Influence of Cellulose Characteristics on Pyrolysis Suitability
Abstract
:1. Introduction
2. Materials and Methods
2.1. Raw Materials
2.2. Cellulose Production and Chemical Composition
2.3. Degree of Polymerization
2.4. Crystallinity
2.5. Thermogravimetric Analysis
2.6. Kinetic Modeling
2.6.1. Ozawa–Flynn–Wall and ASTME-2070 Methods
2.6.2. Friedman Method
2.6.3. Kissinger–Akahira–Sunose (KAS) Method
3. Results and Discussion
3.1. Composition of Cellulose Pulps
3.2. Degree of Polymerization, Crystallinity and Crystal Size of Cellulose Pulps
3.2.1. Degree of Polymerization
3.2.2. Crystallinity and Crystal Size
3.3. TGA and DTG Study of Cellulose Pulps
3.4. Cellulose Pulps Kinetics
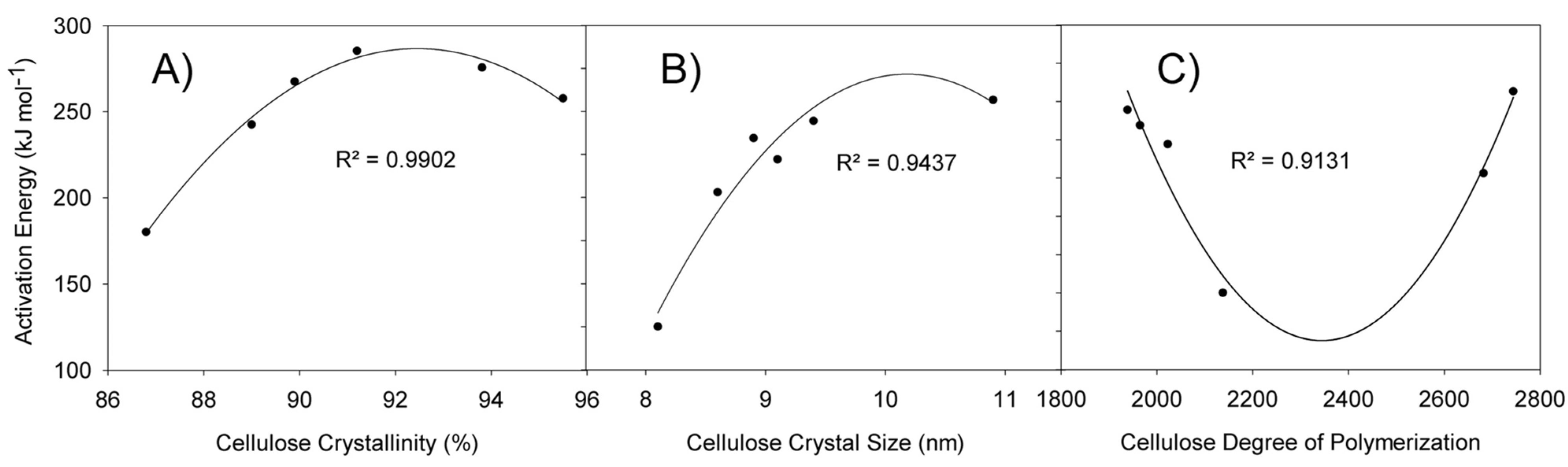
3.5. Kinetic Constants Dependence on Cellulose Characteristics
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Vooradi, R.; Anne, S.B.; Tula, A.K.; Eden, M.R.; Gani, R. Energy and CO2 management for chemical and related industries: Issues, opportunities and challenges. BMC Chem. Eng. 2019, 1, 7. [Google Scholar] [CrossRef]
- De Jong, E.; Higson, A.; Walsh, P.; Wellisch, M.B. Bio-Based Chemicals Value Added Products from Biorefineries. 2012. Available online: https://www.ieabioenergy.com/wp-content/uploads/2013/10/Task-42-Biobased-Chemicals-value-added-products-from-biorefineries.pdf (accessed on 22 August 2021).
