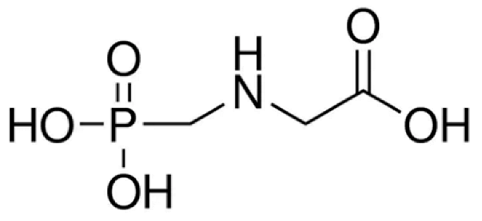
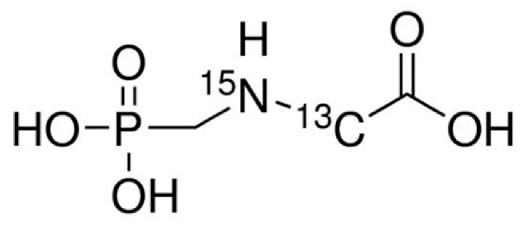
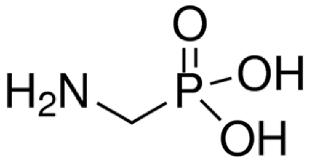
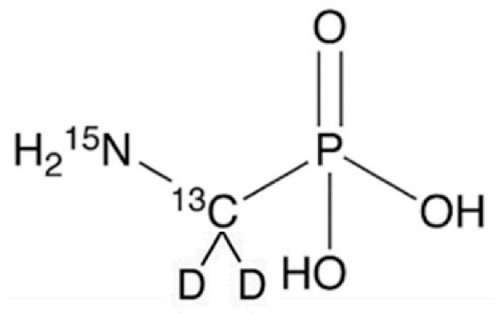
Abstract
Glyphosate-based herbicides are the most widely used pesticides in the world; however, the toxicity of glyphosate (GlyP) toward humans, especially its carcinogenicity, is controversial. The aim of this work was to validate a rapid assay for measuring GlyP and its metabolite aminomethylphosphonic acid (AMPA) in urine for human biomonitoring. The analytes were purified via solid-phase extraction in the presence of isotopically labeled internal standards. An LC-MS/MS assay was developed using a column with a novel hybrid stationary phase combined with anion exchange and hydrophilic interaction liquid chromatography. Detection and quantification were performed using negative electrospray ionization in a hybrid triple quadrupole/linear ion trap mass spectrometer. The retention times for AMPA and GlyP were 1.44 and 7.24 min, respectively. Calibration curves showed a linear dynamic range of up to 40 µg/L, inter- and intra-run precisions <7.5%, and accuracies within 10% of the theoretical concentrations. The limits of quantification were 0.1 µg/L and 0.5 µg/L for GlyP and AMPA, respectively. The matrix effect bias was controlled using internal standards. Successful participation in external quality assurance exercises strengthens the validity of the method. The assay was applied to the measurement of GlyP and AMPA in the urine of 9 urban residents, 26 rural residents, and 12 agricultural workers; while AMPA was mostly not quantifiable, the median GlyP values were 0.1 and 0.34 µg/L in rural residents and workers, respectively. The assay is useful to assess GlyP and AMPA in human urine following different exposure scenarios.
1. Introduction
Glyphosate-based herbicides are wide-spectrum herbicides with no selective effect and are the most widely used pesticides in the world [1]. These types of pesticides, which represent more than 750 formulations, are extensively used in both intensive farming and home gardens [2]. However, the toxicity of glyphosate (GlyP), especially its carcinogenicity, is widely debated; in fact, GlyP has been classified as probably carcinogenic to humans by the International Agency for Research on Cancer [3], but according to the US Environmental Protection Agency (EPA) and the European Chemical Agency (ECHA), GlyP is not classified as a human carcinogen [4,5]. According to the harmonized classification and labeling (CLP) approved by the European Union, this substance is toxic to aquatic life, has long-lasting effects, and causes serious eye damage [6]. In a recent peer review of the risk assessment of GlyP conducted by the European Food and Safety Agency (EFSA) based on its use as a herbicide, no critical areas of concern for mammalian toxicology were identified [7].
In the environment, GlyP has been detected in soil and water [8]; microbial degradation forms an aminomethylphosphonic acid (AMPA), which is also the main metabolite of GlyP in mammals [9]. Human exposure to GlyP and AMPA can occur in occupational settings as well as in the general environment. Professional exposure relates to agricultural workers applying GlyP to crops and pesticide production workers. Environmental exposure relates to the general population of individuals living in the proximity of treated fields, ingesting contaminated foods and water, and/or using glyphosate-based herbicides in non-professional activities, such as gardening. The biological monitoring of exposure has been applied to assess GlyP and AMPA in urine samples. While concentrations of GlyP in professional usage may be up to tens of µg/L, for the general population, the concentrations are typically below 1 µg/L; for AMPA, the concentration is generally much lower [10].
