The Histopathology of Leg Ulcers
Abstract
1. Introduction
2. Venous Leg Ulcers
2.1. Mixed Venous-Arterial Leg Ulcers
2.2. Livedoid Vasculopathy
3. Arterial Leg Ulcers
3.1. Occlusive-Ischemic
3.2. Ischemic Subcutaneous Arteriolosclerosis
3.3. Arteriolosclerotic Ulcer of Martorell
3.4. Calciphylaxis
3.5. Diabetic Leg Ulcers
4. Neuropathic Leg Ulcer
5. Inflammatory Leg Ulcers
5.1. Vasculitis
5.2. Pyoderma Gangrenosum
5.3. Necrobiosis Lipoidica
6. Decubitus
7. Hydroxyurea-Induced Ulcers
8. Ulcerating Skin Tumors
9. Other Causes
10. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Pannier, F.; Rabe, E. Differential diagnosis of leg ulcers. Phlebology 2013, 28 (Suppl. S1), 55–60. [Google Scholar] [CrossRef]
- Mekkes, J.; Loots, M.A.M.; Van Der Wal, A.C.; Bos, J.D. Causes, investigation and treatment of leg ulceration. Br. J. Dermatol. 2003, 148, 388–401. [Google Scholar] [CrossRef]
- Agale, S.V. Chronic Leg Ulcers: Epidemiology, Aetiopathogenesis, and Management. Ulcers 2013, 2013, 1–9. [Google Scholar] [CrossRef]
- Lautenschlager, S.; Eichmann, A. Differential diagnosis of leg ulcers. Curr. Probl. Dermatol. 1999, 27, 259–270. [Google Scholar] [CrossRef] [PubMed]
- Sen, C.K.; Gordillo, G.M.; Roy, S.; Kirsner, R.; Lambert, L.; Hunt, T.K.; Gottrup, F.; Gurtner, G.C.; Longaker, M.T. Human skin wounds: A major and snowballing threat to public health and the economy. Wound Repair Regen. 2009, 17, 763–771. [Google Scholar] [CrossRef]
- Nelzén, O.; Bergqvist, D.; Lindhagen, A. Venous and non-venous leg ulcers: Clinical history and appearance in a population study. Br. J. Surg. 1994, 81, 182–187. [Google Scholar] [CrossRef] [PubMed]
- Moffatt, C.J.; Franks, P.J.; Doherty, D.C.; Martin, R.; Blewett, R.; Ross, F. Prevalence of leg ulceration in a London population. QJM Mon. J. Assoc. Physicians 2004, 97, 431–437. [Google Scholar] [CrossRef] [PubMed]
- Liedberg, E.; Persson, B.M. Increased incidence of lower limb amputation for arterial occlusive disease. Acta Orthop. Scand. 1983, 54, 230–234. [Google Scholar] [CrossRef] [PubMed]
- Bello, Y.M.; Phillips, T.J. Management of venous ulcers. J. Cutan. Med. Surg. 1998, 3 (Suppl. S1), S1-6–12. [Google Scholar]
- Labropoulos, N.; Manalo, D.; Patel, N.P.; Tiongson, J.; Pryor, L.; Giannoukas, A.D. Uncommon leg ulcers in the lower extremity. J. Vasc. Surg. 2007, 45, 568–573.e2. [Google Scholar] [CrossRef]
- Gottrup, F.; Karlsmark, T. Leg ulcers: Uncommon presentations. Clin. Dermatol. 2005, 23, 601–611. [Google Scholar] [CrossRef]
- Körber, A.; Jockenhöfer, F.; Sondermann, W.; Stoffels-Weindorf, M.; Dissemond, J. First manifestation of leg ulcers: Analysis of data from 1000 patients. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2017, 68, 483–491. [Google Scholar] [CrossRef]
- Isoherranen, K.; O’Brien, J.J.; Barker, J.; Dissemond, J.; Hafner, J.; Jemec, G.B.E.; Kamarachev, J.; Läuchli, S.; Montero, E.C.; Nobbe, S.; et al. Atypical wounds. Best clinical practice and challenges. J. Wound Care 2019, 28 (Suppl. S6), S1–S92. [Google Scholar] [CrossRef]
- O’Donnell, T.F.; Passman, M.A.; Marston, W.A.; Ennis, W.J.; Dalsing, M.; Kistner, R.L.; Lurie, F.; Henke, P.K.; Gloviczki, M.L.; Eklöf, B.G.; et al. Management of venous leg ulcers: Clinical practice guidelines of the Society for Vascular Surgery® and the American Venous Forum. J. Vasc. Surg. 2014, 60, 3S–59S. [Google Scholar] [CrossRef]
- Bolton, L.L.; Girolami, S.; Corbett, L.; van Rijswijk, L. The Association for the Advancement of Wound Care (AAWC) venous and pressure ulcer guidelines. Ostomy. Wound Manag. 2014, 60, 24–66. [Google Scholar]
- Senet, P.; Combemale, P.; Debure, C.; Baudot, N.; Machet, L.; Aout, M.; Vicaut, E.; Lok, C. Angio-Dermatology Group Of The French Society Of Dermatology Malignancy and chronic leg ulcers: The value of systematic wound biopsies: A prospective, multicenter, cross-sectional study. Arch. Dermatol. 2012, 148, 704–708. [Google Scholar] [CrossRef] [PubMed]
- Misciali, C.; Dika, E.; Baraldi, C.; Fanti, P.A.; Mirelli, M.; Stella, A.; Bertoncelli, M.; Patrizi, A. Vascular leg ulcers: Histopathologic study of 293 patients. Am. J. Dermatopathol. 2014, 36, 977–983. [Google Scholar] [CrossRef] [PubMed]
- Maibach, H.I.; Shai, A. Wound Healing and Ulcers of the Skin; Springer: New York, NY, USA, 2005; ISBN 978-3-540-21275-1. [Google Scholar]
- Körber, A.; Schadendorf, D.; Dissemond, J. Causes of leg ulcers. Analysis of the data from a dermatologic wound care center. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2009, 60, 483–488. [Google Scholar] [CrossRef] [PubMed]
- Evans, C.J.; Fowkes, F.G.; Ruckley, C.V.; Lee, A.J. Prevalence of varicose veins and chronic venous insufficiency in men and women in the general population: Edinburgh Vein Study. J. Epidemiol. Community Health 1999, 53, 149–153. [Google Scholar] [CrossRef] [PubMed]
- Fritsch, P.; Schwarz, T. Dermatologie Venerologie: Grundlagen, Klinik, Atlas; 3. Vollständig Überarbeitete Auflage; Springer: Berlin/Heidelberg, Germany, 2018; 1217p, ISBN 978-3-662-53646-9. [Google Scholar]
- Eklöf, B.; Rutherford, R.B.; Bergan, J.J.; Carpentier, P.H.; Gloviczki, P.; Kistner, R.L.; Meissner, M.H.; Moneta, G.L.; Myers, K.; Padberg, F.T.; et al. Revision of the CEAP classification for chronic venous disorders: Consensus statement. J. Vasc. Surg. 2004, 40, 1248–1252. [Google Scholar] [CrossRef] [PubMed]
- Neumann, H.A.M.; Cornu-Thénard, A.; Jünger, M.; Mosti, G.; Munte, K.; Partsch, H.; Rabe, E.; Ramelet, A.A.; Streit, M. Evidence-based (S3) guidelines for diagnostics and treatment of venous leg ulcers. J. Eur. Acad. Dermatol. Venereol. JEADV 2016, 30, 1843–1875. [Google Scholar] [CrossRef]
- Scott, H.J.; Coleridge Smith, P.D.; Scurr, J.H. Histological study of white blood cells and their association with lipodermatosclerosis and venous ulceration. Br. J. Surg. 1991, 78, 210–211. [Google Scholar] [CrossRef] [PubMed]
- Wilkinson, L.S.; Bunker, C.; Edwards, J.C.; Scurr, J.H.; Smith, P.D. Leukocytes: Their role in the etiopathogenesis of skin damage in venous disease. J. Vasc. Surg. 1993, 17, 669–675. [Google Scholar] [CrossRef] [PubMed]
