Biorefinery Gets Hot: Thermophilic Enzymes and Microorganisms for Second-Generation Bioethanol Production
Abstract
:1. Introduction
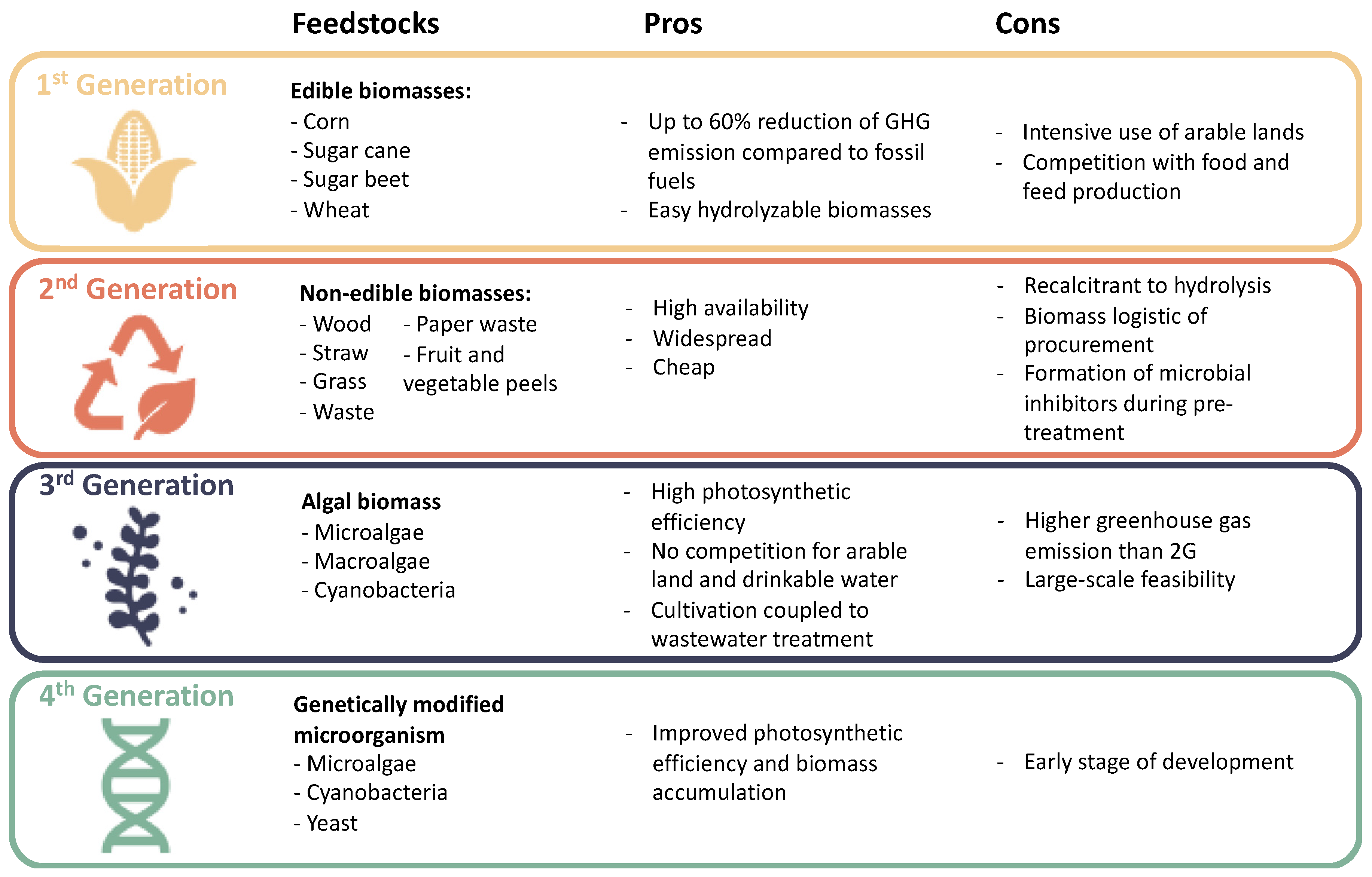
1.1. Need for Alternative Fuels
1.2. Bioethanol Biorefinery: State of the Art and Challenges
1.3. Advantages of Using Thermophiles in Biorefineries
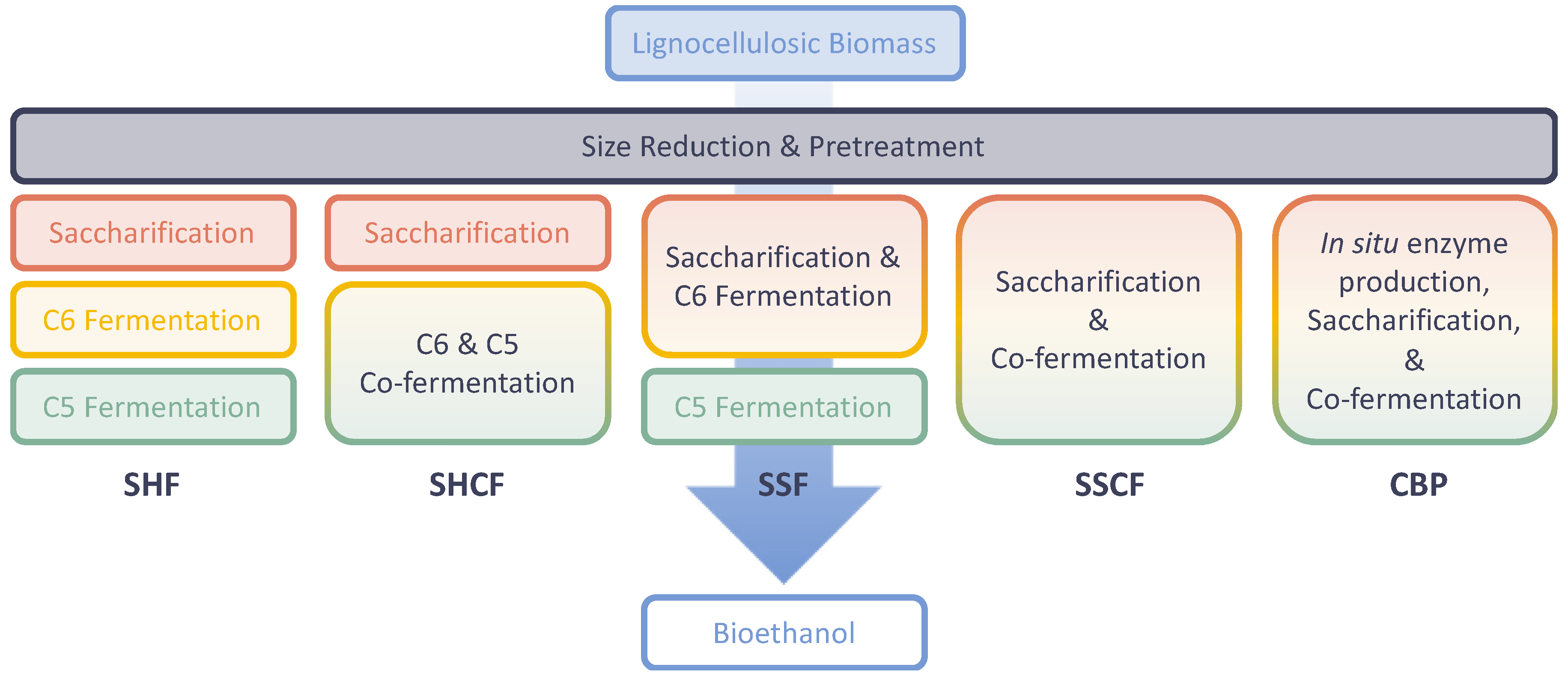
2. Pretreatment of Lignocellulosic Biomasses
3. Enzymatic Hydrolysis
4. Thermophilic Fermentation
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Conflicts of Interest
References
- Tans, P.; Keeling, R. Trends in Atmospheric Carbon Dioxide. Available online: https://gml.noaa.gov/ccgg/trends/ (accessed on 29 July 2021).
- Collins, M.; Knutti, R.; Arblaster, J.; Dufresne, J.-L.; Fichefet, T.; Friedlingstein, P.; Gao, X.; Gutowski, W.J.; Johns, T.; Krinner, G.; et al. Long-term Climate Change: Projections, Commitments and Irreversibility. In Climate Change 2013—The Physical Science Basis; Stocker, T.F., Qin, D., Plattner, G.-K., Tignor, M.M.B., Allen, S.K., Boschung, J., Nauels, A., Xia, Y., Bex, V., Midgley, P.M., Eds.; Intergovernmental Panel on Climate Change; Cambridge University Press: Cambridge, UK, 2013; pp. 1029–1136. ISBN 9781107057991. [Google Scholar]
- Intergovernmental Panel on Climate Change. Climate Change 2014 Mitigation of Climate Change; Cambridge University Press: Cambridge, UK, 2014; ISBN 9781107654815. [Google Scholar] [CrossRef] [Green Version]
- Kang, Q.; Appels, L.; Tan, T.; Dewil, R. Bioethanol from lignocellulosic biomass: Current findings determine research priorities. Sci. World J. 2014, 2014, 298153. [Google Scholar] [CrossRef]
- Sharma, B.; Larroche, C.; Dussap, C.G. Comprehensive assessment of 2G bioethanol production. Bioresour. Technol. 2020, 313, 123630. [Google Scholar] [CrossRef] [PubMed]
- Sharma, D.; Saini, A. Lignocellulosic Ethanol Production from a Biorefinery Perspective; Springer: Singapore, 2020; ISBN 978-981-15-4572-6. [Google Scholar] [CrossRef]
- Thompson, P.B. The agricultural ethics of biofuels: The food vs. fuel debate. Agriculture 2012, 2, 339–358. [Google Scholar] [CrossRef] [Green Version]
- Prasad, S.; Ingle, A.P. Impacts of sustainable biofuels production from biomass. In Sustainable Bioenergy; Rai, M., Ingle, A.P., Eds.; Elsevier: Amsterdam, The Netherlands, 2019; Chapter 12; pp. 327–346. ISBN 978-0-12-817654-2. [Google Scholar] [CrossRef]
- von Lampe, M. Economic Assessment of Biofuel Support Policies. 2008. Available online: https://trid.trb.org/view/864830 (accessed on 1 July 2021).
