Resource and Energy Utilization of Swine Wastewater Treatment: Recent Progress and Future Directions
Abstract
:1. Introduction
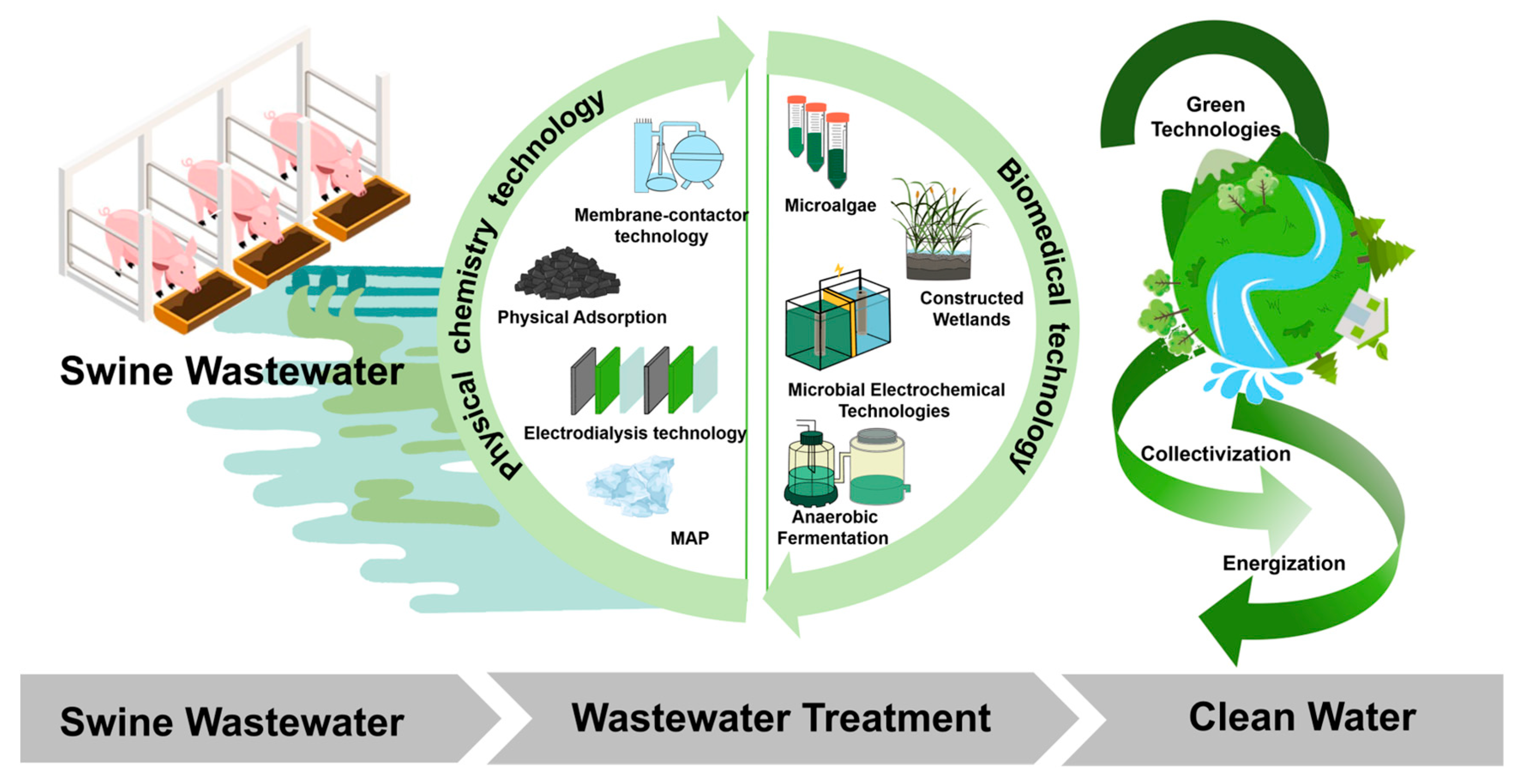
2. Research Status of Wastewater Treatment in Swine Wastewater
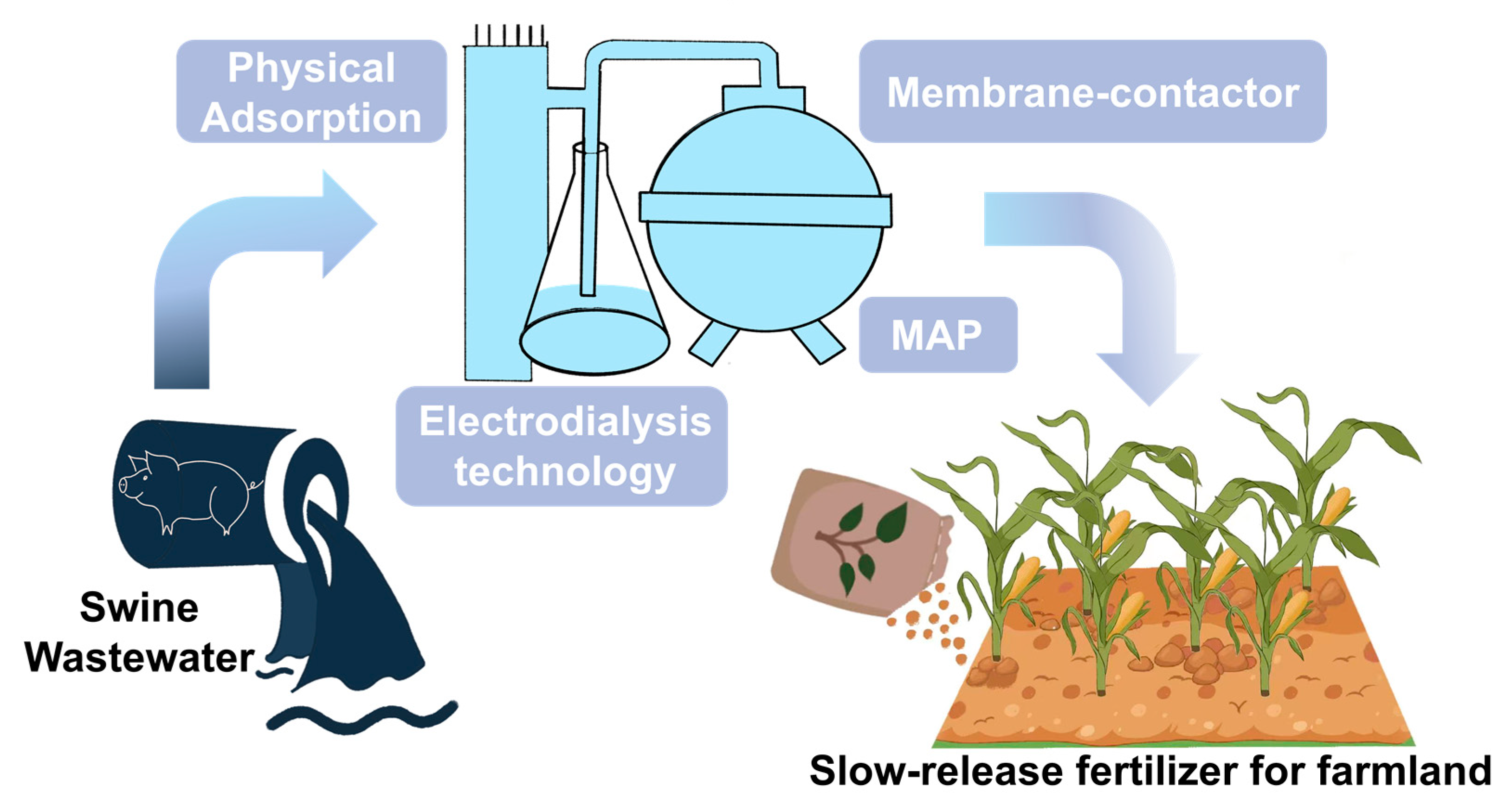
3. Physical and Chemical Technology Treating Swine Wastewater
3.1. Optimization of Materials for Enhancing Physical Adsorption Technology
3.2. Minimum Costs for the Large-Scale Application of Chemical Treatment Technology
3.3. Optimization of Membrane Materials to Exploit the Resource Potential
3.4. Optimization Wastewater Pretreatment Methods
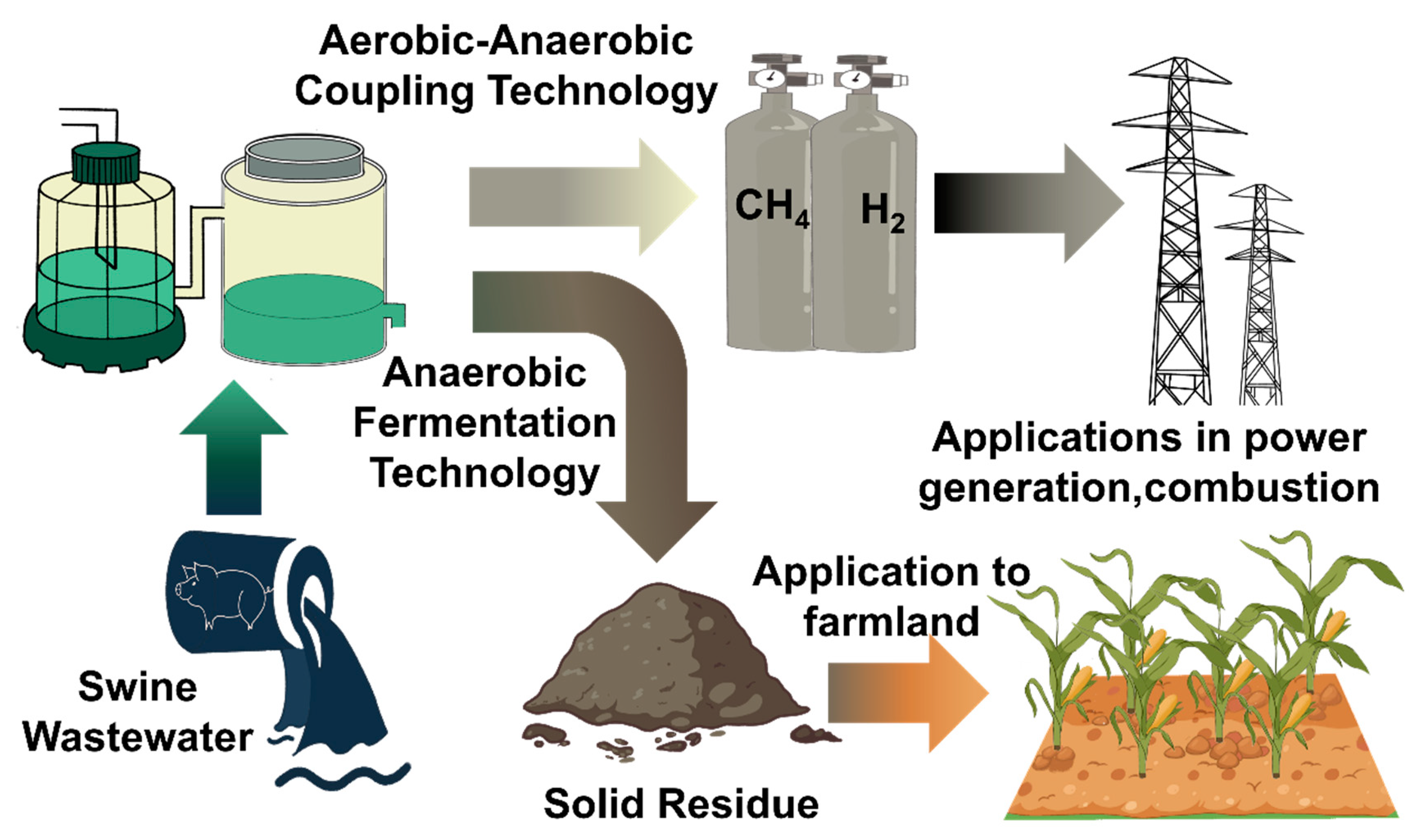
4. Microbial Metabolism for Treating Swine Wastewater
4.1. Optimization of Resource Efficiency to Meet Emission Standards
4.2. Further Optimizing the Resource Efficiency of Anaerobic–Aerobic Coupling Technology
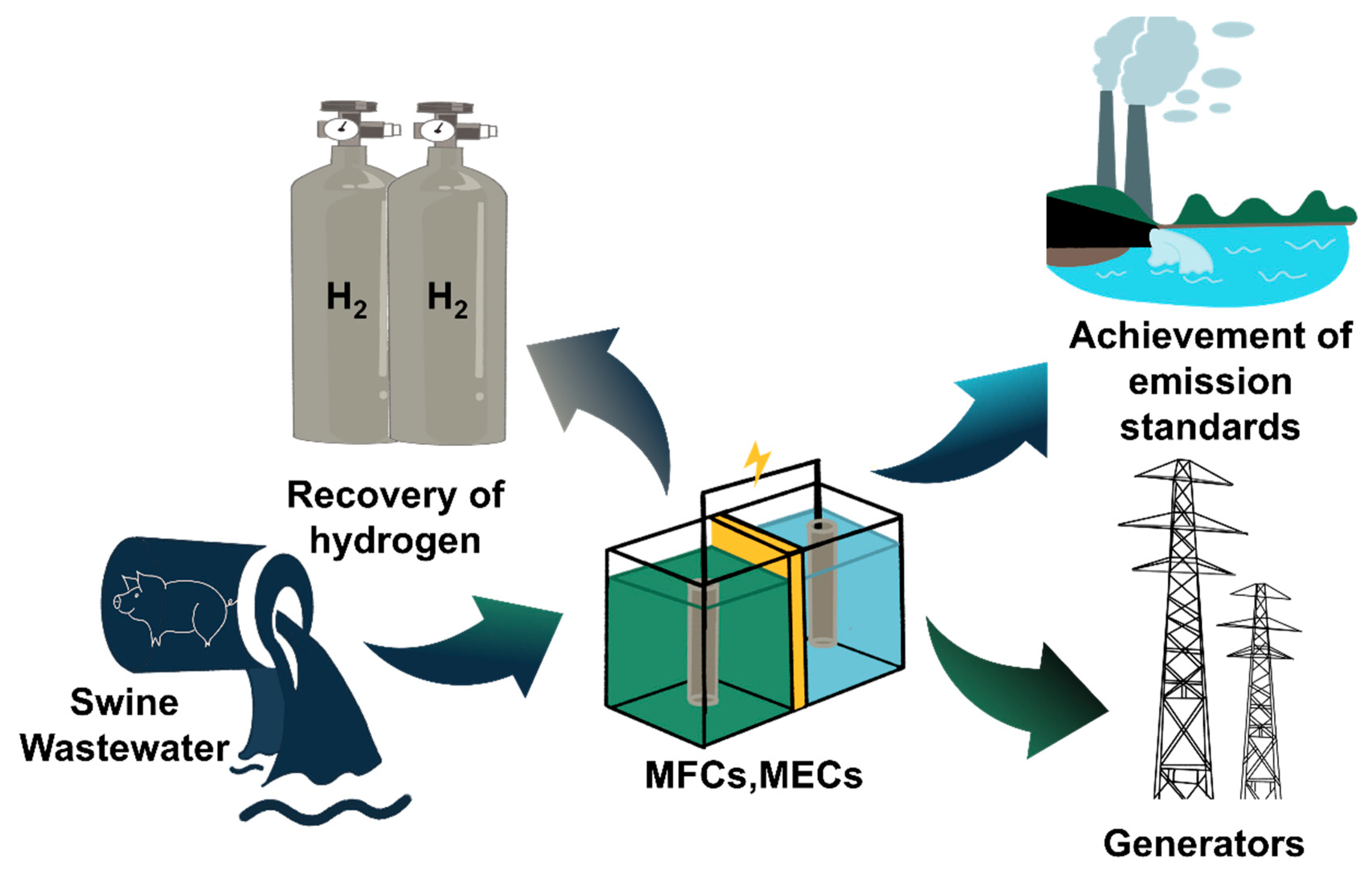
5. Microbial Electrochemical Technologies for Treating Swine Wastewater
5.1. Optimization Electrode Cost and Durability for Optimized Microbial Fuel Cells (MFCs)
5.2. Optimization of Electrode Materials for Microbial Electrolytic Cells (MECs)
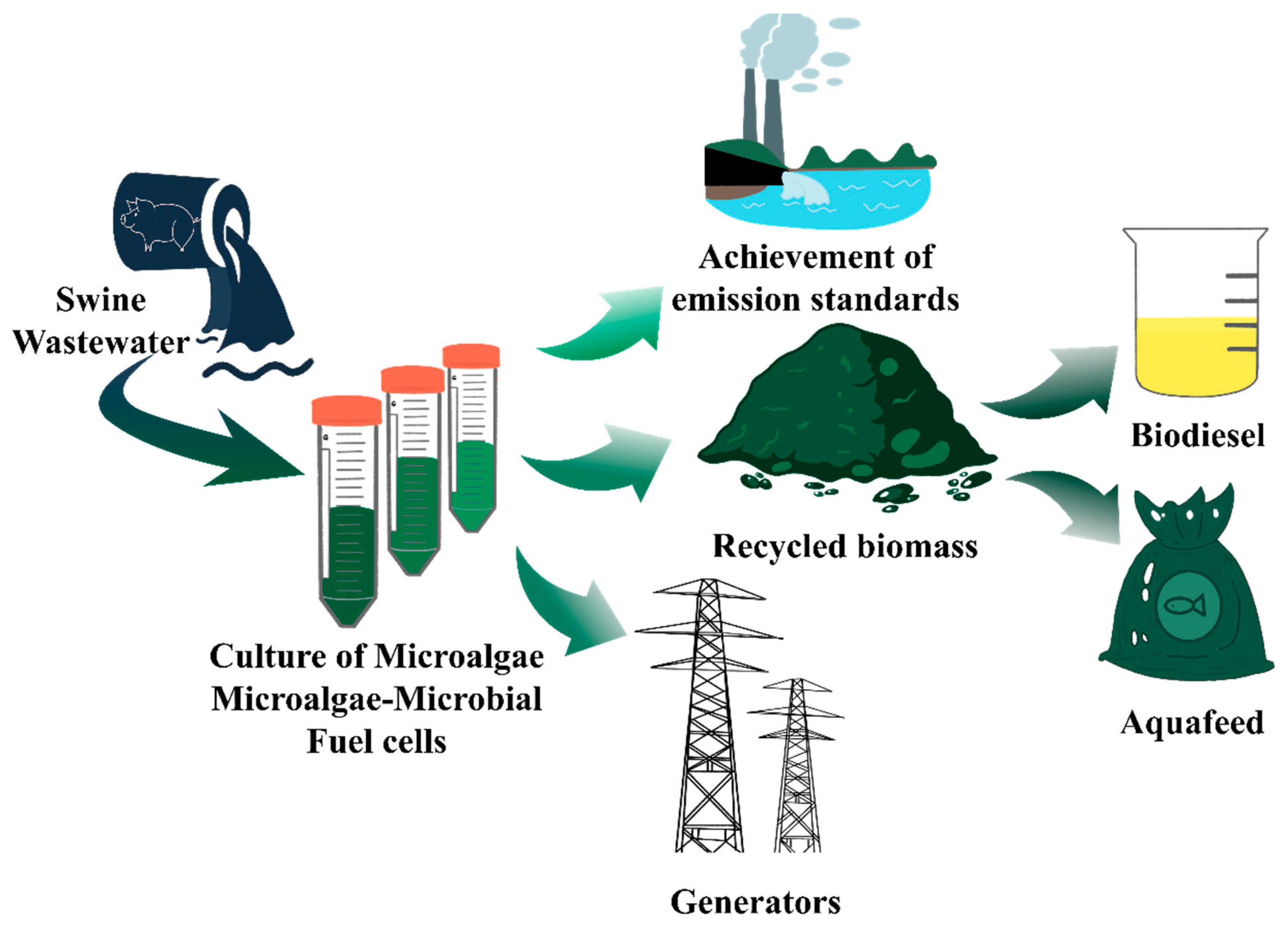
6. Microalgal-Based Technology for Treating Swine Wastewater
6.1. Selection of Microalgal Species and Optimization of Biomass Recovery Methods
| Species | Biomass Productivity (mg L−1 d−1) | TN Removal (%) | NH4+-N Removal (%) | TP Removal (%) | COD Removal (%) | References |
|---|---|---|---|---|---|---|
| C. sorokiniana | 23.4–408.9 | 60–98.6 | 79.1–85 | 64.7–96.4 | 36–93.7 | [76,77,78] |
| C. subellipsoidea | 860 | 75.3 | – | 78 | – | [79] |
| C. vulgaris | 86.1–101.7 | 69.6–80.9 | 91.2 | 64.4–94 | 72.2–95.7 | [80,81,82] |
| Chlamydomonas | 28 | 62.00 | – | 28.00 | – | [83] |
| Chlorella | 48–130 | 74.2–97.6 | 92–95 | 28–97.1 | 66.7–75 | [83,84,85] |
| Diplosphaera | – | 54.5 | – | 82.5 | 70.7 | [86] |
| Monoraphidium | 860 | 65.8–76.7 | – | 37.8–75.2 | 81.5–84 | [86,87] |
| Oleoabundans | 5.1 | – | 37.5 | 26.9 | – | [88] |
| Pyrenoidosa | 7.7–29.9 | – | 65.8–97.6 | 75.4–85.3 | – | [88,89] |
| S. abundans | 970 | 81 | – | 65.9 | 77 | [90] |
| S. obliquus | 11.8 | – | 72.4 | 80.9 | – | [88] |
| S. quadricauda | – | 95.5 | – | 96.4 | 81.9 | [91] |
| Scenedesmus | 7.1–211 | 77.8 | 80–95 | 86.7–94.1 | 26.4–83.3 | [84,92,93] |
| Spirulina | 48.4–115 | 75 | 80 | 86.7 | 68.8 | [84,94] |
6.2. Coupling Microbial Fuel Cells Technique
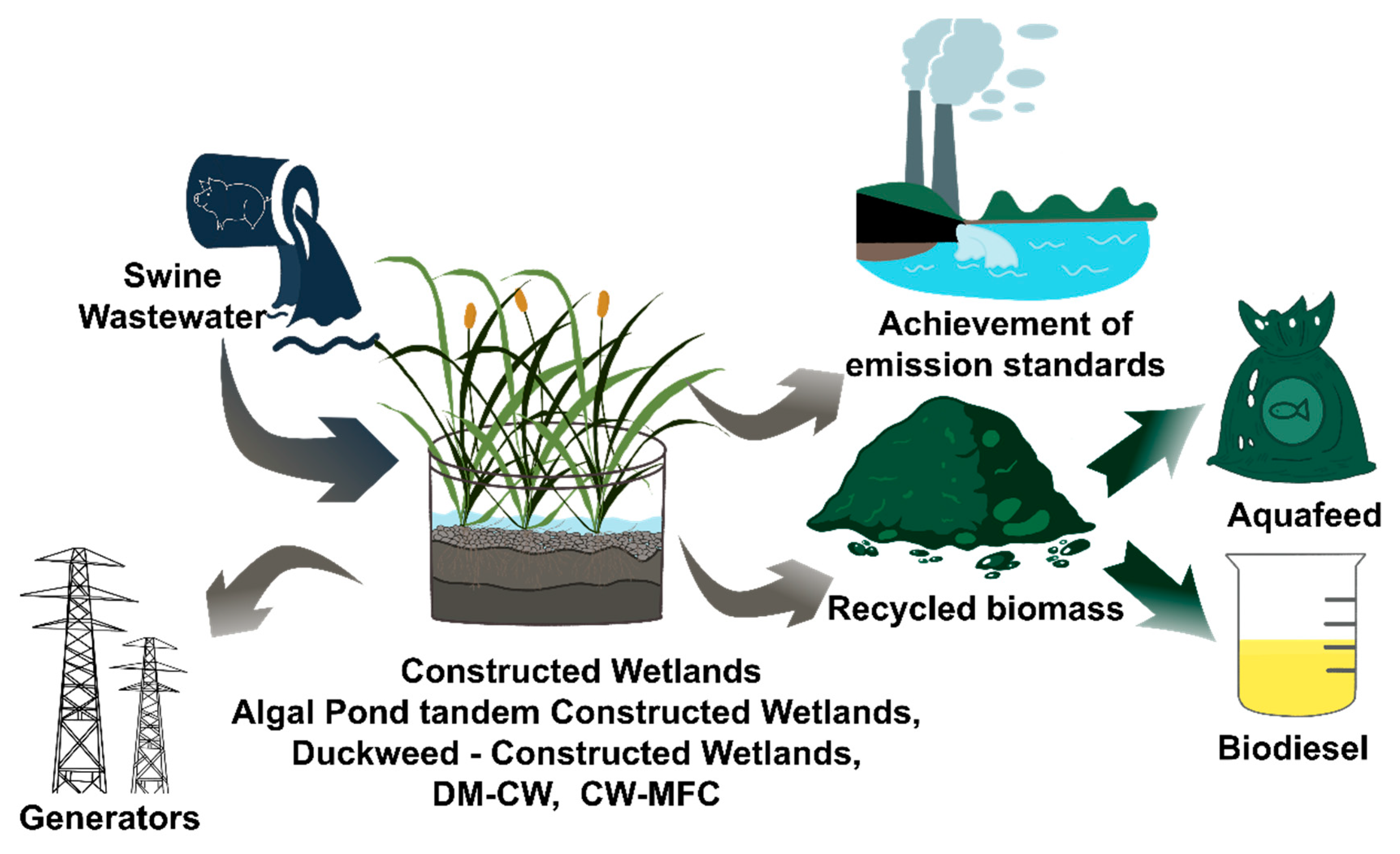
7. Constructed Wetlands for Treating Swine Wastewater
7.1. Optimization of Nitrogen Removal to Improve the Resource Treatment Effect of Constructed Wetlands (CWs)
7.2. Optimization of Microalgae-Based CWs
7.3. Choice of Duckweed or Microalgae for the Purpose of Optimizing CW Operation
8. Envisioning the Future: Optimizing Resource Utilization Techniques for Swine Wastewater
9. Conclusions and Implications
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zhang, K.; Yang, S.; Wang, W.; Luo, H.; Chen, W.; Zhang, X.; Ma, D.; An, X.; Chen, F.; Cheng, L.; et al. Bioelectrochemical processes and cellulosic carbon source enhance the autotrophic and heterotrophic denitrification of low C/N ratio wastewater in tidal flow constructed wetland—Microbial fuel cells. J. Clean. Prod. 2022, 363, 132368. [Google Scholar] [CrossRef]
- Deng, L.; Zheng, D.; Zhang, J.; Yang, H.; Wang, L.; Wang, W.; He, T.; Zhang, Y. Treatment and utilization of swine wastewater—A review on technologies in full-scale application. Sci. Total. Environ. 2023, 880, 163223. [Google Scholar] [CrossRef] [PubMed]
- Park, J.-H.; Ryu, H.-D.; Chung, E.-G.; Oa, S.-W.; Kim, Y.-S. TOC Standards for Sustainably Managing Refractory Organic Matter in Swine Wastewater Effluent. Sustainability 2022, 14, 10092. [Google Scholar] [CrossRef]
- Dong, L.; Qi, Z.; Li, M.; Zhang, Y.; Chen, Y.; Qi, Y.; Wu, H. Organics and nutrient removal from swine wastewater by constructed wetlands using ceramsite and magnetite as substrates. Environ. Chem. Eng. 2021, 9, 104739. [Google Scholar] [CrossRef]
- Deng, F.; Olvera-Vargas, H.; Zhou, M.; Qiu, S.; Sirés, I.; Brillas, E. Critical Review on the Mechanisms of Fe2+ Regeneration in the Electro-Fenton Process_ Fundamentals and Boosting Strategies. Chem. Rev. 2023, 123, 4635–4662. [Google Scholar] [CrossRef]
