Synthesis of Palladium and Copper Nanoparticles Supported on TiO2 for Oxidation Solvent-Free Aerobic Oxidation of Benzyl Alcohol
Abstract
:1. Introduction
2. Materials and Methods
2.1. Preparation of Catalyst
2.2. Catalytic Performance
2.3. Catalyst Recyclability
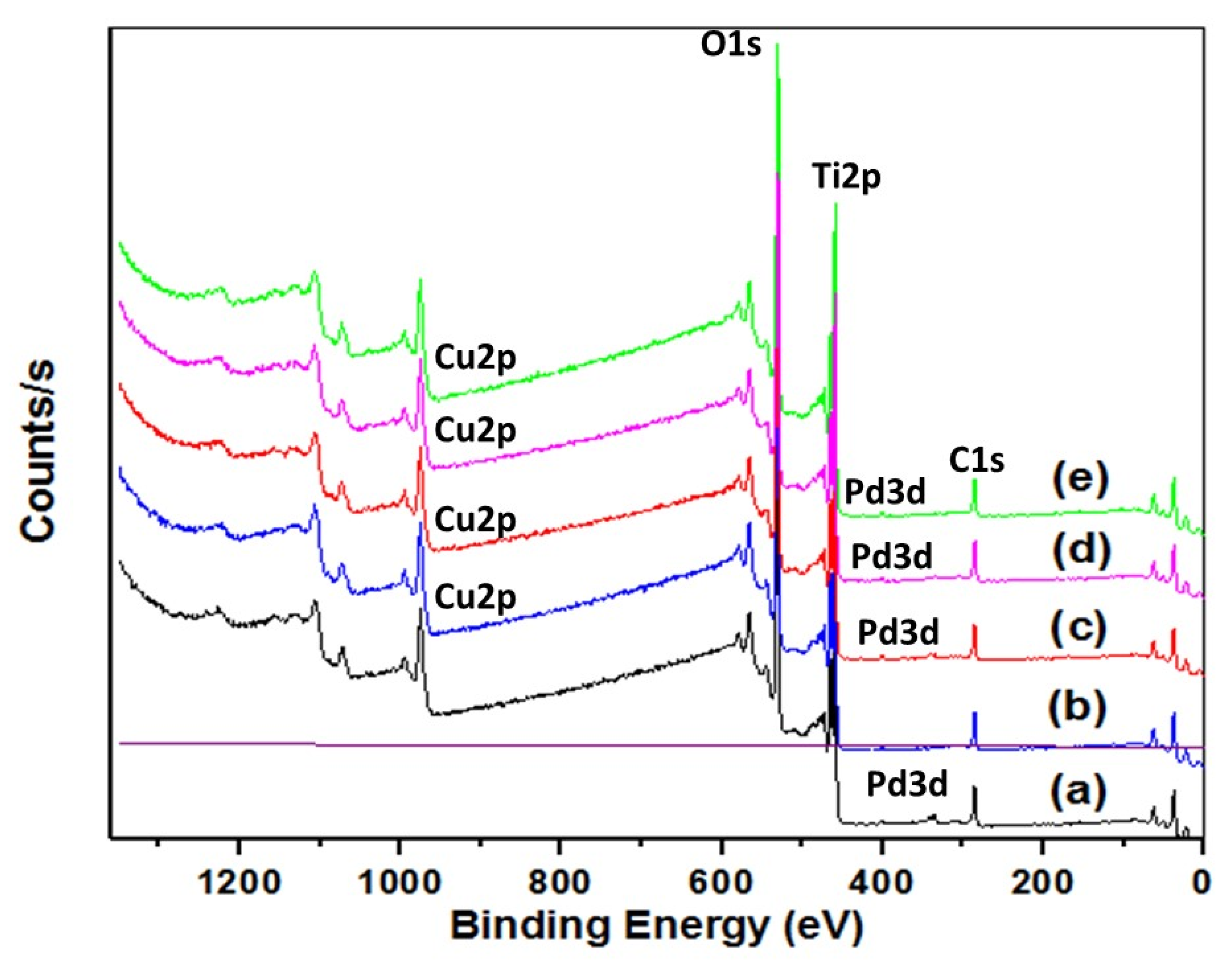
2.4. Catalyst Characterization
3. Results and Discussion
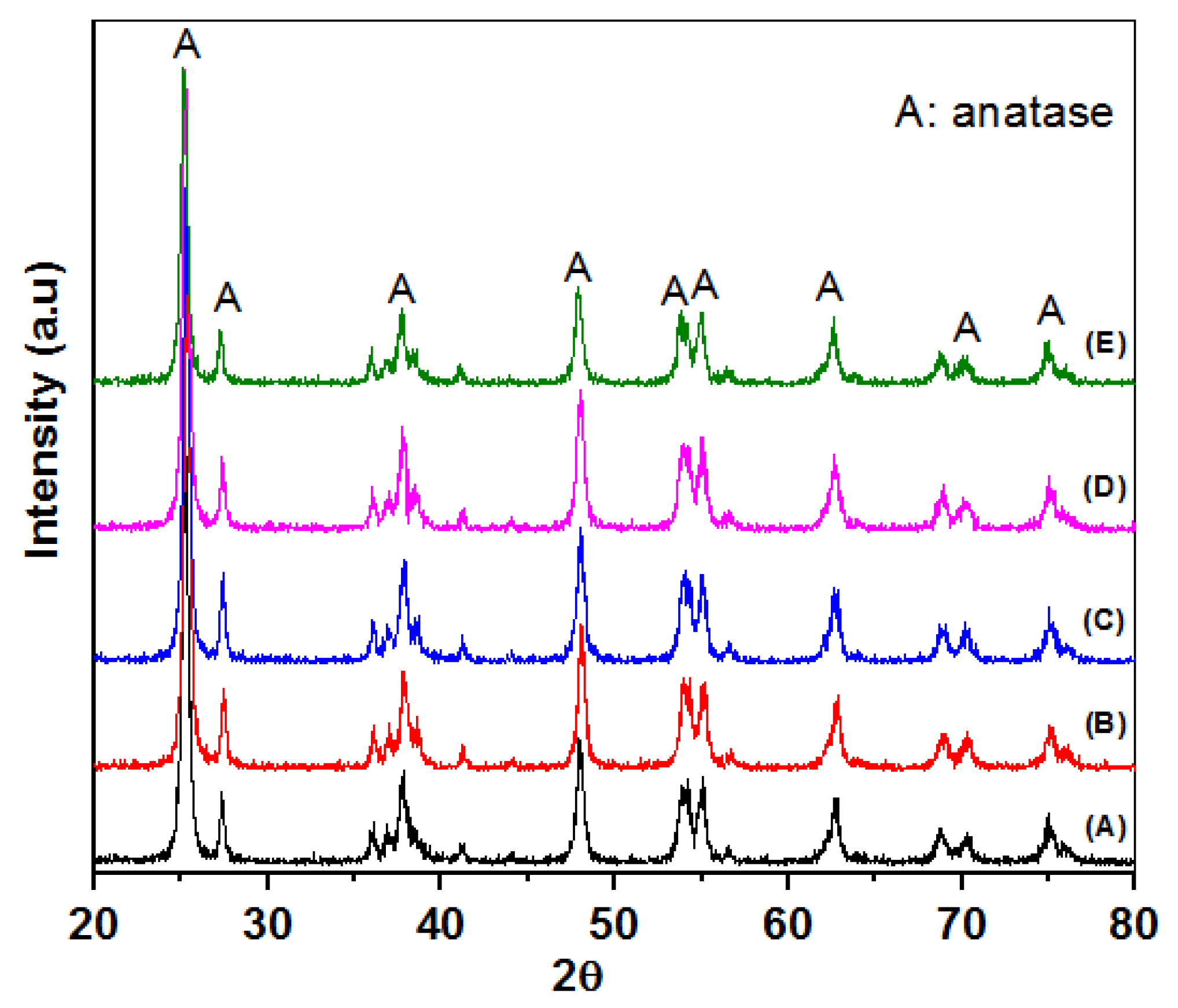
3.1. Materials Charcterization
3.2. Catalytic Activity
3.2.1. Effect of Cu–Pd Ratio Supported on TiO2
3.2.2. Effect of Temperature
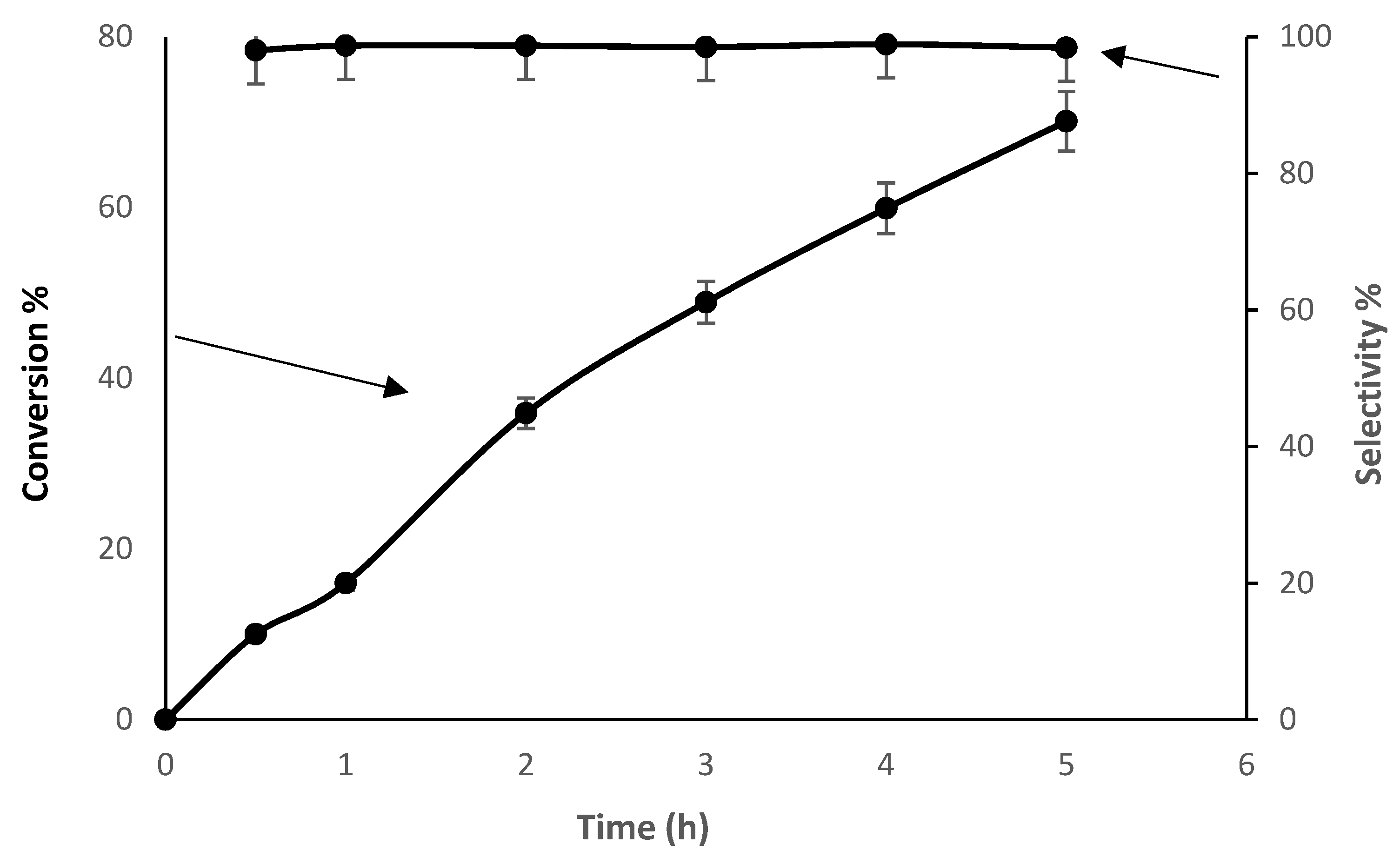
3.2.3. Time Study
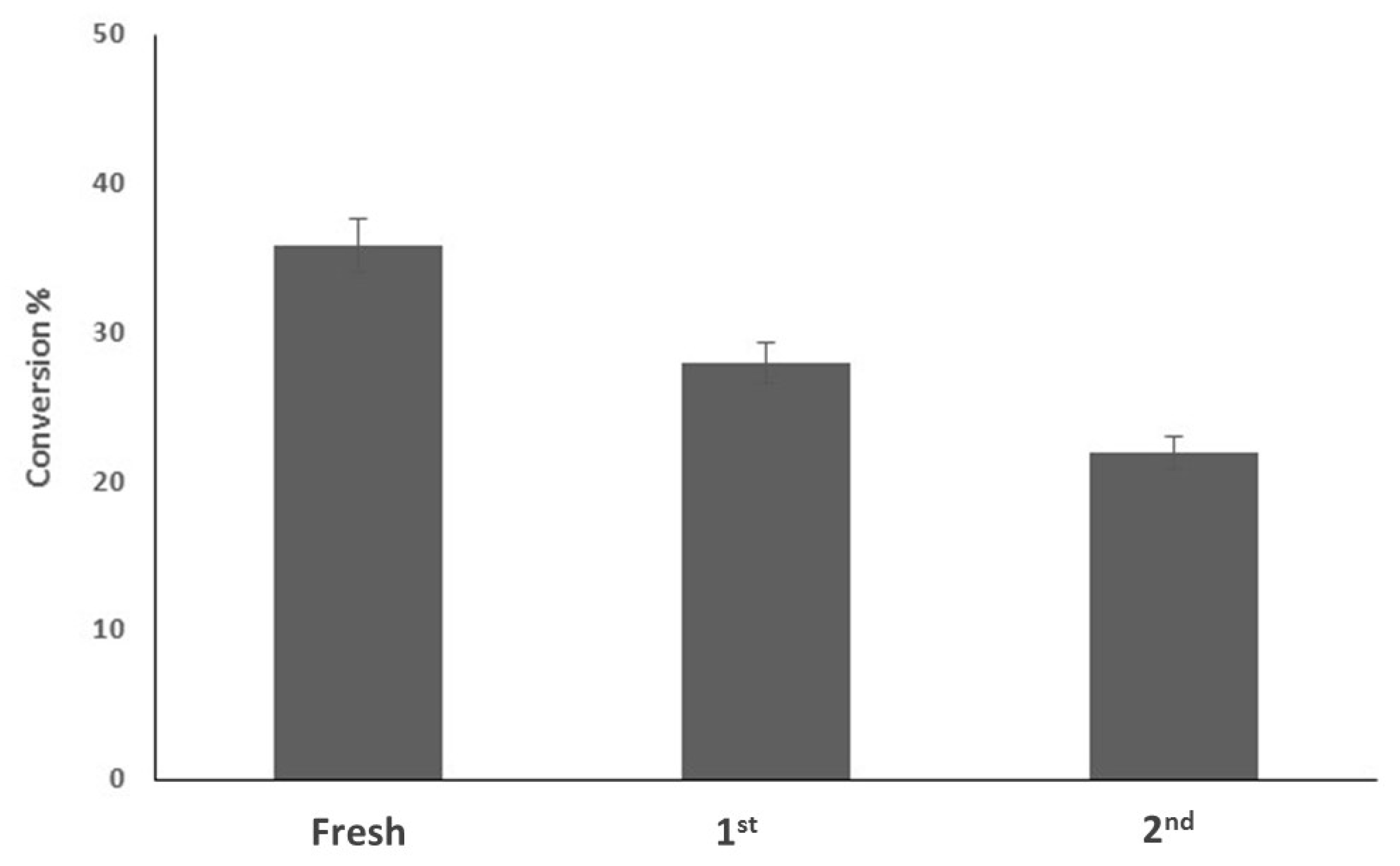
3.2.4. Catalyst Reusability
4. Conclusions
Supplementary Materials
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Jachuck, R.J.J.; Selvaraj, D.K.; Varma, R.S. Process intensification: Oxidation of benzyl alcohol using a continuous isothermal reactor under microwave irradiation. Green Chem. 2006, 8, 29–33. [Google Scholar] [CrossRef]
- Ragupathi, C.; Judith Vijaya, J.; Narayanan, S.; Jesudoss, S.K.; John Kennedy, L. Highly selective oxidation of benzyl alcohol to benzaldehyde with hydrogen peroxide by cobalt aluminate catalysis: A comparison of conventional and microwave methods. Ceram. Int. 2015, 41, 2069–2080. [Google Scholar] [CrossRef]
- Ndolomingo, M.J.; Meijboom, R. Selective liquid phase oxidation of benzyl alcohol to benzaldehyde by tert-butyl hydroperoxide over gamma-Al2O3 supported copper and gold nanoparticles. Appl. Surf. Sci. 2017, 398, 19–32. [Google Scholar] [CrossRef]
