Abstract
Various porous polymer materials have been prepared for the separation of CO2 from mixed gases. However, complex processes, expensive monomers, and costly catalysts are commonly used for their synthesis, making the adsorbents difficult to achieve in industrial applications. Herein, we developed a strategy to fabricate a series of benzene rings containing porous polymer materials (B-PPMs) via a facile condensation reaction of two inexpensive monomers, namely tetraphenylsilane and 1,4-bis(bromomethyl)benzene. The B-PPMs are verified to have accessible surface areas, large pore volumes, and appreciate pore sizes via a series of characterizations. The B-PPM-2 exhibits the best CO2 adsorption amount of 67 cm3·g−1 at 273 K and 1 bar, while the CO2/N2 selectivity can reach 64.5 and 51.9 at 273 K and 298 K, respectively. Furthermore, the adsorbent B-PPM-2 can be completely regenerated after five cycles of breakthrough experiments under mild conditions, which may provide promising candidates for selective capture of CO2 from mixtures.
1. Introduction
Carbon dioxide, being a greenhouse gas, has wrought environmental havoc, severe pollution, weather calamities, and negative impacts on animals and plants and has become an urgent environmental crisis [1,2,3]. Carbon capture and storage is considered a presumable technology that can separate CO2 from fossil fuels, thereby reducing the increasing carbon dioxide emissions into the atmosphere. Generally speaking, the traditional method for removing CO2 is the ‘wet method’, namely chemical adsorption, which uses liquid ammonia solutions, such as monoethanolamine [4,5,6], diethanolamine [7,8], and diisopropanolamine [9,10]. However, solvent absorption methods have the disadvantages of severe equipment corrosion, high regeneration energy consumption, and decreased absorption capacity.
Different from the solvent adsorption process, the usage of solid adsorbents has the advantages of simple preparation, low cost, and environmental friendliness, which have been considered promising alternative methods for the effective removal of CO2. Up to now, a variety of porous materials, including metal oxides [11,12,13], mesoporous silica [14,15], activated carbon [16,17,18,19], porous organic polymers (POPs) [20,21,22,23,24,25,26,27,28], metal organic frameworks (MOFs) [29,30,31], and natural zeolites [32,33] has been prepared for the separation of CO2 from mixed gases such as biogas and natural gas, showing enhanced capture performance. With widespread attention from the scientific and industrial communities, the design and preparation of adsorbent materials with higher adsorption capacity and lower costs have become the focus direction of solid adsorbent research at present.
According to the literature, MOFs exhibit excellent adsorption capacity for CO2 because of their large specific surface area and high pore volume [34,35]. However, most reported MOFs are synthesized via complex processes and expensive monomers, and their structures are prone to collapse under high temperatures, moisture, and other harsh environments, rendering them unsuitable for industrial applications. As a porous solid adsorption material, POPs exhibit more stable physicochemical properties due to their stable covalent bond structure. For example, via condensation reactions of tris(4-aminophenyl)amine (TAPA) and 4,4′,4″-boranetriyltris(2,3,5,6-tetramethylbenzaldehyde) (BTMA) with the presence of a catalyst, Zhai et al. synthesized the porous polymer, BTMA-TAPA-COF, which exhibited large surface areas of 630 m2·g−1 and a good CO2 capture capacity of 42 cm3·g−1 [36]. Nevertheless, it should be stated that the BTMA was prepared from the dangerous and expensive monomer tert-Butyllithium at 195 K under a series of complex reactions. PAF-8 was synthesized via the Yamamoto-type Ullmann reactions from two monomers of tetraphenylsilane and formaldehyde dimethyl acetal without the presence of water and oxygen. The resultant PAF-8 possessed a high surface area of 785 m2·g−1 as well as a good CO2 capacity of 35.5 cm3·g−1 [37]. Despite the numerous solid porous polymer materials that have been synthesized to date, the challenge remains in synthesizing the porous polymer materials from cost-effective monomers via a simple polymerization reaction with the absence of a catalyst.
In the present study, we devised and implemented a strategy to fabricate a series of benzene rings containing porous polymer materials (B-PPMs) via a straightforward condensation reaction involving tetraphenylsilane and 1,4-bis(bromomethyl)benzene (Scheme 1). The polymerization reactions can occur with the absence of any catalysts under mind conditions because of the proper reactivity of the reagent. In addition, the low-cost and readily available monomers without the use of the expensive catalyst enable a good economy and large-scale production of the B-PPMs in the future. The B-PPMs exhibit corresponding accessible surface areas and large pore volumes and appreciate pore sizes achieved by modulating the quantity of 1,4-bis(bromomethyl)benzene. The B-PPMs exhibit remarkable CO2 capture performance, ranging from 38 to 67 cm3·g−1 at 273 K and 1 bar. It is noteworthy that the B-PPM-2 displays outstanding CO2 capture performance with a capacity of 67 cm3·g−1, which surpasses many other reported adsorbents, like BoxPOP-2 (34.7 cm3·g−1) [38], 476-MOF (47.6 cm3·g−1) [39], and A5 Zeolite (30.2 cm3·g−1) [32]. Additionally, the B-PPM-2 exhibits remarkable CO2/N2 selectivity of 64.5 at 273 K and 0.1 bar. Notably, the B-PPM-2 also displays full regeneration performance without any significant decrease in capacity after six consecutive cycles. Furthermore, the adsorbent B-PPM-2 maintains strong separation performance after five cycles of breakthrough experiments, which may provide promising candidates for selective capture of CO2 from CO2/N2 mixtures.

Scheme 1.
Synthetic route of the B-PPM-2.