- Li, L.; Fu, R.; Zou, J.; Wang, S.; Ding, J.; Han, J.; Zhao, M. Research Progress of Iron-Based Catalysts in Ozonation Wastewater Treatment. ACS ES&T Water 2023, 3, 908–922. [Google Scholar]
- Li, L.; Liang, T.; Zhao, M.; Lv, Y.; Song, Z.; Sheng, T.; Ma, F. A review on mycelial pellets as biological carriers: Wastewater treatment and recovery for resource and energy. Bioresour. Technol. 2022, 355, 127200. [Google Scholar] [CrossRef]
- Deng, F.; Jiang, J.; Sirés, I. State-of-the-art review and bibliometric analysis on electro-Fenton process. Carbon Lett. 2023, 33, 17–34. [Google Scholar] [CrossRef]
- SShao, Q.; Zhang, Y.; Liu, Z.; Long, L.; Liu, Z.; Chen, Y.; Hu, X.-M.; Lu, M.; Huang, L.-Z. Phosphorus and nitrogen recovery from wastewater by ceramsite: Adsorption mechanism, plant cultivation and sustainability analysis. Sci. Total. Environ. 2021, 805, 150288. [Google Scholar] [CrossRef] [PubMed]
- Christensen, M.L.; Jakobsen, A.H.; Hansen, C.S.K.; Skovbjerg, M.; Andersen, R.B.M.; Jensen, M.D.; Sundmark, K. Pilot-scale hydrolysis of primary sludge for production of easily degradable carbon to treat biological wastewater or produce biogas. Sci. Total. Environ. 2022, 846, 157532. [Google Scholar] [CrossRef] [PubMed]
- Maurya, R.; Zhu, X.; Valverde-Pérez, B.; Kiran, B.R.; General, T.; Sharma, S.; Sharma, A.K.; Thomsen, M.; Mohan, S.V.; Mohanty, K.; et al. Advances in microalgal research for valorization of industrial wastewater. Bioresour. Technol. 2021, 343, 126128. [Google Scholar] [CrossRef]
- Lee, W.-C.; Chang, C.-C. Effectively Recycling Swine Wastewater by Coagulation-Flocculation of Nonionic Polyacrylamide. Sustainability 2022, 14, 1742. [Google Scholar] [CrossRef]
- Domingues, E.; Lincho, J.; Fernandes, M.J.; Gomes, J.; Martins, R.C. Low-cost materials for swine wastewater treatment using adsorption and Fenton’s process. Environ. Sci. Pollut. Res. Int. 2003. [Google Scholar] [CrossRef] [PubMed]
- Rahman, T.U.; Roy, H.; Islam, R.; Tahmid, M.; Fariha, A.; Mazumder, A.; Tasnim, N.; Pervez, N.; Cai, Y.; Naddeo, V.; et al. The Advancement in Membrane Bioreactor (MBR) Technology toward Sustainable Industrial Wastewater Management. Membranes 2023, 13, 181. [Google Scholar] [CrossRef] [PubMed]
- Vaishnav, S.; Saini, T.; Chauhan, A.; Gaur, G.K.; Tiwari, R.; Dutt, T.; Tarafdar, A. Livestock and poultry farm wastewater treatment and its valorization for generating value-added products: Recent updates and way forward. Bioresour. Technol. 2023, 382, 129170. [Google Scholar] [CrossRef] [PubMed]
- Ghimire, S.; Wang, L.; Zhang, B.; Li, X.; Shahbazi, A. Production and modification of hydrochar from anaerobically digested cattail for adsorbing ammonium and phosphorous in wastewater. Water Sci. Technol. 2021, 84, 1678–1692. [Google Scholar] [CrossRef]
- Li, L.; Han, J.; Huang, X.; Qiu, S.; Liu, X.; Liu, L.; Zhao, M.; Qu, J.; Zou, J.; Zhang, J. Organic pollutants removal from aqueous solutions using metal-organic frameworks (MOFs) as adsorbents: A review. J. Environ. Chem. Eng. 2023, 11, 111217. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, M.; Wu, P.; Zhang, X.; Wang, S.; Yu, Z.; Wang, B. Simultaneous reclaiming phosphate and ammonium from aqueous solutions by calcium alginate-biochar composite: Sorption performance and governing mechanisms. Chem. Eng. J. 2022, 429, 132166. [Google Scholar] [CrossRef]
- Abukhadra, M.R.; Mostafa, M. Effective decontamination of phosphate and ammonium utilizing novel muscovite/phillipsite composite; equilibrium investigation and realistic application. Sci. Total Environ. 2019, 667, 101–111. [Google Scholar] [CrossRef]
- Ro, K.S. Kinetics and Energetics of Producing Animal Manure-Based Biochar. BioEnergy Res. 2016, 9, 447–453. [Google Scholar] [CrossRef]
- Peng, Y.; Sun, Y.; Hanif, A.; Shang, J.; Shen, Z.; Hou, D.; Zhou, Y.; Chen, Q.; Ok, Y.S.; Tsang, D.C. Design and fabrication of exfoliated Mg/Al layered double hydroxides on biochar support. J. Clean. Prod. 2021, 289, 125142. [Google Scholar] [CrossRef]
- Yang, F.; Zhang, S.; Sun, Y.; Tsang, D.C.; Cheng, K.; Ok, Y.S. Assembling biochar with various layered double hydroxides for enhancement of phosphorus recovery. J. Hazard. Mater. 2019, 365, 665–673. [Google Scholar] [CrossRef] [PubMed]