- Zhu, S.; Cen, Y.; Yang, M.; Guo, J.; Chen, C.; Wang, J.; Fan, W. Probing the intrinsic active sites of modified graphene oxide for aerobic benzylic alcohol oxidation. Appl. Catal. B Environ. 2017, 211, 89–97. [Google Scholar] [CrossRef]
- Sheldon, R.A.; van Bekkum, H. Fine Chemicals Through Heterogeneous Catalysis; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2001. [Google Scholar]
- Enache, D.I.; Edwards, J.K.; Landon, P.; Solsona-Espriu, B.; Carley, A.F.; Herzing, A.A.; Watanabe, M.; Kiely, C.J.; Knightand, D.W.; Hutchings, G.J. Solvent-free oxidation of primary alcohols to aldehydes using Au-Pd/TiO2 catalyst. Science 2006, 311, 362–365. [Google Scholar] [CrossRef]
- Yamaguchi, K.; Mizuno, N. Supported ruthenium catalyst for the heterogeneous oxidation of alcohols with molecular oxygen. Angew. Chem. Int. Ed. Engl. 2002, 41, 4538–4542. [Google Scholar] [CrossRef]
- Karimi, B.; Biglari, A.; Clark, J.H.; Budarin, V. Green, transition-metal-free aerobic oxidation of alcohols using a highly durable supported organocatalyst. Angew. Chem. Int. Ed. Engl. 2007, 46, 7210–7213. [Google Scholar] [CrossRef] [PubMed]
- Hudlicky, M. Oxidation in Organic Chemistry; American Chemical Society: Washington, DC, USA, 1990. [Google Scholar]
- Miyamura, H.; Matsubara, R.; Miyazaki, Y.; Kobayashi, S. Aerobic oxidation of alcohols at room temperature and atmospheric conditions catalyzed by reusable gold nanoclusters stabilized by the benzene rings of polystyrene derivatives. Angew. Chem. Int. Ed. Engl. 2007, 46, 4151–4154. [Google Scholar] [CrossRef]
