Morphea Profunda with Tertiary Lymphoid Follicles: Description of Two Cases and Review of the Literature
Abstract
:1. Introduction
2. Case Report
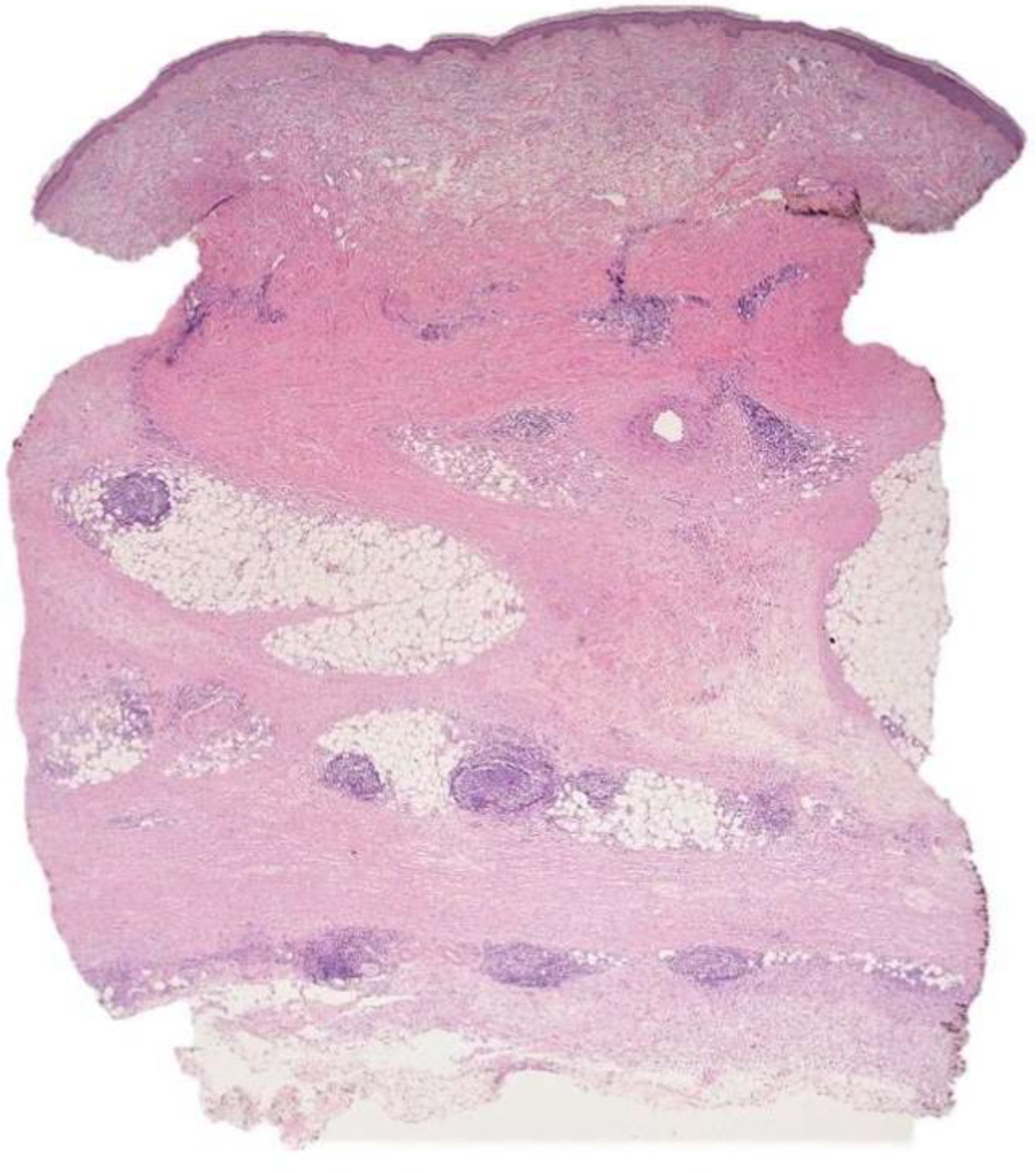
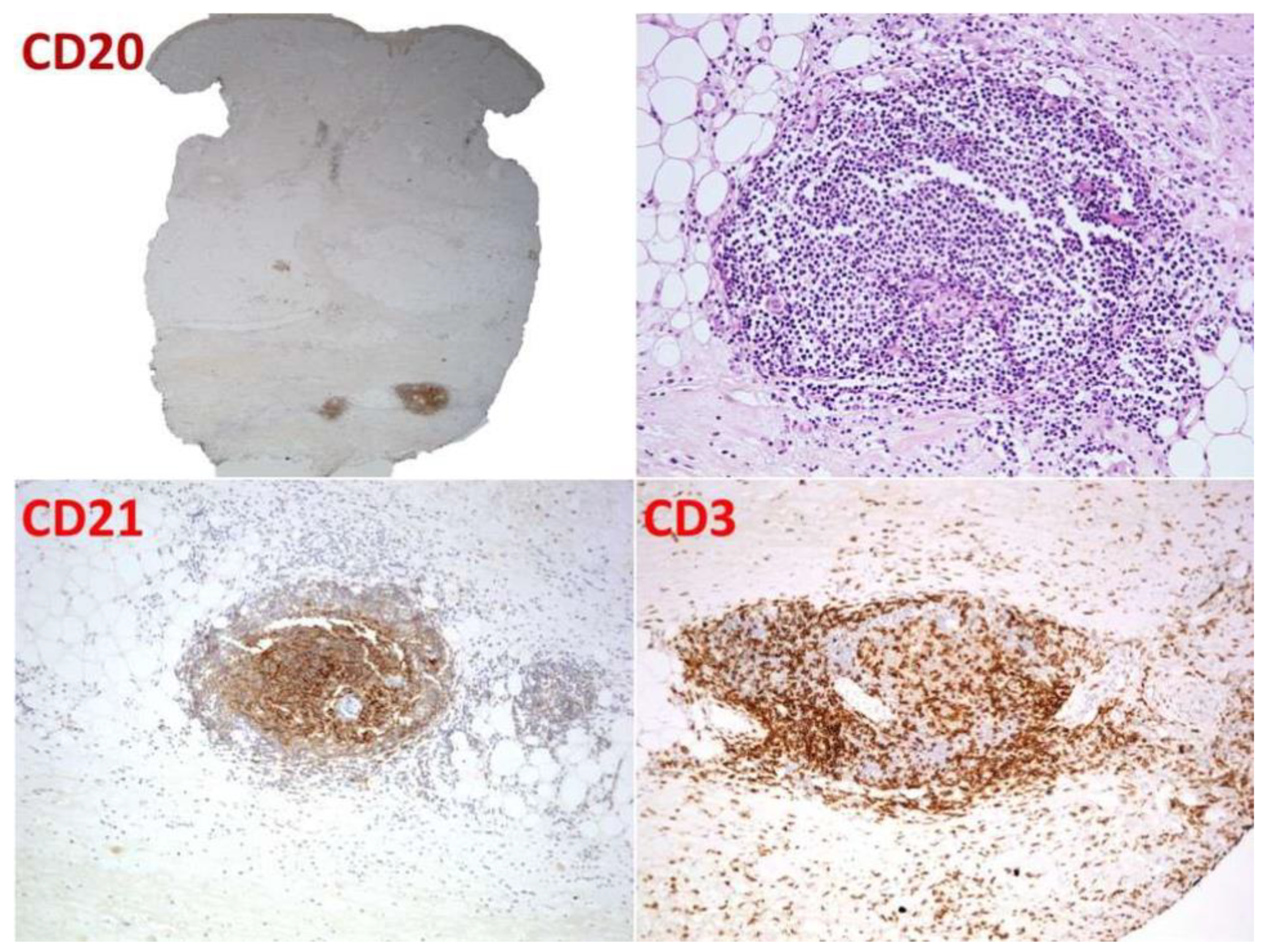
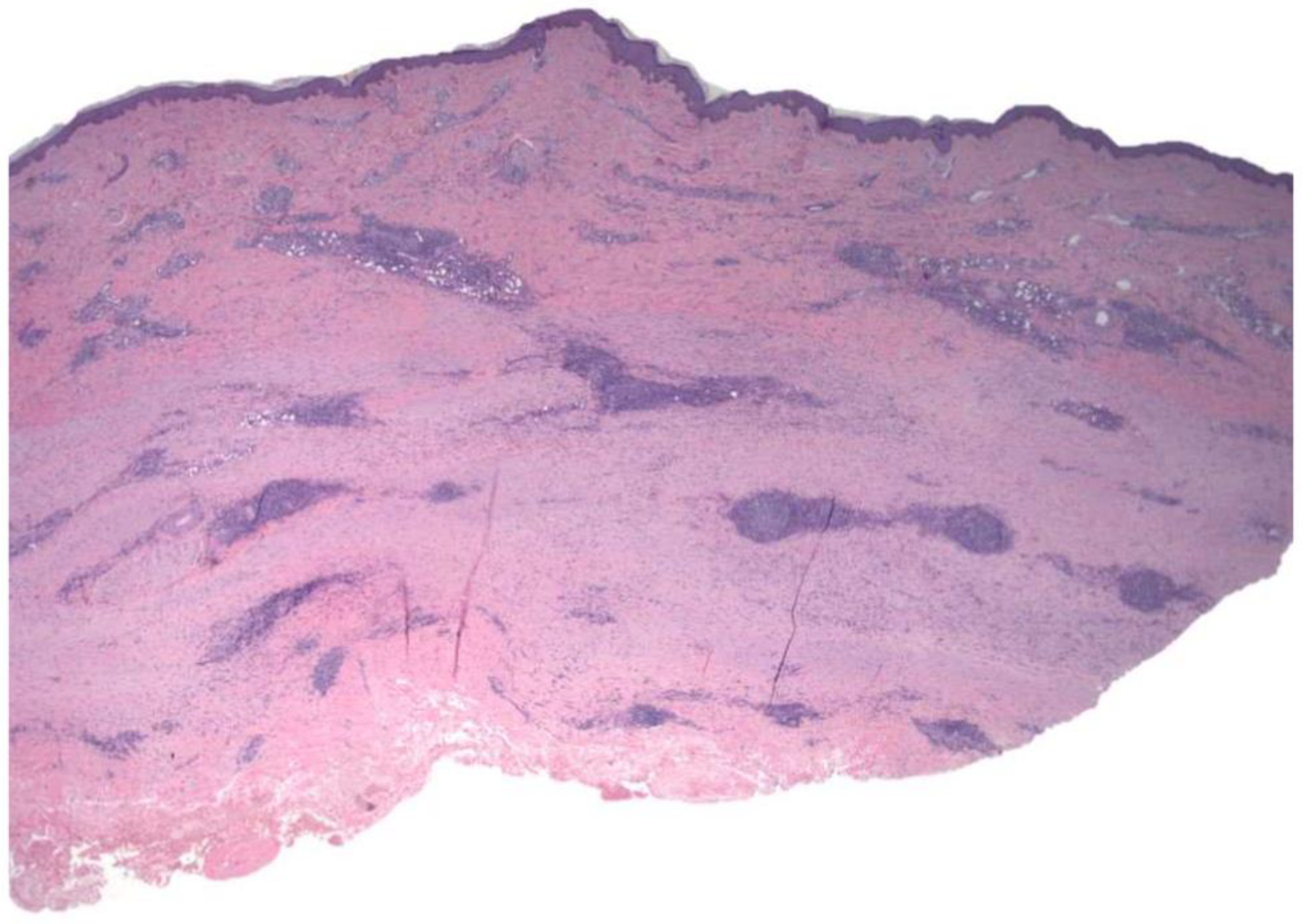
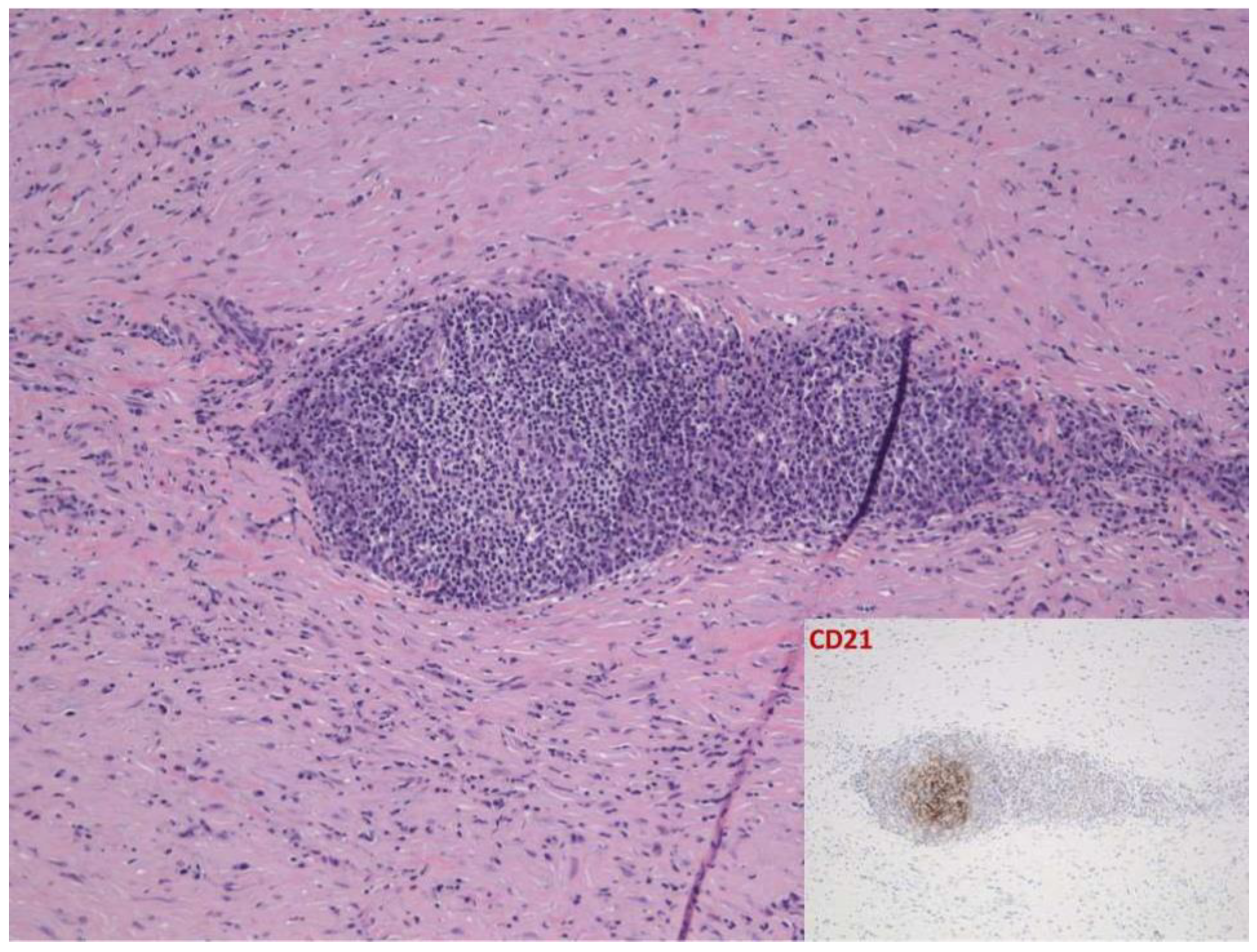
2.1. Case 1
2.2. Case 2
3. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Bielsa, I.; Ariza, A. Deep Morphea. Semin. Cutan. Med. Surg. 2007, 26, 90–95. [Google Scholar] [CrossRef] [PubMed]
- Knobler, R.; Moinzadeh, P.; Hunzelmann, N.; Kreuter, A.; Cozzio, A.; Mouthon, L.; Cutolo, M.; Rongioletti, F.; Denton, C.; Rudnicka, L.; et al. European Dermatology Forum S1-guideline on the diagnosis and treatment of sclerosing diseases of the skin, Part 1: Localized scleroderma, systemic sclerosis and overlap syndromes. J. Eur. Acad. Dermatol. Venereol. 2017, 31, 1401–1424. [Google Scholar] [CrossRef]
- Lodyga, M.; Hinz, B. TGF-β1—A truly transforming growth factor in fibrosis and immunity. Semin. Cell Dev. Biol. 2020, 101, 123–139. [Google Scholar] [CrossRef] [PubMed]