Several analytical methods have been developed for measuring GlyP in human urine, including AMPA. These include the use of enzyme-linked immunosorbent assays [11,12], gas chromatography–mass spectrometry after derivatization [13,14], and liquid chromatography coupled to mass spectrometry (LC-MS/MS), with different sample preparations and chromatographic columns [15,16,17,18,19,20,21,22,23,24]. Due to its high sensitivity and specificity, LC-MS/MS is particularly suitable for measuring low levels of chemicals in urine. However, given the high polarity, amphoteric nature, and low molecular weight of GlyP and AMPA, chromatographic separation may be critical, with very short retention times and the possibility of peak interferences, especially when reverse-phase chromatography is applied. To overcome this issue, alternatives have been proposed, such as sample derivatization [21], ion chromatography [15,20], and hydrophilic interaction liquid chromatography (HILIC). However, some drawbacks are associated with these approaches. While sample derivatization is time-consuming and dirties the column and the mass spectrometer, ion chromatography is associated with signal suppression and low sensitivity, and HILIC is affected by poor peak shape and retention time instability. Recently, novel columns with hybrid stationary phases combined with ion exchange and hydrophilic interaction liquid chromatography have become available. They increase the retention times of GlyP without the need for sample derivatization and ensure good chromatographic performances and column duration [24].
The aim of this study was to validate a sensitive and specific analytical method for the analysis of GlyP and AMPA in urine for the human biological monitoring of exposure based on LC-MS/MS by applying a column with a novel hybrid stationary phase combining anion-exchange and hydrophilic interaction liquid chromatography. This method was applied to determine GlyP and AMPA in urine samples of individuals with different exposures.
2. Materials and Methods
2.1. Chemicals
Analytical standards of GlyP and AMPA (both at a purity of ≥ 99%) were purchased from Sigma–Aldrich (Milan, Italy). For the preparation of the internal standard solution (IS), Glyphosate-2-13C,15N and AMPA-13C,15N,D2 were purchased from Sigma–Aldrich (98% atom 13C and 15N; Milan, Italy) and Cerilliant (99% atom 13C, 98% atom 15N, and 98% atom D; Milan, Italy), respectively. For the mobile phases, standard solutions, assay optimization, and sample preparation, methanol (MeOH), acetonitrile (CH3CN), and formic acid (all LC-MS/MS grade, Sigma–Aldrich, Milan, Italy) were used. Purified water was obtained using a Milli-Q Plus ultra-pure water system (Millipore, Milford, MA, USA).
2.2. Standard, Calibration, and Quality-Control Solutions
Aqueous standard solutions containing both GlyP and AMPA at concentrations of 2500, 250, and 25 µg/L were prepared. An IS solution containing the isotopically labeled analytes, each at a concentration of 2500 µg/L, was prepared in water. Standard and IS solutions were stored at −20 °C in the dark in plastic tubes. Under these conditions, the solutions were stable for up to 6 months.
Calibration solutions containing the analytes, each at concentrations of 0.1, 0.5, 1.0, 2.0, 5.0, 10, 20, and 40 µg/L, and QC solutions at 1.5, 2.5, and 25 µg/L, for low-, medium-, and high-QC, respectively, were prepared by adding suitable amounts of standard solutions to water.
Urine samples from healthy volunteers without known exposure to GlyP were tested for the absence of GlyP and AMPA signals; the blank samples were pooled and used as a matrix to prepare the calibration curves.
Before starting the analytical procedure, the IS solution was added to each calibration solution, QC solution, and unknown sample to the final concentration of 25 µg/L.
2.3. Equipment
For sample purification, a solid phase extraction cartridge (SPE, SampliQ Si-SAX, 500 mg × 3 mL, Agilent Technologies, Cernusco sul Naviglio, Italy) was used. A concentration step was performed using a dry block with nitrogen flow (Reacti-Vap Pierce, Milan, Italy). The analyses were performed using a high-performance liquid chromatograph (Agilent Technologies 1260, Cernusco sul Naviglio, Italy) interfaced with a hybrid triple quadrupole/linear ion trap mass spectrometer (QTRAP 5500; Sciex, Monza, Italy) equipped with an electrospray ionization source (ESI).
2.4. LC-MS/MS Analysis
The LC separation was performed with the column Raptor Polar X, 50 × 2.1 mm, 2.7 µm particle size (Restek, Cernusco sul Naviglio, Italy), kept at 40 °C using a linear gradient obtained with the A phase, 0.5% formic acid in water, and the B phase, 0.5% formic acid in CH3CN, flowing at 500 µL/min. The gradient was programmed as follows: 0–1 min, 65% B isocratic; 1–4 min, from 65% to 10% B; 4–9 min, 10% B isocratic; 9–10 min, from 10% to 65% B; and 10–15 min, 65% B isocratic. The mass spectrometer operated in multiple reaction monitoring (MRM) mode, with a dwell time of 150 ms, using the ESI source in negative ionization mode. The principal ionization source parameters were as follows: gas 1 pressure 80 psi, gas 2 pressure 60 psi, curtain gas pressure 20 psi, heater temperature 600 °C, and entrance potential 10 V. The two most intense MRM transitions for each native analyte were recorded; the most intense transition was used for quantitation, and the other for qualification (Table 1). For each isotopically labeled standard, the most intense ion transition was recorded. Analist® software (version 1.6.3; Sciex, Monza, Italy) was used to set up the method and the analysis batches, while MultiQuant™ software (version 3.0.8664.0; Sciex, Monza, Italy) was used for quantification.