- Stephens, E.; Ross, I.L.; Mussgnug, J.H.; Wagner, L.D.; Borowitzka, M.A.; Posten, C.; Kruse, O.; Hankamer, B. Future prospects of microalgal biofuel production systems. Trends Plant Sci. 2010, 15, 554–564. [Google Scholar] [CrossRef] [PubMed]
- Mayfield, C.A.; Foster, C.D.; Smith, C.T.; Gan, J.; Fox, S. Opportunities, barriers, and strategies for forest bioenergy and bio-based product development in the Southern United States. Biomass Bioenergy 2007, 31, 631–637. [Google Scholar] [CrossRef]
- Stephen, J.D.; Mabee, W.E.; Saddler, J.N. Will second-generation ethanol be able to compete with first-generation ethanol? Opportunities for cost reduction. Biofuels Bioprod. Biorefin. 2012, 6, 159–176. [Google Scholar] [CrossRef]
- Lynd, L.R.; Wyman, C.E.; Gerngross, T.U. Biocommodity engineering. Biotechnol. Prog. 1999, 15, 777–793. [Google Scholar] [CrossRef]
- Chen, H. Biotechnology of Lignocellulose: Theory and Practice; Chemical Industry Press and Springer: Beijing, China, 2014; ISBN 9789400768987. [Google Scholar] [CrossRef]
- O’Sullivan, A.C. Cellulose: The structure slowly unravels. Cellulose 1997, 4, 173–207. [Google Scholar] [CrossRef]
- Karnaouri, A.; Matsakas, L.; Topakas, E.; Rova, U.; Christakopoulos, P. Development of thermophilic tailor-made enzyme mixtures for the bioconversion of agricultural and forest residues. Front. Microbiol. 2016, 7, 177. [Google Scholar] [CrossRef] [Green Version]
- Chen, Y.A.; Zhou, Y.; Liu, D.; Zhao, X.; Qin, Y. Evaluation of the action of Tween 20 non-ionic surfactant during enzymatic hydrolysis of lignocellulose: Pretreatment, hydrolysis conditions and lignin structure. Bioresour. Technol. 2018, 269, 329–338. [Google Scholar] [CrossRef]
- Balat, M.; Balat, H.; Öz, C. Progress in bioethanol processing. Prog. Energy Combust. Sci. 2008, 34, 551–573. [Google Scholar] [CrossRef]
- Lombard, V.; Golaconda Ramulu, H.; Drula, E.; Coutinho, P.M.; Henrissat, B. The carbohydrate-active enzymes database (CAZy) in 2013. Nucleic Acids Res. 2014, 42, 490–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, B.C. Hemicellulose bioconversion. J. Ind. Microbiol. Biotechnol. 2003, 30, 279–291. [Google Scholar] [CrossRef] [PubMed]
- Aulitto, M.; Fusco, S.; Limauro, D.; Fiorentino, G.; Bartolucci, S.; Contursi, P. Galactomannan degradation by thermophilic enzymes: A hot topic for biotechnological applications. World J. Microbiol. Biotechnol. 2019, 35, 32. [Google Scholar] [CrossRef] [Green Version]
- Himmel, M.E.; Ding, S.-Y.; Johnson, D.K.; Adney, W.S.; Nimlos, M.R.; Brady, J.W.; Foust, T.D. Biomass Recalcitrance: Engineering Plants and Enzymes for Biofuels Production. Science 2007, 315, 804–807. [Google Scholar] [CrossRef] [Green Version]
- Demirbas, A. Use of algae as biofuel sources. Energy Convers. Manag. 2010, 51, 2738–2749. [Google Scholar] [CrossRef]
- Shah, S.H.; Raja, I.A.; Rizwan, M.; Rashid, N.; Mahmood, Q.; Shah, F.A.; Pervez, A. Potential of microalgal biodiesel production and its sustainability perspectives in Pakistan. Renew. Sustain. Energy Rev. 2018, 81, 76–92. [Google Scholar] [CrossRef]
- Bharathiraja, B.; Chakravarthy, M.; Ranjith Kumar, R.; Yogendran, D.; Yuvaraj, D.; Jayamuthunagai, J.; Praveen Kumar, R.; Palani, S. Aquatic biomass (algae) as a future feed stock for bio-refineries: A review on cultivation, processing and products. Renew. Sustain. Energy Rev. 2015, 47, 634–653. [Google Scholar] [CrossRef]
- Katiyar, R.; Gurjar, B.R.; Biswas, S.; Pruthi, V.; Kumar, N.; Kumar, P. Microalgae: An emerging source of energy based bio-products and a solution for environmental issues. Renew. Sustain. Energy Rev. 2017, 72, 1083–1093. [Google Scholar] [CrossRef] [Green Version]
- Zuliani, L.; Frison, N.; Jelic, A.; Fatone, F.; Bolzonella, D.; Ballottari, M. Microalgae cultivation on anaerobic digestate of municipalwastewater, sewage sludge and agro-waste. Int. J. Mol. Sci. 2016, 17, 1692. [Google Scholar] [CrossRef]
- Passell, H.; Dhaliwal, H.; Reno, M.; Wu, B.; Ben Amotz, A.; Ivry, E.; Gay, M.; Czartoski, T.; Laurin, L.; Ayer, N. Algae biodiesel life cycle assessment using current commercial data. J. Environ. Manag. 2013, 129, 103–111. [Google Scholar] [CrossRef]
- Mu, D.; Min, M.; Krohn, B.; Mullins, K.A.; Ruan, R.; Hill, J. Life cycle environmental impacts of wastewater-based algal biofuels. Environ. Sci. Technol. 2014, 48, 11696–11704. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pragya, N.; Pandey, K.K. Life cycle assessment of green diesel production from microalgae. Renew. Energy 2016, 86, 623–632. [Google Scholar] [CrossRef]
- Adams, C.; Godfrey, V.; Wahlen, B.; Seefeldt, L.; Bugbee, B. Understanding precision nitrogen stress to optimize the growth and lipid content tradeoff in oleaginous green microalgae. Bioresour. Technol. 2013, 131, 188–194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Soratana, K.; Harper, W.F.; Landis, A.E. Microalgal biodiesel and the Renewable Fuel Standard’s greenhouse gas requirement. Energy Policy 2012, 46, 498–510. [Google Scholar] [CrossRef]
- Alalwan, H.A.; Alminshid, A.H.; Aljaafari, H.A.S. Promising evolution of biofuel generations. Subject review. Renew. Energy Focus 2019, 28, 127–139. [Google Scholar] [CrossRef]
- de Farias Silva, C.E.; Bertucco, A. Bioethanol from microalgae and cyanobacteria: A review and technological outlook. Process Biochem. 2016, 51, 1833–1842. [Google Scholar] [CrossRef]
- Efroymson, R.A.; Dale, V.H.; Langholtz, M.H. Socioeconomic indicators for sustainable design and commercial development of algal biofuel systems. GCB Bioenergy 2017, 9, 1005–1023. [Google Scholar] [CrossRef]
- Robak, K.; Balcerek, M. Current state-of-the-art in ethanol production from lignocellulosic feedstocks. Microbiol. Res. 2020, 240, 126534. [Google Scholar] [CrossRef]
- Soltanian, S.; Aghbashlo, M.; Almasi, F.; Hosseinzadeh-Bandbafha, H.; Nizami, A.S.; Ok, Y.S.; Lam, S.S.; Tabatabaei, M. A critical review of the effects of pretreatment methods on the exergetic aspects of lignocellulosic biofuels. Energy Convers. Manag. 2020, 212, 112792. [Google Scholar] [CrossRef]
- Hamelinck, C.N.; Van Hooijdonk, G.; Faaij, A.P.C. Ethanol from lignocellulosic biomass: Techno-economic performance in short-, middle- and long-term. Biomass Bioenergy 2005, 28, 384–410. [Google Scholar] [CrossRef]
- Glasser, W.G.; Wright, R.S. Steam-assisted biomass fractionation. II. Fractionation behavior of various biomass resources. Biomass Bioenergy 1998, 14, 219–235. [Google Scholar] [CrossRef]
- Sun, D.; Yang, Q.; Wang, Y.; Gao, H.; He, M.; Lin, X.; Lu, J.; Wang, Y.; Kang, H.; Alam, A.; et al. Distinct mechanisms of enzymatic saccharification and bioethanol conversion enhancement by three surfactants under steam explosion and mild chemical pretreatments in bioenergy Miscanthus. Ind. Crops Prod. 2020, 153, 112559. [Google Scholar] [CrossRef]
- Aulitto, M.; Fusco, S.; Nickel, D.B.; Bartolucci, S.; Contursi, P.; Franzén, C.J. Seed culture pre-adaptation of Bacillus coagulans MA-13 improves lactic acid production in simultaneous saccharification and fermentation. Biotechnol. Biofuels 2019, 12, 45. [Google Scholar] [CrossRef] [Green Version]
- Aulitto, M.; Fusco, S.; Bartolucci, S.; Franzén, C.J.; Contursi, P. Bacillus coagulans MA-13: A promising thermophilic and cellulolytic strain for the production of lactic acid from lignocellulosic hydrolysate. Biotechnol. Biofuels 2017, 10, 210. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandel, A.K.; da Silva, S.S.; Singh, O.V. Detoxification of Lignocellulose Hydrolysates: Biochemical and Metabolic Engineering Toward White Biotechnology. Bioenergy Res. 2013, 6, 388–401. [Google Scholar] [CrossRef]
