Elaboration of Highly Modified Stainless Steel/Lead Dioxide Anodes for Enhanced Electrochemical Degradation of Ampicillin in Water
Abstract
1. Introduction
2. Materials and Methods
2.1. Chemicals
2.2. Preparation of Modified PbO2 Electrodes
2.3. Electrode Characterizations
2.3.1. Electrode Morphology and Composition
2.3.2. Electrochemical Properties
2.4. Anodic Oxidation of Ampicillin
2.4.1. Electrolysis
2.4.2. Analytical Methods and Evaluation of Degradation Efficiency
3. Results and Discussion
3.1. Morphological and Structural Characterization of the Anodes Surface
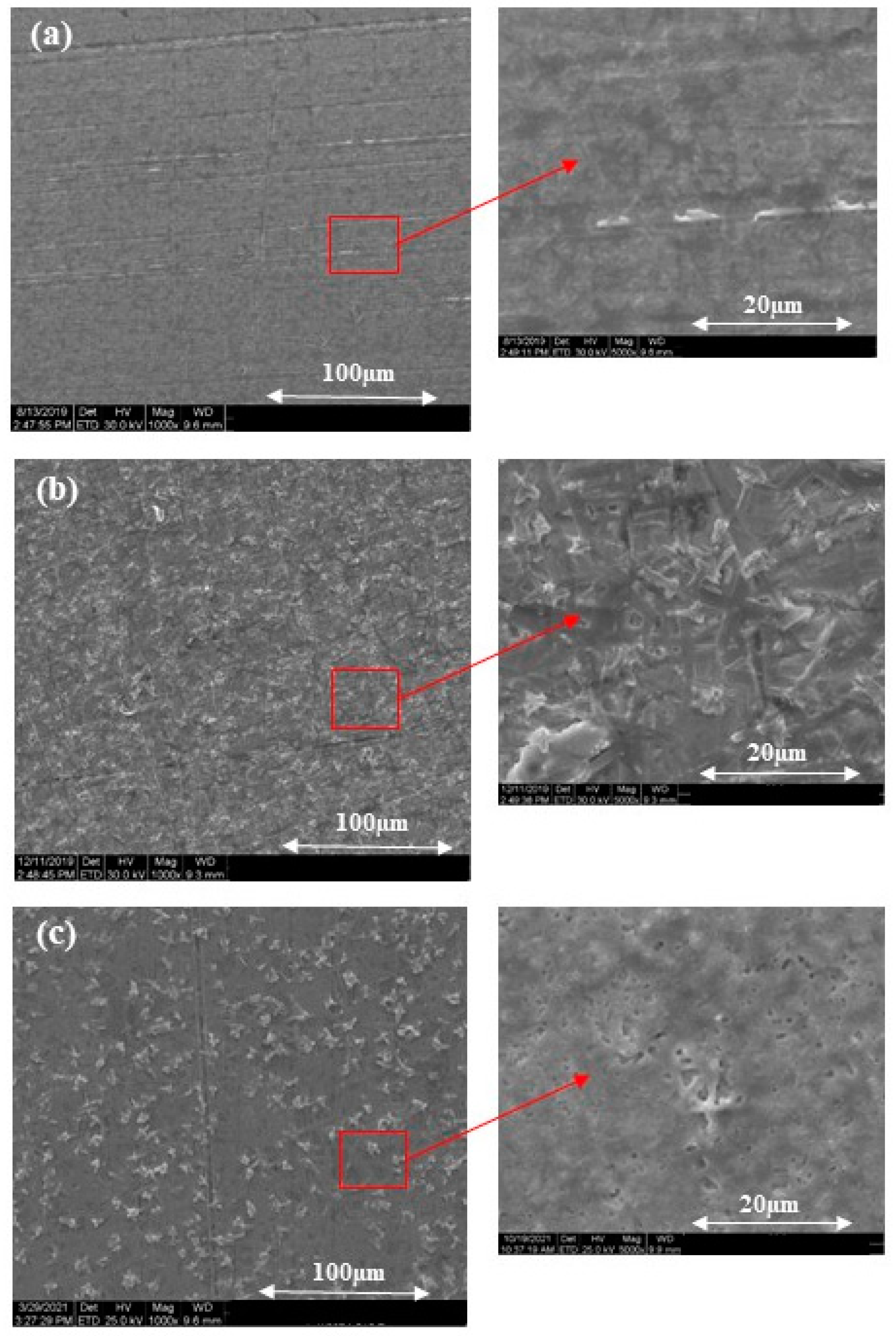
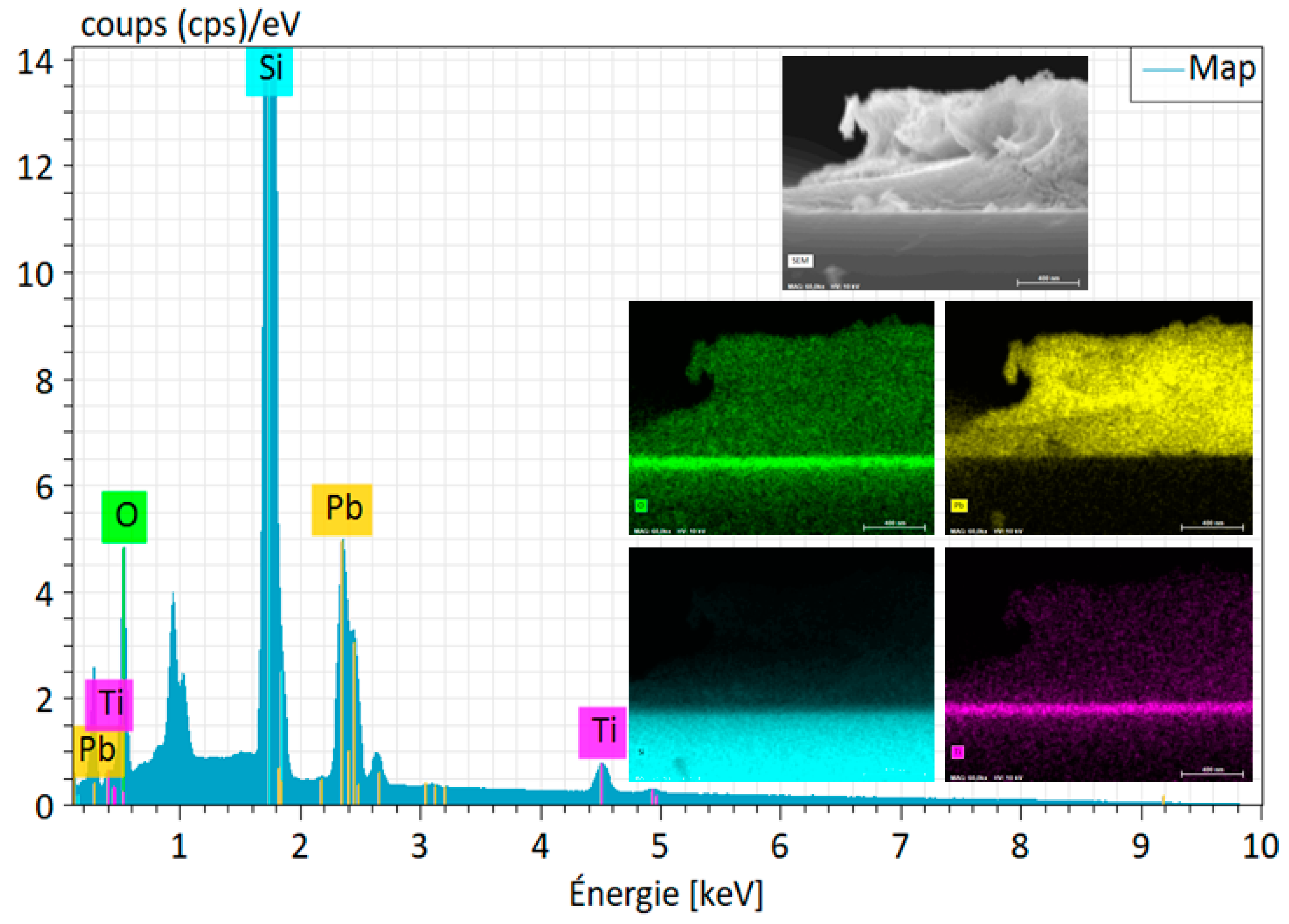
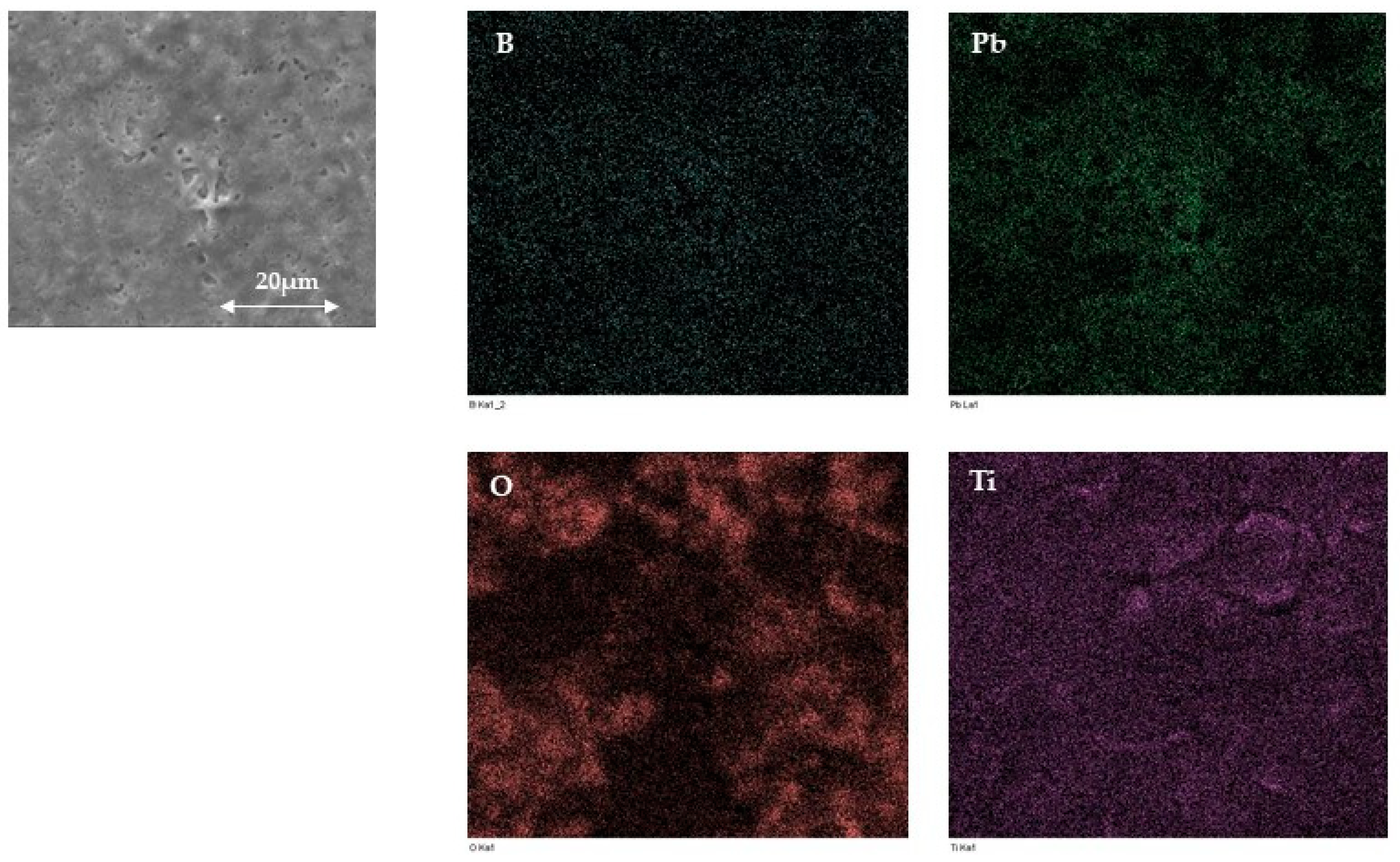
3.1.1. SEM and EDX Analysis
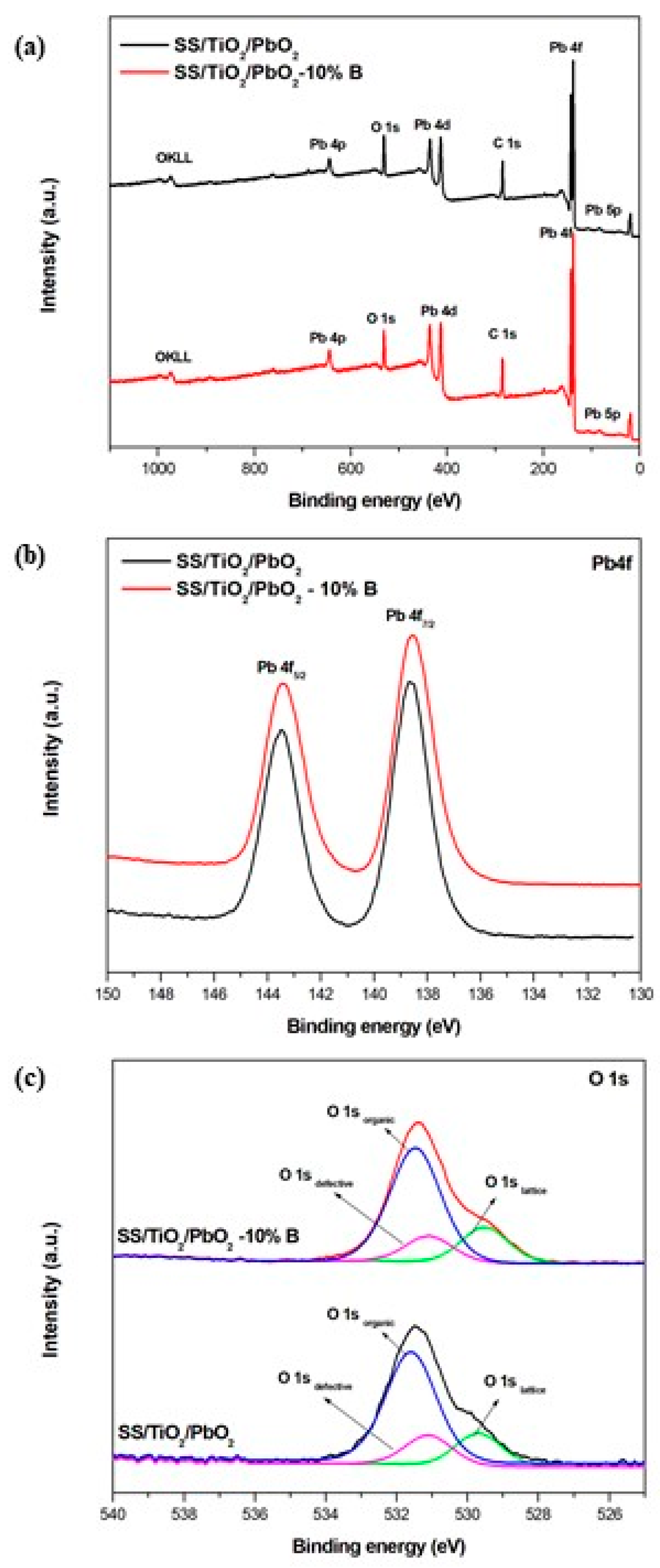
3.1.2. XPS Analysis
3.2. EIS Measurements and Fitting
3.3. Electrochemical Oxidation Performance of Electrodes
3.3.1. AMP Removal
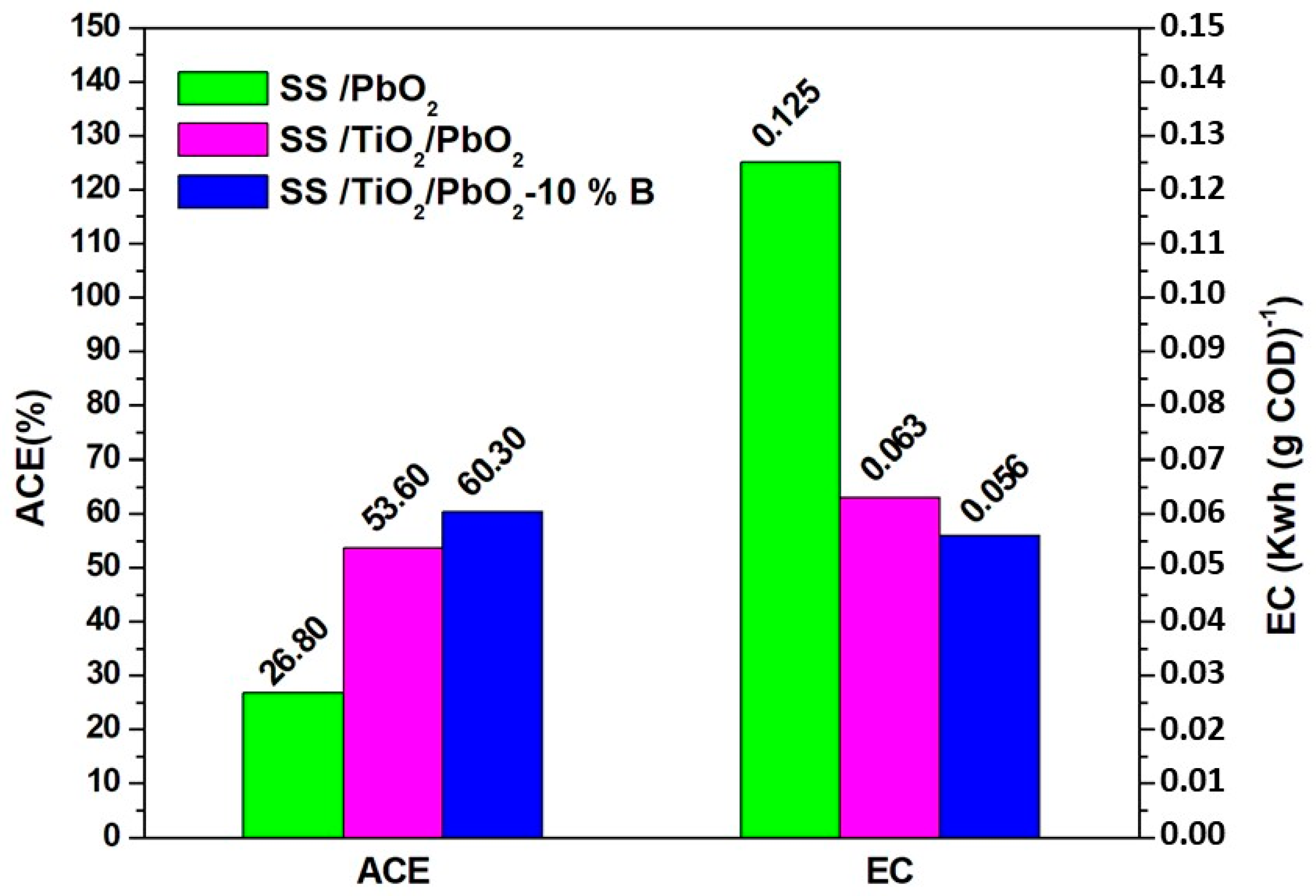
3.3.2. Average Current Efficiency and Energy Evaluation
4. Conclusions
Author Contributions
Funding
Data Availability Statement
Acknowledgments

Conflicts of Interest
References
- Majumder, A.; Gupta, B.; Gupta, A.K. Pharmaceutically Active Compounds in Aqueous Environment: A Status, Toxicity and Insights of Remediation. Environ. Res. 2019, 176, 108542. [Google Scholar] [CrossRef] [PubMed]
- Olvera-Vargas, H.; Gore-Datar, N.; Garcia-Rodriguez, O.; Mutnuri, S.; Lefebvre, O. Electro-Fenton Treatment of Real Pharmaceutical Wastewater Paired with a BDD Anode: Reaction Mechanisms and Respective Contribution of Homogenneous and Heterogenous [Rad]OH. Chem. Eng. J. 2021, 404, 126524. [Google Scholar] [CrossRef]
- Moreira, F.C.; Boaventura, R.A.R.; Brillas, E.; Vilar, V.J.P. Electrochemical Advanced Oxidation Processes: A Review on Their Application to Synthetic and Real Wastewaters. Appl. Catal. B Environ. 2017, 202, 217–261. [Google Scholar] [CrossRef]
- Titchou, F.E.; Zazou, H.; Afanga, H.; Gaayda, J.E.; Ait Akbour, R.; Nidheesh, P.V.; Hamdani, M. An Overview on the Elimination of Organic Contaminants from Aqueous Systems Using Electrochemical Advanced Oxidation Processes. J. Water Process Eng. 2021, 41, 102040. [Google Scholar] [CrossRef]
- Garcia-Segura, S.; Keller, J.; Brillas, E.; Radjenovic, J. Removal of Organic Contaminants from Secondary Effluent by Anodic Oxidation with a Boron-Doped Diamond Anode as Tertiary Treatment. J. Hazard. Mater. 2015, 283, 551–557. [Google Scholar] [CrossRef]
