Reactor Selection for Upgrading Hemicelluloses: Conventional and Miniaturised Reactors for Hydrogenations
Abstract
:1. Introduction
Process of Reactor Selection
- Reaction parameters (stoichiometry of the reaction network, heats of reaction, equilibrium data,…);
- Physical properties (densities, viscosities, solubilities, heat capacities,…);
- Catalyst parameters (reaction rates, selectivity, deactivation characteristics, chemical, mechanical and thermal stability, costs…);
- Reactor parameters (mass and heat transfer coefficients, thermal conductivities, pressure drop, catalyst replacement, design difficulties, simplicity of scale-up, …); and
- Safety parameters (stability and controllability, amount of hazardous inventory,…).
- Particle/interface;
- Reactant injection/removal, mixing as well as contacting; and
- Flow regime.
2. Methods
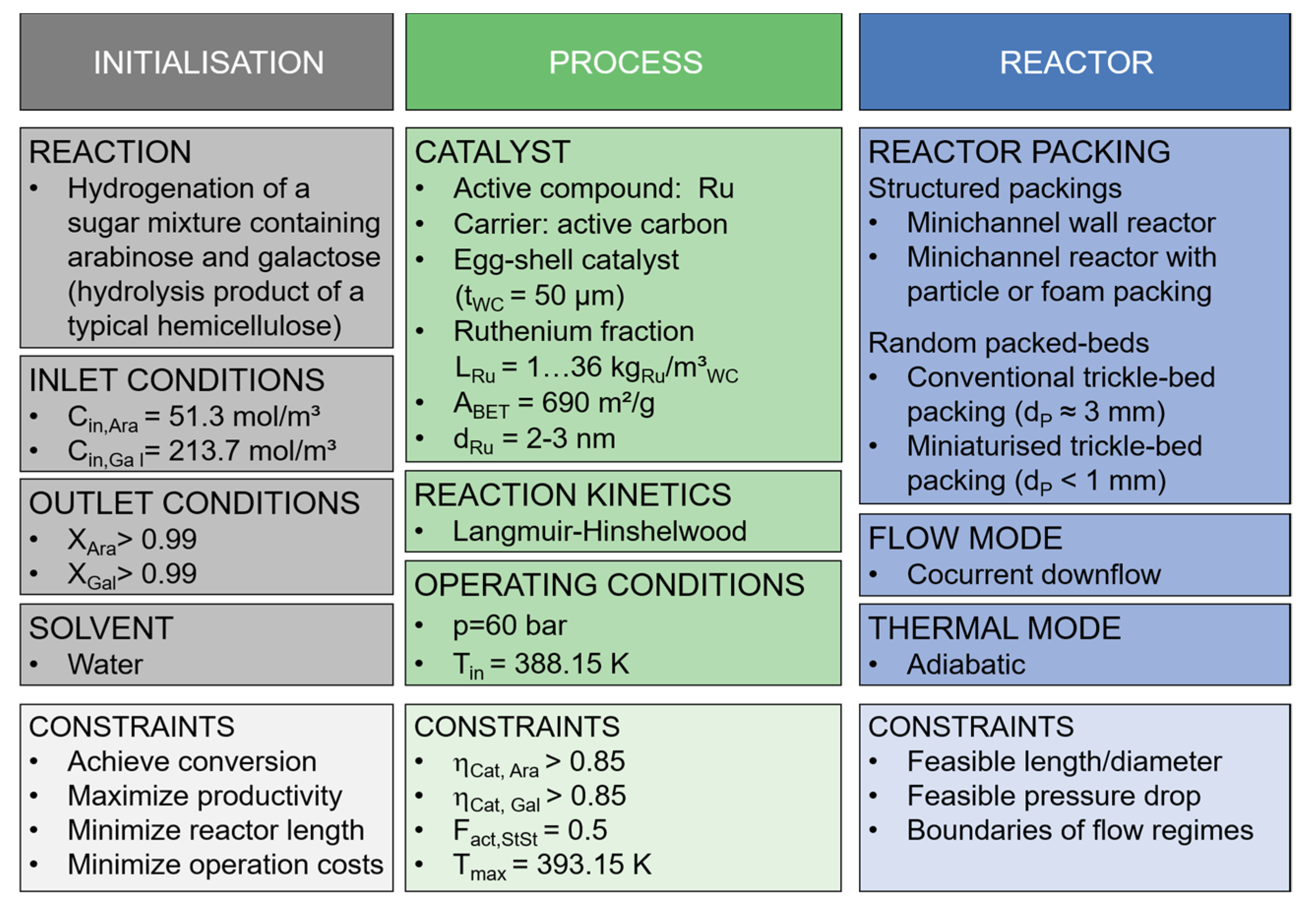
2.1. Definition of the Case Scenario
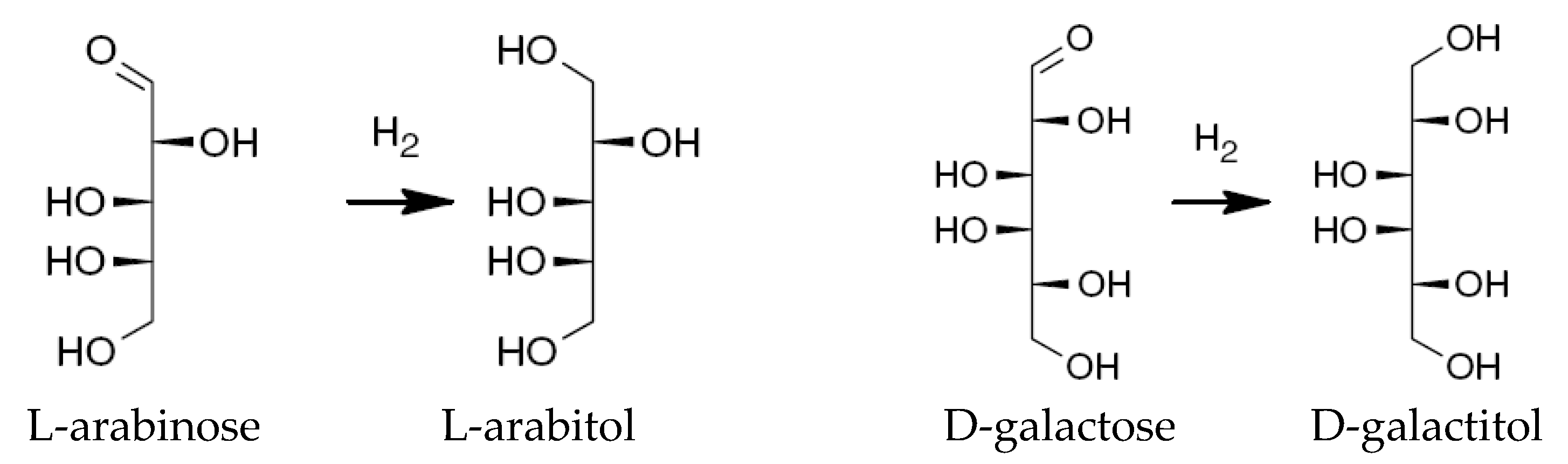
2.1.1. Hydrogenation Process
2.1.2. Process Parameters and Constraints
2.2. Reactor Modelling and Simulation
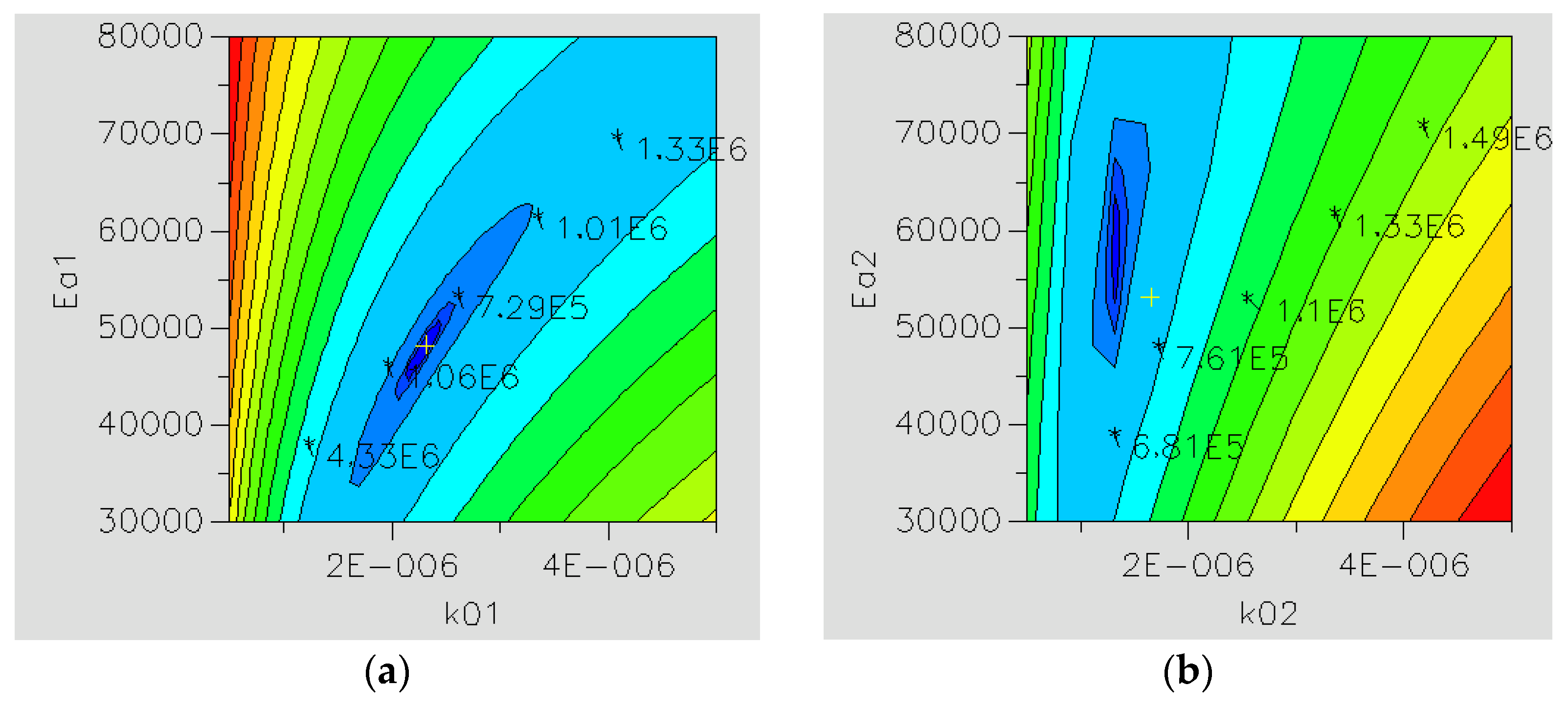
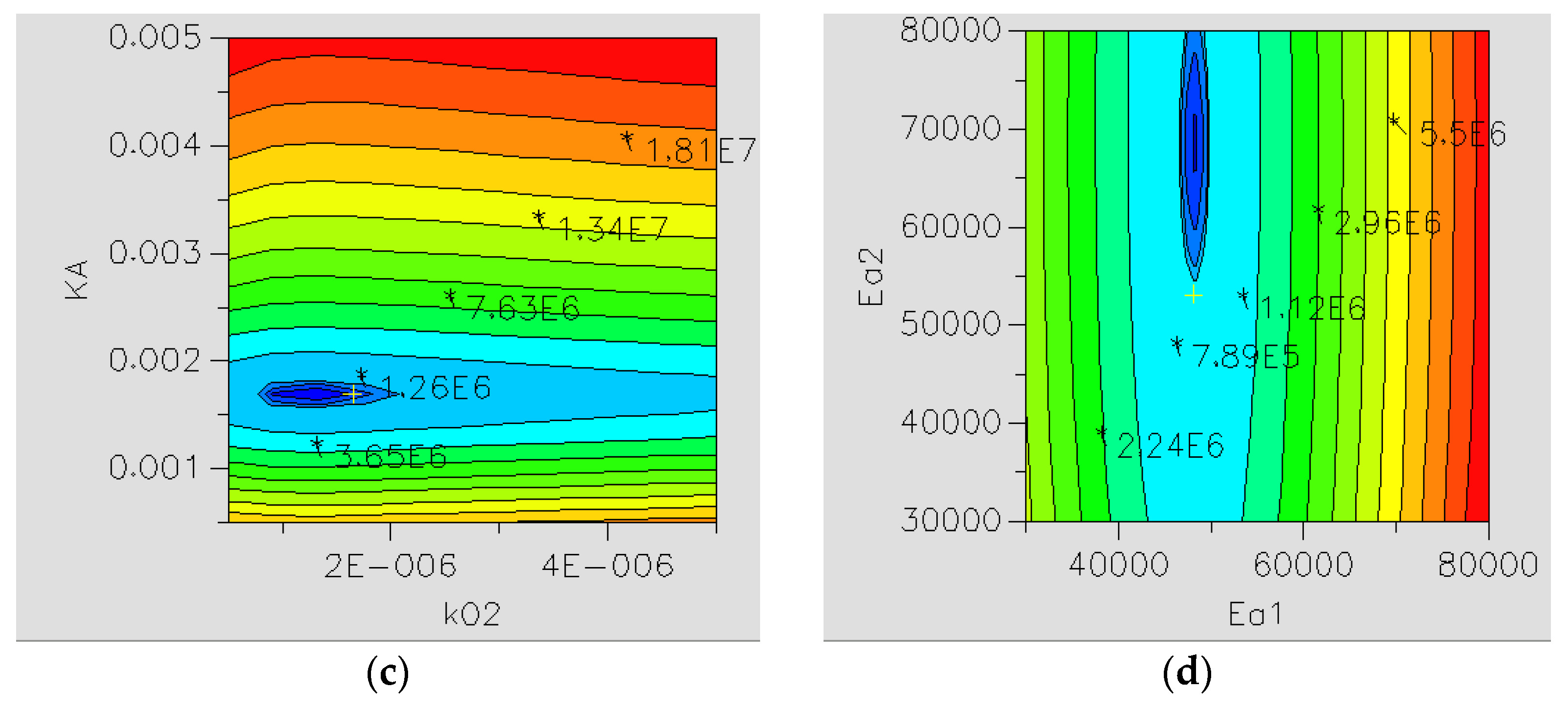
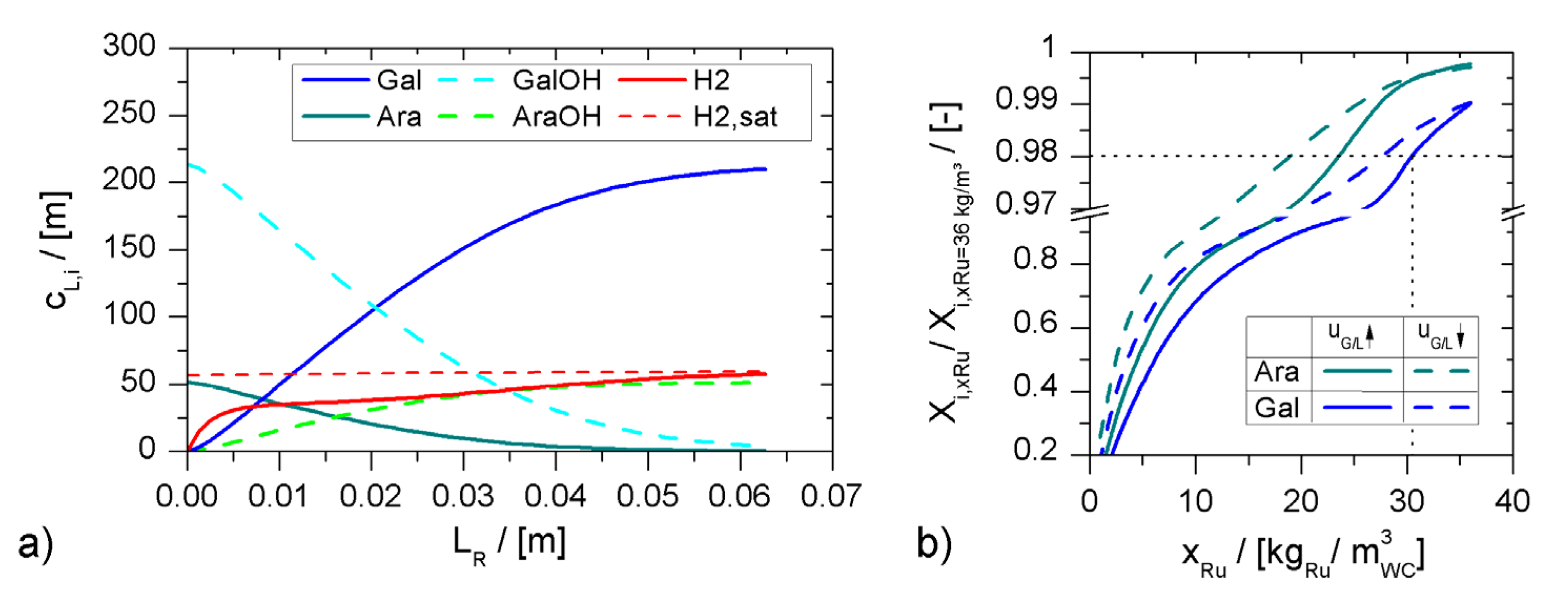
2.2.1. Reaction Kinetics
2.2.2. Reactor Model
Mass Balances
Heat Balances
2.2.3. Model Specifications
Conventional and Miniaturised Trickle-Bed Reactor (TBR and MTBR)
Wall-Coated Minichannel Reactor (MCWR)
Minichannel Reactor Packed with Pellets (MCPR)
Minichannel Reactor Packed with an Open-Celled Foam (MCFR)
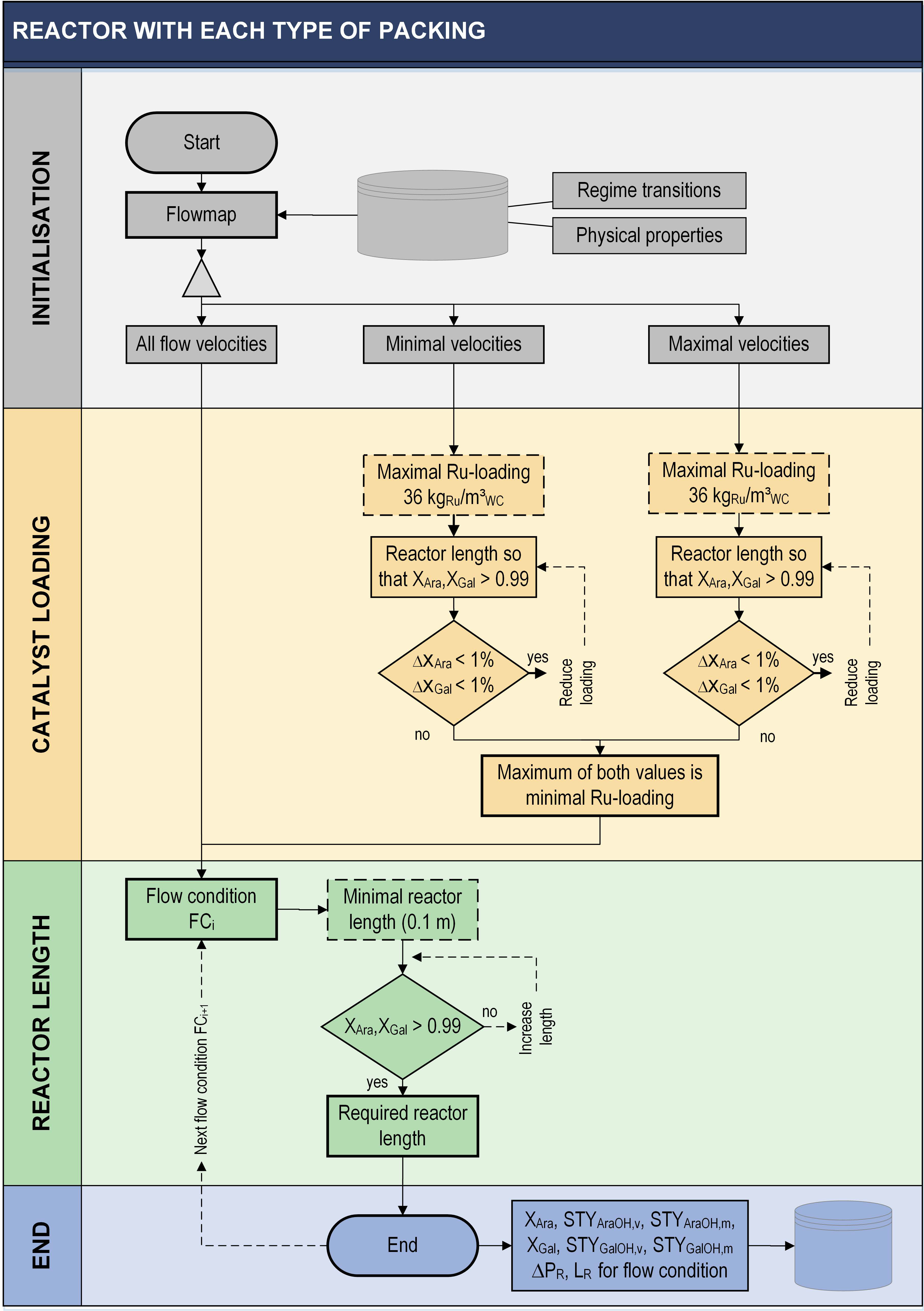
2.3. Evaluation Method
3. Results
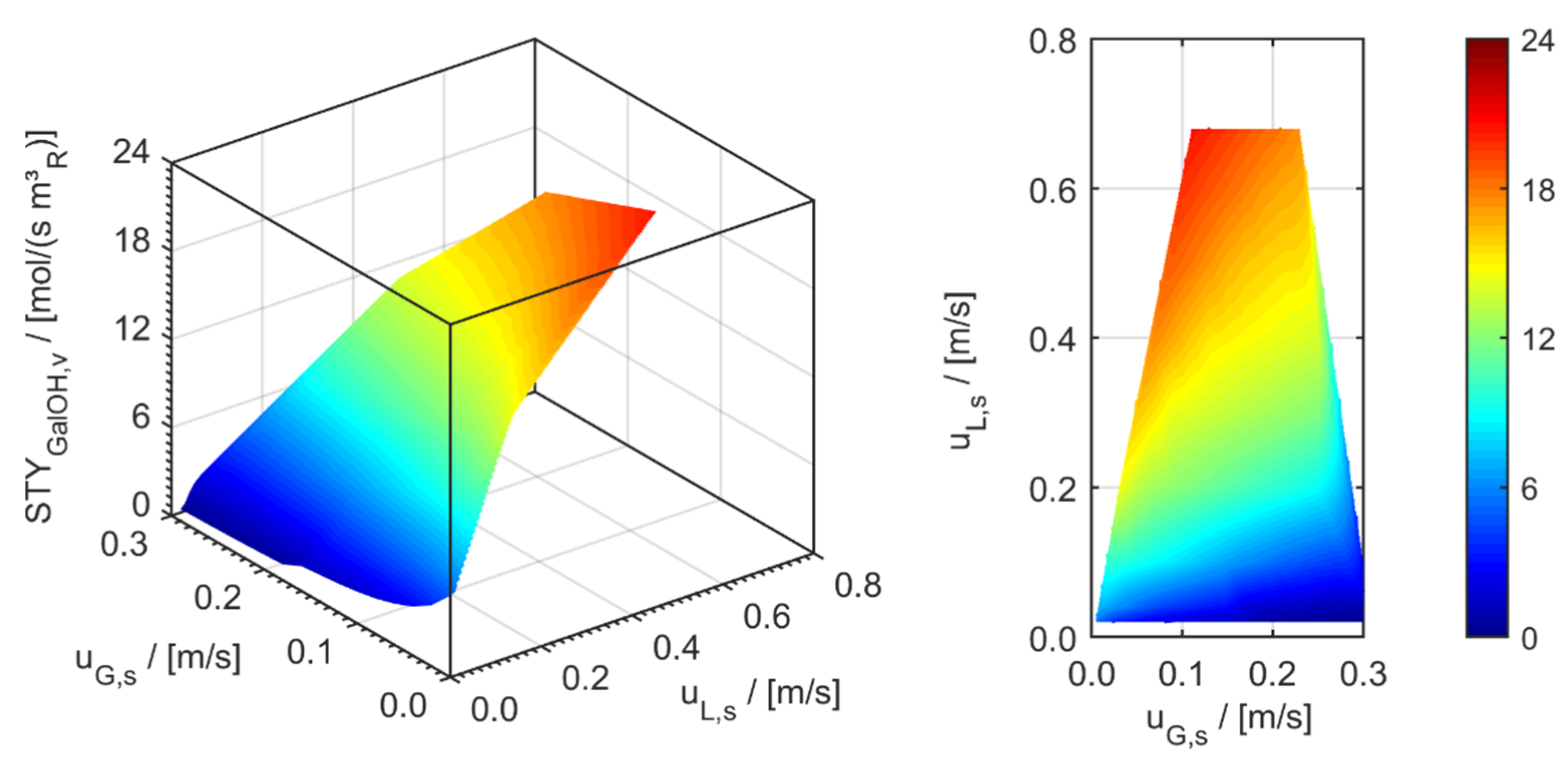
3.1. Minichannel Wall Reactor (MCWR)
3.2. Minichannel Reactor Packed with Catalytic Particles (MCPR)
3.3. Minichannel Reactor with Foam Packing
3.4. Mini Trickle-Bed Reactor
3.5. Trickle-Bed Reactor
4. Selection of a Proper Reactor Configuration
4.1. Quantitative Parameters
4.2. Qualitative Parameters
4.3. Case Study—Production Scenario
5. Summary and Conclusions
Author Contributions
Funding
Conflicts of Interest
Nomenclature
| Area | ||
| Concentration | ||
| Isobaric heat capacity | ||
| Diameter | ||
| Axial dispersion coefficient | ||
| Diffusion coefficient of component i | ||
| Activation energy | ||
| Factor | ||
| Gravitational constant (9.81) | ||
| Mass transfer coefficient | ||
| Adsorption and desorption quasi-equilibria | ||
| Rate constant for reaction j with reaction order n | ||
| Pre-exponential factor for reaction with reaction order n | ||
| Length | ||
| Mass | ||
| Amount of substance | ||
| Order of reaction | ||
| Molar flow rate | ||
| Pressure | ||
| Power | ||
| , R | Radial coordinate, radius | |
| or | Reaction rate per reaction volume or per mass of catalyst/active compound | |
| Ideal gas constant (8.3145) | ||
| Space-time yield | ||
| Time | ||
| Thickness | ||
| Temperature | ||
| Velocity | ||
| Volume | ||
| Volumetric flow rate | ||
| Conversion of educt j () | ||
| Ruthenium loading per reactor volume | ||
| Axial coordinate of reactor |
| Volume inside reactor over volume in boundary layer | ||
| Reaction heat | ||
| Pressure drop | ||
| Void fraction/phase holdup | ||
| Catalyst effectiveness factor | ||
| Thermal conductivity | ||
| Mathematical constant (3.14159…) | ||
| Density, loading | ||
| Stoichiometric coefficient |
| Arabinose | |
| Refers to catalyst activity | |
| Arabitol | |
| Axial | |
| Cylinder | |
| Channel | |
| Distance | |
| Dynamic | |
| Effective | |
| Equivalent | |
| Error | |
| Gas or in the gas phase | |
| Galactose | |
| Gas-liquid | |
| Galactitol | |
| Gas-solid | |
| Hydraulic | |
| Index of reaction | |
| At the reactor inlet | |
| Component j | |
| Liquid or in the liquid phase | |
| Liquid-solid | |
| Related to mass of catalyst | |
| Maximal | |
| Metal (referring to active metal species) | |
| At the reactor outlet | |
| Spherical particle | |
| Cross-sectional | |
| Reaction or reactor | |
| Superficial | |
| Solid or in the solid phase | |
| Slab | |
| Static | |
| Steady state | |
| Total | |
| Related to volume | |
| Washcoat | |
| Referring to saturation |
References
- Walas, S.M. Chemical Process Equipment; Butterworth-Heinemann: Newton, MA, USA, 1990. [Google Scholar]
- Baerns, M.; Behr, A.; Brehm, A. Technische Chemie; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2006; p. 733. [Google Scholar]
- Hessel, V.V. Novel Process Windows—Gate to Maximizing Process Intensification via Flow Chemistry. Chem. Eng. Technol. 2009, 32, 1655–1681. [Google Scholar] [CrossRef]
- Kockmann, N.; Gottsponer, M.; Zimmermann, B.; Roberge, D.M. Enabling Continuous-Flow Chemistry in Microstructured Devices for Pharmaceutical and Fine-Chemical Production. Chem. Eur. J. 2008, 14, 7470–7477. [Google Scholar] [CrossRef] [PubMed]
- Malet-Sanz, L.; Susanne, F. Continuous Flow Synthesis. A Pharma Perspective. J. Med. Chem. 2012, 55, 4062–4098. [Google Scholar] [CrossRef]
- Mills, P.L.; Quiram, D.J.; Ryley, J.F. Microreactor technology and process miniaturization for catalytic reactions—A perspective on recent developments and emerging technologies. Chem. Eng. Sci. 2007, 62, 6992–7010. [Google Scholar] [CrossRef]