Table 1.
Principal LC-MS/MS parameters for analyzing GlyP and AMPA and their internal standards. Molecular structures, MRM transitions (Q1 mass and Q3 mass) for quantifier and qualifier ions, collision energies (CE), and chromatographic retention times are given.
2.5. Sample Preparation
Before analysis, samples (calibrators, QCs, and unknown samples) were purified with SPE cartridges. The workflow was as follows: cartridge conditioning with 2 mL of methanol followed by 2 mL of water; sample loading with 1 mL of sample; cartridge wash with 2 mL of water, followed by 2 mL of methanol and 1 mL of 10% formic acid in methanol; and elution with 1.5 mL of 10% formic acid in methanol. The eluate was evaporated under a gentle stream of nitrogen, with the heating block set at 45 °C. The dried samples were reconstituted with 100 µL of 0.1% formic acid in water, vigorously mixed with a vortex, and then transferred into a plastic insert. At the end, an aliquot of 10 µL was injected in LC-MS/MS for analysis.
2.6. Set Up of the Analytical Sequence
In the routine analysis, the calibration curve and QCs were run with every set of unknown samples. A typical analytical sequence consisted of a calibration curve, followed by unknown samples and three QC (low-, medium-, and high-QC) every ten unknown samples, followed by a second calibration curve.
2.7. Chromatographic Method
2.7.1. Analytical Column Selection and MS/MS Analysis
To define the best chromatographic separation, different columns were tested. They were as follows: reverse phase columns Betasil C18, 150 × 2.1 mm, 5 µm particle size (Thermo Fisher Scientific, Rodano, Italy), Betasil C8, 100 × 2.1 mm, 5 µm particle size (Thermo Fisher Scientific, Rodano, Italy), Zorbax XDB–C8, 150 × 2.1 mm, 3.5 µm particle size (Agilent Technologies, Cernusco sul Naviglio, Italy), and the novel Raptor Polar X, 50 × 2.1 mm, 2.7 µm particle size (Restek, Cernusco sul Naviglio, Italy), combining anion-exchange and a hydrophilic interaction liquid chromatography. Different linear gradient programs, as well as different organic solvents (MeOH or CH3CN), and the addition of acid (no acid; 0.1% formic acid, or 0.5% formic acid), were tested to improve the peak separation, peak shape, retention times, and signal-to-noise ratio. The MS/MS working conditions, such as the ESI and ionization parameters, MRM transitions, and collision energies, were optimized by a direct infusion of standard solutions in water (0.1 mg/L) using a combination of manual and auto-tuning.
2.7.2. SPE Extraction and Purification
SampliQ Si-SAX cartridges were chosen based on the previous use of this stationary phase [16]. To optimize the extraction procedure and the recovery evaluation, low-, medium-, and high-QC in urine (n = 5 for each level) were analyzed in two different batches. In the optimization experiment, after the sample loading, all fractions were collected and analyzed to test the possible leaching of analytes during the washing. The recovery was calculated as the percent ratio between the chromatographic signals of a purified extract versus the signal of GlyP and AMPA in water directly injected into the chromatographic system.
2.8. Assay Validation
2.8.1. Calibration Curve, Limits of Detection and Quantification, Carryover, Mid-Term Stability, Precision, Accuracy, Selectivity, and Matrix Effect
The calibration curve was prepared with one blank and eight non-zero calibration solutions, covering the expected range of concentrations. A least-squares linear regression analysis was applied to interpolate the data pairs, where y was the ratio between the chromatographic peak area and the chromatographic peak area of the corresponding IS, and x was the concentration (µg/L). For method validation, fourteen replicates of each calibration level were analyzed.
The limit of quantification (LOQ) of the assay was calculated according to the following expression:
where SEq is the standard error of the intercept q, and m is the slope of the linear regression. To verify whether the obtained results met the US FDA requirements for the LOQ selection, the precision (expressed as the coefficients of variation, %RSD) and accuracy (calculated as the %Theoretical) were calculated at the LOQ level [25].
LOQ = (5SEq + q)/m
A sample of water was analyzed immediately after the highest point of the calibration curve to test the carryover effect.
Mid-term stability was evaluated as the variability of the calibration curve slopes (n = 14) over a period of six months and estimated as %RSDslope.