- Mahmood, A.; Robinson, G.E.; Powell, L. An improved oxidation of an alcohol using aqueous permanganate and phase-transfer catalyst. Organ. Proc. Res. Dev. 1999, 3, 363–364. [Google Scholar] [CrossRef]
- Thottathil, J.K.; Moniot, J.L.; Mueller, R.H.; Wong, M.K.; Kissick, T.P. Conversion of L-pyroglutamic acid to 4-alkyl-substituted L-prolines. The synthesis of trans-4-cyclohexyl-L-proline. J. Organ. Chem. 1986, 51, 3140–3143. [Google Scholar] [CrossRef]
- Bäckvall, J.-E. Modern Oxidation Methods; Wiley-VCH Verlag GmbH & Co. KGaA: Hoboken, NJ, USA, 2010. [Google Scholar]
- Tojo, G.; Fernandez, M. Oxidation of Alcohols to Aldehydes and Ketones; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Ryland, B.L.; Stahl, S.S. Practical Aerobic Oxidations of Alcohols and Amines with Homogeneous Copper/TEMPO and Related Catalyst Systems. Angew. Chem. Int. Ed. 2014, 53, 8824–8838. [Google Scholar] [CrossRef] [Green Version]
- Villa, A.; Wang, D.; Dimitratos, N.; Su, D.; Trevisan, V.; Prati, L. Pd on carbon nanotubes for liquid phase alcohol oxidation. Catal. Today 2010, 150, 8–15. [Google Scholar] [CrossRef] [Green Version]
- Liu, J.; Zou, S.; Lu, L.; Zhao, H.; Xiao, L.; Fan, J. Room temperature selective oxidation of benzyl alcohol under base-free aqueous conditions on Pt/TiO2. Catal. Commun. 2017, 99, 6–9. [Google Scholar] [CrossRef]