2. Materials and Methods
2.1. Materials Synthesis
Commercial reagents of the tetraphenylsilane, 1,4-bis(bromomethyl)benzene, and tetrahydrofuran (THF) were purchased from Adamas-beta, Shanghai, China, and all the reagents were directly utilized without any further treatment. The ethanol and deionized water were employed for cleaning procedures during the preparation process of the adsorbents.
The minute synthesis route of the B-PPM-2 was drawn in Scheme 1. Then, 1 mmol tetraphenylsilane and 4 mmol 1,4-bis(bromomethyl)benzene were dissolved in the THF (50 mL) in turn. Then, the mixture solution was transferred to a sealed container and kept at constant temperature of 333 K for 48 h with continuous stirring. Upon cooling to room temperature, the filter cake was rinsed alternately with methanol and deionized water until the desired neutralization was achieved. With further vacuum drying overnight for the removal of the traces of methanol and moisture, the obtained residual was donated as B-PPM-2. All the B-PPMs were synthesized via the same reaction as drawn in Scheme 1. The resultant B-PPM-1, B-PPM-3, and B-PPM-4 are prepared successfully via the homologous synthesizing process, which corresponds to above-mentioned 1,4-bis(bromomethyl)benzene reaction molar amount of 8 mmol, 2 mmol, and 1 mmol, respectively.
2.2. Characterization
The fracture and generation of chemical bonds are direct evidence for detecting the successful preparation of materials. Fourier transform infrared (FTIR) spectra of the samples were recorded on a Nicolet Nexus 360 spectrometer (Thermo Nicolet, Waltham, USA). The flakes were uniformly mixed using the adsorbent and potassium bromide in a mass ratio of 1:150 and tested for 32 scans with a wavenumber between 4000 cm−1 and 650 cm−1. The X-ray powder diffraction (XRPD) patterns could characterize crystal structure of the B-PPMs and were recorded using the Bruker D8 Advance diffractometer (Bruker, Billerica, USA). The detailed experiment conditions included the 2θ range of 5–60, the step size of 0.02°, and the scan rate of 1°·min−1. Thermogravimetry (TG) analyses were performed with a TGA209F3 (Netzsch, Bavaria, Germany) apparatus to obtain thermal stability. The B-PPMs were heated from 298 K to 1073 K at a heating rate of 10 K·min−1, with the protective atmosphere of high-purity nitrogen (99.999%) avoiding oxidation. Images of high-resolution transmission electron microscopy (HRTEM) were obtained using a JEM-2010 UHR electron microscope (JEOL, Tokyo, Japan). The scanning electron microscope (SEM) images were observed on a Hitachi S-4800 (Hitachi, Tokyo, Japan). The N2 adsorption–desorption isotherms were conducted to explore the structural properties of the B-PPMs at 77 K with BSD-660 (BSD, Beijing, China). Prior to the isotherm test, the B-PPMs were dried at 383 K under vacuum conditions for 1 h. The samples of 0.1 g were heated in a vacuum-drying oven for 3 h and then quickly transferred to the analyzer tube to prevent contamination from moisture and impurities in the air.
2.3. Adsorption Tests
The ASAP 2020 analyzer (Micromeritics, Atlanta, GA, USA) was employed for the CO2, CH4, and N2 (pure, 99.999%) static adsorption over the B-PPMs at 273 K and 298 K and 0–1 bar. The helium was used to measure the dead volume due to the fact that helium was rarely adsorbed into the B-PPMs. The isosteric heats of adsorption (Qst) were employed to illustrate the interaction between the B-PPMs and the gas molecules and calculated using the Clausius−Clapeyron equation. To predict the selectivity of CO2 over N2 and CH4, the ideal adsorption solution theory (IAST) was employed. The IAST was defined as (xi/yj)/(xj/yi), in which xi and yi (xj and yj) were the molar fractions of component 1 (component 2) in the adsorbed and bulk phases, respectively.
Dynamic breakthrough experiments were critical to further probe the potential of B-PPM-2 in industrial production. Approximately 0.5 g B-PPM-2 were filled into a special glass tube and dried under vacuum for 1 h at 393 K, then submerged in the mixture of ice and water. The CO2/N2 (15/85, v/v) mixture was fed into the system at the rate of 3 mL·min−1 at 273 K and 1 bar. The composition of each component gas at the outlet was precisely recorded every 30 s using automated gas chromatography and drawn as the dynamic breakthrough curves.
3. Results and Discussion
3.1. Characterization
The FT−IR spectra and TG curves of the B-PPMs are listed in Figure 1a and 1b, respectively. The B-PPMs’ successful fabrication can be confirmed via the FT−IR spectra, which are listed in Figure 1a. The –C–Si– bond of the regent tetraphenylsilane is readily recognized by its stretching vibration bands, which are located at 736 cm−1 and 803 cm−1 [40,41]. These two stretching vibration peaks of –C–Si– are observable in the B-PPMs, illustrating the successful introduction of tetraphenylsilane to the B-PPMs. The intense stretching vibration of –C–Br– of the monomer 1,4-bis(bromomethyl)benzene located at 743 cm−1 is weakened in the B-PPMs, exhibiting the break of the –C–Br– during the fabrication process. These results imply the B-PPMs are successfully fabricated via a facile condensation reaction.
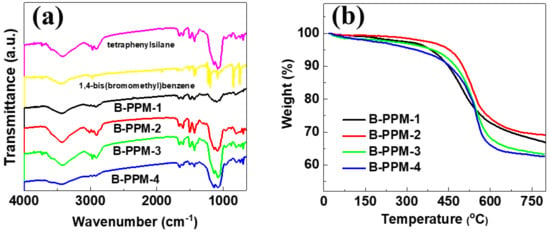
Figure 1.