- Ben Osman, Y.; Le Meins, J.-M.; Bousselmi, L.; Akrout, H.; Berling, D. Effect of Electrode Shape and Deposition Technique on Electrochemical Treatment of Ampicillin in Water. Environ. Technol. Innov. 2021, 23, 101709. [Google Scholar] [CrossRef]
- Sopaj, F.; Rodrigo, M.A.; Oturan, N.; Podvorica, F.I.; Pinson, J.; Oturan, M.A. Influence of the Anode Materials on the Electrochemical Oxidation Efficiency. Application to Oxidative Degradation of the Pharmaceutical Amoxicillin. Chem. Eng. J. 2015, 262, 286–294. [Google Scholar] [CrossRef]
- Jiang, Y.; Zhao, H.; Liang, J.; Yue, L.; Li, T.; Luo, Y.; Liu, Q.; Lu, S.; Asiri, A.M.; Gong, Z.; et al. Anodic Oxidation for the Degradation of Organic Pollutants: Anode Materials, Operating Conditions and Mechanisms. A Mini Review. Electrochem. commun. 2021, 123, 106912. [Google Scholar] [CrossRef]
- Qiao, J.; Xiong, Y. Electrochemical Oxidation Technology: A Review of Its Application in High-Efficiency Treatment of Wastewater Containing Persistent Organic Pollutants. J. Water Process Eng. 2021, 44, 102308. [Google Scholar] [CrossRef]
- Nurhayati, E. A Brief Review on Electro-Generated Hydroxyl Radical for Organic Wastewater Mineralization. J. Sains Teknol. Lingkung. 2012, 4, 24–31. [Google Scholar] [CrossRef]
- Kapałka, A.; Baltruschat, H.; Comninellis, C. Electrochemical Oxidation of Organic Compounds Induced by Electro-Generated Free Hydroxyl Radicals on BDD Electrodes. In Synthetic Diamond Films: Preparation, Electrochemistry, Characterization, and Applications; John Wiley & Sons, Inc.: Hoboken, NJ, USA, 2011; pp. 237–260. [Google Scholar] [CrossRef]
- Elaissaoui, I.; Akrout, H.; Bousselmi, L. Interface Behavior of PbO2 on Pure Lead and Stainless Steel as Anode for Dye Degradation. Desalin. Water Treat. 2016, 57, 16161–16176. [Google Scholar] [CrossRef]
- Zhou, Q.; Zhou, X.; Zheng, R.; Liu, Z.; Wang, J. Application of Lead Oxide Electrodes in Wastewater Treatment: A Review. Sci. Total Environ. 2022, 806, 150088. [Google Scholar] [CrossRef]
- Fu, X.; Han, Y.; Xu, H.; Su, Z.; Liu, L. Electrochemical Study of a Novel High-Efficiency PbO2 Anode Based on a Cerium-Graphene Oxide Co-Doping Strategy: Electrodeposition Mechanism, Parameter Optimization, and Degradation Pathways. J. Hazard. Mater. 2022, 422, 126890. [Google Scholar] [CrossRef]