- Albadi, J.; Alihoseinzadeh, A.; Razeghi, A. Novel metal oxide nanocomposite of Au/CuO-ZnO for recyclable catalytic aerobic oxidation of alcohols in water. Catal. Commun. 2014, 49, 1–5. [Google Scholar] [CrossRef]
- Zhan, G.; Huang, J.; Du, M.; Sun, D.; Abdul-Rauf, I.; Lin, W.; Hong, Y.; Li, Q. Liquid phase oxidation of benzyl alcohol to benzaldehyde with novel uncalcined bioreduction au catalysts: High activity and durability. Chem. Eng. J. 2012, 187, 232–238. [Google Scholar] [CrossRef]
- Ganesamoorthy, S.; Tamizh, M.M.; Shanmugasundaram, K.; Karvembu, R. Immobilization of Ru (III) complex on silica: A heterogenized catalyst for selective oxidation of alcohols in water at room temperature. Tetrahedron Lett. 2013, 54, 7035–7039. [Google Scholar] [CrossRef]
- Friend, C.M.; Xu, B. Heterogeneous Catalysis: A Central Science for a Sustainable Future. Acc. Chem. Res. 2017, 50, 517–521. [Google Scholar] [CrossRef] [PubMed]
- Nishimura, S. Handbook of Heterogeneous Catalytic Hydrogenation for Organic Synthesis; Wiley: New York, NY, USA, 2001. [Google Scholar]
- Astruc, D. Nanoparticles and Catalysis; Wiley: New York, NY, USA, 2008. [Google Scholar] [CrossRef]
- Bilgicli, H.G.; Burhan, H.; Diler, F.; Cellat, K.; Kuyuldar, E.; Zengin, M.; Sen, F. Composites of palladium nanoparticles and graphene oxide as a highly active and reusable catalyst for the hydrogenation of nitroarenes. Microporous Mesoporous Mater. 2020, 296, 110014. [Google Scholar] [CrossRef]