(a) FT−IR spectra and (b) TG curves of the B-PPMs.
Figure 1b shows the TG curves of the B-PPMs, which reflect the thermal stability information. All the samples show two main weight loss processes, and the adsorbed trace water and impurities quickly escape from the pore structure of the B-PPMs at around 150 °C. A sharp weight loss appears, ranging from 450 °C to 600 °C, giving evidence of the high thermal stability of the B-PPMs. It is obviously observed that the B-PPM-2 shows a high initial decomposition temperature of about 450 °C, which is higher than that of B-PPM-1 as well as that of B-PPM-3 and B-PPM-4. Remarkably, the B-PPM-2 manifests a total weight residual of 69%, which is bigger in contrast to that of the B-PPM-1, B-PPM-3, and B-PPM-4 (correspond to 66%, 63%, and 62%, respectively). The difference in the thermal stability is dictated by introducing 1,4-bis(bromomethyl)benzene. The high-quality residue remaining after the heat treatment indicates the successful synthetization of the B-PPMs with a high crosslinking degree from the two simple reactants. The amorphous characteristics of the B-PPMs are validated via the XRD in Figure S1, and no intense diffraction peaks are observed [42].
All four N2 adsorption–desorption isotherms of the B-PPMs are drawn in Figure 2a and the corresponding pore size distribution of the B-PPMs are drawn in Figure 2b. It is obvious that the isotherms of the B-PPMs are assigned to the characteristic IV-type isotherm. At the relative pressure ranging from 0 to 0.05, the B-PPMs exhibit a linear increase in the amount of captured N2, which is relevant to the high porosity. With the relative pressure increase of more than 0.1, the B-PPMs still illustrate a rapid escalation in N2 uptakes along with visible hysteresis loops. These results indicate that the B-PPMs achieve a great deal of micropores and mesopores, which results from the condensation reaction of the two monomers. As the introducing amount of the monomer 1,4-bis(bromomethyl)benzene decreases, the B-PPMs show a marked decline in N2 uptakes. It is believed that the 1,4-bis(bromomethyl)benzene significantly determines the pore structure of the B-PPMs.

Figure 2.
(a) Nitrogen adsorption−desorption isotherms at 77 K; (b) corresponding pore size distributions of B-PPMs.
As listed in Table 1, the calculated specific surface areas of the B-PPMs have been calculated upon the N2 adsorption−desorption isotherms. The B-PPM-1 owns the largest surface area, meaning 680 m2·g−1, surpassing the B-PPM-2, B-PPM-3, and B-PPM-4, which registered surface areas of 593 m2·g−1, 405 m2·g−1, and 309 m2·g−1, respectively. In line with the tendency of the specific surface area, the B-PPM-1 possesses the highest total pore volume of 0.61 cm3·g−1, while the total pore volume of the B-PPM-2, B-PPM-3, and B-PPM-4 range from 0.49 cm3·g−1 to 0.26 cm3·g−1.

Table 1.
Porosity properties of B-PPMs.
As shown in Figure 2b, the corresponding pore size distribution of the B-PPMs illustrates that all the B-PPMs possess abundant micropores, which are favorable for the CO2 molecules immobilization. The B-PPM-1 exposes the ultra-micropores between 0.45 nm to 0.81 nm. Simultaneously, the B-PPM-2 exhibits micropores around 0.52–0.83 nm, slightly less than those observed in B-PPM-1, a consequence of the decreased introduction of the 1,4-bis(bromomethyl)benzene. With decreasing the 1,4-bis(bromomethyl)benzene content, the pore structures of the polymer-PPMs are undeveloped evidently, as previously mentioned. The B-PPM-3 and B-PPM-4 both show fewer pores, which are located around between 0.45 and 0.85 nm and between 0.55 and 0.9 nm, respectively. In accordance with the TG curves, these results indicate that the 1,4-bis(bromomethyl)benzene determines the pore structures of the porous polymer. It is clearly observed that all the B-PPMs mainly reveal microporous structures. Based on previous work, the micropores are regarded as responsible for gas storage and transport [43,44,45]. Consequently, the synthesized B-PPMs with large surface areas and well-distributed micropores possess great potential for use in gas separation and storage.
The surface morphologies and pore structures of the B-PPMs are observed using SEM. As shown in Figure S2, all of the B-PPMs exhibit irregular block morphology with disordered channels and pores. The TEM images of the representative sample B-PPM-2 are displayed in Figure S3, and the images highlight the abundant micropores with the wormhole-like arrangement of B-PPM-2 from macropores to micropores that are hyperconnected.
3.2. Gas Adsorption Performance
The gas capture performance of adsorbents B-PPMs for pure single-component CO2 was investigated and shown in Figure 3a, while Figure 3b,c exhibit the CO2 isosteric heat of the B-PPMs and the IAST selectivity for the B-PPM-2. With the absolute pressure increased from 0 to 1 bar, all the B-PPMs emerge a tendency for the CO2 amount to increase steadily and gently at 273 K. These adsorption isotherms indicate the reality that the accessible pore structures of the B-PPMs have been occupied by a great deal of CO2 molecules. For instance, B-PPM-1 demonstrates a CO2 adsorption capacity of 52 cm3·g−1, primarily attributed to its high surface area of 680 m2·g−1 and total pore volume of 0.61 cm3·g−1. As reported in the literature, a higher specific surface area of the adsorbent always supplies the more available accessible pores and achieves the higher CO2 adsorption capacity [44,46,47]. Consequently, it is beyond question that the insufficient pore structure of the B-PPM-3 and B-PPM-4, characterized by smaller surface area (405 m2·g−1 and 309 m2·g−1) and pore volume (0.34 cm3·g−1 and 0.26 cm3·g−1), lead to the lower CO2 uptake of 41 cm3·g−1 and 38 cm3·g−1.

