Impact of the Smoking Process on Biogenic Amine Levels in Traditional Dry-Cured Chorizo
Abstract
1. Introduction
2. Materials and Methods
2.1. Chorizo Technology and Sampling Procedures
2.2. Physicochemical Analyses
2.3. Sample Preparation and BA Extraction
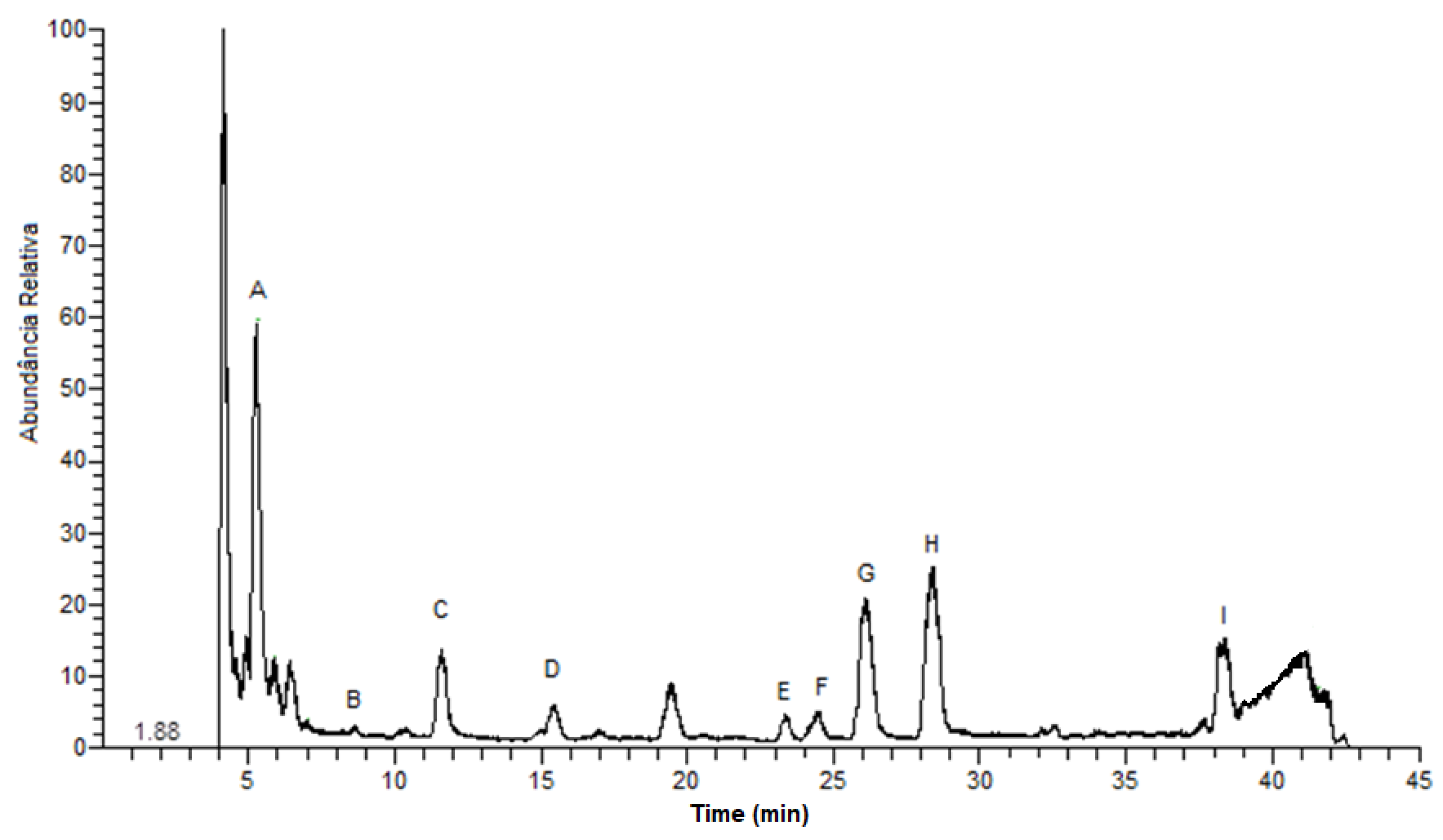
2.4. Chromatographic Analysis by LC-DAD-ESI/MSn
2.5. Chromatographic Analysis by HPLC-DAD
2.6. Statistical Analysis
3. Results and Discussion
3.1. Biogenic Amine Identification
3.2. Biogenic Amine Quantification by HPLC-DAD
3.3. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Patarata, L.; Judas, I.; Silva, J.A.; Esteves, A.; Martins, C. A comparison of the physicochemical and sensory characteristics of alheira samples from different-sized producers. Meat Sci. 2008, 79, 131–138. [Google Scholar] [CrossRef]
- Albano, H.; Henriques, I.; Correia, A.; Hogg, T.; Teixeira, P. Characterization of microbial population of ‘Alheira’ (a traditional Portuguese fermented sausage) by PCR-DGGE and traditional cultural microbiological methods. J. Appl. Microbiol. 2008, 105, 2187–2194. [Google Scholar] [CrossRef] [PubMed]
- Roseiro, L.C.; Gomes, A.; Patarata, L.; Santos, C. Comparative survey of PAHs incidence in Portuguese traditional meat and blood sausages. Food Chem. Toxicol. 2012, 50, 1891–1896. [Google Scholar] [CrossRef] [PubMed]
- Campos, S.D.; Alves, R.C.; Mendes, E.; Costa, A.S.; Casal, S.; Oliveira, M.B. Nutritional value and influence of the thermal processing on a traditional Portuguese fermented sausage (alheira). Meat Sci. 2013, 93, 914–918. [Google Scholar] [CrossRef]
- Ferreira, V.; Barbosa, J.; Vendeiro, S.; Mota, A.; Silva, F.; Monteiro, M.J.; Hogg, T.; Gibbs, P.; Teixeira, P. Chemical and microbiological characterization of alheira: A typical Portuguese fermented sausage with particular reference to factors relating to food safety. Meat Sci. 2006, 73, 570–575. [Google Scholar] [CrossRef] [PubMed]
- Ferreira, V.; Barbosa, J.; Silva, J.; Vendeiro, S.; Mota, A.; Silva, F.; Monteiro, M.J.; Hogg, T.; Gibbs, P.; Teixeira, P. Chemical and microbiological characterisation of “Salpicao de Vinhais” and “Chourica de Vinhais”: Traditional dry sausages produced in the North of Portugal. Food Microbiol. 2007, 24, 618–623. [Google Scholar] [CrossRef]
- Santos, C.; Gomes, A.; Roseiro, L.C. Polycyclic aromatic hydrocarbons incidence in Portuguese traditional smoked meat products. Food Chem. Toxicol. 2011, 49, 2343–2347. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, R.; Gerber, M. Food Processing and the Mediterranean Diet. Nutrients 2015, 7, 7925–7964. [Google Scholar] [CrossRef]
- Simko, P. Determination of polycyclic aromatic hydrocarbons in smoked meat products and smoke flavouring food additives. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2002, 770, 3–18. [Google Scholar] [CrossRef]