- Valdivia, M.; Galan, J.L.; Laffarga, J.; Ramos, J.L. Biofuels 2020: Biorefineries based on lignocellulosic materials. Microb. Biotechnol. 2016, 9, 585–594. [Google Scholar] [CrossRef] [PubMed]
- Zabed, H.; Sahu, J.N.; Boyce, A.N.; Faruq, G. Fuel ethanol production from lignocellulosic biomass: An overview on feedstocks and technological approaches. Renew. Sustain. Energy Rev. 2016, 66, 751–774. [Google Scholar] [CrossRef]
- Toor, M.; Kumar, S.S.; Malyan, S.K.; Bishnoi, N.R.; Mathimani, T.; Rajendran, K.; Pugazhendhi, A. An overview on bioethanol production from lignocellulosic feedstocks. Chemosphere 2020, 242, 125080. [Google Scholar] [CrossRef] [PubMed]
- Olofsson, K.; Bertilsson, M.; Lidén, G. A short review on SSF—An interesting process option for ethanol production from lignocellulosic feedstocks. Biotechnol. Biofuels 2008, 1, 7. [Google Scholar] [CrossRef] [Green Version]
- Bhalla, A.; Bansal, N.; Kumar, S.; Bischoff, K.M.; Sani, R.K. Improved lignocellulose conversion to biofuels with thermophilic bacteria and thermostable enzymes. Bioresour. Technol. 2013, 128, 751–759. [Google Scholar] [CrossRef]
- Shen, J.; Agblevor, F.A. Ethanol production of semi-simultaneous saccharification and fermentation from mixture of cotton gin waste and recycled paper sludge. Bioprocess Biosyst. Eng. 2011, 34, 33–43. [Google Scholar] [CrossRef] [Green Version]
- Shen, J.; Agblevor, F.A. Modeling semi-simultaneous saccharification and fermentation of ethanol production from cellulose. Biomass Bioenergy 2010, 34, 1098–1107. [Google Scholar] [CrossRef]
- Patel, A.; Shah, A.R. Integrated lignocellulosic biorefinery: Gateway for production of second generation ethanol and value added products. J. Bioresour. Bioprod. 2021, 6, 108–128. [Google Scholar] [CrossRef]
- Olguin-Maciel, E.; Singh, A.; Chable-Villacis, R.; Tapia-Tussell, R.; Ruiz, H.A. Consolidated Bioprocessing, an Innovative Strategy towards Sustainability for Biofuels Production from Crop Residues: An Overview. Agronomy 2020, 10, 1834. [Google Scholar] [CrossRef]
- Singh, S.; Shukla, L.; Khare, S.; Nain, L. Detection and Characterization of New Thermostable Endoglucanase from Aspergillus awamori Strain F 18. J. Mycol. Plant Pathol. 2011, 41, 97–103. [Google Scholar]
- Rigoldi, F.; Donini, S.; Redaelli, A.; Parisini, E.; Gautieri, A. Review: Engineering of thermostable enzymes for industrial applications. APL Bioeng. 2018, 2, 011501. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Böhme, B.; Moritz, B.; Wendler, J.; Hertel, T.C.; Ihling, C.; Brandt, W.; Pietzsch, M. Enzymatic activity and thermoresistance of improved microbial transglutaminase variants. Amino Acids 2020, 52, 313–326. [Google Scholar] [CrossRef]
- Espliego, J.M.E.; Saiz, V.B.; Torregrosa-Crespo, J.; Luque, A.V.; Camacho Carrasco, M.L.; Pire, C.; Bonete, M.J.; Martínez-Espinosa, R.M. Extremophile Enzymes and Biotechnology. In Extremophiles; CRC Press: Boca Raton, FL, USA; Taylor & Francis: Abingdon, UK, 2018; pp. 227–248. [Google Scholar] [CrossRef]
- Margaryan, A.; Shahinyan, G.; Hovhannisyan, P.; Panosyan, H.; Birkeland, N.-K.; Trchounian, A. Geobacillus and Anoxybacillus spp. from Terrestrial Geothermal Springs Worldwide: Diversity and Biotechnological Applications. In Extremophiles in Eurasian Ecosystems: Ecology, Diversity, and Applications; Egamberdieva, D., Birkeland, N.-K., Panosyan, H., Li, W.-J., Eds.; Springer: Singapore, 2018; pp. 119–166. ISBN 978-981-13-0329-6. [Google Scholar] [CrossRef]
- Viikari, L.; Alapuranen, M.; Puranen, T.; Vehmaanperä, J.; Siika-aho, M. Thermostable Enzymes in Lignocellulose Hydrolysis. In Biofuels; Olsson, L., Ed.; Springer: Berlin/Heidelberg, Germany, 2007; pp. 121–145. ISBN 978-3-540-73651-6. [Google Scholar] [CrossRef]
- Taylor, M.P.; Eley, K.L.; Martin, S.; Tuffin, M.I.; Burton, S.G.; Cowan, D.A. Thermophilic ethanologenesis: Future prospects for second-generation bioethanol production. Trends Biotechnol. 2009, 27, 398–405. [Google Scholar] [CrossRef] [PubMed]
- Arora, R.; Behera, S.; Kumar, S. Bioprospecting thermophilic/thermotolerant microbes for production of lignocellulosic ethanol: A future perspective. Renew. Sustain. Energy Rev. 2015, 51, 699–717. [Google Scholar] [CrossRef]
- Di Donato, P.; Finore, I.; Poli, A.; Nicolaus, B.; Lama, L. The production of second generation bioethanol: The biotechnology potential of thermophilic bacteria. J. Clean. Prod. 2019, 233, 1410–1417. [Google Scholar] [CrossRef]
- Linares-Pasten, J.; Andersson, M.; Karlsson, E. Thermostable Glycoside Hydrolases in Biorefinery Technologies. Curr. Biotechnol. 2014, 3, 26–44. [Google Scholar] [CrossRef]
- Kucharska, K.; Rybarczyk, P.; Hołowacz, I.; Łukajtis, R.; Glinka, M.; Kamiński, M. Pretreatment of lignocellulosic materials as substrates for fermentation processes. Molecules 2018, 23, 2937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Amin, F.R.; Khalid, H.; Zhang, H.; Rahman, S.; Zhang, R.; Liu, G.; Chen, C. Pretreatment methods of lignocellulosic biomass for anaerobic digestion. AMB Express 2017, 7, 72. [Google Scholar] [CrossRef] [Green Version]
- Baruah, J.; Nath, B.K.; Sharma, R.; Kumar, S.; Deka, R.C.; Baruah, D.C.; Kalita, E. Recent trends in the pretreatment of lignocellulosic biomass for value-added products. Front. Energy Res. 2018, 6, 141. [Google Scholar] [CrossRef]
- Rahmati, S.; Doherty, W.; Dubal, D.; Atanda, L.; Moghaddam, L.; Sonar, P.; Hessel, V.; Ostrikov, K. Pretreatment and fermentation of lignocellulosic biomass: Reaction mechanisms and process engineering. React. Chem. Eng. 2020, 5, 2017–2047. [Google Scholar] [CrossRef]
- Agbor, V.B.; Cicek, N.; Sparling, R.; Berlin, A.; Levin, D.B. Biomass pretreatment: Fundamentals toward application. Biotechnol. Adv. 2011, 29, 675–685. [Google Scholar] [CrossRef]
- Zhang, X.; Yu, H.; Huang, H.; Liu, Y. Evaluation of biological pretreatment with white rot fungi for the enzymatic hydrolysis of bamboo culms. Int. Biodeterior. Biodegrad. 2007, 60, 159–164. [Google Scholar] [CrossRef]
- Martínez, Á.T.; Speranza, M.; Ruiz-Dueñas, F.J.; Ferreira, P.; Camarero, S.; Guillén, F.; Martínez, M.J.; Gutiérrez, A.; Del Río, J.C. Biodegradation of lignocellulosics: Microbial, chemical, and enzymatic aspects of the fungal attack of lignin. Int. Microbiol. 2005, 8, 195–204. [Google Scholar] [CrossRef] [PubMed]
- Kumar, R.; Wyman, C.E. Effects of cellulase and xylanase enzymes on the deconstruction of solids from pretreatment of poplar by leading technologies. Biotechnol. Prog. 2009, 25, 302–314. [Google Scholar] [CrossRef] [PubMed]
- Mandels, M.; Weber, J.; Parizek, R. Enhanced cellulase production by a mutant of Trichoderma viride. Appl. Microbiol. 1971, 21, 152–154. [Google Scholar] [CrossRef] [PubMed]
- Fenel, F.; Zitting, A.J.; Kantelinen, A. Increased alkali stability in Trichoderma reesei endo-1,4-β-xylanase II by site directed mutagenesis. J. Biotechnol. 2006, 121, 102–107. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.-L.; Wang, X.-F.; Niu, J.-L.; Lü, Y.-C.; Guo, P.; Shen, H.-L.; Cui, Z.-J. Composition diversity of the multifunctional bacterium community NSC-7. Huan Jing Ke Xue = Huanjing Kexue 2009, 30, 2112–2117. [Google Scholar]
