Upcycling Textile White Mud to Fabricate MIL-125-Derived Amorphous TiO2@C: Effective Electrocatalyst for Cathodic Reduction of Antibiotics
Abstract
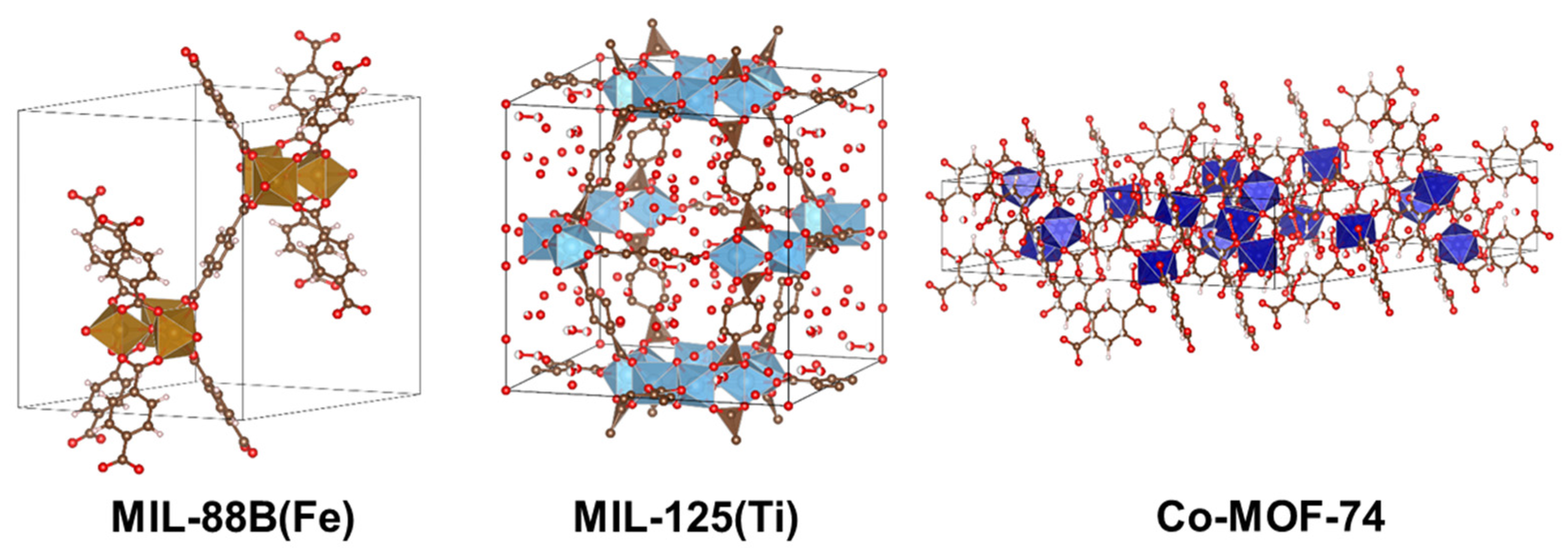
:1. Introduction
2. Materials and Methods
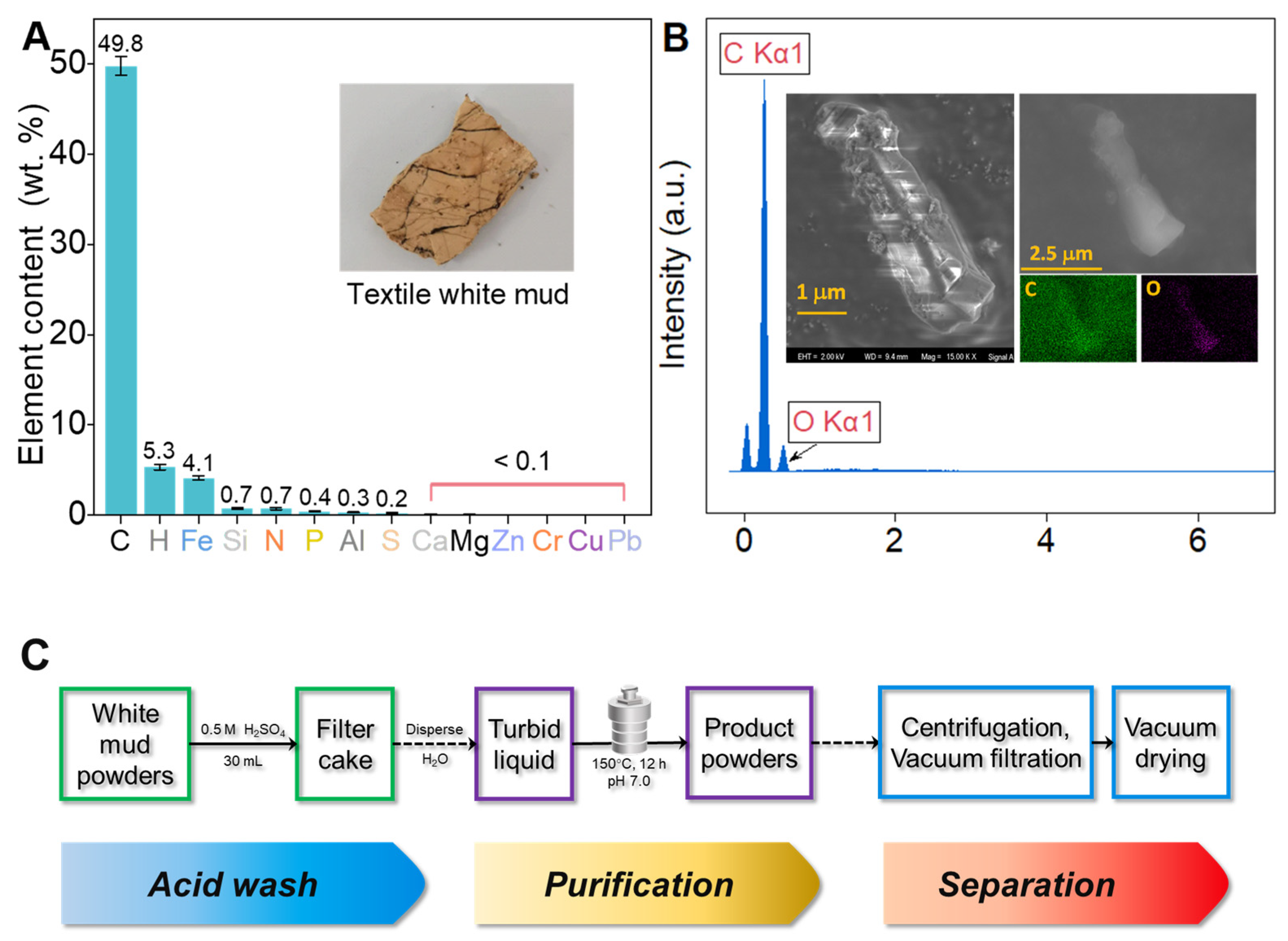
2.1. Textile White Mud and Chemicals
2.2. Fabrication of MIL-125(Ti) and TiO2@C Derived from Textile White Mud
2.3. Experimental Seup of Cathodic Reduction Test
2.4. Characterizations and Analytical Methods
3. Results and Discussion
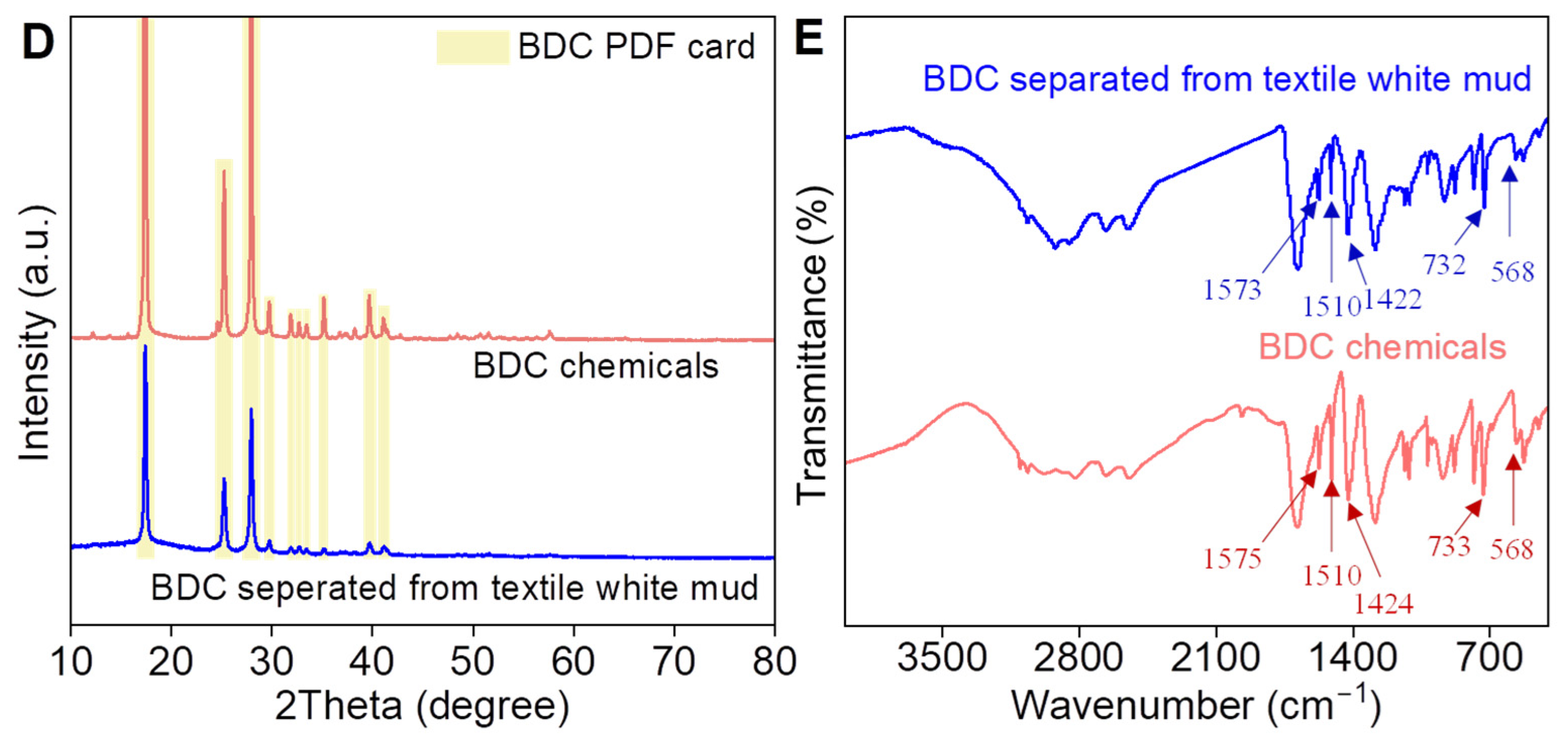
3.1. Separation of 1,4-Dicarboxybenzene from Textile White Mud
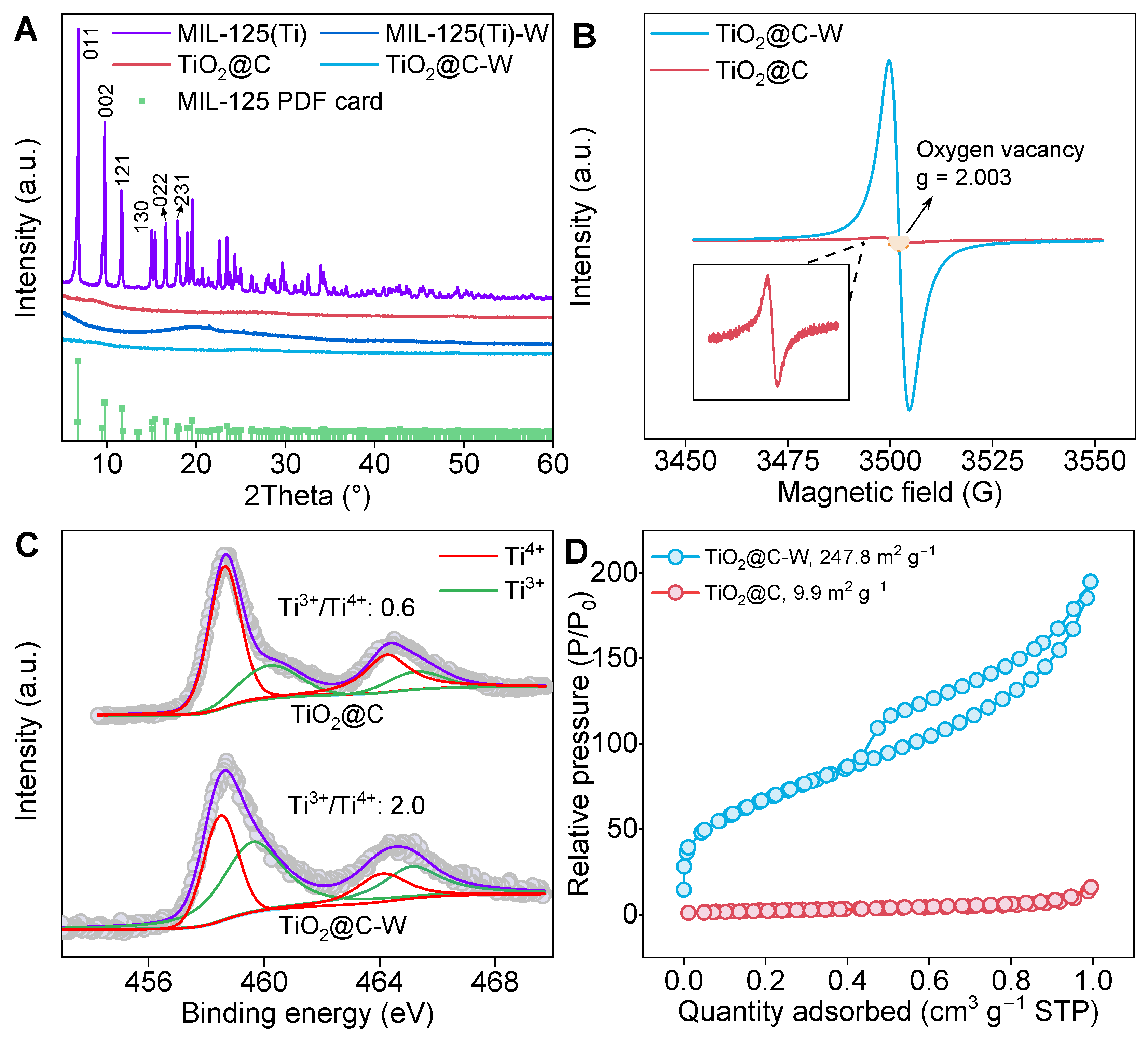
3.2. Fabrication and Structural Characterization
3.3. Cathodic Reduction Performance of TiO2@C-W
3.3.1. Screening of BDC:Ti Molar Ratio and Carbonization Temperature
3.3.2. Comparison with Other Electrocatalysts
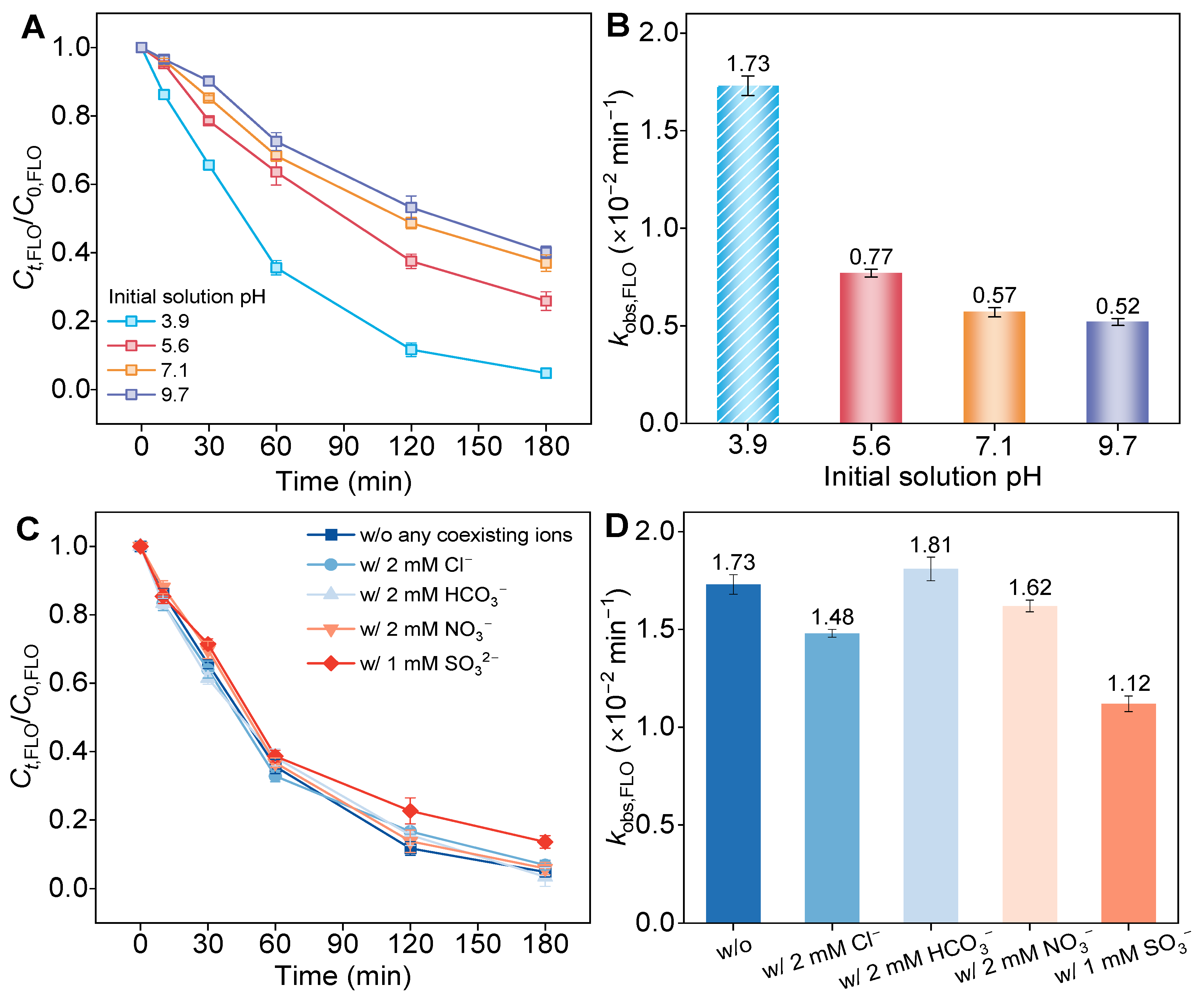
3.4. Effect of Solution pH and Co-Existing Ions on Performance of TiO2@C-W
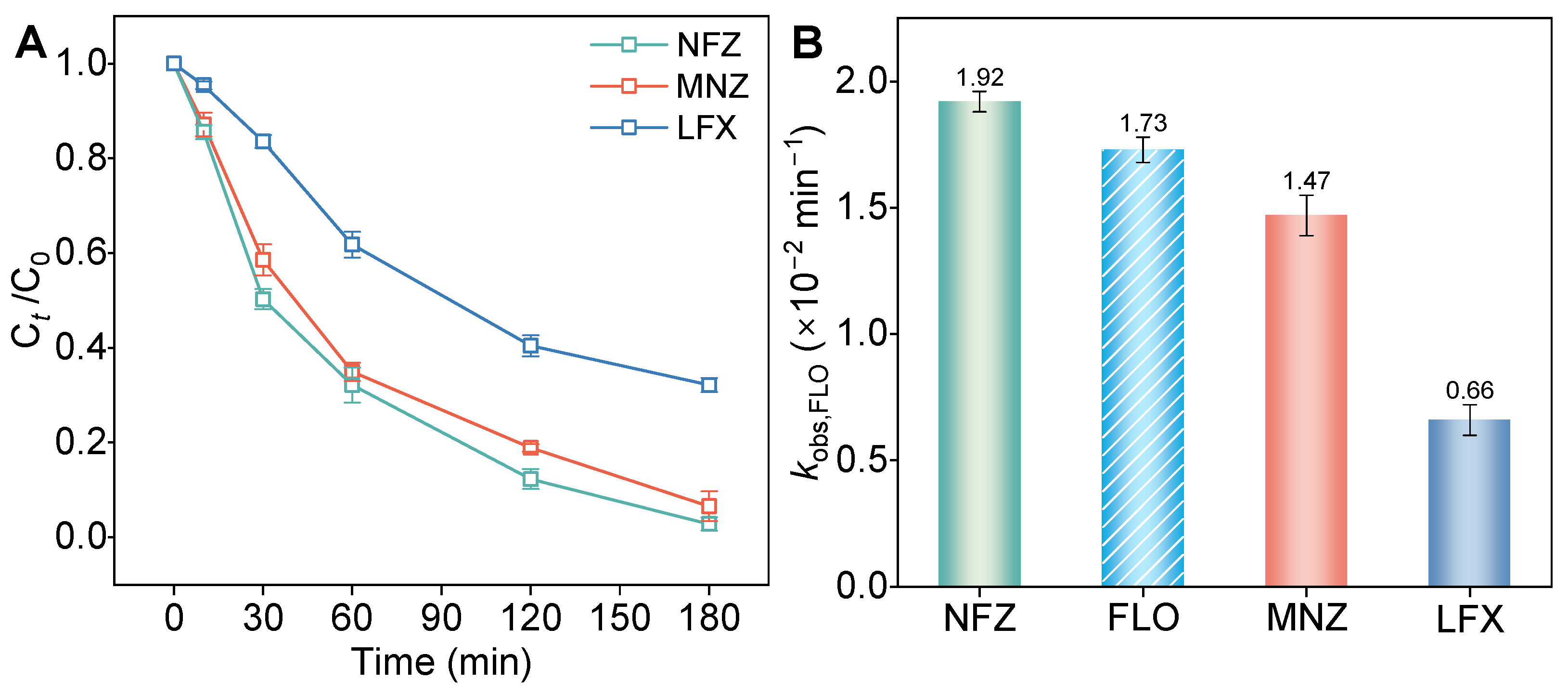
3.5. Cathodic Reduction Performance for Other Antibiotics by TiO2@C-W
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- O’Neill, J. Review on Antimicrobial Resistance. In Antimicrobial Resistance: Tackling a Crisis for the Health and Wealth of Nations; The Wellcome Trust and UK Government: London, UK, 2014. [Google Scholar]
- Jechalke, S.; Heuer, H.; Siemens, J.; Amelung, W.; Smalla, K. Fate and effects of veterinary antibiotics in soil. Trends Microbiol. 2014, 22, 536–545. [Google Scholar] [CrossRef] [PubMed]
- Michael, I.; Rizzo, L.; McArdell, C.S.; Manaia, C.M.; Merlin, C.; Schwartz, T.; Dagot, C.; Fatta-Kassinos, D. Urban wastewater treatment plants as hotspots for the release of antibiotics in the environment: A review. Water Res. 2013, 47, 957–995. [Google Scholar] [CrossRef] [PubMed]
- Sapkota, A.; Sapkota, A.R.; Kucharski, M.; Burke, J.; McKenzie, S.; Walker, P.; Lawrence, R. Aquaculture Practices and Potential Human Health Risks: Current Knowledge and Future Priorities. Environ. Int. 2008, 34, 1215–1226. [Google Scholar] [CrossRef]