- Yu, N.; Wei, J.; Gu, Z.; Sun, H.; Guo, Y.; Zong, J.; Li, X.; Ni, P.; Han, E. Electrocatalysis Degradation of Coal Tar Wastewater Using a Novel Hydrophobic Benzalacetone Modified Lead Dioxide Electrode. Chemosphere 2022, 289, 133014. [Google Scholar] [CrossRef]
- Li, J.; Li, M.; Li, D.; Wen, Q.; Chen, Z. Electrochemical Pretreatment of Coal Gasification Wastewater with Bi-Doped PbO2 Electrode: Preparation of Anode, Efficiency and Mechanism. Chemosphere 2020, 248, 126021. [Google Scholar] [CrossRef] [PubMed]
- Feng, J.; Lan, H.; Tao, Q.; Chen, W.; Dai, Q. Electrochemical Oxidation of a Typical PPCP Wastewater with a Novel High-Efficiency PbO2 Anode Based on NCNSs and Ce Co-Modification: Parameter Optimization and Degradation Mechanism. J. Electroanal. Chem. 2022, 916, 116305. [Google Scholar] [CrossRef]
- Zhou, M.; Dai, Q.; Lei, L.; Ma, C.; Wang, D. Long Life Modified Lead Dioxide Anode for Organic Wastewater Treatment: Electrochemical Characteristics and Degradation Mechanism. Environ. Sci. Technol. 2005, 39, 363–370. [Google Scholar] [CrossRef]
- Zhou, Y.; Li, Z.; Hao, C.; Zhang, Y.; Chai, S.; Han, G.; Xu, H.; Lu, J.; Dang, Y.; Sun, X.; et al. Electrocatalysis Enhancement of α, β-PbO2 Nanocrystals Induced via Rare Earth Er(III) Doping Strategy: Principle, Degradation Application and Electrocatalytic Mechanism. Electrochim. Acta 2020, 333, 135535. [Google Scholar] [CrossRef]
- Fazlinezhad, S.; Jafarzadeh, K.; Shooshtari Gugtapeh, H.; Mirali, S.M. Characterization and Electrochemical Properties of Stable Ni2+ and F- Co-Doped PbO2 Coating on Titanium Substrate. J. Electroanal. Chem. 2022, 909, 116145. [Google Scholar] [CrossRef]
- Zhao, G.; Zhang, Y.; Lei, Y.; Baoying, L.V.; Gao, J.; Zhang, Y.; Li, D. Fabrication and Electrochemical Treatment Application of a Novel Lead Dioxide Anode with Superhydrophobic Surfaces, High Oxygen Evolution Potential, and Oxidation Capability. Environ. Sci. Technol. 2010, 44, 1754–1759. [Google Scholar] [CrossRef] [PubMed]
- Elaissaoui, I.; Akrout, H.; Grassini, S.; Fulginiti, D.; Bousselmi, L. Role of SiOx Interlayer in the Electrochemical Degradation of Amaranth Dye Using SS/PbO2 Anodes. Mater. Des. 2016, 110, 633–643. [Google Scholar] [CrossRef]
