Epibiont Cohabitation in Freshwater Shrimp Neocaridina davidi with the Description of Two Species New to Science, Cladogonium kumaki sp. nov. and Monodiscus kumaki sp. nov., and Redescription of Scutariella japonica and Holtodrilus truncatus †
Abstract
Simple Summary
Abstract
1. Introduction
2. Materials and Methods
2.1. Preliminary Study
2.2. Animals Collection from the Aquarium Trade
2.3. Epibiont Detection and Identification
2.4. Data Analysis
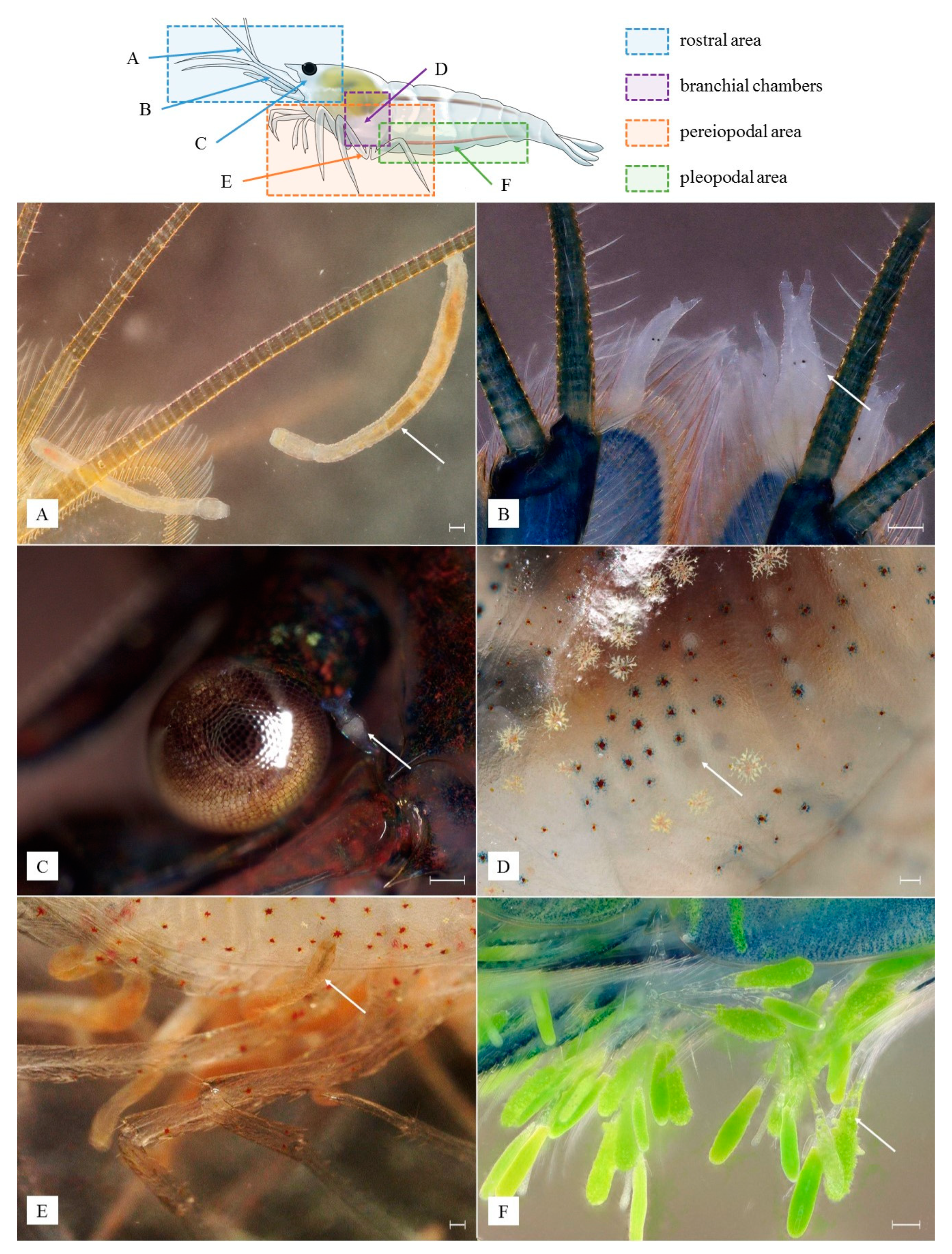
3. Results
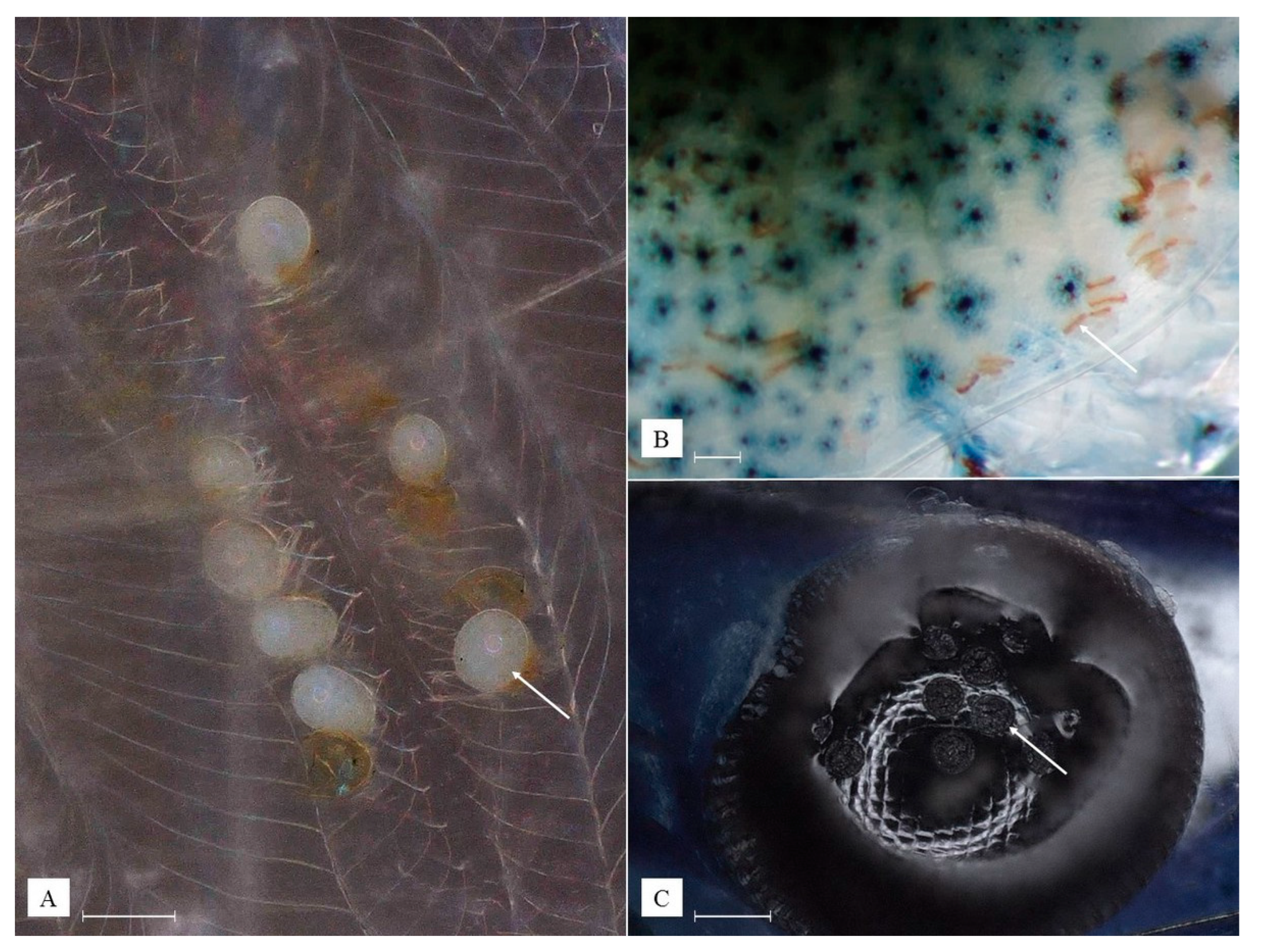
3.1. Identified Epibionts
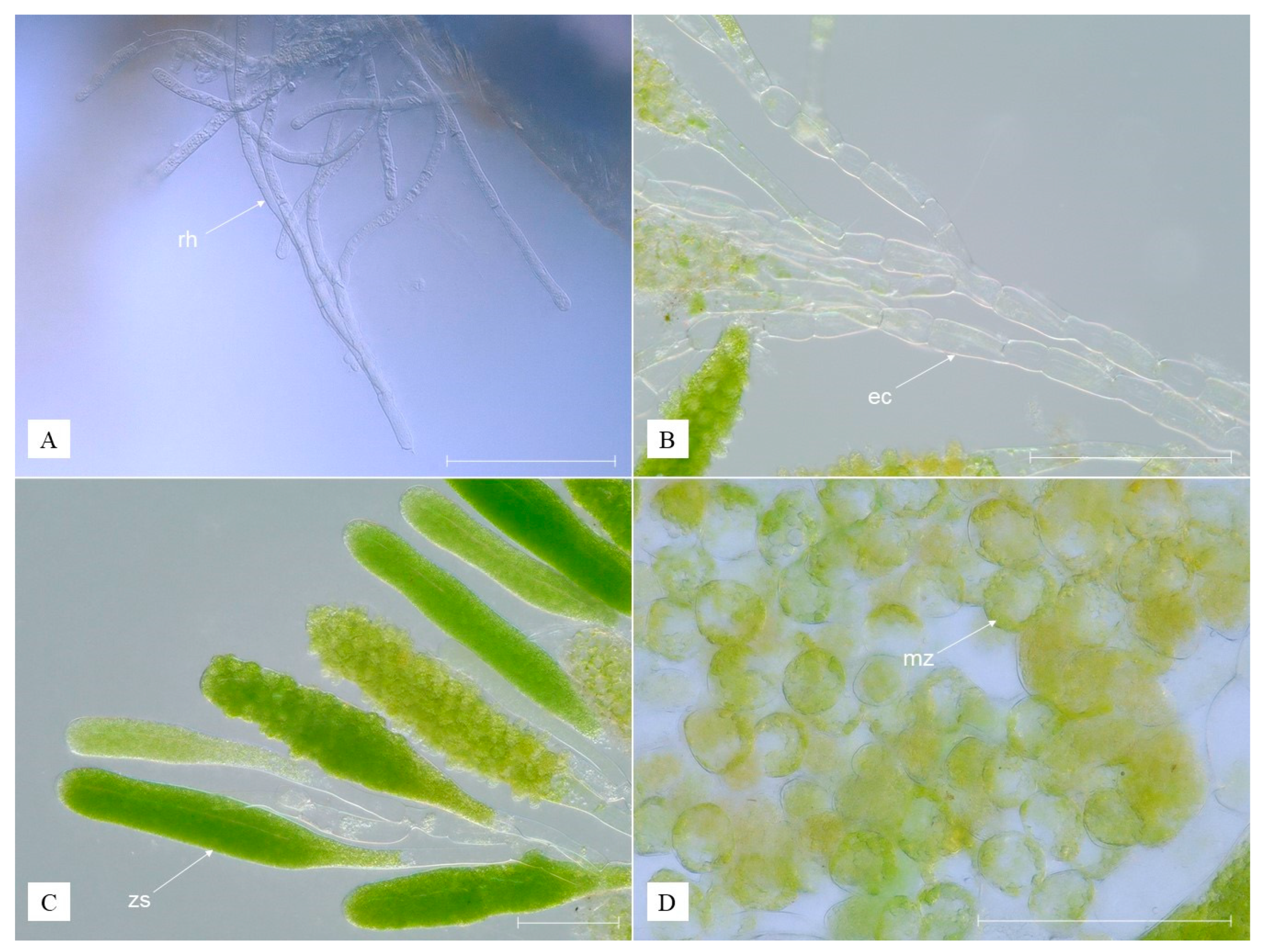
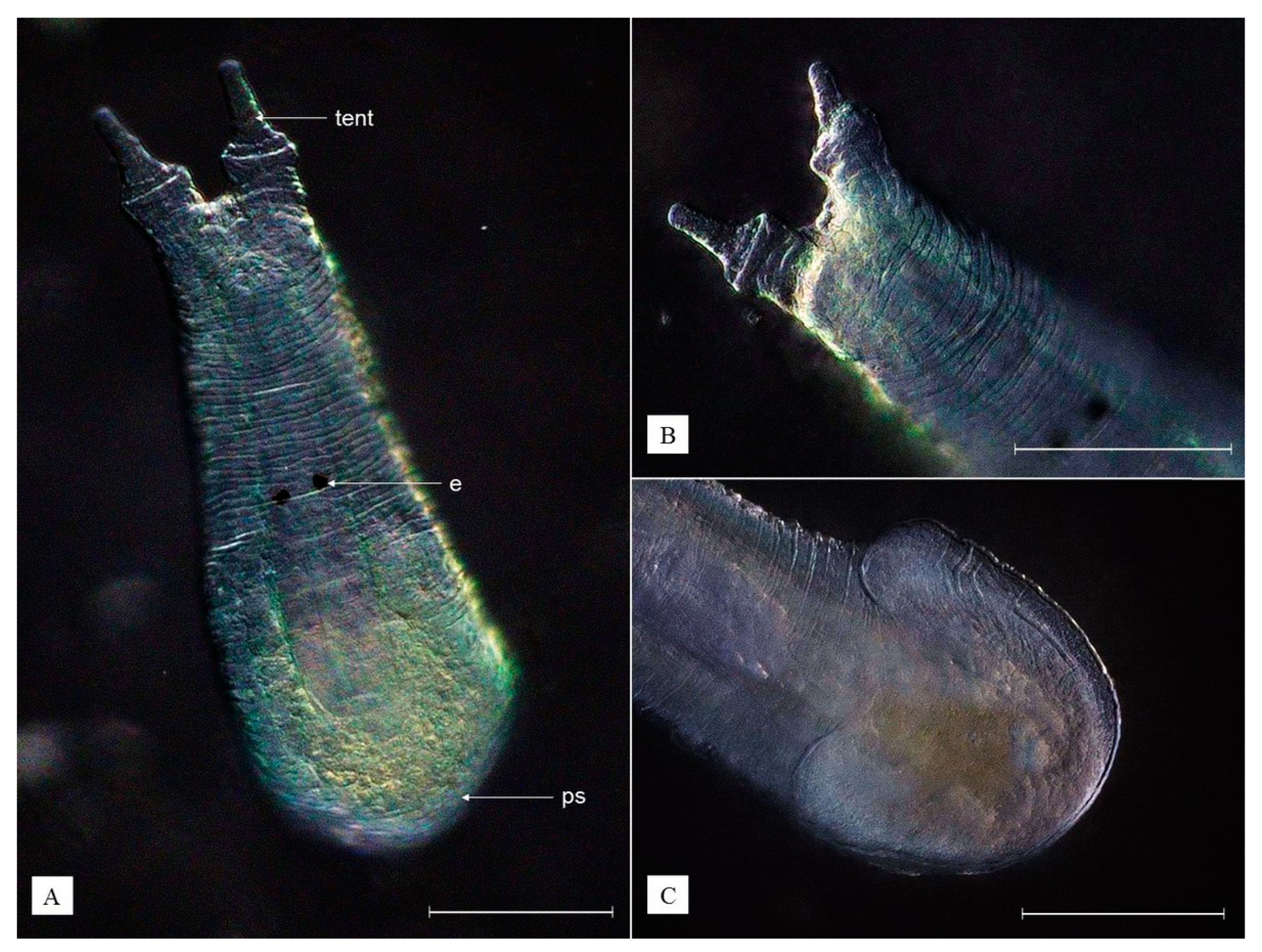
3.2. Description of Species New to Science
- Kingdom Plantae
- Phylum Chlorophyta
- Class Ulvophyceae
- Order Cladophorales
- Family Pithophoraceae
- Kingdom Animalia
- Phylum Platyhelminthes
- Class Rhabditophora
- Order Rhabdocoela
- Family Temnocephalidae
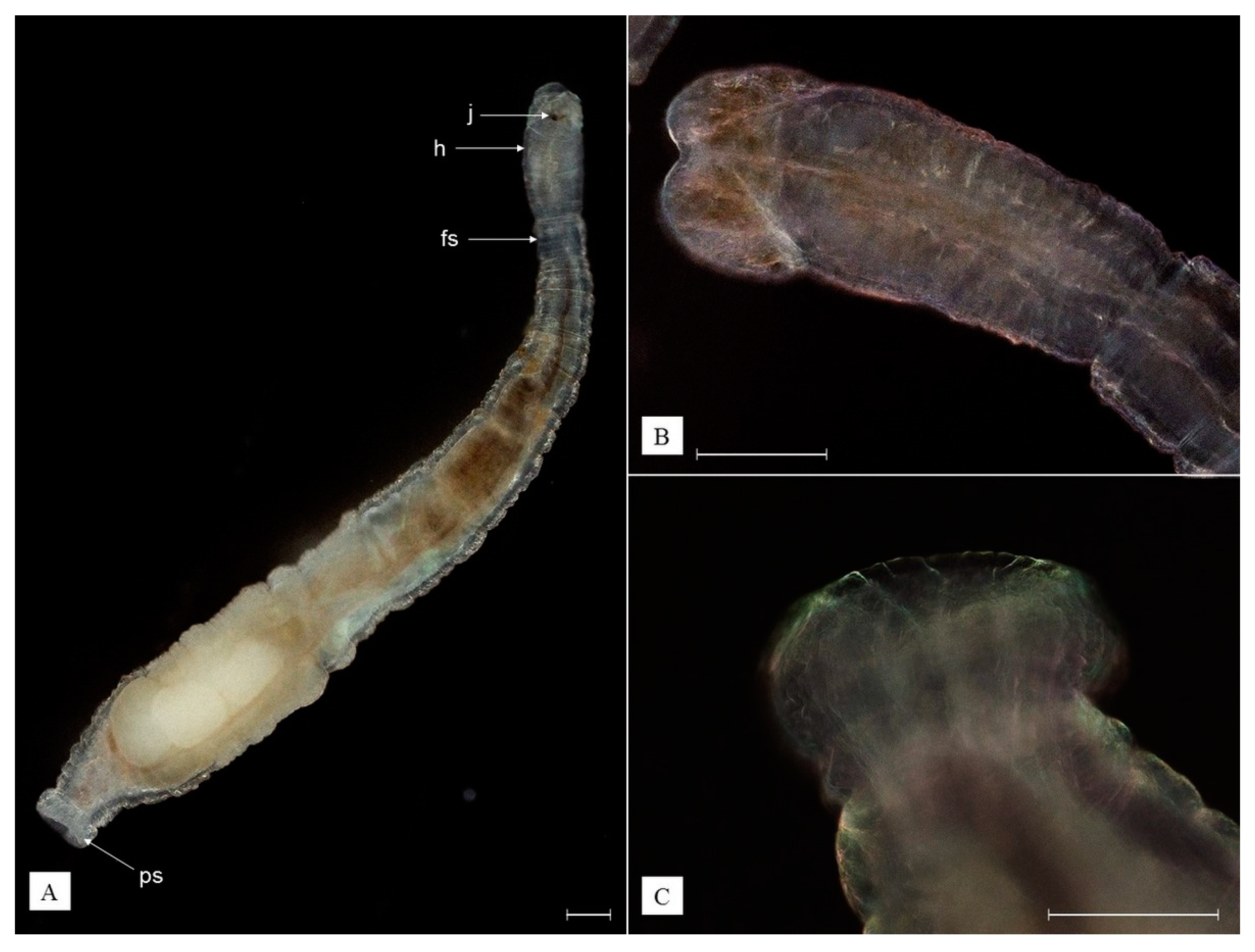
3.3. Redescription of Recorded Epibiotic Species
- Phylum Annelida
- Class Clitellata
- Family Branchiobdellidae
3.4. Prevalence and Mean Intensities of Epibionts
3.5. Microhabitat Preferences, Interactions among Epibionts and Host
4. Discussion
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Padilla, D.K.; Williams, S.L. Beyond ballast water: Aquarium and ornamental trades as sources of invasive species in aquatic ecosystems. Front. Ecol. Environ. 2004, 2, 131–138. [Google Scholar] [CrossRef]
- Duggan, I.C. The freshwater aquarium trade as a vector for incidental invertebrate fauna. Biol. Inv. 2010, 12, 3757–3770. [Google Scholar] [CrossRef]
- Patoka, J.; Bláha, M.; Devetter, M.; Rylková, K.; Čadková, Z.; Kalous, L. Aquarium hitchhikers: Attached commensals imported with freshwater shrimps via the pet trade. Biol. Inv. 2016, 18, 457–461. [Google Scholar] [CrossRef]