- Szterk, A.; Roszko, M.; Malek, K.; Kurek, M.; Zbiec, M.; Waszkiewicz-Robak, B. Profiles and concentrations of heterocyclic aromatic amines formed in beef during various heat treatments depend on the time of ripening and muscle type. Meat Sci. 2012, 92, 587–595. [Google Scholar] [CrossRef]
- Iko Afe, O.H.; Kpoclou, Y.E.; Douny, C.; Anihouvi, V.B.; Igout, A.; Mahillon, J.; Hounhouigan, D.J.; Scippo, M.L. Chemical hazards in smoked meat and fish. Food Sci. Nutr. 2021, 9, 6903–6922. [Google Scholar] [CrossRef] [PubMed]
- Serio, A.; Laika, J.; Maggio, F.; Sacchetti, G.; D’Alessandro, F.; Rossi, C.; Martuscelli, M.; Chaves-Lopez, C.; Paparella, A. Casing Contribution to Proteolytic Changes and Biogenic Amines Content in the Production of an Artisanal Naturally Fermented Dry Sausage. Foods 2020, 9, 1286. [Google Scholar] [CrossRef] [PubMed]
- Suzzi, G.; Gardini, F. Biogenic amines in dry fermented sausages: A review. Int. J. Food Microbiol. 2003, 88, 41–54. [Google Scholar] [CrossRef]
- Kuley, E.; Ozogul, F.; Balikci, E.; Durmus, M.; Ayas, D. The influences of fish infusion broth on the biogenic amines formation by lactic acid bacteria. Braz. J. Microbiol. 2013, 44, 407–415. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Wójcik, W.; Lukasiewicz, M.; Puppel, K. Biogenic amines: Formation, action and toxicity—A review. J. Sci. Food Agric. 2021, 101, 2634–2640. [Google Scholar] [CrossRef] [PubMed]
- Gardini, F.; Ozogul, Y.; Suzzi, G.; Tabanelli, G.; Ozogul, F. Technological Factors Affecting Biogenic Amine Content in Foods: A Review. Front. Microbiol. 2016, 7, 1218. [Google Scholar] [CrossRef] [PubMed]
- Ramos, R.M.; Valente, I.M.; Rodrigues, J.A. Analysis of biogenic amines in wines by salting-out assisted liquid-liquid extraction and high-performance liquid chromatography with fluorimetric detection. Talanta 2014, 124, 146–151. [Google Scholar] [CrossRef]
- Valente, I.M.; Goncalves, L.M.; Rodrigues, J.A. Another glimpse over the salting-out assisted liquid-liquid extraction in acetonitrile/water mixtures. J. Chromatogr. A 2013, 1308, 58–62. [Google Scholar] [CrossRef]
- Douny, C.; Benmedjadi, S.; Brose, F.; Afé, O.H.I.; Igout, A.; Hounhouigan, D.J.; Anihouvi, V.B.; Scippo, M.L. Development of an Analytical Method for the Simultaneous Measurement of 10 Biogenic Amines in Meat: Application to Beninese Grilled Pork Samples. Food Anal. Method 2019, 12, 2392–2400. [Google Scholar] [CrossRef]
- Gu, M.H.; Li, C.; Su, Y.Y.; Chen, L.; Li, S.B.; Li, X.; Zheng, X.C.; Zhang, D.Q. Novel insights from protein degradation: Deciphering the dynamic evolution of biogenic amines as a quality indicator in pork during storage. Food Res. Int. 2023, 167, 112684. [Google Scholar] [CrossRef]