- Ren, L.; Li, Y.; Wang, K.; Ding, K.; Sha, M.; Cao, Y.; Kong, F.; Wang, S. Recovery of phosphorus from eutrophic water using nano zero-valent iron-modified biochar and its utilization. Chemosphere 2021, 284, 131391. [Google Scholar] [CrossRef] [PubMed]
- An, X.; Wu, Z.; Yu, J.; Cravotto, G.; Liu, X.; Li, Q.; Yu, B. Copyrolysis of Biomass, Bentonite, and Nutrients as a New Strategy for the Synthesis of Improved Biochar-Based Slow-Release Fertilizers. ACS Sustain. Chem. Eng. 2020, 8, 3181–3190. [Google Scholar] [CrossRef]
- Luo, Y.; Liu, M.; Chen, Y.; Wang, T.; Zhang, W. Preparation and regeneration of iron-modified nanofibres for low-concentration phosphorus-containing wastewater treatment. R. Soc. Open Sci. 2019, 6, 190764. [Google Scholar] [CrossRef] [PubMed]
- Liu, M.; Liu, C.; Liao, W.; Xie, J.; Zhang, X.; Gao, Z. Impact of biochar application on gas emissions from liquid pig manure storage. Sci. Total. Environ. 2021, 771, 145454. [Google Scholar] [CrossRef]
- Huang, H.; Chen, Y.; Jiang, Y.; Ding, L. Treatment of swine wastewater combined with MgO-saponification wastewater by struvite precipitation technology. Chem. Eng. J. 2014, 254, 418–425. [Google Scholar] [CrossRef]
- Kim, B.; Lee, W.; Lee, H.; Rim, J. Ammonium nitrogen removal from slurry-type swine wastewater by pretreatment using struvite crystallization for nitrogen control of anaerobic digestion. Water Sci. Technol. 2004, 49, 215–222. [Google Scholar] [CrossRef]
- Sancho, I.; Licon, E.; Valderrama, C.; de Arespacochaga, N.; López-Palau, S.; Cortina, J. Recovery of ammonia from domestic wastewater effluents as liquid fertilizers by integration of natural zeolites and hollow fibre membrane contactors. SCISci. Total. Environ. 2017, 584–585, 244–251. [Google Scholar] [CrossRef]
- KKamilya, T.; Majumder, A.; Yadav, M.K.; Ayoob, S.; Tripathy, S.; Gupta, A.K. Nutrient pollution and its remediation using constructed wetlands: Insights into removal and recovery mechanisms, modifications and sustainable aspects. J. Environ. Chem. Eng. 2022, 10, 10744. [Google Scholar] [CrossRef]
- Li, Q.; Wang, S.; Wang, L.; Zhang, L.; Wan, X.; Sun, Z. The Recovery of Phosphorus from Acidic Ultra-High Phosphorous Wastewater by the Struvite Crystallization. Water 2020, 12, 946. [Google Scholar] [CrossRef]
- Song, Y.; Yuan, P.; Zheng, B.; Peng, H.; Yuan, F.; Gao, Y. Nutrients removal and recovery by crystallization of magnesium ammonium phosphate from synthetic swine wastewater. Chemosphere 2007, 69, 319–324. [Google Scholar] [CrossRef]
- Trotta, S.; Adani, F.; Fedele, M.; Salvatori, M. Nitrogen and phosphorus recovery from cow digestate by struvite precipitation: Process optimization to maximize phosphorus recovery. Results Eng. 2023, 20, 101478. [Google Scholar] [CrossRef]
- Ye, Y.; Ngo, H.H.; Guo, W.; Liu, Y.; Chang, S.W.; Nguyen, D.D.; Liang, H.; Wang, J. A critical review on ammonium recovery from wastewater for sustainable wastewater management. Bioresour. Technol. 2018, 268, 749–758. [Google Scholar] [CrossRef] [PubMed]
- Park, J.; Jang, Y.; Lee, W.; Choi, Y. Effect of chemical speciation in boundary layer on performance of ammonia recovery in membrane contactor. Desalination 2023, 558, 116618. [Google Scholar] [CrossRef]
- Rongwong, W.; Bae, T.-H.; Jiraratananon, R. Economic optimization of hollow fiber membrane contactors for ammonia nitrogen recovery from anaerobic digestion effluents. J. Environ. Chem. Eng. 2022, 10, 108631. [Google Scholar] [CrossRef]
- Jang, Y.; Lee, W.; Park, J.; Choi, Y. Recovery of ammonia from wastewater by liquid-liquid membrane contactor: A review. Membr. Water Treat. 2022, 13, 147–166. [Google Scholar]
- AAmaral, M.C.S.; Magalhães, N.C.; Moravia, W.G.; Ferreira, C.D. Ammonia recovery from landfill leachate using hydrophobic membrane contactors. Water Sci. Technol. 2016, 74, 2177–2184. [Google Scholar] [CrossRef]
- Scarazzato, T.; Panossian, Z.; Tenório, J.; Pérez-Herranz, V.; Espinosa, D. A review of cleaner production in electroplating industries using electrodialysis. Clean. Prod. 2017, 168, 1590–1602. [Google Scholar] [CrossRef]
- Lim, S.J.; Kim, T.-H.; Kim, J.-Y.; Shin, I.H.; Kwak, H.S. Enhanced treatment of swine wastewater by electron beam irradiation and ion-exchange biological reactor. Sep. Purif. Technol. 2016, 157, 72–79. [Google Scholar] [CrossRef]