- Raffetto, J.D. Pathophysiology of wound healing and alterations in venous leg ulcers-review. Phlebology 2016, 31, 56–62. [Google Scholar] [CrossRef]
- Jünger, M.; Steins, A.; Hahn, M.; Häfner, H.M. Microcirculatory dysfunction in chronic venous insufficiency (CVI). Microcirculation 2000, 7 Pt 2, S3–S12. [Google Scholar] [CrossRef] [PubMed]
- Brem, H.; Stojadinovic, O.; Diegelmann, R.F.; Entero, H.; Lee, B.; Pastar, I.; Golinko, M.; Rosenberg, H.; Tomic-Canic, M. Molecular markers in patients with chronic wounds to guide surgical debridement. Mol. Med. 2007, 13, 30–39. [Google Scholar] [CrossRef]
- Herrick, S.E.; Sloan, P.; McGurk, M.; Freak, L.; McCollum, C.N.; Ferguson, M.W. Sequential changes in histologic pattern and extracellular matrix deposition during the healing of chronic venous ulcers. Am. J. Pathol. 1992, 141, 1085–1095. [Google Scholar]
- Grey, J.E.; Harding, K.G.; Enoch, S. Venous and arterial leg ulcers. BMJ 2006, 332, 347–350. [Google Scholar] [CrossRef] [PubMed]
- Heising, S.; Giebel, J.; Ostrowitzki, A.-L.; Riedel, F.; Haase, H.; Sippel, K.; Jünger, M. Evaluation of apoptotic cells and immunohistochemical detection of FAS, FAS-L, Bcl-2, Bax, p53 and c-Myc in the skin of patients with chronic venous leg ulcers. Int. J. Mol. Med. 2008, 22, 497–505. [Google Scholar] [CrossRef][Green Version]
- Burnand, K.G.; Whimster, I.; Clemenson, G.; Thomas, M.L.; Browse, N.L. The relationship between the number of capillaries in the skin of the venous ulcer-bearing area of the lower leg and the fall in foot vein pressure during exercise. Br. J. Surg. 1981, 68, 297–300. [Google Scholar] [CrossRef]
- Falanga, V.; Moosa, H.H.; Nemeth, A.J.; Alstadt, S.P.; Eaglstein, W.H. Dermal pericapillary fibrin in venous disease and venous ulceration. Arch. Dermatol. 1987, 123, 620–623. [Google Scholar] [CrossRef]
- Browse, N.L.; Burnand, K.G. The cause of venous ulceration. Lancet Lond. Engl. 1982, 2, 243–245. [Google Scholar] [CrossRef]
- Vanscheidt, W.; Laaff, H.; Wokalek, H.; Niedner, R.; Schöpf, E. Pericapillary fibrin cuff: A histological sign of venous leg ulceration. J. Cutan. Pathol. 1990, 17, 266–268. [Google Scholar] [CrossRef]
- Fernandez, A.P.; Miteva, M.; Roberts, B.; Ricotti, C.; Rouhani, P.; Romanelli, P. Histopathologic analysis of dermal lymphatic alterations in chronic venous insufficiency ulcers using D2-40. J. Am. Acad. Dermatol. 2011, 64, 1123.e1–1123.e12. [Google Scholar] [CrossRef]
- Burg, M.R.; Mitschang, C.; Goerge, T.; Schneider, S.W. Livedoid vasculopathy—A diagnostic and therapeutic challenge. Front. Med. 2022, 9, 1012178. [Google Scholar] [CrossRef] [PubMed]
- Goerge, T. Livedoid vasculopathy. Pathogenesis, diagnosis and treatment of cutaneous infarction. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 2011, 62, 627–634. [Google Scholar] [CrossRef]
- Criado, P.R.; Rivitti, E.A.; Sotto, M.N.; Valente, N.Y.S.; Aoki, V.; Carvalho, J.F.D.; Vasconcellos, C. Livedoid vasculopathy: An intringuing cutaneous disease. An. Bras. Dermatol. 2011, 86, 961–977. [Google Scholar] [CrossRef]
- Alavi, A.; Hafner, J.; Dutz, J.P.; Mayer, D.; Sibbald, R.G.; Criado, P.R.; Senet, P.; Callen, J.P.; Phillips, T.J.; Romanelli, M.; et al. Atrophie Blanche: Is It Associated with Venous Disease or Livedoid Vasculopathy? Adv. Skin Wound Care 2014, 27, 518–524. [Google Scholar] [CrossRef] [PubMed]
- Gray, H.R.; Graham, J.H.; Johnson, W.; Burgoon, C.F. Atrophie blanche: Periodic painful ulcers of lower extremities. A clinical and histopathological entity. Arch. Dermatol. 1966, 93, 187–193. [Google Scholar] [CrossRef] [PubMed]
- Gardette, E.; Moguelet, P.; Bouaziz, J.-D.; Lipsker, D.; Dereure, O.; Le Pelletier, F.; Lok, C.; Maisonobe, T.; Bessis, D.; Conard, J.; et al. Livedoid Vasculopathy: A French Observational Study Including Therapeutic Options. Acta Derm. Venereol. 2018, 98, 842–847. [Google Scholar] [CrossRef] [PubMed]
- Kerk, N.; Goerge, T. Livedoid vasculopathy—A thrombotic disease. VASA Z. Gefasskrankheiten 2013, 42, 317–322. [Google Scholar] [CrossRef]
- Reagin, H.; Marks, E.; Weis, S.; Susa, J. Livedoid Vasculopathy Presenting in a Patient With Sickle Cell Disease. Am. J. Dermatopathol. 2018, 40, 682–685. [Google Scholar] [CrossRef]
- Agirbasli, M.; Göktay, F.; Peker, I.; Gunes, P.; Aker, F.V.; Akkiprik, M. Enhanced mRNA expression of plasminogen activator inhibitor-1 in livedoid vasculopathy lesions. Cardiovasc. Ther. 2017, 35, e12255. [Google Scholar] [CrossRef]
- Becker, A.; Stoffels-Weindorf, M.; Schimming, T.; Dissemond, J. Recurrent leg ulcers due to livedoid vasculopathy: Successful treatment with low-molecular-weight heparin. Dtsch. Med. Wochenschr. 1946 2013, 138, 1458–1462. [Google Scholar] [CrossRef]
- Bard, J.W.; Winkelmann, R.K. Livedo vasculitis. Segmental hyalinizing vasculitis of the dermis. Arch. Dermatol. 1967, 96, 489–499. [Google Scholar] [CrossRef] [PubMed]
- Krishna, S.M.; Moxon, J.V.; Golledge, J. A review of the pathophysiology and potential biomarkers for peripheral artery disease. Int. J. Mol. Sci. 2015, 16, 11294–11322. [Google Scholar] [CrossRef] [PubMed]
- Hardman, R.L.; Jazaeri, O.; Yi, J.; Smith, M.; Gupta, R. Overview of classification systems in peripheral artery disease. Semin. Interv. Radiol. 2014, 31, 378–388. [Google Scholar] [CrossRef] [PubMed]
- Foley, T.R.; Armstrong, E.J.; Waldo, S.W. Contemporary evaluation and management of lower extremity peripheral artery disease. Heart Br. Card. Soc. 2016, 102, 1436–1441. [Google Scholar] [CrossRef] [PubMed]
- Sieggreen, M.Y.; Kline, R.A. Arterial insufficiency and ulceration: Diagnosis and treatment options. Adv. Skin Wound Care 2004, 17, 242–251. [Google Scholar] [CrossRef]
- Hafner, J.; Nobbe, S.; Partsch, H.; Läuchli, S.; Mayer, D.; Amann-Vesti, B.; Speich, R.; Schmid, C.; Burg, G.; French, L.E. Martorell hypertensive ischemic leg ulcer: A model of ischemic subcutaneous arteriolosclerosis. Arch. Dermatol. 2010, 146, 961–968. [Google Scholar] [CrossRef]
- Ong, S.; Coulson, I.H. Normo-renal calciphylaxis: Response to sodium thiosulfate. J. Am. Acad. Dermatol. 2011, 64, e82–e84. [Google Scholar] [CrossRef]