- Bláha, M.; Weiperth, A.; Patoka, J.; Szajbert, B.; Balogh, E.R.; Staszny, Á.; Ferincz, Á.; Lente, V.; Maciaszek, R.; Kouba, A. The pet trade as a source of non-native decapods: The case of crayfish and shrimps in a thermal waterbody in Hungary. Environ. Monit. Assess. 2022, 194, 795. [Google Scholar] [CrossRef]
- Simberloff, D.; Martin, J.-L.; Genovesi, P.; Maris, V.; Wardle, D.A.; Aronson, J.; Courchamp, F.; Galil, B.; García-Berthou, E.; Pascal, M.; et al. Impacts of biological invasions: What’s what and the way forward. Trends. Ecol. Evol. 2013, 28, 58–66. [Google Scholar] [CrossRef] [PubMed]
- Ložek, F.; Patoka, J.; Bláha, M. Another hitchhiker exposed: Diceratocephala boschmai (Platyhelminthes: Temnocephalida) found associated with ornamental crayfish Cherax spp. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 25. [Google Scholar] [CrossRef]
- Klotz, W.; Miesen, F.W.; Hüllen, S.; Herder, F. Two Asian freshwater shrimp species found in a thermally polluted stream system in North Rhine-Westphalia, Germany. Aquat. Inv. 2013, 8, 333–339. [Google Scholar] [CrossRef]
- Maciaszek, R.; Zarzyński, P. Nanoakwarium, Zwierzęta, Technika, Aquascaping, 1st ed.; Galaktyka: Łódź, Poland, 2021; pp. 89–156, 259–277. (In Polish) [Google Scholar]
- Maciaszek, R.; Kamaszewski, M.; Strużyński, W.; Łapa, P. Epibionts of ornamental freshwater shrimps bred in Taiwan. Ann. Warsaw. Univ. Life Sci. SGGW. Anim. Sci. 2018, 57, 133–142. [Google Scholar] [CrossRef]
- Hung, M.S.; Chan, T.Y.; Yu, H.P. Atyid shrimps (Decapoda: Caridea) of Taiwan, with descriptions of three new species. J. Crustacean. Biol. 1993, 13, 481–503. [Google Scholar] [CrossRef]
- Han, C.C.; Hsu, K.C.; Fang, L.S.; Cheng, I.M.; Lin, H.D. Geographical and temporal origins of Neocaridina species (Decapoda: Caridea: Atyidae) in Taiwan. BMC. Genet. 2019, 20, 86. [Google Scholar] [CrossRef]
- Jabłońska, A.; Mamos, T.; Gruszka, P.; Szlauer-Łukaszewska, A.; Grabowski, M. First record and DNA barcodes of the aquarium shrimp, Neocaridina davidi, in Central Europe from thermally polluted River Oder canal, Poland. Knowl. Manag. Aquat. Ecosyst. 2018, 419, 14. [Google Scholar] [CrossRef]
