Abstract
Galli–Galli disease (GGD) is a rare genodermatosis that exhibits autosomal dominant inheritance with variable penetrance. GGD typically manifests with erythematous macules, papules, and reticulate hyperpigmentation in flexural areas. A distinct atypical variant exists, which features brown macules predominantly on the trunk, lower limbs, and extremities, with a notable absence of the hallmark reticulated hyperpigmentation in flexural areas. This review includes a detailed literature search and examines cases since GGD’s first description in 1982. It aims to synthesize the current knowledge on GGD, covering its etiology, clinical presentation, histopathology, diagnosis, and treatment. A significant aspect of this review is the exploration of the genetic, histopathological, and clinical parallels between GGD and Dowling-Degos disease (DDD), which is another rare autosomal dominant genodermatosis, particularly focusing on their shared mutations in the KRT5 and POGLUT1 genes. This supports the hypothesis that GGD and DDD may be different phenotypic expressions of the same pathological condition, although they have traditionally been recognized as separate entities, with suprabasal acantholysis being a distinctive feature of GGD. Lastly, this review discusses the existing treatment approaches, underscoring the absence of established guidelines and the limited effectiveness of various treatments.
Keywords:
hyperpigmentation; acantholysis; keratin-5; skin diseases; genetic; skin diseases; papulosquamous 1. Introduction
Galli–Galli Disease (GGD) is a rare and poorly understood genodermatosis that exhibits an autosomal dominant inheritance pattern with variable penetrance [1,2], although sporadic cases lacking family history have been described [1]. First described by Bardach, Gebhart, and Luger in 1982 [3], GGD was identified in two brothers and has since been classified as part of the reticulate pigmented disorders of the skin (RPDS) group [4].
Clinically, GGD is characterized by erythematous macules and papules with reticulate hyperpigmentation affecting the flexural areas, including the axillary, inguinal, and neck regions. During the inflammatory phase of the disease, itchy, erythematous, vesicular, and/or scaly papules may manifest in the same distribution, as well as on the trunk and proximal extremities [5,6,7]. Comedo-like lesions or pitted acneiform scars on the face may occasionally occur [6,8,9]. An emerging consensus posits a distinct GGD subset characterized by an atypical phenotype. While histopathological features remain consistent with classical GGD, this variant diverges in its presentation of brown macules on the trunk, lower limbs, and extremities, almost absent the hallmark reticulated hyperpigmentation in flexural areas [5].
The clinical phenotype of classic GGD bears a striking resemblance to Dowling-Degos disease (DDD), another rare autosomal dominant genodermatosis characterized by reticulate pigmentary macules of the flexures, comedo-like lesions on the back, neck, and face, and perioral pitted acneiform scars [10,11]. In addition, there have been various reports in the literature that include atypical presentations of DDD or additional uncommon findings [10]. This significant overlap between GGD and DDD has led to ongoing confusion and misdiagnosis [12]. Traditionally, the differentiation has been primarily histopathologic, with suprabasal acantholysis serving as a distinctive feature that is historically attributable to GGD, thereby categorizing it as an acantholytic variant of DDD [5]. Finally, molecular genetic analyses have identified mutations in the KRT5 and POGLUT1 genes in some cases of both GGD and DDD; therefore, GGD is now referred to as a variant of DDD [7].
The aim of this review is to provide a comprehensive synthesis of the existing knowledge on GGD, encompassing its etiology, clinical presentation, histopathology, diagnosis, and treatment options.
A further aim is to substantiate the hypothesis that GGD and DDD may, in fact, constitute two distinct clinical phenotypes of a unique pathological entity.
2. Literature Search
A search of the dermatologic literature was performed using the PubMed search engine to identify any reports of Galli–Galli disease since it was first described in 1982 to September 2022. The following key words and their synonyms were used singly or in combination [Galli–Galli Disease], [acantholytic], [acantholysis], and [Dowling-Degos disease]. The language was not limited to English. Various types of publications were considered for inclusion, such as original articles, reviews, case series and case reports, comment articles, and letters to editors. The reference lists of all articles, reviews, and case reports included were hand-searched to find additional eligible articles.
3. Pathogenesis
Despite the fact that GGD and DDD have very similar clinical aspects to each other, GGD has traditionally been considered to be a separate entity from DDD because suprabasal acantholysis is histopathologically observed only in GGD [12]. Advances in genetics have identified key mutations in genes such as KRT5 and POGLUT1 as pivotal in the pathogenesis of GGD. Intriguingly, these mutations have also been reported in cases of DDD [7,13].
3.1. KRT5
Keratin 5 (KRT5) protein coding gene, which was initially identified in DDD in 2006, is the main gene involved in the pathogenesis of GGD [8]. This gene is located on chromosome 12 (12q13.13), contains nine exons, and is predominantly expressed within the basal cells of the epidermis [14]. Its alteration, which is mainly caused by frameshift mutations, causes premature stop codons and results in haploinsufficiency (an insufficient production of the gene product) [5,14].
KRT5 is a protein that forms intermediate filaments, or heterodimers, with cytokeratin 14 (KRT14) [14,15]. Both proteins play a critical role in attaching keratinocytes together and anchoring the epidermis to the dermis (Figure 1) [14,15,16].
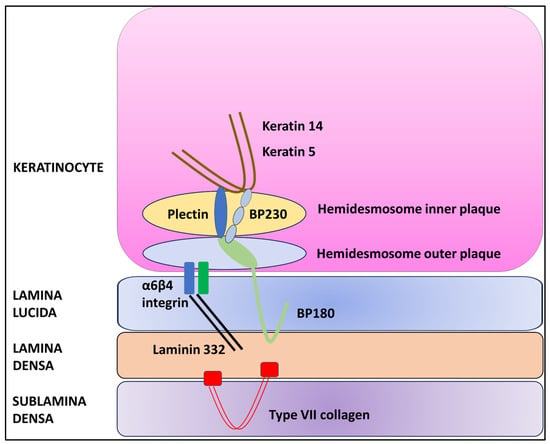
Figure 1.
Schematic diagram of the dermal–epidermal junction.
In the presence of haploinsufficiency, the ratio of KRT5 to KRT14 is altered, resulting in a change in intermediate filament structure and impaired binding to desmosomes and hemidesmosomes and leading to the characteristic histopathologic alteration associated with GGD, i.e., acantholysis [7,14]. However, the precise pathogenetic mechanism that causes acantholysis is still unknown [7,17,18]. In addition, KRT5 plays a key role in epidermal differentiation and the uptake and degradation of melanosomes by keratinocytes [19]. In fact, mutations of the KRT5 gene have been associated with hyperplasia of epidermal ridges and the accumulation of melanin, resulting in the typical reticular hyperpigmentation [14,19].
Mutations in the KRT5 gene were identified in 21 out of 69 patients diagnosed with classical Galli–Galli disease [8,9,18,20,21,22,23,24]. The most common genetic alteration in the KRT5 gene is the c.418dupA missense mutation, which was found in 13 of the 21 patients with a KRT5 mutation [18,21,22,24]. Notably, this specific mutation has also been observed in DDD [5,7,18,21,22,24]. The c.418dupA mutation results in the insertion of an adenosine at position 418, causing a frameshift in the gene sequence [5,8].
Several de novo mutations have been identified in the KRT5 gene over the years in patients with classical GGD, most of which have not been linked to distinct phenotypes [24] An Arabian patient harbored a substitution of thymine for cytosine at position 2 (c.T2C), resulting in the destruction of the NlaIII endonuclease recognition site and a change in the codon of coding mRNA, which resulted in haploinsufficiency [20].
An Asian-American woman was identified as carrying a c.38dupG mutation, which led to the insertion of a guanine at position 38 in exon 1, subsequently causing a premature stop codon and resulting in KRT5 haploinsufficiency [8].
A Caucasian patient had the c.14C > A mutation, which resulted in the substitution of cytosine for adenosine [21].