- Huang, H.; Zhang, D.; Guo, G.; Jiang, Y.; Wang, M.; Zhang, P.; Li, J. Dolomite application for the removal of nutrients from synthetic swine wastewater by a novel combined electrochemical process. Chem. Eng. J. 2018, 335, 665–675. [Google Scholar] [CrossRef]
- Zheng, H.; Tang, F.; Lin, Y.; Xu, Z.; Xie, Z.; Tian, J. Solid-state anaerobic digestion of rice straw pretreated with swine manure digested effluent. J. Clean. Prod. 2022, 348, 131252. [Google Scholar] [CrossRef]
- Chen, Y.; Liu, H.; Zheng, X.; Wang, X.; Wu, J. New method for enhancement of bioenergy production from municipal organic wastes via regulation of anaerobic fermentation process. Appl. Energy 2017, 196, 190–198. [Google Scholar] [CrossRef]
- Tang, H.; Ma, Z.; Qin, Y.; Wu, H.; Xu, X.; Xin, L.; Wu, W. Pilot-scale study of step-feed anaerobic coupled four-stage micro-oxygen gradient aeration process for treating digested swine wastewater with low carbon/nitrogen ratios. Bioresour. Technol. 2023, 380, 129087. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.; Wang, T.; Chen, J.; Guo, J.; Luo, H.; Chen, W.; Mo, Y.; Wei, Z.; Huang, X. The reduction and fate of antibiotic resistance genes (ARGs) and mobile genetic elements (MGEs) in microbial fuel cell (MFC) during treatment of livestock wastewater. J. Contam. Hydrol. 2022, 247, 103981. [Google Scholar] [CrossRef] [PubMed]
- Pan, J.; Ma, J.; Zhai, L.; Liu, H. Enhanced methane production and syntrophic connection between microorganisms during semi-continuous anaerobic digestion of chicken manure by adding biochar. J. Clean. Prod. 2019, 240, 118178. [Google Scholar] [CrossRef]
- Zhao, X.; Meng, X.; Liu, Y.; Bai, S.; Li, B.; Li, H.; Hou, N.; Li, C. Single-cell sorting of microalgae and identification of optimal conditions by using response surface methodology coupled with life-cycle approaches. Sci. Total. Environ. 2022, 832, 155061. [Google Scholar] [CrossRef]
- Escalante-Estrada, V.E.; Garzón-Zúñiga, M.A.; Valle-Cervantes, S.; Páez-Lerma, J.B. Swine Wastewater Treatment for Small Farms by a New Anaerobic-Aerobic Biofiltration Technology. Water Air Soil Pollut. 2019, 230, 145. [Google Scholar] [CrossRef]
- Bastiani, C.D.; Kennedy, D.; Reynolds, A. CFD simulation of anaerobic granular sludge reactors: A review. Water Res. 2023, 242, 120220. [Google Scholar] [CrossRef]
- Gonzalez-Tineo, P.A.; Durán-Hinojosa, U.; Delgadillo-Mirquez, L.R.; Meza-Escalante, E.R.; Gortáres-Moroyoqui, P.; Ulloa-Mercado, R.G.; Serrano-Palacios, D. Performance improvement of an integrated anaerobic-aerobic hybrid reactor for the treatment of swine wastewater. J. Water Process Eng. 2020, 34, 101164. [Google Scholar] [CrossRef]
- Scherson, Y.D.; Woo, S.-G.; Criddle, C.S. Production of Nitrous Oxide From Anaerobic Digester Centrate and Its Use as a Co-oxidant of Biogas to Enhance Energy Recovery. Environ. Sci. Technol. 2014, 48, 5612–5619. [Google Scholar] [CrossRef] [PubMed]
- Weißbach, M.; Drewes, J.E.; Koch, K. Application of the oxidation reduction potential (ORP) for process control and monitoring nitrite in a Coupled Aerobic-anoxic Nitrous Decomposition Operation (CANDO). Chem. Eng. J. 2018, 343, 484–491. [Google Scholar] [CrossRef]
- Terán, R.A.S.; Orozco, C.D.l.M.; Acuña, I.J.G.; Rosales, S.G.; Araujo, G.D.; Arias, H.O.R. Removing Organic Matter and Nutrients from Swine Wastewater after Anaerobic-Aerobic Treatment. Water 2017, 9, 726. [Google Scholar] [CrossRef]
- Nguyen, D.-T.; Taguchi, K. A floating microbial fuel cell: Generating electricity from Japanese rice washing wastewater. Energy Rep. 2020, 6, 758–762. [Google Scholar] [CrossRef]
- Wagner, R.C.; Regan, J.M.; Oh, S.-E.; Zuo, Y.; Logan, B.E. Hydrogen and methane production from swine wastewater using microbial electrolysis cells. Water Res. 2009, 43, 1480–1488. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.; An, J.; Jang, J.K.; Chang, I.S. Determination of optimum electrical connection mode for multi-electrode-embedded microbial fuel cells coupled with anaerobic digester for enhancement of swine wastewater treatment efficiency and energy recovery. Bioresour. Technol. 2020, 297, 122464. [Google Scholar] [CrossRef] [PubMed]
- Nasrabadi, A.M.; Moghimi, M. Experimental investigation of factors affecting the micro microbial fuel cells? main outputs. J. Power Sources 2023, 564, 232871. [Google Scholar] [CrossRef]
