1. Introduction
Production and applications of carbon-based nanomaterials (CNMs) occupies a very important place in nanotechnology research and development. CNMs with different forms including carbon nanotubes (CNTs), carbon nanofibers (CNFs), etc., with their unique physical and chemical properties are still believed to offer solutions for environmental and technical challenges [
1,
2,
3,
4].
Super-hydrophobic CNMs, which exhibit contact angles (CA) > 150°, played serious roles in tackling many technical problems related to chemical and physical natures of surfaces and contacts. Therefore, super-hydrophobic CNMs have been implemented in various applications, including drug delivery materials [
5], adsorbents [
6], antifouling and self-healing membranes [
7], and others [
8,
9]. The hydrophobicity of carbonaceous materials is mainly dependent on the roughness and surface chemistry of the synthesized samples [
8]. CNMs’ growth over other materials can influence the chemistry and roughness of the surface of the synthesized nanomaterial [
9]. Thus, emphasis should be given to synthesize the carbon superstructures containing different types of hybrids to ensure super-hydrophobic characteristics by optimizing its roughness and physiochemical properties.
Until recently, several methods have been used to synthesize CNMs including chemical vapor deposition method (CVD) [
10], carbon arc discharge method (CA) [
11], high-pressure carbon monoxide conversion (HiPco) process [
12], and pulsed laser vaporization technique (PLV) [
13]. The superior quality of produced nanomaterials can be assured using CA and PLV methods. However, the application of CA method is restricted due to the high processing temperature of around 2700 °C needed to evaporate the carbon atoms from solid carbon sources; while the PLV method requires vacuum conditions and continuous graphite target replacement [
14]. Thus, the scale-up of these production processes for commercialization purposes is difficult. Hence, for larger-scale production, the CVD method has gained much more attention than the others [
15]. The CVD method is considered the most suitable synthesis method as it can ensure the product’s high quality and quantity simultaneously [
16]. Materials like silica [
17,
18], alumina [
19,
20], zeolite [
21], and recently MgO [
22,
23] were used as supports for active metals to develop different types of nanostructured carbons like single walled nanotubes (SWNTs), multi walled nanotube (MWNTs), and nanofibers. Nevertheless, powder activated carbon (PAC) is considered to be the most suitable precursor in this regard due to its economic feasibility and unique features, including high thermal stability, high surface area, and prospect for chemical modifications by simple means [
24,
25]. Nano to micro dimensional carbon having different proportions of graphitic or disordered regions in carbon–carbon composites can induce superior properties based on the usage of suitable catalysts and process parameter optimization [
26]. PAC has been used to synthesize carbon nanofibers (CNFs) using acetylene (C
2H
2) and iron (Fe) catalysts in the CVD process [
27,
28]. On the other hand, modification of CNMs structure has been done by other researchers to achieve artificial super-hydrophobic surfaces. Optimization of the super-hydrophobic CNMs synthesis process could be hampered due to the aggregation nature of CNMs and usually, it comprises impurities [
29]. The hybrid PAC–CNMs maintains the chemical compatibility between these two materials and combines the favorable characteristics of both.
Catalysts commonly used for CNTs growth are some transition metals like iron (Fe), cobalt (Co), and nickel (Ni) [
27]. Lots of literature was already devoted to the CNT growth using different metals and their alloys [
30]. Transition metals have empty ‘d’ shells which enable them to interact with hydrocarbons resulting in greater catalytic activity. The metallic particles used in the CVD process serve as seeding agents for the nanotubes, subsequently, they robustly control the configuration and quality of the finally developed materials. For the decomposition of hydrocarbons, transition metals require support for the successful growth of nanotubes. It was found that not only growth rate and diameter are highly influenced by the catalyst type and composition, but also the microstructure and morphology [
31]. Moreover, the synthesis of CNMs from hydrocarbons is affected by the bimetallic catalysts’ synergistic effects [
32,
33,
34]. For example, the growth of CNMs was improved by using bimetallic catalysts such as Fe–Co, Co–Mo, and Fe–Ni [
35,
36,
37,
38,
39]. All the above-mentioned catalysts (single or bimetallic catalysts) were successfully used with substrates other than PAC in most cases due to the difficulties in forming metallic nanoclusters on the surface of PAC with conventional methods.
Combining more than one catalyst at the same time affects the characteristics of the grown CNMs due to the interaction between the substrate surface and different metal clusters. In this study, the combination of bi-catalyst (Fe/Co), which deposited on PAC as a substrate, is considered to produce super-hydrophobic CNMs with high yield and effective performance. Moreover, the reaction is also investigated as an effective process parameter in thermal CVD to decompose acetylene as a carbon source at 650 °C. The optimum carbon yield (CY) and contact angle (CA) were the objective functions used for the production process while the catalyst’s composition and reaction time were regarded as the explanatory variables in the response surface method (RSM) approach. The ultimate goal of this study is to produce super-hydrophobic CNMs to be utilized as material composites for several applications such as sorption [
6], membrane distillation [
40], separation of organic mixtures, purification of water by adsorption techniques, and catalysis [
41].