The intra- and inter-day precision and accuracy were determined by analyzing the low-, medium-, and high-QC solutions three times on the same day, on eight different days over a period of 6 months. The precision was expressed as the relative standard deviation (%RSD). The accuracy was calculated as the percent ratio between the concentration calculated from the calibration curves and the theoretical (spiked) concentrations (%Theoretical).
For the selectivity test, two different urine samples were selected: one blank urine from a subject with no GlyP exposure and one urine from a professionally exposed subject. These urines were added to a mixture of 40 pesticides from different agrochemical categories (5 insecticides, 13 herbicides, and 22 fungicides), chosen from the main pesticides used in the area and including some pesticides not authorized by the EU but persistent in the environment. Three replicates for each sample were prepared and analyzed.
For the matrix effect determination, urine samples from six different donors without known exposure to pesticides were used. QC samples and calibration curves were obtained with the procedure described in Section 2.2. The relative matrix effect (%Matrixrelative) was determined as the inter-matrix precision value (expressed as %RSD) obtained by comparing the QC response in water vs. the QC response in urine. Moreover, the inter-matrix slope range (%Rslope) was calculated via the following formula [26]:
where Maximumslope and Minimumslope are the highest and the lowest slope values of the calibration curves obtained from the different subjects. All calculations were performed both with and without IS adjustment for comparison.
% Rslope = [(Maximumslope − Minimumslope)/Minimumslope] × 100
2.8.2. External Verification
To further verify the method, we participated in the German External Quality Assessment Scheme (G-EQUAS) and certification for occupational–medical and environmental–medical toxicological analyses in biological materials [27]. Three urine samples with unknown concentrations of GlyP in the environmental concentration range were delivered to our laboratory in 2022 and 2023 as part of rounds 69, 70, and 71; they were analyzed with the method described here. To further investigate the validity of our method, another six G-EQUAS samples, corresponding to three previous rounds, were analyzed.
2.9. Method Application
Urine samples from 9 urban residents, 26 rural residents living near the treated crops, and 12 agricultural workers who had mixed, loaded, and applied different pesticides, including GlyP, on crops (mainly corn, wheat, and soy) were analyzed for GlyP and AMPA. Spot urine samples were collected in the morning at the end of the application season (April–May). The samples were stored at −20 °C until analysis. Urinary creatinine was determined using Jaffe’s colorimetric method; the concentrations were in the acceptable range (from 0.39 to 1.25 g/L) [28] for all samples.
3. Results and Discussion
3.1. Method Development
3.1.1. Analytical Column Selection and MS/MS Analysis
By performing tests to select the best chromatographic conditions, we noticed that operating with reverse phase columns (both C8 and C18), the retention time of GlyP and AMPA never exceeded 2 min, regardless of the length of the column. With such short retention times, analytes entered the mass spectrometer ion source together with several other non-retained small molecules and salts from the biological matrix, which caused strong ion suppression and decreased sensitivity. The column finally adopted, the Raptor Polar X, has a stationary phase combining ion exchange and hydrophilic interaction chromatography in a single ligand. Figure 1 shows an example of the chromatogram of the quantifier ions of AMPA and GlyP in a sample of an agricultural worker. The retention time of GlyP is about 7 min, which allows for overcoming the matrix interferences described above and enhances sensitivity.
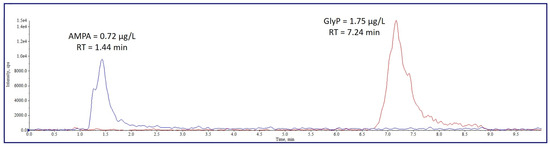
Figure 1.
Chromatogram of the quantifier ions of AMPA and GlyP in a urine sample from an agricultural worker (0.72 µg/L of AMPA and 1.75 µg/L of GlyP).
In Figure 2, an example of the quantifier ion chromatograms of AMPA (a) and GlyP (b) and their internal standards in blank urine and in urine with the analytes at the LOQ are shown; although the retention time of AMPA is still quite short (2 min), the chromatographic signals for both AMPA and GlyP are clearly distinguishable from the noise.
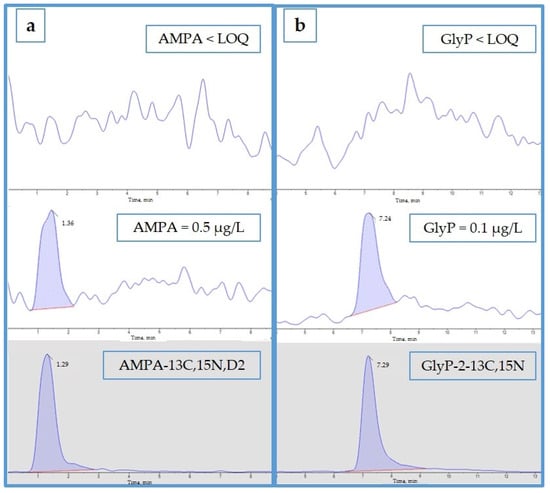
Figure 2.