- Ma, D.; Jiang, Z.-H.; Lay, C.-H.; Zhou, D. Electricity generation from swine wastewater in microbial fuel cell: Hydraulic reaction time effect. Int. J. Hydrogen Energy 2016, 41, 21820–21826. [Google Scholar] [CrossRef]
- Ichihashi, O.; Hirooka, K. Removal and recovery of phosphorus as struvite from swine wastewater using microbial fuel cell. Bioresour. Technol. 2012, 114, 303–307. [Google Scholar] [CrossRef]
- Babanova, S.; Jones, J.; Phadke, S.; Lu, M.; Angulo, C.; Garcia, J.; Carpenter, K.; Cortese, R.; Chen, S.; Phan, T.; et al. Continuous flow, large-scale, microbial fuel cell system for the sustained treatment of swine waste. Water Environ. Res. 2020, 92, 60–72. [Google Scholar] [CrossRef]
- Patwardhan, S.B.; Savla, N.; Pandit, S.; Gupta, P.K.; Mathuriya, A.S.; Lahiri, D.; Jadhav, D.A.; Rai, A.K.; Priya, K.; Ray, R.R.; et al. Microbial Fuel Cell United with Other Existing Technologies for Enhanced Power Generation and Efficient Wastewater Treatment. Appl. Sci. 2021, 11, 10777. [Google Scholar] [CrossRef]
- Wu, X.; Xie, W.; Ye, J.; Sun, D.; Ohnuki, T.; Li, M.; Zhang, X.; Fang, Q.; Tang, Q.; Li, D. Progress in heavy metals-containing wastewater treatment via microbial electrolysis cell: A review. J. Water Process. Eng. 2023, 55, 104228. [Google Scholar] [CrossRef]
- Liu, W.-Z.; Wang, A.-J.; Ren, N.-Q.; Zhao, X.-Y.; Liu, L.-H.; Yu, Z.-G.; Lee, D.-J. Electrochemically assisted biohydrogen production from acetate. Energy Fuels 2008, 22, 159–163. [Google Scholar] [CrossRef]
- Jiang, J.; Lopez-Ruiz, J.A.; Bian, Y.; Sun, D.; Yan, Y.; Chen, X.; Zhu, J.; May, H.D.; Ren, Z.J. Scale-up and techno-economic analysis of microbial electrolysis cells for hydrogen production from wastewater. Water Res. 2023, 241, 120139. [Google Scholar] [CrossRef]
- Samsudeen, N.; Spurgeon, J.; Matheswaran, M. Satyavolu, Simultaneous biohydrogen production with distillery wastewater treatment using modified microbial electrolysis cell. Int. J. Hydrogen Energy 2020, 45, 18266–18274. [Google Scholar]
- Colantonio, N.; Kim, Y. Cadmium (II) removal mechanisms in microbial electrolysis cells. J. Hazard. Mater. 2016, 311, 134–141. [Google Scholar] [CrossRef]
- Wang, C.; Ye, X.; Liu, Y.; Jia, Z.; Cao, C.; Xiao, Q.; Du, J.; Kong, X.; Wu, X.; Chen, Z.; et al. Enhanced anaerobic digestion for degradation of swine wastewater through a Fe/Ni-MOF modified microbial electrolysis cell. J. Clean. Prod. 2022, 380, 134773. [Google Scholar] [CrossRef]
- Beegle, J.R.; Borole, A.P. An integrated microbial electrolysis-anaerobic digestion process combined with pretreatment of wastewater solids to improve hydrogen production. Environ. Sci. Water Res. Technol. 2017, 3, 1073–1085. [Google Scholar] [CrossRef]
- Park, S.-G.; Rajesh, P.; Hwang, M.-H.; Chu, K.H.; Cho, S.; Chae, K.-J. Long-term effects of anti-biofouling proton exchange membrane using silver nanoparticles and polydopamine on the performance of microbial electrolysis cells. Int. J. Hydrogen Energy 2021, 46, 11345–11356. [Google Scholar] [CrossRef]
- Nirmala, N.; Praveen, G.; AmitKumar, S.; SundarRajan, P.; Baskaran, A.; Priyadharsini, P.; SanjayKumar, S.; Dawn, S.; Pavithra, K.G.; Arun, J.; et al. A review on biological biohydrogen production: Outlook on genetic strain enhancements, reactor model and techno-economics analysis. Sci. Total. Environ. 2023, 896, 16514. [Google Scholar] [CrossRef]
- Liu, X.-Y.; Hong, Y.; Zhao, G.-P.; Zhang, H.-K.; Zhai, Q.-Y.; Wang, Q. Microalgae-based swine wastewater treatment: Strain screening, conditions optimization, physiological activity and biomass potential. Sci. Total. Environ. 2022, 807, 151008. [Google Scholar] [CrossRef] [PubMed]
- Kadir, W.N.A.; Lam, M.K.; Uemura, Y.; Lim, J.W.; Lee, K.T. Harvesting and pre-treatment of microalgae cultivated in wastewater for biodiesel production: A review. Energy Convers. Manag. 2018, 171, 1416–1429. [Google Scholar] [CrossRef]
- Cheng, D.; Ngo, H.; Guo, W.; Chang, S.; Nguyen, D.; Kumar, S. Microalgae biomass from swine wastewater and its conversion to bioenergy. Bioresour. Technol. 2018, 275, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, M.; Yang, Y.; Chen, Z.; Chen, Y.; Wen, Y.; Chen, B. Removal of nutrients from undiluted anaerobically treated piggery wastewater by improved microalgae. Bioresour. Technol. 2016, 222, 130–138. [Google Scholar] [CrossRef] [PubMed]
