Blisters and Milia around the Peritoneal Dialysis Catheter: A Case of Localized Bullous Pemphigoid
Abstract
1. Introduction
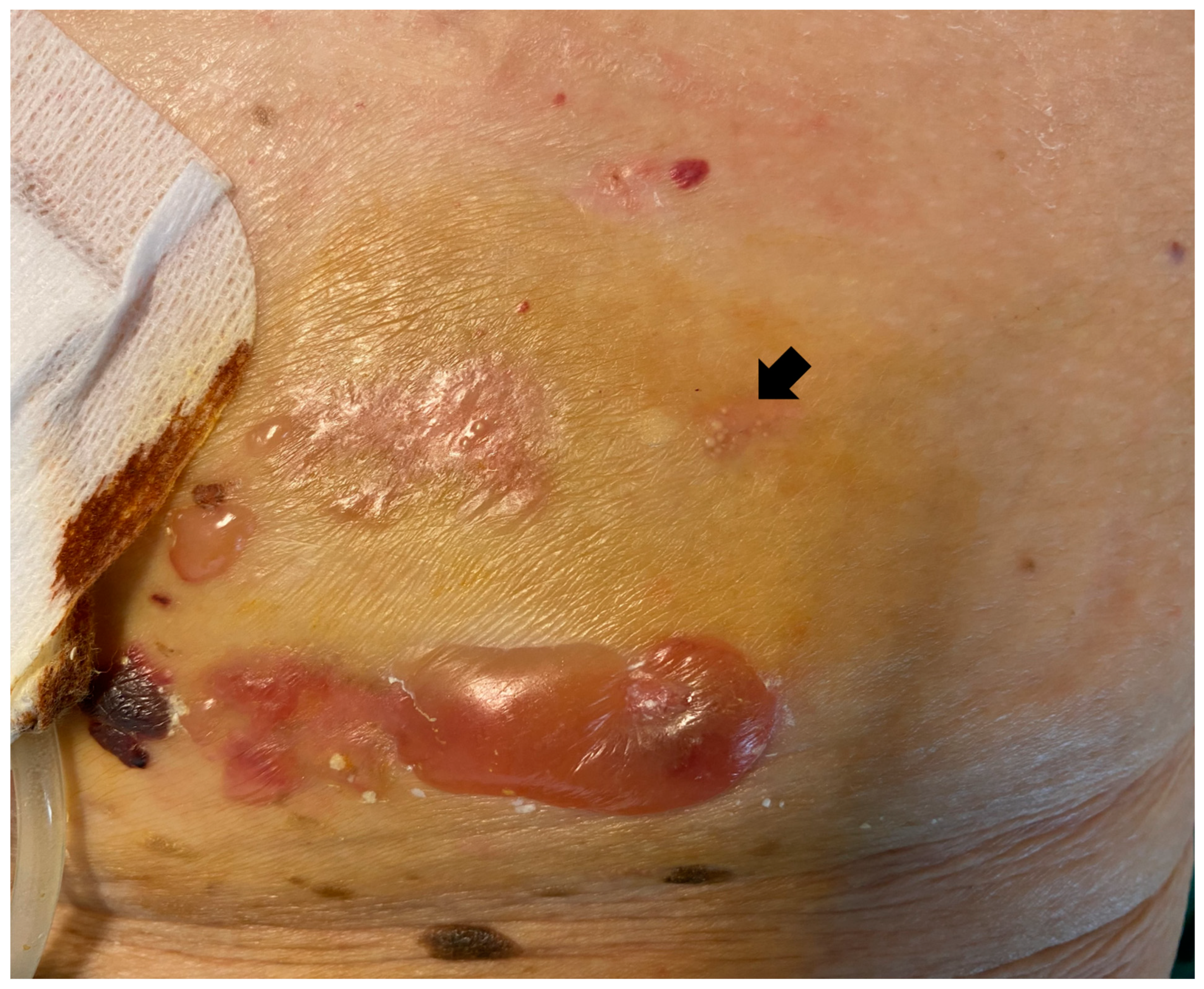
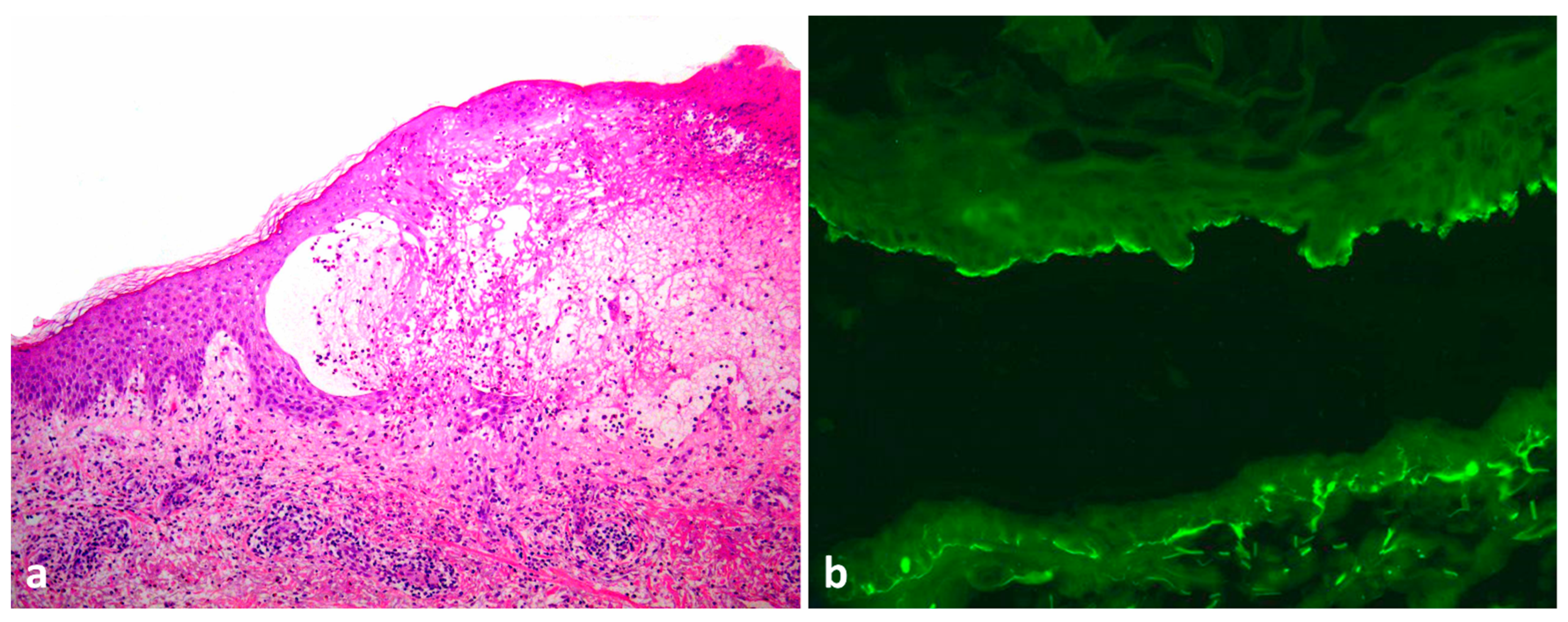
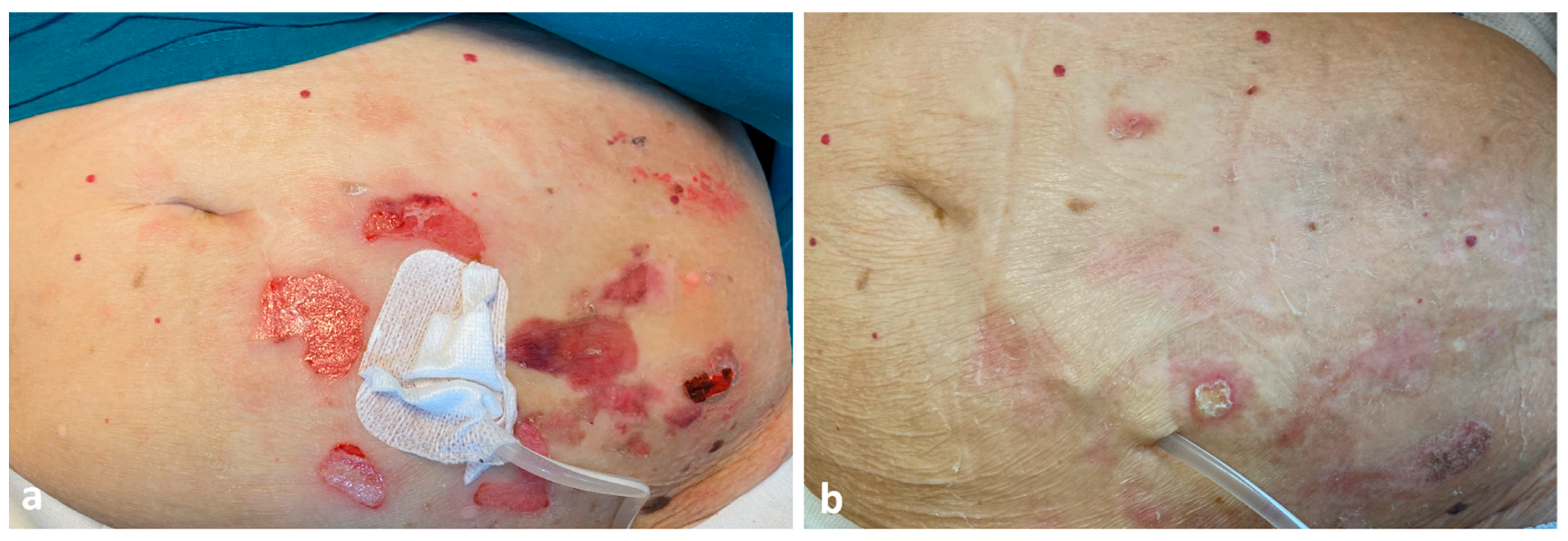
2. Case Report
3. Discussion
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Ständer, S.; Kasperkiewicz, M.; Thaçi, D.; Schmidt, E.; Zillikens, D.; Vorobyev, A.; Ludwig, R.J. Prevalence and presumptive triggers of localized bullous pemphigoid. J. Dermatol. 2021, 48, 1257–1261. [Google Scholar] [CrossRef] [PubMed]
- Korman, N. Bullous pemphigoid. J. Am. Acad. Dermatol. 1987, 16, 907–924. [Google Scholar] [CrossRef]
- Nowsheen, S.; Wieland, C.N.; Camilleri, M.J.; Gibson, L.E.; Lehman, J.S. Peristomal pemphigoid: A single-center retrospective cohort study. J. Am. Acad. Dermatol. 2022, 86, 204–207. [Google Scholar] [CrossRef]
- Michelerio, A.; Croci, G.A.; Vassallo, C.; Brazzelli, V. Hemorrhagic vesiculobullous eruption on the palms and the soles as presentation of dyshidrosiform bullous pemphigoid. JAAD Case Rep. 2017, 4, 61–63. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Freeman, B.D.; Rubin, B.G. Bullous pemphigoid after prosthetic vascular graft placement. Surgery 1998, 124, 112–113. [Google Scholar] [CrossRef]
- Kamada, N.; Ino, N.; Hatamochi, A.; Shinkai, H. A case of bullous pemphigoid in a patient on hemodialysis. J. Dermatol. 1998, 25, 246–249. [Google Scholar] [CrossRef]
- Yesudian, P.D.; Dobson, C.M.; Ahmad, R.; Azurdia, R.M. Trauma-induced bullous pemphigoid around venous access site in a haemodialysis patient. Clin. Exp. Dermatol. 2002, 27, 70–72. [Google Scholar] [CrossRef]