- Dilera, F.; Burhan, H.; Genca, H.; Kuyuldarb, E.; Zengin, M.; Cellat, K.; Senb, F. Efficient preparation and application of monodisperse palladium loaded graphene oxide as a reusable and effective heterogeneous catalyst for suzuki cross-coupling reaction. J. Mol. Liq. 2020, 298, 111967. [Google Scholar] [CrossRef]
- Kakiuchi, N.; Maeda, Y.; Nishimura, T. Uemura, S. Pd(II)−Hydrotalcite-Catalyzed Oxidation of Alcohols to Aldehydes and Ketones Using Atmospheric Pressure of Air. J. Org. Chem. 2001, 66, 6620–6625. [Google Scholar] [CrossRef]
- Chan-Thaw, C.E.; Savara, A.; Villa, A. Selective benzyl alcohol oxidation over Pd catalysts. Catalysts 2018, 8, 431. [Google Scholar] [CrossRef] [Green Version]
- Yang, C.-C.; Yu, Y.-H.; van der Linden, B.; Wu, J.C.S.; Mul, G. Artificial photosynthesis over crystalline TiO2-based catalysts: Fact or fiction? J. Am. Chem. Soc. 2010, 132, 8398–8406. [Google Scholar] [CrossRef]
- Pelton, R.; Geng, X.; Brook, M. Photocatalytic paper from colloidal TiO2-fact or fantasy. Adv. Colloid Interface Sci. 2006, 127, 43–53. [Google Scholar] [CrossRef] [PubMed]
- Saikia, H.; Borah, B.J.; Yamada, Y.; Bharali, P. Enhanced catalytic activity of CuPd alloy nanoparticles towards reduction of nitroaromatics and hexavalent chromium. J. Colloid Interface Sci. 2017, 486, 46–57. [Google Scholar] [CrossRef]
- Diyarbakir, S.; Can, H.; Metin, Ö. Reduced Graphene Oxide-Supported CuPd Alloy Nanoparticles as Efficient Catalysts for the Sonogashira Cross-Coupling Reactions. ACS Appl. Mater. Interfaces 2015, 7, 3199–3206. [Google Scholar] [CrossRef] [PubMed]
