Optimization and Validation of Sensitive UPLC-PDA Method for Simultaneous Determination of Thymoquinone and Glibenclamide in SNEDDs Formulations Using Response Surface Methodology
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials
2.2. Design of Experiment (DoE)
2.3. UPLC Analytical System and Conditions
2.4. Optimization of UPLC Conditions for GB Analysis
2.5. Preparation of Standard Stock Solution, Calibration and Quality Control Samples
2.6. UPLC Analytical Validation
2.6.1. Linearity
2.6.2. Accuracy and Precision
2.6.3. Limit of Detection (LOD) and Limit of Quantification (LOQ)
2.6.4. Robustness
2.7. Preparation and Characterization of SNEDDS
Determination of Drug Content
3. Results
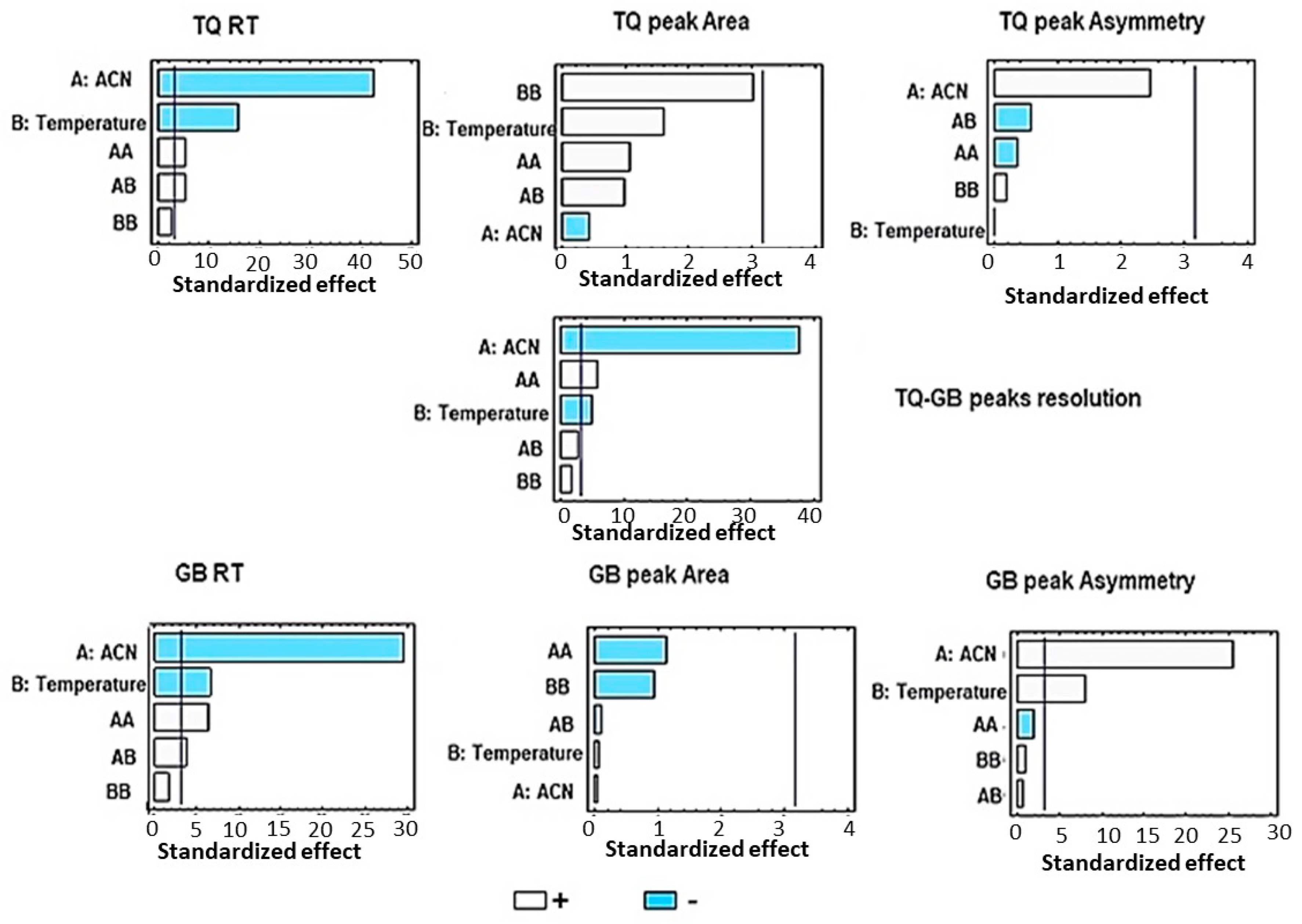
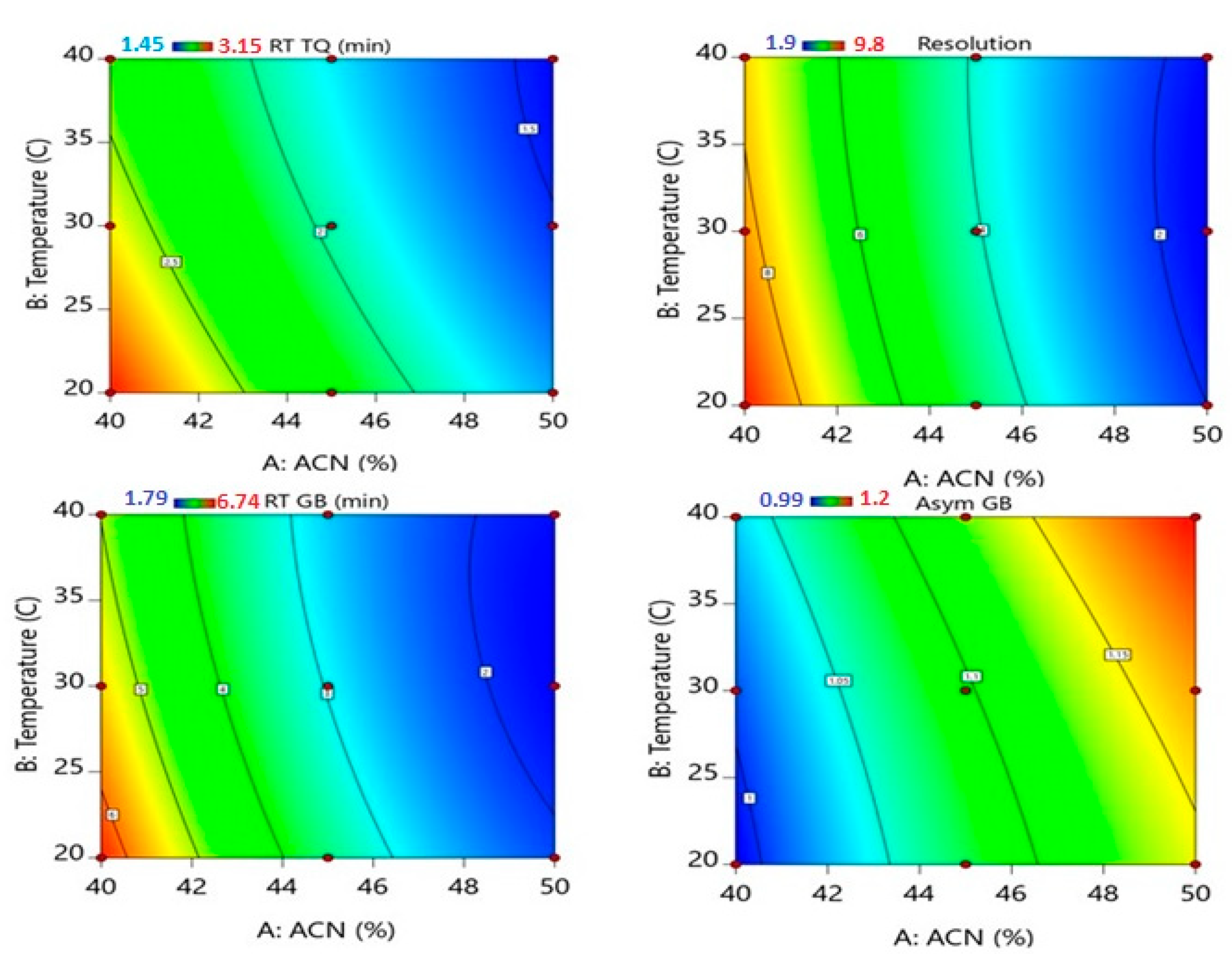
3.1. Effect of Independent Factors on Retention Time (RT)
3.2. Effect of Independent Factors on Peak Area
3.3. Effect of Independent Factors on Peak Symmetry
3.4. Effect of Independent Factors on the Resolution between TQ and GB Peaks
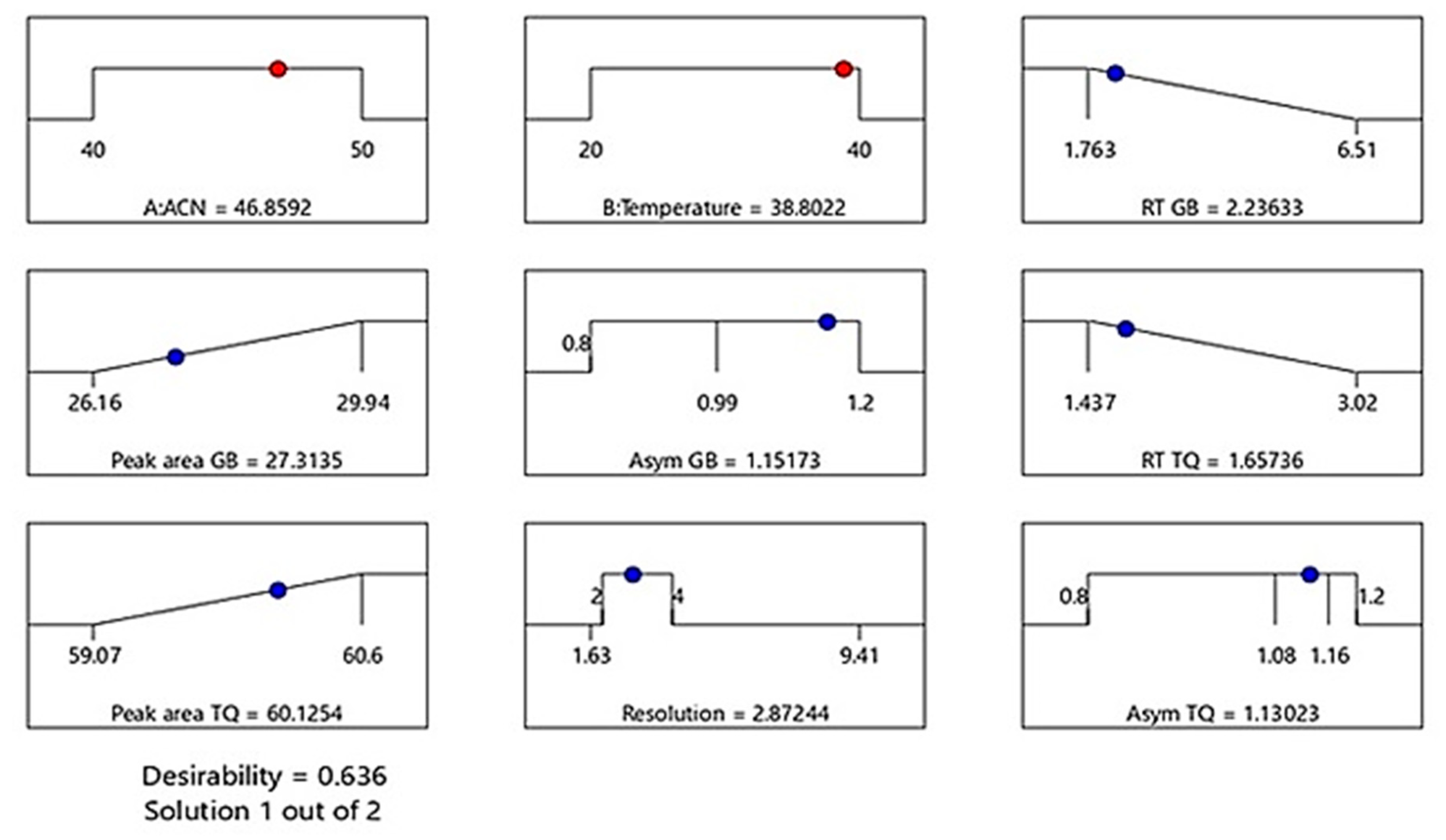
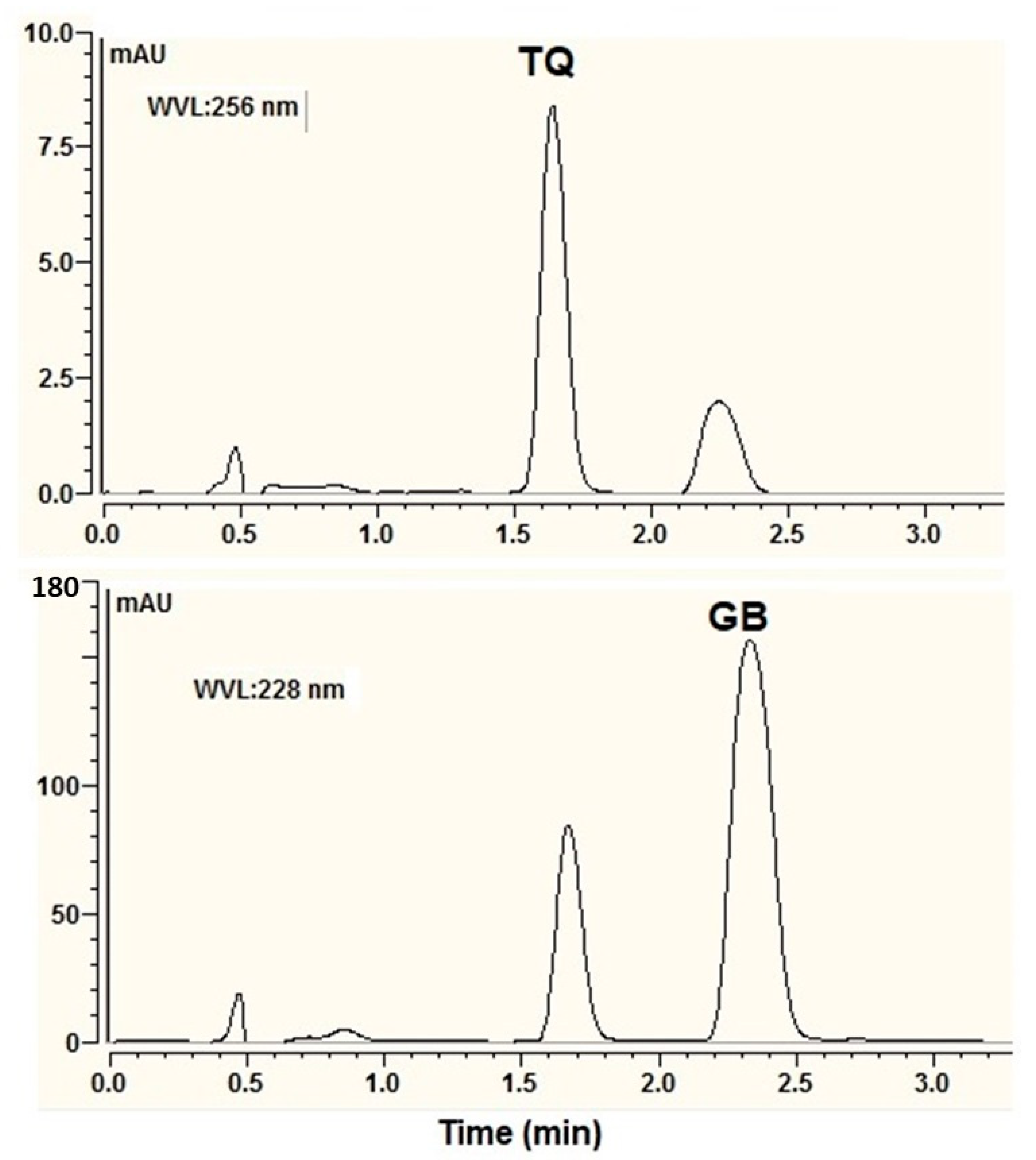
3.5. Optimization of UPLC Conditions for Simultaneous Analysis of TQ and GB
3.6. Validation Method
3.6.1. Linearity
3.6.2. Limit of Detection (LOD) and Limit of Quantification (LOQ)
3.6.3. Accuracy and Precision
3.6.4. Robustness
3.7. Characterization of SNEDD Formula Containing TQ and GB
Physicochemical Properties and Drug Content
4. Discussion
5. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Adibkia, K.; Ghajar, S.; Osouli-Bostanabad, K.; Balaei, N.; Emami, S.; Barzegar-Jalali, M. Novel gliclazide electrosprayed nano-solid dispersions: Physicochemical characterization and dissolution evaluation. Adv. Pharm. Bull. 2019, 9, 231. [Google Scholar] [CrossRef] [PubMed]
- Albertini, B.; Sabatino, M.D.; Melegari, C.; Passerini, N. Formulation of spray congealed microparticles with self-emulsifying ability for enhanced glibenclamide dissolution performance. J. Microencap. 2015, 32, 181–192. [Google Scholar] [CrossRef] [PubMed]
- Tran, N.; Pham, B.; Le, L. Bioactive compounds in anti-diabetic plants: From herbal medicine to modern drug discovery. Biology 2020, 9, 252. [Google Scholar] [CrossRef]