- Proctor, L.; Hawkins, J.M. Continuous Processing in the Pharmaceutical Industry. In Green Chemistry in the Pharmaceutical Industry; Wiley: Hoboken, NJ, USA, 2010; pp. 221–242. [Google Scholar]
- Ehrfeld, W.; Hessel, V.; Löwe, H. Microreactors; Wiley-VCH: Weinheim, Germany, 2000. [Google Scholar]
- Hessel, V. Chemical Micro Process Engineering: Processing and Plants; Wiley-VCH: Weinheim, Germany, 2005. [Google Scholar]
- Doan, L.T.V.; Stavarek, P.; de Bellefon, C. A Method to Identify Best Available Technologies (BAT) for Hydrogenation Reactors in the Pharmaceutical Industry. J. Flow Chem. 2012, 2, 77–82. [Google Scholar] [CrossRef] [Green Version]
- Dudukovic, M. Reaction Engineering for Environmental Benign Processes; Lecture Notes; Washington State University: St. Louis, MO, USA, 2016. [Google Scholar]
- Krishna, R.; Sie, S.T. Strategies for multiphase reactor selection. Chem. Eng. Sci. 1994, 49, 4029–4065. [Google Scholar] [CrossRef]
- Duduković, M.; Mills, P. Scale-up and multiphase reaction engineering. Curr. Opin. Chem. Eng. 2015, 9, 49–58. [Google Scholar] [CrossRef]
- Jacobs, R.; Jansweijer, W. A knowledge-based system for reactor selection. Comput. Chem. Eng. 2000, 24, 1781–1801. [Google Scholar] [CrossRef]
- Salmi, T.; Mikkola, J.-P.; Wärnå, J. Bridging Chemical Reaction Engineering and Reactor Technology; Åbo Akademi University: Turku, Finland, 2009. [Google Scholar]
- Hartman, R.L.; McMullen, J.P.; Jensen, K.F. Deciding Whether to Go with the Flow: Evaluating the Merits of Flow Reactors for Synthesis. Angew. Chem. Int. Ed. 2011, 50, 7502–7519. [Google Scholar] [CrossRef] [PubMed]
- Khadilkar, M.; Wu, Y.; Al-Dahhan, M.; Duduković, M.; Colakyan, M. Comparison of trickle-bed and upflow reactor performance at high pressure: Model predictions and experimental observations. Chem. Eng. Sci. 1996, 51, 2139–2148. [Google Scholar] [CrossRef]
- Lange, R.; Hanika, J.; Stradiotto, D.; Hudgins, R.; Silveston, P. Investigations of periodically operated trickle-bed reactors. Chem. Eng. Sci. 1994, 49, 5615–5621. [Google Scholar] [CrossRef]
- Khadilkar, M.; Al-Dahhan, M.; Duduković, M. Parametric study of unsteady-state flow modulation in trickle-bed reactors. Chem. Eng. Sci. 1999, 54, 2585–2595. [Google Scholar] [CrossRef]
- Selinsek, M.; Bohrer, M.; Vankayala, B.K.; Haas-Santo, K.; Kraut, M.; Dittmeyer, R. Towards a new membrane micro reactor system for direct synthesis of hydrogen peroxide. Catal. Today 2016, 268, 85–94. [Google Scholar] [CrossRef]
- Pashkova, A.; Svajda, K.; Dittmeyer, R. Direct synthesis of hydrogen peroxide in a catalytic membrane contactor. Chem. Eng. J. 2008, 139, 165–171. [Google Scholar] [CrossRef]
- Kertalli, E.; D’Angelo, M.N.; Schouten, J.; Nijhuis, T. Design and optimization of a catalytic membrane reactor for the direct synthesis of propylene oxide. Chem. Eng. Sci. 2015, 138, 465–472. [Google Scholar] [CrossRef]
- Atta, A.; Roy, S.; Nigam, K. Prediction of pressure drop and liquid holdup in trickle bed reactor using relative permeability concept in CFD. Chem. Eng. Sci. 2007, 62, 5870–5879. [Google Scholar] [CrossRef]
- Davis, M.E.; Davis, R.J. Fundmentals of Chelmical Reaction Engineering; Chemical Engineering Series; McGraw-Hill: Boston, MA, USA, 2003. [Google Scholar]
- Levenspiel, O. Chemical Reaction Engineering, 3rd ed.; Wiley: New York, NY, USA, 1998; ISBN 978-0-471-25424-9. [Google Scholar]
- Haase, S.; Weiss, M.; Langsch, R.; Bauer, T.; Lange, R. Hydrodynamics and mass transfer in three-phase composite minichannel fixed-bed reactors. Chem. Eng. Sci. 2013, 94, 224–236. [Google Scholar] [CrossRef]
- Tourvieille, J.-N.; Philippe, R.; de Bellefon, C. Milli-channel with metal foams under an applied gas–liquid periodic flow: External mass transfer performance and pressure drop. Chem. Eng. J. 2015, 267, 332–346. [Google Scholar] [CrossRef]
- Iliuta, I.; Iliuta, M.C.; Thyrion, F.C. Gas-liquid mass transfer in trickle-bed reactors: Gas-side mass transfer. Chem. Eng. Technol. 1997, 20, 589–595. [Google Scholar] [CrossRef]
- Bulsari, A.B.; Saxén, B.; Saxén, H. A chemical reactor selection expert system created by training an artificial neural network. In Advances in Computing and Information, Proceedings of the ICCI ’91: International Conference on Computing and Information, Ottawa, ON, Canada, 27–29 May 1991; Dehne, F., Fiala, F., Koczkodaj, W.W., Eds.; Springer: Berlin/Heidelberg, Germany, 1991; pp. 645–656. [Google Scholar]
- Murzin, D.Y.; Simakova, I. Catalysis in biomass processing. Catal. Ind. 2011, 3, 218–249. [Google Scholar] [CrossRef]
- Mikkola, J.-P.; Sklavounos, E.; King, A.W.T.; Virtanen, P. The Biorefinery and Green Chemistry. In Ionic Liquids in the Biorefinery Concept: Challenges and Perspectives; Royal Society of Chemistry (RSC): London, UK, 2015; pp. P001–P006. [Google Scholar]
- Corma, A.; Iborra, S.; Velty, A. Chemical Routes for the Transformation of Biomass into Chemicals. Chem. Rev. 2007, 107, 2411–2502. [Google Scholar] [CrossRef] [PubMed]
- Murzin, D.Y.; Simakova, I.L. 7.21—Catalysis in Biomass Processing. In Comprehensive Inorganic Chemistry II, 2nd ed.; Poeppelmeier, J.R., Ed.; Elsevier: Amsterdam, The Netherlands, 2013; pp. 559–586. [Google Scholar]
- Alonso, D.M.; Bond, J.Q.; Dumesic, J.A. Catalytic conversion of biomass to biofuels. Green Chem. 2010, 12, 1493–1513. [Google Scholar] [CrossRef]