- Lu, J.; Liu, Q.; Wang, L.; Tu, W.; Chu, H.; Ding, W.; Jiang, S.; Ma, Y.; Shi, X.; Pu, W.; et al. Increased expression of latent TGF-β-binding protein 4 affects the fibrotic process in scleroderma by TGF-β/SMAD signaling. Lab. Investig. 2017, 97, 591–601. [Google Scholar] [CrossRef] [PubMed]
- Grabell, D.; Hsieh, C.; Andrew, R.; Martires, K.; Kim, A.; Vasquez, R.; Jacobe, H. The role of skin trauma in the distribution of morphea lesions: A cross-sectional survey of the Morphea in Adults and Children cohort IV. J. Am. Acad. Dermatol. 2014, 71, 493–498. [Google Scholar] [CrossRef] [Green Version]
- Wohlfahrt, T.; Usherenko, S.; Englbrecht, M.; Dees, C.; Weber, S.; Beyer, C.; Gelse, K.; Distler, O.; Schett, G.; Distler, J.H.W.; et al. Type 2 innate lymphoid cell counts are increased in patients with systemic sclerosis and correlate with the extent of fibrosis. Ann. Rheum. Dis. 2016, 75, 623–626. [Google Scholar] [CrossRef] [Green Version]
- Horsburgh, S.; Todryk, S.; Ramming, A.; Distler, J.H.; O’Reilly, S. Innate lymphoid cells and fibrotic regulation. Immunol. Lett. 2018, 195, 38–44. [Google Scholar] [CrossRef] [PubMed]