- Sun, J.; Ren, J.; Hu, X.; Hou, Y.; Yang, Y. Therapeutic effects of Chinese herbal medicines and their extracts on diabetes. Biomed. Pharmacother. 2021, 142, 111977. [Google Scholar] [CrossRef] [PubMed]
- Tarasevičienė, Ž.; Laukagalis, V.; Paulauskienė, A.; Baltušnikienė, A.; Meškinytė, E. Quality Changes of Cold-Pressed Black Cumin (Nigella sativa L.), Safflower (Carthamus tinctorius L.), and Milk Thistle (Silybum marianum L.) Seed Oils during Storage. Plants 2023, 12, 1351. [Google Scholar] [CrossRef] [PubMed]
- Mahomoodally, M.F.; Aumeeruddy, M.Z.; Legoabe, L.J.; Montesano, D.; Zengin, G. Nigella sativa L. and its active compound thymoquinone in the clinical management of diabetes: A systematic review. Int. J. Mol. Sci 2022, 23, 12111. [Google Scholar] [CrossRef]
- Atta, M.S.; El-Far, A.H.; Farrag, F.A.; Abdel-Daim, M.M.; Al Jaouni, S.K.; Mousa, S.A. Thymoquinone attenuates cardiomyopathy in streptozotocin-treated diabetic rats. Oxidative Med. Cell. Longev. 2018, 2018, 7845681. [Google Scholar] [CrossRef]
- Aldukhayel, A. Prevalence of diabetic nephropathy among Type 2 diabetic patients in some of the Arab countries. Int. J. Health Sci. 2017, 11, 1. [Google Scholar]
- Pari, L.; Sankaranarayanan, C. Beneficial effects of thymoquinone on hepatic key enzymes in streptozotocin–nicotinamide induced diabetic rats. Life Sci. 2009, 85, 830–834. [Google Scholar] [CrossRef] [PubMed]
- Atta, M.S.; Almadaly, E.A.; El-Far, A.H.; Saleh, R.M.; Assar, D.H.; Al Jaouni, S.K.; Mousa, S.A. Thymoquinone defeats diabetes-induced testicular damage in rats targeting antioxidant, inflammatory and aromatase expression. Int. J. Mol. Sci 2017, 18, 919. [Google Scholar] [CrossRef]
- Faisal, L.M.; Abdel-Moneim, A.-M.H.; Alsharidah, A.S.; Mobark, M.A.; Abdellatif, A.A.; Saleem, I.Y.; Al Rugaie, O.; Mohany, K.M.; Alsharidah, M. Thymoquinone lowers blood glucose and reduces oxidative stress in a rat model of diabetes. Molecules 2021, 26, 2348. [Google Scholar] [CrossRef] [PubMed]
- Abdelrazek, H.; Kilany, O.E.; Muhammad, M.A.; Tag, H.M.; Abdelazim, A.M. Black seed thymoquinone improved insulin secretion, hepatic glycogen storage, and oxidative stress in streptozotocin-induced diabetic male Wistar rats. Oxidative Med. Cell. Longev. 2018, 2018, 8104165. [Google Scholar] [CrossRef] [PubMed]
