A Case of Pleomorphic Dermal Sarcoma: Giant Exophytic Tumor of the Medial Canthus
Abstract
:1. Introduction
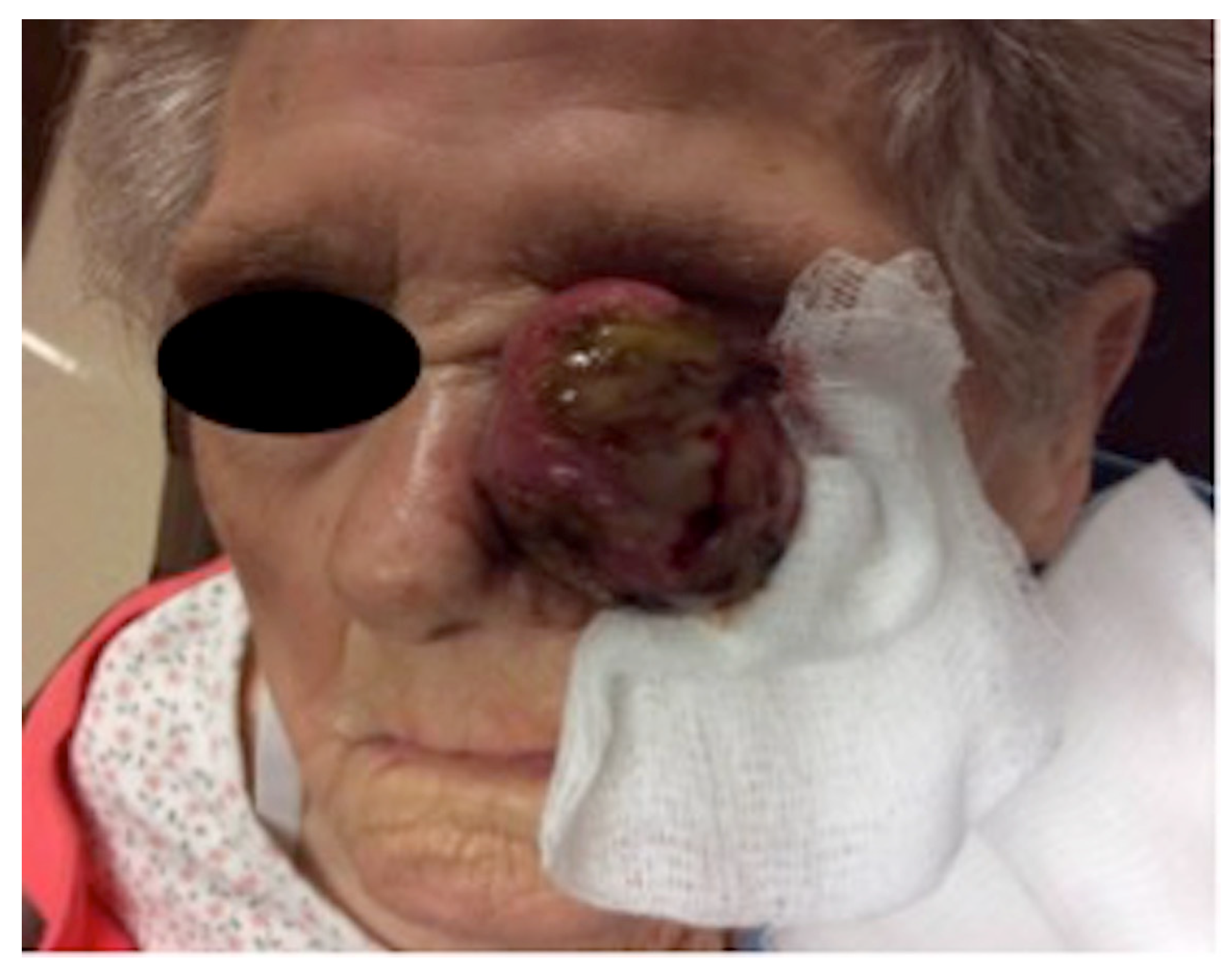
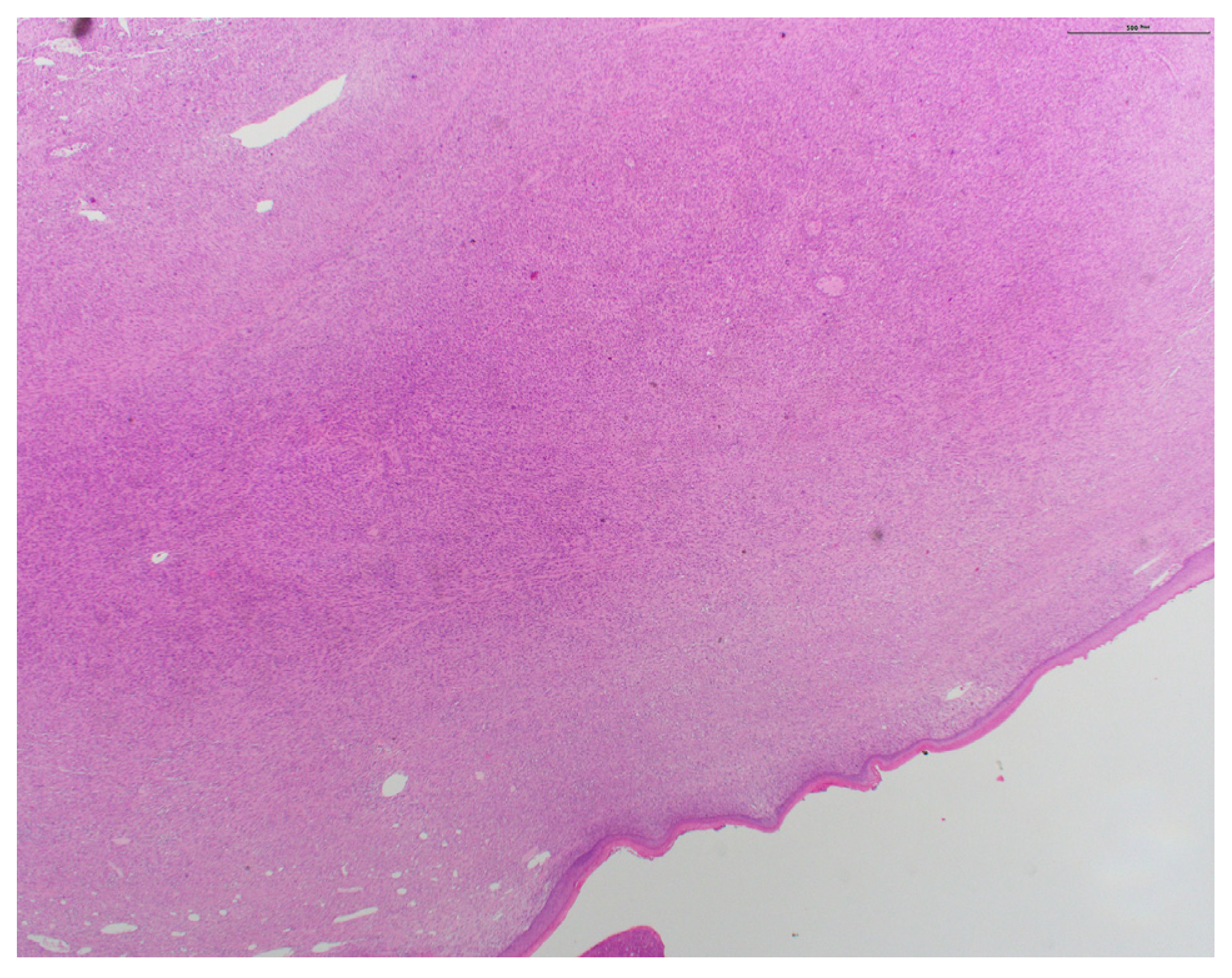
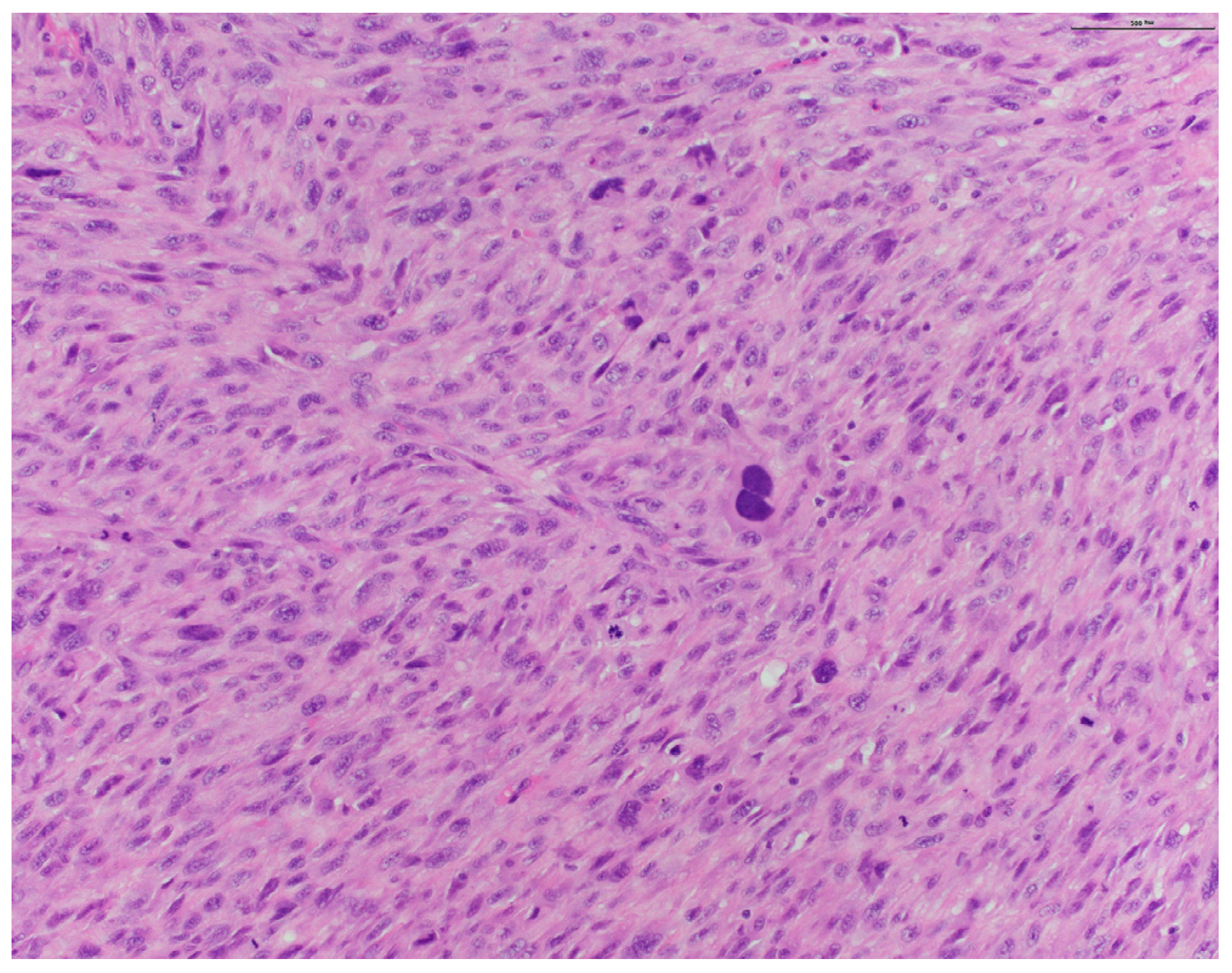
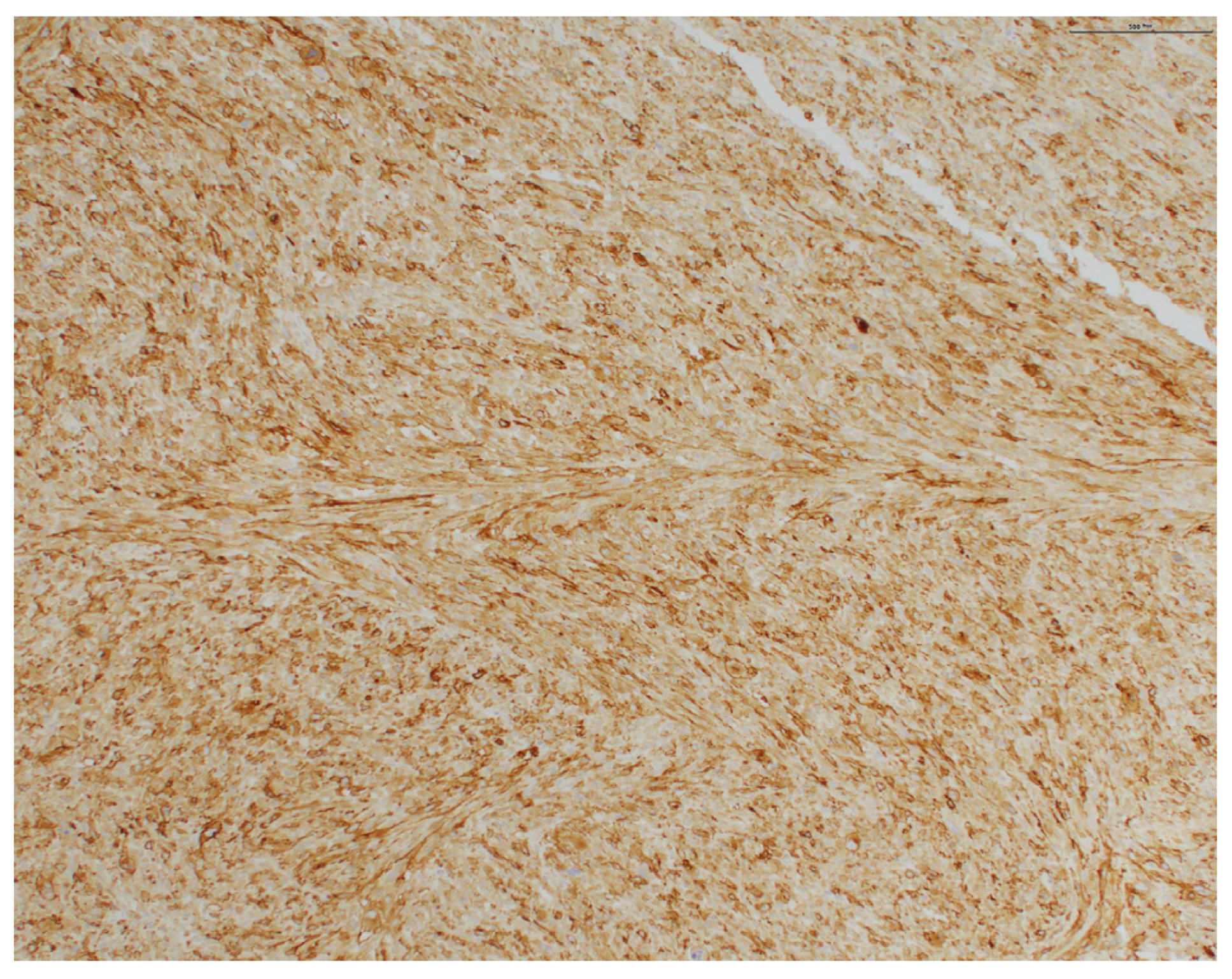
2. Case Presentation
3. Discussion
4. Conclusion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Soleymani, T.; Tyler, H.S. Conception and Management of a Poorly Understood Spectrum of Dermatologic Neoplasms: Atypical Fibroxanthoma, Pleomorphic Dermal Sarcoma, and Undifferentiated Pleomorphic Sarcoma. Curr. Treat. Options Oncol. 2017, 18, 50. [Google Scholar] [CrossRef] [PubMed]
- Miller, K.; Goodlad, J.R.; Brenn, T. Pleomorphic dermal sarcoma: Adverse histologic features predict aggressive behavior and allow distinction from atypical fibroxanthoma. Am. J. Surg. Pathol. 2012, 36, 1317–1326. [Google Scholar] [CrossRef]
