Electrochemical Reduction of CO2 to CO on Hydrophobic Zn Foam Rod in a Microchannel Electrochemical Reactor
Abstract
:1. Introduction
2. Materials and Methods
2.1. Materials and Chemicals
2.2. Preparation of Zinc Foam and Hydrophobic Zinc Foam
2.3. Electrocatalytic Tests and Product Inspection
3. Results and Discussion
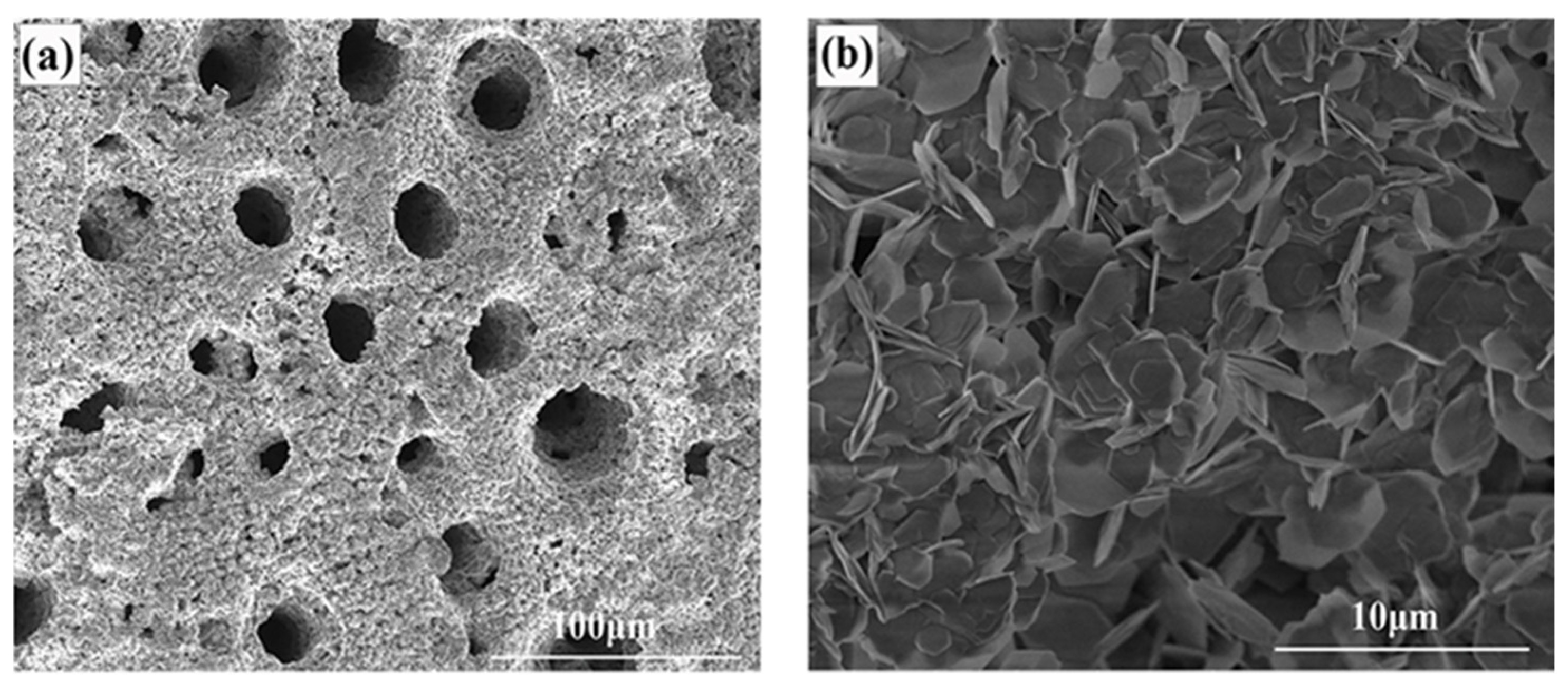
3.1. Characterization of the Electrode
3.2. The Formation Mechanism of the Hydrophobic Layer
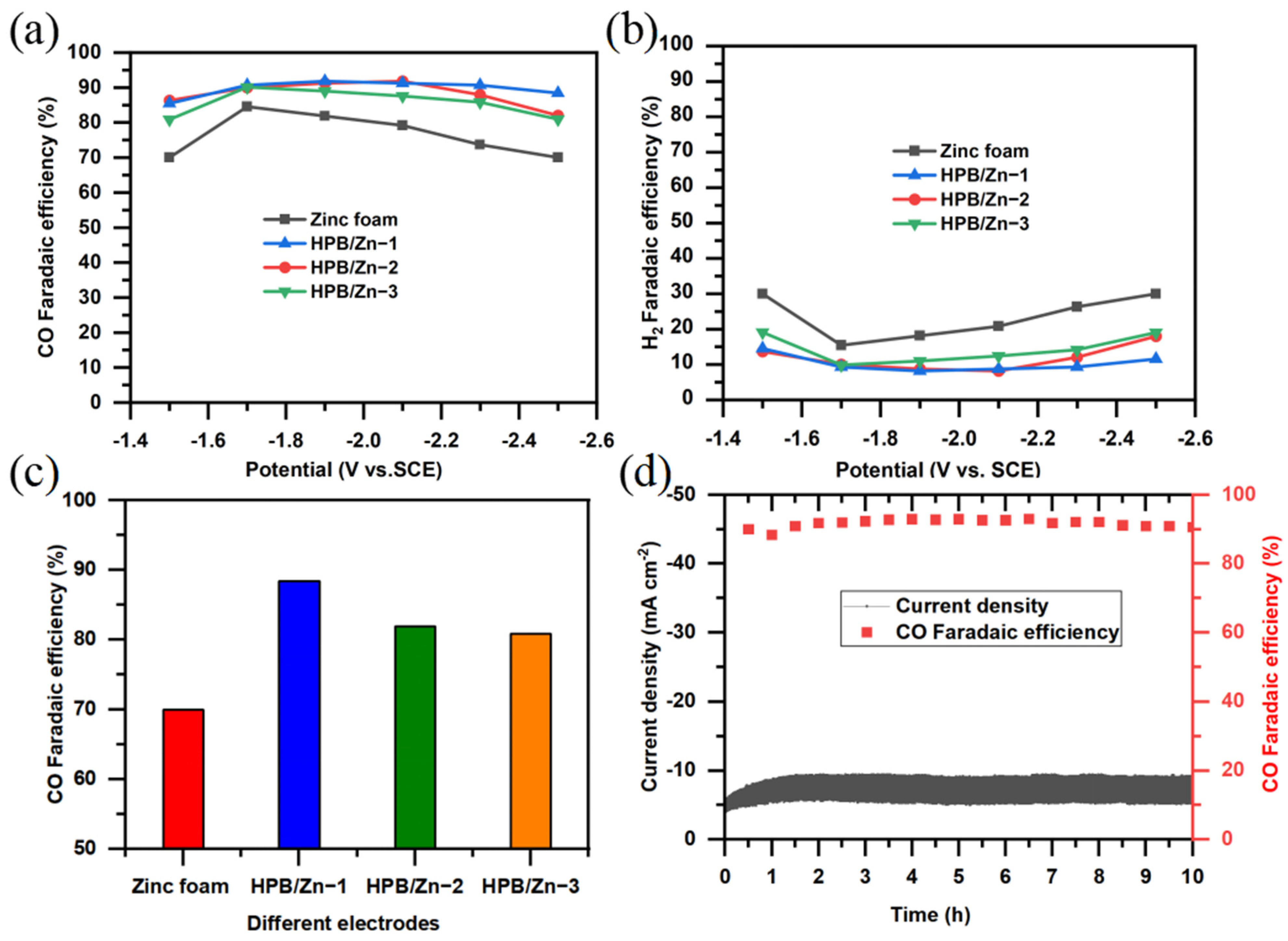
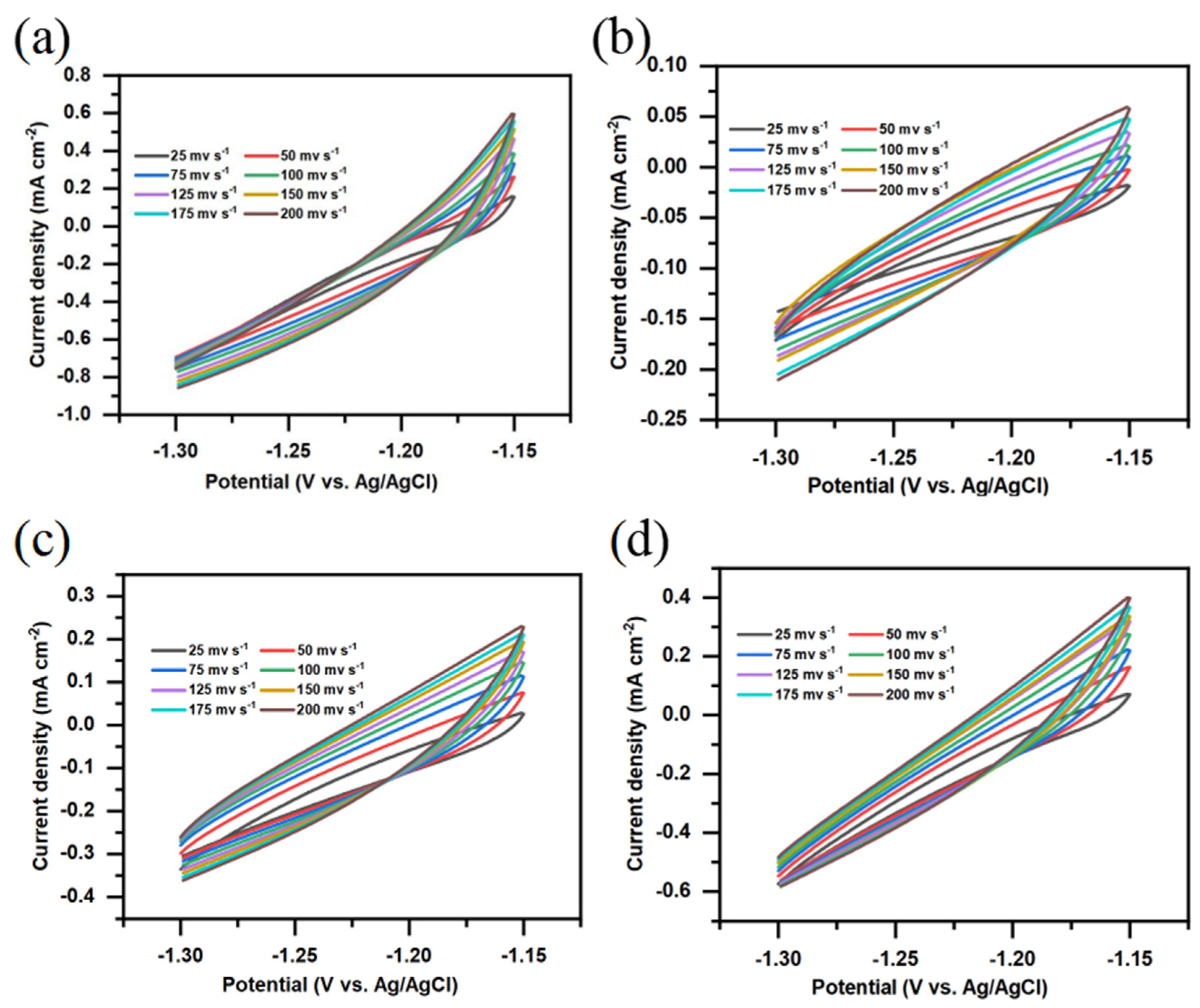
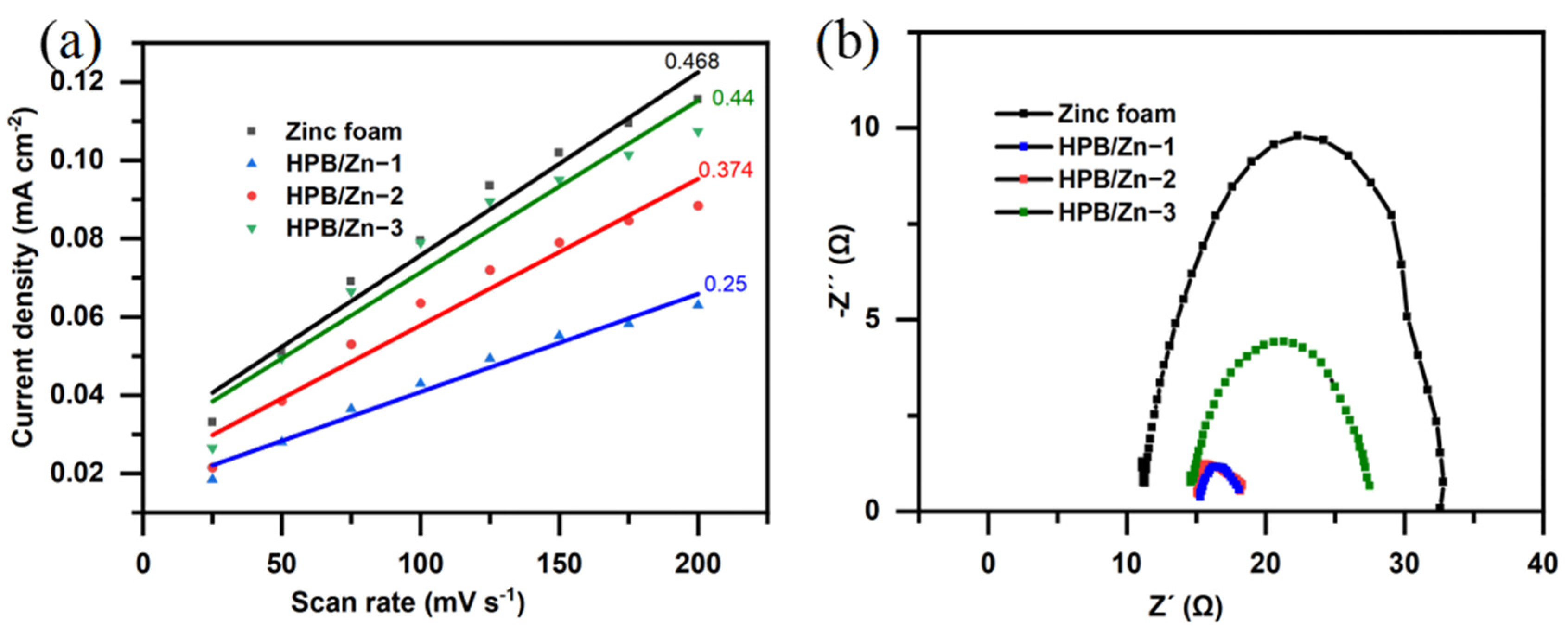
3.3. Performance on CO2 Reduction
4. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Park, G.; Hong, S.; Choi, M.; Lee, S.; Lee, J. Au on highly hydrophobic carbon substrate for improved selective CO production from CO2 in gas-phase electrolytic cell. Catal. Today 2020, 355, 340–346. [Google Scholar] [CrossRef]
- Zhai, P.; Gu, X.; Wei, Y.; Zuo, J.; Chen, Q.; Liu, W.; Jiang, H.; Wang, X.; Gong, Y. Enhanced mass transfer in three-dimensional single-atom nickel catalyst with open-pore structure for highly efficient CO2 electrolysis. J. Energy Chem. 2021, 62, 43–50. [Google Scholar] [CrossRef]
- Luo, W.; Zhang, J.; Li, M.; Züttel, A. Boosting CO Production in Electrocatalytic CO2 Reduction on Highly Porous Zn Catalysts. ACS Catal. 2019, 9, 3783–3791. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.; Li, C.W.; Kanan, M.W. Aqueous CO2 reduction at very low overpotential on oxide-derived Au nanoparticles. J. Am. Chem. Soc. 2012, 134, 19969–19972. [Google Scholar] [CrossRef]