- Cabello, F.C.; Godfrey, H.P.; Tomova, A.; Ivanova, L.; Dölz, H.; Millanao, A.; Buschmann, A.H. Antimicrobial Use in Aquaculture Re-Examined: Its Relevance to Antimicrobial Resistance and to Animal and Human Health. Environ. Microbiol. 2013, 15, 1917–1942. [Google Scholar] [CrossRef]
- Wang, H.; Wang, N.; Wang, B.; Zhao, Q.; Fang, H.; Fu, C.; Tang, C.; Jiang, F.; Zhou, Y.; Chen, Y.; et al. Antibiotics in Drinking Water in Shanghai and Their Contribution to Antibiotic Exposure of School Children. Environ. Sci. Technol. 2016, 50, 2692–2699. [Google Scholar] [CrossRef]
- Chen, H.; Jing, L.; Teng, Y.; Wang, J. Multimedia fate modeling and risk assessment of antibiotics in a water-scarce megacity. J. Hazard. Mater. 2018, 348, 75–83. [Google Scholar] [CrossRef] [PubMed]
- Homem, V.; Santos, L. Degradation and removal methods of antibiotics from aqueous matrices--a review. J. Environ. Manag. 2011, 92, 2304–2347. [Google Scholar] [CrossRef]
- Fagervold, S.K.; Watts, J.E.M.; May, H.D.; Sowers, K.R. Effects of bioaugmentation on indigenous PCB dechlorination activity in sediment microcosms. Water Res. 2011, 45, 3899–3907. [Google Scholar] [CrossRef]
- Agarwal, S.; Al-Abed, S.R.; Dionysiou, D.D.; Graybill, E. Reactivity of substituted chlorines and ensuing dechlorination pathways of select PCB congeners with Pd/Mg bimetallics. Environ. Sci. Technol. 2009, 43, 915–921. [Google Scholar] [CrossRef]
- Agarwal, S.; Al-Abed, S.R.; Dionysiou, D.D. A feasibility study on Pd/Mg application in historically contaminated sediments and PCB spiked substrates. J. Hazard. Mater. 2009, 172, 1156–1162. [Google Scholar] [CrossRef]
- Han, Y.L.; Yan, W.L. Reductive dechlorination of trichloroethene by zero-valent iron nanoparticles: Reactivity enhancement through sulfidation treatment. Environ. Sci. Technol. 2016, 50, 12992–13001. [Google Scholar] [CrossRef] [PubMed]
- Phenrat, T.; Saleh, N.; Sirk, K.; Tilton, R.D.; Lowry, G.V. Aggregation and sedimentation of aqueous nanoscale zerovalent iron dispersions. Environ. Sci. Technol. 2007, 41, 284–290. [Google Scholar] [CrossRef] [PubMed]
- Xu, J.; Tan, L.S.; Baig, S.A.; Wu, D.L.; Lv, X.S.; Xu, X.H. Dechlorination of 2,4-dichlorophenol by nanoscale magnetic Pd/Fe particles: Effects of pH, temperature, common dissolved ions and humic acid. Chem. Eng. J. 2013, 231, 26–35. [Google Scholar] [CrossRef]