2. Materials and Methods
2.1. Materials and Reagents
Iron nitrate Fe (NO3)3·9H2O, cobaltous nitrate hexahydrate Co(NO3)26H2O, PAC, and acetone were purchased from Sigma Aldrich, Kuala Lumpur, Malaysia. Acetylene gas (C2H2), hydrogen gas (H2), and nitrogen gas (N2) were purchased from GasLink Industrial Gases SDN BHD, Selangor, Malaysia. Analytical grade reagents and chemicals were used in this research. Thus, no additional purification step was necessary for conducting the experiments.
2.2. Synthesis of Binary Metal Catalyst
The bimetallic catalysts (iron and cobalt) were first dissolved in acetone using their salts. Then the incipient wetness method was used to deposit the catalyst over the surface of the PAC. Based on
Table 1, different weight percent (
w/w%) of catalyst samples were prepared. The ratios between PAC and catalysts were calculated based on the experimental design matrix provided by the Box-Behnken design (BBD). According to
Table 1, the catalyst solution was prepared by dissolving the desired amount of the catalysts in 5 mL of acetone solution. The mixture was kept inside the universal glass bottle and stirred until all the catalysts salts were completely dissolved.
2.3. Powdered Activated Carbon Impregnation
The metallic catalysts (iron and cobalt) were impregnated on PAC. After the catalysts were dissolved in acetone, PAC (2 g) was mixed with that catalyst–solvent mixture in a glass bottle. The glass bottles containing catalyst dissolved in acetone and PAC were sonicated at 40 kHz and 60 °C to evaporate the solvent. The mixture was further dried at 100 °C overnight using a conventional drying oven. After drying, the mixture was crushed to powder. The powder mixture thus obtained was stored using a desiccator to prevent moisture adsorption and sent for further characterization and application.
2.4. Synthesis of Carbon Nanostructured Materials (CNMs)
The catalysts of desired ratio was mixed with PAC and transferred to a ceramic boat (50 mm OD, 40 mm ID, 1500 mm L). This boat was then inserted inside the CVD reaction tube. The inert atmosphere was created by the flow of nitrogen (N2) gas at a flow rate of 200 mL/min inside the CVD reactor. The sample was first heated at 350 °C for 2 h under this inert blanket condition to prevent burning and ash formation. Hydrogen gas (200 mL/min) was passed at 450 °C for 2 h inside the CVD reactor to ensure the reduction process of the calcined sample. The sample then was cooled down to room temperature. At this stage, the amount of moisture present in the sample was determined by weighing the sample (WC).
Under atmospheric pressure, the reduced catalyst mixture was placed inside the tubular ceramic reactor. Inside the reactor, a mixture of acetylene (50 mL/min) and hydrogen (200 mL/min) gas was flown. For facilitating the growth of CNMs, acetylene to hydrogen gas ratio was kept at 1:4 and the reaction was carried out at 650 °C for different reaction time as provided by the design matrix. After the reaction was completed, the reactor was cooled down using N
2 gas flow (200 mL/min). The weight (WP) of the synthesized CNMs was taken. The carbon yield was calculated using Equation (1).
where
Wp and
Wc are the weight of the sample after and before the reaction, respectively.
2.5. Equipment and Measurements
All the weighing measurements were recorded by using four digits weighing balance (HR-202i, Japan) with a measurement range between 0.001 and 220 g. PAC was mixed with the catalyst solution and the resultant mixture was placed inside the ultrasonic bath model (JAC 2010 P, Gyeonggi-Do, Korea) to ensure proper impregnation of metal catalysts onto the PAC substrate. The path is equipped with three levels of sonication, timer up to 99 min, and heater up to 90 °C. The drying of the PAC and CNMs samples was carried out in a drying oven (Model 600-Memmert, Büchenbach, Germany), where the maximum temperature can be set up to 220 °C. The CVD process was carried out in situ using an OTF-1200-80 mm dual-zone tube furnace for the CNMs growth. The tube furnace contains a fused quartz tube having the dimensions (OD: 80 mm; ID: 72 mm; length: 1000 mm). The heating area was covered with a resistance heating glass wool procured from Isolite Ceramic Fiber Sdn. Bhd., Johor, Malaysia.
