Synthesis of Carboxymethylcellulose–Acrylamide–Montmorillonite Composite Hydrogels for Wastewater Purification
Abstract
:1. Introduction
2. Experimental
2.1. Materials
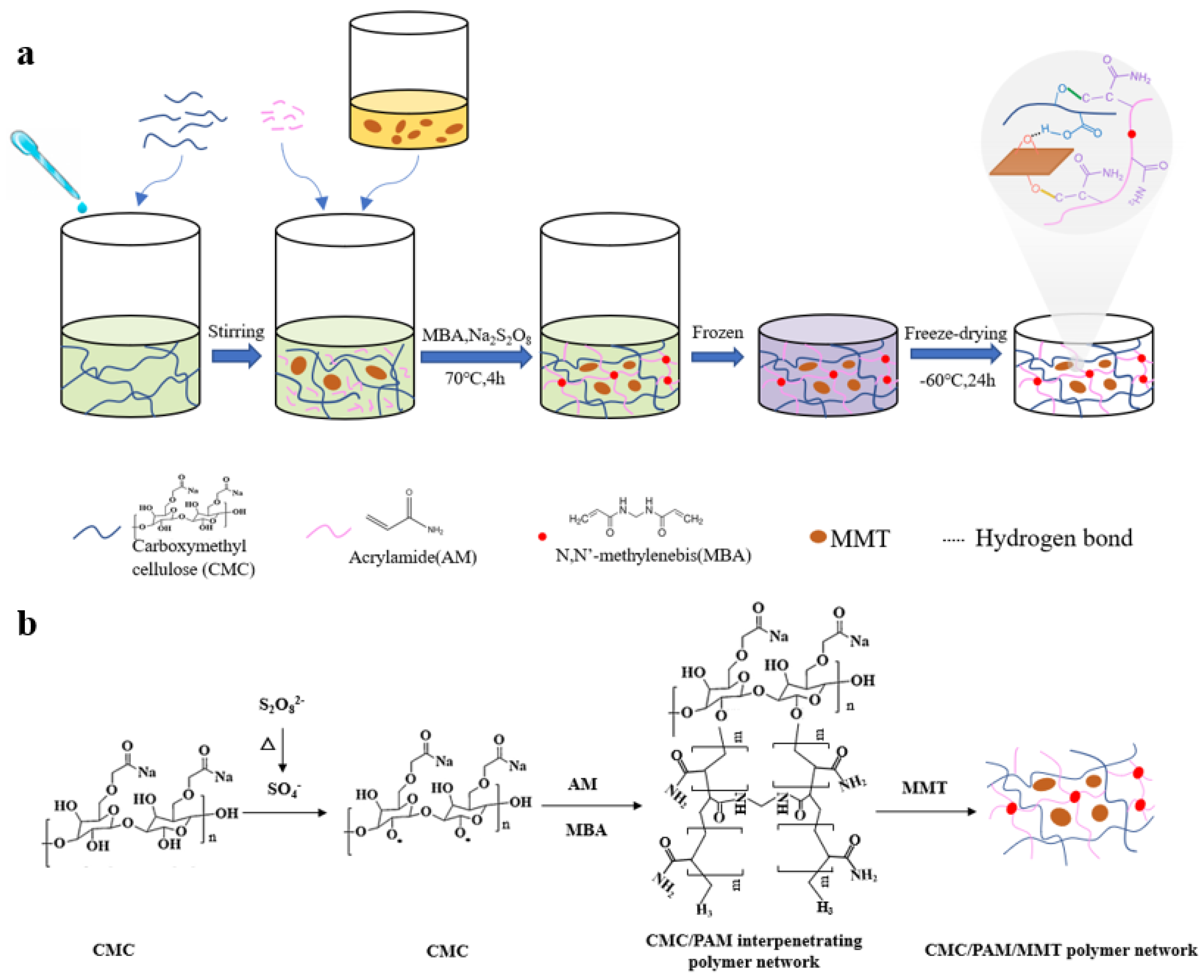
2.2. Preparation of CMC-AM Hydrogel
2.3. Preparation of CMC-AM-MMT Hydrogel
2.4. Characterization
2.5. Adsorption Performance Analysis of Methylene Blue
2.6. Adsorption Performance Analysis of Cr6+ ions
2.7. Calculation Analysis
3. Results and Discussion
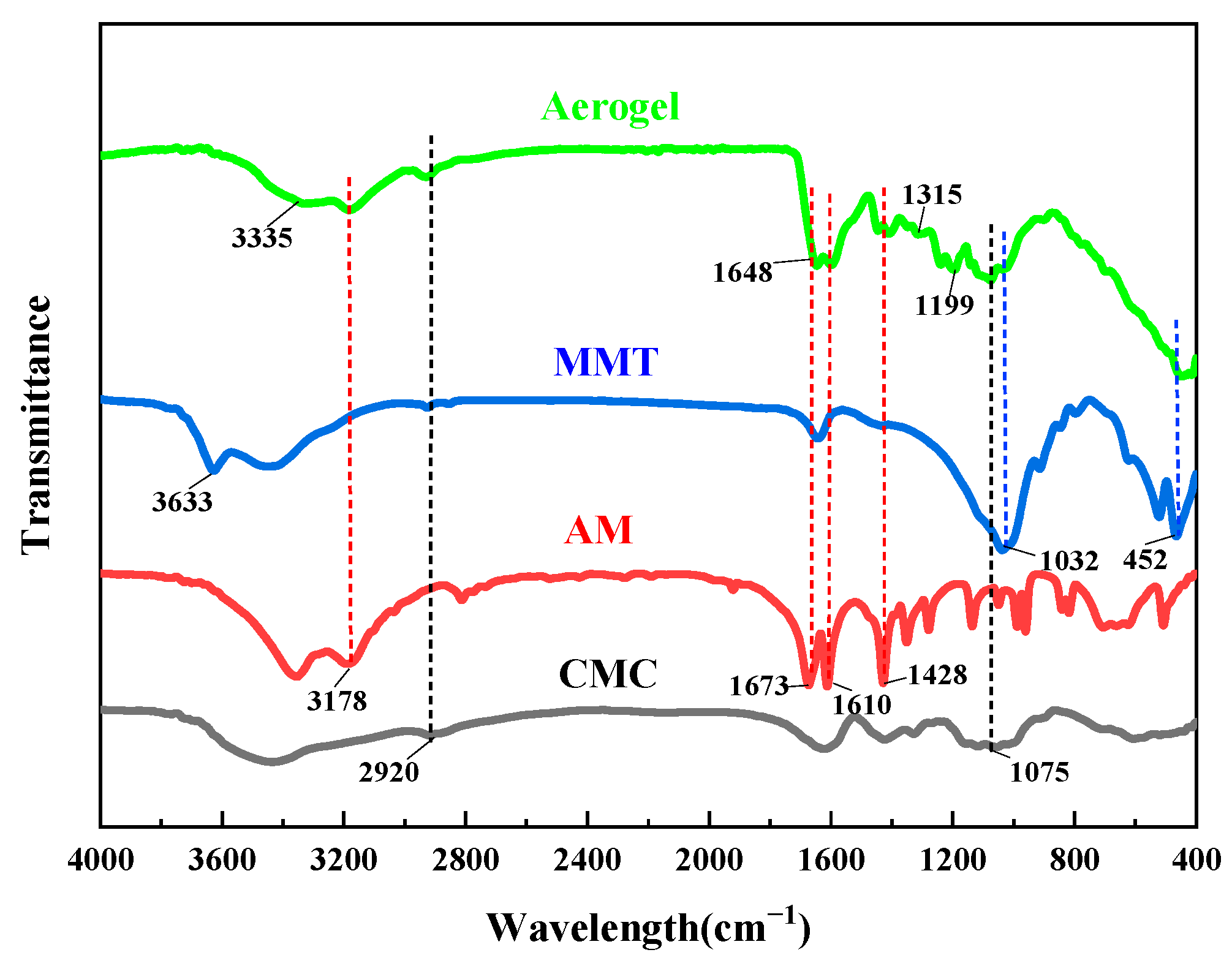
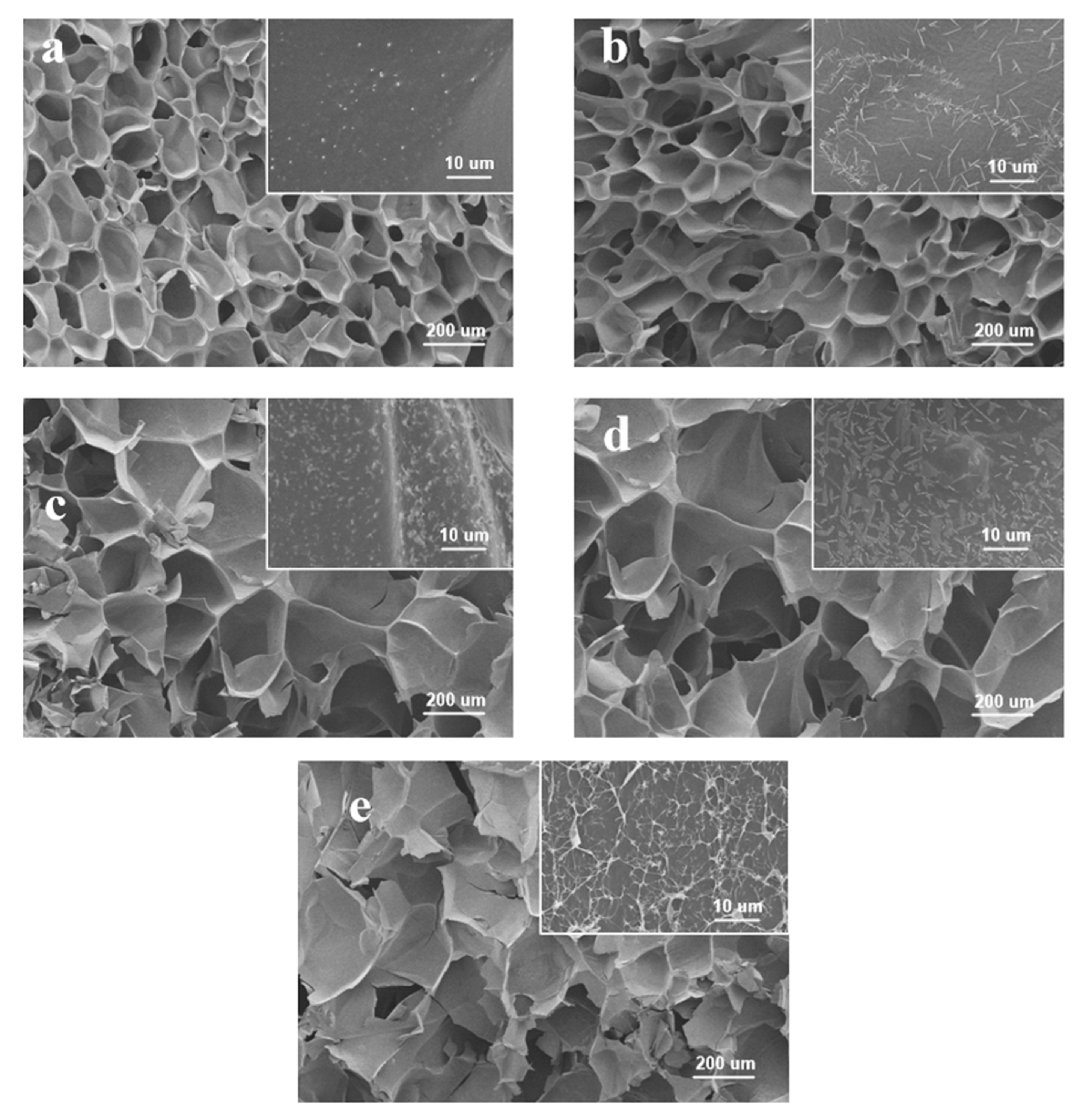
3.1. Characterization of the CMC Hydrogel
3.2. Methylene Blue Removal Performance
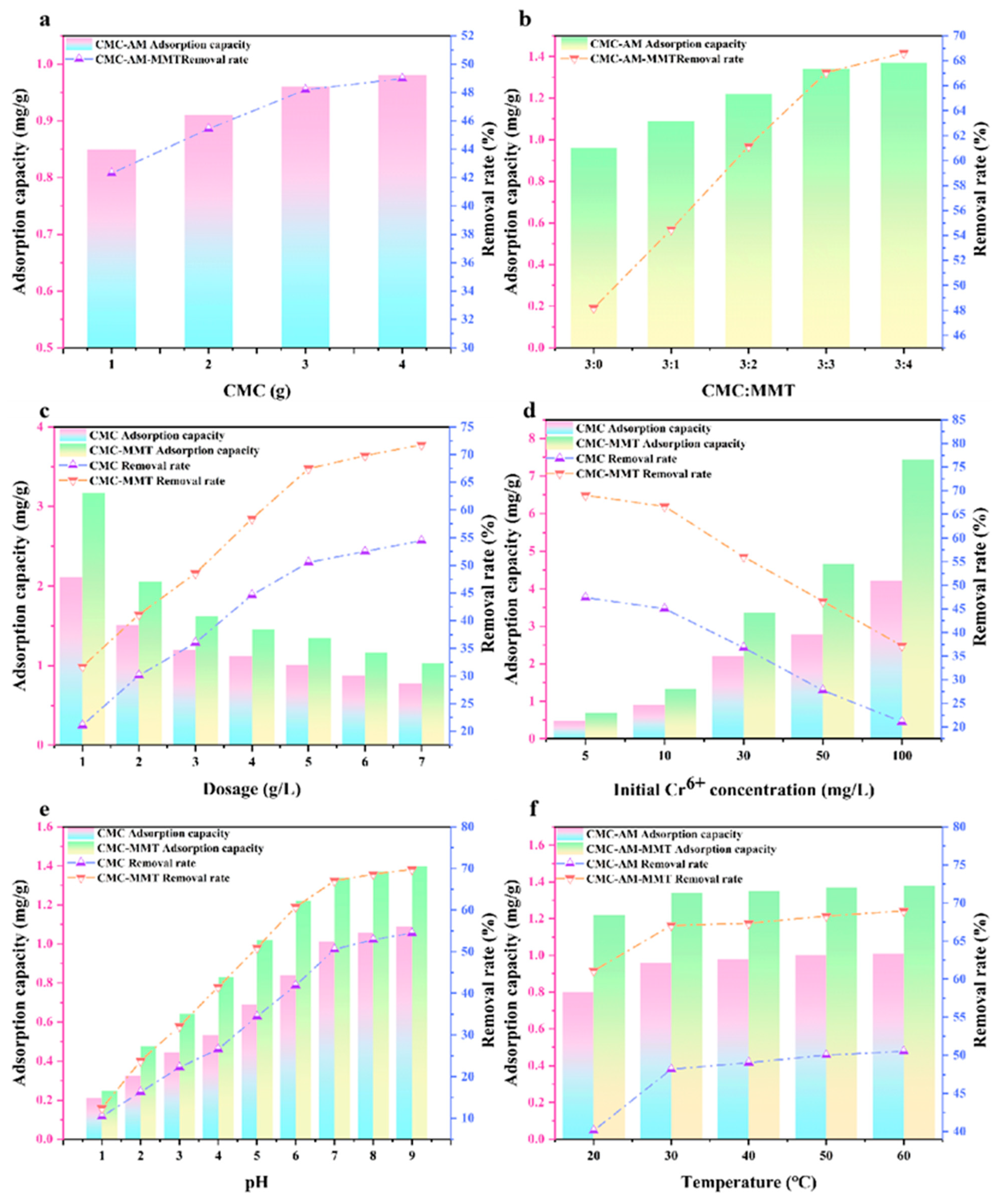
3.2.1. Initial CMC and MMT Content
3.2.2. Dosage of Adsorbent
3.2.3. The Initial Concentration of MB
3.2.4. pH Effect
3.2.5. Adsorption Temperature
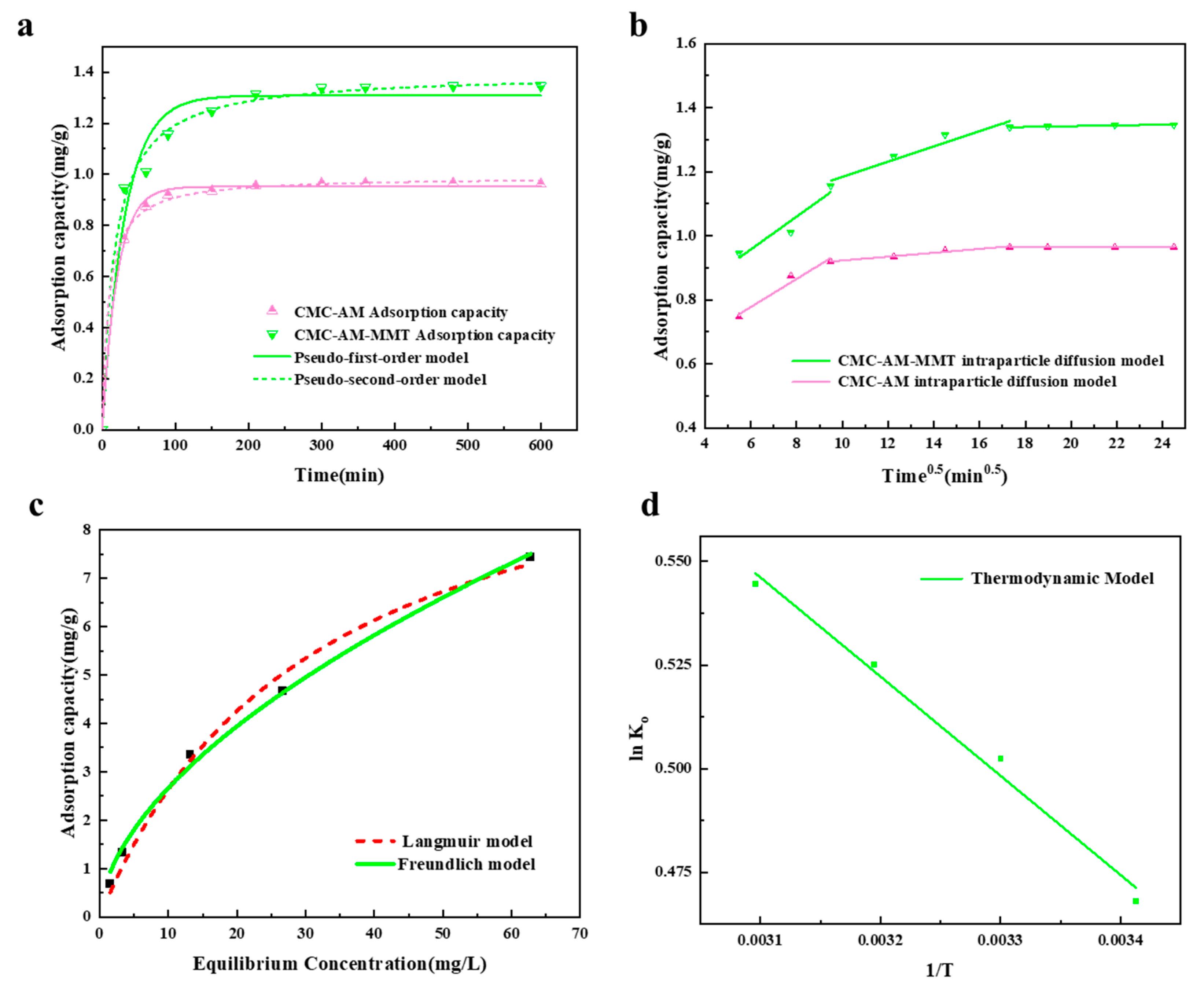
3.2.6. Adsorption Kinetics and Internal Particle Diffusion
3.2.7. Adsorption Isotherm
3.2.8. Thermodynamic Parameters
3.3. Cr6+ Ions Removal Performance
3.3.1. Initial CMC and MMT Content
3.3.2. Dosage of Adsorbent
3.3.3. Initial Concentration of Cr6+
3.3.4. pH Effect
3.3.5. Adsorption Temperature
3.3.6. Adsorption Kinetics and Internal Particle Diffusion
3.3.7. Adsorption Isotherm
3.3.8. Thermodynamic Parameters
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Conflicts of Interest
References
- Zamora-Ledezma, C.; Negrete-Bolagay, D.; Figueroa, F.; Zamora-Ledezma, E.; Ni, M.; Alexis, F.; Guerrero, V.H. Heavy metal water pollution: A fresh look about hazards, novel and conventional remediation methods. Environ. Technol. Innov. 2021, 22, 101504. [Google Scholar] [CrossRef]
- Patel, Y.N.; Patel, M.P. Adsorption of azo dyes from water by new poly (3-acrylamidopropyl)-trimethylammonium chloride-co-N,N-dimethylacrylamide superabsorbent hydrogel—Equilibrium and kinetic studies. J. Environ. Chem. Eng. 2013, 1, 1368–1374. [Google Scholar] [CrossRef]
- Zhao, C.; Liu, G.; Tan, Q.; Gao, M.; Chen, G.; Huang, X.; Xu, X.; Li, L.; Wang, J.; Zhang, Y.; et al. Polysaccharide-based biopolymer hydrogels for heavy metal detection and adsorption. J. Adv. Res. 2023, 44, 53–70. [Google Scholar] [CrossRef] [PubMed]
- Wu, J.; Dong, Z.; Li, X.; Li, P.; Wei, J.; Hu, M.; Geng, L.; Peng, X. Constructing acid-resistant chitosan/cellulose nanofibrils composite membrane for the adsorption of methylene blue. J. Environ. Chem. Eng. 2022, 10, 107754. [Google Scholar] [CrossRef]