- Wu, S.; Qi, Y.; Fan, C.; Dai, B.; Huang, J.; Zhou, W.; He, S.; Gao, L. Improvement of anaerobic biological treatment effect by catalytic micro-electrolysis for monensin production wastewater. Chem. Eng. J. 2016, 296, 260–267. [Google Scholar] [CrossRef]
- Belkheiri, D.; Fourcade, F.; Geneste, F.; Floner, D.; Aït-Amar, H.; Amrane, A. Feasibility of an electrochemical pre-treatment prior to a biological treatment for tetracycline removal. Sep. Purif. Technol. 2011, 83, 151–156. [Google Scholar] [CrossRef]
- Radjenović, J.; Farré, M.J.; Mu, Y.; Gernjak, W.; Keller, J. Reductive electrochemical remediation of emerging and regulated disinfection byproducts. Water Res. 2012, 46, 1705–1714. [Google Scholar] [CrossRef]
- Kong, D.; Liang, B.; Yun, H.; Cheng, H.; Ma, J.; Cui, M.; Wang, A.; Ren, N. Cathodic degradation of antibiotics: Characterization and pathway analysis. Water Res. 2015, 72, 281–292. [Google Scholar] [CrossRef]
- Kong, D.; Liang, B.; Yun, H.; Ma, J.; Li, Z.; Wang, A.; Ren, N. Electrochemical degradation of nitrofurans furazolidone by cathode: Characterization, pathway and antibacterial activity analysis. Chem. Eng. J. 2015, 262, 1244–1251. [Google Scholar] [CrossRef]
- Deng, J.; Hu, X.M.; Gao, E.; Wu, F.; Yin, W.; Huang, L.Z.; Dionysiou, D.D. Electrochemical reductive remediation of trichloroethylene contaminated groundwater using biomimetic iron-nitrogen-doped carbon. J. Hazard. Mater. 2021, 419, 126458. [Google Scholar] [CrossRef]
- Lou, Z.; Yu, C.; Wen, X.; Xu, Y.; Yu, J.; Xu, X. Construction of Pd nanoparticles/two-dimensional Co-MOF nanosheets heterojunction for enhanced electrocatalytic hydrodechlorination. Appl. Catal. B Environ. 2022, 317, 121730. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Cui, D.; Luo, X.; Liang, B.; Yang, L.; Liu, T.; Wang, A.; Luo, S. Ultrafine palladium nanoparticles supported on 3D self-supported Ni foam for cathodic dechlorination of florfenicol. Chem. Eng. J. 2019, 359, 894–901. [Google Scholar] [CrossRef]