- Nasrollahzadeh, M.; Jaleh, B.; Ehsani, A. Preparation of carbon supported CuPd nanoparticles as novel heterogeneous catalysts for the reduction of nitroarenes and the phosphine-free Suzuki–Miyaura coupling reaction. New J. Chem. 2015, 39, 1148–1153. [Google Scholar] [CrossRef]
- Zamaraev, K.I. Catalytic science and technology for environmental issues. Catal. Today 1997, 35, 3–13. [Google Scholar] [CrossRef]
- Kluson, P.; Cerveny, L. Selective hydrogenation over ruthenium catalysts. Appl. Catal. A Gen. 1995, 128, 13–31. [Google Scholar] [CrossRef]
- Kim, K.S.; Gossmann, A.F.; Winograd, N. X-ray photoelectron spectroscopic studies of palladium oxides and the palladium-oxygen electrode. Anal. Chem. 1974, 46, 197–200. [Google Scholar] [CrossRef]
- Mukherjee, P.; Roy, P.S.; Mandal, K.; Bhattacharjee, D.; Dasgupta, S.; Bhattacharya, S.K. Improved catalysis of room temperature synthesized Pd-Cu alloy nanoparticles for anodic oxidation of ethanol in alkaline media. Electrochim. Acta 2015, 154, 447–455. [Google Scholar] [CrossRef]
- Zhang, L.; Persaud, R.; Madey, T.E. Ultrathin metal films on a metal oxide surface: Growth of Au on TiO2 (110). Phys. Rev. B 1997, 56, 10549–10557. [Google Scholar] [CrossRef]
- Li, X.; Feng, J.; Sun, J.; Wang, Z.; Zhao, W. Solvent-Free Catalytic Oxidation of Benzyl Alcohol over Au-Pd Bimetal Deposited on TiO2: Comparison of Rutile, Brookite, and Anatase. Nanoscale Res. Lett. 2019, 14, 394. [Google Scholar] [CrossRef]