- El-Ghobashy, M.A.; Salem, I.A.; El-Dahrawy, W.M.; Salem, M.A. Fabrication of α-MnO2/Fe-Mn binary oxide nanocomposite as an efficient adsorbent for the removal of methylene blue from wastewater. J. Mol. Struct. 2023, 1272, 134118. [Google Scholar] [CrossRef]
- Yan, H.; Lai, C.; Wang, D.; Liu, S.; Li, X.; Zhou, X.; Yi, H.; Li, B.; Zhang, M.; Li, L.; et al. In situ chemical oxidation: Peroxide or persulfate coupled with membrane technology for wastewater treatment. J. Mater. Chem. A 2021, 9, 11944–11960. [Google Scholar] [CrossRef]
- Domingues, E.; Silva, M.J.; Vaz, T.; Gomes, J.; Martins, R.C. Sulfate radical based advanced oxidation processes for agro-industrial effluents treatment: A comparative review with Fenton’s peroxidation. Sci. Total Environ. 2022, 832, 155029. [Google Scholar] [CrossRef]
- Chaturvedi, A.; Rai, B.N.; Singh, R.S.; Jaiswal, R.P. A comprehensive review on the integration of advanced oxidation processes with biodegradation for the treatment of textile wastewater containing azo dyes. Rev. Chem. Eng. 2022, 38, 617–639. [Google Scholar] [CrossRef]
- Wolski, E.A.; González, J.F.; Orozco, A.M.F.; Durruty, I.; Ceretta, M.B. Biodegradation of textile wastewater: Enhancement of biodegradability via the addition of co-substrates followed by phytotoxicity analysis of the effluent. Water Sci. Technol. 2018, 2017, 516–526. [Google Scholar] [CrossRef]
- Rashid, R.; Shafiq, I.; Akhter, P.; Iqbal, M.J.; Hussain, M. A state-of-the-art review on wastewater treatment techniques: The effectiveness of adsorption method. Environ. Sci. Pollut. Res. Int. 2021, 28, 9050–9066. [Google Scholar] [CrossRef]
- Khani, M.H.; Khamseh, A.G. Statistical analysis, equilibrium and dynamic study on the biosorption of strontium ions on Chlorella vulgaris. J. Radioanal. Nucl. Chem. 2023, 332, 3325–3334. [Google Scholar] [CrossRef]
- Khamseh, A.A.G.; Ghorbanian, S.A.; Amini, Y.; Shadman, M.M. Investigation of kinetic, isotherm and adsorption efficacy of thorium by orange peel immobilized on calcium alginate. Sci. Rep. 2023, 13, 8393. [Google Scholar] [CrossRef] [PubMed]
- Kavitha, E.; Poonguzhali, E.; Nanditha, D.; Kapoor, A.; Arthanareeswaran, G.; Prabhakar, S. Current status and future prospects of membrane separation processes for value recovery from wastewater. Chemosphere 2022, 291, 132690. [Google Scholar] [CrossRef] [PubMed]