A Caucasian patient with a segmental phenotype was found to carry a c.476C4T mutation. This mutation caused a substitution of leucine for proline at position 159, resulting in the production of a nonfunctional protein [23].
An Indian family was found to carry a mutation c.10C > T, in which cysteine was replaced by threonine at position 10 [9]. In addition to the classic symptoms, this family exhibited hyperpigmentation on the extremities and face. Furthermore, multiple hypopigmented macules were symmetrically distributed over the trunk.
3.2. POGLUT1
With the development of whole-exome sequencing (WES), mutations in POGLUT1 (encoding protein O-glucosyltransferase 1) have been shown to underlie some cases of GGD/DDD [13,25].
The POGLUT1 gene, which is located on chromosome 3q13.33, encodes a protein that plays a crucial role in posttranslational modifications within the Notch pathway, transferring glucose molecules to serine residues of epidermal growth factor-like (EGF-like) domains within the endoplasmic reticulum [13,26,27]. The addition of the O-glucose residues by POGLUT1 plays a crucial role in the folding of Notch pathway receptor proteins within the endoplasmic reticulum, which is essential for their transport to the cell surface (Figure 2) [26,27,28,29].
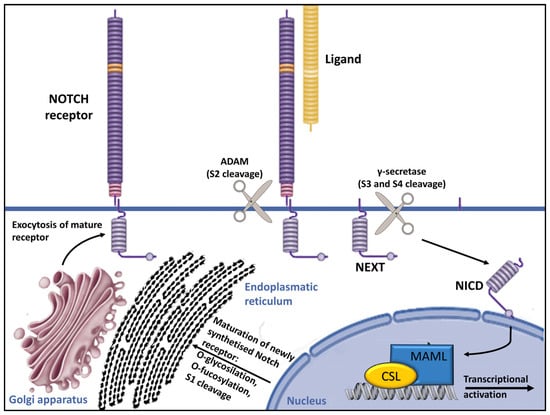
Figure 2.
Overview of Notch signaling. Notch receptors undergo initial translation in the endoplasmic reticulum and are then trafficked to the Golgi apparatus. During trafficking, O-linked and N-linked glycosylations occur. Then, in the Golgi apparatus, Notch receptors are cleaved into heterodimers (S1 cleavage) and transported to the cell membrane. Upon reaching the cell surface, the receptor is activated through ligand binding. This ligand–receptor interaction triggers transendocytosis in the neighboring cell, inducing a conformational alteration in the Notch receptor and exposing the S2 site. This site is cleaved by ADAM metalloproteases (S2 cleavage), generating a membrane-anchored Notch extracellular truncation (NEXT). The S2 cleavage exposes S3 and S4 sites, facilitating further proteolytic cleavage by the γ-secretase complex (S3/S4 cleavage). This results in the liberation of the notch intracellular domain (NICD), which translocates to the nucleus. There, it binds to CBF1/RBP-Jκ/Su(H)/Lag-1 (CSL), which is known as RBP-Jκ in vertebrates. In its basal state, CSL functions as a transcriptional repressor by associating with corepressor (Co-R) proteins. NICD binding converts it into a transcriptional activator by forming a ternary complex with mastermind-like (MAML) proteins, thus initiating the transcription of downstream target genes [29].
The Notch pathway comprises four receptors and five ligands, and it is responsible for regulating skin homeostasis. It affects the proliferation and differentiation of melanocytes and keratinocytes, as well as their interaction. The alteration of this pathway leads to keratinocyte differentiation abnormalities, as evidenced by the increased expression of KRT5 and KRT14 in immunohistochemistry, as well as disturbance in skin pigmentation, as demonstrated by the abnormal migration of melanoblasts and melanocytes to ectopic sites in animal models [13].
In 2014, Basmanav et al. discovered various mutations in the POGLUT1 gene in individuals diagnosed with DDD who exhibited a disseminated pattern of brownish macular and lentiginous lesions on the extremities, trunk, and neck, rather than the typical domination of flexural folds commonly seen in classical DDD [13]. Notably, six of these cases had previously been classified as atypical GGD in an earlier study by one of the Authors [21]. They identified two nonsense mutations (c.11G > A and c.652C > T) and a splice-site mutation (c.798-2A > C) [13]. These mutations led to truncated proteins with compromised stability, subsequently reducing the glycosylation of receptor proteins in the Notch pathway [13].
The observed reduction in glycosylation has been demonstrated in animal models, revealing abnormalities in keratinocyte and melanocyte development and differentiation [13]. The POGLUT1 gene significance in cell differentiation is further supported by immunohistochemical analysis, which shows increased expression in the upper layers (spinous and granular) and a decrease in expression by about 50% in affected individuals. This is consistent with heterozygous mutations leading to a loss of function [13,25,27,30].
Histologically, the skin lesions of individuals with POGLUT1 mutations have showed a digitiform epidermal ridge hyperplasia with pronounced hyperpigmentation at the tips of the rete ridges, focal hypergranulosis, and, notably, some small horn cysts and minor acantholysis.
In recent years, three additional cases of GGD with POGLUT1 mutation were identified: one with a c.1013delTinsAAC [30], one with c.652 > T [4], and one with c.798-2A > C [31].
3.3. A Possible Role of PSENEN?
The presenilin enhancer, gamma-secretase subunit (PENSEN) gene plays a key role in Notch signaling activation, providing instructions for a protein called gamma-secretase subunit PEN-2 (shortened to PEN-2) [32]. It is a transmembrane protease composed of four essential protein subunits (the γ-secretase complex) located in the cell membrane, where it cleaves many different transmembrane proteins. This cleavage is an important step in several chemical signaling pathways that transmit signals from outside the cell into the nucleus, including Notch signaling. (Figure 2) [29,32,33,34,35].
The PSENEN gene, which has been extensively studied in the context of neurodegeneration, has been linked to over 300 mutations primarily associated with early-onset Alzheimer’s disease [32]. Recently, mutations in PSENEN have also been discovered in patients with familial hidradenitis suppurativa (HS) [36]. These mutations result in presenilin dysfunction and impaired cleavage activity, particularly affecting substrates like Notch receptors [37,38].
A cooccurrence of HS and DDD was first mentioned in 1990 [39] and subsequently confirmed in numerous case reports [33]. Pavlovsky et al. described four more individuals with HS–DDD [33] Importantly, the study also identified a founder mutation in the PSENEN gene [33].
Del Mar et al. reported the first case of GGD associated with HS but did not conduct any genetic assays to identify mutations that had been previously reported in such conditions [34]. Although the precise pathogenic mechanism linking GGD and DDD with HS remains unclear, current evidence suggests that mutations in PSENEN may be involved, similarly to alterations in POGLUT1, affecting the Notch signaling pathway [33,38,40,41].
4. Clinical Presentation
To the best of our knowledge, 69 cases of GGD have been described in the literature, including 27 males and 42 females (female-to-male ratio of 1.5) with a mean age at diagnosis of 49 years (range 17–77 years). Difficulty in diagnosing may account for the low prevalence of the dermatosis [5]. In our literature review, a specific age at disease onset and time of diagnosis was provided in 52/69 cases, with an average delay of 15 years in diagnosis. Remarkably, in two cases, the delay exceeded 40 years [21,24]. It is noteworthy that in 15 cases the exact age of onset or diagnosis remained unspecified [30,34,42,43,44,45]. This underscores the challenges in either symptom recognition or the diagnostic process itself.
The disease involves individuals between the second and ninth decades of life, reaching its peak in the third and fifth decade [5,7,45]. The majority of patients were Caucasians (52/63) [1,2,3,4,6,18,19,21,22,23,24,31,42,43,45,46,47,48,49,50,51,52], followed by Indians (6) [9,44], Asians (5) [53], Africans (1) [34], Arabians (1) [20], Asian-Americans (1) [8], Japanese (1) [30], and Latin Americans (1) [54]. In one case the race is unspecified [5].