- Yang, L.; Chen, Z.; Ma, T.; Zhang, S.; Dai, W.; Xiao, X.; Luo, X.; Zou, J.; Tu, X.; Yang, L.; et al. Efficient electrochemical dehalogenation of florfenicol without discharging toxic intermediates via direct electron transfer over electrochromic WO3. Chem. Eng. J. 2021, 412, 127481. [Google Scholar] [CrossRef]
- Liu, H.; Han, J.; Yuan, J.; Liu, C.; Wang, D.; Liu, T.; Liu, M.; Luo, J.; Wang, A.; Crittenden, J.C. Deep Dehalogenation of Florfenicol Using Crystalline CoP Nanosheet Arrays on a Ti Plate via Direct Cathodic Reduction and Atomic H. Environ. Sci. Technol. 2019, 53, 11932–11940. [Google Scholar] [CrossRef]
- Yang, J.; Jiang, S.-F.; Hu, W.-F.; Jiang, H. Highly efficient electrochemical dechlorination of florfenicol by an ultrathin molybdenum disulfide cathode. Chem. Eng. J. 2022, 427, 131600. [Google Scholar] [CrossRef]
- Lou, Z.; Wen, X.; Song, L.; Yan, C.; Chen, H.; Lu, T.; Yu, J.; Xu, X.; Li, J. Oxygen vacancy engineered molecular imprinted TiO2 for preferential florfenicol remediation by electro-reductive approach: Enhanced dehalogenation performance and elimination of antibiotic resistance genes. Appl. Catal. B Environ. 2023, 336, 122923. [Google Scholar] [CrossRef]
- Xiao, L.; Wang, Z.; Guan, J. 2D MOFs and their derivatives for electrocatalytic applications: Recent advances and new challenges. Coord. Chem. Rev. 2022, 472, 214777. [Google Scholar] [CrossRef]
- Munawar, T.; Bashir, A.; Nadeem, M.S.; Mukhtar, F.; Manzoor, S.; Ashiq, M.N.; Khan, S.A.; Koc, M.; Iqbal, F. Scalable synthesis of MOF-derived Nd2O3@C and V2O5@C nanohybrid: Efficient electrocatalyst for OER in alkaline medium. Fuel 2024, 355, 129485. [Google Scholar] [CrossRef]
- Hyoun Ahn, C.; Seok Yang, W.; Jae Kim, J.; Sudha Priyanga, G.; Thomas, T.; Deshpande, N.G.; Seong Lee, H.; Koun Cho, H. Design of hydrangea-type Co/Mo bimetal MOFs and MOF-derived Co/Mo2C embedded carbon composites for highly efficient oxygen evolution reaction. Chem. Eng. J. 2022, 435, 134815. [Google Scholar] [CrossRef]
- Wang, X.; He, J.; Yu, B.; Sun, B.; Yang, D.; Zhang, X.; Zhang, Q.; Zhang, W.; Gu, L.; Chen, Y. CoSe2 nanoparticles embedded MOF-derived Co-N-C nanoflake arrays as efficient and stable electrocatalyst for hydrogen evolution reaction. Appl. Catal. B Environ. 2019, 258, 117996. [Google Scholar] [CrossRef]