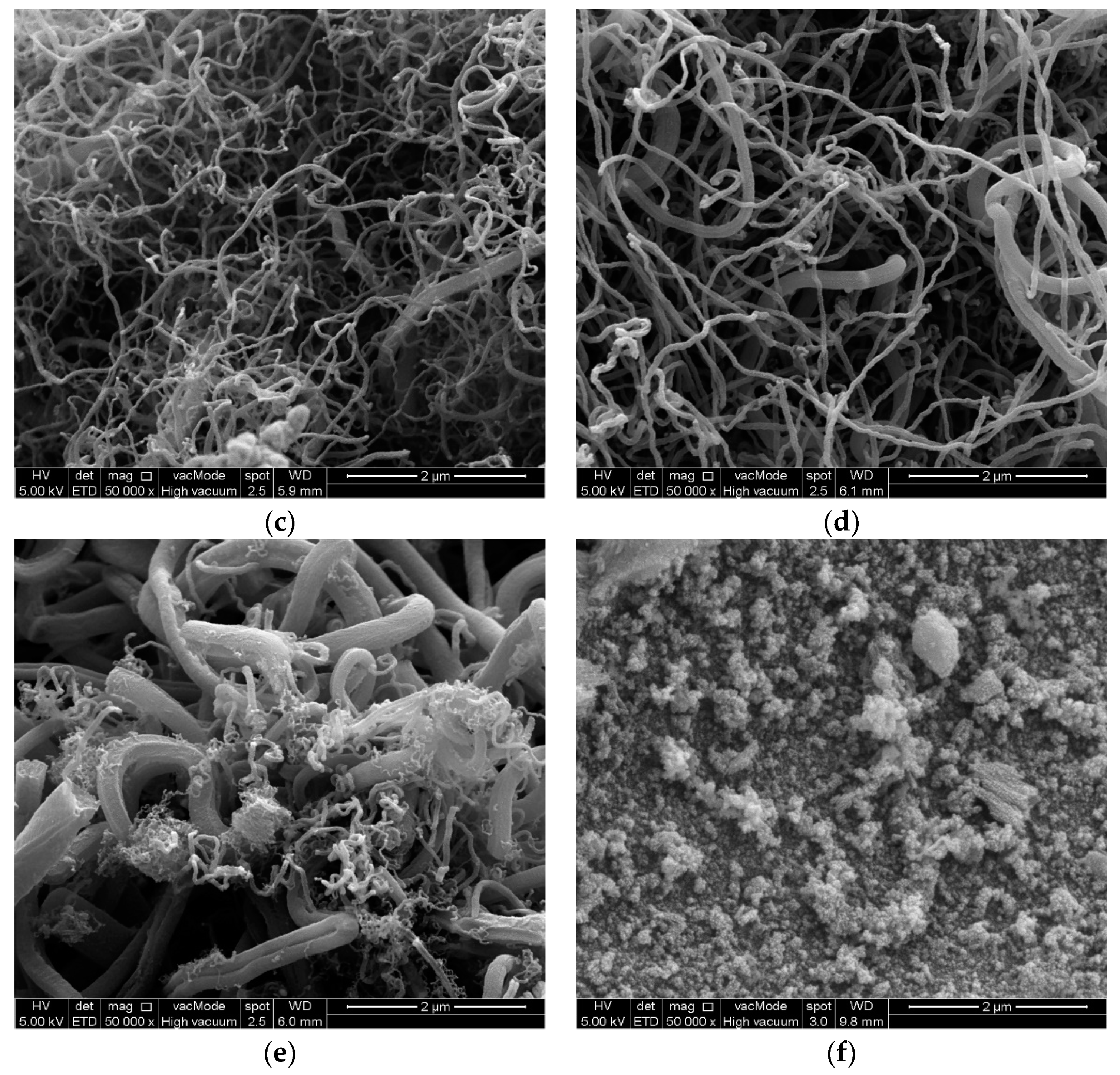
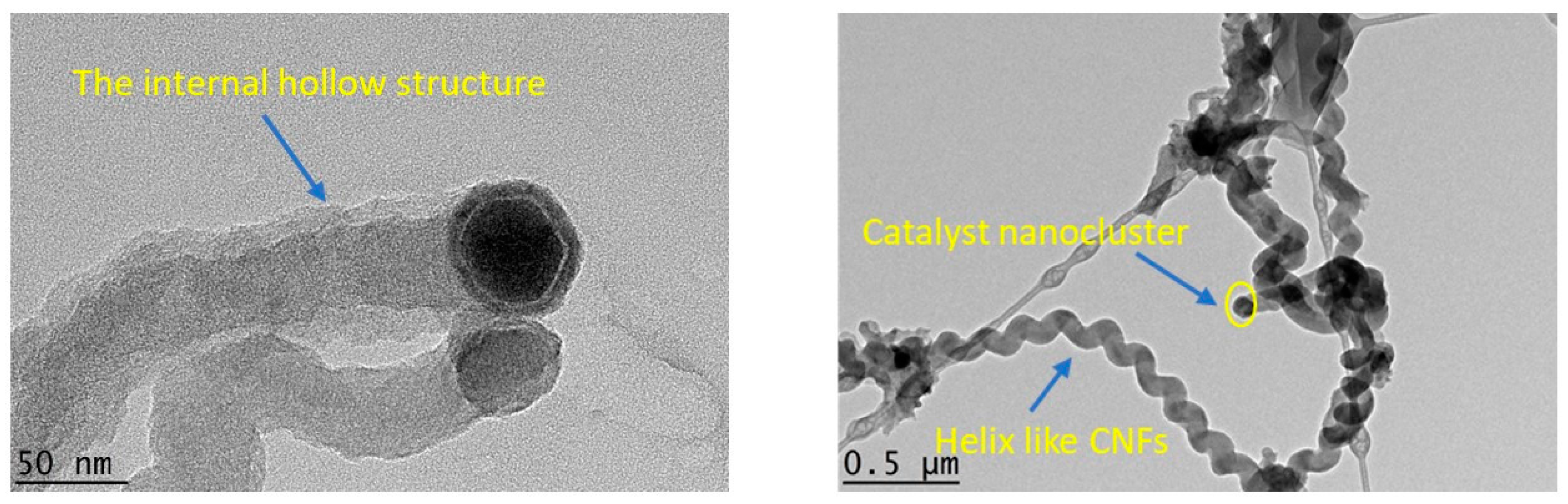
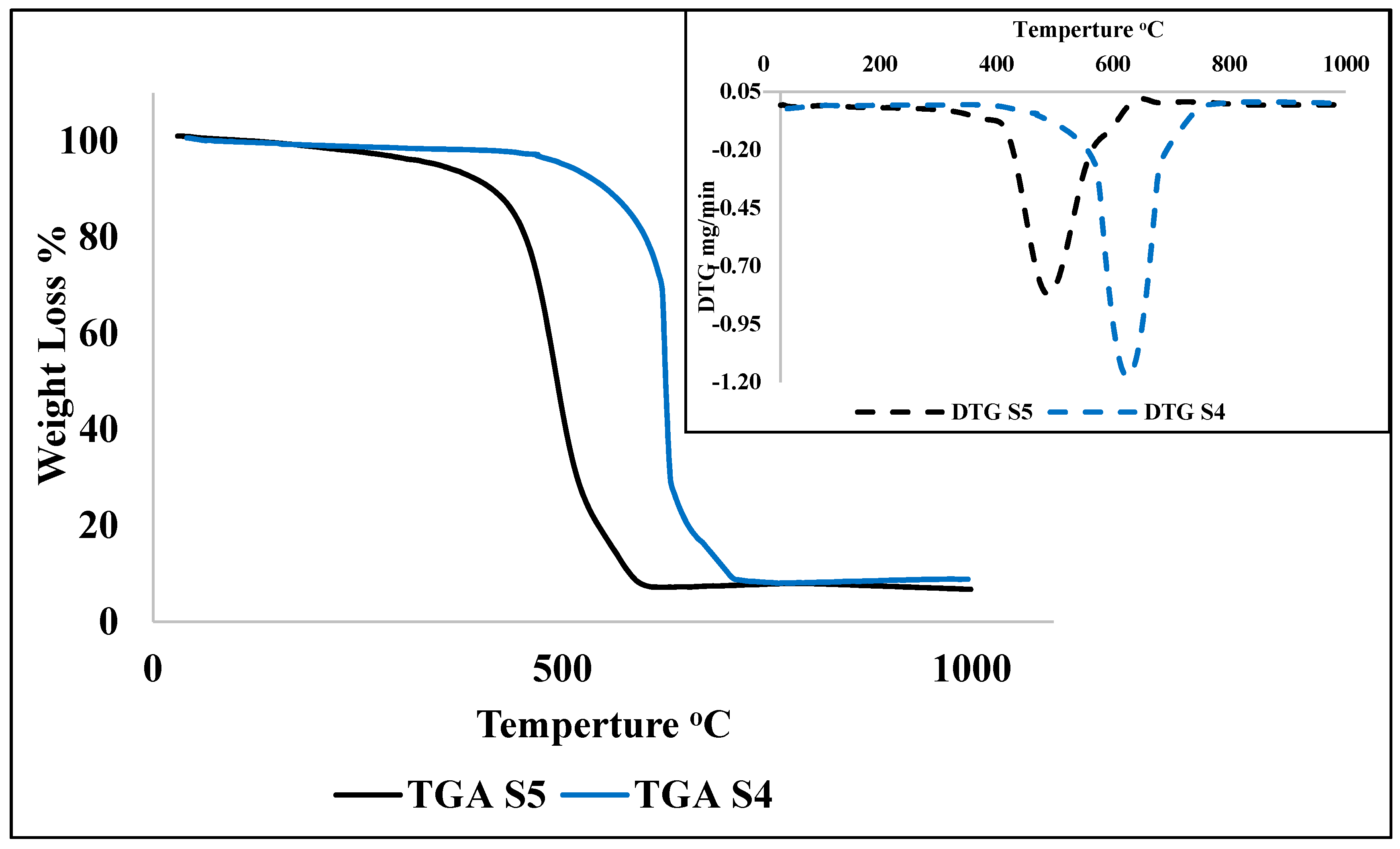
Characterization of the synthesized samples was carried out to classify the type and shape of nanomaterials. Field emission scanning electron microscopic analysis (FESEM-Hitachi-SU8000, Ibaraki, Japan) was used to observe the morphology of the prepared samples. The aluminum stubs were coated by a platinum layer by sputtering and the synthesized sample was placed over it for FESEM