Quantifier ion chromatograms of AMPA (a) and GlyP (b) of a blank urine sample (upper panels), of a blank urine sample added with the analyte at LOQ level (middle panels), and the corresponding labeled IS (lower panels).
Previous experiences could achieve long retention times only after a derivatization step [21], but this required reagents that can dirty the system and a longer, and sometimes cumbersome, procedure for sample preparation. Based on these considerations, we concluded that the proposed column is very convenient as it allowed us to reduce sample handling and the preparation time to obtain a simple method with good sensitivity. Indeed, this is a relevant advantage, especially considering that, in studies including the general population, a large percentage of samples were reported as below the limit of quantification/detection [10].
To improve the peak separation, peak shape, retention times, and signal-to-noise ratio, different chromatographic conditions were tested. The addition of 0.5% aqueous formic acid to both mobile phases was chosen to improve the peak shapes for both GlyP and AMPA.
The principal tuning parameters and chromatographic conditions are reported in Table 1. Signals were registered in a negative ionization mode; for each chemical, the transitions producing the most abundant ion were chosen for quantification (m/z 168 → 63 for GlyP; m/z 110 → 63 for AMPA; m/z 170 → 63 for Glyphosate-2-13C,15N; and m/z 114 → 63 for AMPA-13C,15N,D2).
3.1.2. SPE Extraction and Purification
In the optimization experiment, no signal was found in the loading fraction or during the cartridge wash. The elution volume was finally set at 1.5 mL to allow for the complete elution of both GlyP and AMPA without organic solvent waste.
The recovery of GlyP and AMPA from the urine following the SPE step ranged from 86% to 112%, with lower recovery of AMPA (from 86% to 95%) compared to GlyP (from 92% to 112%); the recovery of IS was similar to that of the respective analyte. Indeed, the correction of the analyte’s signal with the IS resulted in a ratio close to 100% (94% and 93% for GlyP and AMPA, respectively), confirming the importance of using the isotopically labeled analogs to ensure reliable analytical performances.
3.2. Assay Validation
3.2.1. Calibration Curve, Limits of Detection and Quantification, Carryover, Mid-Term Stability, Precision, Accuracy, Selectivity, and Matrix Effect
A summary of the validation parameters is reported in Table 2.

Table 2.
Major parameters of the assay validation, including limit of detection (LOD), limit of quantification (LOQ), matrix effect, and precision and accuracy for quality controls.
Good linearity was found for both analytes, with coefficients of determination (R2) higher than 0.992. The LOQ was 0.1 µg/L for GlyP and 0.5 µg/L for AMPA. These LOQs are in line with or better than those previously reported [10]. At the LOQ, the precision was 2.3% for GlyP and 3.8% for AMPA, while the accuracy was 112% for GlyP and 101% for AMPA. No carryover was found. For all analytes, the mid-term stability of the calibration curve was good, with the %RSDslope up to 5.6%, which is within the range of intra-day precision. The inter- and intra-run precision and accuracy of the assay met the US FDA requirements for the validation of bioanalytical methods (precision, estimated as %RSD < 10%; accuracy between 93 and 108% of the theoretical concentrations) [25].
In the selectivity experiment, no interference in the signal of GlyP and AMPA from other pesticides was highlighted. In the samples of the exposed subjects, the quantification showed good reproducibility (%RSD = 3.7%), with precision in line with those obtained for the intra-day experiment.
For both GlyP and AMPA, a significant matrix effect was found; this was, however, completely overcome using the IS. The variability of the QC obtained in different matrices (%Matrixrelative) ranged from 6.1 to 9.2% without IS correction and from 4.6 to 6.8% with IS. Other studies have confirmed the importance of using isotopically marked analogs to minimize the matrix effect [14,15,16]. Finally, the %Rslope, representing the maximum difference in the slope values obtained using matrices from different individuals, were 14.2 and 29.7% without IS for GlyP and AMPA, respectively. They were reduced to below 3.9% with IS correction. Overall, some matrix effect was observed; however, the use of isotopically labeled IS effectively reduced it and maintained a good analytical performance.
3.2.2. External Verification
The external verification was performed only for GlyP, as it is not available for AMPA. Participation in the G-EQUAS rounds 69, 70, and 71 was evaluated as satisfactory for both the low and high levels. In round 69, we reported concentrations of 0.49 and 1.67 µg/L vs. 0.45 (0.33–0.57) and 1.86 (1.44–2.28) µg/L. In round 70, we reported concentrations of 0.21 and 0.97 µg/L vs. 0.22 (0.16–0.28) and 1.03 (0.76–1.30) µg/L. In round 71, we reported concentrations of 0.36 and 1.51 µg/L vs. 0.33 (0.24–0.42) and 1.58 (1.22–1.94) µg/L (see Figure 3). Also, considering the G-EQUAS samples of three previous rounds, the accuracies ranged from 90 to 110%.