- Li, Y.; Chen, C.; Cao, R.; Pan, Z.; He, H.; Zhou, K. Dual-atom Ag2/graphene catalyst for efficient electroreduction of CO2 to CO. Appl. Catal. B Environ. 2020, 268, 118747. [Google Scholar] [CrossRef]
- Jee, M.S.; Jeon, H.S.; Kim, C.; Lee, H.; Koh, J.H.; Cho, J.; Min, B.K.; Hwang, Y.J. Enhancement in carbon dioxide activity and stability on nanostructured silver electrode and the role of oxygen. Appl. Catal. B Environ. 2016, 180, 372–378. [Google Scholar] [CrossRef]
- Koh, J.H.; Jeon, H.S.; Jee, M.S.; Nursanto, E.B.; Lee, H.; Hwang, Y.J.; Min, B.K. Oxygen Plasma Induced Hierarchically Structured Gold Electrocatalyst for Selective Reduction of Carbon Dioxide to Carbon Monoxide. J. Phys. Chem. C 2014, 119, 883–889. [Google Scholar] [CrossRef]
- Rosen, J.; Hutchings, G.S.; Lu, Q.; Forest, R.V.; Moore, A.; Jiao, F. Electrodeposited Zn Dendrites with Enhanced CO Selectivity for Electrocatalytic CO2 Reduction. ACS Catal. 2015, 5, 4586–4591. [Google Scholar] [CrossRef]
- Jiang, X.; Cai, F.; Gao, D.; Dong, J.; Miao, S.; Wang, G.; Bao, X. Electrocatalytic reduction of carbon dioxide over reduced nanoporous zinc oxide. Electrochem. Commun. 2016, 68, 67–70. [Google Scholar] [CrossRef]