- Duan, X.; Li, J.; Liu, W.; Chang, L.; Yang, C. Fabrication and Characterization of a Novel PbO2 Electrode with a CNT Interlayer. RSC Adv. 2016, 6, 28927–28936. [Google Scholar] [CrossRef]
- Lin, H.; Niu, J.; Ding, S.; Zhang, L. Electrochemical Degradation of Perfluorooctanoic Acid (PFOA) by Ti/SnO2-Sb, Ti/SnO2-Sb/PbO2 and Ti/SnO2-Sb/MnO2 Anodes. Water Res. 2012, 46, 2281–2289. [Google Scholar] [CrossRef]
- Xing, X.; Ni, J.; Zhu, X.; Jiang, Y.; Xia, J. Maximization of Current Efficiency for Organic Pollutants Oxidation at BDD, Ti/SnO2-Sb/PbO2, and Ti/SnO2-Sb Anodes. Chemosphere 2018, 205, 361–368. [Google Scholar] [CrossRef]
- Tang, C.B.; Lu, Y.X.; Wang, F.; Niu, H.; Yu, L.H.; Xue, J.Q. Influence of a MnO2-WC Interlayer on the Stability and Electrocatalytic Activity of Titanium-Based PbO2 Anodes. Electrochim. Acta 2020, 331, 135381. [Google Scholar] [CrossRef]
- Boukhchina, S.; Akrout, H.; Berling, D.; Bousselmi, L. Highly Efficient Modified Lead Oxide Electrode Using a Spin Coating/Electrodeposition Mode on Titanium for Electrochemical Treatment of Pharmaceutical Pollutant. Chemosphere 2019, 221, 356–365. [Google Scholar] [CrossRef]
- Yu, L.; Xue, J.; Zhang, L.; Tang, C.; Guo, Y. Fabrication of a Stable Ti/Pb-TiOxNWs/PbO2 Anode and Its Application in Benzoquinone Degradation. Electrochim. Acta 2021, 368, 137532. [Google Scholar] [CrossRef]
- Zhu, Y.; Jin, K.; Li, H.; Qian, H.; Wang, H.; Zhao, L. A Novel Anode with Anticorrosive Coating for Efficient Degradation of Toluene. Chem. Eng. J. 2018, 334, 206–215. [Google Scholar] [CrossRef]
- Xia, Y.; Bian, X.; Xia, Y.; Zhou, W.; Wang, L.; Fan, S.; Xiong, P.; Zhan, T.; Dai, Q.; Chen, J. Effect of Indium Doping on the PbO2 Electrode for the Enhanced Electrochemical Oxidation of Aspirin: An Electrode Comparative Study. Sep. Purif. Technol. 2020, 237, 116321. [Google Scholar] [CrossRef]
- Dai, Q.; Zhou, J.; Weng, M.; Luo, X.; Feng, D.; Chen, J. Electrochemical Oxidation Metronidazole with Co Modified PbO2 Electrode: Degradation and Mechanism. Sep. Purif. Technol. 2016, 166, 109–116. [Google Scholar] [CrossRef]