It is increasingly evident that two distinct presentations of GGD exist, rather than a single phenotype, as formerly believed [5,21,49]. The first presentation, which is observed in the majority of patients (42/69) and consequently designated as typical, is characterized by progressive symmetrical, reticular, and partly lentiginous pigmentation of the flexures (the neck, axillary, mammary, and inguinal folds) in combination with erythematous brownish macules and papules, which sometimes coalesce into reticulated plaques (Figure 3) [1,2,3,6,8,9,18,20,21,22,24,34,42,46,47,48,49,52,53,54].
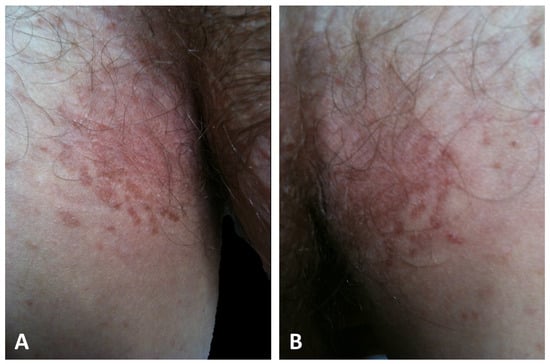
Figure 3.
Typical presentation of GGD with hyperkeratotic papules and erythematous brownish macules that coalesce into patches with a reticulated appearance on the inguinal (A) and axillary folds (B).
The second presentation, which has been identified in a minority of cases (25/69) and hence defined as atypical, is characterized by erythematous macules and papules widely distributed over the trunk, neck, back, abdomen, and limbs without the involvement of the flexural folds (Figure 4) [4,5,19,21,30,43,44,45,50,51,55,56,57].
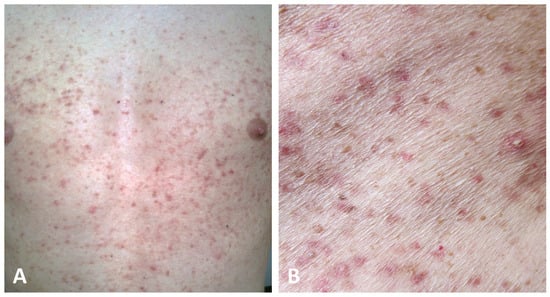
Figure 4.
Atypical presentation of GGD with dissemination of brownish macules and erythematous papules on the trunk with sparing of the folds (A,B).
One patient presented with brown macules in a segmental variation involving her left thigh due to a type-1 segmental inherited disorder resulting in a KRT5 p.P159L mutation [23]. Because the patient exhibited a localized distribution without generalized involvement, it was postulated that this could represent a type 1 segmental manifestation resulting from a postzygotic mutation [58]. In contrast, type 2 mosaicism arises from postzygotic loss of heterozygosity, with loss of the wild-type allele leading to generalized involvement with one segment more affected than the rest of the skin. This event was not observed in this case, as the remaining skin was unaffected [23,58]. Only a single case could not be categorized as typical, atypical, or segmental due to insufficient data [31].
During the inflammatory phase, the maculo-papular lesions become more pronounced and develop into pruritic vesicles, erosions, and crusts (Figure 5) [5,49,54].
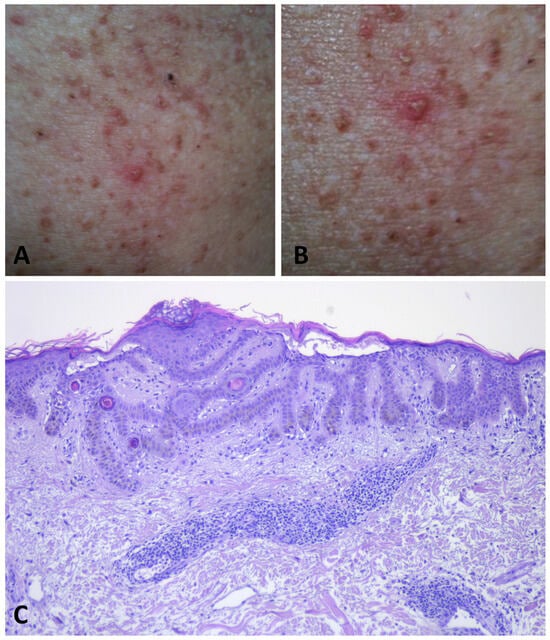
Figure 5.
During flare phases of GGD, multiple vesicles develop, leading to intensely pruritic erosions and crusts on the skin (A,B). Histopathology shows a superficial dermal perivascular lymphocytic infiltrate. Apical acantholysis is apparent in the epidermis (C).
Various etiological factors may trigger the inflammatory response. In a study conducted by Hanneken et al. in 2011, sweat was identified as the primary aggravating factor in 10 of the 18 patients, followed by elevated temperature (9/18) and ultraviolet radiation (7/18), with these three triggers coexisting in four patients [21]. Only in a single case was decreased temperature the possible triggering factor. Moreover, exacerbation following dialysis treatment was first reported in the Literature [45].
In GGD, a notable correlation has been identified between genotype and phenotype. Specifically, mutations in the KRT5 gene have been observed to cause cutaneous involvement primarily in the flexural areas, resulting in the typical clinical manifestation [21]. Among the 43 patients exhibiting the typical presentation, the mutation in the KRT5 gene was tested in 23 individuals, of which 20 were found to have the mutation. The mutation was not tested in the remaining 20 individuals [8,9,18,20,21,22,24]. Interestingly, the sole reported case of segmental GGD, which has been attributed to the type 1 mosaicism phenomenon, was associated with the p.P159L type KRT5 gene mutation [23]. In contrast, among the 24 patients with the atypical presentation, no mutation was detected within the KRT5 gene [4,5,19,21,30,43,44,45,50,51,55,56,57]. These findings were corroborated by Basmanav et al., who identified mutations in the POGLUT1 gene in individuals presenting with atypical GGD [13,21]. Similarly, studies conducted by Kono et al. [30] and Rundle et al. [4] revealed that patients with atypical variants were characterized by mutations in the POGLUT1 gene. Unfortunately, data regarding POGLUT1 mutations in the other cases of atypical GGD are not available [19,43,44,45,49,50,51,55,56,57]. Table 1 summarizes the clinical data of the patients.

Table 1.
Clinical characteristics of the patient with Galli–Galli disease in the literature.
5. Histopathology
Skin biopsy plays a crucial role in diagnosing GGD. Characteristic features consist of filiform, digitated hyperplasia of the epidermal ridges, basal hyperpigmentation of the tips of rete ridges, multiple foci of acantholysis in the upper spinous and/or granular layer, and narrowing of the suprapapillary epidermis with widened granular cell layer [5,7,8,49,50]. Some dyskeratotic keratinocytes may also be seen [3,4,8,30,31,43,45,50,51,55,56]. It should be noted that these findings are not consistently present and may vary depending on the stage of the disease.
During the inflammatory phase, an infiltrate of lymphocytes, histiocytes, and sparse eosinophils is observed in the papillary dermis [5,19,50]. Foci of epidermal spongiosis close to acantholytic foci may eventuate in papulovesicular and scale-crusted lesions (Figure 5C).
During the noninflammatory phase, dermatosis runs an asymptomatic course and may be clinically and histologically indistinguishable from DDD. Histopathologically, acantholysis, if any, is minimal, and there is no evident dermal inflammation (Figure 6).
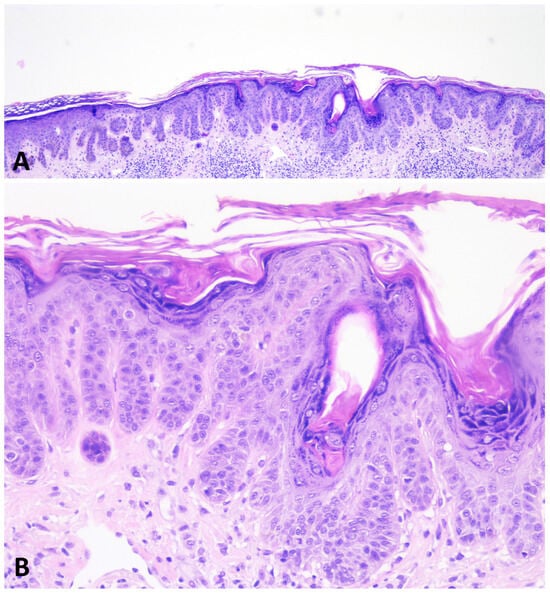
Figure 6.