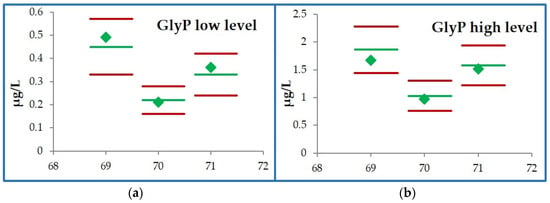
Figure 3.
Results of urinary GlyP in the GEQUAS external verification in rounds 69, 70, and 71: (a) low concentration samples; (b) high concentration samples. The green line represents the mean level; the red lines are the minimum and maximum; our results are reported as green diamonds.
3.3. Method Application
The results of GlyP and AMPA in the urine samples of 47 volunteers are summarized in Table 3.

Table 3.
Results of GlyP and AMPA in urine samples of urban residents, rural residents, and agricultural workers.
In the urban residents, GlyP and AMPA were always below the limit of quantification. In the rural residents, GlyP was above the LOQ in 58% of the samples, with a mean level of 0.13 µg/L, while AMPA was never detected. In the agricultural workers, GlyP was detected in almost all samples (92%), with a mean level of 0.42 µg/L and a maximum of 1.75 µg/L, while AMPA was detected in only two samples (0.50 and 0.72 µg/L). In Figure 4, the box plot distributions of GlyP in the three groups are reported. The median levels follow the order of agricultural workers > rural residents > urban residents (p < 0.001). The levels of GlyP and AMPA detected in these samples are in line with those previously reported for both the exposed and the non-occupationally exposed subjects [10,13,29,30,31].
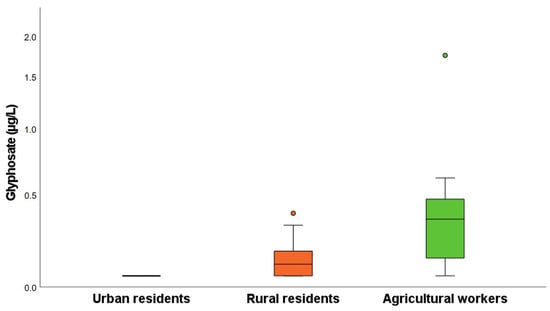
Figure 4.
Levels of GlyP in urine samples of urban residents, rural residents, and agricultural workers.
4. Conclusions
An LC-MS/MS method for determining GlyP and its main metabolite AMPA in human urine was validated in-house, according to international guidelines, and participating in an external verification exercise. Good linearity, precision, and accuracy were obtained. Isotopically labeled internal standards play an essential role in controlling the sources of bias. The high sensitivity of the method allows for the quantification of GlyP and AMPA in the urine samples of both individuals with occupational exposure and those belonging to the general population.
Author Contributions
Conceptualization, E.P., R.M., and S.F.; methodology, E.P. and R.M.; validation, E.P. and R.M.; formal analysis, E.P.; investigation, E.P. and R.M.; data curation, E.P. and R.M.; writing—original draft preparation, E.P.; writing—review and editing, R.M. and S.F.; visualization, E.P. and R.M.; supervision, S.F.; project administration, S.F. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
This study was conducted according to the guidelines of the Declaration of Helsinki. Since ours is a purely observational study, non-pharmacological and non-profit, the approval of an ethics committee was not necessary according to the Italian legislation (Decreto della Direzione Generale della Sanità n.11960 del 13.07.2004 relativo all’approvazione delle linee guida sugli Studi “Osservazionali” o “non interventistici”; Determinazione AIFA—20 marzo 2008. Linee guida per la classificazione e conduzione degli studi osservazionali sui farmaci. (GU n. 76 del 31-3-2008)).
Informed Consent Statement
Informed consent was obtained from all subjects involved in the study. Their privacy is protected according to the Italian regulations in force (D.L.vo 196/2003 e le Linee Guida del Garante, Deliberazione n. 52 del 24/07/2008).
Data Availability Statement
The data presented in this study may be available upon reasonable request from the corresponding author. The data are not publicly available due to privacy and legal restrictions.
Acknowledgments
The authors acknowledge support from the Department of Clinical Sciences and Community Health of the University of Milan through the APC initiative.
Conflicts of Interest
The authors declare no conflict of interest.