- Levitt-Barmats, Y.A.; Yanai, Z.; Cohen, T.M.; Shenkar, N. Life-history traits and ecological characteristics of the ornamental shrimp Neocaridina denticulata (De Haan, 1844), recently introduced into the freshwater systems of Israel. Aquat. Inv. 2019, 14, 4. [Google Scholar] [CrossRef]
- Weiperth, A.; Gábris, V.; Danyik, T.; Farkas, A.; Kuříková, P.; Kouba, A.; Patoka, J. Occurrence of non-native red cherry shrimp in European temperate waterbodies: A case study from Hungary. Knowl. Manag. Aquat. Ecosyst. 2019, 420, 9. [Google Scholar] [CrossRef]
- Onuki, K. The distribution of the invasive shrimp Neocaridina davidi (Decapoda: Caridea: Atyidae) in relation to environmental parameters in a stream at Kunitachi, Tokyo, Japan. Crustac. Res. 2021, 50, 33–39. [Google Scholar] [CrossRef] [PubMed]
- Niwa, N.; Ohtaka, A. Accidental Introduction of Symbionts with Imported Freshwater Shrimps. In Assessment and Control of Biological Invasion Risks; Koike, F., Clout, M.N., Kawamichi, M., De Poorter, M., Iwatsuki, K., Eds.; Shoukadoh Book Sellers: Kyoto, Japan; IUCN: Gland, Switzerland, 2006; pp. 182–186. [Google Scholar]
- Maciaszek, R.; Świderek, W.; Kaliszewicz, A.; Karaban, K.; Szpakowski, B. First report of Scutariella japonica (Matjašič, 1990), a temnocephalid epibiont from South-East Asia, found on introduced ornamental freshwater shrimp in European waters. Knowl. Manag. Aquat. Ecosyst. 2021, 422, 19. [Google Scholar] [CrossRef]
- Kakui, K.; Komai, T. First record of Scutariella japonica (Platyhelminthes: Rhabdocoela) from Hokkaido, Japan, and notes on its host shrimp Neocaridina sp. aff. davidi (Decapoda: Caridea: Atyidae). Aquat. Anim. 2022, 2022, AA2022-1. [Google Scholar]
- Schneider, R.; Prati, S.; Grabner, D.; Sures, B. First report of microsporidians in the non-native shrimp Neocaridina davidi from a temperate European stream. Dis. Aquat. Org. 2022, 150, 125–130. [Google Scholar] [CrossRef] [PubMed]