- Wongwilaiwalin, S.; Rattanachomsri, U.; Laothanachareon, T.; Eurwilaichitr, L.; Igarashi, Y.; Champreda, V. Analysis of a thermophilic lignocellulose degrading microbial consortium and multi-species lignocellulolytic enzyme system. Enzym. Microb. Technol. 2010, 47, 283–290. [Google Scholar] [CrossRef]
- Wang, W.; Yan, L.; Cui, Z.; Gao, Y.; Wang, Y.; Jing, R. Characterization of a microbial consortium capable of degrading lignocellulose. Bioresour. Technol. 2011, 102, 9321–9324. [Google Scholar] [CrossRef]
- Feng, Y.; Yu, Y.; Wang, X.; Qu, Y.; Li, D.; He, W.; Kim, B.H. Degradation of raw corn stover powder (RCSP) by an enriched microbial consortium and its community structure. Bioresour. Technol. 2011, 102, 742–747. [Google Scholar] [CrossRef]
- Haruta, S.; Cui, Z.; Huang, Z.; Li, M.; Ishii, M.; Igarashi, Y. Construction of a stable microbial community with high cellulose-degradation ability. Appl. Microbiol. Biotechnol. 2002, 59, 529–534. [Google Scholar] [CrossRef]
- Strazzulli, A.; Fusco, S.; Cobucci-Ponzano, B.; Moracci, M.; Contursi, P. Metagenomics of microbial and viral life in terrestrial geothermal environments. Rev. Environ. Sci. Biotechnol. 2017, 16, 425–454. [Google Scholar] [CrossRef] [Green Version]
- Graham, J.E.; Clark, M.E.; Nadler, D.C.; Huffer, S.; Chokhawala, H.A.; Rowland, S.E.; Blanch, H.W.; Clark, D.S.; Robb, F.T. Identification and characterization of a multidomain hyperthermophilic cellulase from an archaeal enrichment. Nat. Commun. 2011, 2, 375. [Google Scholar] [CrossRef] [Green Version]
- Elleuche, S.; Schröder, C.; Sahm, K.; Antranikian, G. Extremozymes-biocatalysts with unique properties from extremophilic microorganisms. Curr. Opin. Biotechnol. 2014, 29, 116–123. [Google Scholar] [CrossRef] [PubMed]
- Ebaid, R.; Wang, H.; Sha, C.; Abomohra, A.E.F.; Shao, W. Recent trends in hyperthermophilic enzymes production and future perspectives for biofuel industry: A critical review. J. Clean. Prod. 2019, 238, 117925. [Google Scholar] [CrossRef]
- Cobucci-Ponzano, B.; Ionata, E.; La Cara, F.; Morana, A.; Ferrara, M.C.; Maurelli, L.; Strazzulli, A.; Giglio, R.; Moracci, M. Extremophilic (Hemi)cellulolytic Microorganisms and Enzymes. In Lignocellulose Conversion: Enzymatic and Microbial Tools for Bioethanol Production; Faraco, V., Ed.; Springer: Berlin/Heidelberg, Germany, 2013; pp. 111–130. ISBN 978-3-642-37861-4. [Google Scholar] [CrossRef]
- Banerjee, G.; Car, S.; Scott-Craig, J.S.; Borrusch, M.S.; Walton, J.D. Rapid optimization of enzyme mixtures for deconstruction of diverse pretreatment/biomass feedstock combinations. Biotechnol. Biofuels 2010, 3, 22. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chylenski, P.; Forsberg, Z.; Ståhlberg, J.; Várnai, A.; Lersch, M.; Bengtsson, O.; Sæbø, S.; Horn, S.J.; Eijsink, V.G.H. Development of minimal enzyme cocktails for hydrolysis of sulfite-pulped lignocellulosic biomass. J. Biotechnol. 2017, 246, 16–23. [Google Scholar] [CrossRef] [PubMed]
- Kallioinen, A.; Puranen, T.; Siika-Aho, M. Mixtures of thermostable enzymes show high performance in biomass saccharification. Appl. Biochem. Biotechnol. 2014, 173, 1038–1056. [Google Scholar] [CrossRef]
- Hassan, O.; Ling, T.P.; Maskat, M.Y.; Illias, R.M.; Badri, K.; Jahim, J.; Mahadi, N.M. Optimization of pretreatments for the hydrolysis of oil palm empty fruit bunch fiber (EFBF) using enzyme mixtures. Biomass Bioenergy 2013, 56, 137–146. [Google Scholar] [CrossRef]
- Zhu, J.Y.; Pan, X.J.; Wang, G.S.; Gleisner, R. Sulfite pretreatment (SPORL) for robust enzymatic saccharification of spruce and red pine. Bioresour. Technol. 2009, 100, 2411–2418. [Google Scholar] [CrossRef]
- Youngblood, A.; Zhu, J.; Scott, C.T. Ethanol Production from Woody Biomass: Silvicultural Opportunities for Suppressed Western Conifers. In Integrated Management of Carbon Sequestration and Biomass Utilization Opportunities in a Changing Climate; U.S. Department of Agriculture, Forest Service, Rocky Mountain Research Station: Fort Collins, CO, USA, 2010; pp. 169–184. [Google Scholar]
- Wang, G.S.; Pan, X.J.; Zhu, J.Y.; Gleisner, R.; Rockwood, D. Sulfite pretreatment to overcome recalcitrance of lignocellulose (SPORL) for robust enzymatic saccharification of hardwoods. Biotechnol. Prog. 2009, 25, 1086–1093. [Google Scholar] [CrossRef]
- Sassner, P.; Mårtensson, C.G.; Galbe, M.; Zacchi, G. Steam pretreatment of H2SO4-impregnated Salix for the production of bioethanol. Bioresour. Technol. 2008, 99, 137–145. [Google Scholar] [CrossRef]
- Sternberg, D.; Vuayakumar, P.; Reese, E.T. β-Glucosidase: Microbial production and effect on enzymatic hydrolysis of cellulose. Can. J. Microbiol. 1977, 23, 139–147. [Google Scholar] [CrossRef]
- Bernardi, A.V.; Yonamine, D.K.; Uyemura, S.A.; Dinamarco, T.M. A thermostable Aspergillus fumigatus gh7 endoglucanase over-expressed in pichia pastoris stimulates lignocellulosic biomass hydrolysis. Int. J. Mol. Sci. 2019, 20, 2261. [Google Scholar] [CrossRef] [Green Version]
- Bagewadi, Z.K.; Mulla, S.I.; Ninnekar, H.Z. Purification and characterization of endo β-1,4-d-glucanase from Trichoderma harzianum strain HZN11 and its application in production of bioethanol from sweet sorghum bagasse. 3 Biotech 2016, 6, 101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, C.; Yang, R.; Sun, Y.; Liu, M.; Zhou, L.; Li, D. Identification and Characterization of a Novel Hyperthermostable Bifunctional Cellobiohydrolase- Xylanase Enzyme for Synergistic Effect With Commercial Cellulase on Pretreated Wheat Straw Degradation. Front. Bioeng. Biotechnol. 2020, 8, 296. [Google Scholar] [CrossRef]
- Nascimento, C.V.; Souza, F.H.M.; Masui, D.C.; Leone, F.A.; Peralta, R.M.; Jorge, J.A.; Furriel, R.P.M. Purification and biochemical properties of a glucose-stimulated β-d-glucosidase produced by Humicola grisea var. thermoidea grown on sugarcane bagasse. J. Microbiol. 2010, 48, 53–62. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Wang, M.; Gao, R.; Yu, X.; Chen, G. Synergistic effect of thermostable β-glucosidase TN0602 and cellulase on cellulose hydrolysis. 3 Biotech 2017, 7, 54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cintra, L.C.; da Costa, I.C.; de Oliveira, I.C.M.; Fernandes, A.G.; Faria, S.P.; Jesuíno, R.S.A.; Ravanal, M.C.; Eyzaguirre, J.; Ramos, L.P.; de Faria, F.P.; et al. The boosting effect of recombinant hemicellulases on the enzymatic hydrolysis of steam-treated sugarcane bagasse. Enzym. Microb. Technol. 2020, 133, 109447. [Google Scholar] [CrossRef]
- Gonçalves, G.A.L.; Takasugi, Y.; Jia, L.; Mori, Y.; Noda, S.; Tanaka, T.; Ichinose, H.; Kamiya, N. Synergistic effect and application of xylanases as accessory enzymes to enhance the hydrolysis of pretreated bagasse. Enzym. Microb. Technol. 2015, 72, 16–24. [Google Scholar] [CrossRef] [Green Version]
- Sepulchro, A.G.V.; Pellegrini, V.O.A.; Briganti, L.; de Araujo, E.A.; de Araujo, S.S.; Polikarpov, I. Transformation of xylan into value-added biocommodities using Thermobacillus composti GH10 xylanase. Carbohydr. Polym. 2020, 247, 116714. [Google Scholar] [CrossRef] [PubMed]
- Katsimpouras, C.; Dimarogona, M.; Petropoulos, P.; Christakopoulos, P.; Topakas, E. A thermostable GH26 endo-β-mannanase from Myceliophthora thermophila capable of enhancing lignocellulose degradation. Appl. Microbiol. Biotechnol. 2016, 100, 8385–8397. [Google Scholar] [CrossRef]