References
- Maggi, F.; La Cecilia, D.; Tang, F.H.M.; McBratney, A. The global environmental hazard of glyphosate use. Sci. Total. Environ. 2020, 717, 137167. [Google Scholar] [CrossRef]
- Benbrook, C.M. Trends in glyphosate herbicide use in the United States and globally. Environ. Sci. Eur. 2016, 28, 3. [Google Scholar] [CrossRef] [PubMed]
- International Agency for Research on Cancer (IARC). Some Organophosphate Insecticides and Herbicides-IARC Monographs-Glyphosate; International Agency for Research on Cancer: Lyon, France, 2017; Volume 112, pp. 1–92. [Google Scholar]
- US Environmental Protection Agency (EPA). Glyphosate Issue Paper: Evaluation of Carcinogenic. 2016. Available online: https://www.epa.gov/sites/default/files/2016-09/documents/glyphosate_issue_paper_evaluation_of_carcincogenic_potential.pdf (accessed on 8 September 2023).
- European Chemical Agency (ECHA). Glyphosate Not Classified as a Carcinogen by ECHA. Available online: https://echa.europa.eu/it/-/glyphosate-not-classified-as-a-carcinogen-by-echa (accessed on 8 September 2023).
- European Chemical Agency (ECHA). Hazard Classification & Labelling of Glyphosate. Available online: https://echa.europa.eu/it/substance-information/-/substanceinfo/100.012.726 (accessed on 8 September 2023).
- European Food and Safety Agency (EFSA). Peer Review of the Pesticide Risk Assessment of the Active Substance Glyphosate. Available online: https://www.efsa.europa.eu/en/efsajournal/pub/8164 (accessed on 1 September 2023).
- Kanissery, R.; Gairhe, B.; Kadyampakeni, D.; Batuman, O.; Alferez, F. Glyphosate: Its environmental persistence and impact on crop health and nutrition. Plants 2019, 8, 499. [Google Scholar] [CrossRef] [PubMed]
- Anadón, A.; Martínez-Larrañaga, M.R.; Martínez, M.A.; Castellano, V.J.; Martínez, M.; Martin, M.T.; Nozal, M.J.; Bernal, J.L. Toxicokinetics of glyphosate and its metabolite aminomethyl phosphonic acid in rats. Toxicol. Lett. 2009, 190, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.; Coggins, M.A.; Koch, H.M. Human Biomonitoring of Glyphosate Exposures: State-of-the-Art and Future Research Challenges. Toxics 2020, 8, 60. [Google Scholar] [CrossRef] [PubMed]
- Grau, D.; Grau, N.; Gascuel, Q.; Paroissin, C.; Stratonovitch, C.; Lairon, D.; Devault, D.A.; Di Cristofaro, J. Quantifiable urine glyphosate levels detected in 99% of the French population, with higher values in men, in younger people, and in farmers. Environ. Sci. Pollut. Res. 2022, 29, 32882–32893. [Google Scholar] [CrossRef] [PubMed]
- Rendón-Von Osten, J.; Dzul-Caamal, R. Glyphosate Residues in Groundwater, Drinking Water and Urine of Subsistence Farmers from Intensive Agriculture Localities: A Survey in Hopelchén, Campeche, Mexico. Int. J. Environ. Res. Public Health 2017, 14, 595. [Google Scholar] [CrossRef] [PubMed]
- Conrad, A.; Schröter-Kermani, C.; Hoppe, H.W.; Rüther, M.; Pieper, S.; Kolossa-Gehring, M. Glyphosate in German adults—Time trend (2001 to 2015) of human exposure to a widely used herbicide. Int. J. Hyg. Environ. Health 2017, 220, 8–16. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.; Koslitz, S.; Bury, D.; Brüning, T.; Conrad, A.; Kolossa-Gehring, M.; Coggins, M.A.; Koch, H.M. Sensitive and selective quantification of glyphosate and aminomethylphosphonic acid (AMPA) in urine of the general population by gas chromatography-tandem mass spectrometry. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2020, 1158, 122348. [Google Scholar] [CrossRef] [PubMed]
- Chen, M.X.; Cao, Z.Y.; Jiang, Y.; Zhu, Z.W. Direct determination of glyphosate and its major metabolite, aminomethylphosphonic acid, in fruits and vegetables by mixed-mode hydrophilic interaction/weak anion-exchange liquid chromatography coupled with electrospray tandem mass spectrometry. J. Chromatogr. A 2013, 1272, 90–99. [Google Scholar] [CrossRef]
- Jensen, P.K.; Wujcik, C.E.; McGuire, M.K.; McGuire, M.A. Validation of reliable and selective methods for direct determination of glyphosate and aminomethylphosphonic acid in milk and urine using LC-MS/MS. J. Environ. Sci. Health-Part B Pestic. Food Contam. Agric. Wastes 2016, 51, 254–259. [Google Scholar] [CrossRef]