- Weber, B.; Deinsberger, J.; Hafner, J.; Beltraminelli, H.; Tzaneva, S.; Böhler, K. Localization-mapping of arteriolosclerotic ulcers of Martorell using two-dimensional computational rendering reveals a predominant location on the mid-lateral lower leg. J. Eur. Acad. Dermatol. Venereol. 2020, 35. [Google Scholar] [CrossRef]
- Martorell, F. Leg ulcers hypertonicum. Med. Klin. 1957, 52, 1945–1946. [Google Scholar]
- Hines, E.A.; Farber, E.M. Ulcer of the leg due to arteriolosclerosis and ischemia, occurring in the presence of hypertensive disease (hypertensive-ischemic ulcers). Proc. Staff Meet. Mayo Clin. 1946, 21, 337–346. [Google Scholar]
- Vuerstaek, J.D.D.; Reeder, S.W.I.; Henquet, C.J.M.; Neumann, H.A.M. Arteriolosclerotic ulcer of Martorell. J. Eur. Acad. Dermatol. Venereol. JEADV 2010, 24, 867–874. [Google Scholar] [CrossRef]
- Hafner, J. Calciphylaxis and Martorell Hypertensive Ischemic Leg Ulcer: Same Pattern—One Pathophysiology. Dermatology 2016, 232, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Dagregorio, G.; Guillet, G. A retrospective review of 20 hypertensive leg ulcers treated with mesh skin grafts. J. Eur. Acad. Dermatol. Venereol. 2006, 20, 166–169. [Google Scholar] [CrossRef]
- Pinto, A.P.F.L.; Silva, N.A., Jr.; Osorio, C.T.; Rivera, L.M.; Carneiro, S.; Ramos-e-Silva, M.; Bica, B.E.R.G. Martorell’s Ulcer: Diagnostic and Therapeutic Challenge. Case Rep. Dermatol. 2015, 7, 199–206. [Google Scholar] [CrossRef] [PubMed]
- De Andrés, J.; Villanueva, V.L.; Mazzinari, G.; Fabregat, G.; Asensio, J.M.; Monsalve, V. Use of a Spinal Cord Stimulator for Treatment of Martorell Hypertensive Ulcer. Reg. Anesth. Pain Med. 2011, 36, 83–86. [Google Scholar] [CrossRef]
- Giot, J.-P.; Paris, I.; Levillain, P.; Huguier, V.; Charreau, S.; Delwail, A.; Garcia, M.; Garnier, J.; Bernard, F.-X.; Dagregorio, G.; et al. Involvement of IL-1 and Oncostatin M in Acanthosis Associated With Hypertensive Leg Ulcer. Am. J. Pathol. 2013, 182, 806–818. [Google Scholar] [CrossRef] [PubMed]
- Vuerstaek, J.D. Reply. J. Vasc. Surg. 2007, 46, 615–616. [Google Scholar] [CrossRef]
- Leu, H.J. Hypertensive ischemic leg ulcer (Martorell’s ulcer): A specific disease entity? Int. Angiol. J. Int. Union Angiol. 1992, 11, 132–136. [Google Scholar]
- Kuiper, J.P.; Brakkee, A.J. Skin blood flow measurement in microangiopathic changes. Z. Hautkr. 1985, 60, 1495–1496, 1501–1505. [Google Scholar]
- Henderson, C.A.; Highet, A.S.; Lane, S.A.; Hall, R. Arterial hypertension causing leg ulcers. Clin. Exp. Dermatol. 1995, 20, 107–114. [Google Scholar] [CrossRef]
- Salcido, R.S. Enduring eponyms: The mystery of the Martorell ulcer. Adv. Skin Wound Care 2012, 25, 535. [Google Scholar] [CrossRef]
- Monfort, J.-B.; Cury, K.; Moguelet, P.; Chasset, F.; Bachmeyer, C.; Francès, C.; Barbaud, A.; Senet, P. Cutaneous Arteriolosclerosis Is Not Specific to Ischemic Hypertensive Leg Ulcers. Dermatology 2018, 234, 194–197. [Google Scholar] [CrossRef] [PubMed]
- Hayashi, M. Calciphylaxis: Diagnosis and clinical features. Clin. Exp. Nephrol. 2013, 17, 498–503. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Gallo, D.; Ossorio-García, L.; Linares-Barrios, M. Calcinosis Cutis and Calciphylaxis. Actas Dermosifiliogr. 2015, 106, 785–794. [Google Scholar] [CrossRef] [PubMed]
- Nigwekar, S.U.; Wolf, M.; Sterns, R.H.; Hix, J.K. Calciphylaxis from nonuremic causes: A systematic review. Clin. J. Am. Soc. Nephrol. CJASN 2008, 3, 1139–1143. [Google Scholar] [CrossRef]
- Sowers, K.M.; Hayden, M.R. Calcific uremic arteriolopathy: Pathophysiology, reactive oxygen species and therapeutic approaches. Oxid. Med. Cell. Longev. 2010, 3, 109–121. [Google Scholar] [CrossRef] [PubMed]
- Aihara, S.; Yamada, S.; Uchida, Y.; Arase, H.; Tsuchimoto, A.; Nakano, T.; Taniguchi, M.; Higashi, H.; Kitazono, T.; Tsuruya, K. The Successful Treatment of Calciphylaxis with Sodium Thiosulfate and Hyperbaric Oxygen in a Non-dialyzed Patient with Chronic Kidney Disease. Intern. Med. 2016, 55, 1899–1905. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Colboc, H.; Moguelet, P.; Bazin, D.; Carvalho, P.; Dillies, A.-S.; Chaby, G.; Maillard, H.; Kottler, D.; Goujon, E.; Jurus, C.; et al. Localization, Morphologic Features, and Chemical Composition of Calciphylaxis-Related Skin Deposits in Patients With Calcific Uremic Arteriolopathy. JAMA Dermatol. 2019, 155, 789. [Google Scholar] [CrossRef] [PubMed]
- LoGerfo, F.W.; Coffman, J.D. Current concepts. Vascular and microvascular disease of the foot in diabetes. Implications for foot care. N. Engl. J. Med. 1984, 311, 1615–1619. [Google Scholar] [CrossRef]
- dos Santos, V.P.; Caffaro, R.A.; Pozzan, G.; Saieg, M.A.; Castelli Júnior, V. Comparative histological study of atherosclerotic lesions and microvascular changes in amputated lower limbs of diabetic and non-diabetic patients. Arq. Bras. Endocrinol. Metabol. 2008, 52, 1115–1123. [Google Scholar] [CrossRef] [PubMed]
- Goldenberg, S.; Alex, M.; Joshi, R.A.; Blumenthal, H.T. Nonatheromatous peripheral vascular disease of the lower extremity in diabetes mellitus. Diabetes 1959, 8, 261–273. [Google Scholar] [CrossRef]
- Ferguson, M.W.J.; Herrick, S.E.; Spencer, M.-J.; Shaw, J.E.; Boulton, A.J.M.; Sloan, P. The Histology of Diabetic Foot Ulcers. Diabet. Med. 1996, 13, S30–S33. [Google Scholar] [CrossRef]
- Bowling, F.L.; Reeves, N.D.; Boulton, A.J. Gait-related strategies for the prevention of plantar ulcer development in the high risk foot. Curr. Diabetes Rev. 2011, 7, 159–163. [Google Scholar] [CrossRef]
- Piaggesi, A.; Viacava, P.; Rizzo, L.; Naccarato, G.; Baccetti, F.; Romanelli, M.; Zampa, V.; Del Prato, S. Semiquantitative analysis of the histopathological features of the neuropathic foot ulcer: Effects of pressure relief. Diabetes Care 2003, 26, 3123–3128. [Google Scholar] [CrossRef]
- Fahey, T.J.; Sadaty, A.; Jones, W.G.; Barber, A.; Smoller, B.; Shires, G.T. Diabetes impairs the late inflammatory response to wound healing. J. Surg. Res. 1991, 50, 308–313. [Google Scholar] [CrossRef] [PubMed]