- Pardo, J.; Rodríguez-Serna, M.; Mercader, P.; Fortea, J.M. Localized bullous pemphigoid overlying a fistula for hemodialysis. J. Am. Acad. Dermatol. 2004, 51, 131–132. [Google Scholar] [CrossRef] [PubMed]
- Peruzzo, J.; Dantas, L.D.P.; Zampese, M. Bullous pemphigoid associated with chronic renal allograft rejection. J. Am. Acad. Dermatol. 2013, 68, e192–e193. [Google Scholar] [CrossRef]
- Osipowicz, K.; Kalinska-Bienias, A.; Kowalewski, C.; Wozniak, K. Development of bullous pemphigoid during the haemodialysis of a young man: Case report and literature survey. Int. Wound J. 2017, 14, 288–292. [Google Scholar] [CrossRef]
- Giunzioni, D. Development of Bullous Pemphigoid after Tenckhoff Catheter Placement in a Peritoneal Dialysis Patient. Case Rep. Dermatol. 2020, 12, 42–46. [Google Scholar] [CrossRef]
- Anderson, C.; Mowad, C.; Goff, M.; Pelle, M. Bullous pemphigoid arising in surgical wounds. Br. J. Dermatol. 2001, 145, 670–672. [Google Scholar] [CrossRef]
- Biondo, M.; Fiorentino, C.; Persechino, S.; Tammaro, A.; Koverech, A.; Bartolazzi, A.; Raffa, S.; Canzoni, M.; Picchianti-Diamanti, A.; Di Rosa, R.; et al. May Bacterial Infections Trigger Bullous Pemphigoid? Case Report and Review of Literature. Microorganisms 2021, 9, 1235. [Google Scholar] [CrossRef] [PubMed]
- Bastuji-Garin, S.; Joly, P.; Picard-Dahan, C.; Bernard, P.; Vaillant, L.; Pauwels, C.; Salagnac, V.; Lok, C.; Roujeau, J.C. Drugs associated with bullous pemphigoid. A case-control study. Arch. Dermatol. 1996, 132, 272–276. [Google Scholar] [CrossRef] [PubMed]