- Codina Gironès, V.; Moret, S.; Peduzzi, E.; Nasato, M.; Maréchal, F. Optimal use of biomass in large-scale energy systems: Insights for energy policy. Energy 2017, 137, 789–797. [Google Scholar] [CrossRef]
- Jenkins, B.M.; Baxter, L.L.; Koppejan, J. Biomass Combustion. In Thermochemical Processing of Biomass; John Wiley & Sons, Ltd.: Chichester, UK, 2019; pp. 49–83. [Google Scholar]
- Susmozas, A.; Martín-Sampedro, R.; Ibarra, D.; Eugenio, M.E.; Iglesias, R.; Manzanares, P.; Moreno, A.D. Process Strategies for the Transition of 1G to Advanced Bioethanol Production. Processes 2020, 8, 1310. [Google Scholar] [CrossRef]
- Giuntoli, J.; Agostini, A.; Caserini, S.; Lugato, E.; Baxter, D.; Marelli, L. Climate change impacts of power generation from residual biomass. Biomass Bioenergy 2016, 89, 146–158. [Google Scholar] [CrossRef]
- Islas, J.; Manzini, F.; Masera, O.; Vargas, V. Solid Biomass to Heat and Power. In The Role of Bioenergy in the Bioeconomy; Lago, C., Caldés, N., Lechón, Y., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 145–177. [Google Scholar]
- Chen, D.; Gao, A.; Cen, K.; Zhang, J.; Cao, X.; Ma, Z. Investigation of biomass torrefaction based on three major components: Hemicellulose, cellulose, and lignin. Energy Convers. Manag. 2018, 169, 228–237. [Google Scholar] [CrossRef]
- Hameed, S.; Sharma, A.; Pareek, V.; Wu, H.; Yu, Y. A review on biomass pyrolysis models: Kinetic, network and mechanistic models. Biomass Bioenergy 2019, 123, 104–122. [Google Scholar] [CrossRef]
- Soria, M.A.; Barros, D.; Madeira, L.M. Hydrogen production through steam reforming of bio-oils derived from biomass pyrolysis: Thermodynamic analysis including in situ CO2 and/or H2 separation. Fuel 2019, 244, 184–195. [Google Scholar] [CrossRef]
- Carrier, M.; Auret, L.; Bridgwater, A.; Knoetze, J.H. Using Apparent Activation Energy as a Reactivity Criterion for Biomass Pyrolysis. Energy Fuels 2016, 30, 7834–7841. [Google Scholar] [CrossRef] [Green Version]
- Larraín, T.; Carrier, M.; Radovic, L.R. Structure-reactivity relationship in pyrolysis of plastics: A comparison with natural polymers. J. Anal. Appl. Pyrolysis 2017, 126, 346–356. [Google Scholar] [CrossRef] [Green Version]
- Várhegyi, G. Empirical Models with Constant and Variable Activation Energy for Biomass Pyrolysis. Energy Fuels 2019, 33, 2348–2358. [Google Scholar] [CrossRef]
- Vyazovkin, S. Modification of the integral isoconversional method to account for variation in the activation energy. J. Comput. Chem. 2001, 22, 178–183. [Google Scholar] [CrossRef]
- Capart, R.; Khezami, L.; Burnham, A.K. Assessment of various kinetic models for the pyrolysis of a microgranular cellulose. Thermochim. Acta 2004, 417, 79–89. [Google Scholar] [CrossRef] [Green Version]
- Burnham, A.K.; Zhou, X.; Broadbelt, L.J. Critical Review of the Global Chemical Kinetics of Cellulose Thermal Decomposition. Energy Fuels 2015, 29, 2906–2918. [Google Scholar] [CrossRef] [Green Version]