- Zhang, J.; Yu, H.; Quan, X.; Chen, S.; Zhang, Y. Ceramic membrane separation coupled with catalytic ozonation for tertiary treatment of dyestuff wastewater in a pilot-scale study. Chem. Eng. J. 2016, 301, 19–26. [Google Scholar] [CrossRef]
- Zhao, C.; Zhou, J.; Yan, Y.; Yang, L.; Xing, G.; Li, H.; Wu, P.; Wang, M.; Zheng, H. Application of coagulation/flocculation in oily wastewater treatment: A review. Sci. Total Environ. 2021, 765, 142795. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z. The flocculation mechanism and treatment of oily wastewater by flocculation. Water Sci. Technol. 2017, 76, 2630–2637. [Google Scholar] [CrossRef] [PubMed]
- Benalia, M.C.; Youcef, L.; Bouaziz, M.G.; Achour, S.; Menasra, H. Removal of Heavy Metals from Industrial Wastewater by Chemical Precipitation: Mechanisms and Sludge Characterization. Arab. J. Sci. Eng. 2021, 47, 5587–5599. [Google Scholar] [CrossRef]
- Irawan, C.; Kuo, Y.-L.; Liu, J.C. Treatment of boron-containing optoelectronic wastewater by precipitation process. Desalination 2011, 280, 146–151. [Google Scholar] [CrossRef]
- Sun, W.; Ma, G.; Sun, Y.; Liu, Y.; Song, N.; Xu, Y.; Zheng, H. Effective treatment of high phosphorus pharmaceutical wastewater by chemical precipitation. Can. J. Chem. Eng. 2017, 95, 1585–1593. [Google Scholar] [CrossRef]
- Raghu, S.; Ahmed Basha, C. Chemical or electrochemical techniques, followed by ion exchange, for recycle of textile dye wastewater. J. Hazard. Mater. 2007, 149, 324–330. [Google Scholar] [CrossRef]
- Babiak, P.; Schaffer-Harris, G.; Kainuma, M.; Fedorovich, V.; Goryanin, I. Development of a New Hydrogel Anion Exchange Membrane for Swine Wastewater Treatment. Membranes 2022, 12, 984. [Google Scholar] [CrossRef] [PubMed]
- Chen, D.; Li, Y.; Zhong, X.; Zeng, H.; Chen, Z.; Tang, Z.; Cen, C. Preparation of Novel MOF with Multipolar Pore and Adsorption Properties of VOCs. IOP Conf. Ser. Earth Environ. Sci. 2019, 300, 032008. [Google Scholar] [CrossRef]
- Deng, H.; Pan, T.; Zhang, Y.; Wang, L.; Wu, Q.; Ma, J.; Shan, W.; He, H. Adsorptive removal of toluene and dichloromethane from humid exhaust on MFI, BEA and FAU zeolites: An experimental and theoretical study. Chem. Eng. J. 2020, 394, 124986. [Google Scholar] [CrossRef]
- Aljerf, L. High-efficiency extraction of bromocresol purple dye and heavy metals as chromium from industrial effluent by adsorption onto a modified surface of zeolite: Kinetics and equilibrium study. J. Environ. Manag. 2018, 225, 120–132. [Google Scholar] [CrossRef]