- Bian, X.; Xia, Y.; Zhan, T.; Wang, L.; Zhou, W.; Dai, Q.; Chen, J. Electrochemical Removal of Amoxicillin Using a Cu Doped PbO2 Electrode: Electrode Characterization, Operational Parameters Optimization and Degradation Mechanism. Chemosphere 2019, 233, 762–770. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Xia, Y.; Dai, Q. Electrochemical Degradation of Chloramphenicol with a Novel Al Doped PbO2 Electrode: Performance, Kinetics and Degradation Mechanism. Electrochim. Acta 2015, 165, 277–287. [Google Scholar] [CrossRef]
- He, Y.; Lin, H.; Guo, Z.; Zhang, W.; Li, H.; Huang, W. Recent Developments and Advances in Boron-Doped Diamond Electrodes for Electrochemical Oxidation of Organic Pollutants. Sep. Purif. Technol. 2019, 212, 802–821. [Google Scholar] [CrossRef]
- Carolina Espinoza, L.; Candia-Onfray, C.; Vidal, J.; Salazar, R. Influence of the Chemical Nature of Boron-Doped Diamond Anodes on Wastewater Treatments. Curr. Opin. Solid State Mater. Sci. 2021, 25, 100963. [Google Scholar] [CrossRef]
- Garcia-segura, S.; Vieira, E.; Martínez-huitle, C.A. Role of Sp 3/Sp 2 Ratio on the Electrocatalytic Properties of Boron-Doped Diamond Electrodes: A Mini Review. Electrochem. Commun. 2015, 59, 52–55. [Google Scholar] [CrossRef]
- Rodriguez-Mozaz, S.; Vaz-Moreira, I.; Varela Della Giustina, S.; Llorca, M.; Barceló, D.; Schubert, S.; Berendonk, T.U.; Michael-Kordatou, I.; Fatta-Kassinos, D.; Martinez, J.L.; et al. Antibiotic Residues in Final Effluents of European Wastewater Treatment Plants and Their Impact on the Aquatic Environment. Environ. Int. 2020, 140, 105733. [Google Scholar] [CrossRef]
- Boge, E.A.; Nyongesa, P.; Okoth, P.; Sifuna, A.W. Biofilms and Antimicrobial Susceptibility Profiles of Escherichia Coli Recovered from Wastewater Treatment Plants in Kakamega Municipality, Kenya. Egypt. J. Microbiol. 2021, 56, 25–34. [Google Scholar] [CrossRef]
- Stachurová, T.; Piková, H.; Bartas, M.; Semerád, J.; Svobodová, K.; Malachová, K. Beta-Lactam Resistance Development during the Treatment Processes of Municipal Wastewater Treatment Plants. Chemosphere 2021, 280, 130749. [Google Scholar] [CrossRef]
- Zafar, R.; Bashir, S.; Nabi, D.; Arshad, M. Occurrence and Quantification of Prevalent Antibiotics in Wastewater Samples from Rawalpindi and Islamabad, Pakistan. Sci. Total Environ. 2021, 764, 142596. [Google Scholar] [CrossRef]
- Boukhchina, S. Elaboration Des Anodes Par Différentes Méthodes de Déposition et Leur Application Dans La Dépollution Des Eaux Usées Par Voie Électrochimique. Ph.D. Thesis, Université de Carthage, Carthage, Tunisie, 2021. [Google Scholar]