- Jennette, J.C.; Falk, R.J.; Bacon, P.A.; Basu, N.; Cid, M.C.; Ferrario, F.; Flores-Suarez, L.F.; Gross, W.L.; Guillevin, L.; Hagen, E.C.; et al. 2012 Revised International Chapel Hill Consensus Conference Nomenclature of Vasculitides. Arthritis Rheum. 2013, 65, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Lotti, T.; Ghersetich, I.; Comacchi, C.; Jorizzo, J.L. Cutaneous small-vessel vasculitis. J. Am. Acad. Dermatol. 1998, 39, 667–687, quiz 688–690. [Google Scholar] [CrossRef] [PubMed]
- Marzano, A.V.; Balice, Y.; Papini, M.; Testa, R.; Berti, E.; Crosti, C. Localized Wegener’s granulomatosis: Localized WG. J. Eur. Acad. Dermatol. Venereol. 2011, 25, 1466–1470. [Google Scholar] [CrossRef] [PubMed]
- Nasir, N.; Ali, S.A.; Mehmood Riaz, H.M. Cutaneous Ulcers as Initial Presentation of Localized Granulomatosis with Polyangiitis: A Case Report and Review of the Literature. Case Rep. Rheumatol. 2015, 2015, 1–7. [Google Scholar] [CrossRef]
- Tashtoush, B.; Memarpour, R.; Johnston, Y.; Ramirez, J. Large pyoderma gangrenosum-like ulcers: A rare presentation of granulomatosis with polyangiitis. Case Rep. Rheumatol. 2014, 2014, 850364. [Google Scholar] [CrossRef]
- Daoud, M.S.; Gibson, L.E.; DeRemee, R.A.; Specks, U.; el-Azhary, R.A.; Daniel Su, W.P. Cutaneous Wegener’s granulomatosis: Clinical, histopathologic, and immunopathologic features of thirty patients. J. Am. Acad. Dermatol. 1994, 31, 605–612. [Google Scholar] [CrossRef]
- Barksdale, S.K.; Hallahan, C.W.; Kerr, G.S.; Fauci, A.S.; Stern, J.B.; Travis, W.D. Cutaneous pathology in Wegener’s granulomatosis. A clinicopathologic study of 75 biopsies in 46 patients. Am. J. Surg. Pathol. 1995, 19, 161–172. [Google Scholar] [CrossRef] [PubMed]
- Boudny, C.; Nievergelt, H.; Braathen, L.R.; Simon, D. Wegener’s granulomatosis presenting as pyoderma gangrenosum. J. Dtsch. Dermatol. Ges. J. Ger. Soc. Dermatol. JDDG 2008, 6, 477–479. [Google Scholar] [CrossRef]
- Genovese, G.; Tavecchio, S.; Berti, E.; Rongioletti, F.; Marzano, A.V. Pyoderma gangrenosum-like ulcerations in granulomatosis with polyangiitis: Two cases and literature review. Rheumatol. Int. 2018, 38, 1139–1151. [Google Scholar] [CrossRef]
- Marzano, A.V.; Vezzoli, P.; Berti, E. Skin involvement in cutaneous and systemic vasculitis. Autoimmun. Rev. 2013, 12, 467–476. [Google Scholar] [CrossRef]
- Marzano, A.V.; Raimondo, M.G.; Berti, E.; Meroni, P.L.; Ingegnoli, F. Cutaneous Manifestations of ANCA-Associated Small Vessels Vasculitis. Clin. Rev. Allergy Immunol. 2017, 53, 428–438. [Google Scholar] [CrossRef]
- Kawakami, T.; Soma, Y.; Saito, C.; Ogawa, H.; Nagahuchi, Y.; Okazaki, T.; Ozaki, S.; Mizoguchi, M. Cutaneous manifestations in patients with microscopic polyangiitis: Two case reports and a minireview. Acta Derm. Venereol. 2006, 86, 144–147. [Google Scholar] [CrossRef]
- Höfler, G.; Kreipe, H.H.; Moch, H. Pathologie: Das Lehrbuch: Mit 1.300 Meist Farbigen Abbildungen und rund 150 Tabellen; Elsevier: Amsterdam, The Netherlands, 2019; ISBN 978-3-437-42390-1. [Google Scholar]
- Carlson, J.A. The histological assessment of cutaneous vasculitis. Histopathology 2010, 56, 3–23. [Google Scholar] [CrossRef]
- Frumholtz, L.; Laurent-Roussel, S.; Lipsker, D.; Terrier, B. Cutaneous Vasculitis: Review on Diagnosis and Clinicopathologic Correlations. Clin. Rev. Allergy Immunol. 2020, 61, 181–193. Available online: http://link.springer.com/10.1007/s12016-020-08788-4 (accessed on 21 November 2020). [CrossRef]
- Carlson, J.A.; Chen, K.-R. Cutaneous vasculitis update: Small vessel neutrophilic vasculitis syndromes. Am. J. Dermatopathol. 2006, 28, 486–506. [Google Scholar] [CrossRef]
- Brooklyn, T.; Dunnill, G.; Probert, C. Diagnosis and treatment of pyoderma gangrenosum. BMJ 2006, 333, 181–184. [Google Scholar] [CrossRef] [PubMed]
- Ruocco, E.; Sangiuliano, S.; Gravina, A.; Miranda, A.; Nicoletti, G. Pyoderma gangrenosum: An updated review. J. Eur. Acad. Dermatol. Venereol. 2009, 23, 1008–1017. [Google Scholar] [CrossRef] [PubMed]
- Gameiro, A.; Pereira, N.; Cardoso, J.C.; Gonçalo, M. Pyoderma gangrenosum: Challenges and solutions. Clin. Cosmet. Investig. Dermatol. 2015, 8, 285–293. [Google Scholar] [CrossRef] [PubMed]
- Foessleitner, P.; Just, U.; Kiss, H.; Farr, A. Challenge of diagnosing pyoderma gangrenosum after caesarean section. BMJ Case Rep. 2019, 12, e230315. [Google Scholar] [CrossRef] [PubMed]
- Weenig, R.H.; Davis, M.D.P.; Dahl, P.R.; Su, W.P.D. Skin Ulcers Misdiagnosed as Pyoderma Gangrenosum. N. Engl. J. Med. 2002, 347, 1412–1418. [Google Scholar] [CrossRef] [PubMed]
- Su, W.P.; Schroeter, A.L.; Perry, H.O.; Powell, F.C. Histopathologic and immunopathologic study of pyoderma gangrenosum. J. Cutan. Pathol. 1986, 13, 323–330. [Google Scholar] [CrossRef]
- Bhat, R.M. Pyoderma gangrenosum: An update. Indian Dermatol. Online J. 2012, 3, 7–13. [Google Scholar] [CrossRef]
- Elder, D.E. (Ed.) Lever’s Histopathology of the Skin, 11th ed.; Wolters Kluwer: Philadelphia, PA, USA, 2015; ISBN 978-1-4511-9037-3. [Google Scholar]
- Schwaegerle, S.M.; Bergfeld, W.F.; Senitzer, D.; Tidrick, R.T. Pyoderma gangrenosum: A review. J. Am. Acad. Dermatol. 1988, 18, 559–568. [Google Scholar] [CrossRef] [PubMed]
- English, J.S.C.; Fenton, D.A.; Barth, J.; Gray, W.; Wilkinson, J.D. Pyoderma gangrenosum and leucocytoclastic vasculitis in association with rheumatoid arthritis-a report of two cases. Clin. Exp. Dermatol. 1984, 9, 270–276. [Google Scholar] [CrossRef] [PubMed]
- Wong, E.; Greaves, M.W. Pyoderma gangrenosum and leucocytoclastic vasculitis. Clin. Exp. Dermatol. 1985, 10, 68–72. [Google Scholar] [CrossRef] [PubMed]
- Sibbald, C.; Reid, S.; Alavi, A. Necrobiosis Lipoidica. Dermatol. Clin. 2015, 33, 343–360. [Google Scholar] [CrossRef]
- Tee, S.-I.; Chen, Q.P.; Lim, Y.L. Necrobiosis Lipoidica With Elastophagocytosis on an Unusual Location. Am. J. Dermatopathol. 2014, 36, 741–743. [Google Scholar] [CrossRef]
- Muller, S.A.; Winkelmann, R.K. Necrobiosis lipoidica diabeticorum. A clinical and pathological investigation of 171 cases. Arch. Dermatol. 1966, 93, 272–281. [Google Scholar] [CrossRef]
- Lepe, K.; Riley, C.A.; Salazar, F.J. Necrobiosis lipoidica. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2020. Available online: http://www.ncbi.nlm.nih.gov/books/NBK459318/ (accessed on 19 October 2020).