- Hörhammer, H. A Larch Biorefinery Producing Pulp and Lactic Acid. Ph.D. Thesis, Department of Forest Products Technology, Aalto University, Helsinki, Finland, 2014. [Google Scholar]
- Kusema, B.T.; Hilmann, G.; Mäki-Arvela, P.; Willför, S.; Holmbom, B.; Salmi, T.; Murzin, D.Y. Selective Hydrolysis of Arabinogalactan into Arabinose and Galactose Over Heterogeneous Catalysts. Catal. Lett. 2010, 141, 408–412. [Google Scholar] [CrossRef]
- Degelmann, H.; Kowalczyk, J.D.; Kunz, M.D.; Schüttenhelm, M.D. Verfahren zur Hydrierung von Zuckern; Südzucker AG: Manheim, Germany, 1997. [Google Scholar]
- Chao, J.C.; Huibers, D.T.A. Catalytic Hydrogenation of Glucose to Produce Sorbitol; Hydrocarbon Research, Inc.: Lawrenceville, NJ, USA, 1980. [Google Scholar]
- Aho, A.; Roggan, S.; Eränen, K.; Salmi, T.; Murzin, D.Y. Continuous hydrogenation of glucose with ruthenium on carbon nanotube catalysts. Catal. Sci. Technol. 2014, 5, 953–959. [Google Scholar] [CrossRef]
- Makkee, M.; Kieboom, A.P.; van Bekkum, H. Hydrogenation of d-fructose and d-fructose/d-glucose mixtures. Carbohydr. Res. 1985, 138, 225–236. [Google Scholar] [CrossRef]
- Crezee, E.; Hoffer, B.W.; Berger, R.J.; Makkee, M.; Kapteijn, F.; Moulijn, J.A. Three-phase hydrogenation of d-glucose over a carbon supported ruthenium catalyst—Mass transfer and kinetics. Appl. Catal. A Gen. 2003, 251, 1–17. [Google Scholar] [CrossRef]
- Wisnlak, J.; Simon, R. Hydrogenation of Glucose, Fructose, and Their Mixtures. Ind. Eng. Chem. Prod. Res. Dev. 1979, 18, 50–57. [Google Scholar] [CrossRef]
- Sifontes Herrera, V.A.; Oladele, O.; Kordás, K.; Eränen, K.; Mikkola, J.-P.; Murzin, D.Y.; Salmi, T. Sugar hydrogenation over a Ru/C catalyst. J. Chem. Technol. Biotechnol. 2011, 86, 658–668. [Google Scholar] [CrossRef]
- Dodgson, I. Trends and Opportunities with Modern Hydrogenation Catalysts. In Studies in Surface Science and Catalysis; Guisnet, M., Ed.; Elsevier: Amsterdam, The Netherlands, 1993; pp. 1–19. [Google Scholar]
- Eisenbeis, C.; Güttel, R.; Kunz, U.; Turek, T. Monolith loop reactor for hydrogenation of glucose. Catal. Today 2009, 147, S342–S346. [Google Scholar] [CrossRef]
- Aho, A.; Roggan, S.; Simakova, O.A.; Salmi, T.; Murzin, D.Y. Structure sensitivity in catalytic hydrogenation of glucose over ruthenium. Catal. Today 2015, 241, 195–199. [Google Scholar] [CrossRef]
- Simakova, I.; Demidova, Y.S.; Murzina, E.V.; Aho, A.; Murzin, D.Y. Structure Sensitivity in Catalytic Hydrogenation of Galactose and Arabinose over Ru/C Catalysts. Catal. Lett. 2016, 146, 1291–1299. [Google Scholar] [CrossRef]
- Sifontes Herrera, V.A.; Eränen, K.; Mikkola, J.P.; Wärnå, J.; Murzin, D.; Salmi, T. Sugar Hydrogenation in a Parallel Tube Reactor System Equipped with Ru/C Monoliths, in ICOSCAR-3; Ischia: Naples, Italy, 2009. [Google Scholar]
- Chen, B.; Dingerdissen, U.; Krauter, J.; Rotgerink, H.L.; Möbus, K.; Ostgard, D.; Panster, P.; Riermeier, T.; Seebald, S.; Tacke, T.; et al. New developments in hydrogenation catalysis particularly in synthesis of fine and intermediate chemicals. Appl. Catal. A Gen. 2005, 280, 17–46. [Google Scholar] [CrossRef]
- Sifontes Herrera, V.A. Hydrogenation of L-Arabinose, D-Galactose, D-Maltose and L-Rhamnose; Laboratory of Industrial Chemistry and Reaction Engineering, Åbo Akademi University: Turku, Finland, 2012. [Google Scholar]
- Hackl, F. Reaktionstechnische Modellierung eines Rieselbettreaktors und eines Monolithreaktors für die katalytische Hydrierung von Glucose zu Sorbitol. In Chemical Reaction Engineering and Process Plant; Technische Universität Dresden: Dresden, Germany, 2011. [Google Scholar]
- Le Bas, G. The Molecular Volumes of Liquid Chemical Compounds; Longmans, Green & Co.: New York, NY, USA, 1915. [Google Scholar]
- Matubayasi, N.; Nishiyama, A. Thermodynamic quantities of surface formation of aqueous electrolyte solutions VI. Comparison with typical nonelectrolytes, sucrose and glucose. J. Colloid Interface Sci. 2006, 298, 910–913. [Google Scholar] [CrossRef] [PubMed]
- Origlia, M.; Call, T.; Woolley, E. Apparent molar volumes and apparent molar heat capacities of aqueous d-glucose and d-galactose at temperatures from 278.15 K to 393.15 K and at the pressure 0.35 MPa. J. Chem. Thermodyn. 2000, 32, 847–856. [Google Scholar] [CrossRef]
- Partington, J. An Advanced Treatise on Physical Chemistry. In Fundamental Principals: The Properties of Gases; Longmans, Green & Co.: New York, NY, USA, 1949; Volume I. [Google Scholar]
- Poling, B.E.; Prausnitz, J.M.; O’Connel, J.P. Properties of Gases and Liquids, 5th ed.; McGraw-Hill: New York, NY, USA, 2001. [Google Scholar]
- Wilke, C.R.; Chang, P. Correlation of diffusion coefficients in dilute solutions. AIChE J. 1955, 1, 264–270. [Google Scholar] [CrossRef]
- Salmi, T.; Kuusisto, J.; Warna, J.; Mikkola, J.-P. Alternative sweeteners: La dolce vita. Chim. L’Industria 2006, 88, 90–96. [Google Scholar]
- Salmi, T.; Murzin, D.Y.; Warna, J.; Mikkola, J.-P.; Aumo, J.E.B.; Kuusisto, J.I. Modeling and optimization of complex three-phase hydrogenations and isomerizations under mass-transfer limitation and catalyst deactivation. In Catalysis of Organic Reactions; Schmidt, S.R., Ed.; CRC Press: Boca Raton, FL, USA, 2007; pp. 187–196. [Google Scholar]