- KIMKim, J.Y.; Jung, J.-M.; Jung, S.; Park, Y.-K.; Tsang, Y.F.; Lin, K.-Y.A.; Choi, Y.-E.; Kwon, E.E. Biodiesel from microalgae: Recent progress and key challenges. Prog. Energy Combust. Sci. 2022, 93, 101020. [Google Scholar] [CrossRef]
- Zhao, X.; Chen, J.; Guo, M.; Li, C.; Hou, N.; Bai, S. Constructed wetlands treating synthetic wastewater in response to day-night alterations: Performance and mechanisms. Chem. Eng. J. 2022, 446, 137460. [Google Scholar] [CrossRef]
- Su, K.; Li, X.; Lu, T.; Mou, Y.; Liu, N.; Song, M.; Yu, Z. Screening of the heterotrophic microalgae strain for the reclamation of acid producing wastewater. Chemosphere 2022, 307, 136047. [Google Scholar] [CrossRef]
- Cheah, W.Y.; Show, P.L.; Yap, Y.J.; Zaid, H.F.M.; Lam, M.K.; Lim, J.W.; Ho, Y.-C.; Tao, Y. Enhancing microalga Chlorella sorokiniana CY-1 biomass and lipid production in palm oil mill effluent (POME) using novel-designed photobioreactor. (Reprinted from BIOENGINEERED, 2020). Bioengineered 2019, 11, 61–69. [Google Scholar] [CrossRef]
- Liu, T.; Luo, F.; Wang, Z.; Li, Y. The enhanced biomass and lipid accumulation in Coccomyxa subellipsoidea with an integrated treatment strategy initiated by brewery effluent and phytohormones. World J. Microbiol. Biotechnol. 2018, 34, 25. [Google Scholar] [CrossRef]
- Choi, H.-J. Parametric study of brewery wastewater effluent treatment using Chlorella vulgaris microalgae. Environ. Eng. Res. 2016, 21, 401–408. [Google Scholar] [CrossRef]
- Li, F.; Amenorfenyo, D.K.; Zhang, Y.; Zhang, N.; Li, C.; Huang, X. Cultivation of Chlorella vulgaris in Membrane-Treated Industrial Distillery Wastewater: Growth and Wastewater Treatment. Front. Environ. Sci. 2021, 9, 770633. [Google Scholar] [CrossRef]
- Wu, X.; Cen, Q.; Addy, M.; Zheng, H.; Luo, S.; Liu, Y.; Cheng, Y.; Zhou, W.; Chen, P.; Ruan, R. A novel algal biofilm photobioreactor for efficient hog manure wastewater utilization and treatment. Bioresour. Technol. 2019, 292, 121925. [Google Scholar] [CrossRef] [PubMed]
- Guo, G.; Cao, W.; Sun, S.; Zhao, Y.; Hu, C. Nutrient removal and biogas upgrading by integrating fungal-microalgal cultivation with anaerobically digested swine wastewater treatment. J. Appl. Phycol. 2017, 29, 2857–2866. [Google Scholar] [CrossRef]
- Song, C.; Hu, X.; Liu, Z.; Li, S.; Kitamura, Y. Combination of brewery wastewater purification and CO2 fixation with potential value-added ingredients production via different microalgae strains cultivation. J. Clean. Prod. 2020, 268, 122332. [Google Scholar] [CrossRef]
- Leong, W.-H.; Lim, J.-W.; Lam, M.-K.; Uemura, Y.; Ho, C.-D.; Ho, Y.-C. Co-cultivation of activated sludge and microalgae for the simultaneous enhancements of nitrogen-rich wastewater bioremediation and lipid production. J. Taiwan Inst. Chem. Eng. 2018, 87, 216–224. [Google Scholar] [CrossRef]
- Liu, C.; Subashchandrabose, S.; Ming, H.; Xiao, B.; Naidu, R.; Megharaj, M. Phycoremediation of dairy and winery wastewater using Diplosphaera sp. MM1. MM1. J. Appl. Phycol. 2016, 28, 3331–3341. [Google Scholar] [CrossRef]
- Qiao, T.; Zhao, Y.; Han, B.; Li, T.; Zhao, P.; Xu, J.-W.; Huang, L.; Yu, X. Myo-inositol promotes lipid production and nutrients removal by microalga under molasses wastewater. Renew. Energy 2021, 172, 327–335. [Google Scholar] [CrossRef]
- Tan, X.-B.; Zhang, Y.-L.; Zhao, X.-C.; Yang, L.-B.; Yangwang, S.-C.; Zou, Y.; Lu, J.-M. Anaerobic digestates grown oleaginous microalgae for pollutants removal and lipids production. Chemosphere 2022, 308, 136177. [Google Scholar] [CrossRef]
- Yu, J.; You, X.; Wang, Y.; Jin, C.; Zhao, Y.; Guo, L. Focus on the role of synthetic phytohormone for mixotrophic growth and lipid accumulation by Chlorella pyrenoidosa. Chemosphere 2022, 308, 136558. [Google Scholar] [CrossRef]
- Nayak, J.K.; Ghosh, U.K. Post treatment of microalgae treated pharmaceutical wastewater in photosynthetic microbial fuel cell (PMFC) and biodiesel production. Biomass Bioenergy 2019, 131, 105415. [Google Scholar] [CrossRef]
- Yang, Z.; Pei, H.; Hou, Q.; Jiang, L.; Zhang, L.; Nie, C. Algal biofilm-assisted microbial fuel cell to enhance domestic wastewater treatment: Nutrient, organics removal and bioenergy production. Chem. Eng. J. 2018, 332, 277–285. [Google Scholar] [CrossRef]
