Annular Lichenoid Dermatitis (of Youth)
Abstract
:1. Introduction
2. Epidemiology
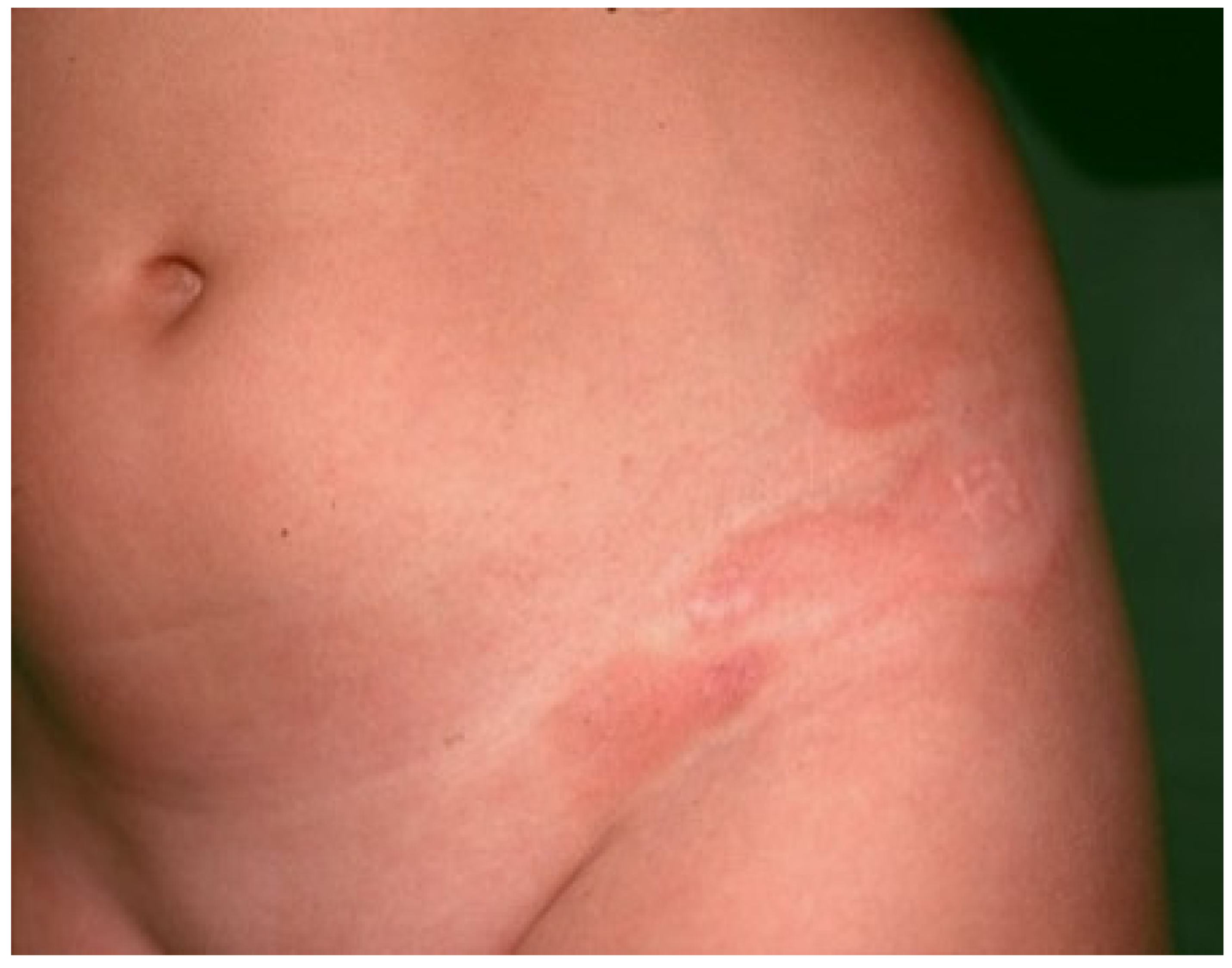
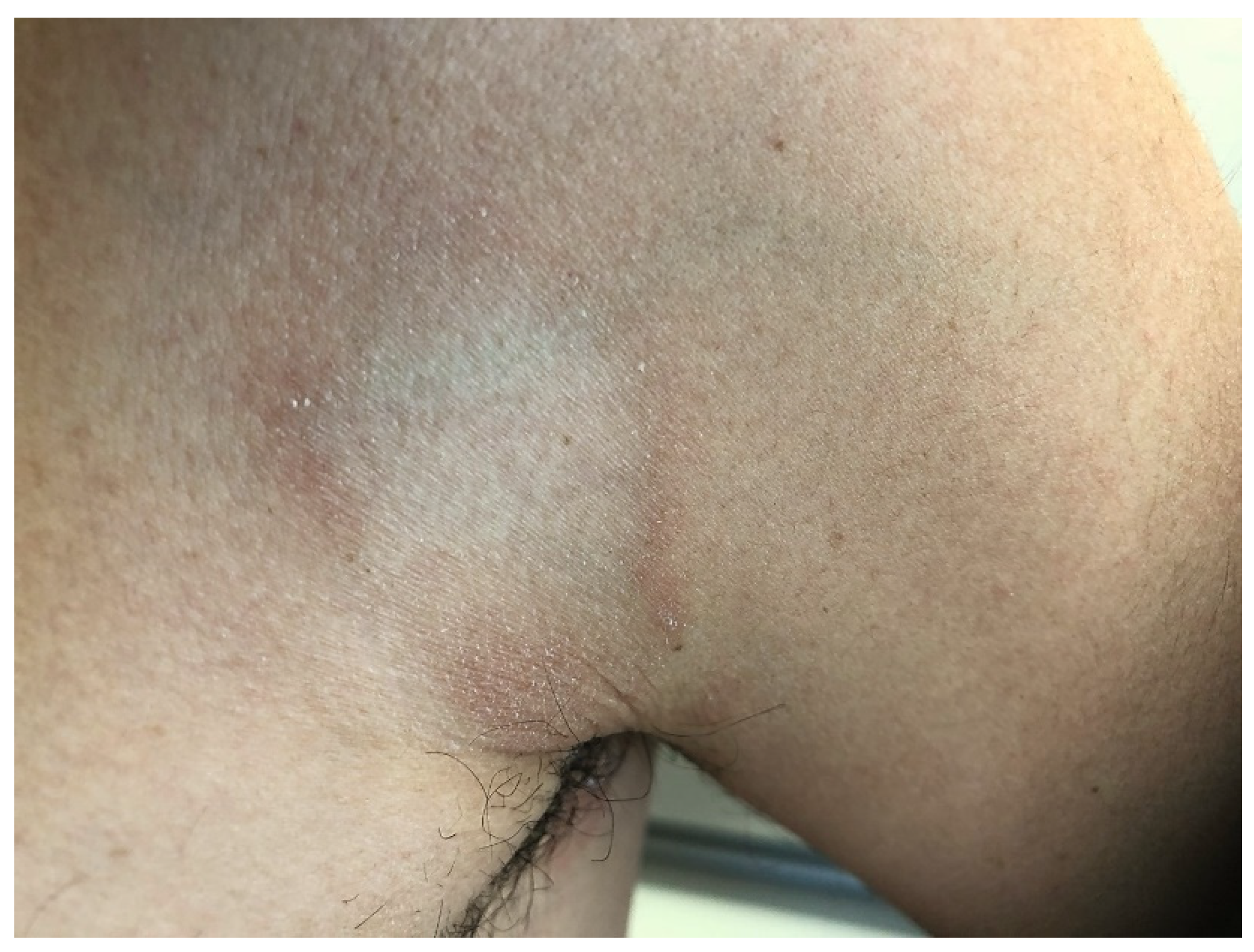
3. Clinical Features
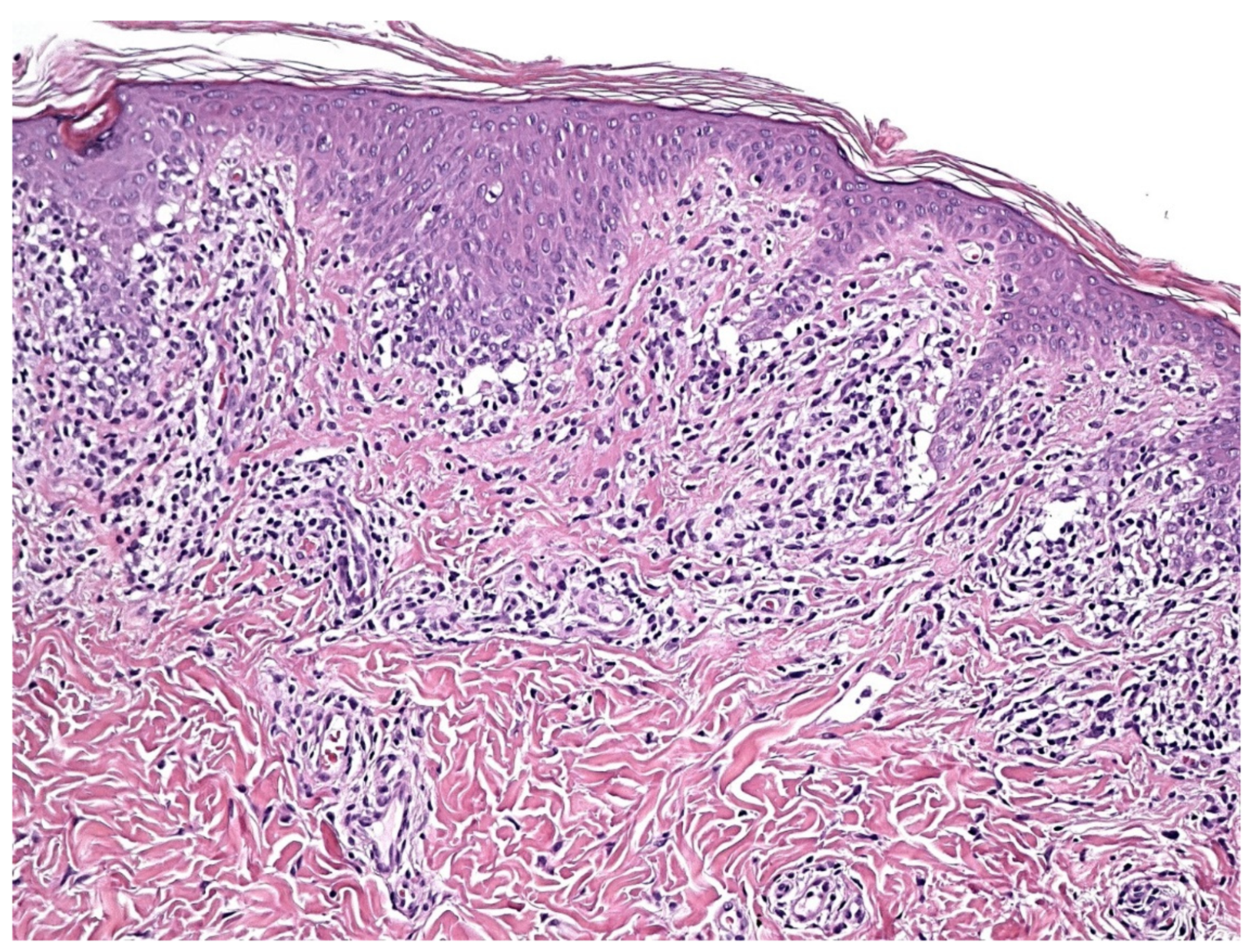
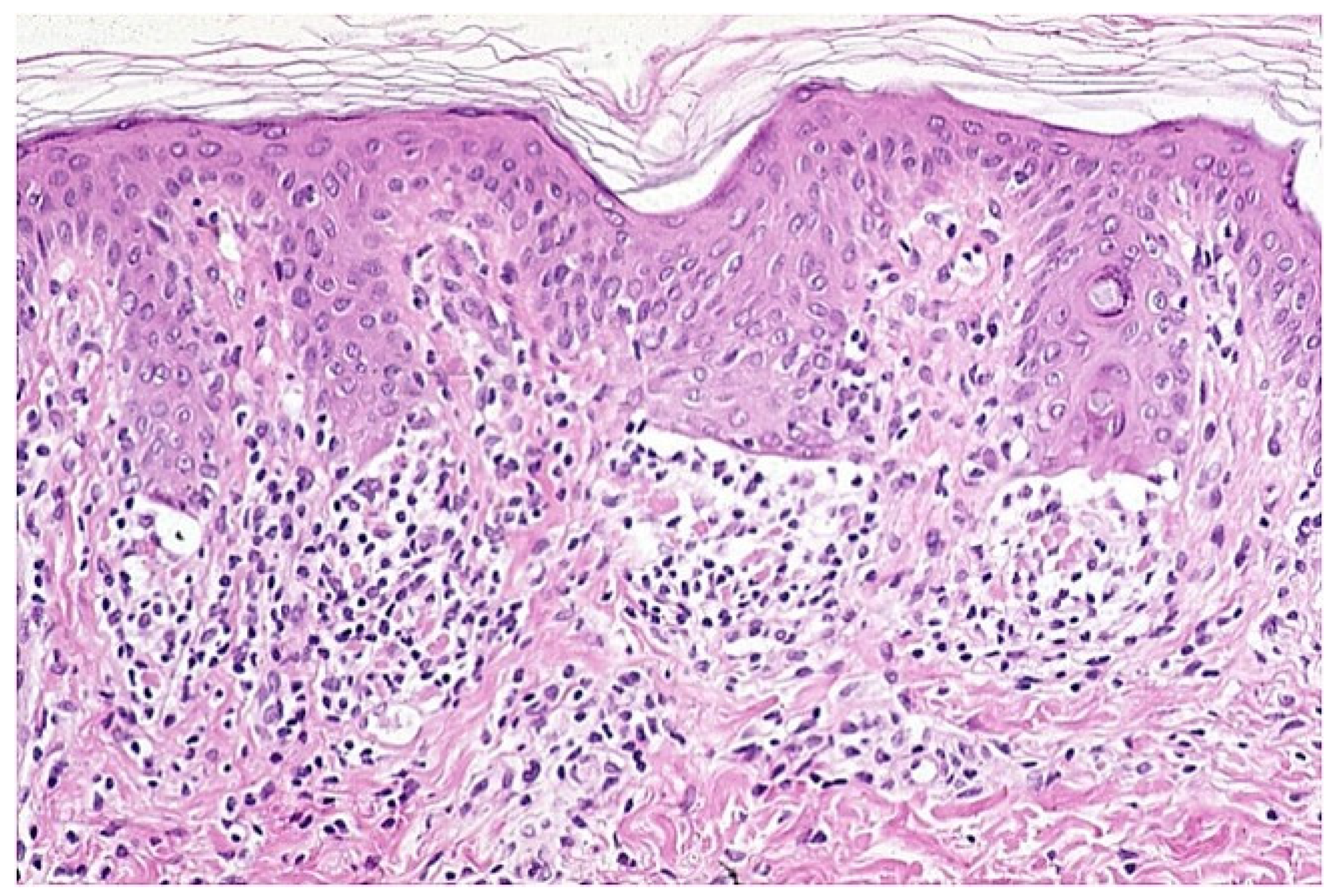
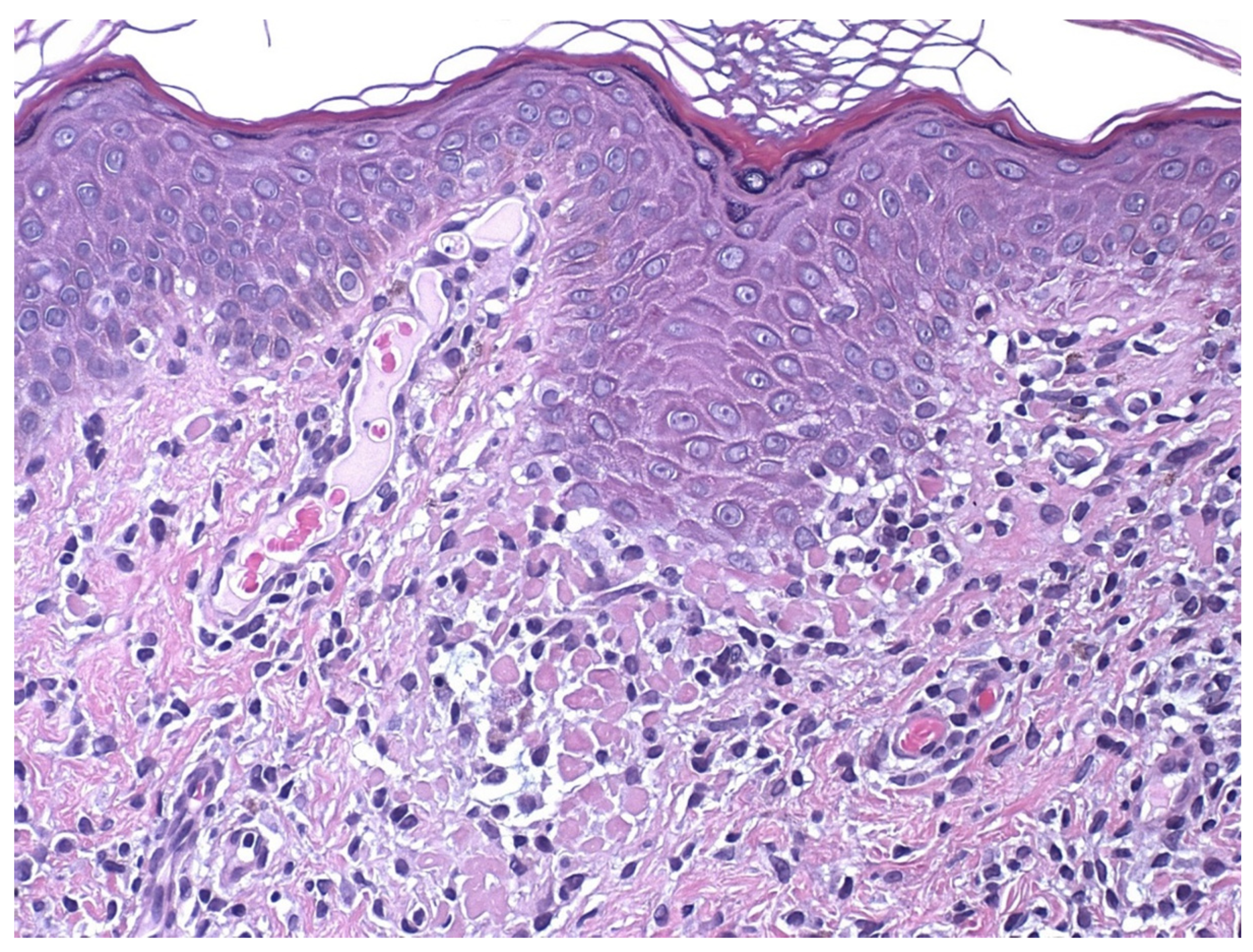
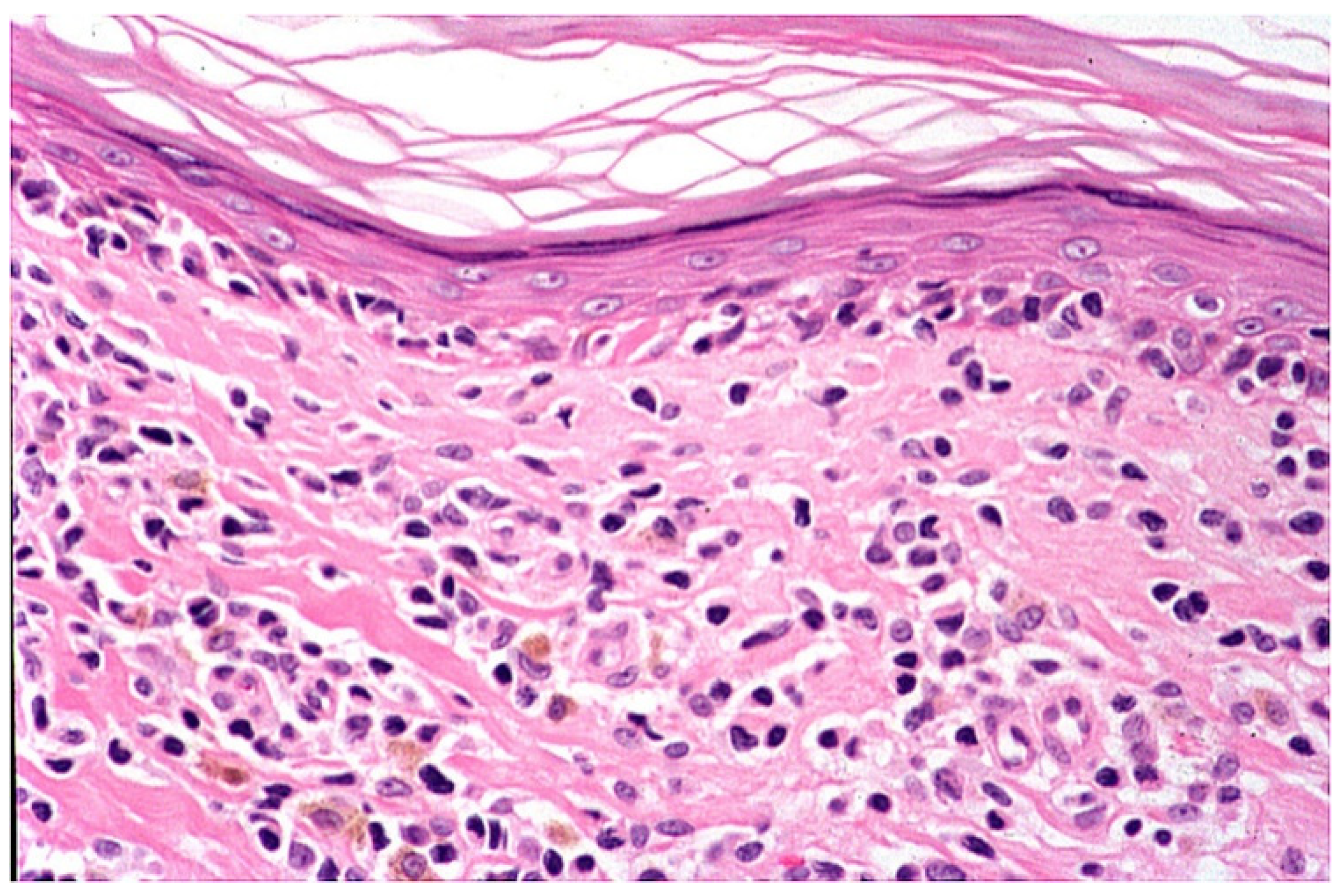
4. Histopathology
5. Immunohistochemical and Molecular Analysis
6. Course
7. Therapy
8. Differential Diagnosis
9. Etiopathogenesis
10. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Annessi, G.; Paradisi, M.; Angelo, C.; Perez, M.; Puddu, P.; Girolomoni, G. Annular lichenoid dermatitis of youth. J. Am. Acad. Dermatol. 2003, 49, 1029–1036. [Google Scholar] [CrossRef]
- De la Torre, C.; Florez, A.; Fernandez-Redondo, V. Negative results of patch testing with standard and textile series in a case of annular lichenoid dermatits of youth. J. Am. Acad. Dermatol. 2005, 53, 172–173. [Google Scholar] [CrossRef]