- Gascon, J.; van Ommen, J.R.; Moulijn, J.A.; Kapteijn, F. Structuring catalyst and reactor—An inviting avenue to process intensification. Catal. Sci. Technol. 2014, 5, 807–817. [Google Scholar] [CrossRef]
- Heibel, A.K.; Lebens, P.J.M. Film Flow Monolithic Reactors. In Structured Catalysts and Reactors; Cybulski, A., Moulijn, J.A., Eds.; Taylor & Francis Group: London, UK, 2006. [Google Scholar]
- Mogalicherla, A.K.; Kunzru, D. Performance of monolithic reactors in film flow. Chem. Eng. Res. Des. 2010, 88, 1057–1066. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Du, P.; Heiszwolf, J.J.; Kapteijn, F.; Moulijn, J.A. Mass transfer characteristics of three-phase monolith reactors. Chem. Eng. Sci. 2001, 56, 6015–6023. [Google Scholar] [CrossRef]
- Kreutzer, M.; Kapteijn, F.; Moulijn, J.A.; Heiszwolf, J.J. Multiphase monolith reactors: Chemical reaction engineering of segmented flow in microchannels. Chem. Eng. Sci. 2005, 60, 5895–5916. [Google Scholar] [CrossRef]
- Al-Dahhan, M.; Larachi, F.; Dudukovic, M.P.; Laurent, A. High-Pressure Trickle-Bed Reactors: A Review. Ind. Eng. Chem. Res. 1997, 36, 3292–3314. [Google Scholar] [CrossRef]
- Dudukovic, P.M.; Larachi, F.; Mills, P.L. Multiphase reactors—Revisited. Chem. Eng. Sci. 1999, 54, 1975–1995. [Google Scholar] [CrossRef]
- Sifontes Herrera, V.A.; Saleem, F.; Kusema, B.T.; Eränen, K.; Salmi, T. Hydrogenation of l-Arabinose and d-Galactose Mixtures Over a Heterogeneous Ru/C Catalyst. Top. Catal. 2012, 55, 550–555. [Google Scholar] [CrossRef]
- Russo, V.; Kilpiö, T.; Di Serio, M.; Tesser, R.; Santacesaria, E.; Murzin, D.Y.; Salmi, T. Dynamic non-isothermal trickle bed reactor with both internal diffusion and heat conduction: Sugar hydrogenation as a case study. Chem. Eng. Res. Des. 2015, 102, 171–185. [Google Scholar] [CrossRef]
- Haase, S.; Bauer, T.; Hilpmann, G.; Lange, M.; Ayubi, M.-M.; Abiev, R. Simultaneous Detection of Hydrodynamics, Mass Transfer and Reaction Rates in a Three-Phase Microreactor. Theor. Found. Chem. Eng. 2020, 54, 48–63. [Google Scholar] [CrossRef]
- Larachi, F.; Laurent, A.; Midoux, N.; Wild, G. Experimental study of a trickle-bed reactor operating at high pressure: Two-phase pressure drop and liquid saturation. Chem. Eng. Sci. 1991, 46, 1233–1246. [Google Scholar] [CrossRef]
- Lange, R.; Schubert, M.; Bauer, T. Liquid Holdup in Trickle-Bed Reactors at Very Low Liquid Reynolds Numbers. Ind. Eng. Chem. Res. 2005, 44, 6504–6508. [Google Scholar] [CrossRef]
- Lappalainen, K.; Alopaeus, V.; Manninen, M.; Aittamaa, J. Improved Hydrodynamic Model for Wetting Efficiency, Pressure Drop, and Liquid Holdup in Trickle-Bed Reactors. Ind. Eng. Chem. Res. 2008, 47, 8436–8444. [Google Scholar] [CrossRef]
- Fu, M.-S.; Tan, C.-S. Liquid holdup and axial dispersion in trickle-bed reactors. Chem. Eng. Sci. 1996, 51, 5357–5361. [Google Scholar] [CrossRef]
- Hochman, J.M.; Effron, E. Two-Phase Cocurrent Downflow in Packed Beds. Ind. Eng. Chem. Fundam. 1969, 8, 63–71. [Google Scholar] [CrossRef]
- Dwivedi, P.N.; Upadhyay, S.N. Particle-Fluid Mass Transfer in Fixed and Fluidized Beds. Ind. Eng. Chem. Process Des. Dev. 1977, 16, 157–165. [Google Scholar] [CrossRef]
- Wild, G.; Larachi, F.; Charpentier, J.C. Heat and Mass Transfer in Gas-Liquid-Solid Fixed Bed Reactors. In Heat and Mass Transfer in Porous Media; Quintard, M.M.T., Ed.; Elsevier: Amsterdam, The Netherlands, 1992. [Google Scholar]
- Burghardt, A.; Bartelmus, G.; Jaroszyński, M.; Kołodziej, A. Hydrodynamics and mass transfer in a three-phase fixed-bed reactor with cocurrent gas—Liquid downflow. Chem. Eng. J. Biochem. Eng. J. 1995, 58, 83–99. [Google Scholar] [CrossRef]
- Haase, S. Minichannel Flow Reactors for Gas-Liquid-Solid Reactions. Ph.D. Thesis, TU Dresden, Dresden, Germany, 2013. [Google Scholar]
- Fries, D.M.; Trachsel, F.; von Rohr, P.R. Segmented gas–liquid flow characterization in rectangular microchannels. Int. J. Multiph. Flow 2008, 34, 1108–1118. [Google Scholar] [CrossRef]
- Kreutzer, M.T.; Kapteijn, F.; Moulijn, J.A.; Kleijn, C.R.; Heiszwolf, J.J. Inertial and interfacial effects on pressure drop of Taylor flow in capillaries. AIChE J. 2005, 51, 2428–2440. [Google Scholar] [CrossRef]
- Langsch, R.; Haase, S.; Lange, R. Hydrodynamik und Stofftransport in einem Perlschnurreaktor für Gas/Flüssig/Fest-Reaktionen. Chem. Ing. Tech. 2013, 85, 642–655. [Google Scholar] [CrossRef]
- Hipolito, A.; Rolland, M.; Boyer, C.; De Bellefon, C. Single Pellet String Reactor for Intensification of Catalyst Testing in Gas/Liquid/Solid Configuration. Oil Gas Sci. Technol. Rev. L’IFP 2010, 65, 689–701. [Google Scholar] [CrossRef] [Green Version]
- Larachi, F.; Grandjean, B. Excel Worksheet Simulators for Packed-Bed Reactors. Available online: http://www.gch.ulaval.ca/flarachi/pbrsimul/pbrsimul.html (accessed on 1 June 2015).
- Haase, S. Characterisation of gas-liquid two-phase flow in minichannels with co-flowing fluid injection inside the channel, part I: Unified mapping of flow regimes. Int. J. Multiph. Flow 2016, 87, 197–211. [Google Scholar] [CrossRef]
- Tourvieille, N.-J.; Philippe, R.; de Bellefon, C. Milli-channel with metal foams under an applied gas–liquid periodic flow: Flow patterns, residence time distribution and pulsing properties. Chem. Eng. Sci. 2015, 126, 406–426. [Google Scholar] [CrossRef]