- Kaatabi, H.; Bamosa, A.O.; Badar, A.; Al-Elq, A.; Abou-Hozaifa, B.; Lebda, F.; Al-Khadra, A.; Al-Almaie, S. Nigella sativa improves glycemic control and ameliorates oxidative stress in patients with type 2 diabe-tes mellitus: Placebo controlled participant blinded clinical trial. PLoS ONE 2015, 10, e0113486. [Google Scholar] [CrossRef] [PubMed]
- Maideen, N.M. Antidiabetic Activity of Nigella Sativa (Black Seeds) and Its Active Constit-uent (Thymoquinone): A Review of Human and Experimental Animal Studies. Chonnam Med. J. 2021, 57, 169–175. [Google Scholar] [CrossRef] [PubMed]
- Heshmati, J.; Namazi, N.; Memarzadeh, M.R.; Taghizadeh, M.; Kolahdooz, F. Nigella sativa oil affects glucose metabolism and lipid concentrations in patients with type 2 diabetes: A randomized, double-blind, placebo-controlled trial. Food Res. Int. 2015, 70, 87–93. [Google Scholar] [CrossRef]
- Haq, N.; Alanazi, F.K.; Alsarra, I.A.; Shakeel, F. Rapid Analysis of Glibenclamide Using an En-vironmentally Benign Stability-Indicating RP-HPLC Method. Iran. J. Pharm. Res. 2014, 13, 863–872. [Google Scholar]
- Ibrahim, M.A.; Alshora, D.H.; Alowayid, M.A.; Alanazi, N.A.; Almutari, R.A. Development and Validation of a Green UPLC Analytical Procedure for Glibenclamide Determination in Pharmaceutical Product Using Response Surface Methodology. Orient. J. Chem. 2022, 38, 865–874. [Google Scholar] [CrossRef]
- Iqbal, M.; Alam, P.; Answer, T. High performance liquid chromatographic method with fluo-rescence detection for the estimation of Thymoquinone in Nigella sativa extracts and mar-keted formulations. Open Access Sci. Rep. 2013, 2, 655–660. [Google Scholar] [CrossRef]
- Ahmad, N.; Ahmed, R.; Amir, M.; Mostafa, A. Ultra-high-performance liquid chromatography-based identification and quantification of thymoquinone in Nigella sa-tiva extract from different geographical regions. Pharmacogn. Mag. 2018, 14, S471–S480. [Google Scholar] [CrossRef]
- Ahmad, A.; Khan, R.M.A.; Alkharfy, K.M. Development and validation of RP-HPLC method for simultaneous estimation of glibenclamide and thymoquinone in rat plasma and its application to pharmacokinetics. Acta Chromatogr. 2015, 27, 435–448. [Google Scholar] [CrossRef]