- Ben Osman, Y. Traitement Électrochimique Des Eaux Usées Chargées En Polluants Émergents Utilisant Des Anodes Innovantes à Coût Maîtrisé. Ph.D. Thesis, Université de Haute-Alsace, Mulhouse, France, 2022. [Google Scholar]
- AFNOR Norme Française NF T 90-101 (Février 2001): Qualité de l’eau: Détermination de La Demande Chimique En Oxygène (DCO); AFNOR: Paris, France, 2001.
- Elaissaoui, I.; Akrout, H.; Bousselmi, L. Electrochemical Degradation of Dye on Lead Dioxide Electrodeposited on Stainless Steel: Effect of Cyclic Voltammetry Parameters. Desalin. Water Treat. 2016, 57, 22120–22132. [Google Scholar] [CrossRef]
- Ghazouani, M.; Akrout, H.; Bousselmi, L. Nitrate and Carbon Matter Removals from Real Effluents Using Si/BDD Electrode. Environ. Sci. Pollut. Res. 2017, 24, 9895–9906. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.; Shang, S.; Li, X. yan Fabrication of Sulfur-Doped TiO2 Nanotube Array as a Conductive Interlayer of PbO2 Anode for Efficient Electrochemical Oxidation of Organic Pollutants. Sep. Purif. Technol. 2021, 258, 118035. [Google Scholar] [CrossRef]
- Kim, K.S.; O’Leary, T.J.; Winograd, N. X-Ray Photoelectron Spectra of Lead Oxides. Anal. Chem. 1973, 45, 2214–2218. [Google Scholar] [CrossRef]
- Velichenko, A.B.; Amadelli, R.; Zucchini, G.L.; Girenko, D.V.; Danilov, F.I. Electrosynthesis and Physicochemical Properties of Fe-Doped Lead Dioxide Electrocatalysts. Electrochim. Acta 2000, 45, 4341–4350. [Google Scholar] [CrossRef]
- Liu, Y.; Liu, H.; Ma, J.; Li, J. Investigation on Electrochemical Properties of Cerium Doped Lead Dioxide Anode and Application for Elimination of Nitrophenol. Electrochim. Acta 2011, 56, 1352–1360. [Google Scholar] [CrossRef]
- Feng, J.; Tao, Q.; Lan, H.; Xia, Y.; Dai, Q. Electrochemical Oxidation of Sulfamethoxazole by Nitrogen-Doped Carbon Nanosheets Composite PbO2 Electrode: Kinetics and Mechanism. Chemosphere 2022, 286, 131610. [Google Scholar] [CrossRef]
- Yu, S.; Hao, C.; Li, Z.; Zhang, R.; Dang, Y.; Zhu, J.J. Promoting the Electrocatalytic Performance of PbO2 Nanocrystals via Incorporation of Y2O3 Nanoparticles: Degradation Application and Electrocatalytic Mechanism. Electrochim. Acta 2021, 369, 137671. [Google Scholar] [CrossRef]