- Durdu, M.; Akyilmaz, M.; Tuncer, I. Annular lichenoid dermatitis of youth. Pediatric Dermatol. 2007, 24, 582–584. [Google Scholar] [CrossRef] [PubMed]
- Kleikamp, S.; Kutzner, H.; Frosch, P.J. Annular lichenoid dermatitis of youth- a further case in a 12-year-old girl. J. Dtsch. Dermatol. Ges. 2008, 6, 653–656. [Google Scholar] [CrossRef]
- Sans, V.; Leaute-Labreze, C.; Vergier, B.; Taieb, A. A further case of Annular lichenoid dermatitis of Youth: Role of the anti-Hepatitis B immunization. Pediatric Dermatol. 2008, 25, 577–579. [Google Scholar] [CrossRef]
- Cesinaro, A.M.; Sighinolfi, P.; Greco, A.; Garagnani, L.; Conti, A.; Fantini, F. Annular lichenoid dermatitis of youth… and beyond: A series of 6 cases. Am. J. Dermatopathol. 2009, 31, 263–267. [Google Scholar] [CrossRef] [PubMed]
- Tsoitis, G.; Kanitakis, J.; Kyamidis, K.; Lefaki, I. Annular lichenoid dermatitis of youth. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1339–1340. [Google Scholar] [CrossRef]