- Ahmed, N.; Abo-zeid, Y.; Sakran, W. Strategies adopted to improve bioavailability of Glibenclamide: Insights on novel delivery systems. J. Adv. Pharm. Res. 2023, 7, 35–49. [Google Scholar] [CrossRef]
- Bhalani, D.V.; Nutan, B.; Kumar, A.; Singh, A.K. Bioavailability enhancement techniques for poorly aqueous soluble drugs and therapeutics. Biomedicines 2022, 10, 2055. [Google Scholar] [CrossRef] [PubMed]
- Kazi, M.; Shahba, A.A.; Alrashoud, S.; Alwadei, M.; Sherif, A.Y.; Alanazi, F.K. Bioactive self-nanoemulsifying drug delivery systems (Bio-SNEDDS) for combined oral delivery of curcumin and piperine. Molecules 2020, 25, 1703. [Google Scholar] [CrossRef] [PubMed]
- Shahba, A.A.; Sherif, A.Y.; Elzayat, E.M.; Kazi, M. Combined Ramipril and Black Seed Oil Dosage Forms Using Bioactive Self-Nanoemulsifying Drug Delivery Systems (BIO-SNEDDSs). Pharmaceuticals 2022, 15, 1120. [Google Scholar] [CrossRef]
- International Federation of Pharmaceutical Manufactures & Associations I. Validation of analytical procedures: Text and methodology, Methodology Q2 (R1). In Proceedings of the International Conference on Harmonization (ICH ’96), Geneva, Switzerland, 6 November 1996. [Google Scholar]
- Shamim, A.; Ansari, M.J.; Aodah, A.; Iqbal, M.; Aqil, M.; Mirza, M.A.; Iqbal, Z.; Ali, A. QbD-Engineered Development and Validation of a RP-HPLC Method for Simultaneous Estimation of Rutin and Ciprofloxacin HCl in Bilosoma Nanoformulation. ACS Omega 2023, 8, 21618–21627. [Google Scholar] [CrossRef] [PubMed]
- Fouad, M.M. RP-UPLC method development and validation for simultaneous estimation of vildagliptin with metformin hydrochloride and ciprofloxacin hydrochloride with dexamethasone sodium phosphate. World J. Pharm. Sci. 2015, 3, 1755–1762. [Google Scholar]
- Ibrahim, M.A.; Alhabib, N.A.; Alshora, D.; Bekhit, M.M.S.; Taha, E.; Mahdi, W.A.; Harthi, A.M. Application of Quality by Design Approach in the Optimization and Development of the UPLC Analytical Method for Determination of Fusidic Acid in Pharmaceutical Products. Separations 2023, 10, 318. [Google Scholar] [CrossRef]
- Li, J.B.; Waters Corporation; Milford, M.A. Effect of Temperature on Column Pressure, Peak Retention Time and Peak Shape. Available online: chrome-extension://efaidnbmnnnibpcajpcglclefindmkaj/https://www.waters.com/webassets/cms/library/docs/watersamd30.pdf (accessed on 22 June 2022).