Figure 3.
(a) CO2 adsorption isotherms of the B-PPMs at 273 K, (b) CO2 isosteric heat of adsorption of the B-PPMs, (c) IAST selectivity of CO2/N2 (15:85) and CO2/CH4 (50:50) on the B-PPM-2 at 273 K.
However, the largest surface area does not have responsibility for ensuring the highest CO2 adsorptive capacity, and the B-PPM-2 demonstrate the highest CO2 adsorptive capacity of 67 cm3·g−1, which is also better than much other literature data (as listed in Table 2), such as 476-MOF (47.6 cm3·g−1) [39], BoxPOP-2 (34.7 cm3·g−1) [38], 13X-PEI-60 (48.2 cm3·g−1) [48], A5 Zeolite (30.2 cm3·g−1) [32], and P2 (67.6 cm3·g−1) [49]. Even more interesting is that the B-PPM-2 possesses a lower surface area (593 m2·g−1) and pore volume (0.34 cm3·g−1) compared with the B-PPM-1. This contrastive result confirms that the surface area is not the sole determinant of CO2 adsorptive capacity. The pore size of B-PPMs is also beneficial to the improvement of the CO2 adsorptive performance. As previous work reported, theoretical calculation indicates that Van der Waal’s interaction between the captured CO2 and the pores of the adsorbents generates powerful heat effects. Along with the reduction in the pore diameters from 2 nm to 0.5 nm, the calculated adsorption heat values range from 18.9 kJ·mol−1 to 29.6 kJ·mol−1 [50]. Similar results are shown in Figure 3b: all the Qst values of the B-PPMs are located around the 26 kJ·mol−1 to 37 kJ·mol−1, exhibiting that the Qst results from the CO2 capacity into the micropore structure of the B-PPMs. It is interesting that the Qst value of the B-PPM-2 is always slightly bigger than that of the B-PPM-1, B-PPM-3, and B-PPM-4 at a certain adsorption capacity, Originating from the more developed micropore structure of the B-PPM-2. When the pore diameter falls below 0.5 nm, the so-called ultra-micropores are rendered useless because the CO2 molecules are prevented from accessing the tiny space [50]. In the case of the B-PPM-1, the micropores with sizes less than 0.5 nm do not help in capturing the CO2 molecular, while the B-PPM-2 is instrumental in immobilizing the CO2 molecular because of the micropore size about 0.52–0.83 nm. Therefore, the efficient improvement of the CO2 adsorptive capacity originates from the synergistic effect of the specific surface area, pore volume, and appropriate micropore size of the B-PPMs.

Table 2.
The adsorption capacity and selectivity for the different adsorbents.
As shown in Figure S4, the adsorption isotherms of CO2, CH4, and N2 on B-PPMs are systematically investigated. Under the influence of a higher temperature of 298 K at 1 bar, the CO2 uptakes of the B-PPMs manifest a marked decline. For example, the adsorption capacity of B-PPM-2 has decreased from 67 cm3·g−1 to 34 cm3·g−1. All four B-PPM polymers show reasonable CO2 adsorptive capacity and barely a few N2 uptakes. The sample B-PPM-2 not only exhibits the best adsorption performance for CO2 but also has the highest adsorption capacity for CH4 (18 cm3·g−1) and N2 (3 cm3·g−1) at 273 K and 1 bar, signifying that the B-PPM-2 has a latent capacity for industrial applications, particularly in the separation of the CO2/N2 and CO2/CH4 gas mixture. The selectivity of CO2/CH4 and CO2/N2 for the B-PPM-2 at 273 K and 298 K were determined using the IAST model, as depicted in Figure 3c and Figure S5. Wherein, the mixture gases have a resemblance with the typical industrial gas composition, with the CO2/CH4 and CO2/N2 ratios of 50/50 and 15/85. At 273 K and 0.1 bar, the CO2/N2 selectivity of the B-PPM -2 is as high as 64.5, making allowances for the relatively few captured amounts of N2. The selectivity value of 64.5 for the B-PPM-2 is higher than much other literature data (as listed in Table 2, e.g., 40 for CAGE [42], 53 for M90_0.5 [51], 34 for P2 [49], 46 for TPI-5 [25], and 47 for AHEP [54]). Compared with some membranes reported in the literature, such as 64 for PPPS/PDMS/PSf (298 K, 5 bar) [55] and 54.8 for Cu-BTC-SC/Pebax (298 K, 1.5 bar) [56], the B-PPM-2 are equally capable of selectively separating the CO2 from the mixture. The CO2/CH4 selectivity of the B-PPM-2 is 6.6 at 273 K and 0.1 bar, which slightly decreases to 5.1 upon the high temperature. Moreover, the IAST selectivity on the B-PPM-2 sample at 273 K and a certain pressure are invariably higher than those at 298 K. For example, at the 0.3 bar, the CO2/N2 selectivity on B-PPM-2 is 16.7 at 273 K, which is higher than 13.4 at 298 K. The results indicate that the B-PPM-2 can produce a marked effect on CO2 purification in the CO2/CH4 and CO2/N2 mixed systems.