- Antal, M.J.; Várhegyi, G.; Jakab, E. Cellulose Pyrolysis Kinetics: Revisited. Ind. Eng. Chem. Res. 1998, 37, 1267–1275. [Google Scholar] [CrossRef] [Green Version]
- Wu, M.; Várhegyi, G.; Zha, Q. Kinetics of cellulose pyrolysis after a pressurized heat treatment. Thermochim. Acta 2009, 496, 59–65. [Google Scholar] [CrossRef] [Green Version]
- Amutio, M.; Lopez, G.; Aguado, R.; Artetxe, M.; Bilbao, J.; Olazar, M. Kinetic study of lignocellulosic biomass oxidative pyrolysis. Fuel 2012, 95, 305–311. [Google Scholar] [CrossRef]
- Cai, J.; Wu, W.; Liu, R.; Huber, G.W. A distributed activation energy model for the pyrolysis of lignocellulosic biomass. Green Chem. 2013, 15, 1331. [Google Scholar] [CrossRef]
- Radojević, M.; Janković, B.; Jovanović, V.; Stojiljković, D.; Manić, N. Comparative pyrolysis kinetics of various biomasses based on model-free and DAEM approaches improved with numerical optimization procedure. PLoS ONE 2018, 13, e0206657. [Google Scholar] [CrossRef] [PubMed]
- Adenson, M.O.; Kelley, M.D.; Elkelany, O.O.; Biernacki, J.J.; Liu, Y. Kinetics of cellulose pyrolysis: Ensuring optimal outcomes. Can. J. Chem. Eng. 2018, 96, 926–935. [Google Scholar] [CrossRef]
- Milosavljevic, I.; Oja, V.; Suuberg, E.M. Thermal Effects in Cellulose Pyrolysis: Relationship to Char Formation Processes. Ind. Eng. Chem. Res. 1996, 35, 653–662. [Google Scholar] [CrossRef]
- Grønli, M.; Antal, M.J.; Várhegyi, G. A Round-Robin Study of Cellulose Pyrolysis Kinetics by Thermogravimetry. Ind. Eng. Chem. Res. 1999, 38, 2238–2244. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Gera, P.; Jha, M.K. Study on Effects of Different Operating Parameters on the Pyrolysis of Biomass: A Review. J. Biofuels Bioenergy 2015, 1, 135. [Google Scholar] [CrossRef]
- Czajka, K.M. The impact of the thermal lag on the interpretation of cellulose pyrolysis. Energy 2021, 236, 121497. [Google Scholar] [CrossRef]
- Kang, K.Y.; Jo, B.M.; Oh, J.S.; Mansfield, S.D. The effects of biopulping on chemical and energy consumptionduring Kraft pulping of hybrid poplar. Wood Fiber Sci. 2003, 35, 594–600. [Google Scholar]
- Ibarra, D.; Camarero, S.; Romero, J.; Martínez, M.J.; Martínez, A.T. Integrating laccase–mediator treatment into an industrial-type sequence for totally chlorine-free bleaching of eucalypt kraft pulp. J. Chem. Technol. Biotechnol. 2006, 81, 1159–1165. [Google Scholar] [CrossRef]
- Jiménez-López, L.; Eugenio, M.E.; Ibarra, D.; Darder, M.; Martín, J.A.; Martín-Sampedro, R. Cellulose nanofibers from a dutch elm disease-resistant ulmus minor clone. Polymers 2020, 12, 2450. [Google Scholar] [CrossRef] [PubMed]
- Fillat, Ú.; Wicklein, B.; Martín-Sampedro, R.; Ibarra, D.; Ruiz-Hitzky, E.; Valencia, C.; Sarrión, A.; Castro, E.; Eugenio, M.E. Assessing cellulose nanofiber production from olive tree pruning residue. Carbohydr. Polym. 2018, 179. [Google Scholar] [CrossRef] [PubMed]
- NRLE TP-510-42618—Determination of structural carbohydrates and lignin in biomass. In Laboratory Analytical Procedure; National Renewable Energy Laboratory: Golden, CO, USA, 2011.