- Huh, W.; Kanitakis, J. Annular lichenoid dermatitis of youth: Report of the first Japanese case and published work review. J. Dermatol. 2010, 37, 531–533. [Google Scholar] [CrossRef] [PubMed]
- Fabroni, C.; Salvini, C.; Piana, S.; Scocco, G.L. Annular lichenoid dermatitis. Clin. Exp. Dermatol. 2010, 35, 921–923. [Google Scholar] [CrossRef] [PubMed]
- Leger, M.C.; Gonzalez, M.E.; Meehan, S.; Schaffer, J.V. Annular lichenoid dermatitis of youth in an American boy. J. Am. Acad. Dermatol. 2013, 68, 155–156. [Google Scholar] [CrossRef]
- Kazlouaskaya, V.; Trager, J.D.; Junkins-Hopkins, J.M. Annular lichenoid dermatits of youth: A separate entity on the spectrum of mycosis fungoides? Case report and review of the literature. J. Cutan. Pathol. 2015, 42, 420–426. [Google Scholar] [CrossRef] [PubMed]
- Di Mercurio, M.; Gisondi, P.; Colato, C.; Schena, D.; Girolomoni, G. Annular lichenoid dermatits of youth: Report of six new cases with review of the literature. Dermatology 2015, 231, 195–200. [Google Scholar] [CrossRef]