- Vaaje-Kolstad, G.; Westereng, B.; Horn, S.J.; Liu, Z.; Zhai, H.; Sørlie, M.; Eijsink, V.G.H. An Oxidative Enzyme Boosting the Enzymatic Conversion of Recalcitrant Polysaccharides. Science 2010, 330, 219–222. [Google Scholar] [CrossRef]
- Cannella, D.; Möllers, K.B.; Frigaard, N.U.; Jensen, P.E.; Bjerrum, M.J.; Johansen, K.S.; Felby, C. Light-driven oxidation of polysaccharides by photosynthetic pigments and a metalloenzyme. Nat. Commun. 2016, 7, 11134. [Google Scholar] [CrossRef] [Green Version]
- Villares, A.; Moreau, C.; Bennati-Granier, C.; Garajova, S.; Foucat, L.; Falourd, X.; Saake, B.; Berrin, J.G.; Cathala, B. Lytic polysaccharide monooxygenases disrupt the cellulose fibers structure. Sci. Rep. 2017, 7, 40262. [Google Scholar] [CrossRef] [PubMed]
- Agrawal, D.; Basotra, N.; Balan, V.; Tsang, A.; Chadha, B.S. Discovery and Expression of Thermostable LPMOs from Thermophilic Fungi for Producing Efficient Lignocellulolytic Enzyme Cocktails. Appl. Biochem. Biotechnol. 2020, 191, 463–481. [Google Scholar] [CrossRef]
- Peciulyte, A.; Samuelsson, L.; Olsson, L.; McFarland, K.C.; Frickmann, J.; Østergård, L.; Halvorsen, R.; Scott, B.R.; Johansen, K.S. Redox processes acidify and decarboxylate steam-pretreated lignocellulosic biomass and are modulated by LPMO and catalase. Biotechnol. Biofuels 2018, 11, 165. [Google Scholar] [CrossRef]
- Scott, B.R.; Huang, H.Z.; Frickman, J.; Halvorsen, R.; Johansen, K.S. Catalase improves saccharification of lignocellulose by reducing lytic polysaccharide monooxygenase-associated enzyme inactivation. Biotechnol. Lett. 2016, 38, 425–434. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.J.; Joo, J.E.; Jeon, S.D.; Hyeon, J.E.; Kim, S.W.; Um, Y.S.; Han, S.O. Enhanced thermostability of mesophilic endoglucanase Z with a high catalytic activity at active temperatures. Int. J. Biol. Macromol. 2016, 86, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Ito, Y.; Ikeuchi, A.; Imamura, C. Advanced evolutionary molecular engineering to produce thermostable cellulase by using a small but efficient library. Protein Eng. Des. Sel. 2013, 26, 73–79. [Google Scholar] [CrossRef]
- Wu, I.; Heel, T.; Arnold, F.H. Role of cysteine residues in thermal inactivation of fungal Cel6A cellobiohydrolases. Biochim. Biophys. Acta—Proteins Proteom. 2013, 1834, 1539–1544. [Google Scholar] [CrossRef] [PubMed]
- Anbar, M.; Gul, O.; Lamed, R.; Sezerman, U.O.; Bayer, E.A. Improved thermostability of Clostridium thermocellum endoglucanase Cel8A by using consensus-guided mutagenesis. Appl. Environ. Microbiol. 2012, 78, 3458–3464. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.J.; Hsiao, Y.Y.; Chen, Y.P.; Ma, T.Y.; Tseng, C.P. Polarity alteration of a calcium site induces a hydrophobic interaction network and enhances Cel9A endoglucanase thermostability. Appl. Environ. Microbiol. 2016, 82, 1662–1674. [Google Scholar] [CrossRef] [Green Version]
- Wijma, H.J.; Floor, R.J.; Jekel, P.A.; Baker, D.; Marrink, S.J.; Janssen, D.B. Computationally designed libraries for rapid enzyme stabilization. Protein Eng. Des. Sel. 2014, 27, 49–58. [Google Scholar] [CrossRef] [Green Version]
- Khersonsky, O.; Lipsh, R.; Avizemer, Z.; Ashani, Y.; Goldsmith, M.; Leader, H.; Dym, O.; Rogotner, S.; Trudeau, D.L.; Prilusky, J.; et al. Automated Design of Efficient and Functionally Diverse Enzyme Repertoires. Mol. Cell 2018, 72, 178–186.e5. [Google Scholar] [CrossRef] [Green Version]
- Rathi, P.C.; Mulnaes, D.; Gohlke, H. VisualCNA: A GUI for interactive constraint network analysis and protein engineering for improving thermostability. Bioinformatics 2015, 31, 2394–2396. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Zhang, X.Z.; Zhang, Z.; Zhang, Y.H.P. Engineering of clostridium phytofermentans endoglucanase Cel5A for improved thermostability. Appl. Environ. Microbiol. 2010, 76, 4914–4917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anbar, M.; Lamed, R.; Bayer, E.A. Thermostability Enhancement of Clostridium thermocellum Cellulosomal Endoglucanase Cel8A by a Single Glycine Substitution. ChemCatChem 2010, 2, 997–1003. [Google Scholar] [CrossRef]
- Dana, C.M.; Saija, P.; Kal, S.M.; Bryan, M.B.; Blanch, H.W.; Clark, D.S. Biased clique shuffling reveals stabilizing mutations in cellulase Cel7A. Biotechnol. Bioeng. 2012, 109, 2710–2719. [Google Scholar] [CrossRef]
- Chang, C.J.; Lee, C.C.; Chan, Y.T.; Trudeau, D.L.; Wu, M.H.; Tsai, C.H.; Yu, S.M.; Ho, T.H.D.; Wang, A.H.J.; Hsiao, C.D.; et al. Exploring the mechanism responsible for cellulase thermostability by structure-guided recombination. PLoS ONE 2016, 11, e0147485. [Google Scholar] [CrossRef] [PubMed]
- Akcapinar, G.B.; Venturini, A.; Martelli, P.L.; Casadio, R.; Sezerman, U.O. Modulating the thermostability of Endoglucanase I from trichoderma reesei using computational approaches. Protein Eng. Des. Sel. 2015, 28, 127–135. [Google Scholar] [CrossRef] [Green Version]
- Goedegebuur, F.; Dankmeyer, L.; Gualfetti, P.; Karkehabadi, S.; Hansson, H.; Jana, S.; Huynh, V.; Kelemen, B.R.; Kruithof, P.; Larenas, E.A.; et al. Improving the thermal stability of cellobiohydrolase Cel7A from Hypocrea jecorina by directed evolution. J. Biol. Chem. 2017, 292, 17418–17430. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aulitto, M.; Fusco, S.; Fiorentino, G.; Limauro, D.; Pedone, E.; Bartolucci, S.; Contursi, P. Thermus thermophilus as source of thermozymes for biotechnological applications: Homologous expression and biochemical characterization of an α-galactosidase. Microb. Cell Fact. 2017, 16, 28. [Google Scholar] [CrossRef] [Green Version]
- Aulitto, M.; Fusco, F.A.; Fiorentino, G.; Bartolucci, S.; Contursi, P.; Limauro, D. A thermophilic enzymatic cocktail for galactomannans degradation. Enzym. Microb. Technol. 2018, 111, 7–11. [Google Scholar] [CrossRef] [Green Version]
- Kong, F.; Yang, J.; Zhen, Z.; Liang, T.; Zhu, D.; Gao, R.; Xie, G. Gene cloning and molecular characterization of a β-glucosidase from Thermotoga naphthophila RUK-10: An effective tool for synthesis of galacto-oligosaccharide and alkyl galactopyranosides. Chem. Res. Chinese Univ. 2015, 31, 774–780. [Google Scholar] [CrossRef]
- Irwin, D.C.; Spezio, M.; Walker, L.P.; Wilson, D.B. Activity studies of eight purified cellulases: Specificity, synergism, and binding domain effects. Biotechnol. Bioeng. 1993, 42, 1002–1013. [Google Scholar] [CrossRef] [PubMed]
- Fonseca, G.G.; Heinzle, E.; Wittmann, C.; Gombert, A.K. The yeast Kluyveromyces marxianus and its biotechnological potential. Appl. Microbiol. Biotechnol. 2008, 79, 339–354. [Google Scholar] [CrossRef] [PubMed]
- Jansen, M.L.A.; Bracher, J.M.; Papapetridis, I.; Verhoeven, M.D.; de Bruijn, H.; de Waal, P.P.; van Maris, A.J.A.; Klaassen, P.; Pronk, J.T. Saccharomyces cerevisiae strains for second-generation ethanol production: From academic exploration to industrial implementation. FEMS Yeast Res. 2017, 17, fox044. [Google Scholar] [CrossRef] [PubMed]
- Kuloyo, O.O.; du Preez, J.C.; del Prado García-Aparicio, M.; Kilian, S.G.; Steyn, L.; Görgens, J. Opuntia ficus-indica cladodes as feedstock for ethanol production by Kluyveromyces marxianus and Saccharomyces cerevisiae. World J. Microbiol. Biotechnol. 2014, 30, 3173–3183. [Google Scholar] [CrossRef] [Green Version]
- Leonel, L.V.; Arruda, P.V.; Chandel, A.K.; Felipe, M.G.A.; Sene, L. Kluyveromyces marxianus: A potential biocatalyst of renewable chemicals and lignocellulosic ethanol production. Crit. Rev. Biotechnol. 2021, 1–22, 1–39. [Google Scholar] [CrossRef]