- Kasaai, M.R. Comparison of various solvents for determination of intrinsic viscosity and viscometric constants for cellulose. J. Appl. Polym. Sci. 2002, 86, 2189–2193. [Google Scholar] [CrossRef]
- Solala, I.; Volperts, A.; Andersone, A.; Dizhbite, T.; Mironova-Ulmane, N.; Vehniäinen, A.; Pere, J.; Vuorinen, T. Mechanoradical formation and its effects on birch kraft pulp during the preparation of nanofibrillated cellulose with Masuko refining. Holzforschung 2012, 66, 477–483. [Google Scholar] [CrossRef]
- Zhang, X.; de Jong, W.; Preto, F. Estimating kinetic parameters in TGA using B-spline smoothing and the Friedman method. Biomass Bioenergy 2009, 33, 1435–1441. [Google Scholar] [CrossRef]
- Fernandez, A.; Saffe, A.; Pereyra, R.; Mazza, G.; Rodriguez, R. Kinetic study of regional agro-industrial wastes pyrolysis using non-isothermal TGA analysis. Appl. Therm. Eng. 2016, 106, 1157–1164. [Google Scholar] [CrossRef]
- Mothé, C.G.; de Miranda, I.C. Study of kinetic parameters of thermal decomposition of bagasse and sugarcane straw using Friedman and Ozawa–Flynn–Wall isoconversional methods. J. Therm. Anal. Calorim. 2013, 113, 497–505. [Google Scholar] [CrossRef]
- Lim, A.C.R.; Chin, B.L.F.; Jawad, Z.A.; Hii, K.L. Kinetic Analysis of Rice Husk Pyrolysis Using Kissinger-Akahira-Sunose (KAS) Method. Procedia Eng. 2016, 148, 1247–1251. [Google Scholar] [CrossRef] [Green Version]
- Brown, M.E.; Dollimore, D.; Galwey, A.K. Reactions in the Solid State. In Comprehensive Chemical Kinetics.; Elsevier: Amsterdam, The Netherlands, 1980. [Google Scholar]
- Ozawa, T. A New Method of Analyzing Thermogravimetric Data. Bull. Chem. Soc. Jpn. 1965, 38, 1881–1886. [Google Scholar] [CrossRef] [Green Version]
- Flynn, J.H.; Wall, L.A. General treatment of the thermogravimetry of polymers. J. Res. Natl. Bur. Stand. Sect. A Phys. Chem. 1966, 70A, 487. [Google Scholar] [CrossRef]
- El-Sayed, S.A.; Mostafa, M.E. Kinetic Parameters Determination of Biomass Pyrolysis Fuels Using TGA and DTA Techniques. Waste Biomass Valorization 2015, 6, 401–415. [Google Scholar] [CrossRef]
- Opfermann, J.R.; Kaisersberger, E.; Flammersheim, H.J. Model-free analysis of thermoanalytical data-advantages and limitations. Thermochim. Acta 2002, 391, 119–127. [Google Scholar] [CrossRef]
- Francis, R.C.; Hausch, D.L.; Granzow, S.G.; Makkonen, H.P.; Kamdem, D.P. Fiber yield for fully bleached kraft pulps from black locust (Robinia pseudoacacia) and silver maple (Acer saccharinum). Holz als Roh-und Werkst. 2001, 59, 49–52. [Google Scholar] [CrossRef]
- Barba, C.; Montané, D.; Rinaudo, M.; Farriol, X. Synthesis and characterization of carboxymethylcelluloses (CMC) from non-wood fibers I.Accessibility of cellulose fibers and CMC synthesis. Cellulose 2002, 9, 319–326. [Google Scholar] [CrossRef]
