef]
- Yang, L.; Li, X.; Li, W.; Yan, X.; Zhang, H. Intensification of interfacial adsorption of dodecylamine onto quartz by ultrasonic method. Sep. Purif. Technol. 2019, 227, 115701. [Google Scholar] [CrossRef]
- De Medeiros, A.R.S.; Baltar, C.A.M. Importance of collector chain length in flotation of fine particles. Miner. Eng. 2018, 122, 179–184. [Google Scholar] [CrossRef]
- Gao, W.; Zhang, Q.; Li, X. Interaction of Ca2+, Mg2+ with dolomite (104) surface and its effect on caproic acid adsorption: DFT calculation. Appl. Surf. Sci. 2023, 614, 156244. [Google Scholar] [CrossRef]
- Liu, W.; Peng, X.; Liu, W.; Tong, K.; Shen, Y.; Zhao, Q.; Zhao, S.; Sun, W. Novel polyhydroxy cationic collector N-(2,3-propanediol)-N-dodecylamine: Synthesis and flotation performance to hematite and quartz. Int. J. Min. Sci. Technol. 2023, 33, 115–122. [Google Scholar] [CrossRef]
- Wei, Z.; Zhang, Q.; Wang, X. New insights on depressive mechanism of citric acid in the selective flotation of dolomite from apatite. Colloids Surf. A Physicochem. Eng. Asp. 2022, 653, 130075. [Google Scholar] [CrossRef]
- Wang, J.-L.; Sun, T.-C. Effects of grain size and regulators on separation of quartz from magnesite. Chin. J. Nonferrous Met. 2008, 18, 2082–2086. [Google Scholar]
- Kruszelnicki, M.; Polowczyk, I.; Kowalczuk, P.B. Insight into the influence of surface wettability on flotation properties of solid particles—Critical contact angle in flotation. Powder Technol. 2024, 431, 119056. [Google Scholar] [CrossRef]
- Huang, Y.; Niu, X.; Liu, G.; Liu, J. Novel chelating surfactant 5-heptyl-1,2,4-triazole-3-thione: Its synthesis and flotation separation of malachite against quartz and calcite. Miner. Eng. 2019, 131, 342–352. [Google Scholar] [CrossRef]
- Xing, Y.; Xu, M.; Gui, X.; Cao, Y.; Babel, B.; Rudolph, M.; Weber, S.; Kappl, M.; Butt, H.-J. The application of atomic force microscopy in mineral flotation. Adv. Colloid Interface Sci. 2018, 256, 373–392. [Google Scholar] [CrossRef]
- Liu, S.; Liu, G.-Y.; Huang, Y.-G.; Zhong, H. Hydrophobic intensification flotation: Comparison of collector containing two minerophilic groups with conventional collectors. Trans. Nonferrous Met. Soc. China 2020, 30, 2536–2546. [Google Scholar] [CrossRef]
- Li, S.; Gao, L.; Wang, J.; Zhou, H.; Liao, Y.; Xing, Y.; Gui, X.; Cao, Y. Polyethylene oxide assisted separation of molybdenite from quartz by flotation. Miner. Eng. 2021, 162, 106765. [Google Scholar] [CrossRef]
- Wang, L.; Wang, G.; Ge, P.; Sun, W.; Tang, H.; Hu, W. Activation mechanisms of quartz flotation with calcium ions and cationic/anionic mixed collectors under alkalescent conditions. Colloids Surf. A Physicochem. Eng. Asp. 2022, 632, 127771. [Google Scholar] [CrossRef]
- Wang, Y.; Cao, Y.; Hu, S.; Wang, J.; Guo, F.; Li, S. Effects of solution pH and polyethylene oxide concentrations on molybdenite–molybdenite, molybdenite–kaolinite, and molybdenite–quartz interaction forces: AFM colloidal probe study. Sep. Purif. Technol. 2022, 280, 119926. [Google Scholar] [CrossRef]
- Li, Z.; Han, Y.; Gao, P.; Wang, H.; Liu, J. The interaction among multiple charged particles induced by cations and direct force measurements by AFM. Colloids Surf. A Physicochem. Eng. Asp. 2020, 589, 124440. [Google Scholar] [CrossRef]