In general, the sorbent regeneration stage is the most critical step of CO2 capture. Numerous regeneration methods have been used in the different regeneration strategies (e.g., PSA, TSA, and VSA) [57,58]. In this case, the method of regeneration in situ is accepted since the porous materials B-PPMs are prepared and tested in the lab. To further investigate the feasibility of the long-term use of the industrial separation of B-PPM-2, consecutive recycling experiments were conducted, as shown in Figure 4a. The adsorption/desorption isotherms of CO2 for the B-PPM-2 were measured for six cycles. It is clear that there is a slight loss of CO2 adsorption capacity during the continuous six cycles. In a single cycle, the adsorption isotherm exhibits a smooth increase, and the desorption isotherm shows the absolute removal of the adsorbed CO2 gases. Following the completion of the entire adsorption/desorption process, a very mild vacuum step is operated at 273 K and maintained for 1 h. After the six cycles, all the adsorption/desorption isotherms are almost coincident, indicating the excellent CO2 adsorption regeneration of the B-PPM-2. Compared with the initial CO2 adsorptive capacity of 67 cm3·g−1, the adsorptive capacity for the sixth cycle is approximately 66.2 cm3·g−1, indicating nearly equivalent performance. These results demonstrate that by only adjusting the operating pressure, CO2 can be entirely released without any residue from the samples. The excellent regeneration will give the B-PPM-2 a passport to the application in the pressure swing adsorption technology (PSA).

Figure 4.
(a) Six adsorption/desorption cycles of CO2 over B-PPM-2 at 273 K and 1 bar. (b) Dynamic breakthrough curves of the B-PPM-2 for CO2/N2 mixture.
Moreover, the price of the porous adsorbents is a crucial factor for the sustained use of industrial separation. The gas separation process over the various solid adsorbents is inherently similar. Therefore, the costs of the monomers and the catalysis mainly determine the overall cost of porous adsorbents. A comparison of the cost of adsorbents based on the price of monomers and catalysts is listed in Table S1. It is important to note that the calculated price of the adsorbents is based on a rough estimation under realistic reaction conditions. Numerous additional factors, such as reaction temperature, duration, multi-level reactions, and pressure, may impact product prices but are not fully considered in this analysis. Consequently, in view of these data, it is evident that the rough price of our material B-PPM-2 is less than that of some reported porous adsorbents.
Dynamic breakthrough experiments are important to further assess the selective adsorption performance of B-PPM-2 in the PSA. As depicted in Figure 4b, in the case of the first dynamic breakthrough experiment for the B-PPM-2, N2 is the first component to break via the fixed bed. This observation is attributed to the poor capture of N2 via B-PPM-2. Moreover, the CO2 breaks the fixed bed over B-PPM-2 at 450 s, which is much longer than 30 s of N2. In the case of the fifth dynamic breakthrough experiment, the N2 molecular escape from the fixed bed as quickly as around 30 s is out of the question. Correspondingly, the CO2 breaks the fixed bed over B-PPM-2 at 420 s, which is a slight fluctuation, compared with the 450 s of the initial one. These results of the dynamic breakthrough curve of the B-PPM-2 are consistent with the single-component adsorption isotherms, indicating more powerful evidence for the potential application over high-efficient CO2 capture from gas mixtures. Altogether, the excellent recycling performance and low price further confirm that our porous material B-PPM-2 is a promising adsorbent for the separation of CO2/N2.
4. Conclusions
In this work, a series of porous polymer B-PPMs originated from the facile polymerization of the tetraphenylsilane and 1,4-bis(bromomethyl)benzene were synthesized successfully. The introduction amount variation of the 1,4-bis(bromomethyl)benzene monomer is important to upgrade the pore structure of the B-PPMs. The B-PPM-2, distinguished by the substantial surface area, generous pore volume, and well-suited micropore size, not only effectuates an outstanding CO2 capture capacity of 67 cm3·g−1 but also displays a remarkable CO2/N2 selectivity of 64.5. We believe that our B-PPMs have potential as competitive candidates for CO2 adsorbents, and this facile synthetic protocol can be further developed to achieve superior porous polymer materials.
Supplementary Materials
The following supporting information can be downloaded at: https://www.mdpi.com/article/10.3390/separations10120581/s1, Figure S1: XRD pattern of the B-PPMs. Figure S2: SEM images of the (a) B-PPM-1, (b) B-PPM-2, (c) B-PPM-3 and (d) B-PPM-4. Figure S3: TEM images (a) 100 nm and (b) 10 nm of the B-PPM-2. Figure S4: CO2, N2, and CH4 adsorption isotherms of the sample (a) B-PPM-1, (b) B-PPM-2, (c) B-PPM-3, and (d) B-PPM-4 at 273 K and 298 K and 1 bar. Figure S5: IAST selectivity of CO2/N2 (15:85) and CO2/CH4 (50:50) on the B-PPM-2 at 298 K.
Author Contributions
Conceptualization, D.X. and J.M.; methodology, X.Y., F.Z., and Z.S.; software, X.Y., F.Z., and J.C.; validation, D.X. and J.M.; writing—original draft preparation, X.Y. and F.Z.; writing—review and editing, D.X. and J.M.; funding acquisition, J.M. All authors have read and agreed to the published version of the manuscript.
Funding
This work was supported by the National Natural Science Foundation of China (No. 22106104 and No. 22308293), Natural Science Foundation of Shandong Province (No. ZR2020QB176), and Jiangsu Agricultural Science and Technology Innovation Fund (No. CX(23)3111).