- Tomašić, V. Application of the monoliths in DeNOx catalysis. Catal. Today 2007, 119, 106–113. [Google Scholar] [CrossRef]
- Tomašić, V.; Jović, F. State-of-the-art in the monolithic catalysts/reactors. Appl. Catal. A Gen. 2006, 311, 112–121. [Google Scholar] [CrossRef]
- Knon, H.; Brennscheidt, T.; Flörchinger, P. Keramische Ultradünnwandträger für zukünftige Emissionsanforderungen. MTZ 2001, 9, 662–666. [Google Scholar] [CrossRef]
- Brück, R.; Müller-Haas, K.; Breuer, J.; Webb, C. Advanced Performance of Metallic Converter Systems Demonstrated on a Production V8 Engine; SAE-Paper 2002-01-0347; Southwest Research Institute: San Antonio, TX, USA, 2002. [Google Scholar]
- Mogalicherla, A.K.; Kunzru, D. Effect of Gas and Liquid Superficial Velocities on the Performance of Monolithic Reactors. Ind. Eng. Chem. Res. 2010, 49, 1631–1641. [Google Scholar] [CrossRef]
- Haase, S. Characterisation of gas-liquid two-phase flow in minichannels with co-flowing fluid injection inside the channel, part II: Gas bubble and liquid slug lengths, film thickness, and void fraction within Taylor flow. Int. J. Multiph. Flow 2017, 88, 251–269. [Google Scholar] [CrossRef]
- Lakhwani, S.; Hughes, K.W. Evaluation of a Stronger Ultra Thin Wall Corning Substrate for Improved Performance; SAE Technical Paper Series; SAE International: Warrendale, PA, USA, 2005. [Google Scholar] [CrossRef]
- Kreutzer, M.T. Hydrodynamics of Taylor Flow in Capillaries and Monolith Reactors; Delft University Press: Delft, The Netherlands, 2003. [Google Scholar]
- Walsh, E.; Muzychka, Y.; Walsh, P.; Egan, V.; Punch, J. Pressure drop in two phase slug/bubble flows in mini scale capillaries. Int. J. Multiph. Flow 2009, 35, 879–884. [Google Scholar] [CrossRef]
- Gupta, R.; Fletcher, D.F.; Haynes, B. On the CFD modelling of Taylor flow in microchannels. Chem. Eng. Sci. 2009, 64, 2941–2950. [Google Scholar] [CrossRef]
- Ganapathy, H.; Al-Hajri, E.; Ohadi, M.M. Phase field modeling of Taylor flow in mini/microchannels, Part II: Hydrodynamics of Taylor flow. Chem. Eng. Sci. 2013, 94, 156–165. [Google Scholar] [CrossRef]
- Klaseboer, E.; Gupta, R.; Manica, R. An extended Bretherton model for long Taylor bubbles at moderate capillary numbers. Phys. Fluids 2014, 26, 032107. [Google Scholar] [CrossRef]
- Wang, X.; Yong, Y.; Yang, C.; Mao, Z.-S.; Li, D. Investigation on pressure drop characteristic and mass transfer performance of gas–liquid flow in micro-channels. Microfluid. Nanofluid. 2014, 16, 413–423. [Google Scholar] [CrossRef]
- Pangarkar, K.; Schildhauer, T.J.; Van Ommen, J.R.; Nijenhuis, J.; Kapteijn, F.; Moulijn, J.A. Structured Packings for Multiphase Catalytic Reactors. Ind. Eng. Chem. Res. 2008, 47, 3720–3751. [Google Scholar] [CrossRef]
- Vaitsis, E.; Chadwick, D.; Alpay, E. Slug Flow Hydrodynamics in the Presence of Catalyst Rods. Chem. Eng. Res. Des. 2004, 82, 653–658. [Google Scholar] [CrossRef]
- Strangio, V.A.; Dautzenberg, F.; Calis, H.P.A.; Gupta, A.V. Fixed Bed Catalytic Bed Reactor; ABB Lummus Global Inc.: Houston, TX, USA, 1999. [Google Scholar]
- Temkin, M.I.; Kulkova, N.V. Ideal-Displacement Laboratory Reactor. Kinetika Kataliz 1969, 10, 461–463. [Google Scholar]
- Bellos, G.; Galtier, P.; Papayannakos, N. Laboratory Reactor for Studying Gaseous and Liquid Phase Reactions; Corning Incooperated: Somerville, MA, USA, 2009. [Google Scholar]
- Kallinikos, L.E.; Papayannakos, N.G. Operation of a Miniscale String Bed Reactor in Spiral Form at Hydrotreatment Conditions. Ind. Eng. Chem. Res. 2007, 46, 5531–5535. [Google Scholar] [CrossRef]
- Satterfield, C.N.; Pelossof, A.A.; Sherwood, T.K. Mass transfer limitations in a trickle-bed reactor. AIChE J. 1969, 15, 226–234. [Google Scholar] [CrossRef]
- Kallinikos, L.E.; Papayannakos, N.G. Intensification of hydrodesulphurization process with a structured bed spiral mini-reactor. Chem. Eng. Process. Process Intensif. 2010, 49, 1025–1030. [Google Scholar] [CrossRef]
- Bauer, T.; Haase, S. Comparison of structured trickle-bed and monolithic reactors in Pd-catalyzed hydrogenation of alpha-methylstyrene. Chem. Eng. J. 2011, 169, 263–269. [Google Scholar] [CrossRef]
- Langsch, R.; Haase, S.; Lange, R. Hydrodynamics and Mass Transfer in a Pellet String Reactor for Gas-Liquid-Solid Reactions. Chem. Ing. Tech. 2013, 85, 642–655. [Google Scholar] [CrossRef]
- Haase, S.; Langsch, R.; Bauer, T.; Lange, R. Impact of Spherical Catalyst Particles on Gas-Liquid Flow Regimes in Minichannels with Square Cross Section. Chem. Ing. Tech. 2014, 86, 467–475. [Google Scholar] [CrossRef]
- Serres, M.; Zanota, M.-L.; Philippe, R.; Vidal, V. On the stability of Taylor bubbles inside a confined highly porous medium. Int. J. Multiph. Flow 2016, 85, 157–163. [Google Scholar] [CrossRef]
- Serres, M.; Schweich, D.; Vidal, V.; Philippe, R. Liquid residence time distribution of multiphase horizontal flow in packed bed milli-channel: Spherical beads versus open cell solid foams. Chem. Eng. Sci. 2018, 190, 149–163. [Google Scholar] [CrossRef]
- Nguyen, T.-S.; Swesi, Y.; Edouard, D.; Fongarland, P. Platelet Millireactor Filled with Open Cell Foam-Supported Pt Nanoparticles for a Three-Phase Catalytic System. Ind. Eng. Chem. Res. 2019, 58, 9352–9361. [Google Scholar] [CrossRef]