- Wang, X.; Fan, M.; Guan, Y.; Liu, Y.; Liu, M.; Karsili, T.N.V.; Yi, J.; Zhou, X.-D.; Zhang, J. MOF-based electrocatalysts for high-efficiency CO2 conversion: Structure, performance, and perspectives. J. Mater. Chem. A 2021, 9, 22710. [Google Scholar] [CrossRef]
- Liu, P.-Y.; Shi, K.; Chen, W.-Z.; Gao, R.; Liu, Z.-L.; Hao, H.; Wang, Y.-Q. Enhanced electrocatalytic nitrogen reduction reaction performance by interfacial engineering of MOF-based sulfides FeNi2S4/NiS hetero-interface. Appl. Catal. B Environ. 2021, 287, 119956. [Google Scholar] [CrossRef]
- Fan, L.; Zhang, L.; Shen, J.; Wang, S.; Chen, H. Study on recovery and refining of TA from alkali reduction wastewater. Desalination 2007, 206, 353–357. [Google Scholar] [CrossRef]
- Hu, S.; Xie, C.; Xu, Y.P.; Chen, X.; Gao, M.L.; Wang, H.; Yang, W.; Xu, Z.N.; Guo, G.C.; Jiang, H.L. Selectivity Control in the Direct CO Esterification over Pd@UiO-66: The Pd Location Matters. Angew. Chem. Int. Ed. 2023, 62, e202311625. [Google Scholar] [CrossRef] [PubMed]
- Hendon, C.H.; Tiana, D.; Fontecave, M.; Sanchez, C.; D’arras, L.; Sassoye, C.; Rozes, L.; Mellot-Draznieks, C.; Walsh, A. Engineering the optical response of the titanium-MIL-125 metal-organic framework through ligand functionalization. J. Am. Chem. Soc. 2013, 135, 10942–10945. [Google Scholar] [CrossRef]
- Wang, D.; Wu, G.; Zhao, Y.; Cui, L.; Shin, C.H.; Ryu, M.H.; Cai, J. Study on the copper(II)-doped MIL-101(Cr) and its performance in VOCs adsorption. Environ. Sci. Pollut. Res. 2018, 25, 28109–28119. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Gao, L.; Li, J.; Lin, J.; Wang, J. Facile synthesis of Cu-BDC/Poly(N-methylol acrylamide) HIPE monoliths via CO2-in-water Emulsion stabilized by metal-organic framework. Polymer 2018, 153, 17–23. [Google Scholar] [CrossRef]
- Jeremias, F.; Lozan, V.; Henninger, S.K.; Janiak, C. Programming MOFs for water sorption: Amino-functionalized MIL-125 and UiO-66 for heat transformation and heat storage applications. Dalton Trans. 2013, 42, 15967–15973. [Google Scholar] [CrossRef]