- Harris, R.B.; Duncan, S.C.; Ecker, R.I.; Winkelmann, R.K. Lymphoid follicles in subcutaneous inflammatory disease. Arch. Dermatol. 1979, 115, 442–443. [Google Scholar] [CrossRef] [PubMed]
- Wong, B.; Piliouras, P.; Mortimore, R.; Zonta, M.; Tucker, S. Lower limb linear morphoea in a pregnant woman with known Graves’ disease and cytomegalovirus immunoglobulin M positivity. Australas. J. Dermatol. 2014, 56, e96–e98. [Google Scholar] [CrossRef]
- Whittaker, S.J.; Smith, N.P.; Jones, R.R. Solitary morphoea profunda. Br. J. Dermatol. 1989, 120, 431–440. [Google Scholar] [CrossRef]
- Dairi, M.; Arnault, J.-P.; Dadban, A.; Lombart, F.; Attencourt, C.; Ortonne, N.; Lok, C.; Chaby, G. Cutaneous lymphoid hyperplasia arising in pre-existing morphea plaques treated with methotrexate. JAAD Case Rep. 2019, 5, 127–130. [Google Scholar] [CrossRef] [PubMed]
- Magro, C.M.; Crowson, A.N.; Harrist, T.J. Atypical Lymphoid Infiltrates Arising in Cutaneous Lesions of Connective Tissue Disease. Am. J. Dermatopathol. 1997, 19, 446–455. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.; Tanzi, C.; Caputo, R.; Alessi, E. Sclerodermic Linear Lupus Panniculitis: Report of Two Cases. Dermatology 2005, 210, 329–332. [Google Scholar] [CrossRef] [PubMed]