- Nurcholis, M.; Lertwattanasakul, N.; Rodrussamee, N.; Kosaka, T.; Murata, M.; Yamada, M. Integration of comprehensive data and biotechnological tools for industrial applications of Kluyveromyces marxianus. Appl. Microbiol. Biotechnol. 2020, 104, 475–488. [Google Scholar] [CrossRef]
- Karim, A.; Gerliani, N.; Aïder, M. Kluyveromyces marxianus: An emerging yeast cell factory for applications in food and biotechnology. Int. J. Food Microbiol. 2020, 333, 108818. [Google Scholar] [CrossRef] [PubMed]
- Madeira, J.V., Jr.; Gombert, A.K. Towards high-temperature fuel ethanol production using Kluyveromyces marxianus: On the search for plug-in strains for the Brazilian sugarcane-based biorefinery. Biomass Bioenergy 2018, 119, 217–228. [Google Scholar] [CrossRef]
- de Araujo Guilherme, A.; Dantas, P.V.F.; Padilha, C.E.A.; dos Santos, E.S.; de Macedo, G.R. Ethanol production from sugarcane bagasse: Use of different fermentation strategies to enhance an environmental-friendly process. J. Environ. Manag. 2019, 234, 44–51. [Google Scholar] [CrossRef]
- Yan, J.; Wei, Z.; Wang, Q.; He, M.; Li, S.; Irbis, C. Bioethanol production from sodium hydroxide/hydrogen peroxide-pretreated water hyacinth via simultaneous saccharification and fermentation with a newly isolated thermotolerant Kluyveromyces marxianu strain. Bioresour. Technol. 2015, 193, 103–109. [Google Scholar] [CrossRef]
- Demain, A.L.; Newcomb, M.; Wu, J.H.D. Cellulase, Clostridia, and Ethanol. Microbiol. Mol. Biol. Rev. 2005, 69, 124–154. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Olson, D.G.; McBride, J.E.; Joe Shaw, A.; Lynd, L.R. Recent progress in consolidated bioprocessing. Curr. Opin. Biotechnol. 2012, 23, 396–405. [Google Scholar] [CrossRef]
- Nisha, M.; Saranyah, K.; Shankar, M.; Saleena, L.M. Enhanced saccharification of lignocellulosic agricultural biomass and increased bioethanol titre using acclimated Clostridium thermocellum DSM1313. 3 Biotech 2017, 7, 35. [Google Scholar] [CrossRef] [Green Version]
- Lynd, L.R.; Guss, A.M.; Himmel, M.E.; Beri, D.; Herring, C.; Holwerda, E.K.; Murphy, S.J.; Olson, D.G.; Paye, J.; Rydzak, T.; et al. Advances in Consolidated Bioprocessing Using Clostridium thermocellum and Thermoanaerobacter saccharolyticum. In Industrial Biotechnology; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2016; pp. 365–394. [Google Scholar] [CrossRef]
- Beri, D.; York, W.S.; Lynd, L.R.; Peña, M.J.; Herring, C.D. Development of a thermophilic coculture for corn fiber conversion to ethanol. Nat. Commun. 2020, 11, 1937. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Du, Y.; Zou, W.; Zhang, K.; Ye, G.; Yang, J. Advances and Applications of Clostridium Co-culture Systems in Biotechnology. Front. Microbiol. 2020, 11, 1937. [Google Scholar] [CrossRef] [PubMed]
- Liberato, V.; Benevenuti, C.; Coelho, F.; Botelho, A.; Amaral, P.; Pereira, N.; Ferreira, T. Clostridium sp. As bio-catalyst for fuels and chemicals production in a biorefinery context. Catalysts 2019, 9, 962. [Google Scholar] [CrossRef] [Green Version]
- Froese, A.G.; Sparling, R. Cross-feeding and wheat straw extractives enhance growth of Clostridium thermocellum-containing co-cultures for consolidated bioprocessing. Bioprocess Biosyst. Eng. 2021, 44, 819–830. [Google Scholar] [CrossRef]
- Xiong, W.; Reyes, L.H.; Michener, W.E.; Maness, P.C.; Chou, K.J. Engineering cellulolytic bacterium Clostridium thermocellum to co-ferment cellulose- and hemicellulose-derived sugars simultaneously. Biotechnol. Bioeng. 2018, 115, 1755–1763. [Google Scholar] [CrossRef]
- Hon, S.; Olson, D.G.; Holwerda, E.K.; Lanahan, A.A.; Murphy, S.J.L.; Maloney, M.I.; Zheng, T.; Papanek, B.; Guss, A.M.; Lynd, L.R. The ethanol pathway from Thermoanaerobacterium saccharolyticum improves ethanol production in Clostridium thermocellum. Metab. Eng. 2017, 42, 175–184. [Google Scholar] [CrossRef]
- Jiang, Y.; Xin, F.; Lu, J.; Dong, W.; Zhang, W.; Zhang, M.; Wu, H.; Ma, J.; Jiang, M. State of the art review of biofuels production from lignocellulose by thermophilic bacteria. Bioresour. Technol. 2017, 245, 1498–1506. [Google Scholar] [CrossRef] [PubMed]
- Bing, R.G.; Sulis, D.B.; Wang, J.P.; Adams, M.W.W.; Kelly, R.M. Thermophilic microbial deconstruction and conversion of natural and transgenic lignocellulose. Environ. Microbiol. Rep. 2021, 13, 272–293. [Google Scholar] [CrossRef] [PubMed]
- Scully, S.M.; Orlygsson, J. Recent advances in second generation ethanol production by thermophilic bacteria. Energies 2015, 8, 1–30. [Google Scholar] [CrossRef] [Green Version]
- Herring, C.D.; Kenealy, W.R.; Joe Shaw, A.; Covalla, S.F.; Olson, D.G.; Zhang, J.; Ryan Sillers, W.; Tsakraklides, V.; Bardsley, J.S.; Rogers, S.R.; et al. Strain and bioprocess improvement of a thermophilic anaerobe for the production of ethanol from wood. Biotechnol. Biofuels 2016, 9, 125. [Google Scholar] [CrossRef] [Green Version]
- Currie, D.H.; Herring, C.D.; Guss, A.M.; Olson, D.G.; Hogsett, D.A.; Lynd, L.R. Functional heterologous expression of an engineered full length CipA from Clostridium thermocellum in Thermoanaerobacterium saccharolyticum. Biotechnol. Biofuels 2013, 6, 32. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, Q.; Hemme, C.L.; Jiang, H.; He, Z.; Zhou, J. Mechanisms of enhanced cellulosic bioethanol fermentation by co-cultivation of Clostridium and Thermoanaerobacter spp. Bioresour. Technol. 2011, 102, 9586–9592. [Google Scholar] [CrossRef]
- Pang, J.; Hao, M.; Li, Y.; Liu, J.; Lan, H.; Zhang, Y.; Zhang, Q.; Liu, Z. Consolidated Bioprocessing Using Clostridium thermocellum and Thermoanaerobacterium thermosaccharolyticum Co-culture for Enhancing Ethanol Production from Corn Straw. BioResources 2018, 13, 8209–8221. [Google Scholar] [CrossRef]
- Pang, J.; Hao, M.; Shi, Y.; Li, Y.; Zhu, M.; Hu, J.; Liu, J.; Zhang, Q.; Liu, Z. Enhancing the ethanol yield from salix using a Clostridium thermocellum and Thermoanaerobacterium thermosaccharolyticum co-culture system. BioResources 2019, 13, 5377–5393. [Google Scholar] [CrossRef]
- Bu, J.; Tian, Q.Q.; Zhu, M.J. Enhanced Biodegradation of Sugar Cane Bagasse by Coculture of Clostridium thermocellum and Thermoanaerobacterium aotearoense Supplemented with CaCO3. Energy Fuels 2017, 31, 9477–9483. [Google Scholar] [CrossRef]
- Singh, N.; Gupta, R.P.; Puri, S.K.; Mathur, A.S. Bioethanol production from pretreated whole slurry rice straw by thermophilic co-culture. Fuel 2021, 301, 121074. [Google Scholar] [CrossRef]
- Liu, Y.; Xie, X.; Liu, W.; Xu, H.; Cao, Y. Consolidated bioprocess for bioethanol production from lignocellulosic biomass using Clostridium thermocellum DSM 1237. BioResources 2020, 15, 8355–8368. [Google Scholar] [CrossRef]
- Blumer-Schuette, S.E.; Brown, S.D.; Sander, K.B.; Bayer, E.A.; Kataeva, I.; Zurawski, J.V.; Conway, J.M.; Adams, M.W.W.; Kelly, R.M. Thermophilic lignocellulose deconstruction. FEMS Microbiol. Rev. 2014, 38, 393–448. [Google Scholar] [CrossRef] [PubMed]
- Chung, D.; Cha, M.; Snyder, E.N.; Elkins, J.G.; Guss, A.M.; Westpheling, J. Cellulosic ethanol production via consolidated bioprocessing at 75 °C by engineered Caldicellulosiruptor bescii. Biotechnol. Biofuels 2015, 8, 163. [Google Scholar] [CrossRef] [Green Version]