- Wei, F.; Liao, D.; Lin, Y.; Hu, C.; Ju, J.; Chen, Y.; Feng, D. Electrochemical Degradation of Reverse Osmosis Concentrate (ROC) Using the Electrodeposited Ti/TiO2-NTs/PbO2 Electrode. Sep. Purif. Technol. 2021, 258, 118056. [Google Scholar] [CrossRef]
- Bogdanowicz, R.; Fabiańska, A.; Golunski, L.; Sobaszek, M.; Gnyba, M.; Ryl, J.; Darowicki, K.; Ossowski, T.; Janssens, S.D.; Haenen, K.; et al. Influence of the Boron Doping Level on the Electrochemical Oxidation of the Azo Dyes at Si/BDD Thin Film Electrodes. Diam. Relat. Mater. 2013, 39, 82–88. [Google Scholar] [CrossRef]
- dos Santos, A.J.; Fortunato, G.V.; Kronka, M.S.; Vernasqui, L.G.; Ferreira, N.G.; Lanza, M.R.V. Electrochemical Oxidation of Ciprofloxacin in Different Aqueous Matrices Using Synthesized Boron-Doped Micro and Nano-Diamond Anodes. Environ. Res. 2022, 204, 112027. [Google Scholar] [CrossRef]
- Hu, Z.; Cai, J.; Song, G.; Tian, Y.; Zhou, M. Anodic Oxidation of Organic Pollutants: Anode Fabrication, Process Hybrid and Environmental Applications. Curr. Opin. Electrochem. 2021, 26, 100659. [Google Scholar] [CrossRef]
- Elmolla, E.S.; Chaudhuri, M. Degradation of the Antibiotics Amoxicillin, Ampicillin and Cloxacillin in Aqueous Solution by the Photo-Fenton Process. J. Hazard. Mater. 2009, 172, 1476–1481. [Google Scholar] [CrossRef]
- Jiang, Y.; Ran, J.; Mao, K.; Yang, X.; Zhong, L.; Yang, C.; Feng, X.; Zhang, H. Recent Progress in Fenton/Fenton-like Reactions for the Removal of Antibiotics in Aqueous Environments. Ecotoxicol. Environ. Saf. 2022, 236, 113464. [Google Scholar] [CrossRef]
- Wirzal, M.D.H.; Yusoff, A.R.M.; Zima, J.; Barek, J. Degradation of Ampicillin and Penicillin G Using Anodic Oxidation. Int. J. Electrochem. Sci. 2013, 8, 8978–8988. [Google Scholar]
- Frontistis, Z.; Mantzavinos, D.; Meriç, S. Degradation of Antibiotic Ampicillin on Boron-Doped Diamond Anode Using the Combined Electrochemical Oxidation—Sodium Persulfate Process. J. Environ. Manag. 2018, 223, 878–887. [Google Scholar] [CrossRef]
- Panizza, M.; Cerisola, G. Influence of Anode Material on the Electrochemical Oxidation of 2-Naphthol: Part 2. Bulk Electrolysis Experiments. Electrochim. Acta 2004, 49, 3221–3226. [Google Scholar] [CrossRef]
- Ghazouani, M.; Akrout, H.; Jellali, S.; Bousselmi, L. Comparative Study of Electrochemical Hybrid Systems for the Treatment of Real Wastewaters from Agri-Food Activities. Sci. Total Environ. 2019, 647, 1651–1664. [Google Scholar] [CrossRef]