analysis. Transmission electron microscopy (TEM) observations were made with a Hitachi-HT7700, Ibaraki, Japan microscope at 120 kV. Synthesized samples were mixed with acetone and the mixture was ultrasonicated. After ultrasonication, a drop of the sample was deposited over the copper grid supported perforated carbon film. The average diameter and particle size distribution of the nanomaterials were calculated using ImageJ software 1.8.0_112. Contact angle (CA) measurement was carried out by KRUSS Goniometer (DSA100). A glass microscope slide (76 × 26 × 1.2 mm) covered with double-sided adhesive tape was used to measure the CA between the nanomaterials and water, wherein a water drop (4 μL) was placed onto the CNMs surface pasted on a tape. The average of triplicate measurements for each sample was taken. The oxidation behavior of the prepared nanomaterials was examined by thermal gravimetric analysis (STA-851, Mettler Toledo, Polaris Parkway Columbus, USA) at a temperature range of 25–800 °C, a heating rate of 10 °C/min, and an oxygen flow rate of 20 mL/min. The Raman spectra of the CNMs were obtained, where the laser power was kept constant at 100 using an Ar + laser (514 nm) and it was focused (50× objective) over the spot having a size of around 1.5–2.0 μm (Renishaw in Via, Sheffield, UK).
2.6. Response Surface Methodology and Process
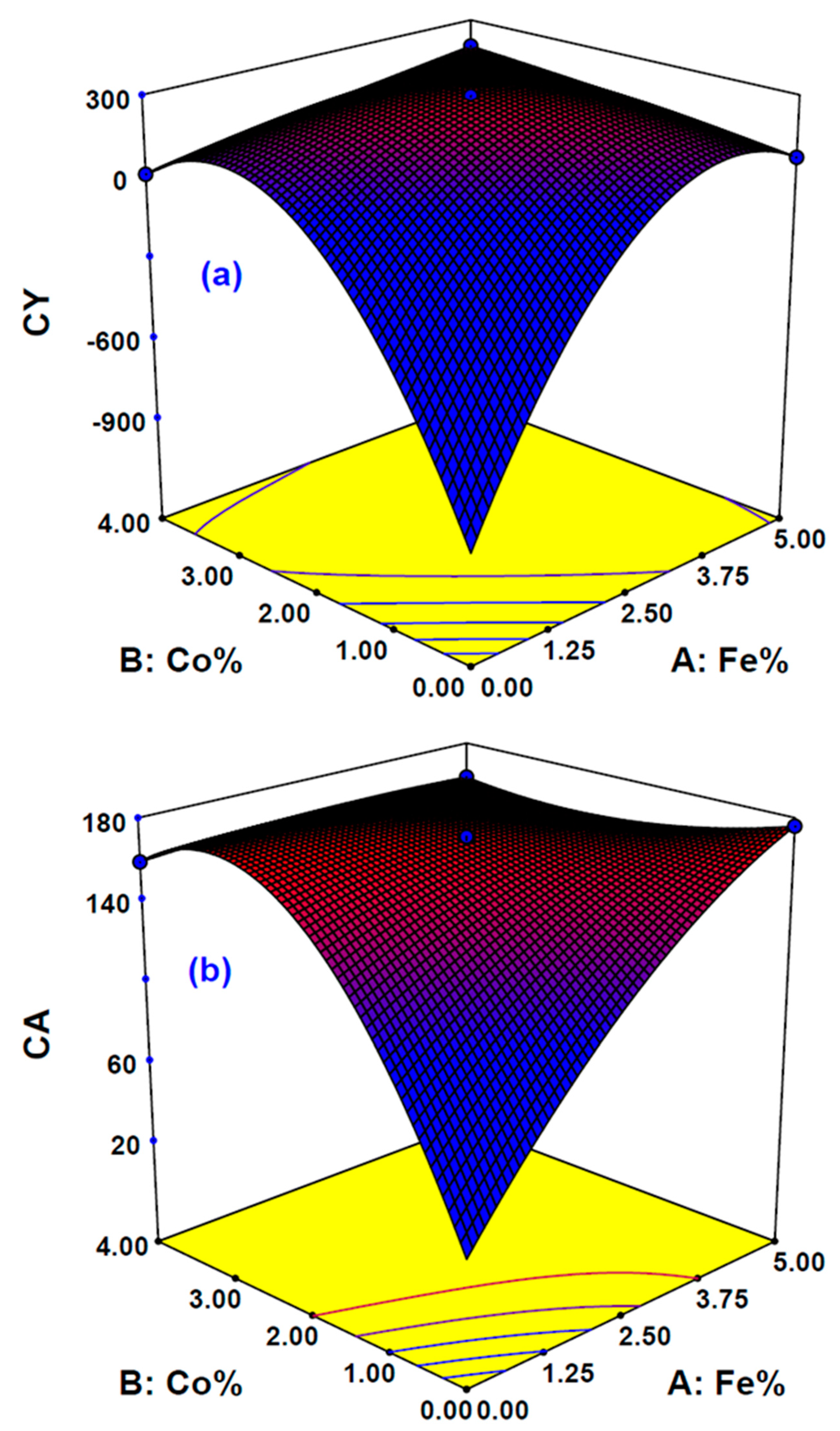
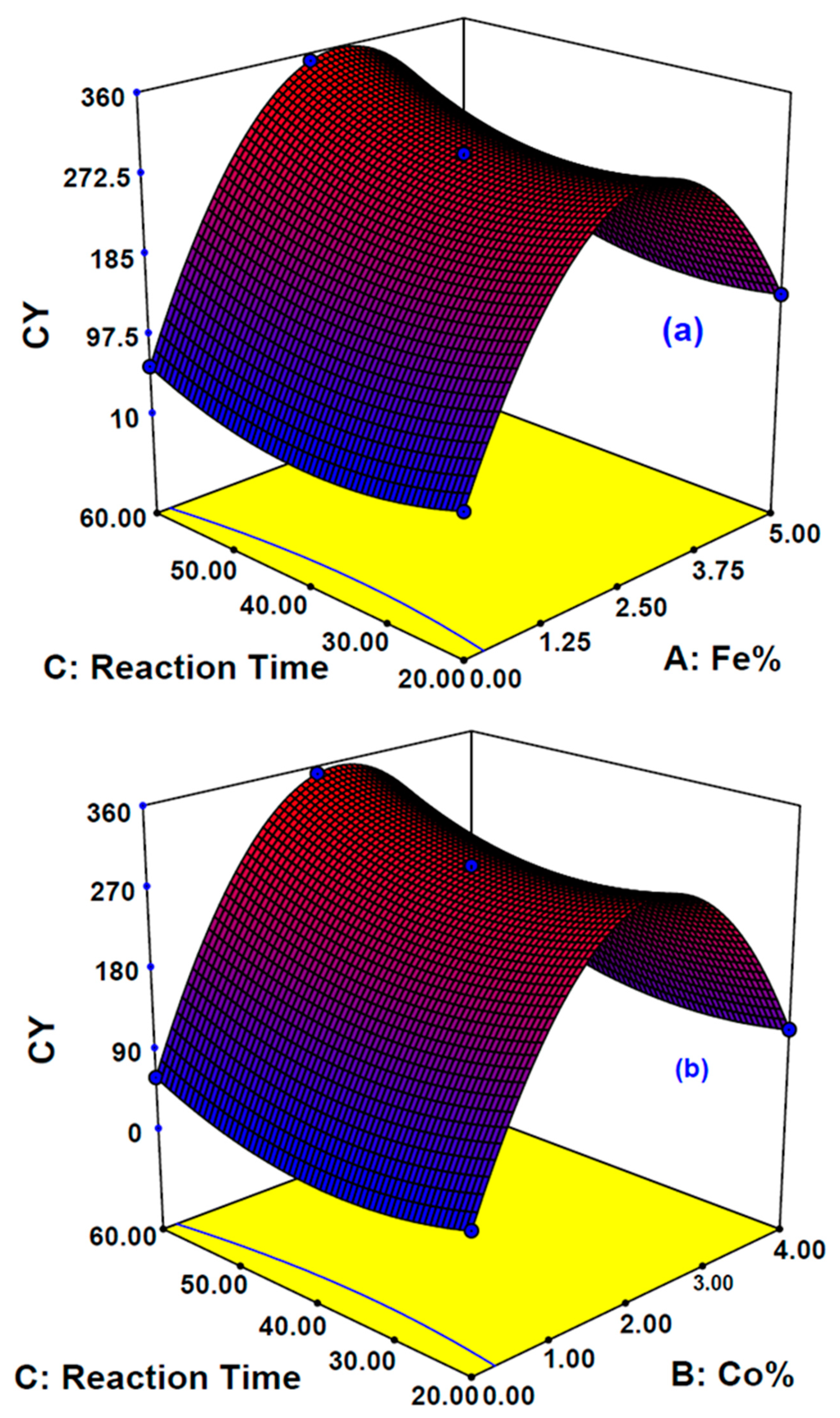
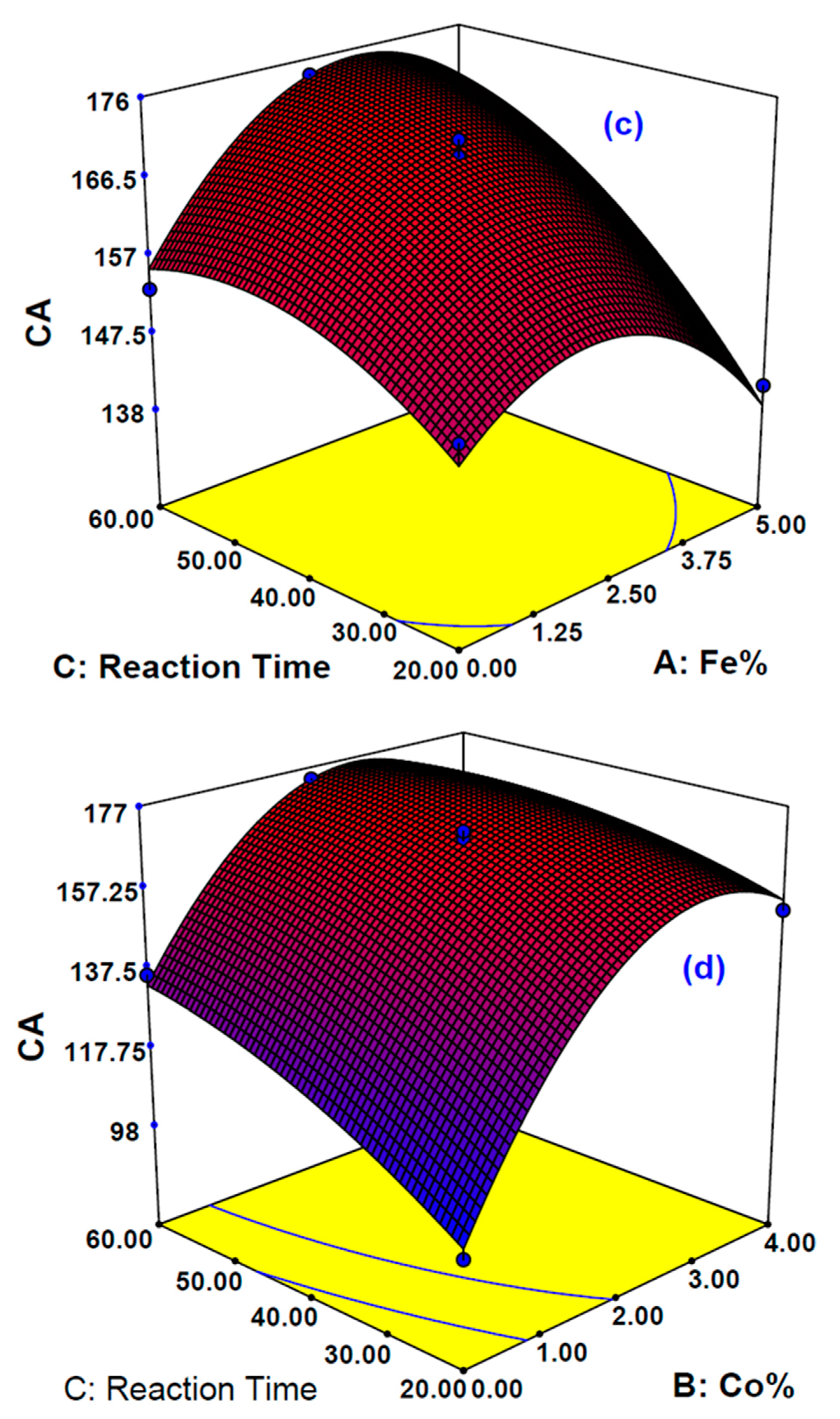