- Yin, Y.; Shi, M.; Ren, Y.; Wang, S.; Hua, M.; Lu, J.; Zhang, W.; Lv, L. Wrinkle structure on multifunctional MOFs to facilitate PPCPs adsorption in wastewater. Chem. Eng. J. 2020, 387, 124196. [Google Scholar] [CrossRef]
- Abd El Salam, H.M. Bio-Sustainable Alternatives Synthesis of Nanoporous Activated Carbon @Al-MOF for the Adsorption of Hazardous Organic Dyes from Wastewater. Water Air Soil Pollut. 2023, 234, 567. [Google Scholar] [CrossRef]
- Karmakar, S.; Roy, D.; De, S. Multicomponent transport model-based scaling up of long-term adsorptive filtration of MOF incorporated mixed matrix hollow fiber membrane: Treatment of textile effluent. Chem. Eng. J. 2021, 403, 125103. [Google Scholar] [CrossRef]
- Wichterle, O.; Lím, D. Hydrophilic Gels for Biological Use. Nature 1960, 185, 117–118. [Google Scholar] [CrossRef]
- Chen, Y.; Wang, T.; Liu, J.; Huang, J.; Zhou, G.; Hu, S. Synthesis of microgel-reinforced double network hydrogel adsorbent and its adsorption on heavy metals. J. Chem. Technol. Biotechnol. 2023, 98, 1260–1268. [Google Scholar] [CrossRef]
- Zhen-Tao, Q.I.; Fang, H.E. Synthesis of novel MTF aerogels with adsorption performance. J. Sol-Gel Sci. Technol. 2019, 94, 582–595. [Google Scholar] [CrossRef]
- Huang, T.; Dai, J.; Yang, J.-h.; Zhang, N.; Wang, Y.; Zhou, Z.-w. Polydopamine coated graphene oxide aerogels and their ultrahigh adsorption ability. Diam. Relat. Mater. 2018, 86, 117–127. [Google Scholar] [CrossRef]
- Ruan, C.; Chen, G.; Ma, Y.; Du, C.; He, C.; Liu, X.; Jin, X.; Chen, Q.; He, S.; Huang, Y. PVA-assisted CNCs/SiO2 composite aerogel for efficient sorption of ciprofloxacin. J. Colloid. Interface Sci. 2023, 630, 544–555. [Google Scholar] [CrossRef] [PubMed]
- Mousavi, S.; Deuber, F.; Petrozzi, S.; Federer, L.; Aliabadi, M.; Shahraki, F.; Adlhart, C. Efficient dye adsorption by highly porous nanofiber aerogels. Colloids Surf. A Physicochem. Eng. Asp. 2018, 547, 117–125. [Google Scholar] [CrossRef]
- Van Tran, V.; Park, D.; Lee, Y.-C. Hydrogel applications for adsorption of contaminants in water and wastewater treatment. Environ. Sci. Pollut. Res. 2018, 25, 24569–24599. [Google Scholar] [CrossRef] [PubMed]
- Yu, F.; Yang, P.; Yang, Z.; Zhang, X.; Ma, J. Double-network hydrogel adsorbents for environmental applications. Chem. Eng. J. 2021, 426, 131900. [Google Scholar] [CrossRef]