- Straub, C.T.; Bing, R.G.; Wang, J.P.; Chiang, V.L.; Adams, M.W.W.; Kelly, R.M. Use of the lignocellulose-degrading bacterium Caldicellulosiruptor bescii to assess recalcitrance and conversion of wild-type and transgenic poplar. Biotechnol. Biofuels 2020, 13, 43. [Google Scholar] [CrossRef] [PubMed]
- Straub, C.T.; Khatibi, P.A.; Wang, J.P.; Conway, J.M.; Williams-Rhaesa, A.M.; Peszlen, I.M.; Chiang, V.L.; Adams, M.W.W.; Kelly, R.M. Quantitative fermentation of unpretreated transgenic poplar by Caldicellulosiruptor bescii. Nat. Commun. 2019, 10, 3548. [Google Scholar] [CrossRef] [PubMed]
- Straub, C.T.; Khatibi, P.A.; Otten, J.K.; Adams, M.W.W.; Kelly, R.M. Lignocellulose solubilization and conversion by extremely thermophilic Caldicellulosiruptor bescii improves by maintaining metabolic activity. Biotechnol. Bioeng. 2019, 116, 1901–1908. [Google Scholar] [CrossRef]
- Zhu, D.; Adebisi, W.A.; Ahmad, F.; Sethupathy, S.; Danso, B.; Sun, J. Recent Development of Extremophilic Bacteria and Their Application in Biorefinery. Front. Bioeng. Biotechnol. 2020, 8, 483. [Google Scholar] [CrossRef]
- Bhalla, A.; Bischoff, K.M.; Sani, R.K. Highly thermostable xylanase production from a thermophilic Geobacillus sp. strain WSUCF1 utilizing lignocellulosic biomass. Front. Bioeng. Biotechnol. 2015, 3, 84. [Google Scholar] [CrossRef]
- Bibra, M.; Rathinam, N.K.; Johnson, G.R.; Sani, R.K. Single pot biovalorization of food waste to ethanol by Geobacillus and Thermoanaerobacter spp. Renew. Energy 2020, 155, 1032–1041. [Google Scholar] [CrossRef]
- Papanek, B.; Biswas, R.; Rydzak, T.; Guss, A.M. Elimination of metabolic pathways to all traditional fermentation products increases ethanol yields in Clostridium thermocellum. Metab. Eng. 2015, 32, 49–54. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tian, L.; Perot, S.J.; Stevenson, D.; Jacobson, T.; Lanahan, A.A.; Amador-Noguez, D.; Olson, D.G.; Lynd, L.R. Metabolome analysis reveals a role for glyceraldehyde 3-phosphate dehydrogenase in the inhibition of C. thermocellum by ethanol. Biotechnol. Biofuels 2017, 10, 276. [Google Scholar] [CrossRef]
- Kim, S.K.; Westpheling, J. Engineering a spermidine biosynthetic pathway in Clostridium thermocellum results in increased resistance to furans and increased ethanol production. Metab. Eng. 2018, 49, 267–274. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.K.; Jin, Y.S.; Choi, I.G.; Park, Y.C.; Seo, J.H. Enhanced tolerance of Saccharomyces cerevisiae to multiple lignocellulose-derived inhibitors through modulation of spermidine contents. Metab. Eng. 2015, 29, 46–55. [Google Scholar] [CrossRef]
- Limayem, A.; Ricke, S.C. Lignocellulosic biomass for bioethanol production: Current perspectives, potential issues and future prospects. Prog. Energy Combust. Sci. 2012, 38, 449–467. [Google Scholar] [CrossRef]
- Sivarathnakumar, S.; Jayamuthunagai, J.; Baskar, G.; Praveenkumar, R.; Selvakumari, I.A.E.; Bharathiraja, B. Bioethanol production from woody stem Prosopis juliflora using thermo tolerant yeast Kluyveromyces marxianus and its kinetics studies. Bioresour. Technol. 2019, 293, 122060. [Google Scholar] [CrossRef] [PubMed]
- de Barros, E.M.; Carvalho, V.M.; Rodrigues, T.H.S.; Rocha, M.V.P.; Gonçalves, L.R.B. Comparison of strategies for the simultaneous saccharification and fermentation of cashew apple bagasse using a thermotolerant Kluyveromyces marxianus to enhance cellulosic ethanol production. Chem. Eng. J. 2017, 307, 939–947. [Google Scholar] [CrossRef]
- Jutakanoke, R.; Tolieng, V.; Tanasupawat, S.; Akaracharanya, A. Ethanol Production from Sugarcane Leaves by Kluyveromyces marxianus S1.17, a Genome-Shuffling Mediated Transformant. BioResources 2017, 12, 1636–1646. [Google Scholar] [CrossRef] [Green Version]
- Jung, Y.R.; Park, J.M.; Heo, S.Y.; Hong, W.K.; Lee, S.M.; Oh, B.R.; Park, S.M.; Seo, J.W.; Kim, C.H. Cellulolytic enzymes produced by a newly isolated soil fungus Penicillium sp. TG2 with potential for use in cellulosic ethanol production. Renew. Energy 2015, 76, 66–71. [Google Scholar] [CrossRef]
- da Silva, F.L.; de Oliveira Campos, A.; dos Santos, D.A.; Batista Magalhães, E.R.; de Macedo, G.R.; dos Santos, E.S. Valorization of an agroextractive residue—Carnauba straw—for the production of bioethanol by simultaneous saccharification and fermentation (SSF). Renew. Energy 2018, 127, 661–669. [Google Scholar] [CrossRef]
- Ganesan, S. Production of Bioethanol from Bamboo using Thermotolerant Yeast with Simultaneous Saccharification and Fermentation Process. Int. J. Curr. Microbiol. Appl. Sci. 2019, 8, 1718–1727. [Google Scholar] [CrossRef]
- Harish Kumar Reddy, Y.; Srijana, M.; Madhusudhan Reddy, D.; Gopal, R. Coculture fermentation of banana agro-waste to ethanol by cellulolytic thermophilic Clostridium thermocellum CT2. Afr. J. Biotechnol. 2010, 9, 1926–1934. [Google Scholar] [CrossRef] [Green Version]
- Lo, J.; Olson, D.G.; Murphy, S.J.L.; Tian, L.; Hon, S.; Lanahan, A.; Guss, A.M.; Lynd, L.R. Engineering electron metabolism to increase ethanol production in Clostridium thermocellum. Metab. Eng. 2017, 39, 71–79. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Eminoǧlu, A.; Murphy, S.J.L.; Maloney, M.; Lanahan, A.; Giannone, R.J.; Hettich, R.L.; Tripathi, S.A.; Beldüz, A.O.; Lynd, L.R.; Olson, D.G. Deletion of the hfsB gene increases ethanol production in Thermoanaerobacterium saccharolyticum and several other thermophilic anaerobic bacteria. Biotechnol. Biofuels 2017, 10, 282. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, H.; Luo, S.; Dai, K.; Qu, C.; Wang, J. Engineering Thermoanaerobacterium aotearoense SCUT27/Δldh with pyruvate formate lyase-activating protein (PflA) knockout for enhanced ethanol tolerance and production. Process Biochem. 2021, 106, 184–190. [Google Scholar] [CrossRef]


| Enzyme [Reference] | Microorganism | Topt (°C) | pHopt | Thermal Stability (Activity %) | Substrate | Result |
|---|---|---|---|---|---|---|
| Endo-1,4-β-glucanaseAf-EGL7 [92] | Aspergillus fumigatus | 55 | 5 | 61% after 24 h at 60 °C | Corncob | The addition of 10 μg of Af-EGL7 to 0.009 FPU of Celluclast® 1.5 L increased the release of reducing sugars by 128% after 72 h (55 °C, pH 5) |
| Rice straw | The addition of 10 μg of Af-EGL7 to 0.009 FPU of Celluclast® 1.5 L increased the release of reducing sugars by 80% after 72 h (55 °C, pH 5) | |||||
| Endo-β-1,4-d-glucanase [93] | Trichoderma harzianum HZN11 | 60 | 5.5 | 66% after 3 h at 65 °C | Alkali pretreated sweet sorghum | 53 U/g mixed with 9 FP U/mL of commercial Trichoderma sps. increased the release of reducing sugars by 54% after 48 h (40 °C, pH 5.5) |
| Sugarcane bagasse | 53 U/g mixed with 9 FP U/mL of commercial Trichoderma sps. increased the release of reducing sugars by 21% after 48 h (40 °C, pH 5.5) | |||||
| Cellobiohydrolase CtCel7 [94] | Chaetomium thermophilum | 60 | 4 | 90% after 3 h at 60 °C | Pretreated wheat straw | The addition of CtCel7 to Sigma-Aldrich cellulase cocktail in 1:1 ratio increased the release of reducing sugars by 63% after 8 h (60 °C, pH 4). |
| β-glucosidase [95] | Humicola grisea var. thermoidea | 50 | 6–7 | 50% after 7 and 14 min at 60 °C in absence or presence of 50 mM glucose | Sugarcane bagasse | The addition of 0.1 U of purified or crude β-glucosidase to 10 FPU T. reesei cellulases increased the saccharification by 50%. (50 °C, pH 5) |
| β-glucosidase TN0602 [96] | Thermotoga naphthophila RUK-10 | 75 | 6 | 50% after 3 h at 90 °C [123] | Corn straw | A maximum increase in released glucose of 30.62% when 0.5 U/mL pf addition TN0602 were added to 0.75 U/mL of commercial cellulase from T. reesei (50 °C, pH 5) |