- Friebe, S.; Mundstock, A.; Unruh, D.; Renz, F.; Caro, J. NH2-MIL-125 as membrane for carbon dioxide sequestration: Thin supported MOF layers contra Mixed-Matrix-Membranes. J. Membr. Sci. 2016, 51, 185–193. [Google Scholar] [CrossRef]
- Liu, C.; Zhang, A.; Pei, D.; Yu, H. Efficient electrochemical reduction of nitrobenzene by defect-engineered TiO2–x single crystal, Environ. Sci. Technol. 2016, 50, 5234–5242. [Google Scholar] [CrossRef]
- Wang, Z.; Wang, K.; Wang, H.; Chen, X.; Dai, W.; Fu, X. The correlation between surface defects and the behavior of hydrogen adsorption over ZnO under UV light irradiation. Catal. Sci. Technol. 2018, 8, 3260–3277. [Google Scholar] [CrossRef]
- Oveisi, M.; Asli, M.A.; Mahmoodi, N.M. MIL-Ti metal-organic frameworks (MOFs) nanomaterials as superior adsorbents: Synthesis and ultrasound-aided dye adsorption from multicomponent wastewater systems. J. Hazard. Mater. 2018, 347, 123–140. [Google Scholar] [CrossRef]
- Abdul Mubarak, N.S.; Foo, K.Y.; Schneider, R.; Abdelhameed, R.M.; Sabar, S. The chemistry of MIL-125 based materials: Structure, synthesis, modification strategies and photocatalytic applications. J. Environ. Chem. Eng. 2022, 10, 106883. [Google Scholar] [CrossRef]
- Yue, K.; Zhang, X.; Jiang, S.; Chen, J.; Yang, Y.; Bi, F.; Wang, Y. Recent advances in strategies to modify MIL-125 (Ti) and its environmental applications. J. Mol. Liq. 2021, 335, 116108. [Google Scholar] [CrossRef]
- Wu, Y.; Gan, L.; Zhang, S.; Jiang, B.; Song, H.; Li, W.; Pan, Y.; Li, A. Enhanced electrocatalytic dechlorination of para-chloronitrobenzene based on Ni/Pd foam electrode. Chem. Eng. J. 2017, 316, 146–153. [Google Scholar] [CrossRef]
- Mao, Z.; Liu, L.; Yang, H.B.; Zhang, Y.; Yao, Z.; Wu, H.; Huang, Y.; Xu, Y.; Liu, B. Atomically dispersed Pd electrocatalyst for efficient aqueous phase dechlorination reaction. Electrochim. Acta 2021, 391, 138886. [Google Scholar] [CrossRef]
- Jiang, G.; Lan, M.; Zhang, Z.; Lv, X.; Lou, Z.; Xu, X.; Dong, F.; Zhang, S. Identification of Active Hydrogen Species on Palladium Nanoparticles for an Enhanced Electrocatalytic Hydrodechlorination of 2,4-Dichlorophenol in Water. Environ. Sci. Technol. 2017, 51, 7599–7605. [Google Scholar] [CrossRef] [PubMed]