- Liao, C.C.; Shin, J.W.; Chen, L.R.; Huang, L.L.; Lin, W.C. First molecular identification of Vorticella sp. from freshwater shrimps in Tainan, Taiwan. Int. J. Parasitol. Parasites. Wildl. 2018, 7, 415–422. [Google Scholar] [CrossRef]
- Bauer, J.; Jung-Schroers, V.; Teitge, F.; Adamek, M.; Steinhagen, D. Association of the alga Cladogonium sp. with a multifactorial disease outbreak in dwarf shrimp (Neocaridina davidi). Dis. Aquat. Org. 2021, 146, 107–115. [Google Scholar] [CrossRef]
- Matjašič, J. Monography of the Family Scutariellidae (Turbellaria, Temnocephalidea), 1st ed.; Academia Scientiarum et Atrium Slovenica: Ljubljana, Slovenia, 1990; pp. 45–91. [Google Scholar]
- Kondrateva, T.; Nikonenkova, T.; Stepanova, N. Using cilioplankton as an indicator of the ecological condition of aquatic ecosystems. Geosciences 2019, 9, 464. [Google Scholar] [CrossRef]
- Maciaszek, R.; Jabłońska, A.; Prati, S.; Świderek, W. First report of freshwater atyid shrimp, Caridina formosae (Decapoda: Caridea) as a host of ectosymbiotic branchiobdellidan, Holtodrilus truncatus (Annelida, Citellata). Knowl. Manag. Aquat. Ecosyst. 2020, 421, 33. [Google Scholar] [CrossRef]
- Hirose, H.; Akiyama, M. A colorless, filamentous chlorophyceous alga, Cladogonium ogishimae gen. et sp. nov., parasitic on freshwater shrimps. Shokubutsugaku Zasshi 1971, 84, 137–140. [Google Scholar] [CrossRef]
- Ohtaka, A.; Chen, R.T. New records of a branchiobdellidan and four microdrile oligochaetes (Annelida: Clitellata) from inland waters of Taiwan. Taiwan J. Biodivers. 2010, 12, 97–110. [Google Scholar]
- Ahn, D.H.; Min, G.S. First report of the branchiobdellidan Holtodrilus truncatus (Annelida: Clitellata) found on the freshwater atyid shrimp Neocaridina sp. from Korea. J. Species. Res. 2016, 5, 459–462. [Google Scholar] [CrossRef]
- R Core Team. A Language and Environment for Statistical Computing; R Foundation for Statistical Computing: Vienna, Austria, 2022; Available online: https://www.R-project.org/ (accessed on 20 February 2023).