Data Availability Statement
Data are contained within this article or Supplementary Material.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Song, K.S.; Fritz, P.W.; Coskun, A. Porous organic polymers for CO2 capture, separation and conversion. Chem. Soc. Rev. 2022, 51, 9831–9852. [Google Scholar] [CrossRef] [PubMed]
- Ozkan, M.; Nayak, S.P.; Ruiz, A.D.; Jiang, W. Current status and pillars of direct air capture technologies. iScience 2022, 25, 103990. [Google Scholar] [CrossRef] [PubMed]
- De Kleijne, K.; Hanssen, S.V.; van Dinteren, L.; Huijbregts, M.A.J.; van Zelm, R.; de Coninck, H. Limits to Paris compatibility of CO2 capture and utilization. One Earth 2022, 5, 168–185. [Google Scholar] [CrossRef]
- Xie, F.; Sun, W.; Pinacho, P.; Schnell, M. CO2 aggregation on monoethanolamine: Observations from rotational spectroscopy. Angew. Chem. Int. Ed. 2023, 62, e202218539. [Google Scholar] [CrossRef] [PubMed]
- Jørsboe, J.K.; Vinjarapu, S.H.B.; Neerup, R.; Møller, A.C.; Jensen, S.; Abildskov, J.; Fosbøl, P. Mobile pilot plant for CO2 capture in biogas upgrading using 30 wt% MEA. Fuel 2023, 350, 128702–128713. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, P.; Wang, Y.; Zhao, Q.; Sun, H. Progress in the separation and purification of carbon hydrocarbon compounds using MOFs and molecular sieves. Separations 2023, 10, 543. [Google Scholar] [CrossRef]
- Ghalib, L.; Abdulkareem, A.; Ali, B.S.; Mazari, S.A. Modeling the rate of corrosion of carbon steel using activated diethanolamine solutions for CO2 absorption. Chin. J. Chem. Eng. 2020, 28, 2099–2110. [Google Scholar] [CrossRef]
- Rashidi, H.; Sahraie, S. Enhancing carbon dioxide absorption performance using the hybrid solvent: Diethanolamine-methanol. Energy 2021, 221, 119799. [Google Scholar] [CrossRef]
- Choi, B.-K.; Kim, S.-M.; Kim, K.-M.; Lee, U.; Choi, J.H.; Lee, J.-S.; Baek, I.H.; Nam, S.C.; Moon, J.-H. Amine blending optimization for maximizing CO2 absorption capacity in a diisopropanolamine–methyldiethanolamine-H2O system using the electrolyte UNIQUAC model. Chem. Eng. J. 2021, 419, 129517. [Google Scholar] [CrossRef]
- Haghtalab, A.; Gholami, V. Carbon dioxide solubility in the aqueous mixtures of diisopropanolamine + l-arginine and diethanolamine + l-arginine at high pressures. J. Mol. Liq. 2019, 288, 111064. [Google Scholar] [CrossRef]
- Seah, G.L.; Wang, L.; Tan, L.F.; Tipjanrawee, C.; Sasangka, W.A.; Usadi, A.K.; McConnachie, J.M.; Tan, K.W. Ordered mesoporous alumina with tunable morphologies and pore sizes for CO2 capture and dye separation. ACS Appl. Mater. Interfaces 2021, 13, 36117–36129. [Google Scholar] [CrossRef] [PubMed]
- Kwon, S.; Yang, H.; Yu, Y.; Choi, Y.; Kim, N.; Kim, G.H.; Ko, K.C.; Na, K. A sustainable carbon-consuming cycle based on sequential activation of CO2 and CH4 using metal oxides. Appl. Catal. B Environ. 2023, 339, 123120. [Google Scholar] [CrossRef]
- Mat, N.; Timmiati, S.N.; Teh, L.P. Recent development in metal oxide-based core-shell material for CO2 capture and utilisation. Appl. Nanosci. 2022, 13, 3797–3817. [Google Scholar] [CrossRef]
- Wang, H.; Liu, X.; Saliy, O.; Hu, W.; Wang, J. Robust amino-functionalized mesoporous silica hollow spheres templated by CO2 bubbles. Molecules 2021, 27, 53. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Huang, L.; Li, S.; Liu, C.; He, H. The capture and catalytic conversion of CO2 by dendritic mesoporous silica-based nanoparticles. Energy Environ. Mater. 2023, 6, e12593. [Google Scholar] [CrossRef]
- Pardakhti, M.; Jafari, T.; Tobin, Z.; Dutta, B.; Moharreri, E.; Shemshaki, N.S.; Suib, S.; Srivastava, R. Trends in solid adsorbent materials development for CO2 capture. ACS Appl. Mater. Interfaces 2019, 11, 34533–34559. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Senkovska, I.; Oschatz, M.; Lohe, M.R.; Borchardt, L.; Heerwig, A.; Liu, Q.; Kaskel, S. Imine-linked polymer-derived nitrogen-doped microporous carbons with excellent CO2 capture properties. ACS Appl. Mater. Interfaces 2013, 5, 3160–3167. [Google Scholar] [CrossRef]
- Li, P.; Xing, C.; Qu, S.; Li, B.; Shen, W. Carbon dioxide capturing by nitrogen-doping microporous carbon. ACS Sustain. Chem. Eng. 2015, 3, 1434–1442. [Google Scholar] [CrossRef]
- Melke, J.; Schuster, R.; Möbus, S.; Jurzinsky, T.; Elsässer, P.; Heilemann, A.; Fischer, A. Electrochemical stability of silica templated polyaniline derived mesoporous N-doped carbons for the design of Pt based oxygen reduction reaction catalysts. Carbon 2019, 146, 44–59. [Google Scholar] [CrossRef]