- Xiao, J.; Gao, M.R.; Liu, S.; Luo, J.L. Hexagonal Zn Nanoplates Enclosed by Zn (100) and Zn (002) Facets for Highly Selective CO2 Electroreduction to CO. ACS Appl. Mater. Interfaces 2020, 12, 31431–31438. [Google Scholar] [CrossRef]
- Lu, Y.; Han, B.; Tian, C.; Wu, J.; Geng, D.; Wang, D. Efficient electrocatalytic reduction of CO2 to CO on an electrodeposited Zn porous network. Electrochem. Commun. 2018, 97, 87–90. [Google Scholar] [CrossRef]
- Xiang, Q.; Li, F.; Wang, J.; Chen, W.; Miao, Q.; Zhang, Q.; Tao, P.; Song, C.; Shang, W.; Zhu, H.; et al. Heterostructure of ZnO Nanosheets/Zn with a Highly Enhanced Edge Surface for Efficient CO2 Electrochemical Reduction to CO. ACS Appl. Mater. Interfaces 2021, 13, 10837–10844. [Google Scholar] [CrossRef]
- Guo, M.; Li, X.; Huang, Y.; Li, L.; Li, J.; Lu, Y.; Xu, Y.; Zhang, L. CO2-Induced Fibrous Zn Catalyst Promotes Electrochemical Reduction of CO2 to CO. Catalysts 2021, 11, 477. [Google Scholar] [CrossRef]
- Raciti, D.; Mao, M.; Park, J.H.; Wang, C. Mass transfer effects in CO2 reduction on Cu nanowire electrocatalysts. Catal. Sci. Technol. 2018, 8, 2364–2369. [Google Scholar] [CrossRef]
- Vennekoetter, J.-B.; Sengpiel, R.; Wessling, M. Beyond the catalyst: How electrode and reactor design determine the product spectrum during electrochemical CO2 reduction. Chem. Eng. J. 2019, 364, 89–101. [Google Scholar] [CrossRef]
- Wakerley, D.; Lamaison, S.; Ozanam, F.; Menguy, N.; Mercier, D.; Marcus, P.; Fontecave, M.; Mougel, V. Bio-inspired hydrophobicity promotes CO2 reduction on a Cu surface. Nat. Mater. 2019, 18, 1222–1227. [Google Scholar] [CrossRef]