- Ohtaka, A.; Gelder, S.R.; Yamato, S.; Chen, R.T.; Nishino, M. Cohabitation of ectosymbiotic Branchiobdellida (Annelida, Clitellata) and Scutariellidae (Platyhelminthes, Rhabditophora, Temnocephalida) on atyid shrimps in Taiwan. Taiwan J. Biodivers. 2015, 17, 253–262. [Google Scholar]
- Fujita, Y.; Kawahara, T.; Niwa, N.; Shokita, R.S. First record of Holtodrilus truncatus (Liang, 1963) (Annelida: Clitellata: Branchiobdellidae) from the Ryukyu Islands. Biol. Mag. Okinawa 2010, 48, 25–33, (In Japanese with English Abstract). [Google Scholar]
- Ohtaka, A.; Gelder, S.R.; Nishino, M.; Ikeda, M.; Toyama, H.; Cui, Y.D.; He, X.B.; Wang, H.Z.; Chen, R.B.; Wang, Z.Y. Distributions of two ectosymbionts, branchiobdellidans (Annelida: Clitellata) and scutariellids (Platyhelminthes: “Turbellaria”: Temnocephalida), on atyid shrimp (Arthropoda: Crustacea) in southeast China. J. Nat. Hist. 2012, 46, 1547–1556. [Google Scholar] [CrossRef]
- Niwa, N.; Archdale, M.V.; Matsuoka, T.; Kawamoto, A.; Nishiyama, H. Microhabitat distribution and behaviour of branchiobdellidan Holtodrilus truncatus found on the freshwater shrimp Neocaridina spp. from the Sugo River, Japan. Cent. Eur. J. Biol. 2014, 9, 80–85. [Google Scholar] [CrossRef]
- Tanaka, K.; Wada, K.; Hamasaki, K. Distribution of Holtodrilus truncatus, a Branchiobdellidan ectosymbiotic on atyid shrimps in the Kii Peninsula, Western Japan, with reference to salinity tolerance and host preference. Zool. Sci. 2016, 33, 154–161. [Google Scholar] [CrossRef]
- Gelder, S.R.; Brinkhurst. R.O. An assessment of the phylogeny of the Branchiobdellida (Annelida: Clitellata), using PAUP. Can. J. Zool. 1990, 68, 1318–1326. [Google Scholar] [CrossRef]
- INC PAS. Alien Species in Poland: Scutariella japonica; Institute of Nature Conservation, Polish Academy of Sciences: Kraków, Poland, 2022; Available online: www.iop.krakow.pl/ias/species/2689 (accessed on 20 February 2023).