- Vazquea-Osorio, M.; Gonzales-Sabin, M.; Gonzalvo-Rodriguez, P.; Rodríguez-Díaz, E. Annular lichenoid dermatitis of youth: A report of 2 cases and a review of the literature. Actas Dermo-Sifiliográficas 2016, 107, e39–e45. [Google Scholar]
- Ulkumen, P.K.; Kocaturk, E.; Gungor, S. Annular lichenoid dermatits of youth in a 15-year-old boy: Topical tacrolimus as a treatment option. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 467. [Google Scholar] [CrossRef]
- Malachowski, S.J.; Creasey, M.; Kinkley, N.; Heaphy, M.R., Jr. Annular lichenoid dermatits of youth: A chronic case managed using pimecrolimus. Pediatric Dermatol. 2016, 33, e360–e361. [Google Scholar] [CrossRef]
- Wilk, M.; Zelger, B.G.; Emberger, M.; Zelger, B. Annular lichenoid dermatitis (of youth). Immunohistochemical and serological evidence for another clinical presentation of Borrelia infection in patients of western Austria. Am. J. Dermatopathol. 2017, 39, 177–180. [Google Scholar] [CrossRef] [PubMed]
- Cesinaro, A.M. Annular lichenoid dermatitis (of youth): Report of a case with lichenplanus-like features. Am. J. Dermatopathol. 2017, 39, 914–915. [Google Scholar] [CrossRef]
- Dubois, D.; Dargent, L.; Ngendahayo, P.; Roquet-Gravy, P.-P. Dermatite annulaire de sujet Jeune: Un case et revue de la litterature. Ann. Dermatol. Venereol. 2018, 145, 365–375. [Google Scholar] [CrossRef]
- Mahmoudi, H.; Ghanadan, A.; Fahim, S.; Moghanlou, S.; Etesami, I.; Daneshpazhooh, M. Annular lichenoid dermatitis of youth: Report on two adult cases and one child. J. Dtsch. Dermatol. Ges. 2019, 17, 1173–1175. [Google Scholar] [CrossRef]
- Stojkovic-Filipovic, J.; Lekic, B.; Brasanac, D.; Lalosevic, J.; Gajic-Veljic, M.; Nikolic, M. Annular lichenoid dermatitis of youth-recurrent case of rare skin disease treated with cyclosporine. Dermatol. Ther. 2020, 33, e13285. [Google Scholar] [CrossRef]
- Auban-Pariente, J.; Santos-Juanes, J.; Vivanco-Allende, B.; Galache-Osuna, C. Dermatitis anular liquenoide de la infancia. An. Pediatría 2021, 95, 209–210. [Google Scholar] [CrossRef]