- Waterlot, C.; Goulas, A. Temperature Effects on Retention and Separation of PAHs in Reversed-Phase Liquid Chromatography Using Columns Packed with Fully Porous and Core-Shell Particles. J. Chem. 2016, 2016, 7294105. [Google Scholar] [CrossRef]
- Pápai, Z.; Pap, T.L. Analysis of peak asymmetry in chromatography. J. Chromatogr. A 2002, 953, 31–38. [Google Scholar] [CrossRef]
- Gotmar, G.; Fornstedt, T.; Guiochon, G. Peak Tailing and Mass Transfer Kinetics in Linear Chromatography—Dependence on the Column Length and the Linear Velocity of the Mobile Phase. J. Chromatogr. 1999, A831, 17–35. [Google Scholar] [CrossRef]
- Spearman, L.; Smith, R.M.; Dube, S. Monitoring effective column temperature by using shape selectivity and hydrophobicity and the effects of mobile phase temperature. J. Chromatogr. A 2004, 1060, 147–151. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Hu, Y.; Carr, P.W. Fast Separations at Elevated Temperatures on Polybutadiene-Coated Zirconia Reversed-Phase Material. Anal. Chem. 1997, 69, 3884–3888. [Google Scholar] [CrossRef] [PubMed]
- Czyrski, A.; Sznura, J. The application of Box-Behnken-Design in the optimization of HPLC separation of fluoroquinolones. Sci Rep. 2019, 9, 19458. [Google Scholar] [CrossRef] [PubMed]
- ’Peak resolution’ in IUPAC Compendium of Chemical Terminology, 3rd ed. International Union of Pure and Applied Chemistry; 2006. Online version 3.0.1. 2019. Available online: https://goldbook.iupac.org/terms/view/P04465 (accessed on 22 June 2022). [CrossRef]
- resolution’ in IUPAC Compendium of Chemical Terminology, 3rd ed. International Union of Pure and Applied Chemistry; 2006. Online version 3.0.1. 2019. Available online: https://goldbook.iupac.org/terms/view/R05317 (accessed on 22 June 2022). [CrossRef]
- Maryutina, T.A.; Savonina, E.Y.; Fedotov, P.S.; Smith, R.M.; Siren, H.; Hibbert, D.B. Terminology of separation methods (IUPAC Recommendations 2017. Pure Appl. Chem. 2018, 90, 181–231. [Google Scholar] [CrossRef]
- Ettre, L.S. Nomenclature for chromatography (IUPAC Recommendations 1993. Pure Appl. Chem. 1993, 65, 819–872. [Google Scholar] [CrossRef]