- Chen, J.; Zhao, K.; Liu, L.; Gao, Y.; Zheng, L.; Liu, M. Modified kaolin hydrogel for Cu2+ adsorption. e-Polymers 2022, 22, 986–996. [Google Scholar] [CrossRef]
- Abdelwahab, H.; Hassan, S.; Mostafa, M.; El Sadek, M. Synthesis and Characterization of Glutamic-Chitosan Hydrogel for Copper and Nickel Removal from Wastewater. Molecules 2016, 21, 684. [Google Scholar] [CrossRef]
- Fekete, T.; Borsa, J.; Takács, E.; Wojnárovits, L. Synthesis of carboxymethylcellulose/starch superabsorbent hydrogels by gamma-irradiation. Chem. Cent. J. 2017, 11, 46. [Google Scholar] [CrossRef]
- Özkahraman, B.; Acar, I.; Emik, S. Removal of Cu2+and Pb2+Ions Using CMC Based Thermoresponsive Nanocomposite Hydrogel. CLEAN—Soil. Air Water 2011, 39, 658–664. [Google Scholar] [CrossRef]
- Wang, N.; Bora, M.; Hao, S.; Tao, K.; Wu, J.; Hu, L.; Liao, J.; Lin, S.; Triantafyllou, M.S.; Li, X. Hyaluronic Acid Methacrylate Hydrogel-Modified Electrochemical Device for Adsorptive Removal of Lead(II). Biosensors 2022, 12, 714. [Google Scholar] [CrossRef]
- Zhang, Y.-H.; Liu, C.-S.; Tian, Y.; Wang, J.; Xin, S.; Sheng, X. An eco-friendly photo-responsive hyaluronic acid-based supramolecular polysaccharide hybrid hydrogels for plant growth regulation and heavy metal ions adsorption. Int. J. Biol. Macromol. 2023, 242, 125194. [Google Scholar] [CrossRef] [PubMed]
- Guilherme, M.R.; Reis, A.V.; Paulino, A.T.; Moia, T.A.; Mattoso, L.H.C.; Tambourgi, E.B. Pectin-based polymer hydrogel as a carrier for release of agricultural nutrients and removal of heavy metals from wastewater. J. Appl. Polym. Sci. 2010, 117, 3146–3154. [Google Scholar] [CrossRef]
- Bhattarai, N.; Gunn, J.; Zhang, M. Chitosan-based hydrogels for controlled, localized drug delivery. Adv. Drug Deliv. Rev. 2010, 62, 83–99. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Li, Q.; Bai, N.; Dong, H.; Mao, D. One-Step Synthesis of Cationic Hydrogel for Efficient Dye Adsorption and Its Second Use for Emulsified Oil Separation. ACS Sustain. Chem. Eng. 2017, 5, 5598–5607. [Google Scholar] [CrossRef]
- Liu, H.; Rong, L.; Wang, B.; Xie, R.; Sui, X.; Xu, H.; Zhang, L.; Zhong, Y.; Mao, Z. Facile fabrication of redox/pH dual stimuli responsive cellulose hydrogel. Carbohydr. Polym. 2017, 176, 299–306. [Google Scholar] [CrossRef] [PubMed]