- Tardío, J.C.; Pinedo, F.; Aramburu, J.A.; Suárez-Massa, D.; Pampín, A.; Requena, L.; Santonja, C. Pleomorphic dermal sarcoma: A more aggressive neoplasm than previously estimated. J. Cutan. Pathol. 2016, 43, 101–112. [Google Scholar] [CrossRef] [PubMed]
- Patterson, J.W.; Hosler, G.A. Tumors of muscle, cartilage, and bone. In Weedon’s Skin Pathology, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2016; pp. 1029–1040. [Google Scholar]
- Kim, J.-I.; Choi, Y.-J.; Seo, H.-M.; Kim, H.-S.; Lim, J.Y.; Kim, D.-H.; Chae, S.W.; Lee, G.-Y.; Kim, W.-S. Case of Pleomorphic Dermal Sarcoma of the Eyelid Treated with Micrographic Surgery and Secondary Intention Healing. Ann. Dermatol. 2016, 28, 632–636. [Google Scholar] [CrossRef]
- O’brien, J.E.; Stout, A.P. Malignant fibrous xanthomas. Cancer 1964, 17, 1445–1455. [Google Scholar] [CrossRef]
- Logan, I.T.; Vroobel, K.M.; le Grange, F.; Perrett, C.M. Pleomorphic dermal sarcoma: Clinicopathological features and outcomes from a 5-year tertiary referral centre experience. Cancer Rep. 2021, 5, e1583. [Google Scholar] [CrossRef] [PubMed]
- Janz, T.A.; Long, B.D.; Joshi, R.R.; Coblens, O.M. Survival differences of low-grade versus high-grade head and neck pleomorphic dermal sarcomas and a review of a scalp case. World J. Otorhinolaryngol. Head Neck Surg. 2022, 9, 74–78. [Google Scholar] [CrossRef] [PubMed]
- Helbig, D.; Klein, S. Immune checkpoint inhibitors for unresectable or metastatic pleomorphic dermal sarcomas. Front. Oncol. 2022, 12, 975342. [Google Scholar] [CrossRef] [PubMed]