- Li, A.; Cao, Q.; Zhou, G.; Schmidt, B.; Zhu, W.; Yuan, X.; Huo, H.; Gong, J.; Antonietti, M. Three-Phase Photocatalysis for the Enhanced Selectivity and Activity of CO2 Reduction on a Hydrophobic Surface. Angew. Chem. 2019, 58, 14549–14555. [Google Scholar] [CrossRef] [PubMed]
- Lin, J.; Tian, J.; Cheng, X.; Tan, J.; Wan, S.; Lin, J.; Wang, Y. Propane ammoxidation over MoVTeNb oxide catalyst in a microchannel reactor. AIChE J. 2018, 64, 4002–4008. [Google Scholar] [CrossRef]
- Yue, J.; Chen, G.; Yuan, Q.; Luo, L.; Gonthier, Y. Hydrodynamics and mass transfer characteristics in gas–liquid flow through a rectangular microchannel. Chem. Eng. Sci. 2007, 62, 2096–2108. [Google Scholar] [CrossRef]
- Zhang, F.; Chen, C.; Tang, Y.; Cheng, Z. CO2 reduction in a microchannel electrochemical reactor with gas-liquid segmented flow. Chem. Eng. J. 2020, 392, 124798. [Google Scholar] [CrossRef]
- Liu, Y.; Xue, J.; Luo, D.; Wang, H.; Gong, X.; Han, Z.; Ren, L. One-step fabrication of biomimetic superhydrophobic surface by electrodeposition on magnesium alloy and its corrosion inhibition. J. Colloid Interface Sci. 2017, 491, 313–320. [Google Scholar] [CrossRef] [PubMed]
- Yang, Z.; Liu, X.; Tian, Y. Fabrication of super-hydrophobic nickel film on copper substrate with improved corrosion inhibition by electrodeposition process. Colloid Surf. A 2019, 560, 205–212. [Google Scholar] [CrossRef] [Green Version]