- Matsuyama-Serisawa, K.; Imai, T.; Nakaso, M.; Serisawa, Y. Reconfirmation of Cladogonium (Chlorophyta, Cladophoraceae) being ecroparasitic on freshwater shrimp. Jpn. J. Phycol. 2014, 62, 1–6. [Google Scholar]
- Fowler-Walker, M.J.; Wernberg, T.; Connell, S.D. Differences in kelp morphology between wave sheltered and exposed localities: Morphologically plastic or fixed traits? Mar. Biol. 2006, 148, 755–767. [Google Scholar] [CrossRef]
- Turland, N.J.; Wiersema, J.H.; Barrie, F.R.; Greuter, W.; Hawksworth, D.L.; Herendeen, P.S.; Knapp, S.; Kusber, W.-H.; Li, D.-Z.; Marhold, K.; et al. International Code of Nomenclature for Algae, Fungi, and Plants (Shenzhen Code) Adopted by the Nineteenth International Botanical Congress Shenzhen, China, July 2017; Regnum Vegetabile 159; Koeltz Botanical Books: Glashütten, Germany, 2018. [Google Scholar]
- Tyler, S.; Artois, T.; Schilling, S.; Hooge, M.; Bush, L.F. World List of Turbellarian Worms: Acoelomorpha, Catenulida, Rhabditophora. Scutariellinae Annandale, 1912. 2006–2022. Accessed through: World Register of Marine Species. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1563346 (accessed on 20 February 2023).
- WoRMS. Holtodrilus Gelder and Brinkhurst, 1990. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=1610321 (accessed on 20 February 2023).
- Boedeker, C.; O’Kelly, C.J.; Star, W.; Leliaert, F. Molecular phylogeny and taxonomy of the Aegagropila clade (Cladophorales, Ulvophyceae), including the description of Aegagropilopsis gen. nov. and Pseudocladophora gen. nov. 1. J. Phycol. 2012, 48, 808–825. [Google Scholar] [CrossRef] [PubMed]
- Guiry, M.D.; Guiry, G.M. AlgaeBase: World-Wide Electronic Publication, National University of Ireland, Galway (Taxonomic Information Republished from AlgaeBase with Permission of M.D. Guiry). Cladogonium H. Hirose & M. Akiyama, 1971. 2023. Available online: https://www.marinespecies.org/aphia.php?p=taxdetails&id=369498 (accessed on 20 February 2023).
- Krkošek, M. Population biology of infectious diseases shared by wild and farmed fish. Can. J. Fish. Aquat. 2017, 74, 620–628. [Google Scholar] [CrossRef]
- Gannon, J.E.; Stemberger, R.S. Zooplankton (especially crustaceans and rotifers) as indicators of water quality. Trans. Am. Microsc. Soc. 1978, 1, 16–35. [Google Scholar] [CrossRef]
- Nicolau, A.; Dias, N.; Mota, M.; Lima, N. Trends in the use of protozoa in the assessment of wastewater treatment. Res. Microbiol. 2001, 152, 621–630. [Google Scholar] [CrossRef]
- Cook, J.A.; Chubb, J.C. Epibionts of Asellus aquaticus (L.) (Crustacea, Isopoda): An SEM study. Freshw. Biol. 1998, 39, 423–438. [Google Scholar] [CrossRef]
- Green, J. Parasites and epibionts of Cladocera. Trans. Zool. Soc. London. 1974, 32, 417–515. [Google Scholar] [CrossRef]
- Vacchiano, E.; Dreisbach, A.; Locascio, D.; Castaneda, L.; Vivian, T.; Buhse, H.E., Jr. Morphogenetic transitions and cytoskeletal elements of the stalked zooid and the telotroch stages in the peritrich ciliate Vorticella convallaria. J. Protozool. 1992, 39, 101–106. [Google Scholar] [CrossRef]
- Gelder, S.R. Zoogeography of Branchiobdellidans (Annelida) and Temnocephalidans (Platyhelminthes) ectosymbiotic on freshwater crustaceans, and their reactions to one another in vitro. Hydrobiologia. 1999, 406, 21–31. [Google Scholar] [CrossRef]
- Lemos, D.; Weissman, D. Moulting in the grow-out of farmed shrimp: A review. Rev. Aquac. 2021, 13, 5–17. [Google Scholar] [CrossRef]
- Pamuru, R.R.; Rosen, O.; Manor, R.; Chung, J.S.; Zmora, N.; Glazer, L.; Aflalo, E.D.; Weil, S.; Tamone, S.L.; Sagi, A. Stimulation of molt by RNA interference of the molt-inhibiting hormone in the crayfish Cherax quadricarinatus. Gen. Comp. Endocr. 2012, 178, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Skelton, J.; Geyer, K.M.; Lennon, J.T.; Creed, R.P.; Brown, B.L. Multi-scale ecological filters shape the crayfish microbiome. Symbiosis 2017, 72, 159–170. [Google Scholar] [CrossRef]
- Ames, C.W.; Helms, B.S.; Stoeckel, J.A. Habitat mediates the outcome of a cleaning symbiosis for a facultatively burrowing crayfish. Freshw. Biol. 2015, 60, 989–999. [Google Scholar] [CrossRef]
- Orlić, K.; Šver, L.; Burić, L.; Kazazić, S.; Grbin, D.; Maguire, I.; Pavić, D.; Hrašćan, R.; Vladušić, T.; Hudina, S.; et al. Cuticle-associated bacteria can inhibit crayfish pathogen Aphanomyces astaci: Opening the perspective of biocontrol in astaciculture. Aquac. 2021, 533, 736112. [Google Scholar] [CrossRef]