- Lee, S.G.; Lee, H.J.; Yoon, M.S.; Kim, D.H. Association of Dipeptidyl Peptidase 4 Inhibitor Use With Risk of Bullous Pemphigoid in Patients With Diabetes. JAMA Dermatol. 2019, 155, 172–177. [Google Scholar] [CrossRef]
- Liu, S.-D.; Chen, W.-T.; Chi, C.-C. Association Between Medication Use and Bullous Pemphigoid: A Systematic Review and Meta-analysis. JAMA Dermatol. 2020, 156, 891–900. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, R.; Haller, H.; Hiss, M. Quiz page september 2012: Erythematous rash around peritoneal dialysis catheter exit site. Am. J. Kidney Dis. 2012, 60, A29–A31. [Google Scholar] [CrossRef]
- McQuillan, R.F.; Chiu, E.; Nessim, S.; Lok, C.E.; Roscoe, J.M.; Tam, P.; Jassal, S.V. A randomized controlled trial comparing mupirocin and polysporin triple ointments in peritoneal dialysis patients: The MP3 Study. Clin. J. Am. Soc. Nephrol. 2012, 7, 297–303. [Google Scholar] [CrossRef]
- Yavascan, O.; Kara, O.D.; Sozen, G.; Aksu, N. Allergic dermatitis caused by povidone iodine: An uncommon complication of chronic peritoneal dialysis treatment. Adv. Perit. Dial. 2005, 21, 131–133. [Google Scholar]
- Piraino, B.; Bernardini, J.; Brown, E.; Figueiredo, A.E.; Johnson, D.; Lye, W.-C.; Price, V.; Ramalakshmi, S.; Szeto, C.C. ISPD position statement on reducing the risks of peritoneal dialysis–related infections. Perit. Dial. Int. 2011, 31, 614–630. [Google Scholar] [CrossRef]
- Patsatsi, A.; Uy, C.D.C.; Murrell, D.F. Multiple milia formation in blistering diseases. Int. J. Womens Dermatol. 2020, 6, 199–202. [Google Scholar] [CrossRef] [PubMed]
- Hisa, T.; Goto, Y.; Taniguchi, S.; Nakanishi, T.; Kakudo, K.; Takigawa, M. Post-bullous milia. Australas. J. Dermatol. 1996, 37, 153–154. [Google Scholar] [CrossRef] [PubMed]