- Abu-Saied, M.A.; Wycisk, R.; Abbassy, M.M.; El-Naim, G.A.; El-Demerdash, F.; Youssef, M.E.; Bassuony, H.; Pintauro, P.N. Sulfated chitosan/PVA absorbent membrane for removal of copper and nickel ions from aqueous solutions—Fabrication and sorption studies. Carbohydr. Polym. 2017, 165, 149–158. [Google Scholar] [CrossRef]
- Godiya, C.B.; Cheng, X.; Li, D.; Chen, Z.; Lu, X. Carboxymethyl cellulose/polyacrylamide composite hydrogel for cascaded treatment/reuse of heavy metal ions in wastewater. J. Hazard. Mater. 2019, 364, 28–38. [Google Scholar] [CrossRef]
- Chen, Y.; Long, Y.; Li, Q.; Chen, X.; Xu, X. Synthesis of high-performance sodium carboxymethyl cellulose-based adsorbent for effective removal of methylene blue and Pb (II). Int. J. Biol. Macromol. 2019, 126, 107–117. [Google Scholar] [CrossRef]
- Stevens, L.; Williams, K.; Han, W.Y.; Drage, T.; Snape, C.; Wood, J.; Wang, J. Preparation and CO2 adsorption of diamine modified montmorillonite via exfoliation grafting route. Chem. Eng. J. 2013, 215–216, 699–708. [Google Scholar] [CrossRef]
- Chen, T.; Yuan, Y.; Zhao, Y.; Rao, F.; Song, S. Preparation of Montmorillonite Nanosheets through Freezing/Thawing and Ultrasonic Exfoliation. Langmuir 2019, 35, 2368–2374. [Google Scholar] [CrossRef]
- Feng, Q.; Chen, X.; Zhao, Y.-q.; Hu, S.-s.; Xia, Z.-w.; Yan, Q.-Z. Preparation of poly(N-isopropylacrylamide)/montmorillonite composite hydrogel by frontal polymerization. Colloid. Polym. Sci. 2017, 295, 883–890. [Google Scholar] [CrossRef]
- Nie, X.; Adalati, A.; Du, J.; Liu, H.; Xu, S.; Wang, J. Preparation of amphoteric nanocomposite hydrogels based on exfoliation of montmorillonite via in-situ intercalative polymerization of hydrophilic cationic and anionic monomers. Appl. Clay Sci. 2014, 97–98, 132–137. [Google Scholar] [CrossRef]
- Irani, M.; Ismail, H.; Ahmad, Z. Preparation and properties of linear low-density polyethylene-g-poly (acrylic acid)/organo-montmorillonite superabsorbent hydrogel composites. Polym. Test. 2013, 32, 502–512. [Google Scholar] [CrossRef]
- Wang, J.; Guo, X. Adsorption kinetic models: Physical meanings, applications, and solving methods. J. Hazard. Mater. 2020, 390, 122156. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Zhan, Y. Adsorption of humic acid from aqueous solution onto unmodified and surfactant-modified chitosan/zeolite composites. Chem. Eng. J. 2012, 200–202, 202–213. [Google Scholar] [CrossRef]