- Bernárdez, C.; Prieto-Torres, L.; Macías, E.; Ramírez-Bellver, J.L.; Haro-Ramos, R.; Diaz-Recuero, J.L.; Requena, L. Concurrent presentation of cutaneous lesions of deep linear morphoea and discoid lupus erythematosus. Lupus 2015, 25, 204–208. [Google Scholar] [CrossRef]
- Umbert, P.; Winkelmann, R.K. Concurrent localized scleroderma and discoid lupus erythematosus. Cutaneous ’mixed’ or ’overlap’ syndrome. Arch. Dermatol. 1978, 114, 1473–1478. [Google Scholar] [CrossRef] [PubMed]
- Štork, J.; Vosmík, F. Lupus erythematosus panniculitis with morphea-like lesions. Clin. Exp. Dermatol. 1994, 19, 79–82. [Google Scholar] [CrossRef]
- Corsiero, E.; Nerviani, A.; Bombardieri, M.; Pitzalis, C. Ectopic Lymphoid Structures: Powerhouse of Autoimmunity. Front. Immunol. 2016, 7, 430. [Google Scholar] [CrossRef] [Green Version]
- Khatri, S.; Torok, K.S.; Mirizio, E.; Liu, C.; Astakhova, K. Autoantibodies in Morphea: An Update. Front. Immunol. 2019, 10, 1487. [Google Scholar] [CrossRef] [PubMed]