- Uchida, S.; Oiso, N.; Koga, H.; Ishii, N.; Okahashi, K.; Matsuda, H.; Hashimoto, T.; Kawada, A. Refractory bullous pemphigoid leaving numerous milia during recovery. J. Dermatol. 2014, 41, 1003–1005. [Google Scholar] [CrossRef] [PubMed]
- Li, P.K.-T.; Chow, K.M.; Van de Luijtgaarden, M.W.; Johnson, D.W.; Jager, K.J.; Mehrotra, R.; Naicker, S.; Pecoits-Filho, R.; Yu, X.Q.; Lameire, N. Changes in the worldwide epidemiology of peritoneal dialysis. Nat. Rev. Nephrol. 2017, 13, 90–103. [Google Scholar] [CrossRef]
- Algoet, C.; Mostinckx, S.; Theate, I.; Vanhooteghem, O. Localized bullous pemphigoid: Four clinical cases and a literature review. Clin. Case Rep. 2020, 8, 516–519. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Michelerio, A.; Tomasini, C. Blisters and Milia around the Peritoneal Dialysis Catheter: A Case of Localized Bullous Pemphigoid. Dermatopathology 2022, 9, 282-286. https://doi.org/10.3390/dermatopathology9030033
Michelerio A, Tomasini C. Blisters and Milia around the Peritoneal Dialysis Catheter: A Case of Localized Bullous Pemphigoid. Dermatopathology. 2022; 9(3):282-286. https://doi.org/10.3390/dermatopathology9030033
Chicago/Turabian StyleMichelerio, Andrea, and Carlo Tomasini. 2022. "Blisters and Milia around the Peritoneal Dialysis Catheter: A Case of Localized Bullous Pemphigoid" Dermatopathology 9, no. 3: 282-286. https://doi.org/10.3390/dermatopathology9030033
APA StyleMichelerio, A., & Tomasini, C. (2022). Blisters and Milia around the Peritoneal Dialysis Catheter: A Case of Localized Bullous Pemphigoid. Dermatopathology, 9(3), 282-286. https://doi.org/10.3390/dermatopathology9030033