- Visciano, P.; Schirone, M.; Paparella, A. An Overview of Histamine and Other Biogenic Amines in Fish and Fish Products. Foods 2020, 9, 1795. [Google Scholar] [CrossRef]
- Martuscelli, M.; Pittia, P.; Casamassima, L.M.; Manetta, A.C.; Lupieri, L.; Neri, L. Effect of intensity of smoking treatment on the free amino acids and biogenic amines occurrence in dry cured ham. Food Chem. 2009, 116, 955–962. [Google Scholar] [CrossRef]
- Rabie, M.A.; Siliha, H.; el-Saidy, S.; el-Badawy, A.A.; Malcata, F.X. Reduced biogenic amine contents in via addition of selected lactic acid bacteria. Food Chem. 2011, 129, 1778–1782. [Google Scholar] [CrossRef]
- Pluta-Kubica, A.; Filipczak-Fiutak, M.; Domagala, J.; Duda, I.; Migdal, W. Contamination of traditionally smoked cheeses with polycyclic aromatic hydrocarbons and biogenic amines. Food Control 2020, 112, 107115. [Google Scholar] [CrossRef]
- Doeun, D.; Davaatseren, M.; Chung, M.S. Biogenic amines in foods. Food Sci. Biotechnol. 2017, 26, 1463–1474. [Google Scholar] [CrossRef]
- Biji, K.B.; Ravishankar, C.N.; Venkateswarlu, R.; Mohan, C.O.; Gopal, T.K. Biogenic amines in seafood: A review. J. Food Sci. Technol. 2016, 53, 2210–2218. [Google Scholar] [CrossRef]
- del Rio, B.; Redruello, B.; Fernandez, M.; Martin, M.C.; Ladero, V.; Alvarez, M.A. The biogenic amine tryptamine, unlike β-phenylethylamine, shows cytotoxicity at concentrations that have been found in foods. Food Chem. 2020, 331, 127303. [Google Scholar] [CrossRef]
- Li, D.W.; Zhang, W.G. Biogenic amines and volatile -nitrosamines in Chinese smoked-cured bacon (Larou) from industrial and artisanal origins. Food Addit. Contam. Part B-Surveill. 2023, 16, 143–160. [Google Scholar] [CrossRef]


| Parameter | Value |
|---|---|
| Water activity (aw) | 0.94 |
| Salt content | 3.2 g 100 g−1 |
| Benzo(a)pyrene | 0.70 µg kg−1 |
| Nitrates | <68 mg NaNO3 kg−1 |
| Nitrites | <11 mg NaNO2 kg−1 |
| Derivatized BA * | Rt (min) | λmax (nm) | [M + H]+ | MS2 (rel.int.) |
|---|---|---|---|---|
| MET + 1DNS | 9.29 | 239, 332 | 265.3 | 250.0 (100%), 171.2 (50%) |
| Ethylamine + 1DNS | 11.61 | 239, 335 | 279.2 | 263.9 (100%), 157.2 (90%) |
| Dimethylamine + 1DNS | 15.05 | 239, 320 | 279.2 | 264.0 (100%), 157.3 (70%) |
| PHE + 1DNS | 23.43 | 245, 335 | 355.3 | 157.2 (100%), 339.9 (70%) |
| ISO-PEN + 1DNS | 24.62 | 239, 335 | 321.3 | 157.2 (100%), 306.0 (30%) |
| PUT + 2DNS | 26.22 | 221, 251 | 555.4 | 304.0 (100%), 220.1 (90%) |
| CAD + 2DNS | 28.48 | 224, 251, 335 | 569.4 | 318.1 (100%), 169.2 (90%) |
| TYR + 2DNS | 38.43 | 227, 248, 344 | 604.3 | 370.0 (100%), 171.1 (40%) |
| Batch | Type of Production | Stage of the Filling Process | MET | PHE | ISO-PEN | PUT | CAD | TYR | Total |
|---|---|---|---|---|---|---|---|---|---|
| A | Non-smoked | Beginning | 0.213 ± 0.066 | 1.14 ± 0.12 | ND | 26.3 ± 9.7 | 261 ± 28 | 88.0 ± 8.2 | 377 ± 45 |