- Arisi, M.; Cavazzana, I.; Cerutti, M.E.; Ferrari, F.; Carabellese, N.; Rossi, M.T.; Tincani, A.; Calzavara-Pinton, P.G.; Franceschini, F. Antibodies against antigens related to Scleroderma in a cohort of patients with Morphea. G. Ital. Dermatol. Venereol. 2016, 153, 451–458. [Google Scholar] [CrossRef]
- Abbas, L.; Joseph, A.; Kunzler, E.; Jacobe, H.T. Morphea: Progress to date and the road ahead. Ann. Transl. Med. 2021, 9, 437. [Google Scholar] [CrossRef]
- Kobayashi, S.; Watanabe, T.; Suzuki, R.; Furu, M.; Ito, H.; Ito, J.; Matsuda, S.; Yoshitomi, H. TGF-β induces the differentiation of human CXCL13-producing CD4+ T cells. Eur. J. Immunol. 2016, 46, 360–371. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, S.; Murata, K.; Shibuya, H.; Morita, M.; Ishikawa, M.; Furu, M.; Ito, H.; Ito, J.; Matsuda, S.; Watanabe, T.; et al. A Distinct Human CD4+ T Cell Subset That Secretes CXCL13 in Rheumatoid Synovium. Arthritis Rheum. 2013, 65, 3063–3072. [Google Scholar] [CrossRef]
- Rao, D.A. T Cells That Help B Cells in Chronically Inflamed Tissues. Front. Immunol. 2018, 9, 1924. [Google Scholar] [CrossRef] [PubMed]