- Lv, K.; Teng, C.; Shi, M.; Yuan, Y.; Zhu, Y.; Wang, J.; Kong, Z.; Lu, X.; Zhu, Y. Hydrophobic and Electronic Properties of the E-MoS2 Nanosheets Induced by FAS for the CO2 Electroreduction to Syngas with a Wide Range of CO/H2 Ratios. Adv. Funct. Mater. 2018, 28, 1802339. [Google Scholar] [CrossRef]
- Weekes, D.M.; Salvatore, D.A.; Reyes, A.; Huang, A.; Berlinguette, C.P. Electrolytic CO2 Reduction in a Flow Cell. Acc. Chem. Res. 2018, 51, 910–918. [Google Scholar] [CrossRef]
- Cai, Z.; Zhang, Y.; Zhao, Y.; Wu, Y.; Xu, W.; Wen, X.; Zhong, Y.; Zhang, Y.; Liu, W.; Wang, H.; et al. Selectivity regulation of CO2 electroreduction through contact interface engineering on superwetting Cu nanoarray electrodes. Nano Res. 2018, 12, 345–349. [Google Scholar] [CrossRef]
- Yue, P.; Fu, Q.; Li, J.; Zhang, L.; Xing, L.; Kang, Z.; Liao, Q.; Zhu, X. Triple-phase electrocatalysis for the enhanced CO2 reduction to HCOOH on a hydrophobic surface. Chem. Eng. J. 2021, 405, 126975. [Google Scholar] [CrossRef]
- Xing, Z.; Hu, L.; Ripatti, D.S.; Hu, X.; Feng, X. Enhancing carbon dioxide gas-diffusion electrolysis by creating a hydrophobic catalyst microenvironment. Nat. Commun. 2021, 12, 136. [Google Scholar] [CrossRef]
- Qiao, J.; Liu, Y.; Hong, F.; Zhang, J. A review of catalysts for the electroreduction of carbon dioxide to produce low-carbon fuels. Chem. Soc. Rev. 2014, 43, 631–675. [Google Scholar] [CrossRef]