- El-Bagary, R.I.; Elkady, E.F.; Tammam, M.H.; Abo-Elmaaty, A. Simultaneous determination of miconazole and hydrocortisone or mometasone using reversed phase liquid chromatography. Eur. J. Chem. 2012, 3, 421–425. [Google Scholar] [CrossRef]



| Independent Factors | Level | Dependent Factors (Response) | |||
|---|---|---|---|---|---|
| −1 | 0 | +1 | |||
| A: ACN (%) | 40 | 45 | 50 | TQ | Y1: Retention time (min) |
| Y2: Peak area (mAU/min) | |||||
| B: Temperature (°C) | 20 | 30 | 40 | Y3: Peak Asymmetry | |
| Y4: Resolution between TQ and GB peaks | |||||
| GB | Y5: Retention time (min) | ||||
| Y6: Peak area (mAU/min) | |||||
| Y7: Peak Asymmetry | |||||
| Response | RT | Peak Area | Assym. | Resolution | ||||
|---|---|---|---|---|---|---|---|---|
| TQ | ||||||||
| Source | p Values | F Value | p Values | F Value | p Values | F Value | p Values | F Value |
| A-ACN | <0.0001 | 1810.31 | 0.6974 | 0.1833 | 0.0895 | 6.14 | <0.0001 | 1417.62 |
| B-Temperature | 0.0006 | 246.45 | 0.2073 | 2.57 | 1.0000 | 0.0001 | 0.0166 | 23.64 |
| AB | 0.0131 | 28.18 | 0.4019 | 0.9485 | 0.6093 | 0.3236 | 0.0696 | 7.67 |
| A2 | 0.0127 | 28.74 | 0.3644 | 1.14 | 0.7444 | 0.1278 | 0.0105 | 33.00 |
| B2 | 0.0752 | 7.17 | 0.0569 | 9.10 | 0.8695 | 0.0320 | 0.2152 | 2.45 |
| Model p value | 0.0002 (significant) | 0.2141 (insignificant) | 0.435 (insignificant) | 0.0003 (significant) | ||||
| GB | ||||||||
| Source | p Values | F Value | p Values | F Value | p Values | F Value | p Values | F Value |
| A-ACN | <0.0001 | 875.92 | 0.9697 | 0.0017 | 0.0001 | 658.29 | <0.0001 | 1417.62 |
| B-Temperature | 0.0070 | 44.06 | 0.9596 | 0.0030 | 0.0041 | 64.29 | 0.0166 | 23.64 |
| AB | 0.0324 | 14.30 | 0.9283 | 0.0095 | 0.5594 | 0.4286 | 0.0696 | 7.67 |
| A2 | 0.0076 | 41.26 | 0.3387 | 1.29 | 0.1612 | 3.43 | 0.0105 | 33.00 |
| B2 | 0.2018 | 2.65 | 0.4166 | 0.8836 | 0.4228 | 0.8571 | 0.2152 | 2.45 |
| Model p value | 0.0006 (significant) | 0.8038 (insignificant) | 0.0009 (significant) | 0.0003 (significant) | ||||
| Run | A ACN (%) | B Temp. (°C) | TQ | GB | |||||
|---|---|---|---|---|---|---|---|---|---|
| Retention Time (min) | Peak Area (mAU/min) | Peak Asymmetry | Peaks Resolution | Retention Time (min) | Peak Area (mAU/min) | Peak Asymmetry | |||
| 1 | 40 | 30 | 2.71 ± 0.05 | 61.45 ± 4.3 | 1.08 ± 0.01 | 8.6 ± 0.035 | 5.73 ± 0.07 | 24.85 ± 5.21 | 1.03 ± 0.04 |