- Lowry, G.V.; Reinhard, M. Pd-Catalyzed TCE Dechlorination in Groundwater: Solute Effects, Biological Control, and Oxidative Catalyst Regeneration. Environ. Sci. Technol. 2000, 34, 3217–3223. [Google Scholar] [CrossRef]
- Wang, H.; Gao, L. Recent developments in electrochemical hydrogen evolution reaction. Curr. Opin. Electrochem. 2018, 7, 7–14. [Google Scholar] [CrossRef]
- Brown, D.; Laboureur, P. The aerobic biodegradability of primary aromatic amines. Chemosphere 1983, 12, 405–414. [Google Scholar] [CrossRef]
- Donlon, B.A.; RazoFlores, E.; Lettinga, G.; Field, J.A. Continuous detoxification, transformation, and degradation of nitrophenols in upflow anaerobic sludge blanket (UASB) reactors. Biotechnol. Bioeng. 1996, 51, 439–449. [Google Scholar] [CrossRef]
- Tazwar, G.; Jain, A.; Devra, V. Oxidative degradation of levofloxacin by water-soluble manganese dioxide in aqueous acidic medium: A kinetic study. Chem. Pap. 2017, 71, 1749–1758. [Google Scholar] [CrossRef]
- Liu, H.; Ding, Y.; Tang, H.; Du, Y.; Zhang, D.; Tang, Y.; Liu, C. Electrocatalytic deep dehalogenation of florfenicol using Fe-doped CoP nanotubes array for blocking resistance gene expression and microbial inhibition during biochemical treatment. Water Res. 2021, 201, 117361. [Google Scholar] [CrossRef]
- Kong, D.; Yun, H.; Cui, D.; Qi, M.; Shao, C.; Cui, D.; Ren, N.; Liang, B.; Wang, A. Response of antimicrobial nitrofurazone-degrading biocathode communities to different cathode potentials. Bioresour. Technol. 2017, 241, 951–958. [Google Scholar] [CrossRef]
- Wang, B.; Lv, X.; Feng, D.; Xie, L.; Zhang, J.; Li, M.; Xie, Y.; Li, J.; Zhou, H. Highly Stable Zr(IV)-Based Metal-Organic Frameworks for the Detection and Removal of Antibiotics and Organic Explosives in Water. J. Am. Chem. Soc. 2016, 138, 6204–6216. [Google Scholar] [CrossRef]
- Chen, J.; Qiu, X.; Fang, Z.; Yang, M.; Pokeung, T.; Gu, F.; Cheng, W.; Lan, B. Removal mechanism of antibiotic metronidazole from aquatic solutions by using nanoscale zero-valent iron particles. Chem. Eng. J. 2012, 181–182, 113–119. [Google Scholar] [CrossRef]