During the noninflammatory phase of GGD, the dermatosis is histologically indistinguishable from DDD. The epidermis shows thin, elongated, branching rete ridges with basal hyperpigmentation. There is mild hyperkeratosis overlying the epidermis (A,B).
In this stage, only digitate hyperplasia of epidermal ridges and melanin deposits in the papillary dermis can be detected. Additional findings that may be observed include follicular comedo-like cysts, hyperkeratosis, dyskeratosis, parakeratosis, acanthosis, and hypergranulosis [5,19,44,50].
6. Diagnosis of Galli–Galli Disease
Currently, there is no specific laboratory test or special staining for GGD. Therefore, diagnosis is based on clinical–pathological correlation and, when possible, genetic analysis [18,49].
Because several conditions may mimic GGD clinically and histopathologically, with often overlapping features, including DDD, Grover disease (GD), and Darier’s disease (DD), a high index of suspicion is required.
DDD is a benign genodermatosis belonging to the group of reticular pigmented dermatoses with autosomal-dominant inheritance patterns. Typically, skin lesions manifest during adulthood as symmetrical macular or reticular hyperpigmentation, as well as noninflammatory reddish-brown papules and plaques on body folds, wrists, face, and chest [10,11]. Hyperkeratotic itchy papules, pitted acneiform scars, comedo-like lesions, hypopigmented maculae, and palmar depressions are possible additional skin manifestations [60,61]. The occasional itch observed in DDD, similarly to GGD, may be related to an increase in the number of mast cells [10].
Both GGD and DDD can manifest in a generalized form featuring multiple small, only partially confluent, reddish-brown papules and plaques, as well as disseminated lentiginous hyperpigmentation over the trunk and extremities. The intriguing report by Wu et al on a family with both flexural and disseminated disease involvement blurred the lines between these two conditions [53]. Remarkably, two biopsies from the same patient yielded different results: a biopsy from the axillary area was diagnosed as GGD, while a biopsy from the abdominal area was identified as DDD.
Differentiation between two entities relies on biopsy. While DDD usually lacks the suprabasal acantholysis observed in GGD [7,12,49,60,61], both conditions share similar features, such as epidermal ridge hyperplasia, hyperkeratosis, epidermal thinning of the epithelium, perivascular lymphocytic infiltrate, melanosis, and dermal fibrosis [12,53,60,61]. The need for repeated skin biopsies from different sites or serial sections to detect acantholysis has been emphasized [50]. Indeed, recent studies have highlighted that patients previously diagnosed with DDD showed foci of epidermal acantholysis on histopathologic sections from skin biopsies, which had been initially missed, relocating these cases within the spectrum of GGD [7]. This has led to the hypothesis that GGD and DDD are not two separate entities, but rather different clinical expressions of the same condition [7,9]. The same genetic background further corroborated this concept [7,24]. Recent genetic findings have revealed loss-of-function mutations in the KRT5 gene in DDD cases [62], and mutations in the POGLUT1 [13] gene have been identified in both DDD and GGD phenotypes.
GD is an acquired condition of unknown origin affecting mostly fair-skinned, elderly males [63] that is characterized by an intensely itchy eruption of discrete papules and vesicles on the trunk and proximal extremities [63,64,65]. The dermatosis is usually self-limiting, but it may persist for years with little response to therapy [63,66]. Clinically, GD may mimic the atypical variant of GGD, and the two conditions exhibit common triggers, such as heat and excessive sweating [63,67]. However, brown macules are not a typical feature of GD. The histopathologic hallmark of the GD is acantholysis, which is often associated with dyskeratosis and gives the lesions an appearance similar to Darier disease, Hailey–Hailey disease, or pemphigus. Spongiotic changes can be observed, as well [68]. In 2010, Fernández-Figueras et al. identified an additional pattern characterized by porokeratosis-like oblique columns of parakeratosis, lesions showing a nevoid or lentiginous silhouette, intraepidermal vesicular lesions, lichenoid changes with basal vacuolization and dyskeratosis, and dysmaturative foci with keratinocyte atypia [69]. Lentiginous elongation of rete ridges, however, is rarely observed in GD, and dyskeratosis is not a common finding in GGD [3,4,8,30,31,43,45,50,51,55,56].
The scientific literature has presented ambiguity concerning the classification of these specific dermatological conditions. In one prominent example, Ackerman provided commentary on a review by Gilchrist [1], wherein he advocated for the reclassification of cases diagnosed as GGD to be instead considered under the umbrella of GD [19,20,46,47,53] and cases of “extensive Grover’s-like eruption with lentiginous freckling” as GGD [43,70].
Moreover, while we have genetic studies recognizing specific mutations in the two forms of GGD, there is a notable absence of extensive genetic research on Grover’s disease. Recent evidence show the presence of ATP2A2 mutations in Grover’s disease [71], while mutations in the KRT5 gene, which are found in GGD, are rarely reported in GD [72]. This supports the idea that hereditary GGD is a distinct entity, while sporadic cases in adults may be part of the broader spectrum of GD.
Darier disease (DD) is a rare autosomal dominant genetic skin condition caused by mutations in the ATP2A2 gene, which encodes SERCA2, a calcium ion transporter within the endoplasmic reticulum [59,73]. These mutations lead to altered desmosomal function with suprabasal acantholysis and dyskeratotic cells [59,73,74]. The dermatosis typically develops during puberty [73,74,75]. Clinical manifestations and disease severity can vary greatly, even within families with the same mutation [73,75,76]. Clinically, DD is characterized by warty plaques formed by coalescing firm, greasy, skin-colored, hyperkeratotic papules and primarily affects the seborrheic areas of the trunk and face [73]. Itching is common, occurring in 80% of patients. The involvement of flexures can lead to hypertrophic, fissured, and malodorous lesions, impacting the patient’s quality of life [73,77]. Triggers are similar to those in Hailey–Hailey disease, including heat, ultraviolet rays, sweat, infection, and mechanical rubbing [73,75]. Other clinical features observed in DD patients include short stature, subungual and palpebral hyperkeratosis, palmoplantar punctiform depressions, leukonychia, and longitudinal erythronychia with nail fragility. DD can also affect the oral cavity, presenting with symptoms such as macroglossia, gingival hyperplasia, leukokeratosis, and cobblestone-like papules [73,75,76]. Histologically, DD is characterized by suprabasal acantholysis and dyskeratotic keratinocytes (corp ronds) in the stratum spinosum and granulosum [73,75,76]. DD patients may experience comorbidities, such as neuropsychiatric disorders, fungal and bacterial infections, and potentially fatal herpes simplex virus reactivation [73,75,76].
7. Dermoscopy
Dermoscopy and reflectance confocal microscopy (RCM) are not usually used for the diagnosis of GGD; however dermoscopy and confocal findings have been described in a 48-year-old woman [56]. Dermoscopy revealed distinct features: one of the hyperkeratotic papules exhibited a central brown, mottled area surrounded by a whitish halo, while a lentigo-like macule displayed a peripheral pseudoreticular pattern with a central brown homogenous area.
To further investigate the condition, RCM imaging was performed [56]. RCM examination of the hyperkeratotic papule showed focal dark clefts with multiple bright, roundish cells at the epidermal level. RCM imaging of the lentigo-like macule revealed multiple, branched, deer antler-like highly refractile structures at the dermal–epidermal junction.
Epidermal dark clefts observed through RCM corresponded to suprabasal acantholysis, while round bright cells indicated dyskeratotic keratinocytes. Additionally, the characteristic elongated rete ridges observed in RCM correlated well with the bright, deer antler-like structures seen in confocal images and the lentigo-like dermoscopic presentation.