- Guo, Z.; Zhang, Y.; Jia, H.; Guo, J.; Xia, M.; Wang, J. Electrochemical Methods for Landfill Leachate Treatment: A Review on Electrocoagulation and Electrooxidation. Sci. Total Environ. 2022, 806, 150529. [Google Scholar] [CrossRef]


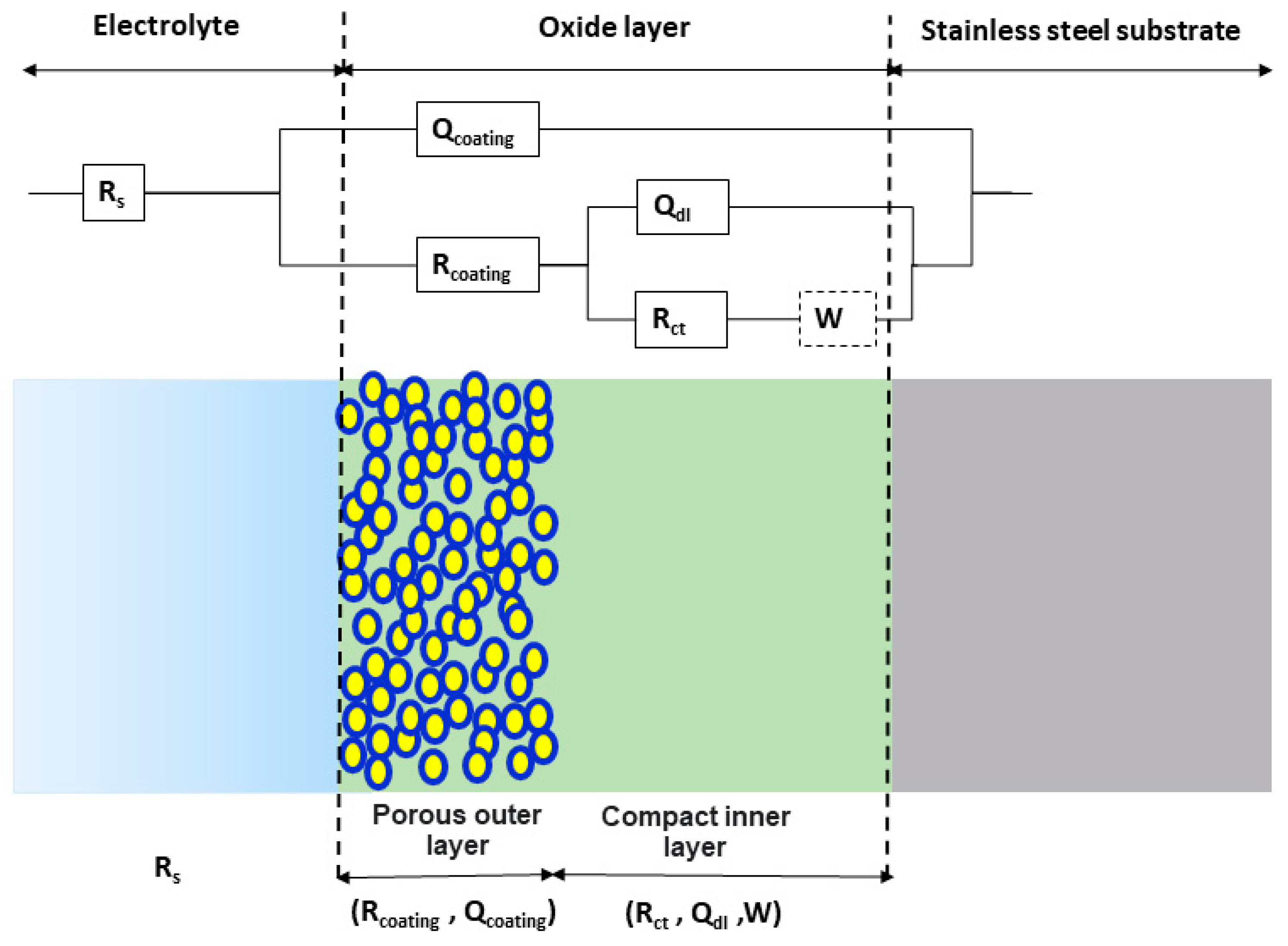
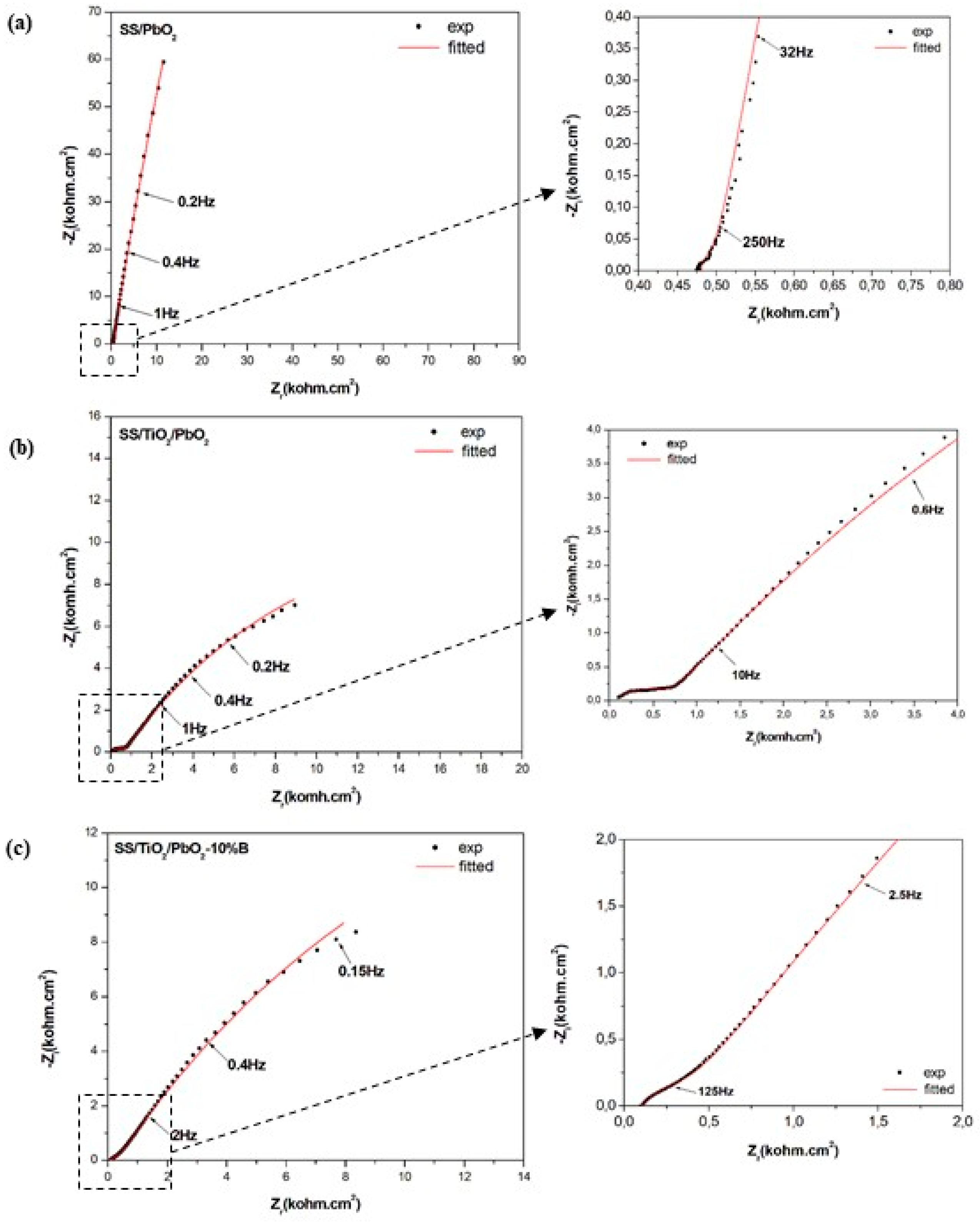

| Electrode | Binding Energy (eV) | O1s Organic (Atom%) | O1s Lattice (Atom%) | O1s Defective (Atom%) | O1s Lattice/O1s Defective (Atom%) | Pb4f7/2/O1s Defective (Atom%) | |
|---|---|---|---|---|---|---|---|
| O1s Lattice | Pb4f7/2 | ||||||
| SS/TiO2/PbO2 | 529.69 | 138.87 | 16.07 | 4.44 | 4.06 | 1.09 | 3.70 |
| SS/TiO2/PbO2-10%B | 529.52 | 138.71 | 17.37 | 4.98 | 3.63 | 1.37 | 4.32 |
| SS/PbO2 | SS/TiO2/PbO2 | SS/TiO2/PbO2-10%B | |
|---|---|---|---|
| Rs (Ω cm2) | 480.6 | 64.71 | 104.5 |
| Q coating (S sn cm−2) | 5.80 × 10−6 | 4.59 × 10−6 | 22.8 × 10−6 |
| n | 1 | 0.59 | 0.68 |
| R coating (Ω cm2) | 68.48 | 659 | 435.9 |
| Qdl (S sn cm−2) | 16.8 × 10−6 | 86.6 × 10−6 | 74.3 × 10−6 |
| n | 0.86 | 0.62 | 0.65 |
| Rct (Ω cm2) | 3.18 × 106 | 3.76 × 104 | 5.64 × 104 |
| W | 4.01 × 10−6 | 1.02 × 104 | - |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ben Osman, Y.; Hajjar-Garreau, S.; Berling, D.; Akrout, H. Elaboration of Highly Modified Stainless Steel/Lead Dioxide Anodes for Enhanced Electrochemical Degradation of Ampicillin in Water. Separations 2023, 10, 5. https://doi.org/10.3390/separations10010005
Ben Osman Y, Hajjar-Garreau S, Berling D, Akrout H. Elaboration of Highly Modified Stainless Steel/Lead Dioxide Anodes for Enhanced Electrochemical Degradation of Ampicillin in Water. Separations. 2023; 10(1):5. https://doi.org/10.3390/separations10010005
Chicago/Turabian StyleBen Osman, Yasmine, Samar Hajjar-Garreau, Dominique Berling, and Hanene Akrout. 2023. "Elaboration of Highly Modified Stainless Steel/Lead Dioxide Anodes for Enhanced Electrochemical Degradation of Ampicillin in Water" Separations 10, no. 1: 5. https://doi.org/10.3390/separations10010005
APA StyleBen Osman, Y., Hajjar-Garreau, S., Berling, D., & Akrout, H. (2023). Elaboration of Highly Modified Stainless Steel/Lead Dioxide Anodes for Enhanced Electrochemical Degradation of Ampicillin in Water. Separations, 10(1), 5. https://doi.org/10.3390/separations10010005