- Lowe, S.; Browne, M.; Boudjelas, S.; De Poorter, M. 100 of the World’s Worst Invasive Alien Species: A Selection from the Global Invasive Species Database; Invasive Species Specialist Group: Auckland, New Zealand, 2000. [Google Scholar]
- Svoboda, J.; Mrugała, A.; Kozubíková-Balcarová, E.; Petrusek, A. Hosts and transmission of the crayfish plague pathogen Aphanomyces astaci: A review. J. Fish. Dis. 2017, 40, 127–140. [Google Scholar] [CrossRef]
- Ramalho, R.O.; Anastácio, P.M. Factors inducing overland movement of invasive crayfish (Procambarus clarkii) in a ricefield habitat. Hydrobiologia 2015, 746, 135–146. [Google Scholar] [CrossRef]
- Hunt, R.; Thomas, J.R.; James, J.; Cable, J. Transmission and terrestrial dispersal of non-native ectosymbionts on invasive crayfish. Hydrobiologia 2018, 820, 135–144. [Google Scholar] [CrossRef]
- Maciaszek, R.; Jabłońska, A.; Prati, S.; Wróblewski, P.; Gruszczyńska, J.; Świderek, W. Marbled crayfish Procambarus virginalis invades a nature reserve: How to stop further introductions? Eur. Zool. J. 2022, 89, 888–901. [Google Scholar] [CrossRef]



| Site Name | Coordinates | ||
|---|---|---|---|
| Taipei, Taiwan | Wai Shuang Hsi River | 25.0624° N | 121.3327° E |
| Roadside ditch 1 | 25.0644° N | 121.3340° E | |
| Roadside ditch 2 | 25.0743° N | 121.3430° E | |
| Cladogonium kumaki sp. nov. (Present Study) | Cladogonium ogishimae (Hirose and Akiyama, 1971) | ||
|---|---|---|---|
| Host species | Neocaridina davidi | Caridina leucosticta Macrobrachium formosense Paratya improvisa | |
| Zoosporangium | Color | Shades of green | Green |
| Surface structure of mature zoosporangia | Highly irregular | Rather flat | |
| Width in µm (mean ± SD) | 19–78 (37.4 ± 19.7) | 130–210 (181.5) | |
| Length in µm (mean ± SD) | 322–540 (458.2 ± 57.5) | 440–790 (561.5) | |
| Zoospores | Color | Green | Green |
| Ciliated | Yes | Yes (4) | |
| Diameter in µm (mean ± SD) | 19.3–35.3 (26.9 ± 3.5) | 8–12 μm | |
| Nuclei | Yes, multiple | Not determined | |
| Erect filament of thallus | Color | Mostly colorless, sometimes partially light green | Colorless |
| Width in µm (mean ± SD) | 7.2–36 (26.5 ± 7.3) | 17–35 (26.5) | |
| Length in µm (mean ± SD) | 107.5–132 (120.3 ± 9.5) | 40–180 (99) | |
| Nuclei | 4–6 | 4–8 | |
| Vacuolation | Yes | Yes | |
| Rhizoidal cells | Color | Colorless | Colorless |
| Width in µm (mean ± SD) | 6.3–9.3 (7.2 ± 0.7) | Not determined | |
| Length in µm (mean ± SD) | 22.1–81.9 (56.3 ± 18.6) | Not determined |
| Monodiscus kumaki sp. nov. (This Study) | Monodiscus parvus (Plate, 1914) * | Monodiscus macbridei (Fernando, 1952) * | Scutariella japonica (Matjašič, 1990) * | |
|---|---|---|---|---|
| Host species | Neocaridina davidi (Bouvier, 1904) | Caridina simoni (Bouvier, 1904) | Caridina sp. | Caridina sp. Neocaridina sp. Paratya sp. |
| Maximum body length when relaxed (mm) | 0.58 | 0.20 | 0.285 | 2.65 |
| Tentacles | Short and conical | Short and conical | Short and conical | With cylindrical base and a warty terminal part |
| Tentacular glands | 3 sorts | 2 sorts | 3 sorts | |
| Pharynx | Ovoid | Ovoid | Ovoid | Pear-shaped |
| Eyes | Not present | Not present | Not present | Present |
| Posterior glands | 2 types | 2 types | 3 types | |
| Sucker | Round | Round | Round | Horse-shoe shaped |
| Source | Sex | N | Epibiont Prevalence in % (n of Infected Individuals) | ||||||
|---|---|---|---|---|---|---|---|---|---|
| Pooled Epibionts | Cladogonium kumaki sp. nov. | Monodiscus kumaki sp. nov. | Scutariella japonica | Holtodrilus truncatus | Rotifers | Ciliates | |||
| Wild | F | 243 | 86 (209) | 28.8 (70) | 35.4 (86) | 34.6 (84) | 68.3(166) | 54.3(132) | 73.3(178) |
| M | 57 | 54.4 (31) | 1.8 (1) | 7.0 (4) | 8.8 (5) | 47.4(27) | 24.6(14) | 43.9 (25) | |
| Aquaculture | F | 269 | 89.2 (240) | 54.6 (147) | 72.5 (195) | 48.0 (129) | 61.0(164) | 61.0(164) | 82.5(222) |
| M | 31 | 58.1 (18) | 29.0 (9) | 51.6 (16) | 19.4 (6) | 41.9(13) | 32.3(10) | 48.4 (15) | |
| Aquaria | F | 257 | 62.6 (161) | 5.1 (13) | 30.0 (77) | 12.8 (33) | 5.8 (15) | 6.6 (17) | 32.7 (84) |
| M | 43 | 67.4 (29) | 4.7 (2) | 25.6 (11) | 18.6 (8) | 11.6 (5) | 7.0 (3) | 34.9 (15) | |
| Total | 900 | 76.4 (688) | 26.9 (242) | 43.2 (389) | 29.4 (265) | 43.3 (390) | 37.8 (340) | 59.9 (539) | |
| Epibiont mean intensity ± SE | |||||||||
| Wild | F | 51.6 ± 6.5 | 116.9 ± 13.0 | 6.6 ± 0.7 | 6.4 ± 0.8 | 5.2 ± 0.4 | - | - | |