8. Therapy
Currently, there is a lack of established guidelines for the treatment of Galli–Galli disease (GGD), and limited evidence exists supporting the effectiveness of any specific treatment options [5,45].
However, certain measures can be recommended to patients, such as avoiding tight clothing that may cause irritation and sun protection to prevent the exacerbation of lesions [45].
Some therapeutic approaches, including vitamin A derivatives and laser therapy, have shown some efficacy [4,22]. Other conventional treatments, such as corticosteroids, emollients, calcineurin inhibitors, antihistamines, phototherapy, and antibiotics, have demonstrated limited efficacy [1,5,55].
Topical corticosteroids: Topical corticosteroids have generally been ineffective in most GGD cases, providing only occasional relief from itching and burning without significant clinical or histopathological improvement [19,31,57,78]. In some instances, the use of topical steroids has even resulted in worsened symptoms [6,34]. However, they are commonly used as an initial treatment during diagnostic investigations and in the early stages of the disease [1,5,19]. Commonly used topical steroids include clobetasol 17-propionate, betamethasone, and triamcinolone cream 0.1% [1,2,4,6,51]. Potential side effects of topical steroids include skin atrophy, telangiectasias, folliculitis, hypertrichosis, striae distensae cutis, and erythema.
Calcineurin inhibitors: Calcineurin inhibitors, such as tacrolimus and pimecrolimus, work at the molecular level by inhibiting cytokine transcription and T lymphocyte activation, thereby regulating the immune response [79,80]. Although there is only one reported case of initial improvement in GGD lesions with off-label use of these inhibitors, subsequent discontinuation was necessary due to cutaneous Candida spp. infections [49]. The side effects of calcineurin inhibitors can include folliculitis, furuncles, dermatitis, burning, irritation, itching, and intertrigo [80].
UVB phototherapy: UVB phototherapy, which utilizes ultraviolet radiation, has rarely been used in GGD due to its limited benefits and extensive side effects [44]. While it may provide some relief from itching, adverse reactions can include erythema, blistering, hyperpigmentation, skin photoaging, increased neoplastic risk, and even the development or exacerbation of GGD [2,19,44].
Laser therapy: Laser therapy, including the Er:YAG laser, pulsed light (IPL), and electrofluorescence, has shown equal efficacy in treating GGD by removing pathological tissue and promoting the regeneration of new epidermal tissue [22,52]. The Er:YAG laser is advantageous due to faster lesion resolution and fewer side effects, such as atrophic scarring and pigmentation changes [52]. An interesting case report from 2011 describes a 68-year-old patient who had chronic itching for 5 years, was diagnosed with GGD, and was treated with the Er:Yag laser at the axillary level in two successive sessions. After anesthesia with prilocaine, a laser with a spot size of 5 mm, a frequency of 8 Hertz, and a pulsed energy of 1200 millijoules was used with excellent results [22]. However, limited availability, high maintenance costs, and potential scarring and dyschromic outcomes in cases with extensive lesions may outweigh the benefits of laser therapy. Nevertheless, it currently represents the best local treatment option for GGD [10,22].
Systemic corticosteroids: Systemic corticosteroids, such as prednisone, have variable effects in GGD, ranging from partial resolution to worsening of lesions, and are typically used during disease flares [1,4]. Common side effects of systemic corticosteroids include hypertension, hyperglycemia, hypokalemia, osteoporosis, capillary fragility, cataracts, and psychiatric changes [81].
Vitamin A derivatives: Vitamin A derivatives, specifically retinoids like isotretinoin, alitretinoin, and acitretin, have shown promise as systemic treatments for GGD [5,82]. These retinoids regulate keratinocyte proliferation and differentiation by binding to the nuclear receptors all-trans retinoic acid receptor (RAR) and all-trans retinoid x receptor (RXR) [82]. Isotretinoin has demonstrated therapeutic effectiveness in treating GGD, with notable improvements observed in itching, rash resolution, reduced extent of rash, and improved erythema after just one month of treatment [51]. Similarly, alitretinoin has shown positive therapeutic outcomes, leading to decreased erosions, resolution of hyperpigmentation, and complete clinical response [6]. However, retinoids have numerous side effects, including facial erythema, pruritus, dermatitis, xerosis, joint pain, headache, fatigue, teratogenic effects, and potential psychiatric symptoms, such as anxiety, depression, and suicidal ideation [82,83].
Antibiotics: Antibiotics, particularly macrolides such as azithromycin, clarithromycin, and erythromycin, have rarely been used in GGD and are primarily employed in the treatment of reticulated pigmentary disorders of the skin [5,78].
Future therapies: Because steroids and retinoids are the first-line treatments for both GGD and Grover’s disease, it has been hypothesized that GGD might benefit from therapies that cause lesion regression in Grover’s disease, such as naltrexone and dupilumab. Naltrexone, a long-acting opioid receptor antagonist used to treat opioid and alcohol dependence [84], is utilized in dermatology at lower dosages (1–5 mg) for conditions often resistant to conventional treatments, e.g., psoriasis guttata, lichen planopilaris, HHD, and GD [84,85,86]. Low-dose naltrexone (LDN) has a shorter binding time and leads to a paradoxical increase in both ligands and receptors, resulting in pain relief and anti-inflammatory action [85]. It may also reduce proinflammatory cytokines and nitric oxide by binding to nonopioid receptors [73,84,85,86].
Dupilumab, a recombinant human IgG4 monoclonal antibody, inhibits the signal transduction of IL-4 and IL-13, which are cytokines involved in Th2-mediated inflammatory diseases [87]. Its off-label use has proven effective for Grover’s disease, which has a Th2-type inflammatory profile [88]. Using dupilumab as monotherapy at a loading dose of 600 mg for the first week, followed by a dose of 300 mg for each week to come for a total of 4 months, led to complete resolution of skin lesions and itching in three patients, suggesting the potential for treating acantholytic dermatoses like GGD [88]. Further research is needed to explore the efficacy of these emerging therapies for GGD and establish more definitive treatment guidelines.
9. Conclusions
GGD remains an intriguing and complex dermatological condition. While it manifests as an inflammatory acantholytic variant of Dowling-Degos disease (DDD), the two share significant clinical attributes, often leading to diagnostic overlaps. The genetic background of GGD is crucial, both for accurate diagnosis and understanding pathogenesis. Given recent advancements, the term “disseminated” is proposed as a more accurate descriptor than “atypical” for GGD phenotypes with diffuse involvement. More broadly, given the numerous overlaps, a proposal to combine GGD and DDD into a single disease entity deserves consideration.
Author Contributions
Conceptualization, A.M., A.G. and C.T.; methodology, A.M. and C.T.; validation, A.M., D.T. and C.T.; formal analysis, A.M.; investigation, A.M. and A.G.; resources, A.M., D.T. and C.T.; data curation, A.M., A.G., D.T. and C.T.; writing—original draft preparation, A.M.; writing—review and editing, A.M., D.T. and C.T.; supervision, C.T. All authors have read and agreed to the published version of the manuscript.
Funding
This research received no external funding.
Institutional Review Board Statement
Not applicable.
Informed Consent Statement
Patients in the study provided consent for publication.
Data Availability Statement
The data presented in this study are available in the literature.
Conflicts of Interest
The authors declare no conflicts of interest.