- Forouzesh, M.A.; Ebadi, A.; Aghaeinejad-Meybodi, A. Degradation of metronidazole antibiotic in aqueous medium using activated carbon as a persulfate activator. Purif. Technol. 2019, 240, 145–151. [Google Scholar] [CrossRef]
- Tran, M.; Fu, C.; Juang, R. Effects of water matrix components on degradation efficiency and pathways of antibiotic metronidazole by UV/TiO2 photocatalysis. J. Mol. Liq. 2019, 276, 32–38. [Google Scholar] [CrossRef]
- Guo, W.; Shi, Y.; Wang, H.; Yang, H.; Zhang, G. Intensification of sonochemical degradation of antibiotics levofloxacin using carbon tetrachloride. Ultrason Sonochem. 2010, 17, 680–684. [Google Scholar] [CrossRef]
- Kansal, S.; Kundu, P.; Sood, S.; Lamba, R.; Umar, A.; Mehta, S.K. Photocatalytic degradation of antibiotic levofloxacin using well-crystalline TiO2 nanoparticles. New J. Chem. 2014, 38, 3220–3226. [Google Scholar]
- Xia, Y.; Dai, Q. Electrochemical degradation of antibiotic levofloxacin by PbO2 electrode: Kinetics, energy demands and reaction pathways. Chemosphere 2018, 205, 215–222. [Google Scholar] [CrossRef]



Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhu, J.; Wen, X.; Feng, Y.; Ren, S.; Lou, Z.; Li, J. Upcycling Textile White Mud to Fabricate MIL-125-Derived Amorphous TiO2@C: Effective Electrocatalyst for Cathodic Reduction of Antibiotics. Separations 2023, 10, 580. https://doi.org/10.3390/separations10120580
Zhu J, Wen X, Feng Y, Ren S, Lou Z, Li J. Upcycling Textile White Mud to Fabricate MIL-125-Derived Amorphous TiO2@C: Effective Electrocatalyst for Cathodic Reduction of Antibiotics. Separations. 2023; 10(12):580. https://doi.org/10.3390/separations10120580
Chicago/Turabian StyleZhu, Jinmei, Xiaofei Wen, Yuanhui Feng, Shuaibing Ren, Zimo Lou, and Jiansheng Li. 2023. "Upcycling Textile White Mud to Fabricate MIL-125-Derived Amorphous TiO2@C: Effective Electrocatalyst for Cathodic Reduction of Antibiotics" Separations 10, no. 12: 580. https://doi.org/10.3390/separations10120580
APA StyleZhu, J., Wen, X., Feng, Y., Ren, S., Lou, Z., & Li, J. (2023). Upcycling Textile White Mud to Fabricate MIL-125-Derived Amorphous TiO2@C: Effective Electrocatalyst for Cathodic Reduction of Antibiotics. Separations, 10(12), 580. https://doi.org/10.3390/separations10120580