- Calonje, J.E.; Brenn, T.; Lazar, A.J.; McKee, P.H. Pathology of the Skin; Elsevier Health Sciences UK: London, UK, 2011; Available online: http://www.myilibrary.com?id=756554 (accessed on 19 October 2020).
- Eason, C.R.; Stovall, C.; Corley, S.B. Ulcerated indurated plaques on the upper extremities. JAAD Case Rep. 2019, 5, 749–751. [Google Scholar] [CrossRef]
- Yen, P.-S.; Wang, K.-H.; Chen, W.-Y.; Yang, Y.-W.; Ho, W.-T. The many faces of necrobiosis lipoidica: A report of three cases with histologic variations. Dermatol. Sin. 2011, 29, 67–71. [Google Scholar] [CrossRef]
- Anders, J.; Heinemann, A.; Leffmann, C.; Leutenegger, M.; Pröfener, F.; von Renteln-Kruse, W. Decubitus ulcers: Pathophysiology and primary prevention. Dtsch. Arzteblatt Int. 2010, 107, 371–381, quiz 382. [Google Scholar] [CrossRef]
- Vandeberg, J. Pressure (decubitus) ulcer: Variation in histopathology?A light and electron microscope study*1. Hum. Pathol. 1995, 26, 195–200. [Google Scholar] [CrossRef]
- Witkowski, J.A.; Parish, L.C. Histopathology of the decubitus ulcer. J. Am. Acad. Dermatol. 1982, 6, 1014–1021. [Google Scholar] [CrossRef]
- Best, P.J. Hydroxyurea-Induced Leg Ulceration in 14 Patients. Ann. Intern. Med. 1998, 128, 29. [Google Scholar] [CrossRef]
- Dissemond, J.; Körber, A. Hydroxyurea-induced ulcers on the leg. CMAJ Can. Med. Assoc. J. J. Assoc. Medicale Can. 2009, 180, 1132. [Google Scholar] [CrossRef]
- Goerge, T.; Schellong, G.; Mesters, R.M.; Berdel, W.E. Mimicry of hydroxyurea-induced leg ulcer by distal vena saphena parva insufficiency. Ann. Hematol. 2012, 91, 471–472. [Google Scholar] [CrossRef]
- Sirieix, M.-E.; Debure, C.; Baudot, N.; Dubertret, L.; Roux, M.-E.; Morel, P.; Frances, C.; Loubeyres, S.; Beylot, C.; Lambert, D.; et al. Leg Ulcers and Hydroxyurea: Forty-one Cases. Arch. Dermatol. 1999, 135, 818–820. Available online: http://archderm.jamanetwork.com/article.aspx?doi=10.1001/archderm.135.7.818 (accessed on 20 October 2020). [CrossRef]
- Kato, N.; Kimura, K.; Yasukawa, K.; Yoshida, K. Hydroxyurea-related leg ulcers in a patient with chronic myelogenous leukemia: A case report and review of the literature. J. Dermatol. 1999, 26, 56–62. [Google Scholar] [CrossRef] [PubMed]
- Quattrone, F.; Dini, V.; Barbanera, S.; Zerbinati, N.; Romanelli, M. Cutaneous ulcers associated with hydroxyurea therapy. J. Tissue Viability 2013, 22, 112–121. [Google Scholar] [CrossRef] [PubMed]
- Daoud, M.S.; Gibson, L.E.; Pittelkow, M.R. Hydroxyurea dermopathy: A unique lichenoid eruption complicating long-term therapy with hydroxyurea. J. Am. Acad. Dermatol. 1997, 36 Pt 1, 178–182. [Google Scholar] [CrossRef] [PubMed]
- Stahl, R.L.; Silber, R. Vasculitic leg ulcers in chronic myelogenous leukemia. Am. J. Med. 1985, 78, 869–872. [Google Scholar] [CrossRef] [PubMed]
- Harris, B.; Eaglstein, W.H.; Falanga, V. Basal cell carcinoma arising in venous ulcers and mimicking granulation tissue. J. Dermatol. Surg. Oncol. 1993, 19, 150–152. [Google Scholar] [CrossRef]