- Bodemer, C.; Belon, M.; Hamel-Teillac, D.; Amoric, J.C.; Fraitag, S.; Prieur, A.M.; De Prost, Y. Scleroderma in children: A retroscpective study of 70 cases. Ann. Dermatol. Venereol. 1999, 126, 691–694. [Google Scholar]
- Bergman, R.; Faclieru, D.; Sahr, D.; Sander, C.A.; Kerner, H.; Ben-Aryeh, Y.; Manov, L.; Hertz, E.; Sabo, E.; Friedman-Birnbaum, R. Immunophenotyping and T-cell receptor g gene rearrangement analysis as an adjunct to the histopathologic diagnosis of mycosis fungoides. J. Am. Acad. Dermatol. 1998, 39, 554–559. [Google Scholar] [CrossRef]
- Bressler, G.S.; Jones, R.E. Erythema annulare centrifugum. J. Am. Acad. Dermatol. 1981, 4, 597–602. [Google Scholar] [CrossRef]
- Peterson, A.O.; Jarrat, M. Annular erythema of infancy. Arch. Dermatol. 1981, 117, 145–148. [Google Scholar] [CrossRef]
- Lee, D.; Lazova, R.; Bolognia, J.L. A figurate papulosquamous variant of infalmmatory vitiligo. Dermatology 2000, 200, 270–274. [Google Scholar] [CrossRef] [PubMed]