- Li, Y.; Wang, H.; Wang, C.; Xu, J.; Ma, S.; Ou, J.; Zhang, J.; Li, G.; Wei, Y.; Ye, M. Atomically precise structure determination of porous organic cage from Ab initio PXRD structure analysis: Its molecular click postfunctionalization and CO2 capture application. ACS Appl. Mater. Interfaces 2020, 12, 17815–17823. [Google Scholar] [CrossRef]
- Hao, G.P.; Li, W.C.; Qian, D.; Lu, A.H. Rapid synthesis of nitrogen-doped porous carbon monolith for CO2 capture. Adv. Mater. 2010, 22, 853–857. [Google Scholar] [CrossRef] [PubMed]
- Zeng, Y.; Zou, R.; Zhao, Y. Covalent organic frameworks for CO2 capture. Adv. Mater. 2016, 28, 2855–2873. [Google Scholar] [CrossRef] [PubMed]
- Ciulla, M.; Canale, V.; Wolicki, R.D.; Pilato, S.; Bruni, P.; Ferrari, S.; Siani, G.; Fontana, A.; Di Profio, P. Enhanced CO2 capture by sorption on electrospun poly (methyl methacrylate). Separations 2023, 10, 505. [Google Scholar] [CrossRef]
- Liu, F.; Zhang, Y.; Wang, S.; Gong, T.; Hua, M.; Qian, J.; Pan, B. Metal-free biomass with abundant carbonyl groups as efficient catalyst for the activation of peroxymonosulfate and degradation of sulfamethoxazole. Chem. Eng. J. 2022, 430, 132767. [Google Scholar] [CrossRef]
- Liebl, M.R.; Senker, J. Microporous functionalized triazine-based polyimides with high CO2 capture capacity. Chem. Mater. 2013, 25, 970–980. [Google Scholar] [CrossRef]
- Ashourirad, B.; Sekizkardes, A.K.; Altarawneh, S.; El-Kaderi, H.M. Exceptional gas adsorption properties by nitrogen-doped porous carbons derived from benzimidazole-linked polymers. Chem. Mater. 2015, 27, 1349–1358. [Google Scholar] [CrossRef]
- Ben, T.; Ren, H.; Ma, S.; Cao, D.; Lan, J.; Jing, X.; Wang, W.; Xu, J.; Deng, F.; Simmons, J.M.; et al. Targeted synthesis of a porous aromatic framework with high stability and exceptionally high surface area. Angew. Chem. Int. Ed. 2009, 48, 9457–9460. [Google Scholar] [CrossRef]
- Li, G.; Zhang, B.; Yan, J.; Wang, Z. Micro- and mesoporous poly(Schiff-base)s constructed from different building blocks and their adsorption behaviors towards organic vapors and CO2 gas. J. Mater. Chem. A 2014, 2, 18881–18888. [Google Scholar] [CrossRef]
- Zhang, H.; Yang, L.M.; Ganz, E. Adsorption properties and microscopic mechanism of CO2 capture in 1,1-Dimethyl-1,2-ethylenediamine-grafted metal-organic frameworks. ACS Appl. Mater. Interfaces 2020, 12, 18533–18540. [Google Scholar] [CrossRef]
- Jiang, Y.; Tan, P.; Qi, S.C.; Liu, X.Q.; Yan, J.H.; Fan, F.; Sun, L.B. Metal-organic frameworks with target-specific active sites switched by photoresponsive motifs: Efficient adsorbents for tailorable CO2 capture. Angew. Chem. Int. Ed. 2019, 58, 6600–6604. [Google Scholar] [CrossRef]
- Rozaini, M.T.; Grekov, D.I.; Bustam, M.A.; Pré, P. Shaping of HKUST-1 via extrusion for the separation of CO2/CH4 in biogas. Separations 2023, 10, 487. [Google Scholar] [CrossRef]
- Wahono, S.K.; Stalin, J.; Addai-Mensah, J.; Skinner, W.; Vinu, A.; Vasilev, K. Physico-chemical modification of natural mordenite-clinoptilolite zeolites and their enhanced CO2 adsorption capacity. Microporous Mesoporous Mater. 2020, 294, 109871–109880. [Google Scholar] [CrossRef]
- Dabbawala, A.A.; Ismail, I.; Vaithilingam, B.V.; Polychronopoulou, K.; Singaravel, G.; Morin, S.; Berthod, M.; Al Wahedi, Y. Synthesis of hierarchical porous Zeolite-Y for enhanced CO2 capture. Microporous Mesoporous Mater. 2020, 303, 110261–110272. [Google Scholar] [CrossRef]
- He, X.; Chen, D.R.; Wang, W.N. Bimetallic metal-organic frameworks (MOFs) synthesized using the spray method for tunable CO2 adsorption. Chem. Eng. J. 2020, 382, 122825–122836. [Google Scholar] [CrossRef]
- Niu, J.; Li, H.; Tao, L.; Fan, Q.; Liu, W.; Tan, M.C. Defect engineering of low-coordinated metal-organic frameworks (MOFs) for improved CO2 access and capture. ACS Appl. Mater. Interfaces 2023, 15, 31664–31674. [Google Scholar] [CrossRef] [PubMed]
- Zhai, L.; Huang, N.; Xu, H.; Chen, Q.; Jiang, D. A backbone design principle for covalent organic frameworks: The impact of weakly interacting units on CO2 adsorption. Chem. Commun. 2017, 53, 4242–4245. [Google Scholar] [CrossRef] [PubMed]
- Cui, P.; Jing, X.F.; Yuan, Y.; Zhu, G.S. Synthesis of porous aromatic framework with Friedel-Crafts alkylation reaction for CO2 separation. Chin. Chem. Lett. 2016, 27, 1479–1484. [Google Scholar] [CrossRef]
- Xu, S.; He, J.; Jin, S.; Tan, B. Heteroatom-rich porous organic polymers constructed by benzoxazine linkage with high carbon dioxide adsorption affinity. J. Colloid Interface Sci. 2018, 509, 457–462. [Google Scholar] [CrossRef]