- Ye, D.; Farriol, X. Improving Accessibility and Reactivity of Celluloses of Annual Plants for the Synthesis of Methylcellulose. Cellulose 2005, 12, 507–515. [Google Scholar] [CrossRef]
- Kim, U.-J.; Eom, S.H.; Wada, M. Thermal decomposition of native cellulose: Influence on crystallite size. Polym. Degrad. Stab. 2010, 95, 778–781. [Google Scholar] [CrossRef]
- Przybysz, K.; Małachowska, E.; Martyniak, D.; Boruszewski, P.; Iłowska, J.; Kalinowska, H.; Przybysz, P. Yield of Pulp, Dimensional Properties of Fibers, and Properties of Paper Produced from Fast Growing Trees and Grasses. BioResources 2018, 13. [Google Scholar] [CrossRef]
- Fengel, D.; Wegener, G. Wood: Chemistry, Ultrastructure, Reactions; Walter de Gruyter: Berlin, Germany, 1989. [Google Scholar]
- Krässig, H.A. Cellulose: Structure, Accessibility, and Reactivity; Krässig Gordon and Breach Science Publishers: Philadelphia, PA, USA, 1993. [Google Scholar]
- Agarwal, U.P.; Reiner, R.R.; Ralph, S.A. Cellulose crystallinity of woods, wood pulps, and agricultural fibers by FT-Raman spectroscopy. In Proceedings of the 16th International Symposium on Wood, Fiber and Pulping Chemistry; Wang, L., Kuan, S., Hou, Q., Cao, C., Si, C., Zhang, H., Eds.; China Light Industry Press: Beijing, China, 2011; Volume II, pp. 69–74. [Google Scholar]
- Jonoobi, M.; Oladi, R.; Davoudpour, Y.; Oksman, K.; Dufresne, A.; Hamzeh, Y.; Davoodi, R. Different preparation methods and properties of nanostructured cellulose from various natural resources and residues: A review. Cellulose 2015, 22, 935–969. [Google Scholar] [CrossRef]
- Martín-Sampedro, R.; Eugenio, M.E.; Fillat, Ú.; Martín, J.A.; Aranda, P.; Ruiz-Hitzky, E.; Ibarra, D.; Wicklein, B. Biorefinery of Lignocellulosic Biomass from an Elm Clone: Production of Fermentable Sugars and Lignin-Derived Biochar for Energy and Environmental Applications. Energy Technol. 2019, 7. [Google Scholar] [CrossRef]
- Gümüskaya, E.; Usta, M.; Kirci, H. The effects of various pulping conditions on crystalline structure of cellulose in cotton linters. Polym. Degrad. Stab. 2003, 81, 559–564. [Google Scholar] [CrossRef]
- Brown, R.C. Thermochemical Processing of Biomass: Conversion into Fuels, Chemicals and Power, 2nd ed.; Wiley Series in Renewable Resources; John Wiley & Sons Inc.: Hoboken, NJ, USA, 2019; p. 4089. [Google Scholar]
- Jin, W.; Singh, K.; Zondlo, J. Pyrolysis Kinetics of Physical Components of Wood and Wood-Polymers Using Isoconversion Method. Agriculture 2013, 3, 12–32. [Google Scholar] [CrossRef] [Green Version]
- Huang, Y.; Li, B.; Liu, D.; Xie, X.; Zhang, H.; Sun, H.; Hu, X.; Zhang, S. Fundamental Advances in Biomass Autothermal/Oxidative Pyrolysis: A Review. ACS Sustain. Chem. Eng. 2020, 8, 11888–11905. [Google Scholar] [CrossRef]