The influence of three sovereign variables, Fe%, Co%, and reaction time on the two responses, carbon yield (CY) and contact angle (CA), were determined. Design-Expert V7.0 software was used and the Box-Behnken design (BBD) was employed by using the response surface methodology (RSM) approach. The influence of the main and combined variables on the desired responses chosen here was examined. A total of 17 experimental runs were proposed by DOE based on BBD with three center points. The independent variable ranges studied were Fe% (0–5%), Co% (0–4%), and reaction time (20–60 min), while gas ratios, temperature, and gas type were fixed at acetylene to hydrogen ratio of 1:4, 650 °C, acetylene gas, respectively. The complete design matrix conforming the actual experimental design and the responses are illustrated by
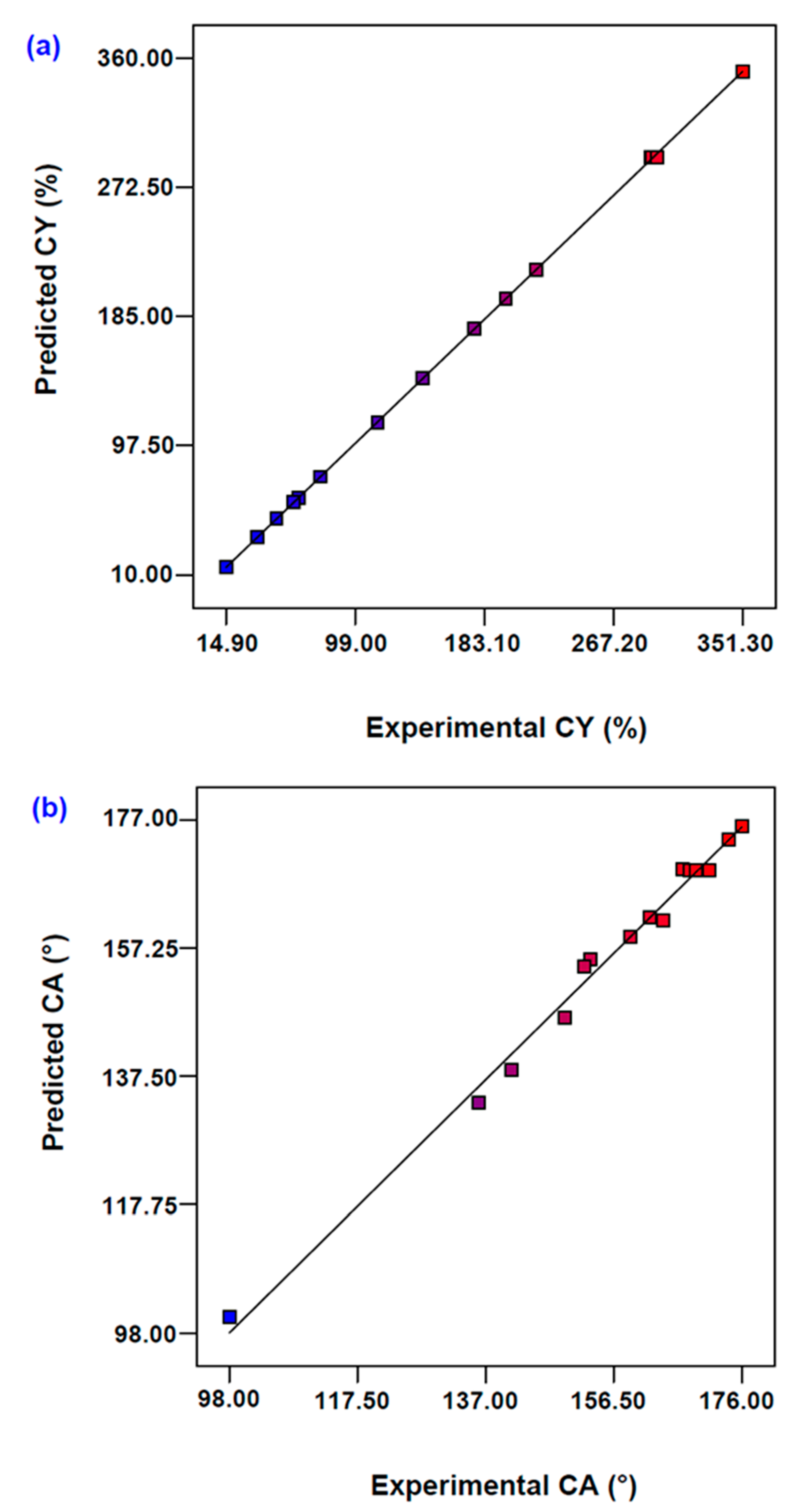
Table 1. The accuracy of the developed models with data reproducibility was observed using the analysis of variance (ANOVA) test. The coefficient of determination (R
2) values was calculated to observe the competency level of the proposed model.