- Sang, L.; Tu, J.; Cheng, H.; Luo, G.; Zhang, J. Hydrodynamics and mass transfer of gas–liquid flow in micropacked bed reactors with metal foam packing. AIChE J. 2020, 66, 1–11. [Google Scholar] [CrossRef]
- Stemmet, C.; van der Schaaf, J.; Kuster, B.; Schouten, J. Solid Foam Packings for Multiphase Reactors. Chem. Eng. Res. Des. 2006, 84, 1134–1141. [Google Scholar] [CrossRef]
- Twigg, M.V.; Richardson, J.T. Fundamentals and Applications of Structured Ceramic Foam Catalysts. Ind. Eng. Chem. Res. 2007, 46, 4166–4177. [Google Scholar] [CrossRef]
- Mohammed, I.; Bauer, T.; Schubert, M.; Lange, R. Hydrodynamic multiplicity in a tubular reactor with solid foam packings. Chem. Eng. J. 2013, 231, 334–344. [Google Scholar] [CrossRef]
- Lali, F. A hydrodynamic study of cylindrical metal foam packings: Residence time distribution and two phase pressure drop. Chem. Eng. Process. Process Intensif. 2017, 115, 1–10. [Google Scholar] [CrossRef]
- Mohammed, I.; Bauer, T.; Schubert, M.; Lange, R. Gas–liquid distribution in tubular reactors with solid foam packings. Chem. Eng. Process. Process Intensif. 2015, 88, 10–18. [Google Scholar] [CrossRef]
- Lali, F.; Gärtner, S.; Haase, S.; Lange, R. Preparation Method for Ruthenium Catalysts Supported by Carbon-Coated Aluminum Foams. Chem. Eng. Technol. 2015, 38, 1353–1361. [Google Scholar] [CrossRef]
- Faridkhou, A.; Tourvieille, J.-N.; Larachi, F. Reactions, hydrodynamics and mass transfer in micro-packed beds—Overview and new mass transfer data. Chem. Eng. Process. Process Intensif. 2016, 110, 80–96. [Google Scholar] [CrossRef]
- Reschetilowski, W. (Ed.) Microreactors in Preparative Chemistry: Practical Aspects in Bioprocessing, Nanotechnology, Catalysis and More; Wiley-VCH Verlag GmbH: Weinheim, Germany, 2013. [Google Scholar]
- Ranade, V.V.; Chaudhari, R.V.; Gunjal, P.R. Chapter 6—Applications and Recent Developments. In Trickle Bed Reactors; Elsevier: Amsterdam, The Netherlands, 2011; pp. 211–256. [Google Scholar]
- Styring, P. Heterogeneous Catalysis in Chemical Micro Reactors. In Microfluidics: History, Theory and Applications; Zimmerman, W.B.J., Ed.; Springer: Berlin/Heidelberg, Germany, 2006. [Google Scholar]
- Kiwi-Minsker, L.; Renken, A. Microstructured reactors for catalytic reactions. Catal. Today 2005, 110, 2–14. [Google Scholar] [CrossRef] [Green Version]
- Jensen, K.F. Flow chemistry-Microreaction technology comes of age. AIChE J. 2017, 63, 858–869. [Google Scholar] [CrossRef]
- Dencic, I.; Hessel, V.; De Croon, M.H.J.M.; Meuldijk, J.; Van Der Doelen, C.W.J.; Koch, K. Recent Changes in Patenting Behavior in Microprocess Technology and its Possible Use for Gas-Liquid Reactions and the Oxidation of Glucose. ChemSusChem 2012, 5, 232–245. [Google Scholar] [CrossRef]
- Jensen, K.F. Microreaction engineering—Is small better? Chem. Eng. Sci. 2001, 56, 293–303. [Google Scholar] [CrossRef]
- Losey, M.W.; Schmidt, A.M.A.; Jensen, K.F. Microfabricated Multiphase Packed-Bed Reactors: Characterization of Mass Transfer and Reactions. Ind. Eng. Chem. Res. 2001, 40, 2555–2562. [Google Scholar] [CrossRef]
- Al-Rifai, N.; Galvanin, F.; Morad, M.; Cao, E.; Cattaneo, S.; Sankar, M.; Dua, V.; Hutchings, G.; Gavriilidis, A. Hydrodynamic effects on three phase micro-packed bed reactor performance—Gold–palladium catalysed benzyl alcohol oxidation. Chem. Eng. Sci. 2016, 149, 129–142. [Google Scholar] [CrossRef] [Green Version]
- Al-Rifai, N.; Cao, E.; Dua, V.; Gavriilidis, A. Microreaction technology aided catalytic process design. Curr. Opin. Chem. Eng. 2013, 2, 338–345. [Google Scholar] [CrossRef]
- Kockmann, N. Transport Phenomena in Micro Process Engineering; Springer: Berlin/Heidelberg, Germany, 2008. [Google Scholar]
- Van Herk, D.; Kreutzer, M.T.; Makkee, M.; Moulijn, J.A. Scaling down trickle bed reactors. Catal. Today 2005, 106, 227–232. [Google Scholar] [CrossRef]
- Sang, L.; Feng, X.; Tu, J.; Xie, B.; Luo, G.; Zhang, J. Investigation of external mass transfer in micropacked bed reactors. Chem. Eng. J. 2020, 393, 124793. [Google Scholar] [CrossRef]
- Moulijn, J.A.; Makkee, M.; Berger, R.J. Catalyst testing in multiphase micro-packed-bed reactors; Criterion for radial mass transport. Catal. Today 2016, 259, 354–359. [Google Scholar] [CrossRef]
- Hommes, A.; ter Horst, A.J.; Koeslag, M.; Heeres, H.J.; Yue, J. Experimental and modeling studies on the Ru/C catalyzed levulinic acid hydrogenation to γ-valerolactone in packed bed microreactors. Chem. Eng. J. 2020, 399, 125750. [Google Scholar] [CrossRef]
- Durante, D.; Kilpiö, T.; Suominen, P.; Herrera, V.S.; Wärnå, J.; Canu, P.; Salmi, T. Modeling and simulation of a small-scale trickle bed reactor for sugar hydrogenation. Comput. Chem. Eng. 2014, 66, 22–35. [Google Scholar] [CrossRef]
- Kilpiö, T.T. Mathematical Modeling of Laboratory Scale Three-Phase Fixed Bed Reactors; Laboratory of Industrial Chemistry and Reaction Engineering, Åbo Akademi University: Turku, Finland, 2013. [Google Scholar]
- Márquez Luzardo, N.M. Hydrodynamics of Multi-Phase Packed Bed Micro-Reactors; Laboratory of Industrial Chemistry and Reaction Engineering, Chemical Engineering Department, TU Delft: Delft, The Netherlands, 2010. [Google Scholar]