- Henrique, M.A.; Flauzino Neto, W.P.; Silvério, H.A.; Martins, D.F.; Gurgel, L.V.A.; da Silva Barud, H.; de Morais, L.C.; Pasquini, D. Kinetic study of the thermal decomposition of cellulose nanocrystals with different polymorphs, cellulose I and II, extracted from different sources and using different types of acids. Ind. Crops Prod. 2015, 76, 128–140. [Google Scholar] [CrossRef] [Green Version]
- Teeri, T.T. Crystalline cellulose degradation: New insight into the function of cellobiohydrolases. Trends Biotechnol. 1997, 15, 160–167. [Google Scholar] [CrossRef]
- Mansfield, S.D.; Meder, R. Cellulose hydrolysis—The role of monocomponent cellulases in crystalline cellulose degradation. Cellulose 2003, 10, 159–169. [Google Scholar] [CrossRef]
- Zickler, G.A.; Wagermaier, W.; Funari, S.S.; Burghammer, M.; Paris, O. In situ X-ray diffraction investigation of thermal decomposition of wood cellulose. J. Anal. Appl. Pyrolysis 2007, 80, 134–140. [Google Scholar] [CrossRef] [Green Version]
- Hidaka, H.; Kim, U.-J.; Wada, M. Synchrotron X-ray fiber diffraction study on the thermal expansion behavior of cellulose crystals in tension wood of Japanese poplar in the low-temperature region. Holzforschung 2010, 64. [Google Scholar] [CrossRef]
- Poletto, M.; Zattera, A.J.; Forte, M.M.C.; Santana, R.M.C. Thermal decomposition of wood: Influence of wood components and cellulose crystallite size. Bioresour. Technol. 2012, 109, 148–153. [Google Scholar] [CrossRef]
- Bílková, L. Application of infrared spectroscopy and thermal analysis to the examination of the degradation of cotton fibers. Polym. Degrad. Stab. 2012, 97, 35–39. [Google Scholar] [CrossRef]
- Špérová, M.; Nasadil, P.; Průšová, A.; Kučerík, J. A hint on the correlation between cellulose fibers polymerization degree and their thermal and thermo-oxidative degradation. J. Therm. Anal. Calorim. 2012, 110, 71–76. [Google Scholar] [CrossRef]
- Mattonai, M.; Pawcenis, D.; del Seppia, S.; Łojewska, J.; Ribechini, E. Effect of ball-milling on crystallinity index, degree of polymerization and thermal stability of cellulose. Bioresour. Technol. 2018, 270, 270–277. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Jiménez, P.E.; Pérez-Maqueda, L.A.; Perejón, A.; Pascual-Cosp, J.; Benítez-Guerrero, M.; Criado, J.M. An improved model for the kinetic description of the thermal degradation of cellulose. Cellulose 2011, 18, 1487–1498. [Google Scholar] [CrossRef]


| Total Lignin (%) | Glucan (%) | Xylan (%) | |
|---|---|---|---|
| Eucalyptus globulus | 1.3 ± 0.1 | 74.5 ± 2.0 | 18.2 ± 0.5 |
| Ulmus minor | 1.3 ± 0.2 | 74.2 ± 1.8 | 19.4 ± 0.9 |
| Linun usitatissimum | 0.2 ± 0.3 | 88.7 ± 0.9 | 5.2 ± 0.4 |
| Olea europaea | 2.5 ± 0.2 | 69.2 ± 1.7 | 20.6 ± 0.8 |
| Robinia pseudoacacia | 1.0 ± 0.3 | 76.4 ± 1.8 | 18.7 ± 0.6 |
| Populus alba | 1.2 ± 0.1 | 74.1 ± 1.8 | 19.1 ± 0.8 |