References
- Gilchrist, H.; Jackson, S.; Morse, L.; Nesbitt, L.T. Galli-Galli disease: A case report with review of the literature. J. Am. Acad. Dermatol. 2008, 58, 299–302. [Google Scholar] [CrossRef]
- Braun-Falco, M.; Volgger, W.; Borelli, S.; Ring, J.; Disch, R. Galli-Galli disease: An unrecognized entity or an acantholytic variant of Dowling-Degos disease? J. Am. Acad. Dermatol. 2001, 45, 760–763. [Google Scholar] [CrossRef]
- Bardach, H.; Gebhart, W.; Luger, T. Genodermatose bei einem Brüderpaar: Morbus Dowling-Degos, Grover, Darier, Hailey-Hailey oder Galli-Galli? [Genodermatosis in a pair of brothers: Dowling-Degos, Grover, Darier, Hailey-Hailey or Galli-Galli disease?]. Hautarzt 1982, 33, 378–383. [Google Scholar]
- Rundle, C.W.; Ophaug, S.; Simpson, E.L. Acitretin therapy for Galli-Galli disease. JAAD Case Rep. 2020, 6, 457–461. [Google Scholar] [CrossRef]
- Yang, A.; Cheung, K.; Kossard, S.; Murrell, D.F. Atypical Disseminated Variant of Galli–Galli Disease: A Review of the Literature. Am. J. Dermatopathol. 2020, 42, 484–490. [Google Scholar] [CrossRef]
- Deenen, N.J.; Damman, J.; Nijsten, T.E.C. Galli–Galli disease successfully treated with alitretinoin. J. Eur. Acad. Dermatol. Venereol. 2019, 33, e232–e233. [Google Scholar] [CrossRef]
- Hanneken, S.; Rütten, A.; Pasternack, S.M.; Eigelshoven, S.; El Shabrawi-Caelen, L.; Wenzel, J.; Braun-Falco, M.; Ruzicka, T.; Nöthen, M.M.; Kruse, R.; et al. Systematic mutation screening of KRT5 supports the hypothesis that Galli-Galli disease is a variant of Dowling-Degos disease. Br. J. Dermatol. 2010, 163, 197–200. [Google Scholar] [CrossRef]
- Reisenauer, A.K.; Wordingham, S.V.; York, J.; Kokkonen, E.W.J.; McLean, W.H.I.; Wilson, N.J.; Smith, F.J.D. Heterozygous frameshift mutation in keratin 5 in a family with Galli-Galli disease. Br. J. Dermatol. 2014, 170, 1362–1365. [Google Scholar] [CrossRef]
- Verma, S.; Pasternack, S.M.; Rutten, A.; Ruzicka, T.; Betz, R.C.; Hanneken, S. The first report of krt5 mutation underlying acantholytic dowling-degos disease with mottled hypopigmentation in an Indian family. Indian J. Dermatol. 2014, 59, 476–480. [Google Scholar] [CrossRef]
- Stephan, C.; Kurban, M.; Abbas, O. Dowling-Degos disease: A review. Int. J. Dermatol. 2021, 60, 944–950. [Google Scholar] [CrossRef]
- Kim, Y.C.; Davis, M.D.P.; Schanbacher, C.F.; Su, W.P.D. Dowling-Degos disease (reticulate pigmented anomaly of the flexures): A clinical and histopathologic study of 6 cases. J. Am. Acad. Dermatol. 1999, 40, 462–467. [Google Scholar] [CrossRef]
- Batycka-Baran, A.; Baran, W.; Hryncewicz-Gwozdz, A.; Burgdorf, W. Dowling-degos disease: Case report and review of the literature. Dermatology 2010, 220, 254–258. [Google Scholar] [CrossRef]
- Basmanav, F.B.; Oprisoreanu, A.M.; Pasternack, S.M.; Thiele, H.; Fritz, G.; Wenzel, J.; Größer, L.; Wehner, M.; Wolf, S.; Fagerberg, C.; et al. Mutations in POGLUT1, encoding protein o-glucosyltransferase 1, cause autosomal-dominant Dowling-Degos Disease. Am. J. Hum. Genet. 2014, 94, 135–143. [Google Scholar] [CrossRef]
- Betz, R.C.; Planko, L.; Eigelshoven, S.; Hanneken, S.; Pasternack, S.M.; Büssow, H.; Van Den Bogaert, K.; Wenzel, J.; Braun-Falco, M.; Rütten, A.; et al. Loss-of-function mutations in the keratin 5 gene lead to Dowling-Degos disease. Am. J. Hum. Genet. 2006, 78, 510–519. [Google Scholar] [CrossRef]
- Bouameur, J.E.; Favre, B.; Fontao, L.; Lingasamy, P.; Begré, N.; Borradori, L. Interaction of plectin with keratins 5 and 14: Dependence on several plectin domains and keratin quaternary structure. J. Investig. Dermatol. 2014, 134, 2776–2783. [Google Scholar] [CrossRef]
- Intong, L.R.A.; Murrell, D.F. Inherited epidermolysis bullosa: New diagnostic criteria and classification. Clin. Dermatol. 2012, 30, 70–77. [Google Scholar] [CrossRef]
- Müller, C.S.; Tremezaygues, L.; Pföhler, C.; Vogt, T. The spectrum of reticulate pigment disorders of the skin revisited. Eur. J. Dermatol. 2012, 22, 596–604. [Google Scholar] [CrossRef]
- Lőrincz, K.; Medvecz, M.; Kiss, N.; Glász-Bóna, A.; Hársing, J.; Lepesi-Benkő, R.; Hatvani, Z.; Mazán, M.; Kárpáti, S.; Wikonkál, N. Confirmation of the role of a KRT5 mutation and successful management of skin lesions in a patient with Galli–Galli disease. Clin. Exp. Dermatol. 2018, 43, 972–974. [Google Scholar] [CrossRef]
- El Shabrawi-Caelen, L.; Rütten, A.; Kerl, H. The expanding spectrum of Galli-Galli disease. J. Am. Acad. Dermatol. 2007, 56, S86–S91. [Google Scholar] [CrossRef]
- Sprecher, E.; Indelman, M.; Khamaysi, Z.; Lugassy, J.; Petronius, D.; Bergman, R. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease. Br. J. Dermatol. 2007, 156, 572–574. [Google Scholar] [CrossRef]
- Hanneken, S.; Rütten, A.; Eigelshoven, S.; Braun-Falco, M.; Pasternack, S.M.; Ruzicka, T.; Nöthen, M.M.; Betz, R.C.; Kruse, R. Klinische und histopathologische Untersuchung anhand einer Fallserie von 18 Patienten [Galli-Galli disease. Clinical and histopathological investigation using a case series of 18 patients]. Hautarzt 2011, 62, 842–851. [Google Scholar] [CrossRef]
- Voth, H.; Landsberg, J.; Reinhard, G.; Refke, M.; Betz, R.C.; Bieber, T.; Wenzel, J. Efficacy of Ablative Laser Treatment in Galli-Galli Disease. Arch. Dermatol. 2011, 147, 317. [Google Scholar] [CrossRef][Green Version]
- Arnold, A.W.; Kiritsi, D.; Happle, R.; Kohlhase, J.; Hausser, I.; Bruckner-Tuderman, L.; Has, C.; Itin, P.H. Type 1 segmental galli-galli disease resulting from a previously unreported keratin 5 mutation. J. Investig. Dermatol. 2012, 132, 2100–2103. [Google Scholar] [CrossRef]
- Schmieder, A.; Pasternack, S.M.; Krahl, D.; Betz, R.C.; Leverkus, M. Galli-Galli disease is an acantholytic variant of Dowling-Degos disease: Additional genetic evidence in a German family. J. Am. Acad. Dermatol. 2012, 66, e250–e251. [Google Scholar] [CrossRef]
- Wilson, N.J.; Cole, C.; Kroboth, K.; Hunter, W.N.; Mann, J.A.; McLean, W.H.I.; Kernland Lang, K.; Beltraminelli, H.; Sabroe, R.A.; Tiffin, N.; et al. Mutations in POGLUT1 in Galli–Galli/Dowling–Degos disease. Br. J. Dermatol. 2017, 176, 270–274. [Google Scholar] [CrossRef]