- Wang, Y.; Wang, D.; Xu, L.; Xue, K.; Zhang, X.; Shi, X.; Liu, C.; Meng, J. Synthesis and utilization of a novel oleate hydroxamic acid collector for the flotation separation of bastnaesite from barite. Miner. Eng. 2023, 204, 108405. [Google Scholar] [CrossRef]
- Liu, W.; Tong, K.; Ding, R.; Liu, W.; Zhao, P.; Sun, W.; Zhao, Q.; Zhao, S. Synthesis of a novel hydroxyl quaternary ammonium collector and its selective flotation separation of quartz from hematite. Miner. Eng. 2023, 200, 108109. [Google Scholar] [CrossRef]
- Yang, B.; Zhu, Z.; Yin, W.; He, J. Effective flotation separation of malachite from quartz with a selective collector: Collection ability, separation performance and adsorption mechanism. J. Mol. Liq. 2022, 368, 120658. [Google Scholar] [CrossRef]
- Jia, K.; Lu, Y.; Liu, J.; Cheng, S.; Liu, S.; Cao, Y.; Li, G. Selective flotation separation of hemimorphite from quartz using the biosurfactant sodium N-lauroylsarcosinate as a novel collector. Miner. Eng. 2023, 198, 108073. [Google Scholar] [CrossRef]
- Huang, Z.; Zhong, H.; Wang, S.; Xia, L.; Zou, W.; Liu, G. Investigations on reverse cationic flotation of iron ore by using a Gemini surfactant: Ethane-1,2-bis(dimethyl-dodecyl-ammonium bromide). Chem. Eng. J. 2014, 257, 218–228. [Google Scholar] [CrossRef]
- Lei, D.; Gui, W.; Zhao, X.; Tian, X.; Xiao, W.; Xue, J.; Wang, Y.; Peng, X. New insight into poor flotation recovery of fine molybdenite: An overlooked phase transition from 2H to 1T MoS2. Sep. Purif. Technol. 2023, 304, 122286. [Google Scholar] [CrossRef]
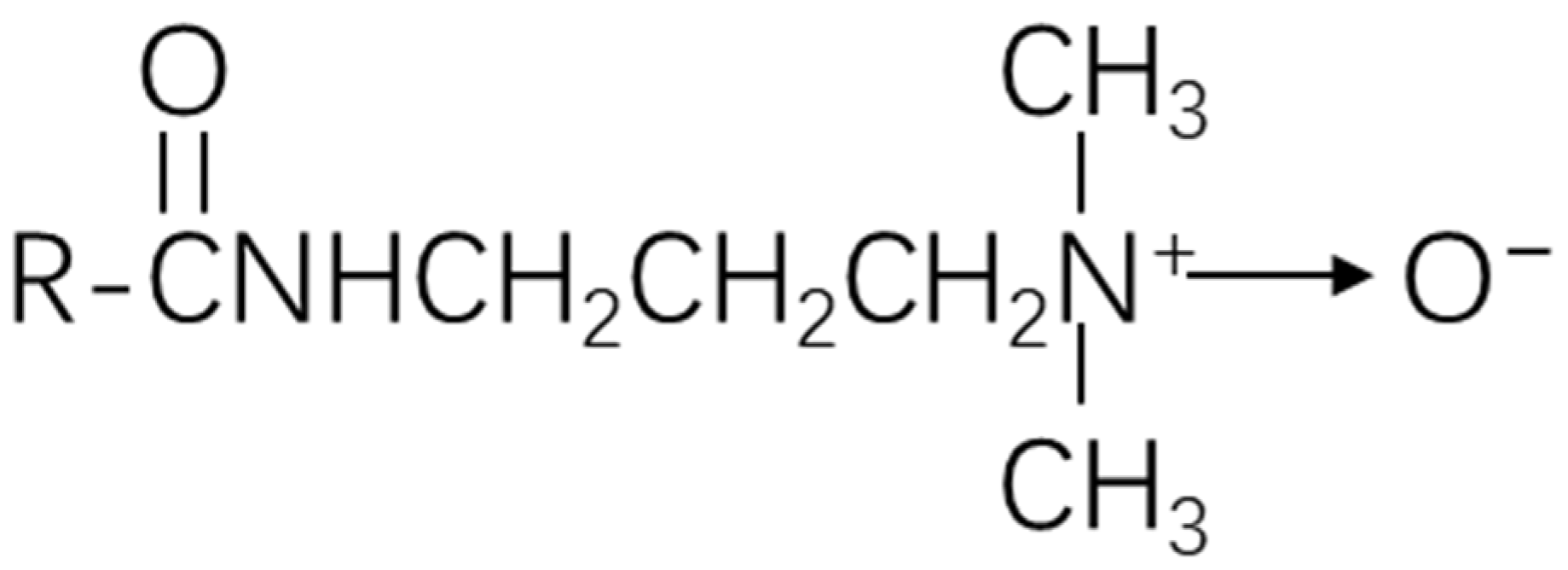



| Sample | MgO | CaO | SiO2 | Al2O3 | SO3 | LOI |
|---|---|---|---|---|---|---|
| Magnesite | 47.28 | 0.31 | 0.36 | 0.08 | 0.03 | 51.94 |
| Quartz | 0.11 | 0.09 | 99.48 | 0.32 | — | — |
| Species | Atomic Concentration (%) | Binding Energy (eV) | |||||
|---|---|---|---|---|---|---|---|
| C 1s | O 1s | Si 2p | N 1s | C 1s | O 1s | Si 2p | |
| Quartz | 17.51 | 48.38 | 30.92 | - | 284.80 | 532.95 | 103.27 |
| Quartz with CPAO | 40.38 | 34.09 | 23.77 | 1.76 | 284.80 | 532.26 | 103.19 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Liu, W.; Zhao, Q.; Zhang, R.; Zhao, P.; Liu, W.; Han, C.; Shen, Y. Study on Selective Adsorption Behavior and Mechanism of Quartz and Magnesite with a New Biodegradable Collector. Separations 2023, 10, 590. https://doi.org/10.3390/separations10120590
Liu W, Zhao Q, Zhang R, Zhao P, Liu W, Han C, Shen Y. Study on Selective Adsorption Behavior and Mechanism of Quartz and Magnesite with a New Biodegradable Collector. Separations. 2023; 10(12):590. https://doi.org/10.3390/separations10120590
Chicago/Turabian StyleLiu, Wenbao, Qiang Zhao, Ruirui Zhang, Panxing Zhao, Wengang Liu, Cong Han, and Yanbai Shen. 2023. "Study on Selective Adsorption Behavior and Mechanism of Quartz and Magnesite with a New Biodegradable Collector" Separations 10, no. 12: 590. https://doi.org/10.3390/separations10120590
APA StyleLiu, W., Zhao, Q., Zhang, R., Zhao, P., Liu, W., Han, C., & Shen, Y. (2023). Study on Selective Adsorption Behavior and Mechanism of Quartz and Magnesite with a New Biodegradable Collector. Separations, 10(12), 590. https://doi.org/10.3390/separations10120590