- Albright, A.R.; Kabat, J.; Li, M.; Raso, F.; Reboldi, A.; Muppidi, J.R. TGFβ signaling in germinal center B cells promotes the transition from light zone to dark zone. J. Exp. Med. 2019, 216, 2531–2545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Cassisa, A.; Vannucchi, M. Morphea Profunda with Tertiary Lymphoid Follicles: Description of Two Cases and Review of the Literature. Dermatopathology 2022, 9, 17-22. https://doi.org/10.3390/dermatopathology9010003
Cassisa A, Vannucchi M. Morphea Profunda with Tertiary Lymphoid Follicles: Description of Two Cases and Review of the Literature. Dermatopathology. 2022; 9(1):17-22. https://doi.org/10.3390/dermatopathology9010003
Chicago/Turabian StyleCassisa, Angelo, and Margherita Vannucchi. 2022. "Morphea Profunda with Tertiary Lymphoid Follicles: Description of Two Cases and Review of the Literature" Dermatopathology 9, no. 1: 17-22. https://doi.org/10.3390/dermatopathology9010003
APA StyleCassisa, A., & Vannucchi, M. (2022). Morphea Profunda with Tertiary Lymphoid Follicles: Description of Two Cases and Review of the Literature. Dermatopathology, 9(1), 17-22. https://doi.org/10.3390/dermatopathology9010003