- Waszczuk, P.; Zelenay, P.; Sobkowski, J. Surface interaction of benzoic acid with a copper electrode. Electrochim. Acta 1995, 40, 5. [Google Scholar] [CrossRef]



| Electrodeposition Time | 0 | 0.5 min | 5 min | 10 min |
|---|---|---|---|---|
| Hydrophobic result | All infiltrated |  |  |  |
| Water contact angle | Almost zero | 136° | 141° | 149° |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zhang, C.; Yan, S.; Lin, J.; Hu, Q.; Zhong, J.; Zhang, B.; Cheng, Z. Electrochemical Reduction of CO2 to CO on Hydrophobic Zn Foam Rod in a Microchannel Electrochemical Reactor. Processes 2021, 9, 1592. https://doi.org/10.3390/pr9091592
Zhang C, Yan S, Lin J, Hu Q, Zhong J, Zhang B, Cheng Z. Electrochemical Reduction of CO2 to CO on Hydrophobic Zn Foam Rod in a Microchannel Electrochemical Reactor. Processes. 2021; 9(9):1592. https://doi.org/10.3390/pr9091592
Chicago/Turabian StyleZhang, Chunxiao, Shenglin Yan, Jing Lin, Qing Hu, Juhua Zhong, Bo Zhang, and Zhenmin Cheng. 2021. "Electrochemical Reduction of CO2 to CO on Hydrophobic Zn Foam Rod in a Microchannel Electrochemical Reactor" Processes 9, no. 9: 1592. https://doi.org/10.3390/pr9091592
APA StyleZhang, C., Yan, S., Lin, J., Hu, Q., Zhong, J., Zhang, B., & Cheng, Z. (2021). Electrochemical Reduction of CO2 to CO on Hydrophobic Zn Foam Rod in a Microchannel Electrochemical Reactor. Processes, 9(9), 1592. https://doi.org/10.3390/pr9091592