- Dudukovic, M.P.; Larachi, F.; Mills, P.L. Multiphase catalytic reactors: A perspective on current knowledge and future trends. Catal. Rev. 2002, 44, 123–246. [Google Scholar] [CrossRef]
- Iliuta, I.; Ortiz-Arroyo, A.; Larachi, F.; Grandjean, B.P.; Wild, G. Hydrodynamics and mass transfer in trickle-bed reactors: An overview. Chem. Eng. Sci. 1999, 54, 5329–5337. [Google Scholar] [CrossRef]
- Atta, A.; Roy, S.; Larachi, F.; Nigam, K.D.P. Cyclic operation of trickle bed reactors: A review. Chem. Eng. Sci. 2014, 115, 205–214. [Google Scholar] [CrossRef]
- Lange, R.; Schubert, M.; Dietrich, W.; Grünewald, M. Unsteady-state operation of trickle-bed reactors. Chem. Eng. Sci. 2004, 59, 5355–5361. [Google Scholar] [CrossRef]
- Lange, R. Modeling and Simulation of Unsteady-State-Operated Trickle-Flow Reactors; Wiley: Hoboken, NJ, USA, 2007; pp. 79–98. [Google Scholar]
- Schubert, M. Performance enhancement of trickle bed reactors. In Chemical Engineering and Plant Design; Technische Universitaet Dresden: Dresden, Germany, 2007. [Google Scholar]
- Iliuta, I.; Larachi, F. Hydrocarbon hydrodesulfurization in vertical, inclined and oscillating trickle beds—Hydrodynamics & reactor performance for offshore petroleum marine applications. Fuel 2016, 186, 35–49. [Google Scholar] [CrossRef]
- Dashliborun, M.A.; Larachi, F.; Schubert, M. Hydrodynamics of gas-liquid cocurrent upflow in oscillating packed beds for offshore marine applications. Chem. Eng. Sci. 2017, 170, 583–596. [Google Scholar] [CrossRef]
- Motamed Dashliborun, A.; Larachi, F.; Schubert, M. Offshore Floating Packed-Bed Reactors: Current Status, Key Challenges and Potential Solutions. In Proceedings of the AIChE—Annual Meeting, San Francisco, CA, USA, 13–18 November 2016; pp. 39–46. [Google Scholar]
- Härting, H.-U.; Berger, R.; Lange, R.; Larachi, F.; Schubert, M. Liquid backmixing in an inclined rotating tubular fixed bed reactor—Augmenting liquid residence time via flow regime adjustment. Chem. Eng. Process. Process Intensif. 2015, 94, 2–10. [Google Scholar] [CrossRef]
- Härting, H.-U.; Bieberle, A.; Lange, R.; Larachi, F.; Schubert, M. Hydrodynamics of co-current two-phase flow in an inclined rotating tubular fixed bed reactor—Wetting intermittency via periodic catalyst immersion. Chem. Eng. Sci. 2015, 128, 147–158. [Google Scholar] [CrossRef]
- Härting, H.-U.; Lange, R.; Larachi, F.; Schubert, M. A novel inclined rotating tubular fixed bed reactor concept for enhancement of reaction rates and adjustment of flow regimes. Chem. Eng. J. 2015, 281, 931–944. [Google Scholar] [CrossRef]
- Fischer Scientific Online Shop. Available online: https://www.fishersci.de/shop/ (accessed on 28 February 2021).
- Vergunst, T.; Kapteijn, F.; Moulijn, J. Preparation of carbon-coated monolithic supports. Carbon 2002, 40, 1891–1902. [Google Scholar] [CrossRef]
- Vergunst, T.; Linders, M.J.G.; Kapteijn, F.; Moulijn, J.A. Carbon-Based Monolithic Structures. Catal. Rev. 2001, 43, 291–314. [Google Scholar] [CrossRef]
- Böttge, D. Werkstoffliche und keramtechnologische Aspekte von funktionalen Beschichtungen auf hochporösen Keramikträgern. In Schriftenreihe Kompetenzen in Keramik; Fraunhofer Verlag: Stuttgart, Germany, 2013; p. 18. [Google Scholar]
- Ma, C. Regeneration of Catalyst for Hydrogenation of Sugars; Archer Daniels Midland Company: Minneapolis, MN, USA, 2015. [Google Scholar]
- Stitt, H.; Marigo, M.; Wilkinson, S.; Dixon, T. How Good is Your Model? “Just because the results are in colour, it doesn’t mean they are right”. Johns. Matthey Technol. Rev. 2015, 59, 74ff. [Google Scholar] [CrossRef]
- Stitt, E. Alternative multiphase reactors for fine chemicals: A world beyond stirred tanks? Chem. Eng. J. 2002, 90, 47–60. [Google Scholar] [CrossRef]
- Murzin, D.Y.; Salmi, T. Catalysis for Lignocellulosic Biomass Processing: Methodological Aspects. Catal. Lett. 2012, 142, 817–829. [Google Scholar] [CrossRef]
- Haase, S.; Bauer, T.; Lange, R. Numbering-up of mini- and microchannel contactors and reactors. Chim. Oggi Chem. Today 2015, 33, 26–30. [Google Scholar]
- Al-Rawashdeh, M.; Fluitsma, L.; Nijhuis, T.; Rebrov, E.; Hessel, V.; Schouten, J. Design criteria for a barrier-based gas–liquid flow distributor for parallel microchannels. Chem. Eng. J. 2011, 181–182, 549–556. [Google Scholar] [CrossRef]
- Al-Rawashdeh, M.; Yu, F.; Nijhuis, T.; Rebrov, E.; Hessel, V.; Schouten, J. Numbered-up gas–liquid micro/milli channels reactor with modular flow distributor. Chem. Eng. J. 2012, 207–208, 645–655. [Google Scholar] [CrossRef] [Green Version]
- De Mas, N.; Günther, A.; Kraus, T.; Schmidt, M.A.; Jensen, K.F. Scaled-out multilayer gas-liquid microreactor with integrated velocimetry sensors. Ind. Eng. Chem. Res. 2005, 44, 8997–9013. [Google Scholar] [CrossRef]
- Haverkamp, V.; Hessel, V.; Lowe, H.; Menges, G.; Warnier, M.J.F.; Rebrov, E.V.; De Croon, M.H.J.M.; Schouten, J.C.; Liauw, M.A. Hydrodynamics and Mixer-Induced Bubble Formation in Micro Bubble Columns with Single and Multiple-Channels. Chem. Eng. Technol. 2006, 29, 1015–1026. [Google Scholar] [CrossRef]
- Al-Rawashdeh, M.; Yue, F.; Patil, N.G.; Nijhuis, A.; Hessel, V.; Schouten, J.C.; Rebrov, E. Designing flow and temperature uniformities in parallel microchannels reactor. AIChE J. 2014, 60, 1941–1952. [Google Scholar] [CrossRef]