- Luo, L.; He, H.; Yang, C.; Wen, S.; Zeng, G.; Wu, M.; Zhou, Z.; Lou, W. Nutrient removal and lipid production by Coelastrella sp. in anaerobically and aerobically treated swine wastewater. Bioresour. Technol. 2016, 216, 135–141. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Zhao, Y.; Zhao, G.; Zhang, H. Nutrient removal and biogas upgrading by integrating freshwater algae cultivation with piggery anaerobic digestate liquid treatment. Appl. Microbiol. Biotechnol. 2015, 99, 6493–6501. [Google Scholar] [CrossRef]
- Krishnamoorthy, S.; Manickam, P.; Muthukaruppan, V. Evaluation of distillery wastewater treatability in a customized photobioreactor using blue-green microalgae—Laboratory and outdoor study. J. Environ. Manag. 2019, 234, 412–423. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.; Hao, Y.; Jiang, J.; Liu, W. Valorization of untreated rice bran towards bioflocculant using a lignocellulose-degrading strain and its use in microalgal biomass harvest. Biotechnol. Biofuels 2017, 10, 90. [Google Scholar] [CrossRef]
- Chen, L.; Li, R.; Ren, X.; Liu, T. Improved aqueous extraction of microalgal lipid by combined enzymatic and thermal lysis from wet biomass of Nannochloropsis oceanica. Bioresour. Technol. 2016, 214, 138–143. [Google Scholar] [CrossRef]
- Husin, M.A.M.; Yasin, N.H.M.; Takriff, M.S.; Jamar, N.H. A review on pretreatment methods for lipid extraction from microalgae biomass. Prep. Biochem. Biotechnol. 2023, 1–16. [Google Scholar] [CrossRef]
- Lee, S.Y.; Khoiroh, I.; Vo, D.-V.N.; Kumar, P.S.; Show, P.L. Techniques of lipid extraction from microalgae for biofuel production: A review. Environ. Chem. Lett. 2021, 19, 231–251. [Google Scholar] [CrossRef]
- Munoz-Cupa, C.; Hu, Y.; Xu, C.; Bassi, A. An overview of microbial fuel cell usage in wastewater treatment, resource recovery and energy production. Sci. Total. Environ. 2021, 754, 142429. [Google Scholar] [CrossRef]
- Wang, Y.; Lin, Z.; Su, X.; Zhao, P.; Zhou, J.; He, Q.; Ai, H. Cost-effective domestic wastewater treatment and bioenergy recovery in an immobilized microalgal-based photoautotrophic microbial fuel cell (PMFC). Chem. Eng. J. 2019, 372, 956–965. [Google Scholar] [CrossRef]
- Ribeiro, V.R.; Osório, H.D.D.; Ulrich, A.C.; Rizzetti, T.M.; Barrios, A.S.; Schneider, R.d.C.d.S.; Benitez, L.B. The use of microalgae-microbial fuel cells in wastewater bioremediation and bioelectricity generation. J. Water Process. Eng. 2022, 48, 102882. [Google Scholar] [CrossRef]
- Hadiyanto, H.; Christwardana, M.; Pratiwi, W.Z.; Purwanto, P.; Sudarno, S.; Haryani, K.; Hoang, A.T. Response surface optimization of microalgae microbial fuel cell (MMFC) enhanced by yeast immobilization for bioelectricity production. Chemosphere 2022, 287, 132275. [Google Scholar] [CrossRef] [PubMed]
- Bai, S.; Chen, J.; Guo, M.; Ren, N.; Zhao, X. Vertical-scale spatial influence of radial oxygen loss on rhizosphere microbial community in constructed wetland. Environ. Int. 2023, 171, 107690. [Google Scholar] [CrossRef] [PubMed]
- Guo, M.; Yang, G.; Meng, X.; Zhang, T.; Li, C.; Bai, S.; Zhao, X. Illuminating plant–microbe interaction: How photoperiod affects rhizosphere and pollutant removal in constructed wetland? Environ. Int. 2023, 179, 108144. [Google Scholar] [CrossRef] [PubMed]
- Cheng, M.; Li, X.; Jiao, X.; Zhao, Z.; Zhang, Y. A Critical Review on Iron-Enhanced Constructed Wetland System: Mechanisms and Application Scope. Water, Air, Soil Pollut. 2022, 233, 524. [Google Scholar] [CrossRef]
- Nguyen, T.; Ngo, H.; Guo, W.; Soda, S.; Vu, N.; Bui, T.; Vo, T.; Bui, X.; Pham, T. White hard clam (Meretrix lyrata) shells media to improve phosphorus removal in lab-scale horizontal sub-surface flow constructed wetlands: Performance, removal pathways, and lifespan. Bioresour. Technol. 2020, 312, 123602. [Google Scholar] [CrossRef] [PubMed]
- Zhao, X.; Meng, X.; Dang, B.; Zhang, T.; Shi, W.; Hou, N.; Yan, Q.; Li, C. Succession dynamics of microbial communities responding to the exogenous microalgae ZM-5 and analysis of the environmental sustainability of a constructed wetland system. Bioresour. Technol. 2023, 371, 128642. [Google Scholar] [CrossRef]
- Sandoval-Herazo, M.; Martínez-Reséndiz, G.; Echeverria, E.F.; Fernández-Lambert, G.; Herazo, L.C.S. Plant Biomass Production in Constructed Wetlands Treating Swine Wastewater in Tropical Climates. Fermentation 2021, 7, 296. [Google Scholar] [CrossRef]