- Phillips, T.J.; Salman, S.M.; Rogers, G.S. Nonhealing leg ulcers: A manifestation of basal cell carcinoma. J. Am. Acad. Dermatol. 1991, 25 Pt 1, 47–49. [Google Scholar] [CrossRef] [PubMed]
- Blank, A.A.; Schnyder, U.W. Squamous cell carcinoma and basal cell carcinoma within the clinical picture of a chronic venous insufficiency in the third stage. Dermatologica 1990, 181, 248–250. [Google Scholar] [CrossRef] [PubMed]
- Lanehart, W.H.; Sanusi, I.D.; Misra, R.P.; O’Neal, B. Metastasizing basal cell carcinoma originating in a stasis ulcer in a black woman. Arch. Dermatol. 1983, 119, 587–591. [Google Scholar] [CrossRef] [PubMed]
- Steffen, C. Marjolin’s ulcer. Report of two cases and evidence that Marjolin did not describe cancer arising in scars of burns. Am. J. Dermatopathol. 1984, 6, 187–193. [Google Scholar] [CrossRef]
- Simmons, M.A.; Edwards, J.M.; Nigam, A. Marjolin’s ulcer presenting in the neck. J. Laryngol. Otol. 2000, 114, 980–982. [Google Scholar] [CrossRef]
- Riess, C. Neoplastic ulcus cruris. Spinocellular carcinoma of the left lower leg with lymph node metastases of the popliteal, inguinal and iliac areas. Hautarzt Z. Dermatol. Venerol. Verwandte Geb. 1989, 40, 592–593. [Google Scholar]
- Tchanque-Fossuo, C.N.; Millsop, J.W.; Johnson, M.A.; Dahle, S.E.; Isseroff, R.R. Ulcerated Basal Cell Carcinomas Masquerading as Venous Leg Ulcers. Adv. Skin Wound Care 2018, 31, 130–134. [Google Scholar] [CrossRef]
- Snyder, R.J.; Stillman, R.M.; Weiss, S.D. Epidermoid cancers that masquerade as venous ulcer disease. Ostomy. Wound Manag. 2003, 49, 63–66. [Google Scholar]
- Johnson, E.L.; Pierpont, Y.N.; Donate, G.; Hiro, M.H.; Mannari, R.J.; Strickland, T.J.; Robson, M.C.; Payne, W.G. Clinical challenge: Cutaneous Kaposi’s sarcoma of the lower extremity. Int. Wound J. 2011, 8, 163–168. [Google Scholar] [CrossRef]
- Boyce, D.E. Non-Hodgkins lymphoma masquerading as a chronic venous leg ulcer. Br. J. Plast. Surg. 1998, 51, 487. [Google Scholar] [CrossRef]
- González-Vela, M.C.; González-López, M.A.; Val-Bernal, J.F.; Mayorga, M.; Armesto, S.; Fernández-Llaca, H. Cutaneous diffuse large B-cell lymphoma of the leg mimicking a chronic venous ulcer. Eur. J. Dermatol. EJD 2007, 17, 92–93. [Google Scholar] [PubMed]
- Garbea, A.; Dippel, E.; Hildenbrand, R.; Bleyl, U.; Schadendorf, D.; Goerdt, S. Cutaneous large B-cell lymphoma of the leg masquerading as a chronic venous ulcer. Br. J. Dermatol. 2002, 146, 144–147. [Google Scholar] [CrossRef] [PubMed]
- Kamal, W.S.A.; Affandi, A.M.; Bhullar, A.; Kamal, W.S.Z. Relapsed lymphoma mimicking venous ulcer: A case report. Med. J. Malays. 2018, 73, 253–254. [Google Scholar]
- De Morentin, H.M.; Dodiuk-Gad, R.P.; Brenner, S. Klinefelter’s syndrome presenting with leg ulcers. Skinmed 2004, 3, 274–278. [Google Scholar] [CrossRef] [PubMed]
- Shanmugam, V.K.; Tsagaris, K.C.; Attinger, C.E. Leg ulcers associated with Klinefelter’s syndrome: A case report and review of the literature. Int. Wound J. 2012, 9, 104–107. [Google Scholar] [CrossRef] [PubMed]