- Tsao, Y.C.; Lai, Y.C.; Liu, H.C.; Liu, R.H.; Lin, D.L. Simultaneous determination and quantitation of paraquat, diquat, glufosinate and glyphosate in postmortem blood and urine by LC-MS-MS. J. Anal. Toxicol. 2016, 40, 427–436. [Google Scholar] [CrossRef] [PubMed]
- Connolly, A.; Jones, K.; Galea, K.S.; Basinas, I.; Kenny, L.; McGowan, P.; Coggins, M. Exposure assessment using human biomonitoring for glyphosate and fluroxypyr users in amenity horticulture. Int. J. Hyg. Environ. Health 2017, 220, 1064–1073. [Google Scholar] [CrossRef] [PubMed]
- Jaikwang, P.; Junkuy, A.; Sapbamrer, R.; Seesen, M.; Khacha-ananda, S.; Mueangkhiao, P.; Wunnapuk, K. A Dilute-and-Shoot LC–MS/MS Method for Urinary Glyphosate and AMPA. Chromatographia 2020, 83, 467–475. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, X.; Huo, Z.; Sun, H.; Zhang, F.; Zhu, B. An ion chromatography tandem mass spectrometry (IC-MS/MS) method for glyphosate and amino methyl phosphoric acid in serum of occupational workers. Microchem. J. 2021, 170, 106614. [Google Scholar] [CrossRef]
- Bressán, I.G.; Llesuy, S.F.; Rodriguez, C.; Ferloni, A.; Dawidowski, A.R.; Figar, S.B.; Giménez, M.I. Optimization and validation of a liquid chromatography-tandem mass spectrometry method for the determination of glyphosate in human urine after pre-column derivatization with 9-fluorenylmethoxycarbonyl chloride. J. Chromatogr. B Anal. Technol. Biomed. Life Sci. 2021, 1171, 122616. [Google Scholar] [CrossRef]
- Martin-Reina, J.; Dahiri, B.; Carbonero-Aguilar, P.; Soria-Dıaz, M.E.; González, A.G.; Bautista, J.; Moreno, I. Validation of a simple method for the determination of glyphosate and aminomethylphosphonic acid in human urine by UPLC-MS/MS. Microchem. J. 2021, 170, 106760. [Google Scholar] [CrossRef]
- Li, Z.-M.; Kannan, K.A.; Tchounwou, B.; Li, Z.-M.; Kannan, K. A Method for the Analysis of Glyphosate, Aminomethylphosphonic Acid, and Glufosinate in Human Urine Using Liquid Chromatography-Tandem Mass Spectrometry. Int. J. Environ. Res. Public Health 2022, 19, 4966. [Google Scholar] [CrossRef]
- Zhang, H.; Dou, J.; Miao, R.; Hu, J.; Huo, Z.; Zhang, F.; Ji, W. An analytical method for the determination of glyphosate and aminomethylphosphoric acid using an anionic polar pesticide column and the application in urine and serum from glyphosate poisoning patients. Anal. Methods 2023, 15, 1668–1673. [Google Scholar] [CrossRef] [PubMed]
- US Food and Drug Administration (FDA). Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Bioanalytical Method Validation Guidance for Industry Biopharmaceutics Contains Nonbinding Recommendations. 2018. Available online: https://www.fda.gov/files/drugs/published/Bioanalytical-Method-Validation-Guidance-for-Industry.pdf (accessed on 6 September 2023).
- Matuszewski, B.K. Standard line slopes as a measure of a relative matrix effect in quantitative HPLC–MS bioanalysis. J. Chromatogr. B 2006, 830, 293–300. [Google Scholar] [CrossRef]
- German External QUality Assessment Scheme (G-EQUAS). The German External Quality Assessment Scheme for Analyses in Biological Materials. Available online: https://app.g-equas.de/web/ (accessed on 6 September 2023).
- Kroll, M.H.; Chesler, R.; Hagengruber, C.; Blank, D.W.; Kestner, J.; Rawe, M. Automated determination of urinary creatinine without sample dilution: Theory and practice. Clin. Chem. 1986, 32, 446–452. [Google Scholar] [CrossRef]
- Lesseur, C.; Pathak, K.V.; Pirrotte, P.; Martinez, M.N.; Ferguson, K.K.; Barrett, E.S.; Nguyen, R.H.N.; Sathyanarayana, S.; Mandrioli, D.; Swan, S.H.; et al. Urinary glyphosate concentration in pregnant women in relation to length of gestation. Environ. Res. 2022, 203, 111811. [Google Scholar] [CrossRef] [PubMed]
- Ruiz, P.; Dualde, P.; Coscollà, C.; Fernández, S.F.; Carbonell, E.; Yusà, V. Biomonitoring of glyphosate and AMPA in the urine of Spanish lactating mothers. Sci. Total Environ. 2021, 801, 149688. [Google Scholar] [CrossRef] [PubMed]
- Stajnko, A.; Snoj Tratnik, J.; Kosjek, T.; Mazej, D.; Jagodic, M.; Eržen, I.; Horvat, M. Seasonal glyphosate and AMPA levels in urine of children and adolescents living in rural regions of Northeastern Slovenia. Environ. Int. 2020, 143, 105985. [Google Scholar] [CrossRef] [PubMed]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).