- Zhou, S.; Xu, S.; Jiang, Y.; Jiang, C.; Wang, D.; Xu, G.; Yang, D.; Wu, S.; Bai, Z.; Zhuang, G.; et al. Enhancing nitrogen removal from anaerobically-digested swine wastewater through integration of Myriophyllum aquaticum and free nitrous acid-based technology in a constructed wetland. Sci. Total. Environ. 2021, 779, 146441. [Google Scholar] [CrossRef]
- Li, M.; Ge, S.; Zhang, J.; Wu, S.; Wu, H.; Zhuang, L.-L. Mechanism and performance of algal pond assisted constructed wetlands for wastewater polishing and nutrient recovery. Sci. Total. Environ. 2022, 840, 156667. [Google Scholar] [CrossRef]
- Li, S.; Qu, W.; Chang, H.; Li, J.; Ho, S.-H. Microalgae-driven swine wastewater biotreatment: Nutrient recovery, key microbial community and current challenges. J. Hazard. Mater. 2022, 440, 129785. [Google Scholar] [CrossRef]
- Yuan, C.; Zhao, F.; Zhao, X.; Zhao, Y. Woodchips as sustained-release carbon source to enhance the nitrogen transformation of low C/N wastewater in a baffle subsurface flow constructed wetland. Chem. Eng. J. 2020, 392, 124840. [Google Scholar] [CrossRef]
- Guštin, S.; Marinšek-Logar, R. Effect of pH, temperature and air flow rate on the continuous ammonia stripping of the anaerobic digestion effluent. Process. Saf. Environ. Prot. 2011, 89, 61–66. [Google Scholar] [CrossRef]
- Xu, J.; Cheng, J.J.; Stomp, A.-M. Growing Spirodela polyrrhiza in Swine Wastewater for the Production of Animal Feed and Fuel Ethanol: A Pilot Study. CLEAN Soil Air Water 2012, 40, 760–765. [Google Scholar] [CrossRef]
- Dinh, T.T.U.; Soda, S.; Nguyen, T.A.H.; Nakajima, J.; Cao, T.H. Nutrient removal by duckweed from anaerobically treated swine wastewater in lab-scale stabilization ponds in Vietnam. Sci. Total. Environ. 2020, 722, 137854. [Google Scholar] [CrossRef] [PubMed]
- Adhikari, U.; Harrigan, T.; Reinhold, D.M. Use of duckweed-based constructed wetlands for nutrient recovery and pollutant reduction from dairy wastewater. Ecol. Eng. 2015, 78, 6–14. [Google Scholar] [CrossRef]
- Bouali, M.; Zrafi, I.; Mouna, F.; Bakhrouf, A. Pilot study of constructed wetlands for tertiary wastewater treatment using duckweed and immobilized microalgae. Afr. J. Microbiol. Res. 2012, 6, 6066–6074. [Google Scholar] [CrossRef]

| Advantage | Disadvantage | Optimization Direction | Resource Recovery Efficiency | |
|---|---|---|---|---|
| Adsorption technology | Lower cost | Recovered element unitary; small scope of application | Optimized adsorption material | 0.48–54.0 mg/L/d |
| Microbial metabolism | Recovery of energy gas | Tailwater cannot be discharged directly | Make tailwater meet discharge standards | 47.9–95.5 mg/L/d |
| Microbial electrochemical | A wide range of raw materials; High productivity | Expensive | Optimize electrode cost | 190–285 mg/L/d |
| Microalgae reactor | Make up for the lower energy density | High environmental load and economic cost | Reduce environmental load and economic cost | 0.54–505 mg/L/d |
| Constructed wetlands | Treatment results are satisfactory | The processing mechanism remains to be studied | Accurately evaluate the operation efficiency of CWs | 168–262 mg/m2/d |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Meng, X.; Jin, M.; Feng, Q.; Sha, A.; Bai, S.; Zhao, X. Resource and Energy Utilization of Swine Wastewater Treatment: Recent Progress and Future Directions. Separations 2023, 10, 591. https://doi.org/10.3390/separations10120591
Meng X, Jin M, Feng Q, Sha A, Bai S, Zhao X. Resource and Energy Utilization of Swine Wastewater Treatment: Recent Progress and Future Directions. Separations. 2023; 10(12):591. https://doi.org/10.3390/separations10120591
Chicago/Turabian StyleMeng, Xiangwei, Ming Jin, Qianzi Feng, Aiqi Sha, Shunwen Bai, and Xinyue Zhao. 2023. "Resource and Energy Utilization of Swine Wastewater Treatment: Recent Progress and Future Directions" Separations 10, no. 12: 591. https://doi.org/10.3390/separations10120591
APA StyleMeng, X., Jin, M., Feng, Q., Sha, A., Bai, S., & Zhao, X. (2023). Resource and Energy Utilization of Swine Wastewater Treatment: Recent Progress and Future Directions. Separations, 10(12), 591. https://doi.org/10.3390/separations10120591