- Zollner, T.M.; Veraart, J.C.; Wolter, M.; Hesse, S.; Villemur, B.; Wenke, A.; Werner, R.J.; Boehncke, W.H.; Jost, S.S.; Scharrer, I.; et al. Leg ulcers in Klinefelter’s syndrome—Further evidence for an involvement of plasminogen activator inhibitor-1. Br. J. Dermatol. 1997, 136, 341–344. [Google Scholar] [PubMed]
- Sen, C.K. Human Wounds and Its Burden: An Updated Compendium of Estimates. Adv. Wound Care 2019, 8, 39–48. [Google Scholar] [CrossRef]
- Simka, M.; Majewski, E. The Social and Economic Burden of Venous Leg Ulcers: Focus on the Role of Micronized Purified Flavonoid Fraction Adjuvant Therapy. Am. J. Clin. Dermatol. 2003, 4, 573–581. [Google Scholar] [CrossRef]
- Fu, X.; Sheng, Z.; Cherry, G.W.; Li, Q. Epidemiological study of chronic dermal ulcers in China. Wound Repair Regen. 1998, 6, 21–27. [Google Scholar] [CrossRef]
- Zeegelaar, J.E.; Stroïnk, A.C.; Steketee, W.H.; Faber, W.R.; van der Wal, A.C.; Komolafe, I.O.O.; Dzamalala, C.; Chibwana, C.; Wendte, J.F.; Zijlstra, E.E. Etiology and incidence of chronic ulcers in Blantyre, Malawi. Int. J. Dermatol. 2006, 45, 933–936. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, C.D.; Florell, S.R.; Bowen, A.R.; Presson, A.P.; Petersen, M.J. Histopathologic vasculitis from the periulcer edge: A retrospective cohort study. J. Am. Acad. Dermatol. 2019, 81, 1353–1357. [Google Scholar] [CrossRef] [PubMed]
- Izadi, K.; Ganchi, P. Chronic wounds. Clin. Plast. Surg. 2005, 32, 209–222. [Google Scholar] [CrossRef]
- Janowska, A.; Dini, V.; Oranges, T.; Iannone, M.; Loggini, B.; Romanelli, M. Atypical Ulcers: Diagnosis and Management. Clin. Interv. Aging 2019, 14, 2137–2143. [Google Scholar] [CrossRef]
- Elston, D.M.; Stratman, E.J.; Miller, S.J. Skin biopsy. J. Am. Acad. Dermatol. 2016, 74, 1–16. [Google Scholar] [CrossRef]
- Deinsberger, J.; Sirovina, S.; Bromberger, S.; Böhler, K.; Vychytil, A.; Meier-Schiesser, B.; Petzelbauer, P.; Beltraminelli, H.; Hafner, J.; Weber, B. Microstructural comparative analysis of calcification patterns in calciphylaxis versus arteriolosclerotic ulcer of Martorell. Eur. J. Dermatol. 2021, 31, 705–711. [Google Scholar] [CrossRef]

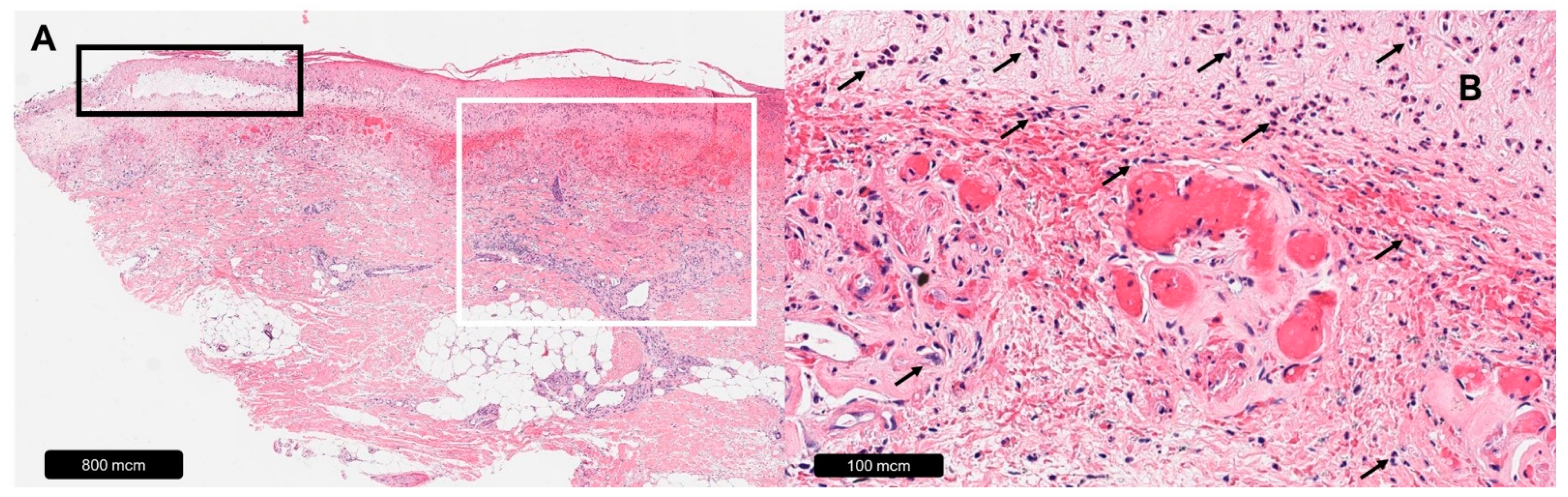
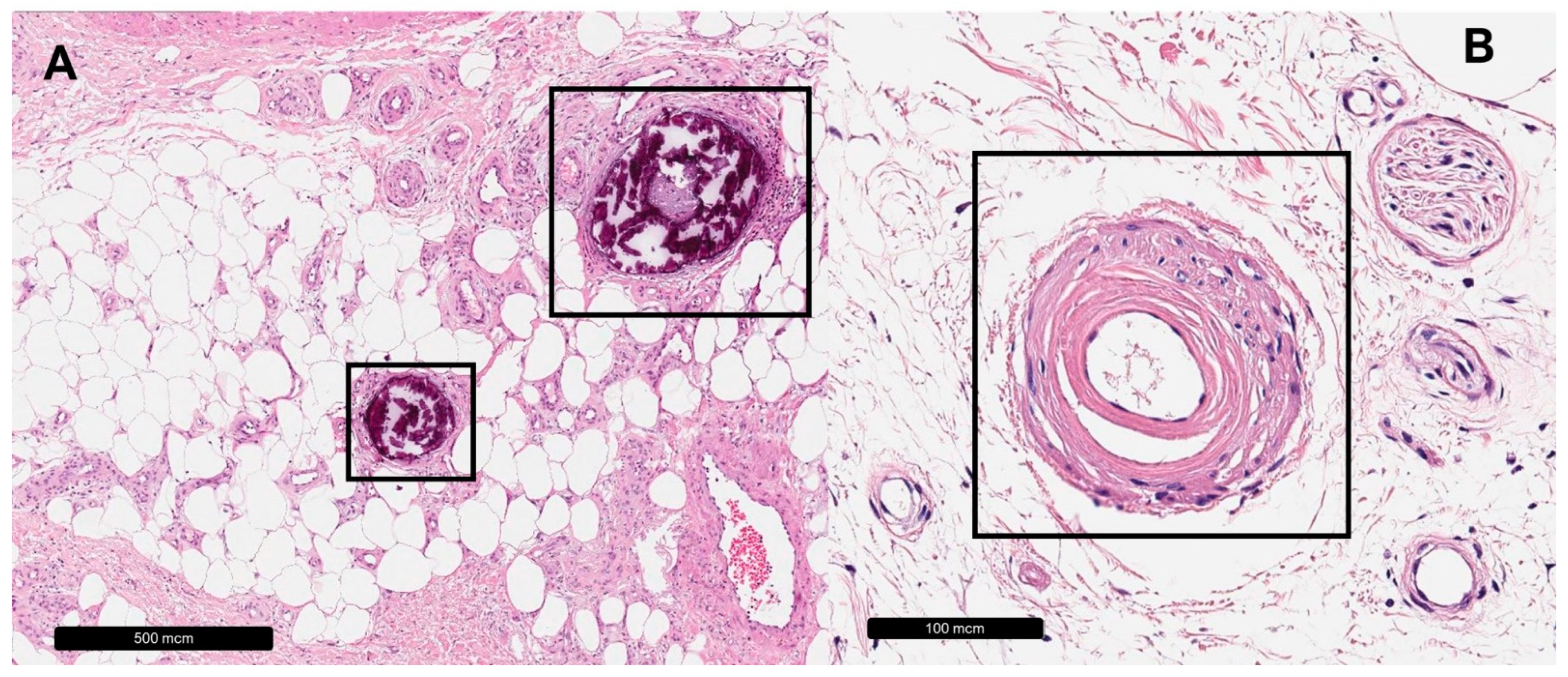
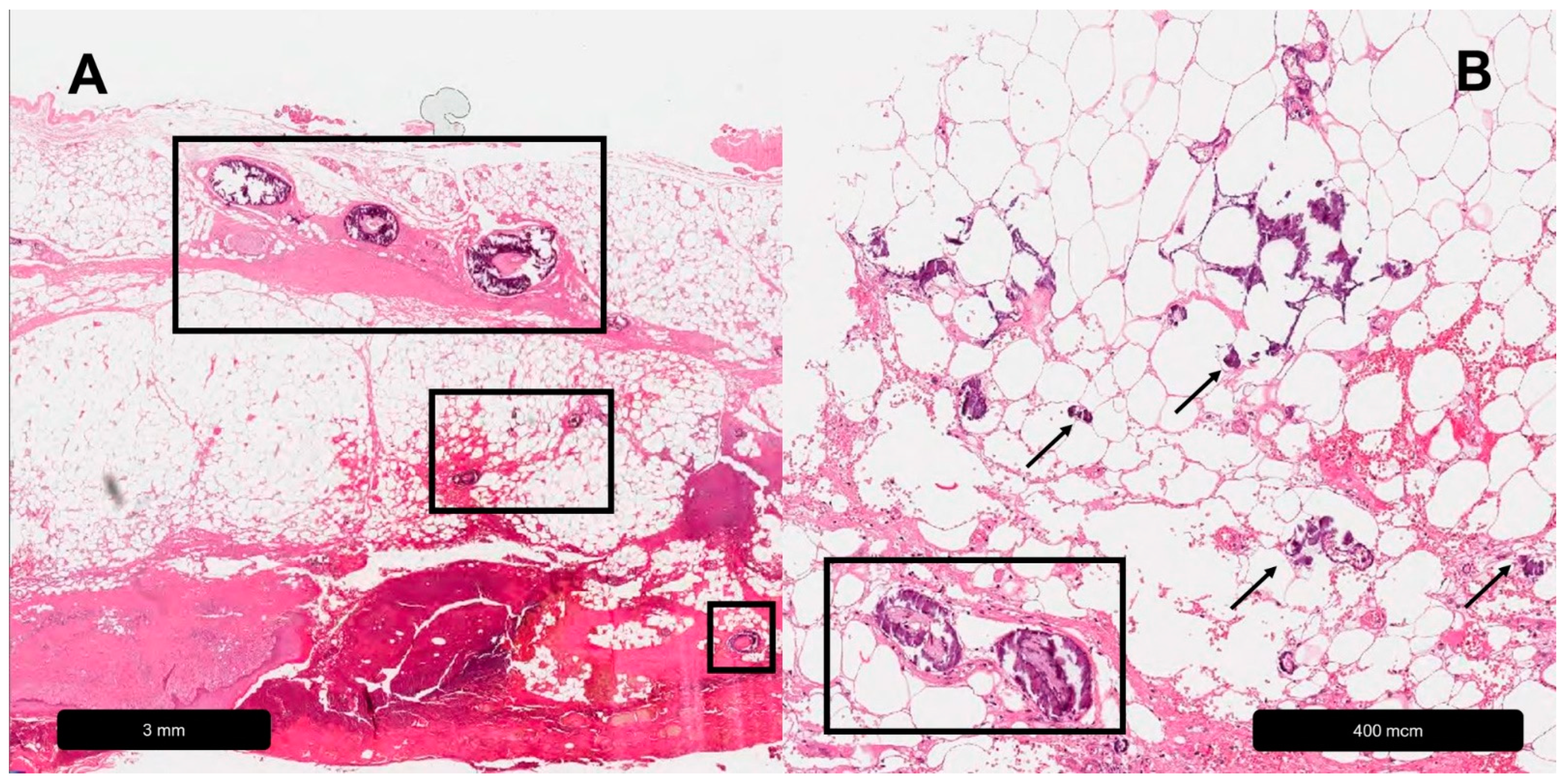
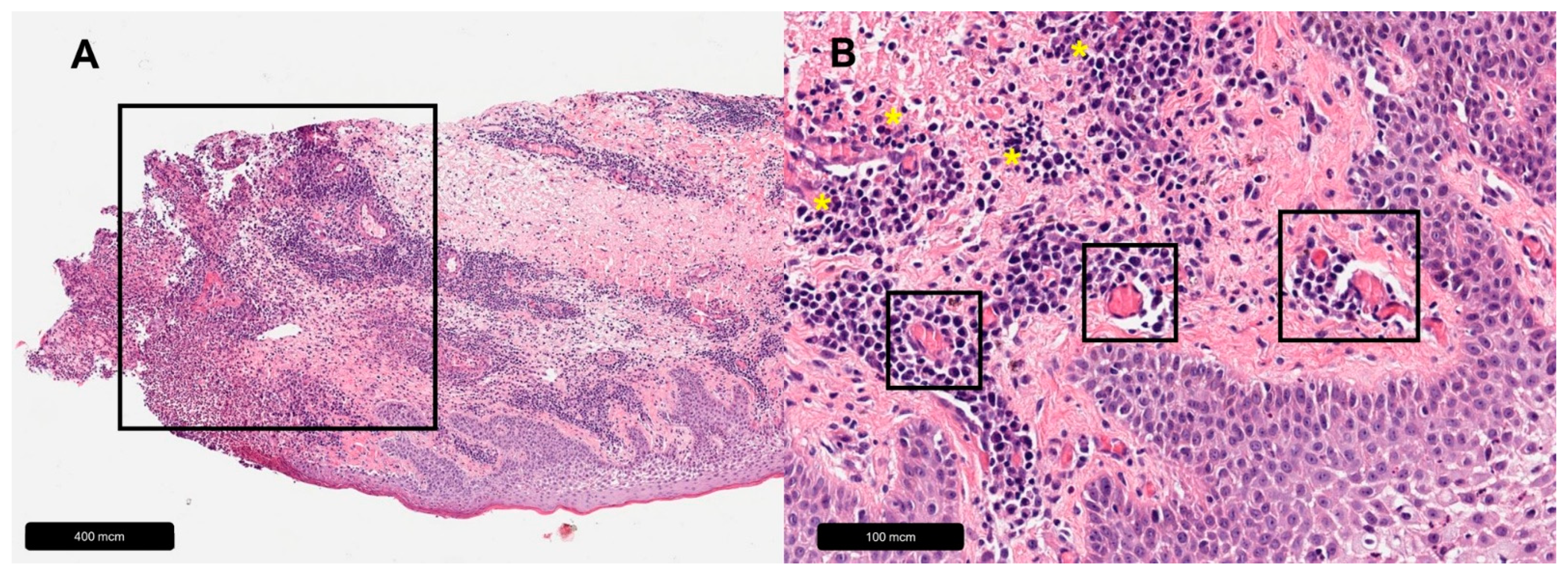
| Venous Leg Ulcer | |
|---|---|
| Location | Malleoli, pretibial |
| Specifics | Late chronic venous disease |
| Epidermis | Spongiosis |
| Hyperkeratosis | |
| Acanthosis | |
| Dermis | Inflammatory cell infiltration |
| Diffuse edema | |
| Hemosiderophages | |
| Dermal sclerosis | |
| Fibrosis | |
| Collagen bundle degeneration | |
| Hypodermis | Lipodermatosclerosis |
| Vessels | Fibrin cuffs |
| Reduced capillary density | |
| Dilated capillaries | |
| Erythrocyte extravasation | |
| Ectatic lymph vessels | |
| Livedoid Vasculopathy | |
|---|---|
| Location | Bilateral |
| Specifics | White scars |
| Epidermis | Spongiosis |
| Atrophy | |
| Dermis | Secondary inflammatory changes |
| Subpapillary plexus thrombosis | |
| Vessels | Endothelial edema |
| Wall thickening | |
| Fibrin thrombi | |
| Subintimal hyalinization | |