- Mehboob, M.Z.; Lang, M. Structure, function, and pathology of protein O-glucosyltransferases. Cell Death Dis. 2021, 12, 71. [Google Scholar] [CrossRef]
- Yu, H.; Takeuchi, H. Protein O-glucosylation: Another essential role of glucose in biology. Curr. Opin. Struct. Biol. 2019, 56, 64–71. [Google Scholar] [CrossRef]
- Li, Z.; Han, K.; Pak, J.E.; Satkunarajah, M.; Zhou, D.; Rini, J.M. Recognition of EGF-like domains by the Notch-modifying O-fucosyltransferase POFUT1. Nat. Chem. Biol. 2017, 13, 757–763. [Google Scholar] [CrossRef]
- Zhou, B.; Lin, W.; Long, Y.; Yang, Y.; Zhang, H.; Wu, K.; Chu, Q. Notch signaling pathway: Architecture, disease, and therapeutics. Signal Transduct. Target. Ther. 2022, 7, 95. [Google Scholar] [CrossRef]
- Kono, M.; Sawada, M.; Nakazawa, Y.; Ogi, T.; Muro, Y.; Akiyama, M. A Japanese case of galli-galli disease due to a previously unreported POGLUT1 mutation. Acta Derm. Venereol. 2019, 99, 458–459. [Google Scholar] [CrossRef]
- Seitz, A.T.; Sterz, H.; Strehlow, V.; Nagel, S.; Dumann, K.; Grunewald, S.; Simon, J.C.; Kunz, M. Full ablative versus fractional ablative laser therapy for Dowling–Degos disease. Lasers Surg. Med. 2018, 51, 321–324. [Google Scholar] [CrossRef]
- Bagaria, J.; Bagyinszky, E.; An, S.S.A. Genetics, Functions, and Clinical Impact of Presenilin-1 (PSEN1) Gene. Int. J. Mol. Sci. 2022, 23, 10970. [Google Scholar] [CrossRef]
- Pavlovsky, M.; Sarig, O.; Eskin-Schwartz, M.; Malchin, N.; Bochner, R.; Mohamad, J.; Gat, A.; Peled, A.; Hafner, A.; Sprecher, E. A phenotype combining hidradenitis suppurativa with Dowling–Degos disease caused by a founder mutation in PSENEN. Br. J. Dermatol. 2018, 178, 502–508. [Google Scholar] [CrossRef]
- Del Mar, M.; González, M.; Sayed, C.; Phadke, P. Hidradenitis Suppurativa Associated with Galli-Galli Disease: Extending the link with Dowling-Degos Disease. JCAD J. Clin. Aesthetic Dermatol. 2020, 13, 46–47. [Google Scholar]
- Wang, Z.; Yan, Y.; Wang, B. γ-Secretase Genetics of Hidradenitis Suppurativa: A Systematic Literature Review. Genet. Artic. Dermatol. 2021, 237, 698–704. [Google Scholar] [CrossRef]
- Wang, B.; Yang, W.; Wen, W.; Sun, J.; Su, B.; Liu, B.; Ma, D.; Lv, D.; Wen, Y.; Qu, T.; et al. γ-Secretase Gene Mutations in Familial Acne Inversa. Science 2010, 330, 1065. [Google Scholar] [CrossRef]
- Zhou, C.; Wen, G.D.; Soe, L.M.; Xu, H.J.; Du, J.; Zhang, J.Z. Novel mutations in PSENEN gene in two Chinese acne inversa families manifested as familial multiple comedones and dowling-degos disease. Chin. Med. J. 2016, 129, 2834–2839. [Google Scholar] [CrossRef]
- Ralser, D.J.; Basmanav, F.B.; Tafazzoli, A.; Wititsuwannakul, J.; Delker, S.; Danda, S.; Thiele, H.; Wolf, S.; Busch, M.; Pulimood, S.A.; et al. Mutations in γ-secretase subunit-encoding PSENEN underlie Dowling-Degos disease associated with acne inversa. J. Clin. Investig. 2017, 127, 1485–1490. [Google Scholar] [CrossRef]
- Weber, L.A.; Kantor, G.R.; Bergfeld, W.F. Reticulate Pigmented Anomaly of the Flexure (DDD): A case report associated with Hidradenitis Suppurativa and Squamous Cell Carcinoma. Cutis 1990, 45, 446–450. [Google Scholar]
- Pink, A.E.; Simpson, M.A.; Brice, G.W.; Smith, C.H.; Desai, N.; Mortimer, P.S.; Barker, J.N.W.N.; Trembath, R.C. PSENEN and NCSTN mutations in familial Hidradenitis suppurativa (Acne inversa). J. Investig. Dermatol. 2011, 131, 1568–1570. [Google Scholar] [CrossRef]
- Peter, D.C.V.; Smith, F.J.D.; Wilson, N.J.; Danda, S. PSENEN Mutation in Coexistent Hidradenitis Suppurativa and Dowling-Degos Disease. Indian Dermatol. Online J. 2020, 12, 147–149. [Google Scholar] [CrossRef]
- Mittag, H.; Rupec, M.; Klingmuller, G. Der Morbus Galli-Galli―eine Entität? Eine klinische, histologische und elektronenmikroskopische Untersuchung. Aktuelle Dermatol. 1986, 12, 41–46. [Google Scholar]
- Cooper, S.M.; Dhittavat, J.; Millard, P.; Burge, S. Extensive Grovers-like eruption with lentiginous «freckling»: Report of two cases. Br. J. Derm. 2004, 150, 350–352. [Google Scholar] [CrossRef]
- Desai, C.; Virmani, N.; Sakhiya, J.; Khopkar, U. An uncommon presentation of Galli-Galli disease. Indian J. Dermatol. Venereol. Leprol. 2016, 82, 720–723. [Google Scholar] [CrossRef]
- Joshi, T.P.; Shaver, S.; Tschen, J. Exacerbation of Galli-Galli Disease Following Dialysis Treatment: A Case Report and Review of Aggravating Factors. Cureus 2021, 13, e15401. [Google Scholar] [CrossRef]
- Rutten, A.; Strauss, T. Galli-Galli disease: A further case report. Aktuelle Derm. 1995, 21, 255–257. [Google Scholar]
- De Deene, A.; Schulze, H. Morbus Galli-Galli:eine variante des morbus Darier? Z. Haut. Geschlechtskr. 1996, 71, 860–862. [Google Scholar]
- Müller, C.S.L.; Pföhler, C.; Tilgen, W. Changing a concept—Controversy on the confusing spectrum of the reticulate pigmented disorders of the skin. J. Cutan. Pathol. 2009, 36, 44–48. [Google Scholar] [CrossRef]
- Mota, R.; Reifenberger, J.; Hanneken, S.; Mühlenstädt, E. Klassische und atypische Präsentation des Morbus Galli-Galli. Hautarzt 2010, 61, 284–286. [Google Scholar] [CrossRef]
- Rongioletti, F.; Fausti, V.; Christana, K.; Montinari, M.; Parodi, A.; Fiocca, R. Atypical variant of Galli-Galli disease (Grover-like eruption with lentiginous freckling) in a liver transplant patient. Am. J. Dermatopathol. 2011, 33, 504–507. [Google Scholar] [CrossRef]
- Dupuy, E.; Alexanian, S.; Hsiao, J. Galli-Galli disease responsive to isotretinoin treatment. Int. J. Dermatol. 2018, 57, 1123–1124. [Google Scholar] [CrossRef]
- Venning, V.L.; Choi-Lombardi, S.; Wong, X.L.; Cheung, K.; Sebaratnam, D.F. A comparison of physical modalities in Galli–Galli disease: Erbium: YAG laser, Intense Pulsed Light and Electrofulguration. Australas. J. Dermatol. 2019, 60, 320–322. [Google Scholar] [CrossRef]
- Wu, Y.-H.; Lin, Y.-C. Generalized Dowling-Degos disease. J. Am. Acad. Dermatol. 2007, 57, 327–334. [Google Scholar] [CrossRef]
- García-Salces, I.; Hörndler, C.; Requena, L. Galli-Galli Disease Presenting as Lichenoid Papules in the Flexures. Actas Dermo-Sifiliográficas 2010, 101, 168–172. [Google Scholar] [CrossRef]