- Ang, G.C.; Roenigk, R.K.; Otley, C.C.; Phillips, K.P.; Weaver, A.L. More Than 2 Decades of Treating Atypical Fibroxanthoma at Mayo Clinic: What have we learned from 91 patients? Dermatol. Surg. 2009, 35, 765–772. [Google Scholar] [CrossRef] [PubMed]
- Meyer, S.N.; Ren, Y.; Taylor, S.; Kiuru, M.; Eisen, D.B. Mohs micrographic surgery versus wide local excision for the treatment of atypical fibroxanthoma: A retrospective cohort analysis. JAAD Int. 2023, 12, 174–176. [Google Scholar] [CrossRef] [PubMed]

Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Moody, R.; Darji, K.; Missall, T.A.; Chow, P.; Behshad, R. A Case of Pleomorphic Dermal Sarcoma: Giant Exophytic Tumor of the Medial Canthus. Dermatopathology 2024, 11, 13-18. https://doi.org/10.3390/dermatopathology11010003
Moody R, Darji K, Missall TA, Chow P, Behshad R. A Case of Pleomorphic Dermal Sarcoma: Giant Exophytic Tumor of the Medial Canthus. Dermatopathology. 2024; 11(1):13-18. https://doi.org/10.3390/dermatopathology11010003
Chicago/Turabian StyleMoody, Rylee, Kavita Darji, Tricia A. Missall, Peter Chow, and Ramona Behshad. 2024. "A Case of Pleomorphic Dermal Sarcoma: Giant Exophytic Tumor of the Medial Canthus" Dermatopathology 11, no. 1: 13-18. https://doi.org/10.3390/dermatopathology11010003
APA StyleMoody, R., Darji, K., Missall, T. A., Chow, P., & Behshad, R. (2024). A Case of Pleomorphic Dermal Sarcoma: Giant Exophytic Tumor of the Medial Canthus. Dermatopathology, 11(1), 13-18. https://doi.org/10.3390/dermatopathology11010003