- Tran, H.N.; You, S.-J.; Chao, H.-P. Thermodynamic parameters of cadmium adsorption onto orange peel calculated from various methods: A comparison study. J. Environ. Chem. Eng. 2016, 4, 2671–2682. [Google Scholar] [CrossRef]
- Jeong, D.; Kim, C.; Kim, Y.; Jung, S. Dual crosslinked carboxymethyl cellulose/polyacrylamide interpenetrating hydrogels with highly enhanced mechanical strength and superabsorbent properties. Eur. Polym. J. 2020, 127, 109586. [Google Scholar] [CrossRef]
- Wang, W.; Ni, J.; Chen, L.; Ai, Z.; Zhao, Y.; Song, S. Synthesis of carboxymethyl cellulose-chitosan-montmorillonite nanosheets composite hydrogel for dye effluent remediation. Int. J. Biol. Macromol. 2020, 165, 1–10. [Google Scholar] [CrossRef]
- Kenawy, E.R.; Azaam, M.M.; El-nshar, E.M. Preparation of carboxymethyl cellulose-g-poly (acrylamide)/montmorillonite superabsorbent composite as a slow-release urea fertilizer. Polym. Adv. Technol. 2018, 29, 2072–2079. [Google Scholar] [CrossRef]


| CMC:MMT | Shrinkage | Density (ρ) g/cm3 | BET m2/g | Porosity (φ) |
|---|---|---|---|---|
| 3:0 | 26.32% | 0.0812 | 1.21 | 93.55% |
| 3:1 | 17.54% | 0.0785 | 1.26 | 93.53% |
| 3:2 | 12.42% | 0.0846 | 2.54 | 93.25% |
| 3:3 | 8.33% | 0.0881 | 3.08 | 93.17% |
| 3:4 | 7.95% | 0.0896 | 2.44 | 92.95% |
| Initial Concentration | CMC:MMT | Pseudo-First-Order | Pseudo-Second-Order | ||||
|---|---|---|---|---|---|---|---|
| Qe | K1 | R2 | Qe | K2 | R2 | ||
| mg/g | min−1 | mg/g | min−1 | ||||
| 60 mg/L | 3:0 | 84.53 | 0.0742 | 0.9993 | 86.11 | 0.0032 | 0.9406 |
| 3:3 | 112.13 | 0.0751 | 0.9998 | 114.02 | 0.0026 | 0.9882 | |
| Initial Concentration | CMC:MMT | KW&M1 | C | R2 | KW&M2 | C | R2 | KW&M3 | C | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/(g·min) | mg/g | mg/(g·min) | mg/g | mg/(g·min) | mg/g | |||||
| 60 mg/L | 3:0 | 1.952 | 65.806 | 0.8965 | 0.206 | 81.571 | 0.966 | 0.046 | 84.201 | 0.958 |
| 3:3 | 2.789 | 86.396 | 0.8366 | 0.190 | 109.225 | 0.951 | 0.064 | 111.367 | 0.942 |
| CMC:MMT | Temperature (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qe | KL | R2 | KF | n | R2 | ||
| mg/g | L/mg | mg/g | |||||
| 3:3 | 303 | 173.73 | 0.0714 | 0.879 | 86.4023 | 4.9443 | 0.9676 |
| Thermodynamic Constant | Temperature (K) | |||
|---|---|---|---|---|
| 293 | 303 | 313 | 323 | |
| Ko | 91.35 | 228.60 | 315.21 | 373.75 |
| (×1000 kJ mol−1) | −10.99 | −13.68 | −14.97 | −15.90 |
| (×1000 kJ mol−1) | 36.14 | 36.14 | 36.14 | 36.14 |
| (J mol−1 K−1) | 162.44 | 162.44 | 162.44 | 162.44 |