| M | 5.0 ± 0.6 | - | 2.0 ± 0.4 | 3.8 ± 1.6 | 4.1 ± 0.5 | - | - | ||
| Aquaculture | F | 123.2 ± 10.7 | 125.0 ± 12.5 | 27.3 ± 1.8 | 11.9 ± 1.0 | 15.1 ± 2.3 | - | - | |
| M | 44.3 ± 11.9 | 40.6 ± 15.1 | 16.0 ± 3.9 | 4.7 ± 2.1 | 11.5 ± 3.2 | - | - | ||
| Aquaria | F | 44.8 ± 4.8 | 58.1 ± 12.6 | 46.7 ± 4.4 | 14.6 ± 2.6 | 3.3 ± 0.6 | - | - | |
| M | 13.2 ± 3.3 | 5.5 ± 0.5 | 19.5 ± 5.8 | 7.1 ± 2.1 | 1.6 ± 0.4 | - | - | ||
| Total | 73.3 ± 4.91 | 114.5 ± 8.7 | 25.6 ± 1.45 | 10.1 ± 0.7 | 9.38 ± 1.0 | - | - | ||
| Variable | Odd Ratios | |||||
|---|---|---|---|---|---|---|
| Cladogonium kumaki sp. nov. | Monodiscus kumaki sp. nov. | Scutariella japonica | Holtodrilus truncatus | Rotifers | Ciliates | |
| Wild | 0.30 (0.21–0.43) | 0.19 (0.13–0.27) | 0.55 (0.39–0.77) | 1.33 (0.95–1.87) | 0.75 (0.54–1.04) | 0.60 (0.41–0.87) |
| Aquaria | 0.05 (0.03–0.08) | 0.17 (0.12–0.25) | 0.20 (0.13–0.29) | 0.05 (0.03–0.08) | 0.05 (0.03–0.08) | 0.13 (0.09–0.19) |
| Males | 0.23 (0.12–0.42) | 0.38 (0.24–0.59) | 0.37 (0.21–0.60) | 0.52 (0.33–0.81) | 0.32 (0.20–0.52) | 0.38 (0.25–0.58) |
| Variable | Odd Ratios | |||
|---|---|---|---|---|
| Cladogonium kumaki sp. nov. | Monodiscus kumaki sp. nov. | Scutariella japonica | Holtodrilus truncatus | |
| Rostral area | 0.28 (0.08–0.79) | 0.29 (0.23–0.36) | 1.27 (0.99–1.63) | 1.63 (1.29–2.06) |
| Pereiopodal area | 9.46 (5.58–17.34) | 0.18 (0.14–0.23) | 0.38 (0.27–0.52) | 1.62 (1.28–2.05) |
| Pleopodal area | 22.62 (13.58–40.99) | 0.36 (0.29–0.45) | 0.51 (0.37–0.68) | 1.00 (0.78–1.29) |
| Spearman’s Rank Correlation of Epibiont Abundances | |||
|---|---|---|---|
| Cladogonium kumaki sp. nov. | Monodiscus kumaki sp. nov. | Scutariella japonica | |
| Monodiscus kumaki sp. nov. | Rho = 0.41, p =< 0.001 | ||
| Scutariella japonica | Rho = 0.39, p =< 0.001 | Rho = 0.16, p =< 0.001 | |
| Holtodrilus truncatus | Rho = 0.33, p =< 0.001 | Rho = −0.16, p = 0.001 | Rho = −0.08, p = 0.044 |
| Epibionts | Egg Production | Shrimp Molting |
|---|---|---|
| Cladogonium kumaki sp. nov. | Rho = −0.26, p =< 0.001 | Rho = −0.22, p =< 0.001 |
| Monodiscus kumaki sp. nov. | Rho = −0.05, p = 0.115 | Rho = −0.31, p =< 0.001 |
| Scutariella japonica | Rho = −0.07, p = 0.054 | Rho = −0.24, p =< 0.001 |
| Holtodrilus truncatus | Rho = −0.01, p = 0.847 | Rho = −0.26, p = 0.847 |
Disclaimer/Publisher’s Note: The statements, opinions and data contained in all publications are solely those of the individual author(s) and contributor(s) and not of MDPI and/or the editor(s). MDPI and/or the editor(s) disclaim responsibility for any injury to people or property resulting from any ideas, methods, instructions or products referred to in the content. |
© 2023 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Maciaszek, R.; Świderek, W.; Prati, S.; Huang, C.-Y.; Karaban, K.; Kaliszewicz, A.; Jabłońska, A. Epibiont Cohabitation in Freshwater Shrimp Neocaridina davidi with the Description of Two Species New to Science, Cladogonium kumaki sp. nov. and Monodiscus kumaki sp. nov., and Redescription of Scutariella japonica and Holtodrilus truncatus. Animals 2023, 13, 1616. https://doi.org/10.3390/ani13101616
Maciaszek R, Świderek W, Prati S, Huang C-Y, Karaban K, Kaliszewicz A, Jabłońska A. Epibiont Cohabitation in Freshwater Shrimp Neocaridina davidi with the Description of Two Species New to Science, Cladogonium kumaki sp. nov. and Monodiscus kumaki sp. nov., and Redescription of Scutariella japonica and Holtodrilus truncatus. Animals. 2023; 13(10):1616. https://doi.org/10.3390/ani13101616
Chicago/Turabian StyleMaciaszek, Rafał, Wiesław Świderek, Sebastian Prati, Chih-Yang Huang, Kamil Karaban, Anita Kaliszewicz, and Aleksandra Jabłońska. 2023. "Epibiont Cohabitation in Freshwater Shrimp Neocaridina davidi with the Description of Two Species New to Science, Cladogonium kumaki sp. nov. and Monodiscus kumaki sp. nov., and Redescription of Scutariella japonica and Holtodrilus truncatus" Animals 13, no. 10: 1616. https://doi.org/10.3390/ani13101616
APA StyleMaciaszek, R., Świderek, W., Prati, S., Huang, C.-Y., Karaban, K., Kaliszewicz, A., & Jabłońska, A. (2023). Epibiont Cohabitation in Freshwater Shrimp Neocaridina davidi with the Description of Two Species New to Science, Cladogonium kumaki sp. nov. and Monodiscus kumaki sp. nov., and Redescription of Scutariella japonica and Holtodrilus truncatus. Animals, 13(10), 1616. https://doi.org/10.3390/ani13101616