- Gomes, J.; Labareda, J.; Viana, I. Galli-Galli disease: A rare acantholytic variant of dowling-degos disease. Case Rep. Med. 2011, 2011, 703257. [Google Scholar] [CrossRef]
- Coelho de Sousa, V.; El-Shabrawi-Caelen, L.; Mendes-Bastos, P.; Oliveira, A. Reflectance confocal microscopy for the diagnosis of Galli-Galli disease. Int. J. Dermatol. 2017, 56, 1501–1504. [Google Scholar] [CrossRef]
- Paolino, G.; Donati, M.; Didona, D.; Panetta, C.; Donati, P. Galli-galli disease presenting as a lentigo-like eruption: A further clinical feature in the wide spectrum of reticulate pigment disorders. Acta Dermatovenerol. Croat. 2017, 25, 300–302. [Google Scholar]
- Happle, R. Mosaicism in Human Skin—Understanding the Patterns and Mechanisms. Arch. Dermatol. 1993, 129, 1460–1470. [Google Scholar] [CrossRef]
- Dhitavat, J.; Fairclough, R.J.; Hovnanian, A.; Burge, S.M. Calcium pumps and keratinocytes: Lessons from Darier’s disease and Hailey-Hailey disease. Br. J. Dermatol. 2004, 150, 821–828. [Google Scholar] [CrossRef]
- Georgescu, E.F.; Stănescu, L.; Popescu, C.F.; Comănescu, M.G.I. Dowling-Degos disease. Rom. J. Morphol. Embryol. 2010, 51, 181–185. [Google Scholar]
- Piccolo, V.; Corneli, P.; Russo, T.; Danielsson, M.; Zalaudek, I.; Argenziano, G. Classic Dowling Degos disease: A rare genodermatosis. Ital. J. Dermatol. Venereol. 2022, 156, 71–72. [Google Scholar] [CrossRef]
- Kumar, S.; Borisov, O.; Maj, C.; Ralser, D.J.; Humbatova, A.; Hanneken, S.; Schmieder, A.; Groß, J.; Maintz, L.; Heineke, A.; et al. Founder Variants in KRT5 and POGLUT1 Are Implicated in Dowling-Degos Disease. J. Investig. Dermatol. 2024, 144, 181–184. [Google Scholar] [CrossRef]
- Aldana, P.C.; Khachemoune, A. Grover disease: Review of subtypes with a focus on management options. Int. J. Dermatol. 2019, 59, 543–550. [Google Scholar] [CrossRef]
- Quirk, C.J.; Heenan, P.J. Grover’s disease: 34 years on. Australas. J. Dermatol. 2004, 45, 83–88. [Google Scholar] [CrossRef]
- Davis, M.D.P.; Dinneen, A.M.; Landa, N.; Gibson, L.E. Grover’s Disease: Clinicopathologic Review of 72 Cases. Mayo Clin. Proc. 1999, 74, 229–234. [Google Scholar] [CrossRef]
- Weaver, J.; Bergfeld, W.F. Grover Disease (Transient Acantholytic Dermatosis). Arch. Pathol. Lab. Med. 2009, 133, 1490–1494. [Google Scholar] [CrossRef]
- Bellinato, F.; Maurelli, M.; Gisondi, P.; Girolomoni, G. Clinical features and treatments of transient acantholytic dermatosis (Grover’s disease): A systematic review. JDDG J. Dtsch. Dermatol. Ges. 2020, 18, 826–833. [Google Scholar] [CrossRef]
- Chalet, M. Transient acantholytic dermatosis: A reevaluation. Arch. Dermatol. 1977, 113, 431–435. [Google Scholar] [CrossRef]
- Fernández-Figueras, M.T.; Puig, L.; Cannata, P.; Cuatrecases, M.; Quer, A.; Ferrándiz, C.; Ariza, A. Grover disease: A reappraisal of histopathological diagnostic criteria in 120 cases. Am. J. Dermatopathol. 2010, 32, 541–549. [Google Scholar] [CrossRef]
- Ackerman, A.B.; Brunhoeber, P. Further references of Galli-Galli disease. J. Am. Acad. Dermatol. 2008, 59, 531–532. [Google Scholar] [CrossRef]
- Seli, D.; Ellis, K.T.; Goldust, M.; Shah, K.; Hu, R.; Zhou, J.; McNiff, J.M.; Choate, K.A. Association of Somatic ATP2A2 Damaging Variants with Grover Disease. JAMA Dermatol. 2023, 159, 745–749. [Google Scholar] [CrossRef]
- Asahina, A.; Ishiko, A.; Saito, I.; Hasegawa, K.; Sawamura, D.; Nakano, H. Grover’s Disease Following Multiple Bilateral Blaschko Lines: A Rare Clinical Presentation with Genetic and Electron Microscopic Analyses. Dermatology 2012, 225, 183–187. [Google Scholar] [CrossRef]
- Rogner, D.F.; Lammer, J.; Zink, A.; Hamm, H. Darier and Hailey-Hailey disease: Update 2021. JDDG J. Dtsch. Dermatol. Ges. 2021, 19, 1478–1501. [Google Scholar] [CrossRef]
- Takagi, A.; Kamijo, M.; Ikeda, S. Darier disease. J. Dermatol. 2016, 43, 275–279. [Google Scholar] [CrossRef]
- Strausburg, M.; Linos, K.; Staser, K.; Mousdicas, N. Dowling-Degos disease co-presenting with Darier disease. Clin. Exp. Dermatol. 2015, 41, 410–412. [Google Scholar] [CrossRef]
- Ho, J.; Bhawan, J. Mimickers of classic acantholytic diseases. J. Dermatol. 2017, 44, 232–242. [Google Scholar] [CrossRef]
- Patel, T.S.; Herrera-Martinez, M. Darier Disease. Mayo Clin. Proc. 2021, 96, 688–689. [Google Scholar] [CrossRef]
- Sardana, K.; Goel, K.; Chugh, S. Reticulate pigmentary disorders. Indian J. Dermatol. Venereol. Leprol. 2013, 79, 17. [Google Scholar] [CrossRef]
- Halloran, P.F. Mechanism of action of the calcineurin inhibitors. Transplant. Proc. 2001, 33, 3067–3069. [Google Scholar] [CrossRef]
- Van, Y.-V.R.; Wald, G.; Lu, C.; Samadi, A.; Wright, M.; Lara, D.; Marano, A.; Otterburn, D.M. The Effect of Topical Tacrolimus on Pedicled Flap Survival. Ann. Plast. Surg. 2020, 85, S118–S121. [Google Scholar] [CrossRef]
- Buchman, A.L. Side Effects of Corticosteroid Therapy. J. Clin. Gastroenterol. 2001, 33, 289–294. [Google Scholar] [CrossRef] [PubMed]
- Beckenbach, L.; Baron, J.M.; Merk, H.F.; Löffler, H.; Amann, P.M. Retinoid treatment of skin diseases. Eur. J. Dermatol. 2015, 25, 384–391. [Google Scholar] [CrossRef]
- David, M.; Hodak, E.; Lowe, N.J. Adverse Effects of Retinoids. Med. Toxicol. 1988, 3, 273–288. [Google Scholar] [CrossRef] [PubMed]
- Sikora, M.; Rakowska, A.; Olszewska, M.; Rudnicka, L. The Use of Naltrexone in Dermatology. Current Evidence and Future Directions. Curr. Drug Targets 2019, 20, 1058–1067. [Google Scholar] [CrossRef] [PubMed]
- Jaros, J.; Lio, P. Low Dose Naltrexone in Dermatology. J. Drugs Dermatol. 2019, 18, 235–238. [Google Scholar]
- Lee, B.; Elston, D.M. The uses of naltrexone in dermatologic conditions. J. Am. Acad. Dermatol. 2019, 80, 1746–1752. [Google Scholar] [CrossRef]
- Harb, H.; Chatila, T.A. Mechanisms of Dupilumab. Clin. Exp. Allergy 2020, 50, 5–14. [Google Scholar] [CrossRef]
- Butler, D.C.; Kollhoff, A.; Berger, T. Treatment of Grover Disease with Dupilumab. JAMA Dermatol. 2021, 157, 353. [Google Scholar] [CrossRef]
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).