- Zhan, B.Z.; White, M.A.; Sham, T.K.; Pincock, J.A.; Doucet, R.J.; Rao, K.V.R.; Robertson, K.N.; Cameron, T.S.J. Zeolite-confined nano-RuO2: A green, selective, and efficient catalyst for aerobic alcohol oxidation. Am. Chem. Soc. 2003, 125, 2195. [Google Scholar] [CrossRef] [PubMed]
- Sun, J.; Han, Y.; Fu, H.; Qu, X.; Xu, Z.; Zheng, S. Au@ Pd/TiO2 with atomically dispersed Pd as highly active catalyst for solvent-free aerobic oxidation of benzyl alcohol. Chem. Eng. J. 2017, 313, 1–9. [Google Scholar] [CrossRef]
- Chen, Y.; Lim, H.; Tang, Q.; Gao, Y.; Sun, T.; Yan, Q.; Yang, Y. Solvent-free aerobic oxidation of benzyl alcohol over Pd monometallic and Au–Pd bimetallic catalysts supported on SBA-16 mesoporous molecular sieves. Appl. Catal. A Gen. 2010, 380, 55–65. [Google Scholar] [CrossRef]

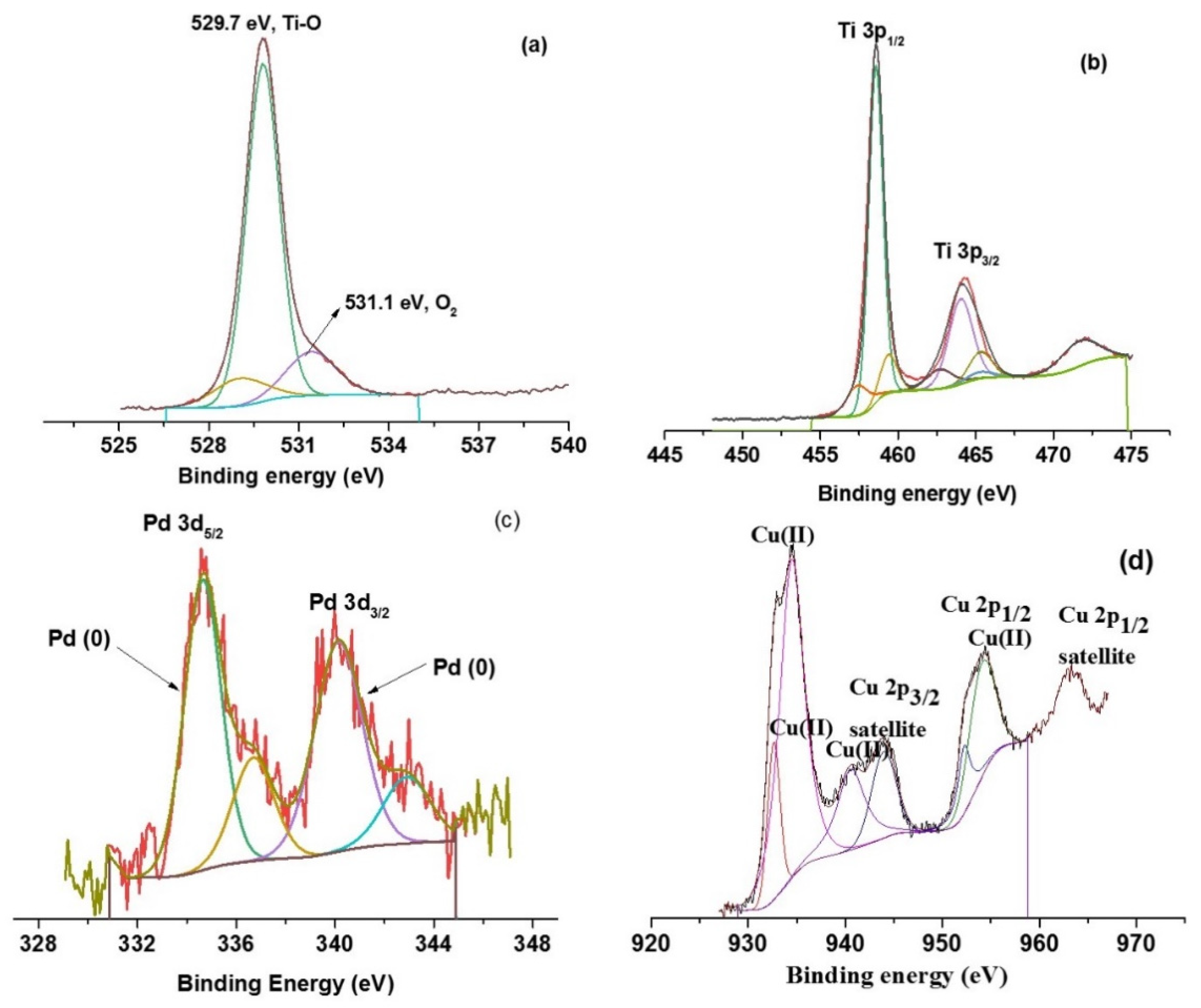
| Sample | Ti2P a | O 1S a | Pd3d a | Cu2p a | Actual At.(%) Pd b | Actual At (%) Cu b |
|---|---|---|---|---|---|---|
| 1%Pd/TiO2 | 459.24 (23.34) | 530.8 (55.64) | 336.1 (0.41) | - | 0.87 | - |
| 1% Cu/TiO2 | 459.27 (22.81) | 530.8 (57.69) | - | 921.2 (0.25) | - | 0.91 |
| 0.75Pd-0.25Cu/TiO2 | 459.33 (21.05) | 530.9 (55.4) | 335.8 (0.27) | 933.2 (0.07) | 0.64 | 0.22 |
| 0.25Pd-0.75Cu/TiO2 | 459.28 (20.84) | 530.9 (58.61) | 335.5 (0.17) | 938.1 (0.21) | 0.21 | 0.69 |
| 0.5Pd-0.5Cu/TiO2 | 459.24 (20.67) | 530.7 (54.47) | 335.7 (0.39) | 937.1 (0.22) | 0.43 | 0.46 |
| Catalyst | Conversion % | Selectivity to Benzaldehyde % | TON a | TOF (h−1) b |