| Degree of Polymerization | Crystallinity (%) | Crystal Size (nm) | |
|---|---|---|---|
| Eucalyptus globulus | 2744 ± 246 | 91.2 ± 1.4 | 10.9 ± 0.4 |
| Ulmus minor | 2682 ± 412 | 89.0 ± 1.1 | 8.6 ± 0.1 |
| Linun usitatissimum | 2023 ± 351 | 95.5 ± 0.1 | 9.1 ± 0.4 |
| Olea europaea | 2138 ± 138 | 86.8 ± 0.6 | 8.1 ± 0.4 |
| Robinia pseudoacacia | 1965 ± 311 | 89.9 ± 2.9 | 8.9 ± 0.3 |
| Populus alba | 1939 ± 397 | 93.8 ± 0.2 | 9.4 ± 0.4 |
| Kinetic Method | R2 | F-Test | |
|---|---|---|---|
| ASTME-2070 | 0.877 | 16,707.22 | |
| Friedman | 0.996 | 4494.01 | |
| Eucalyptus globulus | Ozawa-Flynn-Wall | 0.998 | 805.53 |
| Kissinger-Akahira-Sunose | 0.967 | 2142.13 | |
| ASTME-2070 | 0.904 | 19,899.81 | |
| Friedman | 0.996 | 7990.41 | |
| Ulmus minor | Ozawa-Flynn-Wall | 0.999 | 3137.64 |
| Kissinger-Akahira-Sunose | 0.999 | 4544.63 | |
| ASTME-2070 | 0.912 | 522.26 | |
| Friedman | 0.996 | 18,205.87 | |
| Linun usitatissimum | Ozawa-Flynn-Wall | 0.999 | 424.28 |
| Kissinger-Akahira-Sunose | 0.999 | 522.26 | |
| ASTME-2070 | 0.914 | 150.52 | |
| Friedman | 0.994 | 15.68 | |
| Olea europaea | Ozawa-Flynn-Wall | 0.998 | 19.29 |
| Kissinger-Akahira-Sunose | 0.998 | 17.00 | |
| ASTME-2070 | 0.958 | 134.78 | |
| Friedman | 0.995 | 13.59 | |
| Robinia pseudoacacia | Ozawa-Flynn-Wall | 0.996 | 18.40 |
| Kissinger-Akahira-Sunose | 0.997 | 12.00 | |
| ASTME-2070 | 0.907 | 346.06 | |
| Friedman | 0.990 | 38.51 | |
| Populus alba | Ozawa-Flynn-Wall | 0.997 | 18.00 |
| Kissinger-Akahira-Sunose | 0.997 | 16.18 |
| α | Ea (kJ mol−1) | Log A (log (1/s)) | |
|---|---|---|---|
| Eucalyptus globulus | 0.82 | 285.30 | 21.86 |
| Ulmus minor | 0.85 | 242.42 | 16.39 |
| Linun usitatissimum | 0.80 | 257.67 | 18.80 |
| Olea europaea | 0.75 | 180.07 | 13.04 |
| Robinia pseudoacacia | 0.84 | 267.51 | 21.50 |
| Populus alba | 0.83 | 275.58 | 18.74 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Eugenio, M.E.; Ruiz-Montoya, M.; Martín-Sampedro, R.; Ibarra, D.; Díaz, M.J. Influence of Cellulose Characteristics on Pyrolysis Suitability. Processes 2021, 9, 1584. https://doi.org/10.3390/pr9091584
Eugenio ME, Ruiz-Montoya M, Martín-Sampedro R, Ibarra D, Díaz MJ. Influence of Cellulose Characteristics on Pyrolysis Suitability. Processes. 2021; 9(9):1584. https://doi.org/10.3390/pr9091584
Chicago/Turabian StyleEugenio, María E., Mercedes Ruiz-Montoya, Raquel Martín-Sampedro, David Ibarra, and Manuel J. Díaz. 2021. "Influence of Cellulose Characteristics on Pyrolysis Suitability" Processes 9, no. 9: 1584. https://doi.org/10.3390/pr9091584
APA StyleEugenio, M. E., Ruiz-Montoya, M., Martín-Sampedro, R., Ibarra, D., & Díaz, M. J. (2021). Influence of Cellulose Characteristics on Pyrolysis Suitability. Processes, 9(9), 1584. https://doi.org/10.3390/pr9091584