| Endoxylanase (HXYN2) [97] | Humicola grisea var thermoidea, | 60 | 6.5 | 65% after 48 h at 50 °C | Steam-exploded corn straw | Addition of hemicellulases cocktail (600 U/g HXYN2, 11.5 U/g HXYLA and 0.32 U/g ABF3) to 5 FPU/g Accellerase 1500 enhanced the glucose yield by 14.6% in simultaneous reaction and by 50% in sequential reactions after 48 h, (50 °C, pH 5) |
| β-xylosidase (HXYLA) [97] | 50 | 7 | 20% after 48 h at 50 °C | |||
| α-l-arabinofuranosidase (AFB3) [97] | Penicillium pupurogenum | 50 | 5 | 25% after 48 h at 50 °C | ||
| XynZ-C and Xyn11A [98] | Clostridium thermocellum Thermobifida fusca | 75 | 7 | 96% after 18h at 75 °C in 10% glycerol [124] | Pretreated bagasse | 2-fold increase in the concentration of reducing sugars, when both xylanases, were added to T. reesei cellulase in 50:50 ratio (50 °C, pH 6) |
| Xylanase (TcXyn10A) [99] | Thermobacillus composti | 65 | 6–8 | 40% after 8 h at 65 °C | Sugarcane bagasse | Improvement of hydrolysis Accellerase® 1500 cocktail hydrolysis by 15.35 % increase in xylose release and 4.38% glucose release after 24 h in 1:1 ratio (50 °C pH 5) |
| Endo-β-mannanase MtMan26A [100] | Myceliophthora thermophila | 60 | 6 | 50% after 14.4 h at 60 °C | Pretreated beechwood sawdust | Release of total reducing sugars and glucose improved by 13 and 12%, as a supplement to Celluclast® 1.5 L and Novozyme® 188 in 1:1 ratio (50 °C pH 5) |
| LPMO PMO9D_SCYTH [104] | Scytalidium thermophilum | 60 | 7 | 50% after 60.58 h at 60 °C | Bagasse | 18.9 and 17.5% yield increase from acid or alkali-treated bagasse respectively (Cellic CTec2, 9:1 ratio, 50 °C pH 5) |
| Rice straw | 28.7 and 22.1% yield increase from acid or alkali-treated rice straw respectively (Cellic CTec2, 9:1 ratio, 50 °C pH 5) | |||||
| LPMO PMO9D_MALCI [104] | Malbranchea cinnamomea | 50 | 9 | 50 after 144 h at 50 °C | Bagasse | 21.3 and 23.6% yield increase from acid or alkali-treated bagasse respectively (Cellic CTec2, 9:1 ratio, 50 °C pH 5) |
| Rice straw | 28.8 and 13.6% yield increase from acid or alkali respectively (Cellic CTec2, 9:1 ratio, 50 °C pH 5) |
| Strain [Reference] | Feedstock | Temperature (°C) | Total Fermentation Time (a) | Process Scheme | Maximum Ethanol Titer (b) | Maximum Ethanol Yield (c) |
|---|---|---|---|---|---|---|
| K. marxianus MTCC 1389 [168] | Prosopis juliflora woody stems | 41 | 72 h (72 h) | SSF | 21.45 g/L | 0.67 g/g |
| K. marxianus ATCC 36,907 [169] | Cashew apple bagasse | 40 | 72 h (~28 h) | SSF | 68 g/L | 80.70% |
| K. marxianus S1.17 [170] | Sugarcane leaves | 40 | 132 h (~48 h) | SSF | 5.59 g/L | 0.10 g/g dry weight |
| K. marxianus K213 [133] | Water hyacinth | 42 | 24 h (24 h) | SSF | 7.34 g/L | 0.16 g/g biomass |
| K. marxianus KCTC7001 [171] | Empty palm fruit bunches | 42 | ~28 h | SSF | 7.80% | |
| K. marxianus ATCC 36,907 [172] | Carnauba straw residue | 45 | 48 h (12 h) | SSF | 7.52 g/L | 75.29% |
| K. marxianus ATCC 36,907 [132] | Sugarcane bagasse | 43 | 24 h | SSF | 4.18 g/100 g biomass | |
| K. marxianus TY16 [173] | Bamboo | 42.5 | 108 h (108 h) | SSF | 26.04 g/L | |
| C. thermocellum DSM 1313 [136] | Rice husk | 60 | 120 h | CBP | 1 g/L | |
| C. thermocellum [136] | Sugarcane bagasse | 60 | 120 h | CBP | 1.21 g/L | |
| C. thermocellum ATCC 27,405 and T. thermosaccharolyticum DSM 571 [150] | Corn straw | 55 | 168 h (120 h) | CBP | 0.45 g/L | 11.20% |
| C. thermocellum ATCC 27,405 and T. thermosaccharolyticum DSM 571 [151] | Salix | 55 | 168 h | CBP | 0.2 g/L | 11.10% |
| C. thermocellum CT2 and Clostridium thermosaccharolyticum HG8 [174] | Banana Agro-waste | 60 | 120 h | CBP | 0.41 g/g substrate | |
| C. thermocellum [152] | Sugarcane bagasse | 55 | 168 h | CBP | 10.60 mM | |
| Clostridium sp. DBT-IOC-C19 and Thermoanaerobacter sp. DBT-IOC-X2 [153] | Rice straw slurry | 60 | 144 h (144 h) | CBP | 142 mM | 48% |
| C. thermocellum DSM 1237 [154] | Sugarcane bagasse | 60 | 60 h (~28 h) | CBP | 0.86 g/L | 83.30% |
| Engineered C. bescii [156] | Switchgrass | 75 | 50 h (15 h) | CBP | ~2 mM | |
| C. bescii MACB 1058 [158] | Poplar (transgenic) | 65 | 168 h | CBP | 18.3 mM | |
| G. thermoglucosidasius and T. ethanolicus [162] | Food waste | 60 | 120 h (120 h) | CBP | 18.4 g/L | 0.24 g/g sugar |
| Strain [Reference] | Protein Function (Gene) | Source Organism | Effect |
|---|---|---|---|
| C. thermocellum DSM1313 [142] | Xylose isomerase (xylA), xylulokinase (xylB). | T. ethanolicus | Enabled xylose co-fermentation. |
| C. thermocellum DSM1313 [143] | Bifunctional alcohol dehydrogenases (adhE), NADH-dependent reduced ferredoxin:NADP+ oxidoreductase complex (nfnA/B), and NADPH- dependent alcohol dehydrogenase (adhA) | T. saccharolyticum | Enhanced ethanol production. |
| C. thermocellum DSM1313 Δhpt ΔhydG [163] | Lactate dehydrogenase (ldh), pyruvate-formate lyase (pfl), and phosphotransacetylase and acetate kinase (pta-ack) | Self, knockout | Enhanced ethanol production. |
| C. thermocellum [164] | Gglyceraldehyde 3-phosphate dehydrogenase (gapdh) | T. saccharolyticum | Enhanced ethanol tolerance. |
| C. thermocellum [165] | Spermidine synthase (speE) | Self, overexpression | Enhanced acetate and HMF tolerance. |
| C. thermocellum [175] | Ion-translocating reduced ferredoxin: NAD+ oxidoreductase (rnf) and hydrogenase maturation gene (hydG) | Self, overexpression, and knockout, respectively | Enhanced ethanol production. |
| T. thermosaccharolyticum [138] | A-d-xylosidase (α-Xylp_1211), α-l-galactosidases (α-l-Galp_687 and 697), β-d-xylosidase (β-Xylp_1710), and α-l-arabinofuranosidases (α-Araf_996 and 1120) | Herbinix spp. | Conferred ability to deconstruct GAX component of corn fiber |
| T. saccharolyticum, T. thermosaccharolyticum, T. xylanolyticum, T. mathranii, and C. thermocellum [176] | Hydrogenase (hfsB) | Self, knockout | Enhanced ethanol production. |
| Thermoanaerobacterium aotearoense SCUT27/Δldh [177] | Pyruvate formate lyase-activating protein A (pflA) | Self, knockout | Enhanced ethanol tolerance and production |
| C. bescii Δldh [156] | Bi-functional acetyl-CoA thioesterase/ alcohol dehydrogenase (adhB) and bi-functional acetaldehyde/alcohol dehydrogenase (adhE) | Thermoanaerobacter pseudethanolicus 39E | Enabled ethanol fermentation at high temperatures. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zuliani, L.; Serpico, A.; De Simone, M.; Frison, N.; Fusco, S. Biorefinery Gets Hot: Thermophilic Enzymes and Microorganisms for Second-Generation Bioethanol Production. Processes 2021, 9, 1583. https://doi.org/10.3390/pr9091583
Zuliani L, Serpico A, De Simone M, Frison N, Fusco S. Biorefinery Gets Hot: Thermophilic Enzymes and Microorganisms for Second-Generation Bioethanol Production. Processes. 2021; 9(9):1583. https://doi.org/10.3390/pr9091583
Chicago/Turabian StyleZuliani, Luca, Annabel Serpico, Mario De Simone, Nicola Frison, and Salvatore Fusco. 2021. "Biorefinery Gets Hot: Thermophilic Enzymes and Microorganisms for Second-Generation Bioethanol Production" Processes 9, no. 9: 1583. https://doi.org/10.3390/pr9091583
APA StyleZuliani, L., Serpico, A., De Simone, M., Frison, N., & Fusco, S. (2021). Biorefinery Gets Hot: Thermophilic Enzymes and Microorganisms for Second-Generation Bioethanol Production. Processes, 9(9), 1583. https://doi.org/10.3390/pr9091583