| 2 | 45 | 30 | 1.982 ± 0.04 | 62.01 ± 3.21 | 1.11 ± 0.07 | 4.15 ± 0.02 | 3.17 ± 0.06 | 24.81 ± 2.18 | 1.15 ± 0.03 |
| 3 | 40 | 40 | 2.44 ± 0.07 | 62.71 ± 2.81 | 1.09 ± 0.02 | 7.92 ± 0.18 | 5.01 ± 0.02 | 24.10 ± 4.84 | 1.03 ± 0.05 |
| 4 | 50 | 30 | 1.59 ± 0.06 | 63.08 ± 3.71 | 1.15 ± 0.01 | 1.9 ± 0.05 | 1.97 ± 0.03 | 23.45 ± 3.41 | 1.19 ± 0.01 |
| 5 | 50 | 40 | 1.45 ± 0.03 | 63.07 ± 2.71 | 1.14 ± 0.02 | 1.68 ± 0.06 | 1.79 ± 0.02 | 24.15 ± 2.74 | 1.2 ± 0.01 |
| 6 | 40 | 20 | 3.15 ± 0.02 | 63.21 ± 4.15 | 1.1 ± 0.01 | 9.86 ± 0.15 | 6.74 ± 0.07 | 24.08 ± 1.89 | 1.01 ± 0.01 |
| 7 | 45 | 40 | 1.84 ± 0.03 | 62.78 ± 2.87 | 1.14 ± 0.03 | 3.96 ± 0.07 | 2.83 ± 0.04 | 23.74 ± 2.71 | 1.14 ± 0.03 |
| 8 | 45 | 20 | 2.91 ± 0.08 | 63.21 ± 3.42 | 1.15 ± 0.05 | 4.84 ± 0.11 | 3.56 ± 0.09 | 23.93 ± 2.81 | 1.09 ± 0.02 |
| 9 | 50 | 20 | 1.76 ± 0.09 | 62.74 ± 4.18 | 1.15 ± 0.01 | 2.08 ± 0.21 | 2.18 ± 0.15 | 24.15 ± 1.89 | 1.16 ± 0.01 |
| Optimized Independent Parameters | Response | ||||
|---|---|---|---|---|---|
| Type | Desirability | Predicted | Observed | ||
| ACN (A): 46.86% | TQ | Y4: Retention time (min) | Minimum | 1.66 | 1.67 ± 0.004 |
| Y5: Peak area (mAU/min) | Maximum | 60.13 | 59.13 ± 0.042 | ||
| Y6: Peak Asymmetry | Minimum | 1.13 | 1.13 ± 0.01 | ||
| Y7: Peak Resolution | In range 2–4 | 2.87 | 2.92 ± 0.013 | ||
Temperature (B): 38.80 °C | GB | Y1: Retention time (min) | Minimum | 2.24 | 2.33 ± 0.008 |
| Y2: Peak area (mAU/min) | Maximum | 27.31 | 26.26 ± 0.0.064 | ||
| Y3: Peak Asymmetry | Minimum | 1.15 | 1.16 ± 0.006 | ||
| Nominal Concentration (ppm) | TQ | GB | ||
|---|---|---|---|---|
| % Recovery | % RSD | % Recovery | % RSD | |
| 0.5 | 96.054 | 0.231 | 96.523 | 1.067 |
| 1 | 95.862 | 0.241 | 98.719 | 1.872 |
| 20 | 98.981 | 0.146 | 99.767 | 0.117 |
| 50 | 99.974 | 0.042 | 99.970 | 0.196 |
| Analytes | Nominal Concentration (ppm) | Intraday (Measured Concentration; RSD %) | Inter-Day (Measured Concentration; RSD %) | ||
|---|---|---|---|---|---|
| Day-1 | Day-2 | Day-3 | |||
| TQ | 0.5 | 0.481; 0.231 | 0.481; 0.231 | 0.487; 0.672 | 0.456; 0.566 |
| 1 | 0.958; 0.241 | 0.958; 0.241 | 0.958; 0.241 | 0.941; 0.721 | |
| 20 | 19.796; 0.146 | 19.796; 0.146 | 19.882; 0.201 | 19.229; 0.763 | |