| End | 0.241 ± 0.057 | ND | ND | ND | 52.2 ± 1.7 | 17.7 ± 1.7 | 70.1 ± 0.5 | ||
| Smoked | Beginning | 0.489 ± 0.026 | 14.9 ± 1.4 | 1.91 ± 0.07 | 372 ± 85 | 570 ± 83 | 281 ± 29 | 1239 ± 192 | |
| End | 0.488 ± 0.169 | 0.689 ± 0.255 | 1.62 ± 0.07 | 181 ± 27 | 263 ± 43 | 230 ± 50 | 677 ± 116 | ||
| B | Non-smoked | Beginning | 0.274 ± 0.053 | 0.209 ± 0.116 | ND | ND | ND | ND | 0.482 ± 0.159 |
| End | 0.182 ± 0.057 | ND | ND | ND | 36.9 ± 10.1 | 19.3 ± 3.0 | 56.4 ± 12.9 | ||
| Smoked | Beginning | 0.524 ± 0291 | 36.4 ± 2.9 | 1.89 ± 0.53 | 1372 ± 70 | 261 ± 68 | 199 ± 64 | 1871 ± 147 | |
| End | 0.698 ± 0.101 | 33.9 ± 1.4 | 2.20 ± 0.20 | 837 ± 113 | 701 ± 159 | 175 ± 41 | 1749 ± 307 |
| MET | PHE | ISO-PEN | PUT | CAD | TYR | Total | |
|---|---|---|---|---|---|---|---|
| p-values | |||||||
| Batch | 0.481 | <0.05 * | 0.704 | <0.05 * | 0.582 | 0.720 | 0.289 |
| Type | <0.05 * | <0.05 * | <0.05 * | <0.05 * | <0.05 * | <0.05 * | <0.05 * |
| Stage of filling | 0.760 | 0.464 | 0.958 | 0.387 | 0.981 | 0.524 | 0.454 |
| MET | PHE | ISO-PEN | PUT | CAD | TYR | Total | ||
|---|---|---|---|---|---|---|---|---|
| p-values | ||||||||
| Type | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | <0.001 * | |
| Stage of filling | 0.589 | 0.260 | 0.814 | 0.238 | 0.974 | 0.297 | 0.108 | |
| Type × Stage of filling | 0.239 | 0.362 | 0.814 | 0.278 | 0.472 | 0.955 | 0.446 | |
| RSD | 0.133 | 10.9 | 0.255 | 354 | 194 | 68.2 | 379 | |
| r2 | 0.647 | 0.547 | 0.945 | 0.430 | 0.382 | 0.598 | 0.775 | |
| Adjusted r2 | 0.594 | 0.479 | 0.937 | 0.345 | 0.289 | 0.538 | 0.741 | |
| Mean values for the main effects | ||||||||
| Type | Non-smoked | 0.228 | 0.451 | nd | 6.59 | 64.3 | 31.3 | 126 |
| Smoked | 0.550 | 21.5 | 1.93 | 525 | 337 | 181 | 1384 | |
| Stage of filling | Beginning | 0.375 | 13.3 | 0.952 | 345 | 202 | 119 | 872 |
| End | 0.402 | 8.65 | 0.975 | 187 | 199 | 93 | 638 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valente, I.M.; Sousa, C.S.; Guido, L.F. Impact of the Smoking Process on Biogenic Amine Levels in Traditional Dry-Cured Chorizo. Separations 2023, 10, 585. https://doi.org/10.3390/separations10120585
Valente IM, Sousa CS, Guido LF. Impact of the Smoking Process on Biogenic Amine Levels in Traditional Dry-Cured Chorizo. Separations. 2023; 10(12):585. https://doi.org/10.3390/separations10120585
Chicago/Turabian StyleValente, Inês M., Cláudia S. Sousa, and Luís F. Guido. 2023. "Impact of the Smoking Process on Biogenic Amine Levels in Traditional Dry-Cured Chorizo" Separations 10, no. 12: 585. https://doi.org/10.3390/separations10120585
APA StyleValente, I. M., Sousa, C. S., & Guido, L. F. (2023). Impact of the Smoking Process on Biogenic Amine Levels in Traditional Dry-Cured Chorizo. Separations, 10(12), 585. https://doi.org/10.3390/separations10120585