- Halevy, S.; Shai, A. Lichenoid drug eruption. J. Am. Acad. Dermatol. 1993, 29, 249–255. [Google Scholar] [CrossRef]
- Ackerman, A.B. Scleroderma. In Histologic Diagnosis of Infalmmatory Skin Diseases, 2nd ed.; Ackerman, A.B., Ed.; William and Wilkins: Baltimore, MD, USA, 1997; pp. 706–708. [Google Scholar]

| Reference (First Author) | Number of Cases | Therapy | Remission |
|---|---|---|---|
| Annessi | 23 | Tcs (17) | Complete |
| Scs (1) | Complete | ||
| A (4) | No remission | ||
| SE (2) | Partial | ||
| Ph (2) | Complete | ||
| De la Torre | 1 | Tcs | Complete |
| Durdu | 1 | Tcs | Complete |
| Kleikamp | 1 | Tcs and T | Complete |
| Cesinaro | 3 | Tcs (2) | Complete |
| T (1) | |||
| Huh | 1 | Tcs | Partial |
| Fabroni | 1 | Tcs | Complete |
| Leger | 1 | Tcs and Scs | Complete |
| Kazlouskaya | 1 | Tcs | Complete |
| Di Mercurio | 6 | Tcs (6) | Complete |
| T (2) | Complete | ||
| Osorio | 2 | - | Spontaneous remission |
| Ulkumen | 1 | T | Complete |