|---|---|---|---|---|
| Pd/TiO2 | 92.2 | 88.3 | 193.02 | 196.51 |
| Cu/TiO2 | 1.1 | 99.9 | 0.354 | 0.708 |
| 0.75Pd-0.25Cu/TiO2 | 92.7 | 96.2 | 131.91 | 263.81 |
| 0.25Pd-0.75Cu/TiO2 | 11.4 | 98.2 | 98.90 | 49.43 |
| 0.5Pd-0.5Cu/TiO2 | 59.9 | 98.7 | 227.30 | 113.65 |
| Temperature °C | Conversion % | Selectivity % | |
|---|---|---|---|
| Benzaldehyde | Toluene | ||
| 50 | 3.3 | 44.2 | 0.08 |
| 80 | 8.5 | 66.6 | 0.12 |
| 100 | 12.9 | 99.1 | 0.3 |
| 110 | 26.1 | 98.7 | 0.7 |
| 120 | 35.9 | 98.7 | 1 |
| 130 | 67.1 | 96.6 | 2.9 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alshammari, H.M. Synthesis of Palladium and Copper Nanoparticles Supported on TiO2 for Oxidation Solvent-Free Aerobic Oxidation of Benzyl Alcohol. Processes 2021, 9, 1590. https://doi.org/10.3390/pr9091590
Alshammari HM. Synthesis of Palladium and Copper Nanoparticles Supported on TiO2 for Oxidation Solvent-Free Aerobic Oxidation of Benzyl Alcohol. Processes. 2021; 9(9):1590. https://doi.org/10.3390/pr9091590
Chicago/Turabian StyleAlshammari, Hamed M. 2021. "Synthesis of Palladium and Copper Nanoparticles Supported on TiO2 for Oxidation Solvent-Free Aerobic Oxidation of Benzyl Alcohol" Processes 9, no. 9: 1590. https://doi.org/10.3390/pr9091590
APA StyleAlshammari, H. M. (2021). Synthesis of Palladium and Copper Nanoparticles Supported on TiO2 for Oxidation Solvent-Free Aerobic Oxidation of Benzyl Alcohol. Processes, 9(9), 1590. https://doi.org/10.3390/pr9091590