| Arterial-Ischemic Leg Ulcer | |
|---|---|
| Location | Toes, plantar |
| Specifics | Demarcated lesions |
| Epidermis | Necrosis |
| Epidermal thinning | |
| Dermis | Sclerosis |
| Necrosis | |
| Vessels | Thrombosis |
| Arteriolosclerotic Ulcer of Martorell | |
|---|---|
| Location | Lateral, lower leg |
| Specifics | Disproportional pain |
| Epidermis | Necrosis |
| Acanthosis | |
| Dermis | Necrosis |
| Vessels | Media hypertrophy |
| Stenosis | |
| Calcification | |
| Sub-endothelial hyalinosis | |
| Thrombosis | |
| Subintimal hyalinization | |
| Calciphylaxis | |
|---|---|
| Location | Lower leg |
| Specifics | Disproportional pain |
| Epidermis | Necrosis |
| Dermis | Necrosis |
| Calcium deposits | |
| Hypodermis | Diffuse calcification |
| Vessels | Fibrosis |
| Intima hyperplasia | |
| Media calcification | |
| Thrombosis | |
| Neuropathic Leg Ulcer | |
|---|---|
| Location | Plantar |
| Specifics | Diabetes-associated |
| Epidermis | Hyperkeratosis |
| Epidermal thinning | |
| Dermis | Leukocyte infiltration |
| Degraded extracellular matrix | |
| Cellular debris | |
| Fibrosis | |
| Necrosis | |
| Vessels | Wall thickening |
| Pyoderma Gangrenosum | |
|---|---|
| Location | Lower leg |
| Specifics | Deep necrotic ulcers |
| Epidermis | Necrosis |
| Dermis | Neutrophil infiltration |
| Vessels | PVLI |
| Endothelial swelling | |
| Secondary vasculitis | |
| Necrobiosis Lipoidica | |
|---|---|
| Location | Anterior, lower leg |
| Specifics | “Layered” histology |
| Epidermis | Necrosis |
| Dermis | Degenerated collagen |
| Histiocytes | |
| Multinucleated giant cells | |
| Leukocyte infiltration | |
| Decubitus | |
|---|---|
| Location | Heel, malleoli |
| Specifics | Histology type-dependent |
| Epidermis | - |
| Dermis | Fibrosis |
| Leukocyte infiltration | |
| Atypical fibroblasts | |
| Edema | |
| Vessels | PVLI |
| Occlusion | |
| Hydroxyurea-Induced Ulcers | |
|---|---|
| Location | Malleoli |
| Specifics | After hydroxyurea administration |
| Epidermis | Spongiosis |
| Atrophy | |
| Hyperplasia | |
| Dermis | Fibrosis |
| Vessels | Endothelial edema |
| Wall thickening | |
| Thrombosis | |
| PVLI | |
| Secondary vasculitis | |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2024 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Hofmann, A.G.; Deinsberger, J.; Oszwald, A.; Weber, B. The Histopathology of Leg Ulcers. Dermatopathology 2024, 11, 62-78. https://doi.org/10.3390/dermatopathology11010007
Hofmann AG, Deinsberger J, Oszwald A, Weber B. The Histopathology of Leg Ulcers. Dermatopathology. 2024; 11(1):62-78. https://doi.org/10.3390/dermatopathology11010007
Chicago/Turabian StyleHofmann, Amun Georg, Julia Deinsberger, André Oszwald, and Benedikt Weber. 2024. "The Histopathology of Leg Ulcers" Dermatopathology 11, no. 1: 62-78. https://doi.org/10.3390/dermatopathology11010007
APA StyleHofmann, A. G., Deinsberger, J., Oszwald, A., & Weber, B. (2024). The Histopathology of Leg Ulcers. Dermatopathology, 11(1), 62-78. https://doi.org/10.3390/dermatopathology11010007