- Devatine, A.; Chaumat, H.; Guillaume, S.; Tchibouanga, B.T.; Martínez, F.D.; Julcour, C.; Billet, A.-M. Hydrodynamic study of a monolith-type reactor for intensification of gas-liquid applications. Chem. Eng. Process. Process Intensif. 2017, 122, 277–287. [Google Scholar] [CrossRef] [Green Version]
- Stitt, E.H. Multiphase Reactors. In Proceedings of the XIX International Conference on Chemical Reactors, Vienna, Austria, 5–9 September 2010. [Google Scholar]
- Dornack, C.; Dietz, W.; Schütt, F. Reststoffe der Papierindustrie: Ungenutzte Biomasse? Depotech: Leoben, Austria, 2014; p. 4. [Google Scholar]
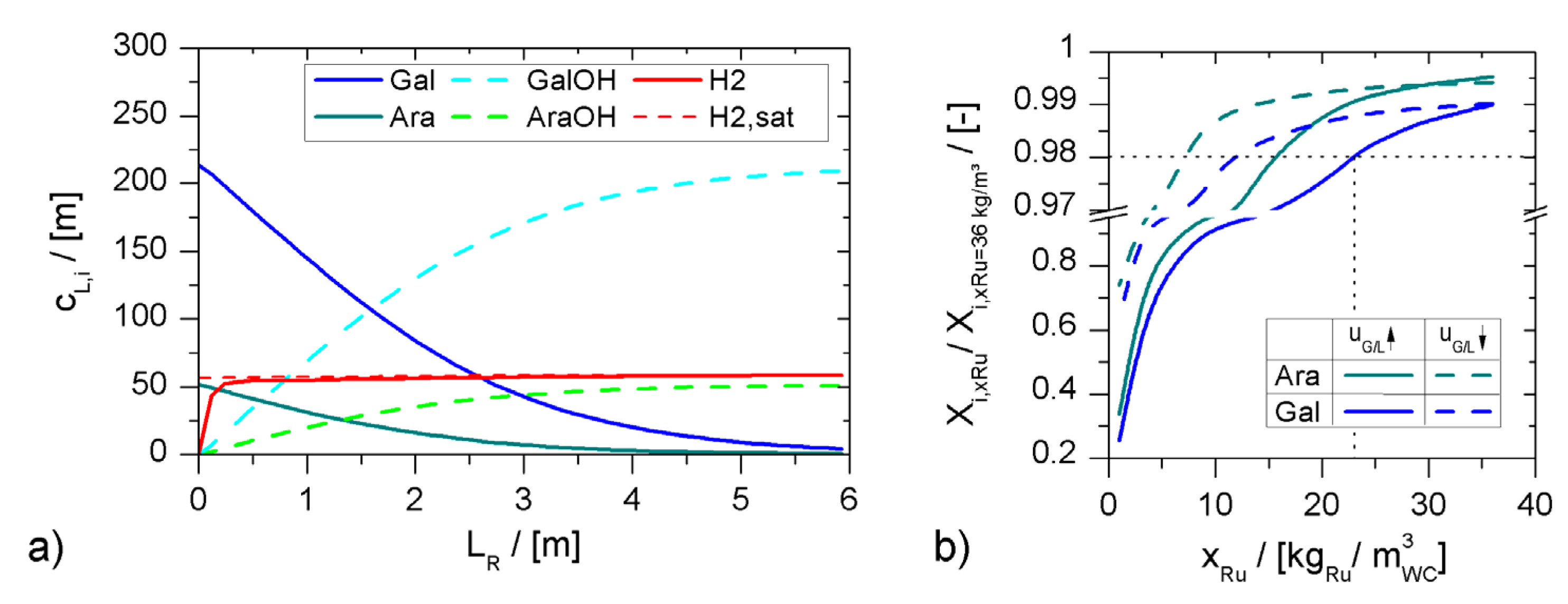


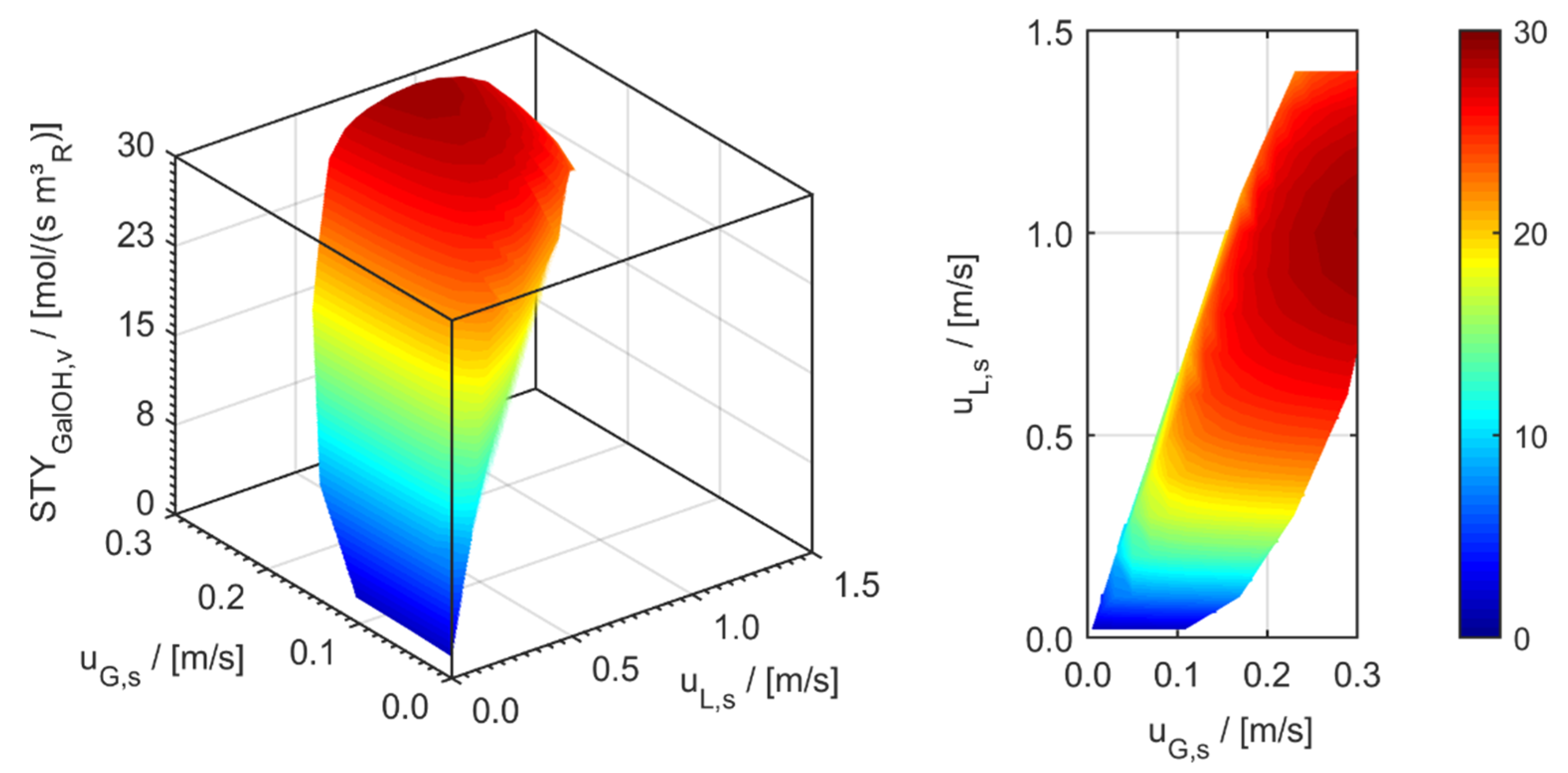
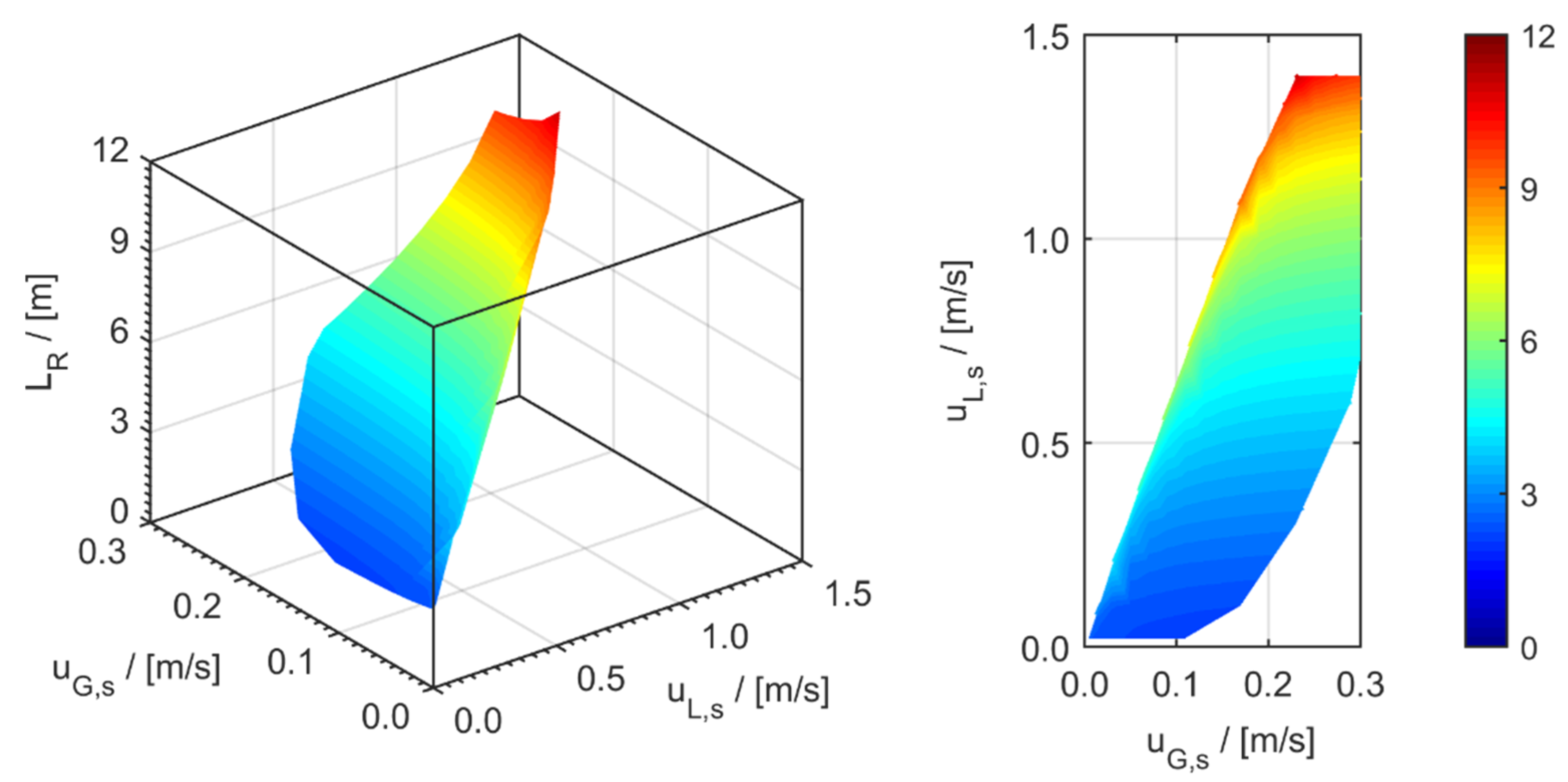

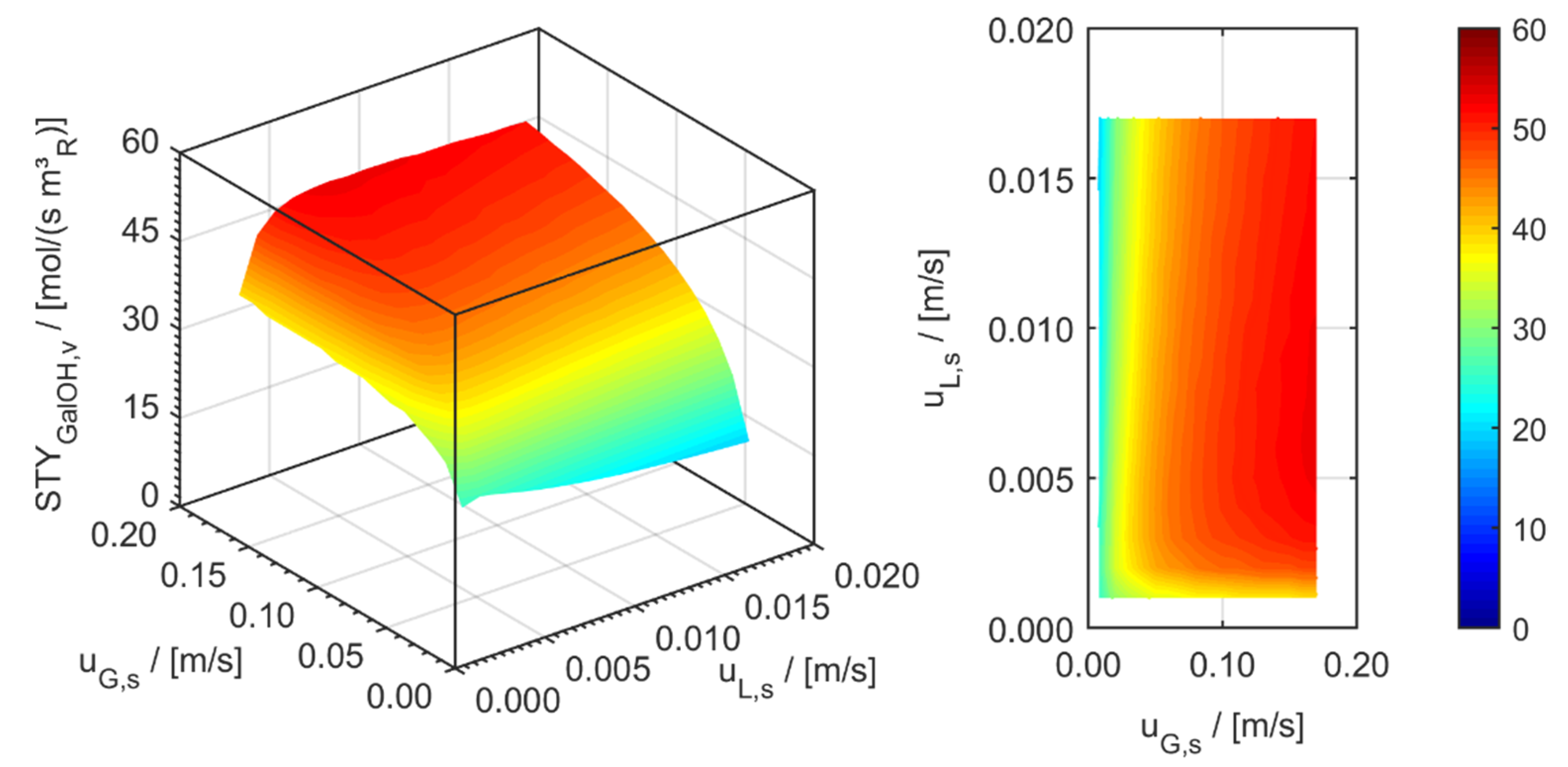
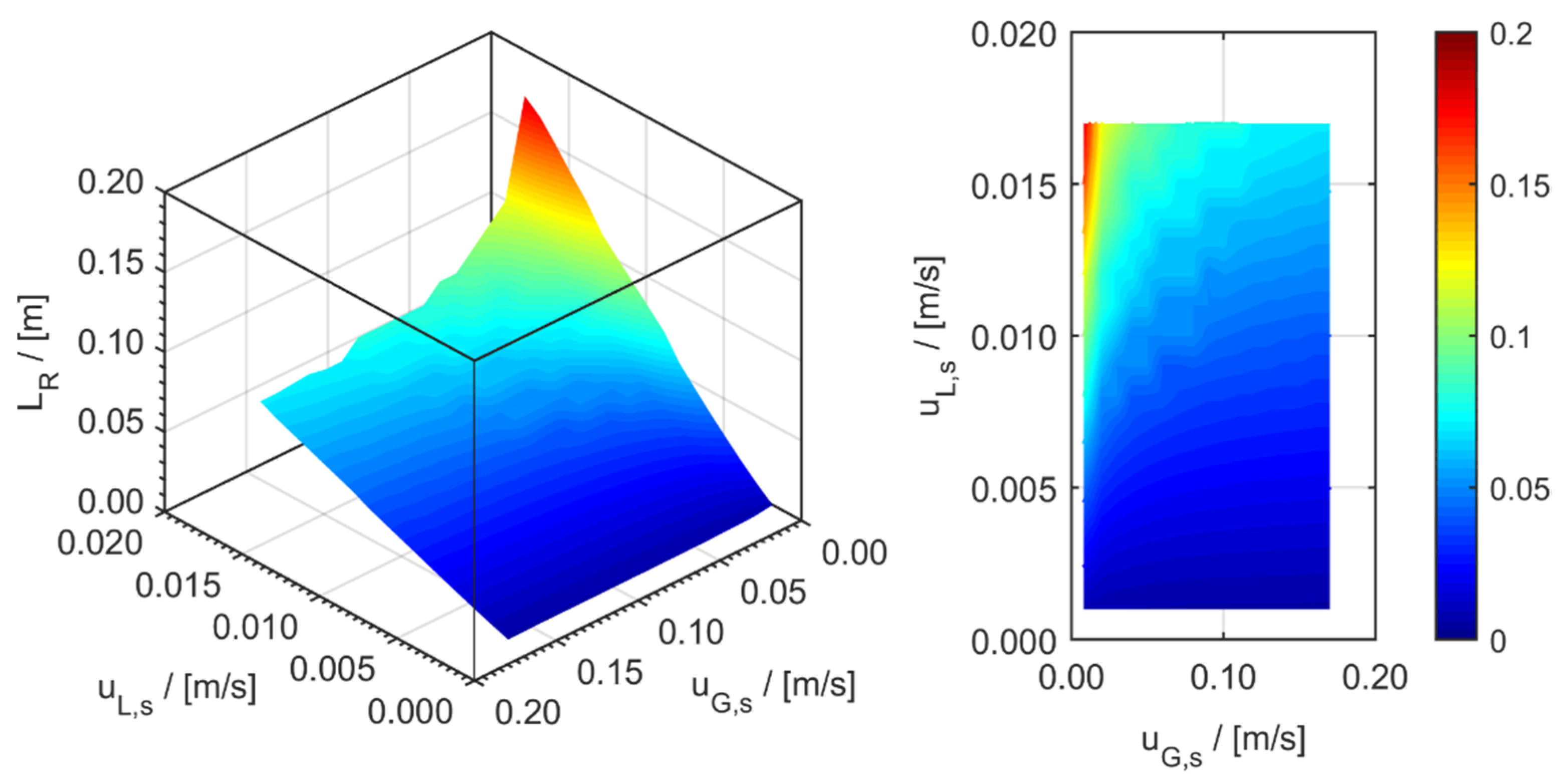
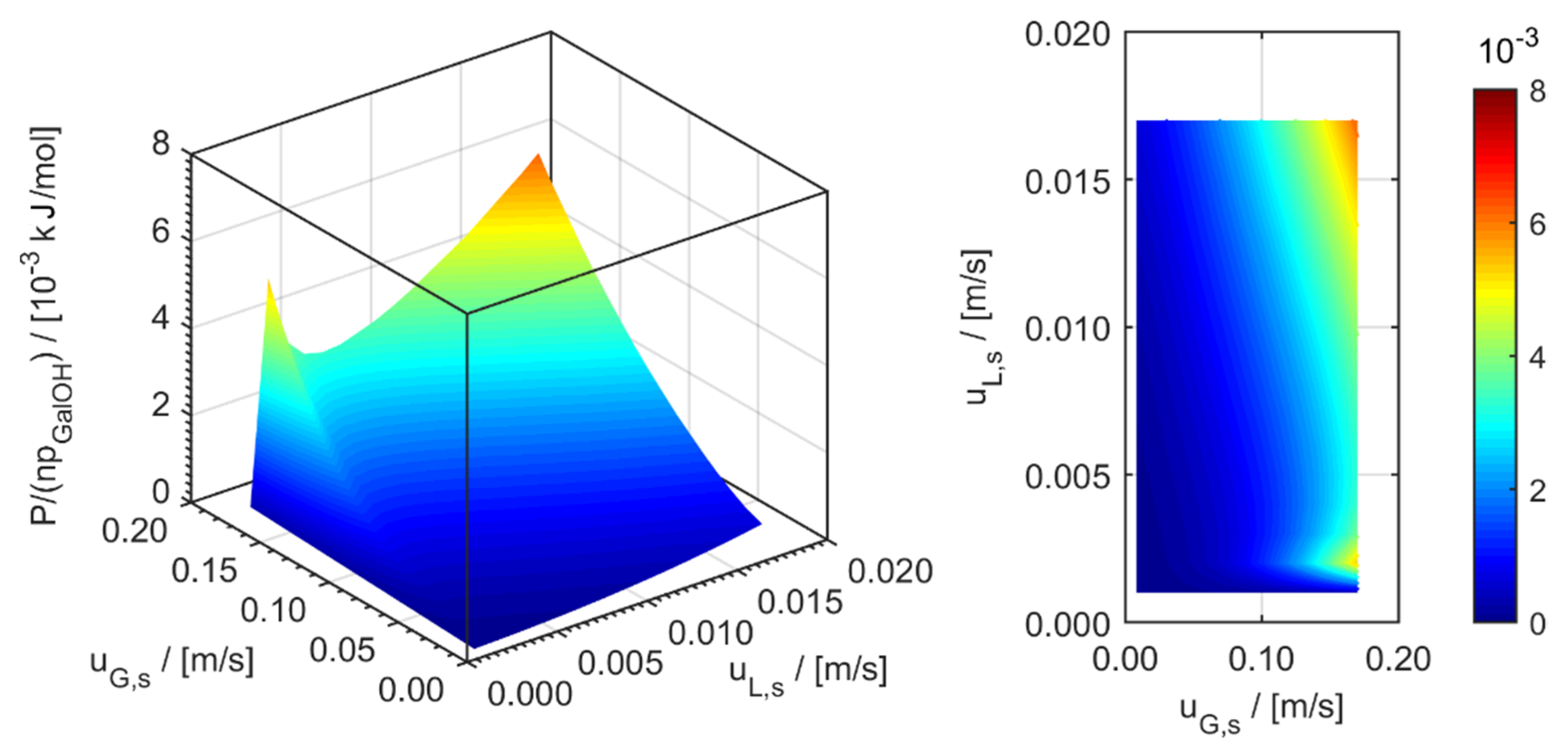
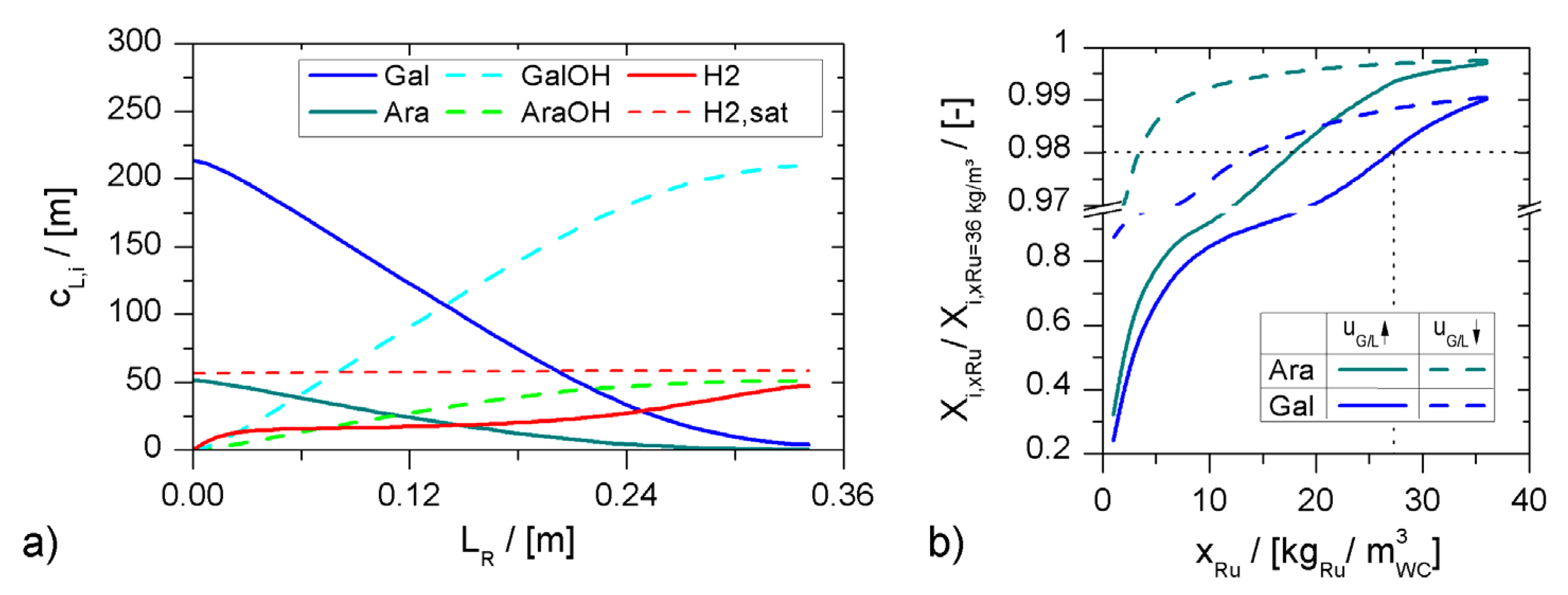
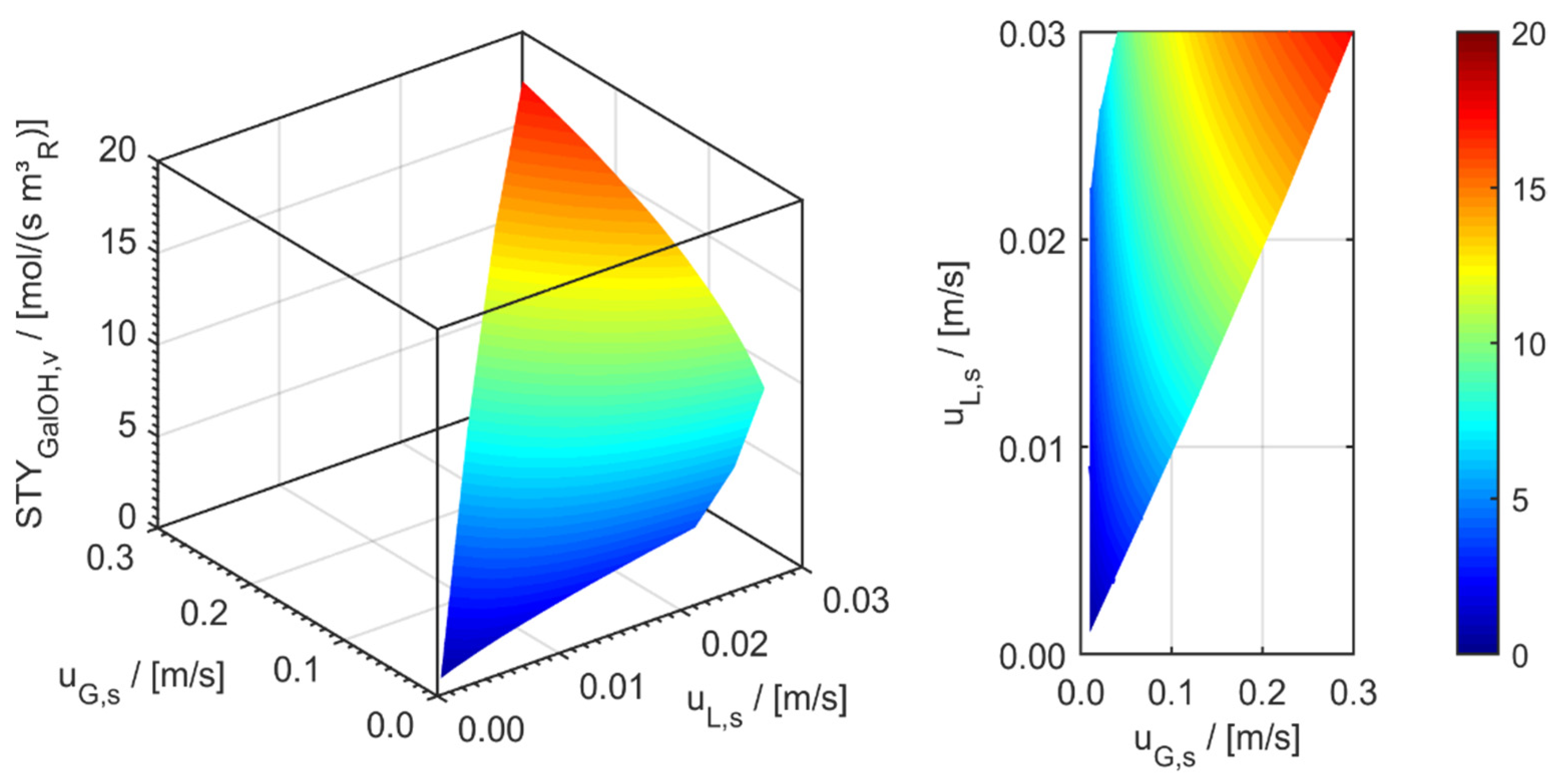

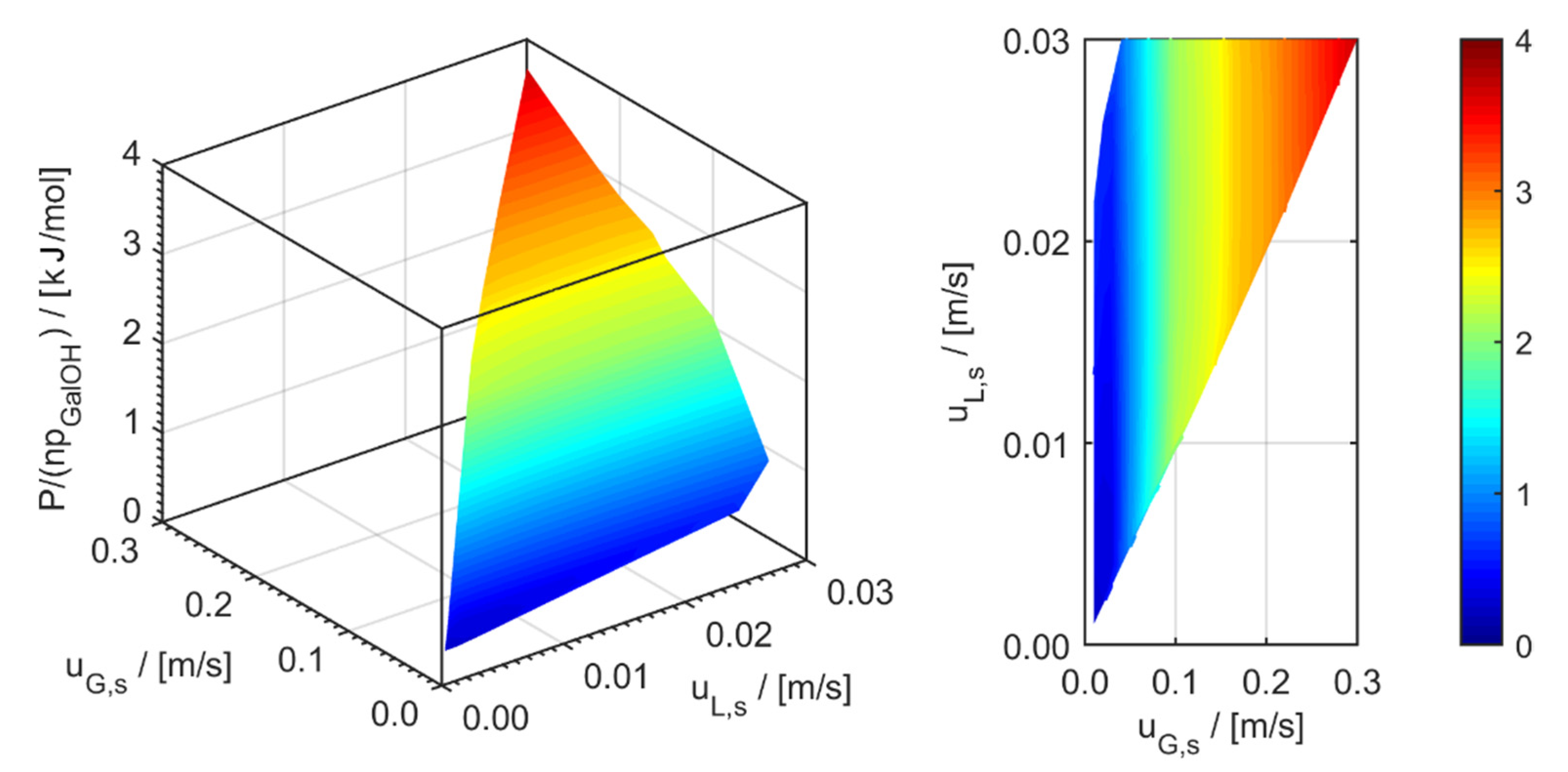

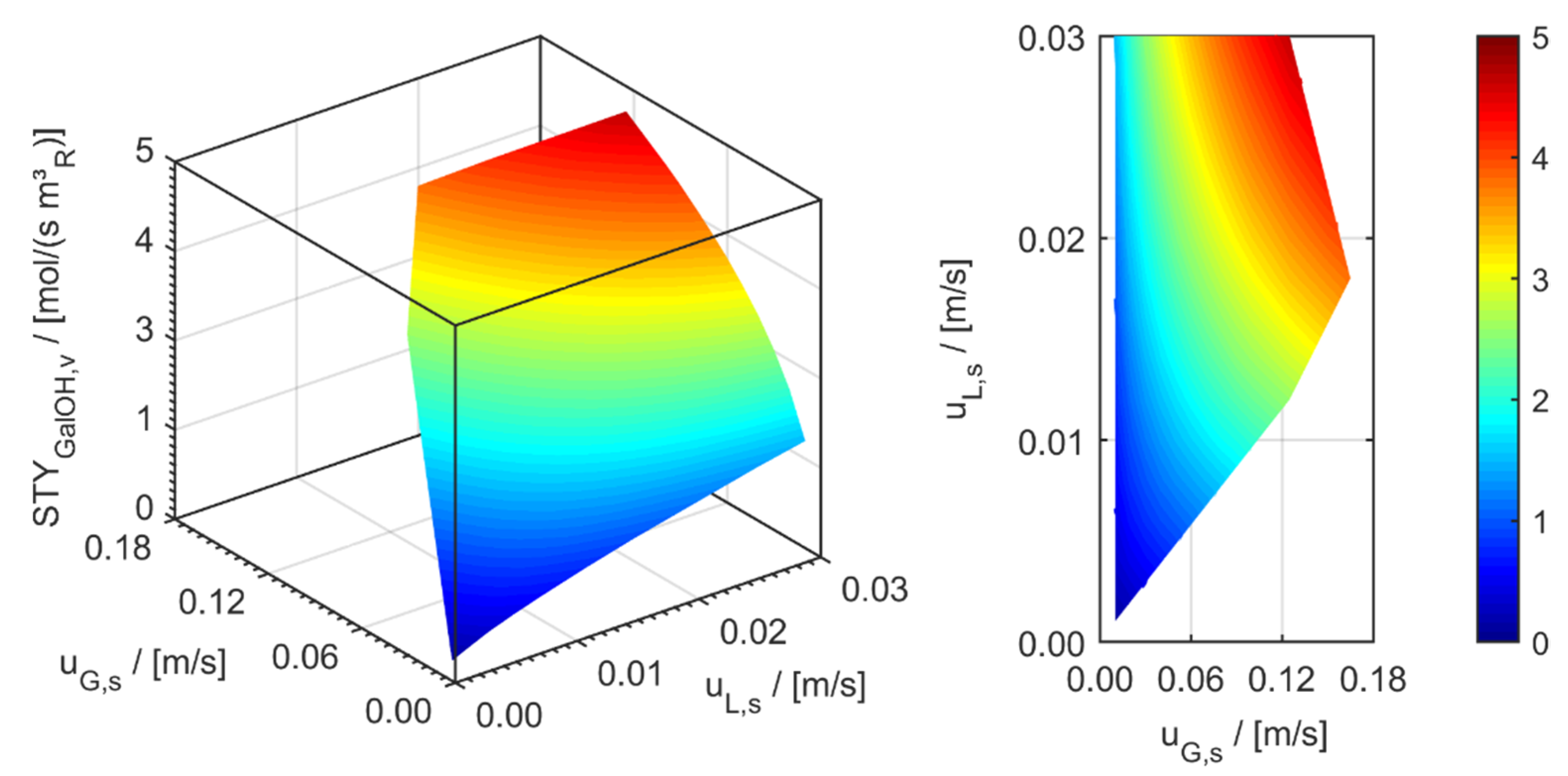
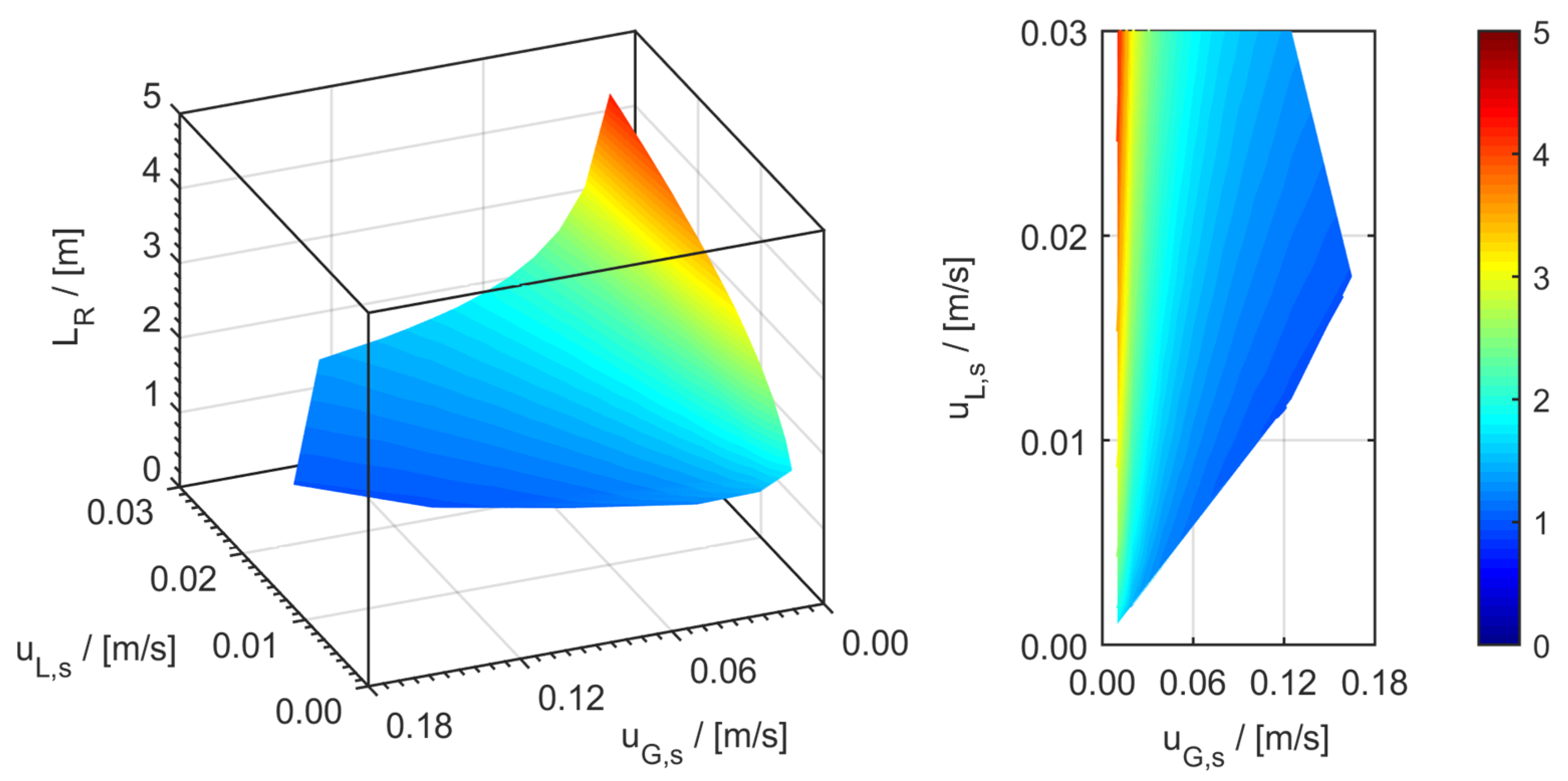

 minichannel wall reactor,
minichannel wall reactor,  minichannel reactor packed with particles,
minichannel reactor packed with particles,  minichannel reactor with foam packing, and trickle-bed reactors:
minichannel reactor with foam packing, and trickle-bed reactors:  conventional and
conventional and  miniaturised at flow conditions creating (a) maximal space-time yields, (b) minimal reactor lengths, and (c) minimal specific energy consumptions in the adiabatic hydrogenation of arabinose/galactose mixtures (pin = 6 MPa, Tin = 115 °C).
miniaturised at flow conditions creating (a) maximal space-time yields, (b) minimal reactor lengths, and (c) minimal specific energy consumptions in the adiabatic hydrogenation of arabinose/galactose mixtures (pin = 6 MPa, Tin = 115 °C).
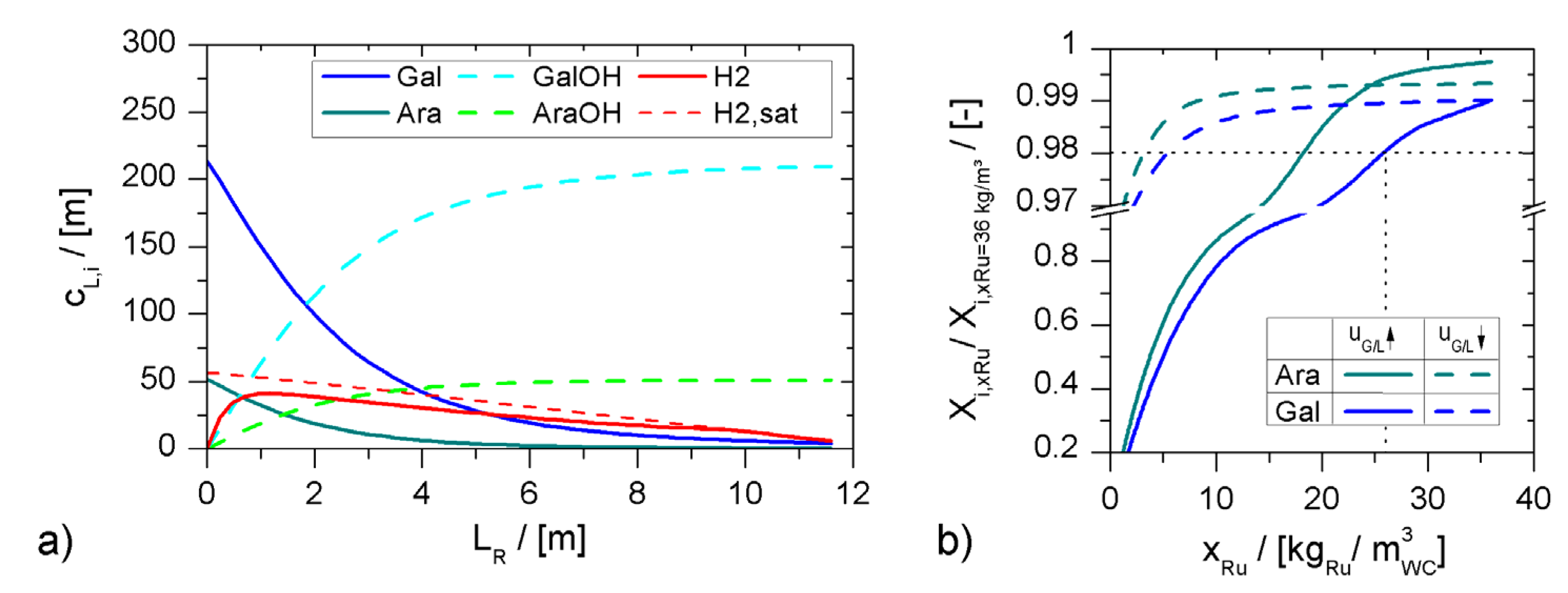
 minichannel wall reactor,
minichannel wall reactor,  minichannel reactor packed with particles,
minichannel reactor packed with particles,  minichannel reactor with foam packing, and trickle-bed reactors:
minichannel reactor with foam packing, and trickle-bed reactors:  conventional and
conventional and  miniaturised at flow conditions creating (a) maximal space-time yields, (b) minimal reactor lengths, and (c) minimal specific energy consumptions in the adiabatic hydrogenation of arabinose/galactose mixtures (pin = 6 MPa, Tin = 115 °C).
miniaturised at flow conditions creating (a) maximal space-time yields, (b) minimal reactor lengths, and (c) minimal specific energy consumptions in the adiabatic hydrogenation of arabinose/galactose mixtures (pin = 6 MPa, Tin = 115 °C).
| Parameter | Value | Relative Error (%) |
|---|---|---|
| Reactor 1 | Flow Condition for | ||||
|---|---|---|---|---|---|
| MCWR | Max. | 0.54 | |||
| Min. | |||||
| Min. | |||||
| Overall Min./Max. | |||||
| MCPR | Max. | ||||
| Min. | |||||
| Min. | |||||
| Overall Min./Max. | |||||
| MCFR | Max. | 52.9 | 6.96 | ||
| Min. | 38.9 | 5.11 | |||
| Min. | 25.1 | 3.29 | |||
| Overall Min./Max. | 52.9 | 6.96 | |||
| MTBR | Max. | 17.3 | 3.11 | 0.37 | 3.68 |
| Min. | 7.73 | 1.38 | 0.33 | 2.35 | |
| Min. | 0.40 | 0.07 | 0.53 | 0.28 | |
| Overall Min./Max. | 17.3 | 3.11 | 0.33 | 0.28 | |
| TBR | Max. | 4.65 | 3.64 | 1.37 | 0.15 |
| Min. | 2.58 | 2.02 | 0.98 | 0.15 | |
| Min. | 1.63 | 1.28 | 1.30 | 0.14 | |
| Overall Min./Max. | 4.65 | 3.64 | 0.98 | 0.14 |
| Criteria | Parameter | MCWR | MCPR | MCFR | TBR | MTBR |
|---|---|---|---|---|---|---|
| Catalyst 1 | Preparation | Lab-scale | Industrial | Lab-scale | Industrial | Industrial |
| Replacement | Moderate (exchange packing) | Moderate (exchange particles) | Moderate (exchange packing) | Simple | Simple | |
| Process 2 | Blocking risk | Small | Medium | Small | Small | High |
| Experience | Lab-scale | Lab-scale | Lab-scale | Industrial | Industrial |
| Reactor | ||||||
|---|---|---|---|---|---|---|
| MCWR | 20.5 | 47 | 5.8 | 12.9 | 8050 | 4.1 |
| MCPR | 29.2 | 33 | 6 | 10.6 | 5460 | 89.3 |
| MCFR | 52.9 | 18 | 0.02 | 108 | 188,000 | 0.03 |
| MTBR | 17.3 | 55 | 0.37 | 44 | - | 28.1 |
| 4.65 | 205 | 1.37 | 44 | - | 1.1 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Haase, S.; de Araujo Filho, C.A.; Wärnå, J.; Murzin, D.Y.; Salmi, T. Reactor Selection for Upgrading Hemicelluloses: Conventional and Miniaturised Reactors for Hydrogenations. Processes 2021, 9, 1558. https://doi.org/10.3390/pr9091558
Haase S, de Araujo Filho CA, Wärnå J, Murzin DY, Salmi T. Reactor Selection for Upgrading Hemicelluloses: Conventional and Miniaturised Reactors for Hydrogenations. Processes. 2021; 9(9):1558. https://doi.org/10.3390/pr9091558
Chicago/Turabian StyleHaase, Stefan, Cesar A. de Araujo Filho, Johan Wärnå, Dmitry Yu. Murzin, and Tapio Salmi. 2021. "Reactor Selection for Upgrading Hemicelluloses: Conventional and Miniaturised Reactors for Hydrogenations" Processes 9, no. 9: 1558. https://doi.org/10.3390/pr9091558
APA StyleHaase, S., de Araujo Filho, C. A., Wärnå, J., Murzin, D. Y., & Salmi, T. (2021). Reactor Selection for Upgrading Hemicelluloses: Conventional and Miniaturised Reactors for Hydrogenations. Processes, 9(9), 1558. https://doi.org/10.3390/pr9091558