| 50 | 49.987; 0.042 | 49.987; 0.042 | 50.648; 0.153 | 50.304; 0.900 | |
| GB | 0.5 | 0.483; 1.067 | 0.483; 1.067 | 0.489; 1.468 | 0.485; 0.275 |
| 1 | 0.987; 1.872 | 0.987; 1.872 | 1.031; 0.194 | 1.016; 0.811 | |
| 20 | 19.953; 0.117 | 19.953; 0.117 | 20.060; 1.059 | 20.001; 0.426 | |
| 50 | 49.985; 0.196 | 49.985; 0.196 | 50.337; 0.178 | 51.492; 0.153 | |
| Parameters | TQ | GB | ||||
|---|---|---|---|---|---|---|
| Flow Rate (mL/min) | Peak Area | Retention Time | Peak Asymmetry | Peak Area | Retention Time | Peak Asymmetry |
| 0.28 | 0.460 | 0.057 | 0.518 | 0.695 | 0.089 | 0.998 |
| 0.3 | 0.146 | 0.23 | 0.884 | 0.117 | 0.343 | 0.517 |
| 0.32 | 0.050 | 0.283 | 1.34 | 0.163 | 0.512 | 0.491 |
| UV wavelength (nm) (TQ/GB) | ||||||
| 254/226 | 0.469 | 0.106 | 0.892 | 1.198 | 0.321 | 0.499 |
| 256/228 | 0.146 | 0.23 | 0.884 | 0.117 | 0.343 | 0.517 |
| 258/230 | 0.315 | 0.283 | 0.892 | 1.057 | 0.261 | 0.499 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshora, D.H.; Ibrahim, M.A.; Sherif, A.Y. Optimization and Validation of Sensitive UPLC-PDA Method for Simultaneous Determination of Thymoquinone and Glibenclamide in SNEDDs Formulations Using Response Surface Methodology. Separations 2023, 10, 577. https://doi.org/10.3390/separations10110577
Alshora DH, Ibrahim MA, Sherif AY. Optimization and Validation of Sensitive UPLC-PDA Method for Simultaneous Determination of Thymoquinone and Glibenclamide in SNEDDs Formulations Using Response Surface Methodology. Separations. 2023; 10(11):577. https://doi.org/10.3390/separations10110577
Chicago/Turabian StyleAlshora, Doaa Hasan, Mohamed Abbas Ibrahim, and Abdelrahman Y. Sherif. 2023. "Optimization and Validation of Sensitive UPLC-PDA Method for Simultaneous Determination of Thymoquinone and Glibenclamide in SNEDDs Formulations Using Response Surface Methodology" Separations 10, no. 11: 577. https://doi.org/10.3390/separations10110577
APA StyleAlshora, D. H., Ibrahim, M. A., & Sherif, A. Y. (2023). Optimization and Validation of Sensitive UPLC-PDA Method for Simultaneous Determination of Thymoquinone and Glibenclamide in SNEDDs Formulations Using Response Surface Methodology. Separations, 10(11), 577. https://doi.org/10.3390/separations10110577