| Initial Concentration | CMC:MMT | Pseudo-First-Order | Pseudo-Secondary | ||||
|---|---|---|---|---|---|---|---|
| Qe | K1 | R2 | Qe | K2 | R2 | ||
| mg/g | min−1 | mg/g | min−1 | ||||
| 10 mg/L | 3:0 | 0.95 | 0.0485 | 0.9972 | 0.99 | 0.1124 | 0.9313 |
| 3:3 | 1.31 | 0.0055 | 0.9716 | 1.39 | 0.0423 | 0.9408 | |
| Initial Concentration | CMC:MMT | KW&M1 | C | R2 | KW&M2 | C | R2 | KW&M3 | C | R2 |
|---|---|---|---|---|---|---|---|---|---|---|
| mg/(g·min) | mg/g | mg/(g·min) | mg/g | mg/(g·min) | mg/g | |||||
| 10 mg/L | 3:0 | 0.0438 | 0.515 | 0.9605 | 0.0145 | 0.862 | 0.9738 | 0.0061 | 0.963 | 0.9526 |
| 3:3 | 0.0512 | 0.650 | 0.9141 | 0.0237 | 0.947 | 0.9329 | 0.0011 | 1.320 | 0.8922 |
| CMC:MMT | Temperature (K) | Langmuir | Freundlich | ||||
|---|---|---|---|---|---|---|---|
| Qe | KL | R2 | KF | n | R2 | ||
| mg/g | L/mg | mg/g | |||||
| 3:3 | 303 | 10.95 | 0.0318 | 0.9564 | 0.73 | 1.7771 | 0.9931 |
| Thermodynamic Constant | Temperature (K) | |||
|---|---|---|---|---|
| 293 | 303 | 313 | 323 | |
| Ko | 1.59 | 1.65 | 1.69 | 1.72 |
| (×1000 kJ mol−1) | −1.14 | −1.26 | −1.36 | −1.46 |
| (×1000 kJ mol−1) | 1.98 | 1.98 | 1.98 | 1.98 |
| (J mol−1 K−1) | 10.70 | 10.70 | 10.70 | 10.70 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xue, Y.; Zhong, S.; Wang, K.; Dong, Q.; Huang, Y.; Zhang, R.; Wang, L.; Jiang, T. Synthesis of Carboxymethylcellulose–Acrylamide–Montmorillonite Composite Hydrogels for Wastewater Purification. Separations 2023, 10, 582. https://doi.org/10.3390/separations10120582
Xue Y, Zhong S, Wang K, Dong Q, Huang Y, Zhang R, Wang L, Jiang T. Synthesis of Carboxymethylcellulose–Acrylamide–Montmorillonite Composite Hydrogels for Wastewater Purification. Separations. 2023; 10(12):582. https://doi.org/10.3390/separations10120582
Chicago/Turabian StyleXue, Yuxuan, Sai Zhong, Kuanwen Wang, Qianrui Dong, Yue Huang, Rui Zhang, Lei Wang, and Tengyao Jiang. 2023. "Synthesis of Carboxymethylcellulose–Acrylamide–Montmorillonite Composite Hydrogels for Wastewater Purification" Separations 10, no. 12: 582. https://doi.org/10.3390/separations10120582
APA StyleXue, Y., Zhong, S., Wang, K., Dong, Q., Huang, Y., Zhang, R., Wang, L., & Jiang, T. (2023). Synthesis of Carboxymethylcellulose–Acrylamide–Montmorillonite Composite Hydrogels for Wastewater Purification. Separations, 10(12), 582. https://doi.org/10.3390/separations10120582