- Wang, X.; Chen, M.; Du, M. A clear insight into the distinguishing CO2 capture by two isostructural Dy(III)-carboxylate coordination frameworks. Inorg. Chem. 2016, 55, 6352–6354. [Google Scholar] [CrossRef]
- Li, Z.; Gao, W.; Meng, A.; Geng, Z.; Gao, L. Large-scale synthesis and raman and photoluminescence properties of single crystalline β-sic nanowires periodically wrapped by amorphous SiO2 nanospheres 2. J. Phys. Chem. C 2009, 113, 91–96. [Google Scholar] [CrossRef]
- Zhong, B.; Kong, L.; Zhang, B.; Yu, Y.; Xia, L. Fabrication of novel hydrophobic SiC/SiO2 bead-string like core-shell nanochains via a facile catalyst/template-free thermal chemical vapor deposition process. Mater. Chem. Phys. 2018, 217, 111–116. [Google Scholar] [CrossRef]
- Buyukcakir, O.; Seo, Y.; Coskun, A. Thinking outside the cage: Controlling the extrinsic porosity and gas uptake properties of shape-persistent molecular cages in nanoporous polymers. Chem. Mater. 2015, 27, 4149–4155. [Google Scholar] [CrossRef]
- Tian, Y.; Zhu, G. Porous aromatic frameworks (PAFs). Chem. Rev. 2020, 120, 8934–8986. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Chen, H.; Wang, S.; Guo, W.; Wang, T.; Suo, X.; Jiang, D.E.; Zhu, X.; Popovs, I.; Dai, S. Transformation strategy for highly crystalline covalent triazine frameworks: From staggered AB to eclipsed AA stacking. J. Am. Chem. Soc. 2020, 142, 6856–6860. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Sun, N.; Wei, W. Facile and controllable synthesis of ordered mesoporous carbons with tunable single-crystal morphology for CO2 capture. Carbon 2020, 161, 629–638. [Google Scholar] [CrossRef]
- Shi, X.; Xiao, H.; Azarabadi, H.; Song, J.; Wu, X.; Chen, X.; Lackner, K.S. Sorbents for the direct capture of CO2 from ambient air. Angew. Chem. Int. Ed. 2020, 59, 6984–7006. [Google Scholar] [CrossRef]
- Saning, A.; Dubadi, R.; Chuenchom, L.; Dechtrirat, D.; Jaroniec, M. Microporous carbons obtained via solvent-free mechanochemical processing, carbonization and activation with potassium citrate and zinc chloride for CO2 adsorption. Separations 2023, 10, 304. [Google Scholar] [CrossRef]
- Karka, S.; Kodukula, S.; Nandury, S.V.; Pal, U. Polyethylenimine-modified zeolite 13X for CO2 capture: Adsorption and kinetic studies. ACS Omega 2019, 4, 16441–16449. [Google Scholar] [CrossRef]
- Qiao, Y.; Zhan, Z.; Yang, Y.; Liu, M.; Huang, Q.; Tan, B.; Ke, X.; Wu, C. Amine or azo functionalized hypercrosslinked polymers for highly efficient CO2 capture and selective CO2 capture. Mater. Today Commun. 2021, 27, 102338. [Google Scholar] [CrossRef]
- Qi, S.C.; Liu, Y.; Peng, A.Z.; Xue, D.M.; Liu, X.; Liu, X.Q.; Sun, L.B. Fabrication of porous carbons from mesitylene for highly efficient CO2 capture: A rational choice improving the carbon loop. Chem. Eng. J. 2019, 361, 945–952. [Google Scholar] [CrossRef]
- Politakos, N.; Barbarin, I.; Cantador, L.S.; Cecilia, J.A.; Mehravar, E.; Tomovska, R. Graphene-based monolithic nanostructures for CO2 capture. Ind. Eng. Chem. Res. 2020, 59, 8612–8621. [Google Scholar] [CrossRef]
- Vieira, R.B.; Moura, P.A.S.; Vilarrasa-García, E.; Azevedo, D.C.S.; Pastore, H.O. Polyamine-grafted magadiite: High CO2 selectivity at capture from CO2/N2 and CO2/CH4 mixtures. J. CO2 Util. 2018, 23, 29–41. [Google Scholar] [CrossRef]
- Liu, S.; Jin, Y.; Bae, J.-S.; Chen, Z.; Dong, P.; Zhao, S.; Li, R. CO2 derived nanoporous carbons for carbon capture. Microporous Mesoporous Mater. 2020, 305, 110356. [Google Scholar] [CrossRef]
- Zhang, Z.; Wang, K.; Atkinson, J.D.; Yan, X.; Li, X.; Rood, M.J.; Yan, Z. Sustainable and hierarchical porous enteromorpha prolifera based carbon for CO2 capture. J. Hazard. Mater. 2012, 229–230, 183–191. [Google Scholar] [CrossRef] [PubMed]
- Sheng, M.; Dong, S.; Qiao, Z.; Li, Q.; Wang, Z. Large-scale preparation of multilayer composite membranes for post-combustion CO2 capture. J. Membr. Sci. 2021, 636, 119595. [Google Scholar] [CrossRef]
- Xu, S.; Huang, H.; Guo, X.; Qiao, Z.; Zhong, C. Highly selective gas transport channels in mixed matrix membranes fabricated by using water-stable Cu-BTC. Sep. Purif. Technol. 2021, 257, 117979. [Google Scholar] [CrossRef]
- Dhoke, C.; Zaabout, A.; Cloete, S.; Amini, S. Review on reactor configurations for adsorption-based CO2 capture. Ind. Eng. Chem. Res. 2021, 60, 3779–3798. [Google Scholar] [CrossRef]
- Raganati, F.; Miccio, F.; Ammendola, P. Adsorption of carbon dioxide for post-combustion capture: A review. Energy Fuels 2021, 35, 12845–12868. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).