| Malakhowski | 1 | Tcs and Pim | Complete |
| Wilk | 12 | Tcs (4) | Complete |
| A (2) | No remission | ||
| Cesinaro | 1 | Tcs | Complete |
| Debois | 1 | Tcs and Pim | Complete |
| Sans | 1 | Tcs | Complete |
| Mahmoudi | 3 | Tcs and T (3) | complete |
| Stojkovic-Filipovic | 1 | Cy | Complete |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Annessi, G.; Annessi, E. Annular Lichenoid Dermatitis (of Youth). Dermatopathology 2022, 9, 23-31. https://doi.org/10.3390/dermatopathology9010004
Annessi G, Annessi E. Annular Lichenoid Dermatitis (of Youth). Dermatopathology. 2022; 9(1):23-31. https://doi.org/10.3390/dermatopathology9010004
Chicago/Turabian StyleAnnessi, Giorgio, and Emanuele Annessi. 2022. "Annular Lichenoid Dermatitis (of Youth)" Dermatopathology 9, no. 1: 23-31. https://doi.org/10.3390/dermatopathology9010004
APA StyleAnnessi, G., & Annessi, E. (2022). Annular Lichenoid Dermatitis (of Youth). Dermatopathology, 9(1), 23-31. https://doi.org/10.3390/dermatopathology9010004





